Abstract
Background
The number of elderly patients with pancreatic cancer is growing, however clinical data on the short-term outcomes, rate of adjuvant chemotherapy, and survival in these patients are limited and we therefore performed a nationwide analysis.
Methods
Data from the prospective Dutch Pancreatic Cancer Audit were analyzed, including all patients undergoing pancreatic cancer resection between January 2014 and December 2016. Patients were classified into two age groups: <75 and ≥75 years. Major complications (Clavien–Dindo grade 3 or higher), 90-day mortality, rates of adjuvant chemotherapy, and survival were compared between age groups. Factors associated with start of adjuvant chemotherapy and survival were evaluated with logistic regression and multivariable Cox regression analysis.
Results
Of 836 patients, 198 were aged ≥75 years (24%) and 638 were aged <75 years (76%). Median follow-up was 38 months (interquartile range [IQR] 31–47). Major complications (31% vs. 28%; p = 0.43) and 90-day mortality (8% vs. 5%; p = 0.18) did not differ. Adjuvant chemotherapy was started in 37% of patients aged ≥75 years versus 69% of patients aged <75 years (p < 0.001). Median overall survival (OS) was 15 months (95% confidence interval [CI] 14–18) versus 21 months (95% CI 19–24; p < 0.001). Age ≥75 years was not independently associated with OS (hazard ratio 0.96, 95% CI 0.79–1.17; p = 0.71), but was associated with a lower rate of adjuvant chemotherapy (odds ratio 0.27, 95% CI 0.18–0.40; p < 0.001).
Conclusions
The rate of major complications and 90-day mortality after pancreatic resection did not differ between elderly and younger patients; however, elderly patients were less often treated with adjuvant chemotherapy and their OS was shorter.
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-022-11831-7.
Pancreatic cancer is a devastating disease, frequently affecting older people. In 2019, nearly half of all patients with pancreatic ductal adenocarcinoma (PDAC) in The Netherlands were aged ≥75 years at the time of diagnosis.1 Due to aging of the population, combined with an increased life expectancy in industrial countries, the number of elderly patients with pancreatic cancer is growing.2
Optimal treatment for pancreatic cancer consists of surgical resection in combination with chemotherapy;3–5 however, pancreatic surgery is associated with high postoperative complication rates.6,7 Although it is commonly felt that age alone should not be a contraindication for resection of pancreatic cancer, surgeons are generally hesitant to perform these major surgical procedures in the elderly.8–13 Previous studies have suggested that older patients have a higher risk of major postoperative complications due to comorbid conditions and functional impairment.3,14–18 It has also been shown that older patients may be less likely to receive adjuvant chemotherapy due to frailty, even though chemotherapy is associated with improved survival.19–21 However, the independent impact of age on clinical outcomes remains controversial.8,22–27 Furthermore, most previous studies on pancreatic surgery in the elderly were performed in selected patients from single-center studies with small study populations and without correction for frailty.8,14,15,19,22,24–26,28–31 A reflection of daily clinical practice in terms of short- and long-term outcomes of elderly patients after resection for pancreatic cancer on a nationwide scale is lacking.
In The Netherlands, efforts have been made to improve outcomes after pancreatic cancer resection. In 2013, a nationwide clinical audit—the Dutch Pancreatic Cancer Audit (DPCA)—was established for quality assessment of perioperative care in pancreatic surgery.32 Over the last decade, pancreatic surgery has been centralized and regional partnerships have emerged. It is stated that patients benefit from centralization due to increased resection rates and reduced morbidity rates.3,33–35 Furthermore, multidisciplinary team meetings have been initiated to carefully screen patients on frailty and surgical risk, aiming to improve selection of patients for optimal treatment, while also paying attention to prehabilitation in order to get patients fit for surgery.36,37 It is likely that these improvements have also benefited elderly patients with pancreatic cancer. We therefore performed the current study with the aim to investigate short-term outcomes, the rate of adjuvant chemotherapy, and survival in elderly patients undergoing pancreatic cancer resection in a recent nationwide cohort in The Netherlands.
Methods
Study Design
This was a post hoc analysis of the DPCA prospective database. All patients undergoing resection for histologically proven PDAC between January 2014 and December 2016 in all 17 centers collaborating in the Dutch Pancreatic Cancer Group were included,38,39 including patients with resectable and borderline resectable PDAC. There were no exclusion criteria. We adhered to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.40
Data Collection
Prospective baseline and perioperative data were retrieved from the available prospective database. Data on ethnicity were not included in the database. Additional data on frailty characteristics, follow-up, treatment, and survival were collected retrospectively from hospital records. The Charlson Comorbidity Index (CCI) was calculated using the MDCalc CCI calculator,41 and TNM status was assessed according to the 8th Edition of the American Joint Committee on Cancer (AJCC) guidelines.42 Resection margins were considered positive if tumor cells were present within 1 mm of the resection margins, apart from the anterior surface.43 Frailty characteristics consisted of polypharmacy (use of five or more medicaments at the time of diagnosis),44 preoperative anemia (female hemoglobin <7.4 mmol/L, male hemoglobin <8.1 mmol/L),45 decreased renal function,46,47 CCI ≥244, body mass index (BMI) <18.5 or ≥31,23 and American Society of Anesthesiologists (ASA) score ≥3.44,48,49 A decreased renal function was, in terms of the preoperative estimated glomerular filtration rate (eGFR), defined as mild (60–89 mL/min/1.73 m2), mild to moderate (45–59 mL/min/1.73 m2), moderate to severe 30–44 mL/min/1.73 m2, or severe 15–29 mL/min/1.73 m2.46,47
Outcomes
Outcomes of interest were major complications, intensive care unit (ICU) readmission, 90-day mortality, rate of adjuvant chemotherapy (completion of at least one cycle), recurrence rate, disease-free survival (DFS), and overall survival (OS). Major complications were defined as Clavien–Dindo grade 3 or higher.50 PDAC recurrence had to be either pathologically proven or suspected through cross-sectional imaging, preferably confirmed by consensus at a multidisciplinary meeting. DFS was defined as the time from the date of resection to the date of diagnosis of PDAC recurrence, while OS was defined as the time from the date of surgery to either death from any cause or last follow-up. If survival data were missing, patients were censored at the date of last follow-up.
Statistical Analyses
Missing data were considered missing at random and were therefore managed by multiple imputation according to a Markov chain Monte Carlo method (5 imputations, 10 iterations).51 Parametric continuous variables were reported as mean with standard deviation (SD) and compared using the Student’s t-test; non-parametric continuous variables were presented as median with interquartile range (IQR) and compared using the Mann-Whitney U-test; and categorical variables were reported as frequencies and compared using the Chi-square test.
Patients were divided into two age groups: <75 and ≥75 years. In the DPCA, the median age of patients with PDAC in The Netherlands is 68 years, with the population aging over time.1,52 Therefore, this study defined patients aged over 75 years as the true elderly. Univariate analysis was performed to compare major complications, ICU readmission, and 90-day mortality between both age groups. OS and DFS were evaluated using the Kaplan–Meier analysis, compared using the log-rank test, and presented as median with 95% confidence intervals (CIs). To minimize the influence of postoperative mortality on the results of long-term survival, OS and DFS were also assessed in patients without 90-day mortality. The influence of age ≥ 75 years on OS was assessed using multivariable Cox proportional hazard analyses and reported as hazard ratios (HRs) with 95% CIs, adjusted for potential confounders. These included sex, BMI, preoperative serum carbohydrate antigen (CA) 19–9, frailty characteristics, location and size of the tumor, microscopic perineural invasion, tumor differentiation, and number of positive lymph nodes. Stratified analyses were performed for patients who received adjuvant chemotherapy and patients who did not. Multivariable logistic regression analyses, adjusted for potential confounders, were performed to assess the association between age ≥ 75 years and administration and completion of adjuvant chemotherapy. A sensitivity analysis for patients aged ≥ 80 years was also performed. Results are given as odds ratios (ORs) with 95% CIs. A two-tailed p-value < 0.05 was considered to indicate statistical significance. Statistics were performed using R version 1.3.1093 (Bell Laboratories, Windsor, NH, USA) with the ‘survival’, ‘ggplot’, and ‘mice’ packages.
Results
A total of 836 patients were included (electronic supplementary material [ESM] Appendix Table 1), of whom 638 (76%) were aged < 75 years and 198 (24%) were aged ≥ 75 years. In patients aged < 75 years, the median age was 66 years (IQR 58–70), and in patients aged ≥ 75 years, the median age was 78 years (IQR 76–80). Patient and tumor characteristics of both groups are summarized in Table 1. The median overall follow-up period was 38 months (IQR 31–47).
Table 1.
Patient, tumor, and treatment characteristics of 638 patients aged <75 years and 198 patients aged ≥75 years after resection for pancreatic cancer
| Age < 75 years [N = 638] |
Age ≥ 75 years [N = 198] |
p-valuea | |
|---|---|---|---|
| Male sex | 353 (55) | 106 (54) | 0.72 |
| BMI < 18.5 or ≥ 31 | 74 (12) | 17 (9) | 0.28 |
|
Charlson Comorbidity Index < 2 ≥ 2 |
390 (61) 248 (39) |
82 (41) 116 (59) |
<0.001 |
|
ASA classification I–II III–IV |
512 (80) 126 (20) |
131 (66) 67 (34) |
<0.001 |
|
ECOG performance score at primary diagnosis 0–1 2–4 |
563 (88) 75 (12) |
164 (83) 34 (17) |
0.05 |
| Preoperative serum log CA19-9 [median (IQR)] | 120 (30–480) | 151 (29–539) | 0.09 |
| Preoperative bilirubin, µmol/L [median (IQR)] | 24 (9–89) | 23 (9–75) | 0.91 |
|
Preoperative eGFR, mL/min/1.73 m2 Normal (> 90) Mildly decreased (60–89) Mildly to moderately decreased (45–59) Moderately to severely decreased (30–45) Severely decreased (<30) |
193 (30) 337 (53) 73 (11) 32 (5) 3 (0) |
41 (21) 125 (63) 20 (10) 10 (5) 3 (1) |
0.03 |
| Preoperative anemia | 335 (52) | 106 (53) | 0.88 |
|
Number of medicaments < 5 ≥ 5 |
403 (63) 235 (37) |
107 (54) 91 (46) |
0.03 |
| Neoadjuvant chemotherapy | 53 (8) | 9 (5) | 0.11 |
|
Method of surgery Open Laparoscopic Robot |
582 (91) 51 (8) 5 (1) |
175 (88) 22 (11) 1 (1) |
0.38 |
|
Type of resection Pancreatoduodenectomy Distal pancreatectomy Total pancreatectomy |
523 (82) 89 (14) 26 (4) |
159 (80) 31 (16) 8 (4) |
0.83 |
|
Tumor location Head Body/tail |
543 (85) 95 (15) |
166 (84) 32 (16) |
0.75 |
| Vascular resection | 175 (27) | 53 (27) | 0.88 |
| Microscopic perineural invasion | 554 (87) | 172 (87) | 0.99 |
| Microscopic lymphovascular invasion | 421 (66) | 122 (62) | 0.31 |
| Tumor size, cmb [mean ± SD] | 3.2 ± 1.3 | 3.2 ± 1.1 | 0.75 |
|
Tumor differentiation Well/moderate Poor |
433 (68) 204 (32) |
130 (71) 58 (29) |
0.32 |
| Total number of resected lymph nodes [median (IQR)] | 16 (11–21) | 12 (9–18) | <0.001 |
| Number of positive lymph nodes [median (IQR)] | 2 (0–4) | 2 (0–4) | 0.57 |
|
TNM stage, AJCC 7th edition ≤ Stage 2a ≥ Stage 2b |
62 (10) 576 (90) |
16 (8) 182 (92) |
0.53 |
|
Resection margin status R0 >1.0 mm R1 ≤ 1.0 mm |
325 (51) 313 (49) |
86 (44) 112 (56) |
0.08 |
Data are expressed as n (%) unless otherwise specified
Percentages may not sum to 100 because of rounding
aThe data were statistically analyzed between both groups using the Chi-square test for categorical variables and Fisher’s exact test when groups consisted of fewer than five patients. The t-test was used for normally distributed continuous variables, and the Wilcoxon rank test was used for non-normally distributed continuous variables
bMaximum diameter of the tumor
SD standard deviation, BMI body mass index, ASA American Society of Anesthesiologists, ECOG Eastern Cooperative Oncology Group, CA19-9 carbohydrate antigen 19-9, IQR interquartile range, eGFR estimated glomerular infiltration rate, AJCC American Joint Committee on Cancer
Short-Term Outcomes
In 62 patients (31%) aged ≥ 75 years and 179 patients (28%) aged < 75 years, one or more major complications occurred (p = 0.43) [Table 2]. Readmission to the ICU was necessary for 40 patients (20%) versus 82 patients (13%) [p = 0.01]. In addition, 90-day mortality occurred in 16 patients (8%) versus 33 patients (5%) [p = 0.18]. In a post hoc multivariable logistic regression analysis adjusted for frailty, sex, BMI, and location and size of the tumor, age (< 75 years vs. ≥ 75 years) was also not associated with major complications or 90-day mortality (OR 1.07, 95% CI 0.74–1.53, p = 0.72; and 1.39, 95% CI 0.72–2.69, p = 0.32, respectively).
Table 2.
Univariate analysis of short- and long-term outcomes of 638 patients aged <75 years and 198 patients aged ≥75 years after resection for pancreatic cancer
| Age < 75 years [N = 638] |
Age ≥ 75 years [N = 198] |
p-valuea | |
|---|---|---|---|
| Major complications | 179 (28) | 62 (31) | 0.43 |
| Length of hospital stay, days [median (IQR)] | 11 (8–15) | 14 (9–20) | < 0.001 |
| Adjuvant chemotherapyc | 429 (69) | 71 (37) | < 0.001 |
|
Type of adjuvant chemotherapyb,d Gemcitabine monotherapy FOLFIRINOX Other Unknown |
408 (95) 5 (1) 2 (0) 10 (2) |
66 (93) 1 (1) 1 (1) 0 (0) |
< 0.001 |
| No. of cycli of adjuvant chemotherapy [median (IQR)]b,e | 6 (4–6) | 6 (3–6) | 0.009 |
| ≥80% of prescribed cycles completedb,e | 288 (73) | 36 (64) | 0.23 |
| 90-day mortality | 33 (5) | 16 (8) | 0.18 |
| Overall survival, months [median (95% CI)] | 21 (19–24) | 15 (14–18) | < 0.001 |
| Disease-free survival, [median (95% CI)]f | 16 (14–17) | 12 (10–14) | < 0.001 |
| Recurrencef | 435 (81) | 122 (81) | 0.99 |
Data are expressed as n (%) unless otherwise specified
Percentages may not sum to 100 because of rounding
IQR nterquartile range, FOLFIRINOX 5-fluorouracil, leucovorin, irinotecan, oxaliplatin chemotherapy, CI confidence interval
aThe data were statistically analyzed between both groups using the Chi-square test for categorical variables and Fisher’s exact test when groups consisted of fewer than five patients. The t-test was used for normally distributed continuous variables, and the Wilcoxon rank test was used for non-normally distributed continuous variables
bCalculated in a subset of patients who started with adjuvant chemotherapy (429 patients aged < 75 years vs. 71 patients aged ≥ 75 years)
c28 missing
d7 missing
e49 missing
f145 missing
Adjuvant Chemotherapy
Adjuvant chemotherapy was started in 71 patients aged ≥ 75 years (37%) versus 429 patients aged < 75 years (69%) [p < 0.001]. Once chemotherapy had commenced, ≥80% of the prescribed cycles were completed in 36 (64%) versus 288 (73%) patients (p = 0.23) [Table 2]. Multivariable analysis showed that age ≥ 75 years was independently associated with start of adjuvant chemotherapy (OR 0.27, 95% CI 0.18–0.40; p < 0.001) [Table 3]. Furthermore, adjuvant chemotherapy was less often administered to patients with a CCI score ≥ 2 (OR 0.62, 95% CI 0.43–0.90; p = 0.01) or major complications (OR 0.21, 95% CI 0.15–0.30; p < 0.001). With regard to frailty, significantly more elderly who did not receive adjuvant chemotherapy had high CCI (≥ 2) and ASA (≥ 3) scores compared with elderly who did receive adjuvant chemotherapy (68 vs. 44%, and 41 vs. 20%, respectively) [ESM Appendix Table 2]. Once started with adjuvant chemotherapy, age ≥ 75 years was not significantly associated with the completion of ≥ 80% of the prescribed cycles (OR 0.69, 95% CI 0.37–1.29; p = 0.24) [Table 3]. Stratified Kaplan–Meier curves for older versus younger patients who started with adjuvant chemotherapy showed an OS of 25 months (95% CI 18–37) and 28 months (95% CI 25–31) [p = 0.18], respectively, and a DFS of 17 months (95% CI 13–25) and 19 months (95% CI 16–21) [p = 0.069], respectively (Figs. 3 and 4).
Table 3.
Multivariable logistic regression analysis to assess the independent impact of age ≥75 years on start and completion ≥80% of adjuvant chemotherapy in 836 patients after resection of pancreatic cancer
| Start adjuvant chemotherapy | Completion ≥80% of adjuvant chemotherapy | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age (≥75 vs. <75 years) | 0.27 | 0.18–0.40 | < 0.001 | 0.69 | 0.37–1.29 | 0.24 |
| Sex (male vs. female) | 0.32 | 0.93–1.87 | 0.12 | 0.65 | 0.40–1.06 | 0.09 |
| Charlson Comorbidity Index (≥2 vs. <2) | 0.62 | 0.43–0.90 | 0.01 | 0.68 | 0.42–1.11 | 0.12 |
| Polypharmacia (≥5 vs. <5 medicaments) | 0.79 | 0.55–1.14 | 0.21 | 1.16 | 0.70–1.92 | 0.56 |
| Anemia (yes vs. no) | 0.88 | 0.53–1.47 | 0.63 | 1.13 | 0.58–2.18 | 0.73 |
| BMI (<18.5 or ≥31 vs. 18.5–31) | 1.28 | 0.75–2.20 | 0.37 | 0.75 | 0.38–1.48 | 0.41 |
|
Renal dysfunction, eGFR (mL/min/1.73 m2) Mildly decreased (60–89) Mildly to moderately decreased (45–59) Moderately to severely decreased (30–45) Severely decreased (<30) |
0.99 0.78 0.90 1.04 |
0.61–1.63 0.39–1.58 0.28–2.83 0.04–24.66 |
0.98 0.49 0.86 0.98 |
0.73 0.85 1.13 – |
0.39–1.35 0.33–2.20 0.12–10.29 – |
0.33 0.74 0.91 0.99 |
| Major complications (yes vs. no) | 0.21 | 0.15–0.30 | < 0.001 | 1.80 | 0.98–3.30 | 0.06 |
| Location tumor (body/tail vs. head) | 0.84 | 0.53–1.32 | 0.45 | 1.44 | 0.71–2.90 | 0.31 |
| Tumor size | 0.96 | 0.83–1.10 | 0.52 | 0.89 | 0.74–1.08 | 0.24 |
| Tumor differentiation (poor vs. well/moderate) | 0.78 | 0.53–1.15 | 0.22 | 0.99 | 0.60–1.63 | 0.98 |
| Preoperative log CA19-9 | 0.98 | 0.89–1.08 | 0.67 | 0.97 | 0.86–1.11 | 0.69 |
| Positive resected lymph nodes | 1.00 | 0.95–1.05 | 0.92 | 1.00 | 0.93–1.08 | 0.93 |
| Resection margin status (R1 vs. R0) | 0.71 | 0.50–1.01 | 0.05 | 1.53 | 0.97–2.41 | 0.07 |
| Neural invasion (yes vs. no) | 0.94 | 0.50–1.74 | 0.84 | 0.98 | 0.50–1.91 | 0.95 |
OR odds ratio, CI confidence interval, BMI body mass index, eGFR estimated glomerular filtration rate, CA19-9 carbohydrate antigen 19-9
Fig. 3.
Overall survival in 71 patients aged ≥ 75 years and in 429 patients aged < 75 years after resection for adjuvant chemotherapy
Fig. 4.
Disease-free survival in 71 patients aged ≥ 75 years and in 429 patients aged < 75 years after resection for adjuvant chemotherapy
Disease Recurrence and Survival
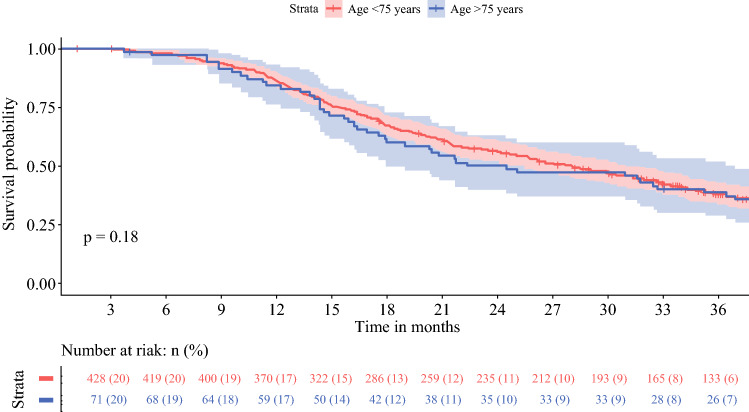
Both age groups developed recurrence in 81% of patients (122 patients ≥75 years of age versus 435 patients <75 years of age; p = 0.99) [Table 2]. However, the median DFS was 12 months (95% CI 10–14 months) for patients aged ≥75 years versus 16 months (95% CI 14–17 months) for patients aged <75 years (p < 0.001). OS was 15 months (95% CI 14–18 months) and 21 months (95% CI 19–24 months) for patients aged ≥75 and <75 years, respectively (p < 0.001) [Figs. 1 and 2].
Fig. 1.
Overall survival of 638 patients aged < 75 years and 198 patients aged ≥ 75 years after resection for pancreatic cancer
Fig. 2.
Disease-free survival of 638 patients aged < 75 years and 198 patients aged ≥ 75 years after resection for pancreatic cancer
In the analysis excluding patients with 90-day mortality, the DFS was 12 months (95% CI 11–14 months) for patients aged ≥ 75 years and 16 months (95% CI 14–17 months) for patients aged < 75 years (p < 0.001), while the OS was 17 months (95% CI 14–20 months) and 23 months (95% CI 21–26 months) for older and younger patients, respectively (p < 0.001).
When adjusted for potential confounders, including frailty characteristics, age ≥ 75 years was not independently associated with either DFS (HR 0.99, 95% CI 0.79–1.23; p = 0.90) or OS (HR 0.96, 95% CI 0.79–1.17; p = 0.71) [Table 4]. Major complications were associated with lower OS (HR 1.39, 95% CI 1.17–1.66; p < 0.001). Certain tumor characteristics (tumor size, tumor differentiation, serum CA19-9, number of resected (positive) lymph nodes, resection margin status, and neural invasion) were also significantly associated with lower DFS and OS (Table 4). Start of adjuvant chemotherapy was associated with improved DFS (HR 0.56, 95% CI 0.46–0.68; p < 0.001) and OS (HR 0.45, 95% CI 0.37–0.53).
Table 4.
Multivariable Cox regression analysis to assess the independent impact of age ≥ 75 years on overall survival and disease-free survival in 836 patients after resection of pancreatic cancer
| Overall survival | Disease-free survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (≥ 75 vs. < 75 years) | 0.96 | 0.79–1.17 | 0.71 | 0.99 | 0.79–1.23 | 0.90 |
| Sex (male vs. female) | 0.95 | 0.80–1.12 | 0.51 | 0.93 | 0.78–1.12 | 0.45 |
| Charlson Comorbidity Index (≥ 2 vs. < 2) | 1.10 | 0.91–1.32 | 0.33 | 1.10 | 0.90–1.33 | 0.36 |
| Polypharmacia (≥ 5 vs. < 5 medicaments) | 0.94 | 0.78–1.13 | 0.49 | 0.92 | 0.75–1.12 | 0.40 |
| Anemia (yes vs. no) | 1.13 | 0.96–1.34 | 0.15 | 0.99 | 0.83–1.19 | 0.95 |
| BMI (< 18.5 or ≥31 vs. 18.5–31) | 1.10 | 0.85–1.43 | 0.48 | 0.99 | 0.75–1.30 | 0.92 |
|
Renal dysfunction, eGFR (mL/min/1.73 m2) Mildly decreased (60–89) Mildly to moderately decreased (45–59) Moderately to severely decreased (30–45) Severely decreased (< 30) |
1.01 0.94 0.92 1.91 |
0.84–1.21 0.71–1.26 0.62–1.35 0.74–4.96 |
0.93 0.69 0.66 0.18 |
1.01 0.90 0.89 0.94 |
0.83–1.22 0.67–1.23 0.59–1.35 0.25–3.60 |
0.95 0.52 0.59 0.93 |
| Major complications (yes vs. no) | 1.39 | 1.17–1.66 | < 0.001 | 1.08 | 0.89–1.31 | 0.46 |
| Location tumor (body/tail vs. head) | 0.98 | 0.77–1.24 | 0.87 | 0.93 | 0.72–1.20 | 0.57 |
| Tumor size | 1.13 | 1.06–1.21 | < 0.001 | 1.14 | 1.06–1.22 | < 0.001 |
| Tumor differentiation (poor vs. well/moderate) | 1.39 | 1.16–1.65 | < 0.001 | 1.40 | 1.16–1.68 | < 0.001 |
| Preoperative log CA19-9 | 1.05 | 1.01–1.09 | 0.03 | 1.07 | 1.02–1.11 | < 0.01 |
| Positive resected lymph nodes | 1.06 | 1.04–1.09 | < 0.001 | 1.08 | 1.05–1.11 | < 0.001 |
| Resection margin status (R1 vs. R0) | 1.27 | 1.08–1.50 | < 0.01 | 1.34 | 1.13–1.60 | < 0.001 |
| Neural invasion (yes vs. no) | 1.71 | 1.29–2.26 | < 0.001 | 1.60 | 1.20–2.13 | < 0.01 |
| Adjuvant chemotherapy (yes vs. no) | 0.45 | 0.37–0.53 | < 0.001 | 0.56 | 0.46–0.68 | < 0.001 |
HR hazard ratio, CI confidence interval, BMI body mass index, eGFR estimated glomerular filtration rate
Sensitivity Analysis Octogenerians
In addition, a sensitivity analysis of all patients aged ≥80 years was performed. Results of the univariate analysis of 53 patients aged ≥80 years versus 783 patients aged <80 years are presented in ESM Appendix Table 3. In 16 patients (30%) aged ≥80 years, one of more major complications occurred compared with 225 patients (29%) aged < 80 years (p = 0.94). Ninety-day mortality was 13% (n = 7) in the older patients compared with 5% (n = 42) in the younger patients (p = 0.03). In the multivariable logistic regression analysis, assessed for frailty, age was not independently associated with 90-day complication-related mortality (ESM Appendix Table 4). Adjuvant chemotherapy was started in 5 patients (10%) aged ≥80 years and 495 patients (65%) aged <80 years (p < 0.001). None of the older patients completed ≥ 80% of the prescribed cycles. Patients aged ≥80 years showed a median DFS of 10 months (95% CI 7–20 months) compared with 15 months (95% CI 14–16 months) in the younger patients (p < 0.01). Median OS was 14 months (95% CI 12–20 months) and 20 months (95% CI 18–22 months) [p < 0.001], respectively.
Discussion
This nationwide study found that short-term outcomes after pancreatic resection, including the incidence of major complications and 90-day mortality, were not significantly different for patients aged ≥ 75 years compared with younger patients; however, long-term survival was shorter in the elderly patients. It was also observed that elderly less often received adjuvant chemotherapy after resection for pancreatic cancer.
The evidence for beneficial outcomes after resection for elderly patients with pancreatic cancer is not straightforward. Most of the studies reporting on elderly are performed in selected patients from single-center studies with small study groups, and the representativeness of these outcomes in a general population can be questioned.8,14,15,19,22,24–26,28–31 Furthermore, the direct impact of age as an independent predictor for short-term postsurgical outcomes remains controversial.8,22–27 In the current study, the rates of major complications and 90-day mortality were lower compared with previous studies, and not significantly different between both age groups.8,14,15,19,24,25,29–31 It appears that nowadays the risks of performing pancreatic cancer surgery in patients aged ≥ 75 years are comparable with that of younger patients, also on a nationwide scale.
Equally important, the impact of age on long-term oncological outcomes after resection of pancreatic cancer remains ambiguous. Previous studies on OS in elderly reported no statistically significant difference between older and younger patients (median 9–26 vs. 12–24 months), and age was not independently associated with OS in multivariable analysis.8, 15,19,24,31 Moreover, DFS was comparable between both age groups (median 7–13 vs. 15 months).19,53 Our results show significant differences in median OS and DFS for patients aged ≥ 75 years when compared with patients aged <75 years, i.e. 15 versus 21 months (p < 0.001) and 12 versus 16 months (p < 0.001). This difference might be explained by the fact that consecutive patients were included, as the nationwide registry is obligatory and has been validated so as to not miss any cases. Consequently, the risk of selection bias in this study has been decreased. Moreover, the relatively low rate of adjuvant chemotherapy in elderly that was observed in this cohort might explain the shorter long-term survival. In terms of frailty, this could be explained by the worse CCI and ASA scores in elderly patients who did not receive chemotherapy in the adjuvant setting. However, more importantly, it should be considered that the decision whether or not to start with adjuvant chemotherapy in elderly is made differently with regard to their life expectancy compared with younger patients. In this perspective, not all elderly patients may want to undergo adjuvant treatment. On the other hand, in elderly who did receive adjuvant chemotherapy, OS and DFS were comparable with that in younger patients who also received adjuvant chemotherapy. Most patients in this cohort were treated with adjuvant gemcitabine. With current 5-fluorouracil, leucovorin, irinotecan, oxaliplatin (FOLFIRINOX) chemotherapy regimens, survival outcomes are improving;54 however, FOLFIRINOX is associated with considerable more toxicity than gemcitabine.54 For frail elderly patients, reduced-dose chemotherapy or the use of modified FOLFIRINOX could provide a solution.55,56 To increase the potential for receiving chemotherapeutical regimens, a neoadjuvant chemotherapy approach could also be a suitable alternative for this patient group, given that they were considered to be fit enough for pancreatic resection.57–59
Previous studies have reported that frailty is a prognostic factor for postoperative morbidity and mortality after major abdominal surgery.60,61 After pancreatic resection, it has also been shown that frailty is associated with an increased incidence of major complications and death, as well as worse survival outcomes.36,49,62,63 Several validated scoring systems can be used to assess frailty, based on a multidimensional approach of physical, mental, and social status.64,65 In this study, we used frailty characteristics derived from the physical domain, since preoperative mental and social assessments were not yet standardized in clinical practice during the study period.23,44–49 Evaluation of outcomes with regard to a complete preoperative frailty assessment could be the focus for future research.
The analysis presented in this study, using a multicenter, nationwide prospective patient cohort, provides valuable insights into the short- and long-term outcomes of elderly after pancreatic resection in a recent real-world patient population. The inclusion period (2014–2016) comprises a period in which nationwide improvements in perioperative care for pancreatic cancer patients have been implemented in The Netherlands. For less physically fit patients, prehabilitation, i.e. preoperative exercise training to optimize functional deficits, nutritional interventions, psychological support, and coaching towards lifestyle changes, has been shown to reduce the risk of postoperative morbidity and is more frequently applied.36,37 Furthermore, it has been suggested that treatment at specialized centers and accounting for comorbidities in the decision-making process leads to improved outcomes for elderly.14,22,23,25 A previous Dutch study demonstrated an increase in pancreatic resections among elderly patients (≥ 75 years) over a time period between 2005 and 2013, resulting in decreased postoperative mortality in high-volume hospitals.33 Therefore, this study concluded that elderly could benefit from centralization when undergoing pancreatic resection.
This study has some limitations. First, data regarding elderly patients with resectable PDAC who did not undergo a resection, including insights into the decision-making process, were not available. Second, although a prospective database was used for baseline and perioperative data, data on follow-up and recurrence treatment were collected retrospectively; hence, only objective characteristics on physical frailty could be collected. It was not possible to obtain detailed information on mental and social frailty scores because this was not always reported in the electronic patient files. Nevertheless, in contrast to most studies, we did adjust for the frailty characteristics that were available. Third, the definition of true elderly remains uncertain. In this study, patients aged 75 years or older were assumed as the true elderly, however, different cut-offs in age have been suggested.66 Some studies propose octogenerians (aged ≥ 80 years) as the true elderly. We therefore performed a sensitivity analysis in octogenerians and the results were in line with the main analyses. Although the rate of postoperative complications did not differ, there was a marked increase in 90-day mortality for patients aged ≥ 80 years. In multivariable logistic regression analysis, age was not independently associated with 90-day complication-related mortality when assessed for frailty. This suggests that failure to rescue rates may be increased in octogenerians due to frailty, and caution should be maintained in the decision making on resection in these patients. Fourth, considerations in the shared decision-making process that led to either the start or omission of adjuvant chemotherapy could not always be identified accurately. This could have given more insights into the reasons to refrain from adjuvant treatment in elderly.
Conclusion
Following recent advancements in pancreatic cancer care, short-term outcomes after resection for pancreatic cancer did not differ between older and younger patients; however, only one-third of the elderly received adjuvant chemotherapy. Survival was shorter in elderly patients. These real-life data from a nationwide, multicenter cohort provide new insights for future shared-decision making on surgery and adjuvant chemotherapy for pancreatic cancer in elderly patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This research did not receive any grants from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
Anne Claire Henry, Thijs J. Schouten, Lois A. Daamen, Marieke S. Walma, Peter Noordzij, Geert A. Cirkel, Maartje Los, Marc G. Besselink, Olivier R. Busch, Bert A. Bonsing, Koop Bosscha, Ronald M. van Dam, Sebastiaan Festen, Bas Groot Koerkamp, Erwin van der Harst, Ignace H.J.T. de Hingh, Geert Kazemier, Mike S. Liem, Vincent E. de Meijer, Vincent B. Nieuwenhuijs, Daphne Roos, Jennifer M.J. Schreinemakers, Martijn W.J. Stommel, I. Quintus Molenaar, and Hjalmar C. van Santvoort have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
I. Quintus Molenaar and Hjalmar C. van Santvoort share senior authorship.
References
- 1.Netherlands Cancer Registry (NCR): Dutch cancer figures. 2019. Available at: http://www.cijfersoverkanker.nl/. Accessed 10 Sep 2020.
- 2.Beltrame V, Gruppo M, Pastorelli D, et al. Outcome of pancreaticoduodenectomy in octogenerians: single institution’s experience and review of the literature. J Visc Surg. 2015;152:279–284. doi: 10.1016/j.jviscsurg.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 3.van der Geest LG, Besselink MG, van Gestel YR, Busch OR, de Hingh IH, de Jong KP, Lemmens VE. Pancreatic cancer surgery in elderly patients: balancing between short-term harm and long-term benefit. A population-based study in the Netherlands. Acta Oncol. 2016;55(3):278–285. doi: 10.3109/0284186X.2015.1105381. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Bachet JB. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 5.Latenstein AEJ, van der Geest LGM, Bonsing BA, et al. Dutch Pancreatic cancer group: nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer. 2020;125:83–93. doi: 10.1016/j.ejca.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Krautz C, Nimptsch U, Weber GF, Mansky T, Grutzmann R. Effect of hospital volume on inhospital morbidity and mortality following pancreatic surgery in Germany. Ann Surg. 2018;267:411–417. doi: 10.1097/SLA.0000000000002248. [DOI] [PubMed] [Google Scholar]
- 7.Lidsky ME, Sun Z, Nussbaum DP, Adam MA, Speicher PJ, Blazer DG., 3rd Going the extra mile: improved survival for pancreatic cancer patients traveling to high-volume centers. Ann Surg. 2017;266(2):333–338. doi: 10.1097/SLA.0000000000001924. [DOI] [PubMed] [Google Scholar]
- 8.Shamali A, DeAth HD, Jaber B, Abuawad M, Barbaro S, Hamaday Z, Hilal MA. Elderly patients have similar short term outcomes and five-year survival compared to younger patients after pancreaticoduodenectomy. Int J Surg. 2017;45:138–143. doi: 10.1016/j.ijsu.2017.07.106. [DOI] [PubMed] [Google Scholar]
- 9.Paiella S, De Pastena M, Pollini T, et al. Pancreaticoduodenectomy in patients ≥ 75 years of age: Are there any differences with other age ranges in oncological and surgical outcomes? Results from a tertiary referral center. World J Gastroenterol. 2017;23(17):3077–3083. doi: 10.3748/wjg.v23.i17.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SY, Fink MA, Perini M, et al. Age 80 years and over is not associated with increased morbidity and mortality following pancreaticoduodenectomy. ANZ J Surg. 2018;88(5):E445–E450. doi: 10.1111/ans.14039. [DOI] [PubMed] [Google Scholar]
- 11.Tan E, Song J, Lam S, D'Souza M, Crawford M, Sandroussi C. Postoperative outcomes in elderly patients undergoing pancreatic resection for pancreatic adenocarcinoma: a systematic review and meta-analysis. Int J Surg. 2019;72:59–68. doi: 10.1016/j.ijsu.2019.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Satoi S, Yamamoto T, Uchida K, et al. Optimal treatment for octogenerians with resectable and borderline resectable pancreatic ductal adenocarcinoma: a multicenter retrospective study. Pancreas. 2020;49(6):837–844. doi: 10.1097/MPA.0000000000001579. [DOI] [PubMed] [Google Scholar]
- 13.Lu L, Zhang X, Tang G, et al. Pancreaticoduodenectomy is justified in a subset of elderly patients with pancreatic ductal adenocarcinoma: a population-based retrospective cohort study of 4,283 patients. Int J Surg. 2018;53:262–268. doi: 10.1016/j.ijsu.2018.03.054. [DOI] [PubMed] [Google Scholar]
- 14.Sukharamwala P, Thoens J, Szuchmacher M, et al. Advanced age is a risk factor for post-operative complications and mortality after a pancreaticoduodenectomy: a meta-analysis and systematic review. HPB (Oxford). 2012;14(10):649–657. doi: 10.1111/j.1477-2574.2012.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renz BW, Khali PN, Mikhailov M, et al. Pancreaticoduodenectomy for adenocarcinoma of the pancreatic head is justified in elderly patients: a retrospective cohort study. Int J Surg. 2016;28:118–125. doi: 10.1016/j.ijsu.2016.02.064. [DOI] [PubMed] [Google Scholar]
- 16.Marmor S, Burke EE, Virnig BA, et al. A comparative analysis of survival outcomes between pancreatectomy and chemotherapy for elderly patients with adenocarcinoma of the pancreas. Cancer. 2016;122:3378–3385. doi: 10.1002/cncr.30199. [DOI] [PubMed] [Google Scholar]
- 17.Chen YT, Ma FH, Wang CF, Zhao DB, Zhang YW, Tian YT. Elderly patients had more severe postoperative complications after pancreatic resection: a retrospective analysis of 727 patients. World J Gastroenterol. 2018;24(7):844–851. doi: 10.3748/wjg.v24.i7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casadei R, Ricci C, Lazzarini E, Taffurelli G, D’Ambra M, Mastroroberto M. Pancreatic resection in patients 80 years or older: a meta-analysis and systematic review. Pancreas. 2014;43(8):1208–1218. doi: 10.1097/MPA.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe Y, Shinkawa T, Endo S, Abe Y, Nishihara K, Nakano T. Long-term outcomes after pancreatectomy for pancreatic ductal adenocarcinoma in elderly patients: special reference to postoperative adjuvant chemotherapy. World J Surg. 2018;42(8):2617–2626. doi: 10.1007/s00268-018-4496-y. [DOI] [PubMed] [Google Scholar]
- 20.Shin SH, Park Y, Hwang DW, et al. Prognostic value of adjuvant chemotherapy following pancreaticoduodenectomy in elderly patients with pancreatic cancer. Anticancer Res. 2019;39(2):1005–1012. doi: 10.21873/anticanres.13206. [DOI] [PubMed] [Google Scholar]
- 21.Malik AK, Lamarca A, Siriwardena AK, et al. The influence of patients' age on the outcome of treatment for pancreatic ductal adenocarcinoma. Pancreas. 2020;49(2):201–207. doi: 10.1097/MPA.0000000000001486. [DOI] [PubMed] [Google Scholar]
- 22.Riall TS, Reddy DM, Nealon WH, Goodwin JS. The effect of age on short-term outcomes after pancreatic resection: a population-based study. Ann Surg. 2008;248(3):459–467. doi: 10.1097/SLA.0b013e318185e1b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan L, Chang M, Wang J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: a systematic review and meta-analysis. Age Ageing. 2021;50:1118–1128. doi: 10.1093/ageing/afab039. [DOI] [PubMed] [Google Scholar]
- 24.Scurtu R, Bachellier P, Oussoultzoglou E, Rosso E, Maroni R, Jaeck D. Outcome after pancreaticoduodenectomy for cancer in elderly patients. J Gastrointest Surg. 2006;10(6):813–822. doi: 10.1016/j.gassur.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Riediger H, Krueger CM, Makowiec F, Adam U. Impact of age and comorbidity on perioperative mortality after 250 pancreatic head resections. Zentralbl Chir. 2016;141(3):270–276. doi: 10.1055/s-0041-111520. [DOI] [PubMed] [Google Scholar]
- 26.Lee DY, Schwartz JA, Wexelman B, Kirchoff D, Yang KC, Attiyeh F. Outcomes of pancreticoduodenectomy for pancreatic malignancy in octogenarians: an American College of Surgeons national surgical Quality improvement program analysis. Am J Surg. 2014;207(4):540–548. doi: 10.1016/j.amjsurg.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 27.Makary MA, Winter JM, Cameron JL, et al. Pancreaticoduodenectomy in the very elderly. J Gastrointest Surg. 2006;10(3):347–356. doi: 10.1016/j.gassur.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Min Park H, Park SJ, Han SS, Hoon Kim S. Surgery for elderly patients with resectable pancreatic cancer, a comparison with non-surgical treatments: a retrospective study outcomes of resectable pancreatic cancer. BMC Cancer. 2019;19(1):1090. doi: 10.1186/s12885-019-6255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feilhauer K, Hennig R, Lenz S, Köninger J. Pancreatic resection in the elderly: is the risk justified? Chirurg. 2015;86(7):670–675. doi: 10.1007/s00104-014-2869-9. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira-Cunha M, Malde DJ, Aldouri A, Morris-Stiff G, Menon KV, Malvern Smith A. Results of pancreatic surgery in the elderly: is age a barrier? HPB. 2013;15(1):24–30. doi: 10.1111/j.1477-2574.2012.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bathe OF, Caldera LH, Francheschi D, et al. Radical resection in periampullary tumors in the elderly: evaluation of long-term results. World J Surg. 2000;24(3):353–358. doi: 10.1007/s002689910056. [DOI] [PubMed] [Google Scholar]
- 32.Van Rijssen LB, Groot Koerkamp B, Zwart MJ, et al. Nationwide prospective audit of pancreatic surgery: design, accuracy, and outcomes of the dutch pancreatic cancer audit. HPB. 2017;19(10):919–926. doi: 10.1016/j.hpb.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 33.van der Geest LG, van Rijssen LB, Molenaar IQ, et al. Volume-outcome relationships in pancreatoduodenectomy for cancer. HPB. 2016;18(4):317–324. doi: 10.1016/j.hpb.2016.01.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;246(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 35.De Wilde RF, Besselink MGH, van der Tweel I, et al. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg. 2012;99(3):404–410. doi: 10.1002/bjs.8664. [DOI] [PubMed] [Google Scholar]
- 36.Augustin T, Burstein MD, Schneider EB, et al. Frailty predicts risk of life-threatening complications and mortality after pancreatic resections. Surgery. 2016;160(4):987–996. doi: 10.1016/j.surg.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Berkel AEM, Bongers BC, Kotte H, et al. Effects of Community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of a randomized clinical trial. Ann Surg. 2022;275(2):e299–e306. doi: 10.1097/SLA.0000000000004702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dutch Pancreatic Cancer Audit (DPCA). 2021. Available at: http://www.dica.nl/dpca. Accessed 1 Oct 2020.
- 39.van Rijssen LB, Koerkamp BG, Zwart MJ, Bonsing BA, Bosscha K, van Dam RM, Wolfgang CL. Nationwide prospective audit of pancreatic surgery: design, accuracy, and outcomes of the dutch pancreatic cancer audit. Hpb. 2017;19(10):919–926. doi: 10.1016/j.hpb.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Walker G, Charlson Comorbidity Index (CCI) Calculator. MdCALC. 2005. Available at: https://www.mdcalc.com/charlson-comorbidity-index-cci. Accessed 13 Nov 2020.
- 42.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer Staging Manual (7th ed). New York, NY: Springer; 2010.
- 43.Federatie Medische Specialisten. Richtlijn Pancreascarcinoom. 2019. Available at: https://richtlijnendatabase.nl/richtlijn/pancreascarcinoom/startpagina.html. Accessed 1 Mar 2021.
- 44.Kurnat-Thoma EL, Murray MT, Juneau P. Frailty and Determinants of Health Among Older Adults in the United States 2011–2016. J Aging Health. 2021; 8982643211040706. [DOI] [PMC free article] [PubMed]
- 45.WHO criteria. 2011. Available at: https://www.who.int/vmnis/indicators/haemoglobin.pdf. Accessed 14 Oct 2021.
- 46.Kidney Disease Improving Global Outcomes guidelines. 2012. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. Accessed 24 Oct 2021.
- 47.Wilhelm-Leen ER, Hall YN, et al. Frailty and chronic kidney disease: the third national health and nutrition evaluation survey. Am J Med. 2009;122(664–671):e662. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kahlmeyer A, Fiebig C, Mueller M, et al. Geriatric assessments can predict functional outcome and mortality after urological tumor surgery. Urol Int. 2021:1-10. [DOI] [PubMed]
- 49.Mogal H, Vermilion SA, Dodson R, et al. Modified Frailty Index predicts morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2017;24(6):1714–1721. doi: 10.1245/s10434-016-5715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59(10):1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Smits CHM, van den Beld HK, Aartsen MJ, Schroots JJF. Aging in the Netherlands: state of the art and science. Gerontologist. 2014;54(3):335–343. doi: 10.1093/geront/gnt096. [DOI] [PubMed] [Google Scholar]
- 53.Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, Uesaka K. Impact of patient age on the postoperative survival in pancreatic head cancer. Ann Surg Oncol. 2017;24(11):3220–3228. doi: 10.1245/s10434-017-5994-0. [DOI] [PubMed] [Google Scholar]
- 54.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 55.Go S, Lee S, Bae WK, et al. Modified FOLFIRINOX versus S-1 as second-line chemotherapy in gemcitabine-failed metastatic pancreatic cancer patients: a randomised controlled trial (MPACA-3) Eur J Cancer. 2021;157:21–30. doi: 10.1016/j.ejca.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Hall PS, Swinson D, Cairns DA, et al. Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: The GO2 phase 3 randomized clinical trial. JAMA Oncol. 2021;7(6):869–877. doi: 10.1001/jamaoncol.2021.0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klaiber U, Leonhardt CS, Strobel O, Tjaden C, Hackert T, Neoptolemos JP. Neoadjuvant and adjuvant chemotherapy in pancreatic cancer. Langenbecks Arch Surg. 2018;403(8):917–932. doi: 10.1007/s00423-018-1724-8. [DOI] [PubMed] [Google Scholar]
- 58.Lambert A, Schwarz L, Ducreux M, Conroy T. Neoadjuvant treatment strategies in resectable pancreatic cancer. Cancers. 2021;13(18):4724. doi: 10.3390/cancers13184724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamad A, Brown ZJ, Ejaz AM, Dillhoff M, Cloyd JM. Neoadjuvant therapy for pancreatic ductal adenocarcinoma: opportunities for personalizes cancer care. World J Gastroenterol. 2021;27(27):4383–4394. doi: 10.3748/wjg.v27.i27.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaw JF, Budiansky D, Shariff F, McIsaac D. The association of frailty with outcomes after cancer surgery: a systematic review and metaanalysis. Ann Surg Oncol. Epub. 2022 doi: 10.1245/s10434-021-11321-2. [DOI] [PubMed] [Google Scholar]
- 61.George EL, Hall DE, Youk A, et al. Association between patient frailty and postoperative mortality across multiple noncardiac surgical specialties. JAMA Surg. 2021;156(1):e205152. doi: 10.1001/jamasurg.2020.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamada S, Shimada M, Morine Y, Imura S, Ikemoto T, Saito Y, et al. Significance of frailty in prognosis after surgery in patients with pancreatic ductal adenocarcinoma. World J Surg Oncol. 2021;19:94. doi: 10.1186/s12957-021-02205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guyton RL, Jr, Mosquera C, Spaniolas K, Fitzgerald TL. Association of increasing frailty with detrimental outcomes after pancreatic resection. Am Surg. 2018;84(4):512–519. doi: 10.1177/000313481808400423. [DOI] [PubMed] [Google Scholar]
- 64.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin SH, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. doi: 10.1186/s12877-016-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orimo H. Reviewing the definition of elderly. Nihon Ronen Igakkai Zasshi. 2006;43(1):27–34. doi: 10.3143/geriatrics.43.27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.