Abstract
Background:
Primary laterality of colorectal cancer is thought to be associated with differences in outcomes. Liver metastasis is the most common site of solitary colorectal cancer spread. However, how primary colorectal cancer laterality affects outcomes in colorectal liver metastasis remains unclear.
Methods:
The Colorectal Liver Operative Metastasis International Collaborative (COLOMIC) of operative hepatectomy cases for colorectal liver metastasis was compiled from five participating institutions. This included consecutive cases from 2000–2018 at all sites. A total of 884 patients were included in this study. Univariate, multivariate, and Kaplan-Meier analyses were performed.
Results:
Patients with left-sided versus right-sided cancers had significantly better overall survival: 49.4 vs. 41.8 months (p<0.05). Patients with KRAS mutations had significantly worse median overall survival compared to KRAS wild-type (43.6 vs 56.1 months; p<0.001). In left-sided cancers, KRAS mutations were associated with significantly worse median overall survival compared to KRAS wild-type cancers (43.6 vs 56.6 months; p<0.01). This association was absent in patients with right-sided primary tumors. Multivariate Cox regression analysis revealed different variable sets (non-overlapping) were associated with overall survival, when comparing left-sided and right-sided cancers.
Discussion:
Understanding how primary tumor laterality and related biological aspects affect long-term outcomes can potentially inform treatment decisions for patients with colorectal liver metastases.
INTRODUCTION
Colorectal cancers are one of the most common malignancies and leading causes of cancer deaths worldwide [1]. Colorectal Liver Metastases (CLM) are the most frequent site of solitary colorectal cancer metastatic spread, in approximately 30% of cases [2, 3], likely due to portal venous drainage [4]. It has been proposed that biological differences between left and right colorectal cancers affect outcomes [5], likely due to the different embryologic origins of the left and right colon [6]. This phenomenon is likely multifactorial, and mutations in Kirsten rat sarcoma viral oncogene homologue (KRAS) and B-Raf proto-oncogene, serine/threonine kinase (BRAF) are thought to play a role in prognosis [7–10]. Additionally, outcomes of colorectal cancer patients treated with the epidermal growth factor receptor inhibitor cetuximab plus chemotherapy have been shown to be dependent on primary tumor laterality [11].
How primary colorectal cancer laterality affects outcomes in CLM remains unclear [12–15], and was not included in the most widely-used prognostic clinical score by Fong et al. [16]. Right-sided primary tumors have more often been found to carry a worse prognoses, but the results have not been uniform: A recent meta-analysis found 21 studies concluded left-sided tumors had better OS, but 17 studies found no statistically significant difference in OS between left and right sided tumors [17]. Previous attempts have been largely limited to single-center retrospective studies with relatively small sets of patients, or population registry-based retrospective reviews.
Using a large set of CLM hepatectomy cases from an international multicenter database from five hepatobiliary institutions, the Colorectal Liver Operative Metastasis International Collaborative (COLOMIC), we hypothesized there is a differential association of primary tumor laterality with long-term outcomes after curative-intent surgical treatment of CLM, and that mutations in KRAS influence this effect.
METHODS
A database of CLM hepatectomy cases was compiled from an international collaborative of five institutions (Wake Forest Baptist Medical Center, Mayo Clinic Florida, University of California San Francisco, Yale New Haven Hospital, and The University of Hong Kong, which we call COLOMIC: Colorectal Liver Operative Metastasis International Collaborative. Institutional Review Board approval was obtained for this project at each participating institution. This database included consecutive cases from 2000–2018 (n=1004) at all participating institutions. Patients must have received a curative-intent hepatectomy operation (major or minor, including those that may have also incorporated ablations), but patients who received ablation-only procedures were excluded from the database. All technical methods of liver resection (crush-clamp, energy device, or hybrid), were included. Major and minor hepatectomies with anatomic and non-anatomic resections were included. Wedge resections solely for diagnostic biopsy purposes were excluded. Patients who underwent multiple hepatectomy operations were excluded from this analysis; these patients are fewer in number and may be different from the patients who receive a single hepatectomy, so the decision was made to include only patients who received a single hepatectomy throughout their clinical courses. Our cohort excluded patients who had two-stage hepatectomies (i.e. two sequential liver resections), and included only 3 patients who had Associating Liver Partition and Portal vein Ligation for Staged hepatectomy (ALPPS) procedures. Of these, 2 patients had left-sided primary cancers and 1 had a right-sided primary cancer. After exclusion criteria were applied, the final number of included cases in this study was n=884. Since there is no consensus in the literature on whether rectal cancers are best grouped with left-sided colon cancers or considered separately [17], we chose to group rectal and left-sided cancers together due to their shared hindgut embryologic origin, and to optimize our analyses by focusing on left-right pathophysiologic differences. We defined right-sided colon tumors as those arising between the cecum and proximal two-thirds of the transverse colon, and left-sided colon cancers as those arising distal to this point and including the rectum. Bilateral colon cancers included at least one tumor on the left and another on the right, found within 3 months of initial diagnosis.
Basic demographic information including age, sex, and race were recorded. Follow-up information, dates of most-recent patient contacts, detection of recurrences, and deaths were recorded, and these were used to calculate overall survival (OS) and recurrence-free survival (RFS). We define recurrence of disease as recurrence at any anatomic site (including but not limited to local hepatic recurrence), and RFS is defined as absence of clinical or radiographic recurrence at any anatomic site after curative intent surgery. Baseline health characteristics and comorbidities were recorded, including global functional status (Independent, Partially-Dependent, or Totally-Dependent) per established definitions [18]. Charlson-Deyo Comorbidity Index was calculated for all patients [19], and variables of this score were recorded independently for each patient (Presence/absence of: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular accident, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus, hemiplegia, chronic kidney disease, leukemia, lymphoma, AIDS). The American Society of Anesthesiologists (ASA) Classification score was recorded for each patient at the time of surgery [20]. Other patient characteristics were recorded as well, including body mass index (BMI), smoking history (absent vs. past/current use), presence of extrahepatic disease on pre-operative imaging, peak carcinoembryonic enzyme (CEA) level and peak bilirubin during post-operative hospitalization course. Median follow up time was calculated using an established method [21].
Tumor/pathologic characteristics recorded were number of hepatic lesions at the time of operation, KRAS gene status (wild-type vs. mutated), BRAF gene status (wild-type vs. mutated), microsatellite instability (MSI) (high/unstable vs. low/stable), and parenchymal margin status (R0, R1, or R2). KRAS, BRAF, and MSI were performed at the discretion of the pathologist. Intraoperative intervention modality (hepatic resection vs. resection plus ablation) and estimated blood loss was recorded. Intraoperative and post-operative transfusions of red blood cells were recorded. Chemotherapy treatment—neoadjuvant, adjuvant, neoadjuvant-plus-adjuvant, or none—was recorded.
The Chi-Square (χ2) and Analysis of Variance (ANOVA) tests were used to compare baseline patient characteristics between laterality groups. Kaplan-Meier analysis with the Log Rank-test was used to determine find differences in median OS and DFS between groups.
The Cox proportional hazards regression method was used to perform univariate analyses on patient, tumoral, operative, and treatment characteristics. To detect variables independently associated with significant changes in OS, we then performed a best-fit multivariate stepwise Cox proportional hazards regression model [22], initially including variables that had a p-values of <0.10 detected on prior univariate analysis. The analysis was not possible for the bilateral primary tumor class due to low number of patients (n=12). BRAF gene status and Microsatellite instability (MSI) were excluded in the multivariate analysis due to low numbers of patients for which these tests were performed. Backward elimination was then performed until only variables with p-values of <0.01 remained. KRAS was then added to the final model to avoid bottlenecking, due to this test being recorded in approximately half of the patient population. Post hoc analysis showed the additional step of adding KRAS did not change the overall results for the other significant variables, for all patients as well as for each primary tumor laterality.
RESULTS
For patients in this dataset, colorectal primary cancers were right-sided in 251 patients, left-sided in 608 patients, bilateral in 13, and unknown in 12 (Table 1). Median age of patients at hepatectomy operation was 61 years; with right-sided being older (62 years) than left-sided (60 years) or bilateral (59 years) primary tumors (ANOVA F=5.1, p<0.01). Median follow-up time for the entire cohort was 60.1 months following the hepatectomy operation. There were no significant differences in sex (χ2=4.18, p=0.12), BMI (ANOVA F=0.3, p=0.71), or racial compositions (χ2=6.84, p=0.08) between groups. Baseline health and comorbidities of patients who were treated for left-sided, right-sided, or bilateral primary colorectal tumors were statistically similar, in terms of global functional status (χ2=1.15, p=0.56), Charlson-Deyo scores (ANOVA F=0.3, p=0.72), and ASA Scores (χ2=4.24, p=0.24). Median times between initial colorectal cancer diagnosis and hepatectomy were 10.2 months for left-sided colorectal cancers, 8.7 months for right-sided colorectal cancers, and 14.1 months for bilateral colorectal cancers. Concomitant liver resections were performed in 1 of 13 bilateral (7.7%), 120 of 608 (19.7%) left-sided, and 57 of 251 (22.7%) right-sided colorectal cancers. There was no significant difference in the proportion of concomitant liver resections between left- and right-sided colorectal cancers (χ2=0.95, p=0.33). There was a significant difference in KRAS gene status between laterality treatment groups (χ2=10.0, p<0.01), with the right-sided tumor group having significantly more mutants (50.0%) compared to the left-sided tumor group (33.3%). For KRAS wild-types the median time between initial cancer diagnosis and hepatectomy was 9.9 months, and for KRAS-mutants this median time was 9.5 months. Chemotherapy treatment strategies did not significantly differ between tumor laterality groups (χ2=4.10, p=0.66), with the most commonly-used approach overall being neoadjuvant plus adjuvant therapy in 37.3% of patients, followed by adjuvant therapy only (29.1%), neoadjuvant therapy only (14.9%), and no chemotherapy (18.7%).
Table 1.
Descriptive statistics
| Characteristic | (Statistical Test) | All Colorectal Primary Lateralities | Left-Sided Primary Colorectal Cancers | Right-Sided Primary Colorectal Cancers | Bilateral Primary Colorectal Cancers | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Patients, n= | 884 | 608 | 251 | 13 | |||||
| Age at Operation, median years ± SD | (ANOVA: F = 5.1, p<0.01) | 61 | ±11.4 | 60 | ±11.1 | 62 | ±11.8 | 59 | ±11.2 |
| Body Mass Index, mean ± SD | (ANOVA: F = 0.3, p = 0.71) | 26.7 | ±5.8 | 26.6 | ±5.8 | 26.9 | ±5.6 | 26.7 | ±5.0 |
| Sex, n= (%) | (χ2 4.18, p = 0.12) | ||||||||
| Male | 362 | (41.6%) | 248 | (40.8%) | 112 | (44.6%) | 2 | (16.7%) | |
| Female | 509 | (58.4%) | 360 | (59.2%) | 139 | (55.4%) | 10 | (83.3%) | |
| Race, n= (%) | (χ2 6.84 p = 0.08) | ||||||||
| White | 525 | (60.2%) | 360 | (59.2%) | 156 | (62.2%) | 9 | (69.2%) | |
| Asian | 226 | (25.9%) | 167 | (27.5%) | 56 | (22.3%) | 3 | (23.1%) | |
| African-American | 77 | (8.8%) | 47 | (7.7%) | 30 | (12.0%) | 0 | (0.0%) | |
| Other/Hispanic | 42 | (4.8%) | 33 | (5.4%) | 9 | (3.6%) | 0 | (0.0%) | |
| Unknown | 2 | (0.2%) | 1 | (0.2%) | 0 | (0.0%) | 1 | (7.7%) | |
| Global Functional Status, n= (%) | (χ2 1.15, p = 0.56) | ||||||||
| Independent | 687 | (78.9%) | 475 | (78.1%) | 202 | (80.5%) | 10 | (83.3%) | |
| Partially-Dependent | 177 | (20.3%) | 127 | (20.9%) | 48 | (19.1%) | 2 | (16.7%) | |
| Totally-Dependent | 7 | (0.8%) | 6 | (1.0%) | 1 | (0.4%) | 0 | (0.0%) | |
| Charlson-Deyo Score, mean ± SD | (ANOVA: F = 0.3, p = 0.72) | 8.57 | ±1.56 | 8.54 | ±1.72 | 8.64 | ±1.07 | 8.58 | ±0.90 |
| ASA Physical Status Score, n= (%) | (χ2 4.24, p = 0.24) | ||||||||
| I | 39 | (4.5%) | 29 | (4.8%) | 9 | (3.6%) | 1 | (8.3%) | |
| II | 303 | (35.2%) | 220 | (36.7%) | 78 | (31.2%) | 5 | (41.7%) | |
| III | 488 | (56.7%) | 331 | (55.3%) | 151 | (60.4%) | 6 | (50.0%) | |
| IV | 31 | (3.6%) | 19 | (3.2%) | 12 | (4.8%) | 0 | (0.0%) | |
| KRAS Gene Status, n= (%) | (χ2 10.0, p<0.01) | ||||||||
| Wild-Type | 256 | (61.8%) | 196 | (66.7%) | 58 | (50.0%) | 2 | (50.0%) | |
| Mutated | 158 | (38.2%) | 98 | (33.3%) | 58 | (50.0%) | 2 | (50.0%) | |
| Chemotherapy Strategy, n= (%) | (χ2 4.10, p = 0.66) | ||||||||
| None | 163 | (18.7%) | 105 | (17.3%) | 55 | (21.9%) | 3 | (23.1%) | |
| Neoadjuvant-Only | 130 | (14.9%) | 90 | (14.8%) | 39 | (15.5%) | 1 | (7.7%) | |
| Adjuvant-Only | 254 | (29.1%) | 179 | (29.4%) | 70 | (27.9%) | 5 | (38.5%) | |
| Neoadjuvant-plus-Adjuvant | 325 | (37.3%) | 234 | (38.5%) | 87 | (34.7%) | 4 | (30.8%) | |
Abbreviations: ANOVA, Analysis of Variance; ASA, American Society of Anesthesiologists; SD, Standard Deviation; χ2, Chi- Square.
Note: Category totals may not add up to totals due to individual exclusions when variables are not recorded/unknown. Bolded statistical test signifies p-value of <0.05.
In terms of post-hepatectomy median OS, patients with left-sided primary colon tumors did significantly better: 49.4 months vs. 41.8 months (Log Rank χ2=4.094, p<0.043), respectively (Figure 1). Bilateral colorectal primary tumors trended toward lower post-hepatectomy median OS (34.5 months) compared to left-sided and right-sided primaries: p=0.102 and p=0.053, respectively (Figure 1). Early post-operative mortality, as defined by death within 30 days of hepatectomy from any cause, occurred in 29 patients from the total group (3.4%), and these cases were included in all analyses. In terms of recurrence-free survival (RFS), there were no significant differences in outcomes for left-sided, right-sided, or bilateral primary colon cancers: 12.1 months, 9.4 months, and 18.8 months, respectively; with p>0.20 for all pairwise comparisons (Supplemental Figure 1S).
Figure 1.

Overall survival after hepatectomy for colorectal liver metastasis, by laterality of primary colorectal cancer
On Cox proportional hazards univariate analysis for the overall group with all lateralities combined, there were several factors for which significant correlations with OS were detected (Table 2). For some variables, significant correlation with OS was dependent on primary tumor laterality. Some variables were significantly associated with OS for the left-sided primary tumor group, whereas this association was absent for right-sided tumors: BMI, ASA class, number of hepatic lesions, KRAS gene status, BRAF gene status, operative intervention modality, and Clavien-Dindo score (including grade V). For other variables, there were associations of right-sided tumors with OS that were absent for left-sided tumors: Peak CEA level, and intraoperative red blood cell transfusions. This analysis could not be performed on the bilateral primary colorectal cancer group for several variables, due to low number of patients in this group (Low degrees of freedom). Variables found in this univariate analysis with p<0.10 for each patient group were then used as the starting point for multivariate analysis.
Table 2.
Univariate cox proportional hazards regression analysis
| All Primary Cancer Lateralities | Left-Sided Primary Colorectal Cancer | Right-Sided Primary Colorectal Cancer | Bilateral Primary Colorectal Cancers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 844 | n= 591 | n= 241 | n = 12 | |||||||||
| H.R. | p-value | (95% C.I.) | H.R. | p-value | (95% C.I.) | H.R. | p-value | (95% C.I.) | H.R. | p-value | (95% C.I.) | |
| Baseline Patient Characteristics | ||||||||||||
| Gender (Female vs. Male) | 1.13 | 0.20 | (0.94–1.37) | 1.19 | 0.14 | (0.94–1.49) | 0.94 | 0.73 | (0.67–1.32) | #REF! | #REF! | #REF! |
| Age (in increments of 5 years) | 1.03 | 0.11 | (0.99–1.08) | 1.04 | 0.14 | (0.99–1.09) | 1.00 | 0.93 | (0.93–1.07) | #REF! | #REF! | #REF! |
| Body Mass Index (BMI in increments of 5 units) | 1.11 | 0.025 | (1.01–1.21) | 1.11 | 0.046 | (1.00–1.23) | 1.10 | 0.30 | (0.92–1.30) | #REF! | #REF! | #REF! |
| ASA Class (III/IV vs. I/II) | 1.33 | <0.01 | (1.10–1.61) | 1.33 | 0.016 | (1.06–1.68) | 1.30 | 0.15 | (0.91–1.87) | #REF! | #REF! | #REF! |
| Diabetes (Present vs. Absent) | 0.96 | 0.78 | (0.74–1.26) | 0.99 | 0.94 | (0.72–1.37) | 0.88 | 0.61 | (0.55–1.42) | Low DoF | ||
| Functional Status (Partially or Fully-Dependent vs. Independent) | 1.05 | 0.66 | (0.84–1.32) | 1.02 | 0.90 | (0.77–1.34) | 1.15 | 0.52 | (0.75–1.74) | #REF! | #REF! | #REF! |
| Smoking History (Present vs. Absent) | 0.95 | 0.58 | (0.77 –1.15) | 0.99 | 0.93 | (0.77–1.26) | 0.90 | 0.56 | (0.63–1.29) | #REF! | #REF! | #REF! |
| Extrahepatic Disease on Preoperative Imaging (Present vs. Absent) | 1.60 | <0.01 | (1.19–2.15) | 1.53 | 0.024 | (1.06–2.22) | 1.74 | 0.034 | (1.04–2.90) | #REF! | #REF! | #REF! |
| Tumor Characteristics | ||||||||||||
| Peak Carcinoembryonic Enzyme Level (CEA ng/mL) | 1.02 | 0.015 | (1.00–1.03) | 1.01 | 0.23 | (0.99–1.03) | 1.03 | 0.018 | (1.01 –1.06) | 1.94 | 0.35 | (0.48–7.92) |
| Number of Hepatic Lesions | 1.11 | <0.001 | (1.06 –1.15) | 1.14 | <0.001 | (1.08–1.20) | 1.06 | 0.15 | (0.98 –1.15) | 0.73 | 0.66 | (0.18–2.93) |
| KRAS gene status (Mutated vs. Wild-Type) | 1.67 | <0.001 | (1.24–2.24) | 1.72 | <0.01 | (1.20–2.47) | 1.42 | 0.21 | (0.83–2.43) | Low DoF | ||
| BRAF gene status (Mutated vs. Wild-Type) | 3.82 | <0.01 | (1.48–9.85) | 3.88 | 0.030 | (1.14–13.23) | 2.59 | 0.22 | (0.57–11.84) | Low DoF | ||
| Microsatellite Instability (High vs Low) | 0.92 | 0.84 | (0.39 – 2.17) | 1.17 | 0.75 | (0.44–3.09) | 0.36 | 0.33 | (0.05 – 2.81) | Low DoF | ||
| Laterality of Primary Colon Cancer | 0.047 | N/A | N/A | N/A | ||||||||
| Bilateral vs. LeftSided | 0.56 | 0.195 | (0.23–1.35) | N/A | N/A | N/A | ||||||
| Bilateral vs. RightSided | 0.45 | 0.083 | (0.18 –1.11) | N/A | N/A | N/A | ||||||
| Lefts vs. Right | 0.81 | 0.044 | (0.66–0.99) | N/A | N/A | N/A | ||||||
| Operative Characteristics | ||||||||||||
| Intervention Modality (Resection vs. Resection with Ablation) | 0.65 | <0.001 | (0.50–0.83) | 0.59 | <0.001 | (0.44–0.79) | 0.88 | 0.62 | (0.52–1.48) | 0.21 | 0.20 | (0.02–2.32) |
| Estimated Blood Loss (Intraoperative), in 100 cc increments | 1.01 | 0.029 | (1.00–1.03) | 1.01 | 0.33 | (0.99–1.02) | 1.02 | 0.029 | (1.00–1.04) | 0.97 | 0.89 | (0.64–1.48) |
| Intraoperative Red Blood Cells Transfusion (Yes vs. No) | 1.56 | <0.001 | (1.22–2.00) | 1.37 | 0.054 | (0.99–1.88) | 1.84 | <0.01 | (1.23–2.76) | Low DoF | ||
| Postoperative Red Blood Cells Transfusion (Yes vs. No) | 1.25 | 0.16 | (0.91–1.72) | 0.99 | 0.96 | (0.62–1.57) | 1.61 | 0.034 | (1.04 – 2.51) | Low DoF | ||
| Peak Bilirubin Level (mg/dL) | 0.77 | 0.059 | (0.59–1.01) | 0.75 | 0.079 | (0.54–1.03) | 0.92 | 0.78 | (0.53 –1.61) | 0.43 | 0.50 | (0.04–4.88) |
| Pathologic Margin Status | 0.0027 | 0.031 | 0.021 | Low DoF | ||||||||
| R1 vs. R0 | 1.63 | <0.001 | (1.23 – 2.18) | 1.57 | <0.01 | (1.12 – 2.19) | 1.76 | 0.050 | (1.00–3.09) | Low DoF | ||
| R2 vs. R0 | 1.41 | 0.34 | (0.70–2.83) | 0.87 | 0.78 | (0.32 –2.34) | 3.43 | 0.037 | (1.08–10.94) | Low DoF | ||
| R1 vs. R2 | 1.16 | 0.69 | (0.55–2.45) | 1.80 | 0.26 | (0.64–5.04) | 0.51 | 0.30 | (0.15 –1.81) | Low DoF | ||
| Post-Operative Morbidity/ Mortality (Clavien-Dindo Grades 0 through V) | 1.11 | <0.01 | (1.03 –1.19) | 1.10 | 0.035 | (1.01 –1.21) | 1.10 | 0.13 | (0.97 –1.26) | 1.08 | 0.87 | (0.46–2.53) |
| Post-Operative Morbidity (Clavien-Dindo Grades 0 through IV) | 1.03 | 0.42 | (0.96–1.11) | 1.03 | 0.61 | (0.93 –1.13) | 1.03 | 0.63 | (0.90 –1.18) | 1.08 | 0.87 | (0.46–2.53) |
| Recurrence of Cancer (Yes vs. No; At any postoperative timepoint) | 2.37 | <0.001 | (1.89–2.96) | 2.31 | <0.001 | (1.76–3.04) | 2.32 | <0.001 | (1.56–3.45) | 4.90 | 0.16 | (0.53–44.93) |
| Treatment Characteristics | ||||||||||||
| Neoadjuvant Chemotherapy (Given vs. Not Given) | 1.09 | 0.34 | (0.91–1.32) | 1.12 | 0.35 | (0.89–1.40) | 1.07 | 0.71 | (0.76–1.49) | 0.43 | 0.45 | (0.05–3.82) |
| Adjuvant Chemotherapy (Given vs. Not Given) | 0.77 | 0.01 | (0.63–0.94) | 0.78 | 0.038 | (0.61 – 0.99) | 0.87 | 0.42 | (0.61 –1.23) | 0.08 | 0.033 | (0.01 – 0.82) |
| Pairwise Comparisons Treatment Characteristics | ||||||||||||
| Adjuvant-Only vs. Neoadjuvant-plus-Adjuvant | 0.83 | 0.11 | (0.65–1.04) | 0.81 | 0.14 | (0.61 –1.07) | 0.86 | 0.49 | (0.56–1.32) | Low DoF | ||
| Adjuvant-Only vs. Neoadjuvant-Only | 0.69 | 0.019 | (0.51 – 0.94) | 0.69 | 0.051 | (0.47 –1.00) | 0.80 | 0.41 | (0.47 –1.36) | Low DoF | ||
| Adjuvant-Only vs. None | 0.68 | <0.01 | (0.52–0.89) | 0.68 | 0.022 | (0.49–0.95) | 0.77 | 0.29 | (0.48–1.25) | 0.20 | 0.19 | (0.02 –2.25) |
| Neoadjuvant-plus-Adjuvant vs. Neoadjuvant-Only | 0.84 | 0.24 | (0.63–1.12) | 0.85 | 0.38 | (0.59–1.22) | 0.93 | 0.78 | (0.56–1.55) | Low DoF | ||
| Neoadjuvant-plus-Adjuvant vs. None | 0.83 | 0.14 | (0.64–1.07) | 0.84 | 0.28 | (0.62–1.15) | 0.90 | 0.64 | (0.57–1.42) | Low DoF | ||
| Neoadjuvant vs. None | 0.98 | 0.91 | (0.71 –1.35) | 0.99 | 0.96 | (0.66–1.48) | 0.96 | 0.89 | (0.55–1.68) | Low DoF | ||
Abbreviations: ASA, American Society of Anesthesiology; BMI, body mass index; CEA, carcinoembryonic antigen; DoF, Degrees of Freedom; H.R. Hazard Ratio.
Note: Low DoF, i.e. Low Degrees of Freedom (n < 10), indicates statistical calculation was not possible due to small sample size. Bolded statistical test signifies p-value of <0.05.
On Kaplan-Meier analysis, patients with KRAS mutations had significantly worse median overall survival compared to KRAS-wild-type (43.6 months vs 56.1 months; Log Rank χ2= 11.7, p<0.001) for all colon cancer primary lateralities combined (Figure 2A). For patients with left-sided primary tumors, KRAS mutations were also associated with significantly worse median overall survival: 43.6 months vs 56.6 months; Log Rank χ2= 8.859; p<0.01 (Figure 2B). This association was absent in patients with right-sided primary tumors, with median OS 43.3 months vs 46.0 months for KRAS mutants versus wild-types; Log Rank χ2=1.616; p=0.204 (Figure 2C). Measuring recurrence-free survival (RFS), KRAS gene status did not appear to have an effect for all lateralities combined (χ2=1.41, p=0.235), nor for left-sided (χ2=1.121, p=0.290) or right-sided (χ2=0.062, p=0.803) primary colorectal cancers (Supplemental Figure 2S). Although all patients in our cohort were ultimately Stage IV, by definition through having liver metastases, we noted colorectal cancers that were Stage I at the time of initial diagnosis, were associated with significantly better survival, in the entire cohort and in left-sided primary cancer (Supplemental Figure 3S). There were not sufficient numbers of Stage I initial primary cancers to draw this conclusion about right-sided colorectal cancers, or for bilateral synchronous colorectal cancers. Initial Stages II, II, and IV cancers did not differ significantly from each other in pairwise comparisons, in the entire cohort or when stratified by primary tumor laterality categories.
Figure 2.

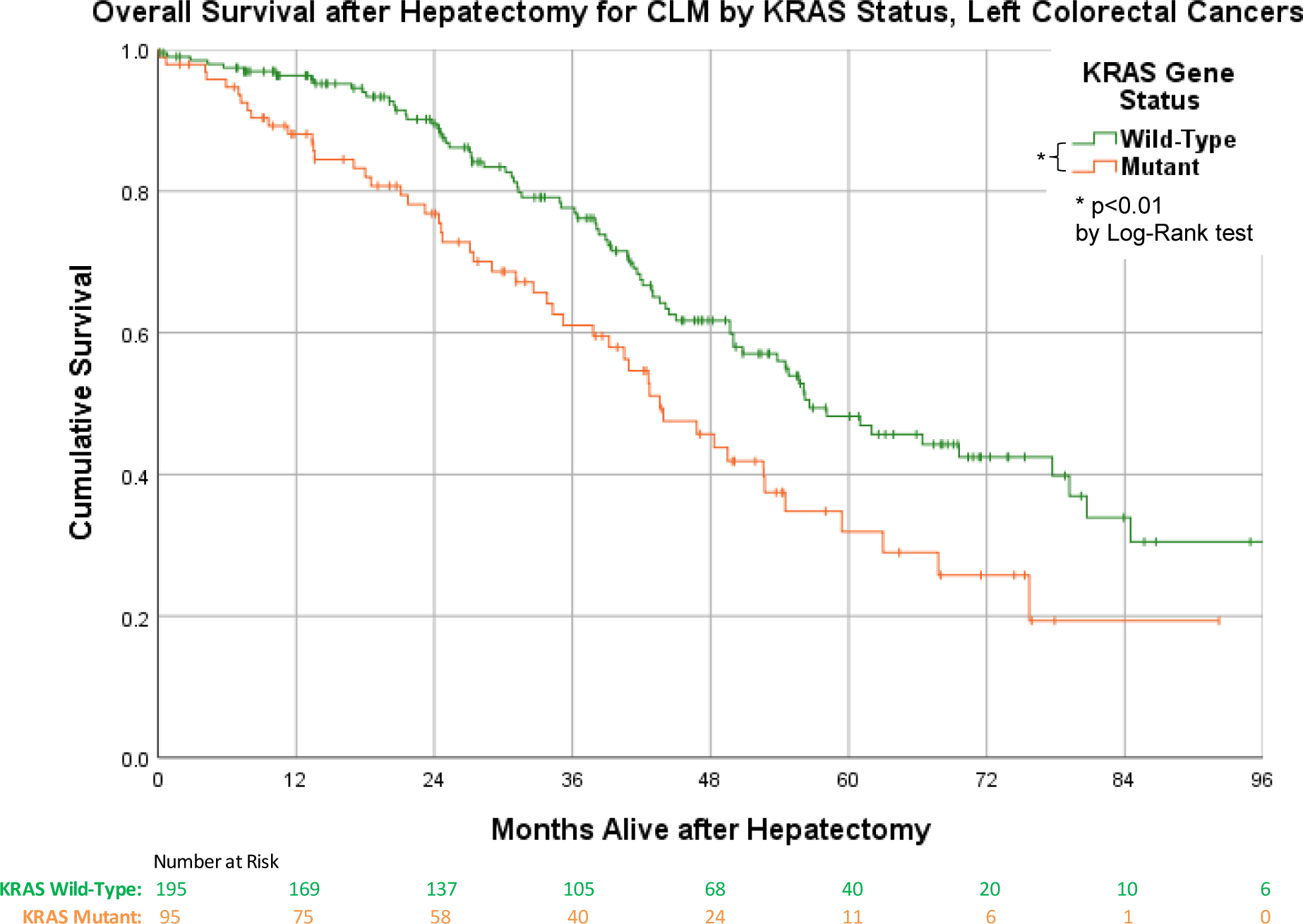

a. Overall survival after hepatectomy for CLM, by KRAS status. b. Overall survival after hepatectomy for CLM for left-sided colorectal cancers, by KRAS status. c. Overall survival after hepatectomy for CLM for right-sided colorectal cancers, by KRAS status
For our multivariate Cox regression analysis using OS as the endpoint, variables demonstrating p<0.10 on univariate analysis (Table 2) for each tumor laterality class were included used as initial covariates for our model (Table 3). When all primary tumor lateralities were grouped together, our model demonstrated: pre-operative extrahepatic disease on imaging, increasing number of hepatic lesions, KRAS gene mutations, and intraoperative red blood cell transfusions, were each independently-associated with worse outcomes. For left-sided primary tumor patients, only increasing number of hepatic lesions and KRAS gene mutations were independently associated with worse outcomes. For right-sided primary tumor patients, KRAS mutations were not significantly associated with worse outcomes. Pathologic parenchymal margins R1 or R2 were independently-associated with worse outcomes only for right-sided primary cancers. Pairwise comparisons showed significantly better outcomes for any approach that included adjuvant chemotherapy versus any approach not including adjuvant chemotherapy (Table 3, bottom).
Table 3.
Multivariate cox proportional hazards stepwise regression model analysis
| All Primary Cancer Lateralities | Left-Sided Primary Colorectal Cancer | Right-Sided Primary Colorectal Cancer | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 532 | n = 388 | n = 209 | |||||||||||||
| Included in Model? | Significant in final Model? | H.R. | p-value | (95% C.I.) | Included in Model? | Significant in final Model? | H.R. | p-value | (95% C.I.) | Included in Model? | Significant in final Model? | H.R. | p-value | (95% C.I.) | |
| Baseline Patient Characteristics | |||||||||||||||
| Body Mass Index (BMI in increments of 5 units) | Yes | No | Yes | No | No | N/A | |||||||||
| ASAClass (III/IVvs. I/ Yes II) | No | Yes | No | No | N/A | ||||||||||
| Extrahepatic Disease on Pre-operative Imaging (Present vs. Absent) | Yes | Yes | 2.06 | 0.0012 | (1.33–3.18) | Yes | No | Yes | No | ||||||
| Tumor Characteristics | |||||||||||||||
| Carcinoembryonic Enzyme Level (CEA ng/mL) | Yes | No | No | N/A | Yes | Yes | 1.04 | 0.0075 | (1.01 –1.06) | ||||||
| Number of Hepatic Lesions | Yes | Yes | 1.19 | <0.0001 | (1.11–1.27) | Yes | Yes | 1.17 | <0.0001 | (1.09–1.26) | No | N/A | |||
| KRAS gene status (Mutated vs. Wild- Type) | Yes | Yes | 1.72 | 0.0022 | (1.22–2.43) | Yes | Yes | 2.00 | <0.0001 | (1.37–2.91) | Yes | No | |||
| Operative Characteristics | |||||||||||||||
| Intervention Modality (Resection vs. Resection with Ablation) | Yes | No | Yes | No | No | N/A | |||||||||
| Estimated Blood Loss (Intraoperative), in 100 cc increments | Yes | No | No | N/A | Yes | No | |||||||||
| Intraoperative Red Blood Cells Transfusion (Yes vs. No) | Yes | Yes | 1.99 | 0.0033 | (1.26–3.16) | Yes | No | Yes | Yes | 2.00 | 0.0019 | (1.29–3.09) | |||
| Postoperative Red Blood Cells Transfusion (Yes vs. No) | No | N/A | No | N/A | Yes | No | |||||||||
| Post-Operative peak Bilirubin (mg/dL) | No | N/A | Yes | No | No | N/A | |||||||||
| Pathologic Margins: R1 vs. R0 | Yes | No | Yes | No | Yes | Yes | 2.38 | 0.011 | (1.22–4.65) | ||||||
| Pathologic Margins: R2 vs. R0 | Yes | No | Yes | No | Yes | Yes | 3.86 | 0.024 | (1.19–12.5) | ||||||
| Pathologic Margins: R1 vs. R2 | Yes | No | Yes | No | No | ||||||||||
| Pairwise Comparisons Treatment Characteristics | |||||||||||||||
| Adjuvant-Only vs. Neoadjuvant-plus- Adjuvant | Yes | No | No | No | N/A | ||||||||||
| Adjuvant-Only vs. Neoadjuvant-Only | Yes | Yes | 0.46 | 0.0038 | (0.27 –0.78) | Yes | Yes | 0.47 | 0.0098 | (0.26–0.68) | No | N/A | |||
| Adjuvant-Only vs. None | Yes | Yes | 0.43 | 0.0024 | (0.25–0.74) | Yes | Yes | 0.37 | 0.0014 | (0.20–0.68) | No | N/A | |||
| Neoadjuvant-plus- Adjuvant vs. Neoadjuvant-Only | Yes | Yes | 0.56 | 0.015 | (0.35–0.89) | Yes | No | No | N/A | ||||||
| Neoadjuvant-plus- Adjuvant vs. None | Yes | Yes | 0.53 | 0.010 | (0.32–0.86) | Yes | Yes | 0.49 | 0.011 | (0.28–0.85) | No | N/A | |||
| Neoadjuvant vs. None | Yes | No | Yes | No | No | N/A | |||||||||
Abbreviations: ASA, American Society of Anesthesiology; BMI, body mass index; CEA, carcinoembryonic antigen; H.R. Hazard Ratio.
Note: A stepwise Cox proportional hazards regression model was performed, initially including variables with p < 0.10 detected on prior univariate analysis. Backward elimination was then performed until only variables with p < 0.01 remained. Bolded statistical test signifies p-value of <0.05.
DISCUSSION
Using our large multi-center database of CLM patients who underwent hepatectomy operations, we examined the association between primary colon cancer laterality and outcomes. Between laterality groups, measured patient characteristics were not significantly different in terms of sex, race, BMI, and baseline health (in terms of functional status, Charlson-Deyo score, and ASA classification). The significant differences in ages between lateralities (median age of right-sided primary cancer patients was 62 versus 60 for left-sided primary cancer patients) is likely attributable to tumor biology, and is similarly present in almost all other published studies looking at laterality of CLM patients [17]. Despite this two-year age differential between groups, it did not carry a significant association with OS on univariate analysis. In our entire cohort, we found median OS in CLM was significantly better after hepatectomy for left-sided compared to right-sided primary colorectal cancers.
In our collaborative group, KRAS was mutated at a significantly higher rate in right-sided primary cancer patients. Interestingly, mutated KRAS status was associated with worse OS in the overall population and in the left-sided primary cancer group, but not in the right-sided primary cancer group. This result was seen on Kaplan-Meier analysis, univariate analysis, as well as multivariate analysis. Thus, our results confirm the findings of the Johns Hopkins group, and the International Genetic Consortium for Colorectal Liver Metastasis [7, 23]. Our work differs from this most recent publication by Margonis et al. [23] in several important but complementary ways: (1) Our study included rectal primaries whereas their study excluded these cases, (2) We stratified our results by KRAS mutation, whereas they stratified their results based on primary tumor location, and (3) Our univariate and multivariate analyses had some differences in the included variables, due to distinctions in collected variables between our respective databases. However, despite these differences in study designs, the overall conclusions were concordant between our studies.
Interestingly, our multivariate analysis found multiple additional differences between the left- and right-sided primary cancer groups in terms of independently-associated prognostic factors. For left sided colon cancers, increasing number of hepatic lesions and KRAS mutation status were predictors of worse OS. For right-sided tumors, higher CEA levels, resection margin status, and intraoperative PRBC transfusions, were predictors of worse OS. Although a direct comparison was not performed, it is interesting to note that two of these factors were shared with the Fong et al. prediction score [16] for outcomes after hepatic resection for CLM: number of hepatic tumors, and high CEA score. However, the correlation was laterality-dependent: The number of hepatic tumors was not predictive of OS in right-sided primary cancers, and high CEA score was not predictive of OS in left-sided primary cancers. When primary tumor laterality is taken into account, additional factors related to the interplay between tumor biology, cancer immunology, and treatment responses, become increasingly relevant. This may explain why hepatic resection parenchymal margins of R1 or R2 (versus R0), and intraoperative blood transfusions, were associated with worse OS—but only for right-sided primary cancers. A related finding was that adjuvant chemotherapy was independently associated with better OS when all primary tumor lateralities were considered together, regardless of whether neoadjuvant chemotherapy is given.
KRAS mutations likely have a complex interplay with genetic and biologic factors that manifest themselves differently within different anatomical regions of the colon. And these interrelated factors continue to influence cancer behavior, as it spreads beyond the walls of the colon. Thus, we propose all colorectal primary cancers with liver metastases should be tested for KRAS mutational status. Doing so will provide valuable prognostic information to the patient and guide the treatment algorithm. In particular, KRAS mutations confer a poorer prognosis, overall and for left-sided colon cancers. In light of this, this subset of patients may warrant a more extended neoadjuvant therapy regimen to gauge tumor biology prior to resection.
Our study has several limitations, including the retrospective design, which is prone to the potential biases common to all retrospective studies. However, our database is strengthened by including a diverse set of institutional participants, using an international participant group that increases patient heterogeneity; thus our results may have a closer approximation of the general population compared to single-center studies. Another limitation in our study was KRAS mutation sequences were not recorded in our database, and it is known that specific mutations likely behave differently [24]. BRAF mutations and MSI may also play a role, but these tests were not performed routinely or frequently enough in our study to draw any conclusions. We also chose to group rectal cancers together with left-sided cancers, based on our interpretation of available literature [17], but analyzing these groups separately is also an acceptable approach. We acknowledge that KRAS mutations may possibly affect rectal cancers differently than left-sided colon cancers. Additionally, our database included parenchymal margin status but not vascular margin status, which may also be an important pathologic prognostic factor. Finally, our database only includes CLM patients who received surgical treatment, and not the overall metastatic colorectal cancer population with liver involvement, and this does not allow a comparison with the denominator patient population. However, the purpose of our database was to study outcomes specifically within the curative-intent CLM paradigm, accepting the selection bias inherent with such an analysis.
Our findings are important because understanding the contributing factors of primary tumor laterality, and related biological aspects, on long-term survival can inform treatment decisions for patients with CLM being considered for hepatectomy. We speculate that treatment strategies for CLM will have to be individualized based on primary tumor laterality and mutation status. In the future, prospective trials with intention-to-treat analyses will be needed to find the most appropriate treatment algorithm and agents which account for primary tumor laterality and tumor mutational status.
Supplementary Material
Supported in part by:
Wake Forest University Comprehensive Cancer Center Biostatistics shared resource funded via the NCI grant award P30CA012197.
Footnotes
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was presented at the Americas Hepato-Pancreato-Biliary Association 2021 Annual Meeting in Miami, FL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017: 66:683–691. [DOI] [PubMed] [Google Scholar]
- 2.Ohlsson B, Tranberg KG, Lundstedt C, Ekberg H, Hederstrom E. Detection of hepatic metastases in colorectal cancer: a prospective study of laboratory and imaging methods. Eur J Surg 1993: 159:275–281. [PubMed] [Google Scholar]
- 3.Finlay IG, McArdle CS. Occult hepatic metastases in colorectal carcinoma. Br J Surg 1986: 73:732–735. [DOI] [PubMed] [Google Scholar]
- 4.Sheth KR, Clary BM. Management of hepatic metastases from colorectal cancer. Clin Colon Rectal Surg 2005: 18:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nitsche U, Stogbauer F, Spath C, Haller B, Wilhelm D, Friess H, Bader FG. Right Sided Colon Cancer as a Distinct Histopathological Subtype with Reduced Prognosis. Dig Surg 2016: 33:157–163. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita S, Brudvik KW, Kopetz SE et al. Embryonic Origin of Primary Colon Cancer Predicts Pathologic Response and Survival in Patients Undergoing Resection for Colon Cancer Liver Metastases. Ann Surg 2018: 267:514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki K, Margonis GA, Wilson A et al. Prognostic Implication of KRAS Status after Hepatectomy for Colorectal Liver Metastases Varies According to Primary Colorectal Tumor Location. Ann Surg Oncol 2016: 23:3736–3743. [DOI] [PubMed] [Google Scholar]
- 8.Schirripa M, Bergamo F, Cremolini C et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br J Cancer 2015: 112:1921–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umeda Y, Nagasaka T, Mori Y et al. Poor prognosis of KRAS or BRAF mutant colorectal liver metastasis without microsatellite instability. J Hepatobiliary Pancreat Sci 2013: 20:223–233. [DOI] [PubMed] [Google Scholar]
- 10.Amikura K, Akagi K, Ogura T, Takahashi A, Sakamoto H. The RAS mutation status predicts survival in patients undergoing hepatic resection for colorectal liver metastases: The results from a genetic analysis of all-RAS. J Surg Oncol 2018: 117:745–755. [DOI] [PubMed] [Google Scholar]
- 11.von Einem JC, Heinemann V, von Weikersthal LF et al. Left-sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild-type metastatic colorectal cancer treated with cetuximab plus chemotherapy: an analysis of the AIO KRK-0104 trial. J Cancer Res Clin Oncol 2014: 140:1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupre A, Malik HZ, Jones RP, Diaz-Nieto R, Fenwick SW, Poston GJ. Influence of the primary tumour location in patients undergoing surgery for colorectal liver metastases. Eur J Surg Oncol 2018: 44:80–86. [DOI] [PubMed] [Google Scholar]
- 13.Creasy JM, Sadot E, Koerkamp BG et al. The Impact of Primary Tumor Location on Long-Term Survival in Patients Undergoing Hepatic Resection for Metastatic Colon Cancer. Ann Surg Oncol 2018: 25:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasser E, Braunwarth E, Riedmann M et al. Primary tumour location affects survival after resection of colorectal liver metastases: A two-institutional cohort study with international validation, systematic meta-analysis and a clinical risk score. PLoS One 2019: 14:e0217411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marques MC, C Ribeiro HS, Costa WL Jr et al. Is primary sidedness a prognostic factor in patients with resected colon cancer liver metastases (CLM)?. J Surg Oncol 2018: 117:858–863. [DOI] [PubMed] [Google Scholar]
- 16.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999: 230:309–18; discussion 318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bingham G, Shetye A, Suresh R, Mirnezami R. Impact of primary tumour location on colorectal liver metastases: A systematic review. World J Clin Oncol 2020: 11:294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(2014). User Guide for the 2014 ACS-NSQIP Participant Use Data File. Available: https://www.facs.org/-/media/files/quality-programs/nsqip/nsqip_puf_userguide_2014.ashx [accessed June 24, 2021].
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987: 40:373–383. [DOI] [PubMed] [Google Scholar]
- 20.Saklad M Grading of Patients for Surgical Procedures. Anesthesiology 1941: 2:281–284. [Google Scholar]
- 21.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996: 17:343–346. [DOI] [PubMed] [Google Scholar]
- 22.(2018). Variable selection techniques for the Cox proportional hazards model: A comparative study. Available: https://core.ac.uk/download/pdf/152600668.pdf[accessed June 22, 2021].
- 23.Margonis GA, Amini N, Buettner S et al. The Prognostic Impact of Primary Tumor Site Differs According to the KRAS Mutational Status: A Study By the International Genetic Consortium for Colorectal Liver Metastasis. Ann Surg 2021: 273:1165–1172. [DOI] [PubMed] [Google Scholar]
- 24.Margonis GA, Kim Y, Spolverato G et al. Association Between Specific Mutations in KRAS Codon 12 and Colorectal Liver Metastasis. JAMA Surg 2015: 150:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


