Summary
Objective:
The mechanisms underlying ovarian dysfunction in Polycystic Ovary Syndrome (PCOS) have not been definitively established. Our objective was to perform a detailed examination of ovarian responses to recombinant follicle stimulating hormone (rFSH) in women with PCOS and controls.
Design:
This prospective, cross-over, dose-response study included three rFSH stimulation periods. Each stimulation period involved three consecutive, daily, subcutaneous injections of rFSH administered at a single dose. Low, medium, and high rFSH doses were weight-adjusted, corresponding to 0.5, 1.1, and 2.2 IU/kg/d, respectively. Stimulation periods occurred in randomized order and were separated by eight-week washouts.
Patients:
Thirty participants (8 PCOS, 22 controls) were studied. PCOS was defined by oligomenorrhea and clinical or biochemical androgen excess, excluding other etiologies of ovulatory dysfunction.
Measurements:
Blood samples were obtained for hormone measurements before- and 24 hours after- each rFSH injection.
Results:
Participants with PCOS had significantly greater BMI, AFC, and circulating testosterone, AMH, and LH concentrations compared to controls participants. Baseline estradiol (E2) concentrations were similar in both groups. At the lowest dose of rFSH, PCOS participants did not demonstrate E2 increments, whereas a significant increase occurred in controls. rFSH-induced E2 production per follicle was significantly reduced in PCOS participants compared to controls at all rFSH doses. Increasing T and decreasing AMH concentrations were associated with augmented E2 production per follicle.
Conclusions:
Women with PCOS exhibited diminished initial E2 responses to rFSH compared to controls. These findings suggest that the mechanism of anovulation in PCOS may involve altered ovarian response to gonadotropins.
Keywords: polycystic ovary syndrome, testosterone, estradiol, follicle stimulating hormone, antimüllerian hormone, ovarian function, ovarian follicle
Introduction
Polycystic ovary syndrome (PCOS) is a complex disorder characterized primarily by reproductive and metabolic dysfunction. It is the most common endocrine condition in reproductive-aged women (1,2). Reproductive features of PCOS include excess ovarian androgen production, chronic anovulation, and polycystic ovarian morphology. Notably, up to 74% of women with PCOS experience infertility resulting from ovulatory dysfunction (3). Although PCOS is the leading cause of anovulatory infertility, the mechanisms underlying ovulatory failure have not been definitively established (4). Several considerations have been suggested including deficiency or inhibition of ovarian aromatase activity, insufficient FSH signaling, premature follicle luteinization, insulin resistance, and excess ovarian androgen production.
Another potential mechanism contributing to follicle dysfunction in PCOS involves altered granulosa cell (GC) responsiveness to FSH. Several in vitro studies have revealed that cultured GCs removed from follicles of women with PCOS exhibited enhanced E2 secretion following FSH stimulation compared with that observed in cells from follicles of ovulatory women (5–7). Consistent with these findings, a dose-response study conducted in women with PCOS demonstrated significant increases in serum E2 following administration of FSH (8). In contrast, some studies have demonstrated that in women with PCOS undergoing ovulation induction with gonadotropin stimulation, ovarian hormone secretion and follicular development were reduced compared to those observed in ovulatory women (9,10). These variable clinical findings are not necessarily surprising given the logistical difficulty in performing extended, multi-dose response studies in women. In addition, many reported clinical investigations were not primarily designed to address follicle responses to a predetermined range of gonadotropin dose. Moreover, in these studies the role of obesity in PCOS was not precisely addressed and may have been complicit in altered GC function. Several reports have noted reduced ovarian responsiveness to both exogenous gonadotropin and clomiphene citrate administration in obese women with PCOS (11–14).
Given the inconsistent results of prior studies investigating granulosa cell dysfunction in PCOS, we proposed to carefully examine ovarian responses to FSH administration in women with PCOS and women with normal ovarian function. A dose-response study of E2 production following weight-adjusted, subcutaneous, recombinant FSH (rFSH) administration at doses commonly used clinically was performed. We hypothesized that early ovarian responsiveness would be reduced in women with PCOS relative to ovulatory controls.
Materials and Methods
Study Design and Setting
This was a prospective cross-over dose-response study. Participants were recruited through University of California, San Diego Health outpatient clinics and advertisements in the San Diego area from September 2017 to July 2019. The study protocol was registered with ClinicalTrials.gov (NCT03252223), was approved by the Human Research Protection Program at the University of California, San Diego (IRB #170684), and conforms to the US Federal Policy for the Protection of Human Subjects. Written informed consent was obtained from each individual prior to participation. The main study procedures were performed at the Altman Clinical and Translational Research Institute at the University of California, San Diego.
Participants
Eight participants with PCOS and 22 ovulatory control (OC) participants with regular menstrual cycles were enrolled in this study. Participants were aged 18 – 37 years, with BMI between 18 and 37. OC subjects reported regular menstrual cycles with luteal symptomatology and lacked signs and symptoms of endocrinopathy. PCOS participants were oligomenorrheic or amenorrheic and demonstrated evidence of androgen excess either biochemically (serum T > 55 ng/mL) and/or clinically (Ferriman-Gallwey score > 8). Alternative etiologies of ovulatory dysfunction and hyperandrogenemia were excluded by history, physical, and laboratory evaluation. Each individual with PCOS exhibited ultrasonographic evidence of bilaterally enlarged ovaries with 20 or more follicles per ovary measuring 2-9 mm in diameter (15). Volunteers with diabetes mellitus or known thyroid, renal, hepatic, cardiac dysfunction were excluded. No participant received any hormone medication or metformin within two months of initiating the study.
Among the 8 participants with PCOS, six completed the study and two withdrew after completing the first rFSH dose-response assessment. Of the 22 enrolled OC participants, 16 completed the study and six withdrew after providing consent (four after completion of the first dose-response assessment, and two after completing two dose-response protocols). The most common reasons for withdrawing from the study were desired initiation of hormonal contraception and scheduling difficulties.
Procedures
Participants were admitted to the Altman Clinical and Translational Research Institute at the University of California, San Diego on the days of testing. Women with PCOS were studied on a random day. OC participants were studied during the mid-follicular phase (cycle days 5 – 9) in order to approximate basal estradiol levels in PCOS subjects (16,17). None of the participants with PCOS experienced recent ovulation, as evidenced by the absence of recent menstrual bleeding for 2 months before the study, absence of a corpus luteum visualized at baseline ultrasound, and serum progesterone (P4) level less than 1.0 ng/mL at baseline sample.
Each subject underwent rFSH dose-response testing at three different doses assigned randomly and separated by a washout interval of eight weeks. At baseline prior to each stimulation phase, height and weight were measured for calculation of Body Mass Index (BMI) and weight-adjusted rFSH dose. After baseline studies were obtained, rFSH (GONAL-f, EMD Serono) was injected subcutaneously every 24 hours for three days at weight-adjusted doses of 0.5 IU/kg/d, 1.1 IU/kg/d, and 2.2 IU/kg/d, rounded to the nearest 1.0 IU. Doses were selected to be equivalent to 37.5, 75, and 150 IU for a person weighing 70 kg (average-weight for a woman in the United States according to the Centers for Disease Control and Prevention) (18). Each rFSH dose was administered between 8 and 10 am. Blood samples were obtained for daily hormone measurements immediately before and 24 ± 2 hours after each rFSH injection.
Three-dimensional, transvaginal ultrasonography (Voluson E8 Expert; GE Healthcare, Chicago, IL) using a 4- to 9-MHz probe (RIC5-9-D Endocavity transducer; GE Healthcare, Chicago, IL) was performed immediately before commencing, and approximately 24 hours after receiving the final dose of rFSH during each stimulation cycle to evaluate each ovary using the SonoAVC™ software. Assessment of ovarian morphology included the total antral follicle count (AFC) of both ovaries in each subject as well as the size of each follicle as determined by its diameter in 1-mm increments. Images were also assessed for development of a lead follicle (defined as a new follicle greater than 10mm in average diameter occurring after rFSH stimulation).
Hormone Measurements
Serum testosterone (T) concentrations were measured using liquid chromatography with tandem mass spectrometry (LC-MS/MS) according to previously published methods (19). AC Extraction Plate™ (AC Plate), from Tecan Schweiz, was used with automated liquid handling for LC-MS/MS sample preparation. Method performance was excellent, with a reportable range of 4–1560 ng/dL (0.14–54.13 nmol/L), between-day CV <6%, mean accuracy for CRM of <4.0% bias, and mean bias of 1.3% vs 4 other LC-MS/MS methods for determination of serum testosterone levels.
Serum FSH, LH, E2, AMH, P4, and 17-hydroxyprogesterone concentrations were measured by the Ligand Assay and Analysis Core Laboratory of the University of Virginia Center for Research in Reproduction. LH and FSH were measured by chemiluminescent immunoassay (Immulite 2000, Siemens Healthcare, Tarrytown, NY). Assay characteristics for FSH (Cat # L2KFS2) were as follows: sensitivity = 0.1 mIU/ml; intra-assay coefficient of variation (CV) = 3.2%; inter-assay CV = 4.1%. For LH (Cat # L2KLH2), sensitivity = 0.1 mIU/ml; intra-assay CV = 3.9%; inter-assay CV = 6.7%. For P4, sensitivity = 0.1 ng/mL; intra-assay CV = 4.4%, and inter-assay CV 5.8%. E2 was measured by radioimmunoassay, using a commercial kit (MP Biomedicals; Orangeburg, NY; Cat #07-238102). Assay characteristics were as follows: sensitivity =10 pg/mL; intra-assay CV = 7.9%; inter-assay CV = 9.8%. AMH was measured by enzyme-linked immunosorbent assay using a commercial kit (ANSH, Webster, TX; Cat # AL-105). Assay characteristics were as follows: sensitivity = 0.16 ng/mL; intra-assay CV = 3.2%; inter-assay CV = 7.0%. 17-hydroxyprogesterone was measured by enzyme-linked immunosorbent assay, using a commercial kit (ALPCO, Salem, NH, Cat # 20-17OHU-E01). Assay characteristics were as follows: sensitivity = 0.034 ng/mL; intra-assay CV = 7.9%; inter-assay CV = 9.8%.
Statistical Analysis
Descriptive statistics are presented n (%) for categorical variables. A χ2 test was used to compare categorical variables between the two participant groups. A Fisher’s exact test was used if the frequency was small. For continuous variables, normality was assessed using the Shapiro Wilk test. Normally distributed continuous variables are presented as mean ± standard error of the mean (SEM), while non-normally distributed variables are presented as median (range). The primary outcome of interest in the study was granulosa cell function, as defined by peak serum E2 level. The secondary outcome was estradiol production per antral follicle. Within group changes in absolute serum E2 from baseline to 72-hour peak were compared with Wilcoxon signed-rank tests or paired student’s t-tests, depending on normality. E2 production per follicle was compared between PCOS and OC groups using Mann Whitney U or independent sample t-tests. Differences in E2 response were analyzed using linear mixed effects modeling with random intercept structure while adjusting for age, BMI, basal T concentration, and AMH level. Analysis of variance was used to evaluate the significance of predictors with more than two levels. For all comparisons, two-tailed significance level was set using an alpha level of 0.05. All analyses were performed using R software, version 4.0.2 (R Project for Statistical Computing).
Based on prior data demonstrating peak E2 response following intramuscular Human Menopausal Gonadotropin administration to be 311 ± 55 pg/mL in ovulatory women, and 128 ± 30 pg/mL in participants with PCOS (9), a sample size of 10 total participants (5 per group) was estimated to have 95% power to be able to a similar difference in E2 response between PCOS and OC groups in this study.
Results
Study Cohort
Baseline demographics and clinical information for all participants in this study are displayed in Table 1. The median age of our participants was 24.0 years (range 18.1 – 36.6 years). Overall, the study population was diverse, including over one-third reporting non-white race (16.7% Asian and 10.0% Black). Nearly 30% reported Hispanic ethnicity. Age, race, and ethnic distribution did not differ between PCOS and OC groups.
Table 1.
Cohort Baseline Characteristics
| PCOS (n = 8) | OC (n = 22) | P-value | |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Age (years) | 22.9 (18.1 – 29.1) | 26.8 (18.6 – 36.6) | 0.22 |
|
| |||
| Race | 0.66 | ||
| White | 5 (62.5%) | 17 (77.3%) | |
| Black | 1 (12.5%) | 2 (9.1%) | |
| Asian/Pacific Islander | 2 (25%) | 3 (13.6%) | |
|
| |||
| Ethnicity | 0.39 | ||
| Hispanic | 1 (12.5%) | 7 (31.8%) | |
| Non-Hispanic | 7 (87.5%) | 15 (68.2%) | |
|
| |||
| Biometric Characteristics | |||
|
| |||
| Weight (kg) | 72.0 (54.8 – 119.3) | 56.1 (46.7 – 85.6) | 0.10 |
|
| |||
| Body Mass Index | 29.4 (21.3 – 36.3) | 22.9 (18.0 – 32.5) | 0.05 |
|
| |||
| Antral Follicle Count | 59.5 (31 – 76) | 24.5 (12 – 52) | <0.01 |
|
| |||
| Basal Hormone Values | |||
|
| |||
| Follicle Stimulating Hormone (IU/L) | 5.0 (4.7 – 9.3) | 7.0(3.6 – 10.9) | 0.14 |
|
| |||
| Luteinizing Hormone (IU/L) | 11.3 (4.3 – 15.8) | 4.8 (2.2 – 9.5) | <0.01 |
|
| |||
| Estradiol (pg/mL) | 78.6 (40.9 – 141.7) | 66.2 (29.7 – 148.8) | 0.34 |
|
| |||
| Total Testosterone (ng/dL) | 39.6 (21.6 – 56.8) | 23.5 (12.1 – 36.0) | <0.01 |
|
| |||
| 17-hydroxyprogesterone (ng/mL) | 1.0(0.7 – 1.6) | 0.8 (0.5 – 1.5) | 0.19 |
|
| |||
| Antimüllerian Hormone (ng/mL) | 12.6 (8.1 – 38.7) | 6.1 (1.9 – 15.4) | <0.01 |
Number (n), median (range), and number (percent) for participant baseline characteristics. Bold type indicates significant differences between Ovulatory Control (OC) and Polycystic Ovary Syndrome (n=8) participants at baseline, with a p-value < 0.05.
Baseline Clinical Data
Basal serum hormone levels are shown in Table 1. Consistent with the diagnosis, participants with PCOS had significantly greater BMI, AFC, and circulating T, LH, and AMH concentrations compared to OC participants. The mean baseline FSH and E2 values were similar between groups. One amenorrheic PCOS subject, after completing testing at two FSH doses, was incidentally found to have a P4 level 1.4 ng/mL on baseline evaluation, indicating recent ovulation. Accordingly, the ensuing rFSH stimulation cycle was excluded from the analysis below.
Serum Estradiol Responses to FSH Stimulation
The daily change in E2 response over 72-hours at each weight-based rFSH dose is shown for OC and PCOS participants in Fig. 1. In OC participants, E2 responses to low- and medium-dose rFSH stimulation were qualitatively similar, while a marked increase was noted after high-dose stimulation. In participants with PCOS, there appeared to be no increase in E2 level following administration of low-dose rFSH, although increases were noted after low- and medium-dose stimulation.
Figure 1.
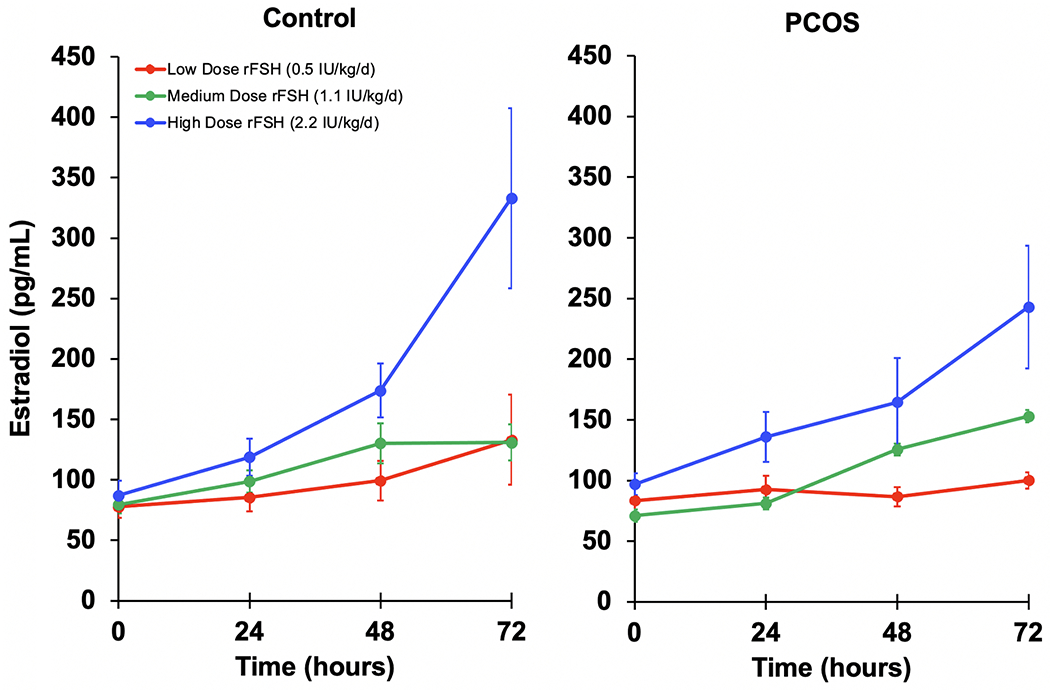
Daily incremental change in rFSH-induced in mean (± SE) serum estradiol (E2) levels by participant group and rFSH dose. Ovulatory Control group (OC, n=22) and Polycystic Ovary Syndrome (PCOS, n=8).
As shown in Fig. 2, OC participants exhibited significant increases in peak serum E2 from baseline to peak at 72 hours following daily administration of rFSH at low dose (baseline 77.8 ± 8.9 pg/ml to peak 135.1 ± 35.3 pg/ml; P<0.01), medium dose (79.5 ± 6.9 pg/ml to 146.6 ± 16.5 pg/ml, P<0.01), and high dose (88.2 ± 11.4 pg/ml to 316.8 ± 70.5 pg/ml, P<0.001). In contrast, among PCOS participants, statistically significant increases in serum E2 levels were not detected after administration of low dose rFSH (79.6 ± 13.1 pg/ml to 100.7 ± 9.1 pg/ml, P>0.05) (Fig. 2a). However, peak serum E2 levels in PCOS participants did increase significantly from baseline after medium dose (71.1 ± 8.3 pg/ml to 153.0 ± 23.9 pg/ml, P<0.01) and high dose stimulation (95.1 ± 10.5 pg/ml to 250.9 ± 59.1, P=0.04) (Fig. 2b and 2c).
Figure 2.
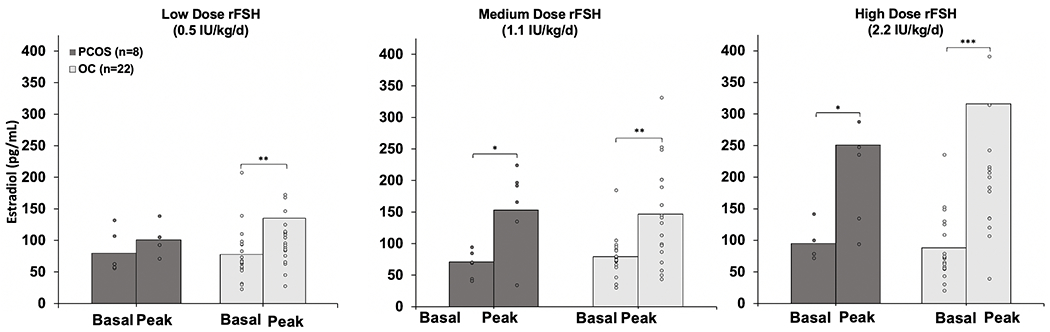
Mean (± SE) baseline and peak serum E2 levels after administration of rFSH in Ovulatory Control (OC, n=22) and Polycystic Ovary Syndrome (PCOS, n=8) participants at doses of low (0.5 IU/kg/d), medium (1.1 IU/kg/d), and high (2.2 IU/kg/d). Significant change from baseline to peak is denoted by asterisks. *, P<0.05; **, P<0.01; ***, P<0.001.
In multivariable analysis, peak E2 response was associated with FSH dose, PCOS diagnosis, BMI, and basal E2 concentration after adjusting for the effects of age and AMH level (Table 2). Among patient characteristics, PCOS diagnosis had the largest magnitude effect on FSH responsiveness, with PCOS participants on average having mean peak E2 levels 176 pg/mL lower than OC participants. Conversely, increasing BMI and basal E2 levels were correlated with augmented FSH responsiveness, although the magnitude of these effects was modest. As expected, high-dose FSH dosing resulted in higher peak E2 production than low-dose stimulation.
Table 2.
Difference in Peak E2 associated with patient characteristics
| Patient Characteristic | Difference in Peak E2 (95% CI) | P-value |
|---|---|---|
|
| ||
| FSH Dose | ||
| Low Dose | (reference) | |
| Medium Dose | 27.4 (−62.6, 117.4) | 0.55 |
| High Dose | 153.1 (60.7, 245.5) | <0.01 |
|
| ||
| Diagnosis | ||
| Ovulatory Controls | (reference) | |
| Polycystic Ovary Sundrome | −175.5 (−308.2, −42.9) | 0.01 |
|
| ||
| Age (per year) | −3.3 (−10.5, 3.9) | 0.37 |
|
| ||
| Body Mass Index (per point) | 10.5 (2.0, 19.1) | 0.02 |
|
| ||
| Total Testosteronea (per ng/mL) | 4.4 (−0.6, 9.4) | 0.08 |
|
| ||
| Antimüllerian Hormonea (per ng/mL) | 0.3 (−6.3, 6.9) | 0.93 |
|
| ||
| Estradiola (per pg/mL) | 1.6 (0.6, 2.6) | <0.01 |
Pooled analysis (n=30) of the difference in Peak E2 associated with patient variables as determined by linear mixed effects modeling with random intercept structure while adjusting for age, body mass index, total testosterone concentration, and antimüllerian hormone level.
Baseline levels. Bold text indicates a statistically significant difference with a p-value < 0.05.
Ovarian Follicle Characteristics Before and After rFSH Stimulation
When compared to OC participants, those with PCOS had markedly greater follicle number at baseline, as expected. Total AFC was positively correlated with serum AMH in both groups (P < 0.001). Consistent with our previously reported findings (20), analysis of follicles by 1-mm increments revealed significantly increased the number of follicles at 2.0- to 2.9-mm through 6.0- to 6.9-mm diameter in women with PCOS compared with controls (Supplemental Fig. 1a). At larger sizes, total follicle numbers were not statistically different when compared between groups.
Among the 6 PCOS subjects who underwent all three rFSH stimulations, three developed a lead follicle greater than 10mm once in three stimulation cycles (two following medium dose and one following low dose stimulation). One participant developed a pre-ovulatory follicle in two of three cycles (following medium and high dose stimulation). The remaining two subjects did not develop a dominant follicle in response to any of the rFSH stimulation doses and no PCOS subject developed a lead follicle in all three subjects. In contrast, 100% of OC subjects who completed all three stimulation periods developed a pre-ovulatory follicle at least once during the study, with 65% developing a lead follicle within 72 hours of FSH initiation at all three doses. One of the six PCOS participants who underwent low dose rFSH stimulating developed a lead follicle, compared to 90.9% of OC subjects (P < 0.01). The rate of lead follicle development following medium rFSH stimulation in the PCOS group was 50%, compared to 86.4% of the OC subjects (P > 0.05). Following high dose stimulation, 33.3% PCOS participants developed a lead follicle compared to 86.4% in the OC group (P = 0.02).
Consistent with greater AFC in the PCOS group, E2 production per follicle was reduced 2- to 3-fold in PCOS participants compared to their OC counterparts at each of the three rFSH doses administered (Fig. 3). In multivariable analysis, follicular E2 production was predicted by FSH dose, PCOS diagnosis, AMH, and basal T concentration after adjusting for the effects of age and BMI (Table 3). As seen with our primary outcome of peak E2 concentration, PCOS diagnosis had a large negative effect on FSH responsiveness, with PCOS subjects producing less E2 per follicle than OC participants. Interestingly, basal T concentration was positively correlated with E2 production per follicle, while serum AMH level was negatively correlated.
Figure 3.
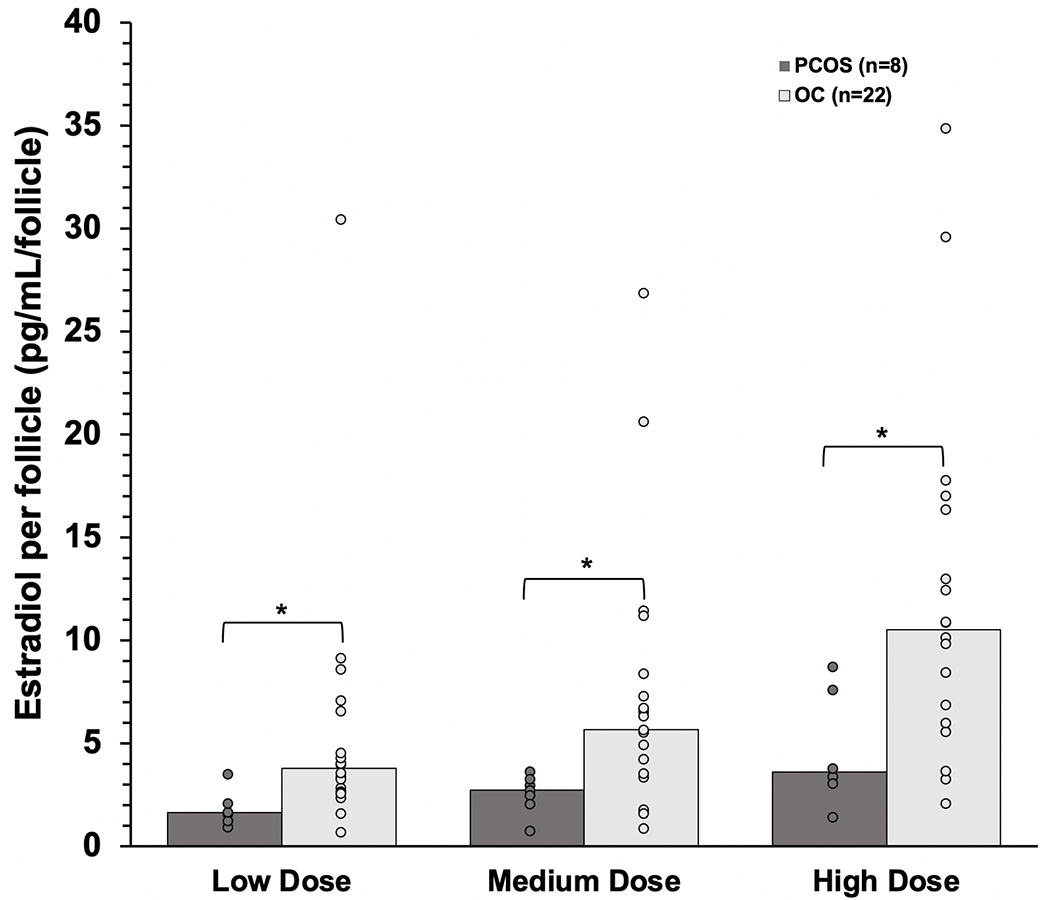
Between group comparison of rFSH-induced peak estradiol (E2) production per follicle in Ovulatory Control (OC, n=22) and Polycystic Ovary Syndrome (PCOS, n=8) participants after low dose (0.53 IU/kg/d), medium dose (1.1 IU/kg/d), and high dose (2.2 IU/kg/d) stimulation. Significant differences between groups are denoted by asterisks. *, P<0.05; **, P<0.01; ***, P<0.001.
Table 3.
Difference in E2 production per follicle associated with patient characteristics (Adjusted Analysis)
| Patient Characteristic | Difference in E2 production per follicle (95% CI) | P-value |
|---|---|---|
|
| ||
| FSH Dose | ||
| Low Dose | (reference) | |
| Medium Dose | 1.2 (−2.0, 4.4) | 0.47 |
| High Dose | 5.2 (2.0, 8.5) | <0.01 |
|
| ||
| Diagnosis | ||
| Ovulatory Controls | (reference) | |
| Polycystic Ovary Syndrome | −4.9 (−9.7, −0.3) | 0.04 |
|
| ||
| Age (per year) | 0.0 (−0.2, 0.3) | 0.85 |
|
| ||
| Body Mass Index (per point) | −0.1 (−0.4, 0.2) | 0.63 |
|
| ||
| Total Testosteronea (per ng/mL) | 0.2 (0.04, 0.38) | 0.02 |
|
| ||
| Antimüllerian Hormonea (per ng/mL) | −0.4 (−0.6, −0.1) | <0.01 |
Pooled, adjusted analysis (n=30) of the difference in Peak E2 production per follicle (pg/mL/follicle) associated with patient variables as determined by linear mixed effects modeling with random intercept structure while adjusting for age, body mass index, total testosterone concentration, and antimüllerian hormone level.
Baseline levels. Bold text indicates a statistically significant difference with a p-value < 0.
Discussion
The results of our study have demonstrated that early GC responses to the lowest FSH dose administered were undetectable in women with PCOS, compared to significant increases of E2 observed in ovulatory women treated similarly. Notably, E2 production per follicle was significantly reduced in participants with PCOS compared to OC at all doses of FSH. Overall, E2 responsiveness was positively correlated with increasing FSH dose and BMI after adjusting for the effects of age and AMH level. We further found that higher T concentrations and lower AMH levels were associated with augmented E2 production per follicle. These findings may provide some insight into the etiology of follicle dysfunction in PCOS and initial ovarian responses to FSH administration in women with PCOS undergoing ovulation induction.
It has been well recognized that ovulatory failure in women with PCOS is associated with arrest of follicle development at the mid-antral stage. This disruption of follicle maturation is accompanied by in vitro evidence of abnormal GC responses to FSH. While basal E2 production by cultured PCOS GCs has been reported to be greater than that exhibited by normal GCs, exposure to saturating doses of FSH failed to sustain commensurate increases of E2 over periods of prolonged stimulation (21). The precise mechanisms underlying this diminished E2 response to the highest dose of FSH by PCOS GCs was unclear, although the presence of increased FSH receptor binding in PCOS GCs as well as ample bioactive FSH in PCOS follicular fluid suggests down regulation or a defect in FSH signaling (5,21,22). In keeping with the concept of abnormal GC function in this disorder, altered steroid production in GCs obtained from individual follicles dissected from PCOS ovaries have been described (23,24). Moreover, greater E2 production from PCOS GCs appeared to be advanced by follicle size compared to cells from comparably sized follicles from ovulatory women (7,25). Collectively, these in vitro findings demonstrated that E2 production by PCOS GCs tends to be greater and occurs at an earlier stage of development than that of normal GCs from comparable sized follicles.
These in vitro findings have been complemented by in vivo clinical studies that have shown increased E2 responses to gonadotropin stimulation in women with PCOS compared to ovulatory women. In our previous study, a relatively greater increase in E2 production was achieved in PCOS participants compared to control participants following a single intravenous administration of FSH at a dose of 150 IU, whereas E2 responses to 37.5 IU and 75 IU produced equivalent E2 increments among PCOS women and controls (8). Higher E2 production in PCOS participants was attributed to increased GC responsiveness as well as greater follicle number. In the current study, we employed subcutaneous FSH dosing similar to those commonly used clinically for ovarian stimulation during ovulation induction. Our experimental design allowed us to discern a threshold dose at which E2 production in PCOS women was negligible compared to a corresponding significant increase observed in OC. Whether a prolonged duration of low dose FSH administration may have been necessary to induce E2 production at such a low dose is unclear.
Our results also appear to contrast with prior studies in women with PCOS demonstrating an association between obesity and reduced ovarian responsiveness to both exogenous gonadotropin stimulation and to modest increases in endogenous FSH induced by clomiphene citrate (11–14,26). Indeed, in our study when rFSH was administered in weight-based fashion, increasing BMI was similarly associated with enhanced total E2 secretion in participants with PCOS. However, administration of rFSH at the lowest dose revealed a distinctive and significant lack of E2 response in the PCOS group. This finding may reflect that previous studies were not designed to test granulosa cell responses to minimal amounts of FSH, particularly at doses utilized in ovarian stimulation protocols for ovulation induction. Alternatively, intraovarian, metabolic, or other endocrine factors may alone, or synergistically with weight, influence ovarian responses to FSH stimulation.
We found that T augmented- and AMH suppressed- E2 production on an individual follicle level. The finding that basal T concentration was positively correlated with per follicle E2 production is consistent with previous studies show that androgens enhance estrogen responses to FSH stimulation in GCs from rodents and non-human primates (27–31). This facilitatory effect of T on GC responsiveness is underscored by colocalization of androgen receptor and FSH receptor on GCs of growing follicles (32). Importantly, induction of FSH receptor expression has also been demonstrated in GCs of non-human primates treated with T in vivo (32). In contrast, the negative correlation between AMH and follicular E2 production observed in the present study is consistent with resistance to gonadotropin stimulation observed in women with PCOS in vivo (12), and with in vitro evidence of reduced aromatase expression and activity induced by AMH exposure (33,34).
Our study is limited by a relatively low number of PCOS participants. However, each individual participant was studied longitudinally, minimizing the risk of confounding by allowing each subject to serve as their own control. In addition, we did not include assessment of circulating levels of insulin that may have influenced GC production of E2. The adjustment for weight in our study design may not have removed any influence imposed by hyperinsulinemia. Our study design included comparison of E2 responses to rFSH in PCOS subjects to those of ovulatory women in the mid-follicular phase of their cycles. This goal of this strategy was to match serum E2 levels in both groups, thereby providing a consistent baseline value. In addition, testing performed in this phase of the normal cycle increased the likelihood of an E2 response to low doses of FSH administered subcutaneously. Finally, the relative distribution of early- and mid-antral follicles appeared to be similar in both groups, which only underscored the lesser E2 response in the PCOS group.
In summary, our results show in women with PCOS, low dose rFSH stimulation failed to induce observable increases in E2 production, suggesting altered GC responsiveness in this disorder. Moreover, we found that ovarian responsiveness to FSH is positively correlated with BMI and basal T concentration, while exhibiting an inverse relationship with AMH concentration. Taken together, these associations suggest abnormal GC function in women with PCOS, as demonstrated by their diminished E2 responses to FSH stimulation in this study. Future investigation is warranted to evaluate responsiveness to extended FSH administration to determine whether enhanced ovarian stimulation may be eventually achieved by prolonged exposure to gonadotropins.
Supplementary Material
Acknowledgements:
This work was partially supported by the National Institutes of Health Grant UL1TR001442 of CTSA, Grant P50 HD012303, Grant K12 HD001259, and Grant T32 HD007203. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- 3.Goldzieher JW, Axelrod LR. Clinical and Biochemical Features of Polycystic Ovarian Disease. Fertil Steril. 1963;14:631–653. [DOI] [PubMed] [Google Scholar]
- 4.Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14(4):367–378. [DOI] [PubMed] [Google Scholar]
- 5.Erickson GF, Magoffin DA, Cragun JR, Chang RJ. The effects of insulin and insulin-like growth factors-I and -II on estradiol production by granulosa cells of polycystic ovaries. J Clin Endocrinol Metab. 1990;70(4):894–902. [DOI] [PubMed] [Google Scholar]
- 6.Mason HD, Willis DS, Holly JM, Franks S. Insulin preincubation enhances insulin-like growth factor-II (IGF-II) action on steroidogenesis in human granulosa cells. J Clin Endocrinol Metab. 1994;78(5):1265–1267. [DOI] [PubMed] [Google Scholar]
- 7.Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83(11):3984–3991. [DOI] [PubMed] [Google Scholar]
- 8.Coffler MS, Patel K, Dahan MH, et al. Evidence for abnormal granulosa cell responsiveness to follicle-stimulating hormone in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(4):1742–1747. [DOI] [PubMed] [Google Scholar]
- 9.DeFazio J, Meldrum DR, Lu JK, et al. Acute ovarian responses to a long-acting agonist of gonadotropin-releasing hormone in ovulatory women and women with polycystic ovarian disease. Fertil Steril. 1985;44(4):453–459. [DOI] [PubMed] [Google Scholar]
- 10.Caruso A, Fortini A, Fulghesu AM, et al. Ovarian sensitivity to follicle-stimulating hormone during the follicular phase of the human menstrual cycle and in patients with polycystic ovarian syndrome. Fertil Steril. 1993;59(1):115–120. [DOI] [PubMed] [Google Scholar]
- 11.White DM, Hardy K, Lovelock S, Franks S. Low-dose gonadotropin induction of ovulation in anovulatory women: still needed in the age of IVF. Reproduction. 2018;156(1):F1–F10. [DOI] [PubMed] [Google Scholar]
- 12.Koninger A, Sauter L, Edimiris P, et al. Predictive markers for the FSH sensitivity of women with polycystic ovarian syndrome. Hum Reprod. 2014;29(3):518–524. [DOI] [PubMed] [Google Scholar]
- 13.Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest. 1976;57(5):1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobo RA, Gysler M, March CM, Goebelsmann U, Mishell DR Jr., Clinical and laboratory predictors of clomiphene response. Fertil Steril. 1982;37(2):168–174. [PubMed] [Google Scholar]
- 15.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110(3):364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nestler JE, Powers LP, Matt DW, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72(1):83–89. [DOI] [PubMed] [Google Scholar]
- 17.Robinson S, Kiddy D, Gelding SV, et al. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin Endocrinol (Oxf). 1993;39(3):351–355. [DOI] [PubMed] [Google Scholar]
- 18.Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007-2010. Vital Health Stat 11. 2012(252):1–48. [PubMed] [Google Scholar]
- 19.Stone Judith A, Fitzerald Robert L. Automated Sample Preparation Enables LC-MS/MS as a Routine Diagnostic Analysis for Serum Testosterone. The Journal of Applied Laboratory Medicine. 2017;2(1):33–46. [DOI] [PubMed] [Google Scholar]
- 20.Homer MV, Toloubeydokhti T, Lawson MA, Garzo G, Duleba AJ, Chang RJ. Individual 17-Hydroxyprogesterone Responses to hCG Are Not Correlated With Follicle Size in Polycystic Ovary Syndrome. J Endocr Soc. 2019;3(4):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson GF, Magoffin DA, Garzo VG, Cheung AP, Chang RJ. Granulosa cells of polycystic ovaries: are they normal or abnormal? Hum Reprod. 1992;7(3):293–299. [DOI] [PubMed] [Google Scholar]
- 22.Almahbobi G, Anderiesz C, Hutchinson P, McFarlane JR, Wood C, Trounson AO. Functional integrity of granulosa cells from polycystic ovaries. Clin Endocrinol (Oxf). 1996;44(5):571–580. [DOI] [PubMed] [Google Scholar]
- 23.Comim FV, Teerds K, Hardy K, Franks S. Increased protein expression of LHCG receptor and 17alpha-hydroxylase/17-20-lyase in human polycystic ovaries. Hum Reprod. 2013;28(11):3086–3092. [DOI] [PubMed] [Google Scholar]
- 24.Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab. 2001;86(3):1318–1323. [DOI] [PubMed] [Google Scholar]
- 25.Mason HD, Willis DS, Beard RW, Winston RM, Margara R, Franks S. Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluid. J Clin Endocrinol Metab. 1994;79(5):1355–1360. [DOI] [PubMed] [Google Scholar]
- 26.Guedikian AA, Lee AY, Grogan TR, et al. Reproductive and metabolic determinants of granulosa cell dysfunction in normal-weight women with polycystic ovary syndrome. Fertil Steril. 2018;109(3):508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tetsuka M, Hillier SG. Androgen receptor gene expression in rat granulosa cells: the role of follicle-stimulating hormone and steroid hormones. Endocrinology. 1996;137(10):4392–4397. [DOI] [PubMed] [Google Scholar]
- 28.Harlow CR, Shaw HJ, Hillier SG, Hodges JK. Factors influencing follicle-stimulating hormone-responsive steroidogenesis in marmoset granulosa cells: effects of androgens and the stage of follicular maturity. Endocrinology. 1988;122(6):2780–2787. [DOI] [PubMed] [Google Scholar]
- 29.Kirilovas D, Naessen T, Bergstrom M, et al. Effects of androgens on aromatase activity and 11C-vorozole binding in granulosa cells in vitro. Acta Obstet Gynecol Scand. 2003;82(3):209–215. [PubMed] [Google Scholar]
- 30.Chan WK, Tan CH. FSH-induced aromatase activity in porcine granulosa cells: non-competitive inhibition by non-aromatizable androgens. J Endocrinol. 1986;108(3):335–341. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal SK, Judd HL, Magoffin DA. A mechanism for the suppression of estrogen production in polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81 (10):3686–3691. [DOI] [PubMed] [Google Scholar]
- 32.Weil SJ, Vendola K, Zhou J, et al. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83(7):2479–2485. [DOI] [PubMed] [Google Scholar]
- 33.di Clemente N, Ghaffari S, Pepinsky RB, et al. A quantitative and interspecific test for biological activity of anti-mullerian hormone: the fetal ovary aromatase assay. Development. 1992;114(3):721–727. [DOI] [PubMed] [Google Scholar]
- 34.Pellatt L, Rice S, Dilaver N, et al. Anti-Mullerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil Steril. 2011;96(5):1246–1251 e1241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


