Introduction
Guidelines from the American College of Physicians (ACP) recommend use of a thiazide diuretic, alkali citrate therapy, or allopurinol in patients with kidney stone disease, who suffer recurrences despite increased fluid intake.1 The ACP guidelines do not endorse 24-hour urine testing prior to prescribing one of these preventive pharmacological therapy (PPT) agents. Such an empiric approach to stone prevention is supported by emerging data that show comparable rates of stone-related events among patients on PPT, regardless of whether 24-hour urine testing was performed prior to prescribing.2,3
Importantly, the ACP guidelines also do not differentiate between the three classes of PPT agents. Rather the guidelines view them as “monolithic” with respect to their effectiveness. However, there are compelling reasons to believe that their effects may differ. For instance, thiazide diuretics can cause hypocitraturia,4 which is a risk factor for calcium stone formation.5 What is more, alkali citrate therapy increases urine pH, which, if not monitored, could alter one’s calcium phosphate stone risk.6 Further, the benefit of allopurinol is unclear in patients with normal urine uric acid excretion, or those with high urine calcium excretion.7
In the absence of any ongoing or planned clinical trials to directly compare the empiric use of these PPT agents with one another, we conducted an observational study. Specifically, we used medical claims data to identify working-age adults with physician-coded diagnoses of kidney stone disease who were prescribed a thiazide diuretic, alkali citrate therapy, or allopurinol without prior 24-hour urine testing. We then evaluated for differences in the frequency of emergency department (ED) visits, hospitalization, and stone-directed surgery among these patients over a three-year time period based on the class of PPT agent prescribed.
Methods
Data source and study population
We used Optum’s de-identified Clinformatics® Data Mart Database (2008-2018), which is a commercial and Medicare Advantage U.S. database that captures all inpatient, outpatient, ED, and pharmacy encounters for an estimated 83 million beneficiaries. Figure 1 is a flow diagram illustrating our cohort construction.
Figure 1.
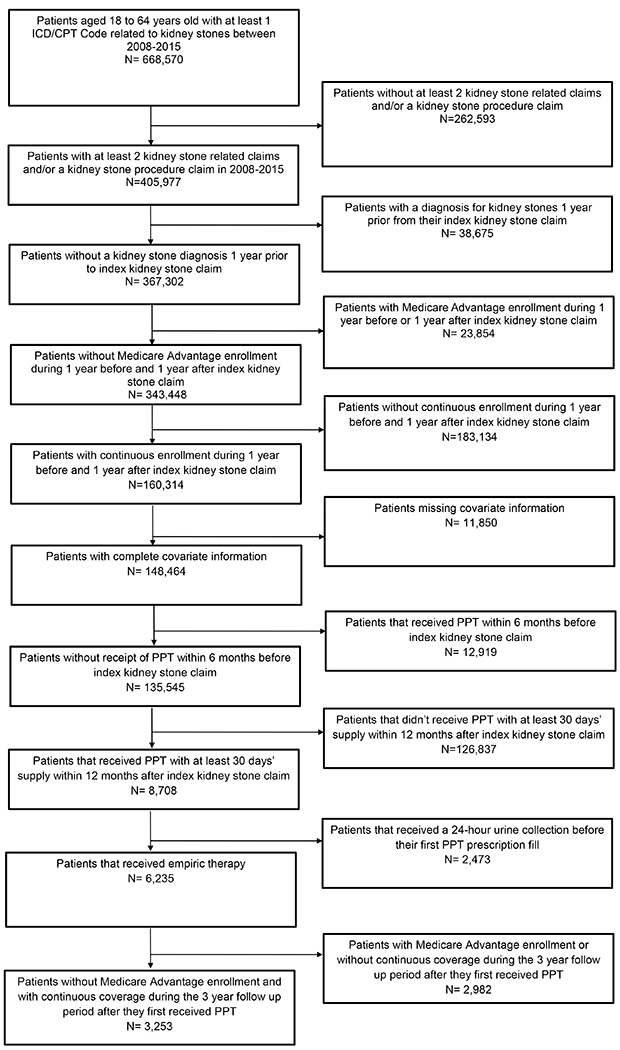
Flowchart for cohort selection
We identified patients aged 18 to 64 years with at least one kidney stone procedure or two kidney stone-related encounters between January 1, 2008, to December 31, 2015. Please see Supplementary Table 1 for a complete list of the Current Procedural Terminology (CPT) and International Classification of Diseases (ICD) codes that we used for this identification. For those identified by kidney stone-related encounters, we indexed based on the earlier of the two encounters. We excluded patients who were insured by Medicare and those without continuous enrollment during the year prior to their index stone encounter to ensure availability of claims for comorbidity adjustment. To make certain of adequate follow-up, we additionally excluded beneficiaries with Medicare enrollment and without continuous enrollment during the three years following receiving PPT.
Distinguishing patients receiving empiric PPT
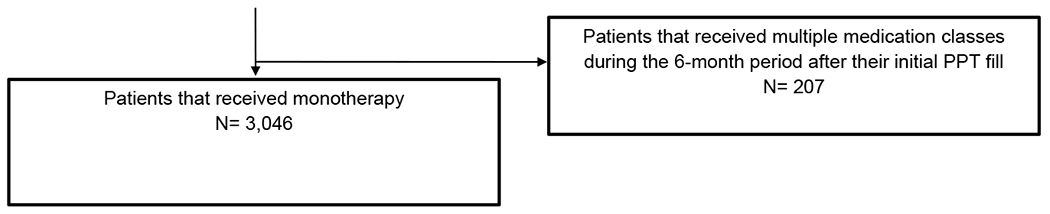
We used appropriate National Drug Codes to identify the subset that had a prescription fill for at least 30 days’ supply of a PPT agent (i.e., thiazides, alkali citrate, or allopurinol) within 12 months of the date after their index stone encounter. A complete list of medications that we considered for PPT can be found in Supplementary Table 2. To guard against PPT prescription fills unrelated to a kidney stone diagnosis, we excluded patients who were prescribed a PPT agent within six months prior to their index stone encounter. Given that our focus was on patients who received empiric PPT, we excluded those who underwent 24-hour urine testing before their first PPT prescription fill using the CPT code for a 24-hour urine oxalate (83945), which is highly specific for kidney stone evaluation.2,8 We excluded patients receiving multiple PPT classes within the first six months after the initial PPT fill in order to facilitate comparisons between different PPT monotherapies.
Assessing kidney stone-related events
We determined recurrence-free probability of a stone-related ED visit, hospitalization, or surgery up to three years after the initial PPT prescription fill. We did not count any events within the first six months after PPT initiation, as these encounters would be unlikely attributable to treatment failure in such a short timeframe. We used relevant place of service codes in the Optum database to identify ED visits and hospitalizations associated with any diagnosis of kidney stone disease during the encounter (see Supplementary Table 1).9,10 We identified stone-related surgery using both CPT and ICD procedure codes (see Supplementary Table 1).
Statistical analysis
For our initial analytic step, we compared patients receiving empiric thiazides, alkali citrate, or allopurinol over a variety of sociodemographic characteristics, including age, gender, race/ethnicity, education, and region of residence. We also examined for differences based on their level of comorbid illness (as defined by the Charlson Index11), PPT adherence (defined as >80% days covered from start of PPT to six months12), and whether there were concurrent diagnoses putting the patient at higher risk for kidney stone recurrence (see Supplementary Table 3).7 We made comparisons using chi-square tests for categorical variables and one-way ANOVA tests for continuous variables. We adjusted our P-values to account for multiple comparisons using the Holm–Bonferroni method.
Next, we compared unadjusted frequencies of stone-related ED visit, hospitalization, both individually and overall, among patients receiving thiazide, alkali citrate, or allopurinol empiric PPT. We then fit a multivariable logistic regression model to estimate the odds of a stone-related event at three years of follow-up, adjusting for the patient factors described above. Given the correlated nature of our data (patients nested within providers), we calculated robust standard errors using the Huber White sandwich estimator.13 From these models, we determined adjusted frequencies of our outcomes by computing predicted population marginal means at each level of the variable of interest.
Finally, we performed sensitivity analyses to test the robustness of our findings. In particular, we refit our regression models, relaxing our continuous medication coverage requirements. To do this, we allowed for up to 14 days and 30 days of gaps in medication coverage.
Finally, for the most common medications for thiazides (hydrochlorothiazide, n=744), alkali citrate (potassium citrate, n=611), and allopurinol, we evaluated for an association between the daily dosage prescribed within each medication subclass and our outcomes of interest.
We conducted all analyses using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC). We performed two-sided significance testing with alpha set at 0.05. The Institutional Review Board at the University of Michigan Health System deemed that this study was exempt from its oversight.
Results
In total, there were 3,046 patients on empiric PPT, of whom 1,834 were prescribed a thiazide, 654 with alkali citrate, and 558 with allopurinol. Table 1 shows the cohort characteristics by PPT class. Patients on allopurinol were less commonly female (13% versus 43% with alkali citrate and 45% with thiazides, p<0.01) and were more likely to have comorbid conditions predisposing to kidney stone formation (44% vs 27% with alkali citrate and 24% with thiazides, p<0.01). Rates of PPT adherence were higher among those on allopurinol and thiazides compared to alkali citrate (allopurinol and thiazides each 38% vs 13% for alkali citrate, p<0.01). Additional differences were observed among the groups by age at diagnosis, race/ethnicity, education, and region of residence.
Table 1.
Comparing patient characteristics by empiric medication class
| Characteristic | Allopurinol (n=558) | Alkali Citrate (n=654) | Thiazides(n=1,834) | P-Value |
|---|---|---|---|---|
| Age in years (%) | <0.01 | |||
| 18 to 34 | 36 (6) | 81 (12) | 124 (7) | |
| 35 to 44 | 106 (19) | 152 (23) | 362 (20) | |
| 45 to 54 | 202 (36) | 213 (33) | 708 (39) | |
| 55 to 64 | 214 (38) | 208 (32) | 640 (35) | |
| Female gender (%) | 74 (13) | 280 (43) | 820 (45) | <0.01 |
| Race/ethnicity (%) | <0.01 | |||
| White | 427 (77) | 487 (74) | 1,371 (75) | |
| Black | 50 (9) | 50 (8) | 215 (12) | |
| Other | 81 (15) | 117 (18) | 248 (14) | |
| Education (%) | 0.02 | |||
| High school or less | 155 (28) | 182 (28) | 514 (28) | |
| Some college | 302 (54) | 347 (53) | 1,058 (58) | |
| College or more | 101 (18) | 125 (19) | 262 (14) | |
| Region of residence (%) | <0.01 | |||
| East North Central | 77 (14) | 83 (13) | 282 (15) | |
| East South Central | 24 (4) | 19 (3) | 91 (5) | |
| Middle Atlantic | 22 (4) | 60 (9) | 100 (5) | |
| Mountain | 58 (10) | 48 (7) | 120 (7) | |
| New England | 14 (3) | 26 (4) | 50 (3) | |
| Pacific | 66 (12) | 60 (9) | 130 (7) | |
| South Atlantic | 161 (29) | 216 (33) | 595 (32) | |
| West North Central | 50 (9) | 51 (8) | 170 (9) | |
| West South Central | 86 (15) | 91 (14) | 296 (16) | |
| Charlson comorbidity index, mean (SD) | 0.4 (1.1) | 0.4 (1.1) | 0.4 (1.0) | 0.96 |
| Adherent (%) | 213 (38) | 86 (13) | 690 (38) | <0.01 |
| High Risk Stone Former(%) | 245 (44) | 176 (27) | 431 (24) | <0.01 |
Abbreviation: SD, standard deviation. IQR, interquartile range.
Unadjusted and adjusted rates of kidney stone-related events up to three years are shown in Figure 2 by PPT class. The unadjusted rate of any stone event was lowest for the thiazide group (14.8%) compared to allopurinol (21.0%) and alkali citrate (21.9%; allopurinol vs thiazide p=0.001, alkali citrate vs thiazide p<0.001). These differences favoring thiazides persisted even after adjustment for patient factors (thiazides 14.8%, allopurinol and alkali citrate each 20.4%; allopurinol vs thiazides p=0.004, alkali citrate vs thiazide p=0.001). Put differently, thiazides, when compared to allopurinol, were associated with 32% lower odds of a subsequent stone event by three years (OR 0.68, 95% CI 0.53-0.88). No such association was observed when comparing alkali citrate to allopurinol (OR 1.00, 95% CI 0.75-1.34).
Figure 2.
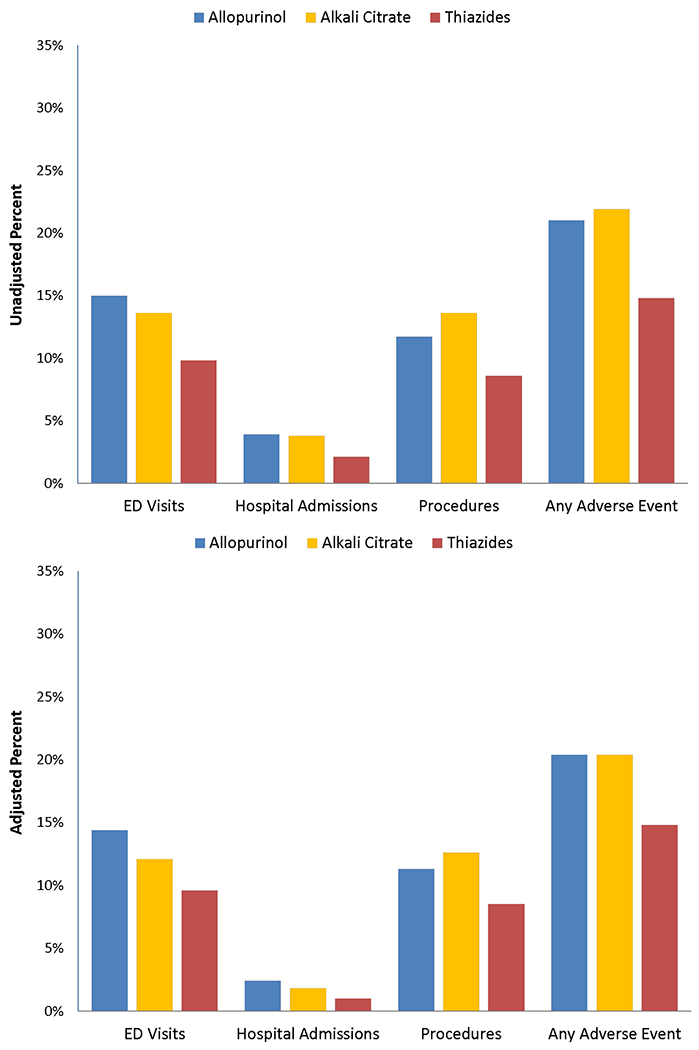
Unadjusted and adjusted rates of symptomatic stone recurrence over 3 years comparing empiric preventive pharmacologic monotherapies.
Supplementary Table 5 shows that, when examining individual stone-related outcomes, the thiazide PPT cohort had the lowest adjusted probability of stone-related ED visits (9.6% vs 14.4% allopurinol and 12.1% alkali citrate), hospital admissions (1.0% vs 2.4% allopurinol and 1.8% alkali citrate), and procedures (8.5% vs 11.3% allopurinol and 12.6% alkali citrate). Sensitivity analysis adjusting for receiving six months continuous days supply defined as ≤14 days, or ≤30 days had similar findings (Supplementary Table 6). Comparing those on a daily dose of hydrochlorothiazide ≤12.5mg, no significant differences in the subsequent odds of a stone event were observed at higher daily doses (see Supplementary Table 7). However, when comparing potassium citrate daily dosing of <20mEq daily, there was a positive dose response with higher doses (p-value for linear trend 0.03), indicating a higher odds of a subsequent stone event at higher doses (see Supplementary Table 7). No differences were seen in outcomes comparing allopurinol ≥300mg daily to the <300mg daily (see Supplementary Table 7)
Discussion
In this study comparing empiric PPT monotherapy among patients with kidney stone disease, there were several important findings. First, compared to alkali citrate and allopurinol, thiazides represent the most common medication class prescribed overall and among those with high-risk comorbid conditions predisposing to kidney stone risk. The relatively high prevalence of allopurinol prescriptions is surprising given the modest evidence demonstrating the drug is useful for prevention of stones. Second, rates of medication adherence to PPT use up to six months were lowest with alkali citrate, while they were comparable for thiazides and allopurinol. Third, after accounting for patient factors and medication adherence, thiazide use was associated with a lower risk of a subsequent stone event when compared to allopurinol use, whereas no significant differences were observed comparing alkali citrate to allopurinol.
No prior studies have compared the effectiveness of PPT across medication classes on subsequent stone events. Evidence supporting the effectiveness of empiric approaches to PPT have been drawn from alkali citrate and thiazide trials that did not require low urinary citrate and high urine calcium as respective inclusion criteria.14–19 A prior study of patients with calcium oxalate stones and higher urine calcium comparing potassium citrate versus hydrochlorothiazide showed favorable changes in urinary parameters with both interventions, but did not examine kidney stone recurrence.20 While alkali citrate is commonly used as PPT in the setting of hypocitraturia and low urine pH,21,22 in this study where the patients did not receive 24-hour urine testing, we did not observe a benefit of alkali citrate over allopurinol. Higher daily doses of potassium citrate were associated with a higher odds of a subsequent stone event – though this finding should not be over interpreted and warrants further investigation, since the clinical indication for higher alkali doses is not readily available from this dataset. It is possible that 24-hour urine testing helps select ideal candidates for alkali citrate use, since it can raise urine pH and promote calcium phosphate stone formation.
The findings of this study are relevant in clinical scenarios when 24-hour urine testing is not available or there is a lack of clinical expertise to interpret and act on its results. While the AUA guidelines recommend 24-hour urine testing in high risk or any motivated patient, there are no statements on whether empiric therapy is appropriate.21 A prior study reported a 7.4% prevalence of 24-hour urine testing among patients with high-risk comorbid conditions at risk for kidney stone recurrence,23 and when performed, 24-hour urine testing is beneficial specifically among patients with a history of multiple stone events is beneficial compared to empiric therapy.24 On the other hand, establishing empiric PPT approaches to kidney stone prevention may lower the barrier for clinicians to initiate treatment, especially in the primary care setting. Additionally, thiazides are widely available, cheap, accepted by clinicians for decades, and in this study appear effective at the lowest doses given.
There are several limitations to this study. The administrative dataset lacks detail beyond kidney stone diagnosis and procedures codes. Information including dietary interventions, prior stone episodes, stone analyses and serum laboratory results are not available. Due to the observational design, there may be unmeasured differences between the different PPT groups and residual confounding. Patients receiving thiazides and allopurinol often had respective hypertension and gout diagnoses within 12 months of the first prescription (83% and 49%, respectively), suggesting that patients may have received these medications to treat other chronic conditions rather primarily for kidney stone disease. Our adherence assessment up to 6 months based on prescription fills assumed the provider did not discontinue or modify the therapy during that timeframe, and that the patients took the medications for each medication fill. Since some forms of alkali citrate can be obtained without a prescription, our data would not have captured those who were taking over-the-counter alkali citrate. Additionally, these data from working-age adults may not be generalizable to other populations, including older populations and those who lack insurance coverage. Further studies are needed to confirm the dose response relationships in the empiric setting.
Future efforts could examine the effectiveness of empiric combination therapy. Two trials of patients with recurrent calcium stones comparing thiazide-based combinations versus thiazide monotherapy did not find differences in stone recurrence risk.25,26 A potential side effect of thiazide use is hypokalemia and hypocitraturia, both of which can be prevented with potassium supplementation.4 Thus, combination thiazide and potassium citrate therapy may comprise the ideal empiric PPT. Future studies can also examine longer periods of follow-up (e.g. 5 years or longer), since kidney stone recurrence does not occur in the short term. Additionally, since PPT are typically prescribed long term with potential side effects, further studies could be performed to examine optimal dosing strategies and the role of continuous versus intermittent treatment.
Conclusions
In this study of working age adults with kidney stone disease, empiric PPT with thiazides is associated with lower rates of subsequent stone-related events by three years compared to alkali citrate or allopurinol. These findings are consistent when accounting for patient factors and medication adherence. These data suggest that empiric thiazides may be preferred for stone recurrence prevention when 24-hour urine testing is not available.
Supplementary Material
Table 2.
Odds of a subsequent stone event by 3 years comparing empiric thiazides and alkali citrate to allopurinol.
| Among patients on Monotherapy N = 3,046 | Odds Ratio | 95% CI |
|---|---|---|
| Medication class (referent Allopurinol) | ||
| Thiazides | 0.68 | (0.53 to 0.88) |
| Alkali Citrate | 1.00 | (0.75 to 1.34) |
Acknowledgements
Supported by National Institutes of Health Grant 1R01DK121709-01A1.
Contributor Information
Ryan S. Hsi, Department of Urology, Vanderbilt University Medical Center.
Phyllis L. Yan, Dow Division of Health Services Research, Department of Urology, University of Michigan.
Joseph J. Crivelli, Department of Urology, University of Alabama at Birmingham School of Medicine.
David S. Goldfarb, Nephrology Section, VA New York Harbor Healthcare System, Division of Nephrology, New York University Langone Medical Center.
Vahakn Shahinian, Dow Division of Health Services Research, Department of Urology, University of Michigan; Division of Nephrology, Department of Internal Medicine, University of Michigan.
John M. Hollingsworth, Dow Division of Health Services Research, Department of Urology, University of Michigan.
References
- 1.Qaseem A, Dallas P, Forciea MA, et al. : Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161: 659–67. [DOI] [PubMed] [Google Scholar]
- 2.Hsi RS, Yan PL, Goldfarb DS, et al. : Comparison of Selective Versus Empiric Pharmacologic Preventative Therapy With Kidney Stone Recurrence. Urology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samson PC, Holt SK, Hsi RS, et al. : The Association Between 24-Hour Urine and Stone Recurrence Among High Risk Kidney Stone Formers: A Population Level Assessment. Urology 2020; 144: 71–76. [DOI] [PubMed] [Google Scholar]
- 4.Nicar MJ, Peterson R and Pak CY: Use of potassium citrate as potassium supplement during thiazide therapy of calcium nephrolithiasis. J Urol 1984; 131: 430–433. [DOI] [PubMed] [Google Scholar]
- 5.Pak CY: Citrate and renal calculi: an update. Miner Electrolyte Metab 1994; 20: 371–377. [PubMed] [Google Scholar]
- 6.Doizi S, Poindexter JR, Pearle MS, et al. : Impact of Potassium Citrate vs Citric Acid on Urinary Stone Risk in Calcium Phosphate Stone Formers. J Urol 2018; 200: 1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fink HA, Wilt TJ, Eidman KE, et al. : Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med 2013; 158: 535–43. [DOI] [PubMed] [Google Scholar]
- 8.Ellison JS, Kaufman SR, Kraft KH, et al. : Underuse of 24-hour urine collection among children with incident urinary stones: a quality-of-care concern? Urology 2014; 84: 457–461. [DOI] [PubMed] [Google Scholar]
- 9.Hollingsworth JM, Norton EC, Kaufman SR, et al. : Medical expulsive therapy versus early endoscopic stone removal for acute renal colic: an instrumental variable analysis. J Urol 2013; 190: 882–887. [DOI] [PubMed] [Google Scholar]
- 10.Dauw CA, Kaufman SR, Hollenbeck BK, et al. : Expulsive therapy versus early endoscopic stone removal in patients with acute renal colic: a comparison of indirect costs. J Urol 2014; 191: 673–677. [DOI] [PubMed] [Google Scholar]
- 11.Quan H, Li B, Couris CM, et al. : Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–682. [DOI] [PubMed] [Google Scholar]
- 12.Dauw CA, Yi Y, Bierlein MJ, et al. : Medication Nonadherence and Effectiveness of Preventive Pharmacological Therapy for Kidney Stones. J Urol 2016; 195: 648–52. [DOI] [PubMed] [Google Scholar]
- 13.White H: A Heteroskedasticity-Consistent Covariance Matrix Estimator And A Direct Test For Heteroskedasticity.; 1980.
- 14.Ettinger B, Pak CY, Citron JT, et al. : Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol 1997; 158: 2069–73. [DOI] [PubMed] [Google Scholar]
- 15.Soygur T, Akbay A and Kupeli S: Effect of potassium citrate therapy on stone recurrence and residual fragments after shockwave lithotripsy in lower caliceal calcium oxalate urolithiasis: a randomized controlled trial. J Endourol 2002; 16: 149–52. [DOI] [PubMed] [Google Scholar]
- 16.Laerum E and Larsen S: Thiazide prophylaxis of urolithiasis. A double-blind study in general practice. Acta Med Scand 1984; 215: 383–9. [PubMed] [Google Scholar]
- 17.Ettinger B, Citron JT, Livermore B, et al. : Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. J Urol 1988; 139: 679–84. [DOI] [PubMed] [Google Scholar]
- 18.Ala-Opas M, Elomaa I, Porkka L, et al. : Unprocessed bran and intermittent thiazide therapy in prevention of recurrent urinary calcium stones. Scand J Urol Nephrol 1987; 21: 311–4. [DOI] [PubMed] [Google Scholar]
- 19.Ahlstrand C, Sandvall K, Tiselius HG: Prophylactic treatment of calcium stone formers with hydrochlorothiazide and magnesium. Proceedings of the Sixth European Symposium on Urolithiasis. Linköping University; 1995: 195–197. [Google Scholar]
- 20.Solak V, Gökce Mİ and Yaman Ö: Potassium citrate vs. hydrochlorothiazide to reduce urinary calcium excretion in calcium oxalate stone patients with hypercalciuria: a prospective randomized study. Int Urol Nephrol 2021. [DOI] [PubMed] [Google Scholar]
- 21.Pearle MS, Goldfarb DS, Assimos DG, et al. : Medical management of kidney stones: AUA guideline. J Urol 2014; 192: 316–24. [DOI] [PubMed] [Google Scholar]
- 22.Skolarikos A, Straub M, Knoll T, et al. : Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur Urol 2015; 67: 750–63. [DOI] [PubMed] [Google Scholar]
- 23.Milose JC, Kaufman SR, Hollenbeck BK, et al. : Prevalence of 24-hour urine collection in high risk stone formers. J Urol 2014; 191: 376–80. [DOI] [PubMed] [Google Scholar]
- 24.Hsi RS, Yan PL, Crivelli JJ, et al. : Comparison of Selective vs Empiric Pharmacologic Preventive Therapy of Kidney Stone Recurrence With High-Risk Features. Urology 2022: S0090-4295(22)00140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Rodriguez A, Arrabal-Martin M, Garcia-Ruiz MJ, et al. : [The role of thiazides in the prophylaxis of recurrent calcium lithiasis]. Actas Urol Esp 2006; 30: 305–9. [DOI] [PubMed] [Google Scholar]
- 26.Borghi L, Meschi T, Guerra A, et al. : Randomized prospective study of a nonthiazide diuretic, indapamide, in preventing calcium stone recurrences. J Cardiovasc Pharmacol 1993; 22 Suppl 6: S78–86. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


