Abstract
Background:
Hidradenitis suppurativa (HS) is an inflammatory skin disease with dysregulation of the IL-17 axis. Recently we reported clinical benefit of brodalumab, a human anti-IL-17 receptor A (IL-17RA) monoclonal antibody, in moderate-to-severe HS.
Objectives:
To characterize the molecular response to brodalumab in HS skin and serum, and to identify biomarkers of treatment response.
Methods:
Ten participants that received 210 mg/1.5mL brodalumab subcutaneously at week 0, 1, 2, 4 and every 2 weeks after were included in this molecular profiling study (NCT03960268). RNA-sequencing and immunohistochemistry of nonlesional, perilesional and lesional HS skin biopsies, and Olink high throughput proteomics of serum at baseline, week 4 and week 12 were assessed.
Results:
At week 12, brodalumab led to a decrease of overall inflammation, and improvement of psoriasis-, keratinocyte- and neutrophil-related pathways. Despite perilesional and lesional skin having no differentially expressed genes at baseline, treatment response was best assessed in perilesional skin. In serum, brodalumab treatment decreased pathways involved in neutrophil inflammation. Patients with higher baseline expression of neutrophil-associated Lipocalin-2 (LCN2) in the skin and IL-17A in the serum demonstrated greater decreases of HS-related inflammatory cytokines as measured in skin biopsies at week 12.
Conclusions:
IL-17RA inhibition by brodalumab impacts several pathogenic inflammatory axes in HS. Perilesional skin provides a valid and robust assessment of treatment response. Expression of LCN2 in skin and IL-17A in serum may be used as biomarkers to stratify patients that may have a superior molecular response to brodalumab =.
INTRODUCTION:
Hidradenitis Suppurativa (HS) is a complex inflammatory skin disease presenting with painful nodules and abscesses with progression to malodorous draining tunnels. HS has a significant disease burden and a more profound impact on patients’ quality of life compared to other systemic skin diseases1–3. Effective therapeutics remain limited, with TNF inhibitor adalimumab as the only U.S. Food Drug Administration (FDA) approved biologic for the disease, however only achieving a clinical response (HiSCR) response of 42–59%4. Despite increased research progress in the past several years, identification and development of novel therapeutics remains hindered by our relatively incomplete understanding of the molecular pathogenesis of HS5. The morphologic heterogeneity of the disease (which include nodules, abscesses, tunnels, and fibrotic regions) as well as a lack of a standardized consensus in assessing the molecular response in therapeutic studies (i.e., which regions to biopsy) add to the challenge of HS research6. Furthermore, molecular and cellular biomarkers of disease activity have remained elusive in HS. One study identified a robust decrease in transcriptomic B cell signature in HS lesional biopsies following treatment with adalimumab and a decrease in B cell lymphocyte chemoattractant CXCL13, while another studied identified a reduction in frequency of T-helper (Th) 17 cells7,8. However, other studies of apremilast and ustekinumab did not identify a biomarker of treatment response despite clinical efficacy9,10.
Recent work identified increased expression of IL-17A and IL-17C and greater infiltration of Th17 cells in HS11–18 19. Considering the evidence for the role of IL-17 signaling in disease pathogenesis, therapeutic targeting of IL-17A pathway in HS has been explored in small cohort studies20–23. A recent phase II double-blind, placebo-controlled randomized trial of bimekizumab, a monoclonal antibody targeting both IL-17A and IL-17F, also showed efficacy, with 46% of treated patients achieving HISCR75 at week 1224. Given the elevated levels of both IL-17A/C in HS, targeting IL-17 Receptor A (IL-17RA), a shared subunit of the receptor complex, is another attractive therapeutic avenue25–30. We have recently conducted a clinical study of brodalumab, a human monoclonal antibody targeting IL-17RA, in HS and reported promising clinical results, with 100% of participants reaching HiSCR31–34. There were no grade 2/3 adverse events, and the treatment was well-tolerated, despite previous reports of Crohn’s disease exacerbations and association with development of infections)34,35.
Here, we characterize the molecular impact of IL-17RA antagonism in HS using a pathway approach. This is the first study to systematically compare treatment response in lesional, perilesional and nonlesional HS skin in the setting of a therapeutic intervention.
MATERIALS AND METHODS:
Study cohort
This study was conducted following approval by the Institutional Review Board. Informed consent in accordance with the Declaration of Helsinki was obtained from all patients. Samples were included from an open-label cohort study (NCT03960268). Ten patients with moderate-to-severe (Hurley Stage 2 and 3) who completed 12 weeks of treatment were enrolled in this molecular characterization study, with complete inclusion and exclusion criteria as previously described34. Patients with HS ≥18 years of age were eligible to participate. Patients were required to have a negative result for hepatitis B virus surface antigen, hepatitis C virus antibody, Human Immunodeficiency Virus (HIV), QuantiFERON Gold tuberculosis and pregnancy tests. Patients with inflammatory bowel disease were excluded from this study. Patients were required to undergo a washout period of 5 half-lives from previous immunomodulating/biologic therapies. Brodalumab 210 mg/1.5mL was administered at week 0, 1, 2, 4 and every two weeks after until the study end point.
Skin biopsy analysis
Punch biopsies of nonlesional, perilesional and lesional HS skin were collected under ultrasound guidance as previously described14,34,36. Biopsies were collected prior to treatment (baseline) and at week 12, with an optional week 4 biopsy. Biopsies were processed and IHC was performed as previously described14. Epidermal thickness and the number of positive cells per field were counted using ImageJ, V1.42 software. RNA was extracted using Qiagen miRNeasy Mini kit (Qiagen). RNA-sequencing (RNA-seq) was performed using NovaSeq 6000 (Illumina), and reads were aligned to the reference genome (GRCh37), (GEO#GSE189266). Differential expression was estimated with DESeq2 and VST to log2 transform the normalized counts37. Differentially expressed genes were defined as fold change (FCH) of ≥ |1.5| and false discovery rate ≤0.5.
Serum analysis
Blood samples were centrifuged, and serum was stored at −80°C. Samples were analyzed using Olink high throughput proteomics (Cardiometabolic, Cardiovascular II, Cardiovascular III and Inflammation panels) as previously described13. Quality control of Olink data was performed using the standard quality control pipeline38. Differentially expressed proteins were defined as fold change (FCH) of ≥ |1.5| and p value ≤0.05.
Statistical analysis
RNA-seq and Olink statistical analysis were performed in R language (www.R-project.org; R foundation, Vienna, Austria) using publicly available packages (www.bioconductor.org). Means of each group and differences between the groups were estimated using least square means. Hypothesis testing was undertaken using the general framework for linear mixed-effect models of limma package. Timepoint and tissue were considered as fixed factors while random effect related to subjects was included. Unsupervised clustering was performed with the Euclidean distance and average agglomeration. RNAseq data p values were corrected for multiplicity using Benjamini-Hochberg procedure while for OLINK data p value adjustment was not performed. Correlation between differentially expressed genes (DEGs) in perilesional and lesional skin was accomplished using Pearson correlation on log2-transformed expression values. Correlation between mRNA expression in skin and Olink expression in serum with the decrease in molecular markers in skin (in logFCH) was achieved using Spearman correlations on log2-transformed expression values. Correlation among Olink expression in serum was performed using Pearson correlations on log2-transformed expression values. Enrichment analyses were completed using Ingenuity Pathway Analysis and PANTHER. Statistical significance between IHC cellular infiltration at baseline, week 4 and week 12 was assessed using a 2-way ANOVA in perilesional and lesional HS skin. Dunnett statistical hypothesis testing was used to correct for multiple comparisons.
RESULTS
To ascertain the molecular response following brodalumab treatment, we studied biopsy tissues and serum from 10 patients who completed 12 weeks of treatment. We collected nonlesional, perilesional and lesional biopsies from patients prior to treatment (baseline), at week 4 (optional for skin biopsies) and week 12. Lesional skin was biopsied at an edge of an active inflammatory nodule, while perilesional and nonlesional skin were biopsied on visually relatively healthy appearing skin 2cm and 10cm away in the same anatomic region36. Details of the clinical results from this study have been previously described34. A cohort of 10 HS subjects allowed us to detect post- vs pre-treatment effect size equal to 1 with 95% significance level and 80% power using a two-tail related samples t-test, which is smaller than the effect size equal to 1.64 (p-value=0.0056) for IL-17A previously reported for the comparison of HS to healthy controls39.
Molecular resolution of HS transcriptome with brodalumab treatment
We investigated brodalumab-induced changes in HS transcriptome (HSTR). RNA-sequencing was performed on whole-tissue skin biopsies and criteria of FCH≥|1.5| and FDR ≤ 0.05 was utilized for differentially expressed genes (DEGs). HS transcriptome was defined as the DEGs between lesional (HSTR-LS) or perilesional (HSTR-PL) skin and healthy appearing nonlesional skin. HSTR-LS had 923 down- and 913 up-regulated DEGs, whereas HSTR-PL had 819 down- and 835- upregulated DEGs. Lesional and perilesional samples were both highly inflamed at baseline and no DEGs were detected between lesional and perilesional skin (Figure 1A–D). We identified a significant correlation between genes in lesional and perilesional skin (r=0.94, p<1E-04, Figure 1A). Treatment yielded an 88.3% reduction in HSTR-PL compared to 55.1% reduction in HSTR-LS week 12 (p<0.001) (Figure 1E–F). At 12 weeks, the odds ratio of HS lesional compared to perilesional transcriptome reduction was 6.11.
Figure 1: Perilesional hidradenitis suppurativa (HS) skin shares overlapping transcriptomic profile with lesional skin at baseline but exhibits more changes with treatment response.
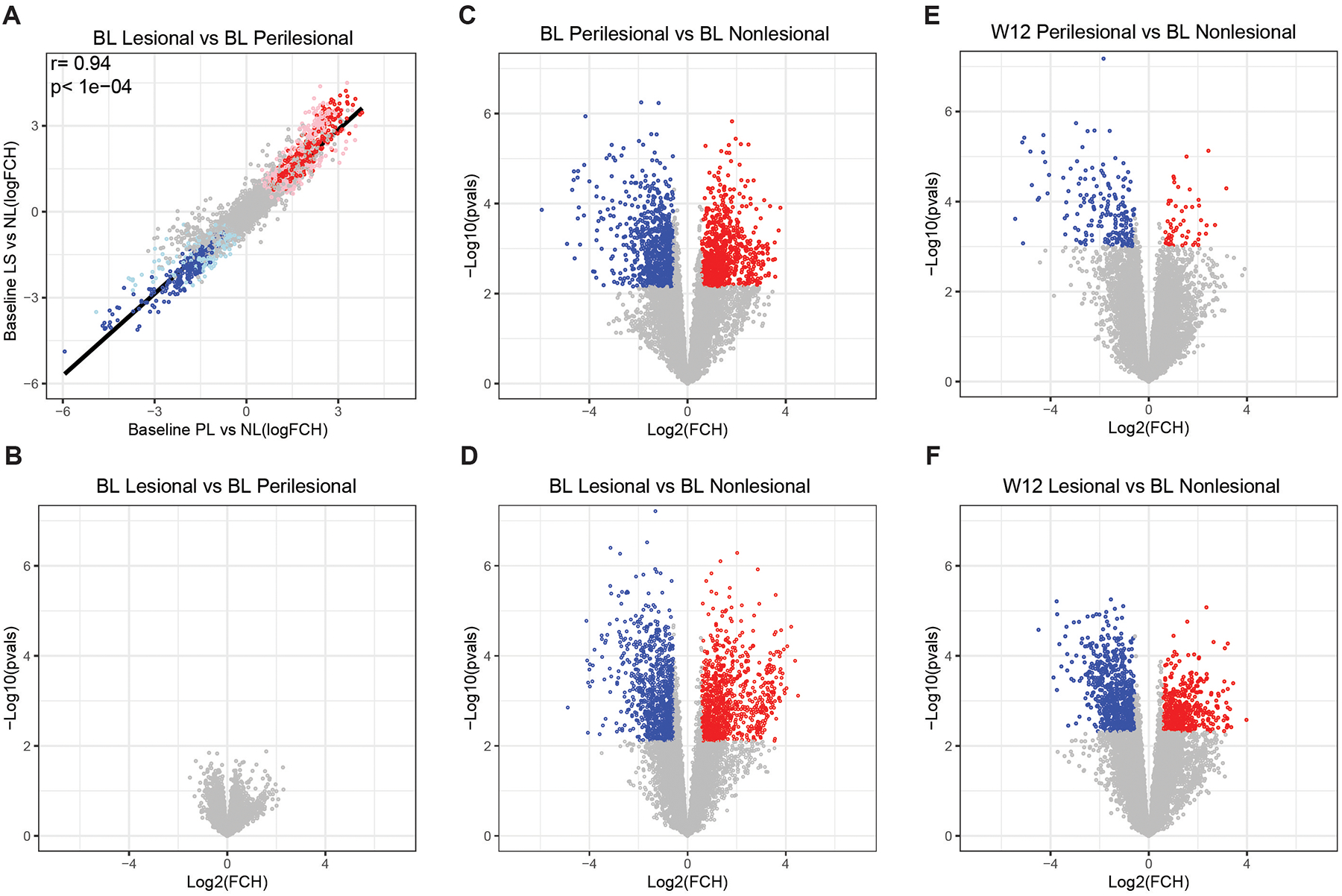
A) Correlation of differentially expressed genes (DEGs) as defined by fold change ≥ |1.5| and false discovery rate ≤ 0.05. Red circles indicate upregulated DEGs while blue circles indicate downregulated DEGs in baseline (BL) lesional (LS) and perilesional (PL) skin relative to nonlesional (NL) skin. r is Pearson correlation. Grey circles indicate genes that are not differentially expressed. Volcano plot of differentially expressed genes in B) lesional versus perilesional skin C) perilesional versus nonlesional skin and D) lesional versus nonlesional skin at baseline. Modulation of DEGs by brodalumab at week 12 in E) perilesional and F) lesional versus baseline nonlesional skin.
Given that treatment response was better detected in HSTR-PL, we focused subsequent analysis on perilesional skin. DEGs modulated with treatment included neutrophilic (CSF3, CD177), Th1 (CXCL11, STAT4), Th17 (IL21R), Th2 (GATA3) and innate interferon pathway related (IFI16, IL18, MX1, MX2, IFITM3, ISG20) markers as well as B cell chemoattractant CXCL13 (Supplemental Figure S1). Using gene-set variation analysis (GSVA) we quantified the behavior of previously curated gene-sets and observed decreases in the general inflammatory response and pathways related to psoriasis, Th1, Th17 and IL-12/IL-23 signaling (Figure 2A). IL-17 signaling is involved in keratinocyte proliferation, differentiation and feed-forward inflammation in which Th17-derived IL-17A/F can lead to increased production of other inflammatory cytokines including IL-36, CCL20, CXCL chemokines and S100 proteins while keratinocyte derived IL-17C can amplify this signaling by leading to increased secretion of IL-17A by Th17 cells8,27,40,41. We thus asked whether previously defined keratinocyte signaling pathways are also decreased with treatment and observed a reduction of multiple keratinocyte-related axes (Figure 2A). Clinically, patients had a reduction in purulent drainage from dermal tunnels34. Given that tunnels are involved in neutrophilic inflammation, we asked whether neutrophilic related pathways are also downregulated with brodalumab treatment and observed a significant decrease of neutrophil-related pathways in perilesional skin13,14 (Figure 2B). By Ingenuity Pathway Analysis (IPA), the top down-regulated pathways with brodalumab treatment included T-cell activation, phagosome formation, pattern recognition of bacteria and virus, wound healing, STAT3 and GM-CSF signaling, neuroinflammation, and the role for IL-17A in psoriasis (p < 0.00001) A strong reduction in B-cell receptor signaling was also detected (Supplemental Figure S2).
Figure 2: Decreased inflammatory pathways in hidradenitis suppurativa (HS) perilesional and lesional skin following treatment with brodalumab.
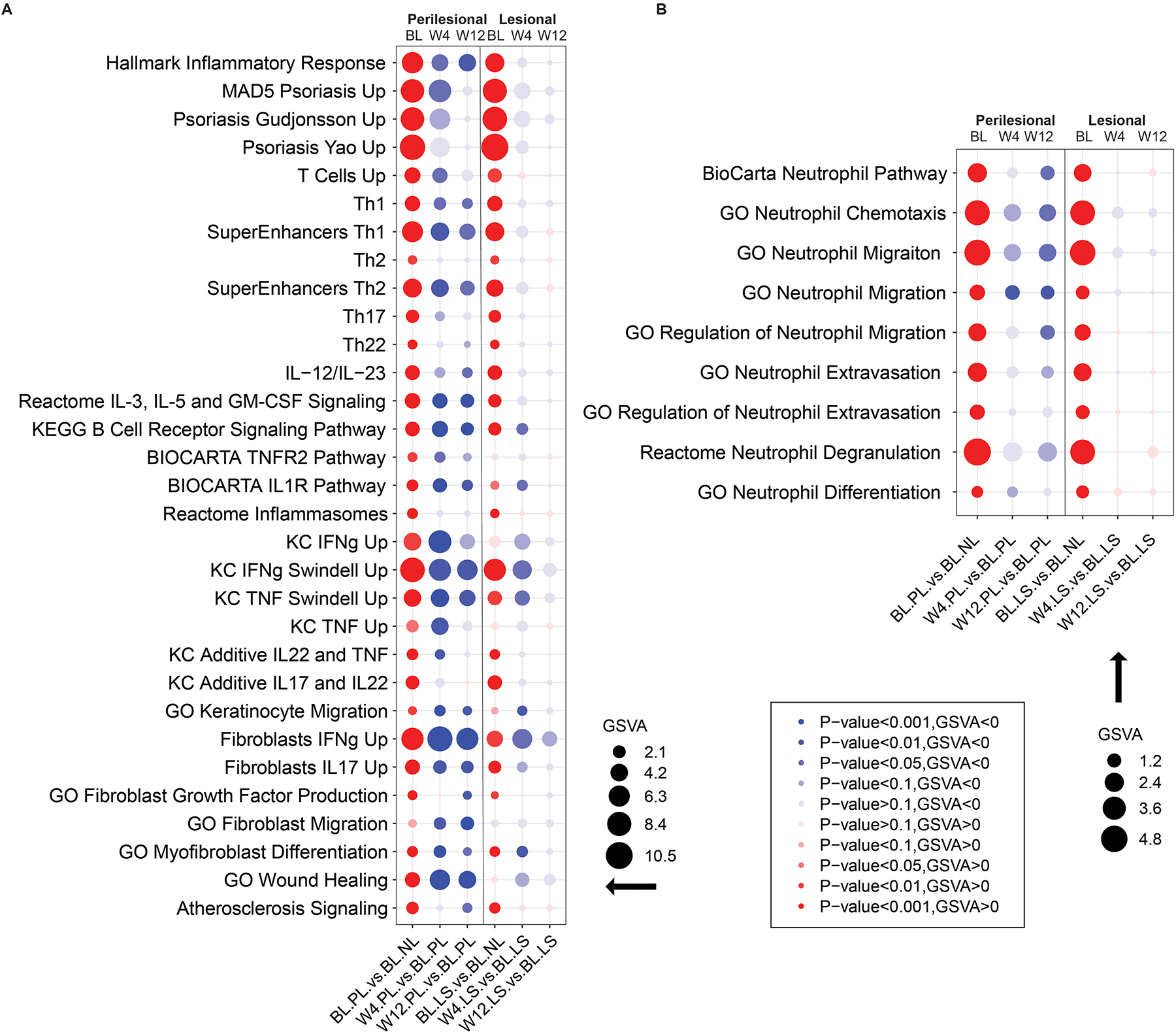
A) Decrease of overall inflammatory pathways, known psoriasis pathways and pathways involved in keratinocyte proliferation at week 4 and week 12 following treatment B) Decrease of neutrophil-associated pathways following treatment with brodalumab. W=week, BL=baseline, NL=nonlesional PL= perilesional LS=lesional skin. The size of the bubble diameter is proportional to the gene-set variation analysis (GSVA) score and the bubble color intensity indicates significance (p value) while red and blue indicates up or down regulation respectively.
Treatment response is better appreciated in perilesional skin
Treatment improvement measures the extent to which posttreatment perilesional and lesional skin transcripts approach baseline nonlesional levels, wherein 100% improvement corresponds to baseline nonlesional level of transcripts in posttreatment perilesional and lesional skin.
We thus calculated the improvement for pathways modulated with treatment40. There was modest improvement from baseline at Week 4. No significant differences between improvement in perilesional and lesional skin were observed (Supplemental Figure S3). At week 12, there was a significantly higher improvement observed in perilesional compared to lesional skin (Figure 3A). Given that perilesional and lesional skin were equally inflamed and had no DEGs at baseline, this data further suggests that a better treatment response is observed in perilesional skin. There was a gradual increase of improvement from week 4 to week 12 in multiple pathways in perilesional skin, including neutrophil-related pathways (neutrophil chemotaxis, neutrophil migration, degranulation), psoriasis related pathways and the atherosclerosis signaling pathway (Figure 3B).
Figure 3: Treatment response is best observed in perilesional skin at week 12.
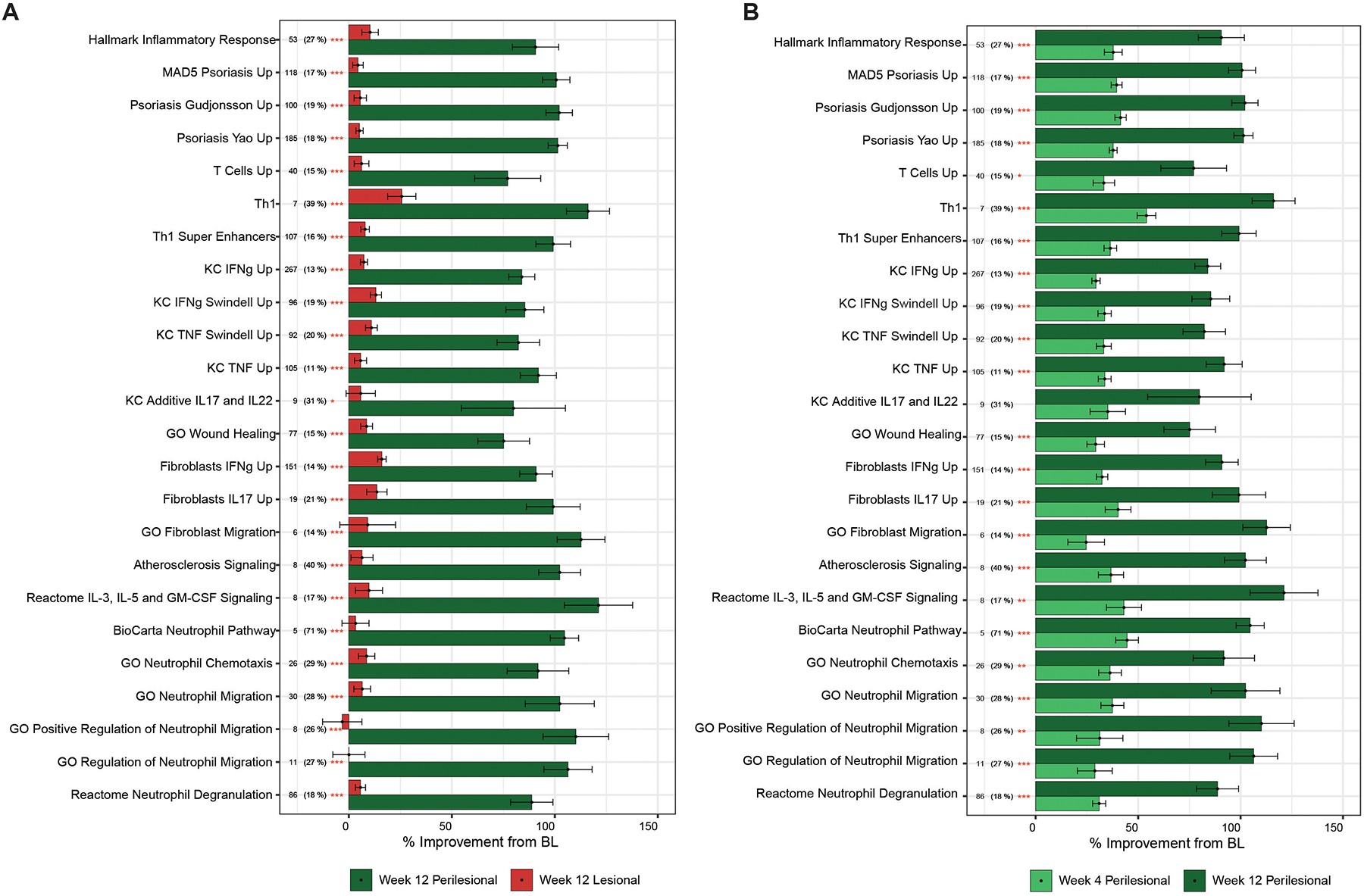
Means of percentage improvement at A) week 12 between perilesional and lesional skin, and B) improvement in perilesional skin between week 4 and week 12. N (%) = number of genes in the hidradenitis suppurativa transcriptome shared with pathway (percentage of total gene in pathway). Stars denote significance with * p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001.
Treatment with brodalumab leads to a decreased inflammatory infiltration in skin
We then assessed the histopathologic changes following brodalumab treatment. Consistent with our previous investigations, we did not find a significant difference between histological properties of lesional and perilesional skin at baseline42. We observed a reduction in epidermal thickness in both lesional and perilesional skin at week 4 and week 12. There was a significant reduction of CD3 and CD11c infiltrates compared to baseline in both perilesional and lesional skin. Epidermal infiltration of CD11c observed in lesional skin at baseline resolved with treatment (Figure 4).
Figure 4: Histological analysis demonstrates decreased inflammatory infiltration in hidradenitis suppurativa (HS) lesional and perilesional skin following treatment with brodalumab.
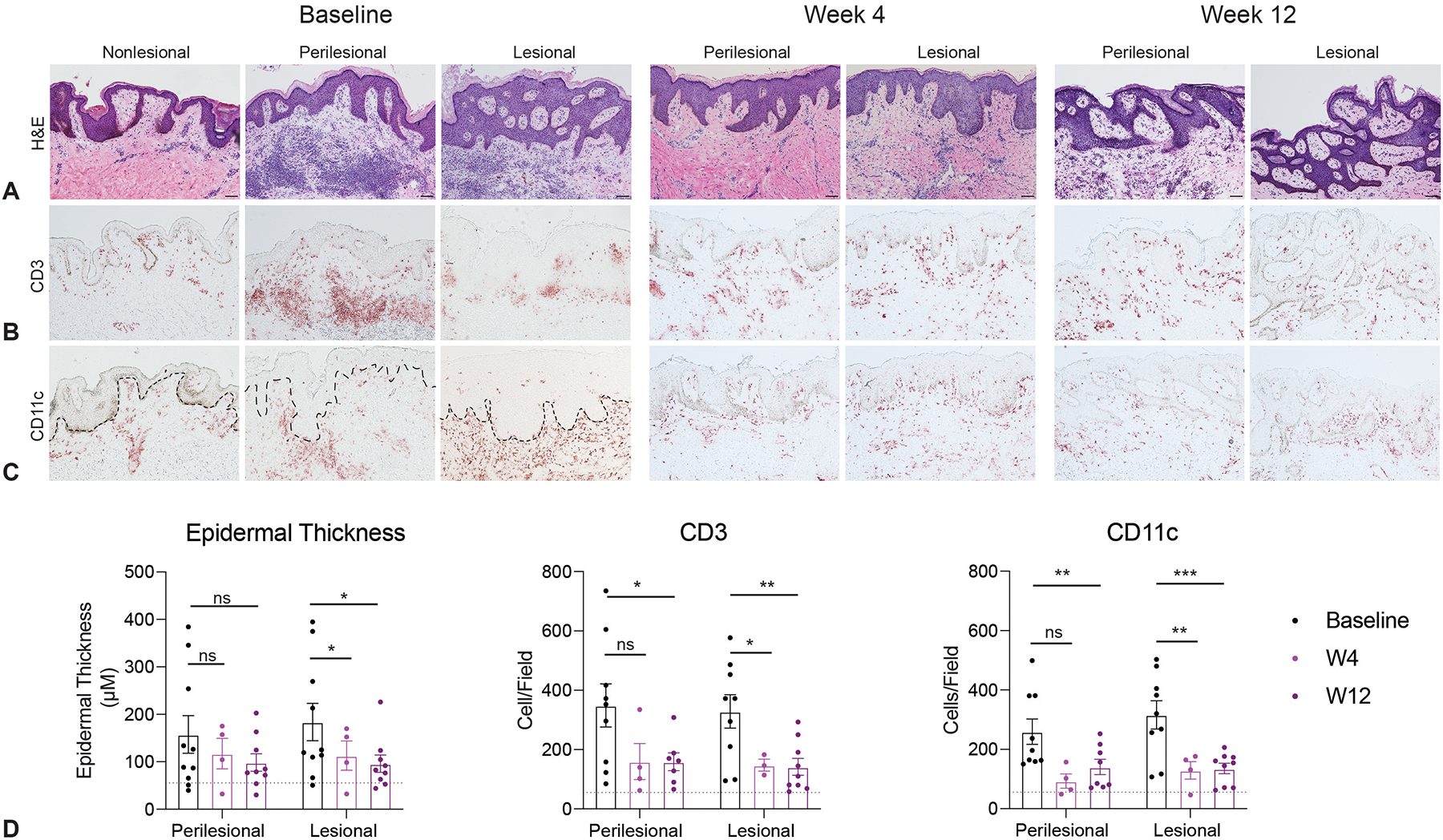
A) Hematoxylin and Eosin (H&E) stain demonstrates a mild reduction in epidermal thickness in lesional skin. There is also comparable reduction of B) CD3+ infiltration, and C) CD11c+ infiltration in both perilesional and lesional skin. Scale bar = 100 μM. D) Quantification of total inflammatory infiltration across the patient cohort (n=10 patients). Dashed line represents infiltrate quantification of nonlesional skin at baseline. Error bars show standard error of mean (SEM), with two-way ANOVA analysis. Stars denote significance with * p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001.
Brodalumab leads to a decrease of granulocyte-related pathways in serum
Using Olink high throughput proteomics, we assessed 368 inflammation-, cardiovascular- and cardiometabolic-related biomarkers in serum. Increases in several inflammatory cytokines in HS were detected at baseline, including TNF, IL-6, and IL-8 elevations. TNF and IL-8 were strongly decreased by 12 weeks of treatment. As many of the measured proteins are inter-correlated (Supplementary Figure S4) due to cytokine interactions, we analyzed the brodalumab treatment response mainly by pathways to establish statistical significance of protein changes. This pathway analysis of all downregulated proteins at both week 4 and week 12 showed an enrichment (ie improvement) in pathways related to TNF response, neutrophil (granulocyte chemotaxis, neutrophil migration, granulocyte migration) and general inflammatory responses (myeloid leukocyte migration, leukocyte chemotaxis, inflammatory response) (Figure 5, Supplemental Figure S5). We note that IL-17A levels were markedly elevated after brodalumab administration, likely reflecting reduced receptor binding mediated by brodalumab.
Figure 5: Assessment of pathways modulated in serum by brodalumab treatment.
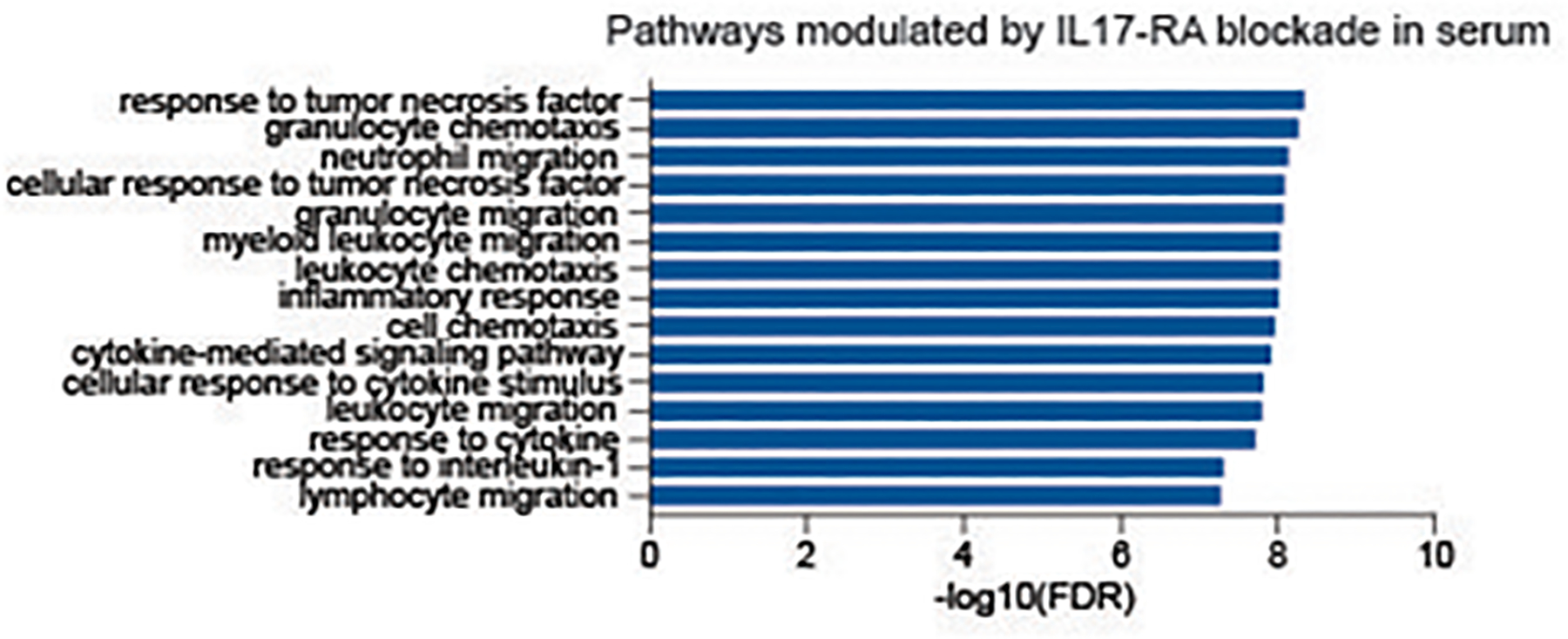
Enrichment analysis of all proteins downregulated with treatment at week 4 and 12 based on PANTHER-Slim Gene Ontology biological processes. Stars denote significance with * p ≤ 0.1 ** p ≤ 0.05 *** p ≤ 0.01 **** p ≤ 0.001
Higher expression of LCN2 in skin and IL-17A in serum correlates with a greater decrease of inflammatory cytokines with treatment
HS heterogeneity presents a challenge to both therapeutic management and molecular assessment of a treatment response. We therefore asked whether we could identify a biomarker to stratify patients that may have a superior molecular response to IL-17RA blockade. Given the decrease of neutrophilic signatures in both skin and blood following treatment with brodalumab, we focused on neutrophil-associated biomarkers. Neutrophil gelatinase-associated lipocalin-2 (LCN2) is expressed in neutrophils and can be used to identify a more inflamed and neutrophilic subtype in skin and serum42–44. Consistent with this, patients with higher LCN2 levels in lesional skin at baseline had a greater decrease of inflammatory markers implicated in HS pathogenesis (IL1B, IL36G, LCN2, TNF) in perilesional skin at Week 12 (Figure 6A). IL-17A is an inducer of LCN245–47. Since that IL-17A serum quantification is commercially available, we asked whether we could use baseline IL-17A expression to stratify patients that will have a greater molecular response in the skin. Patients with higher IL-17A expression in serum had a greater decrease in inflammatory cytokines in perilesional skin at week 12 (Figure 6B,). Taken together, these data suggest that patients with high baseline expression of neutrophil-related LCN2 in the skin or IL-17A in the serum may have a greater molecular response to treatment with brodalumab.
Figure 6: Correlation of biomarkers with molecular disease activity at week 12.
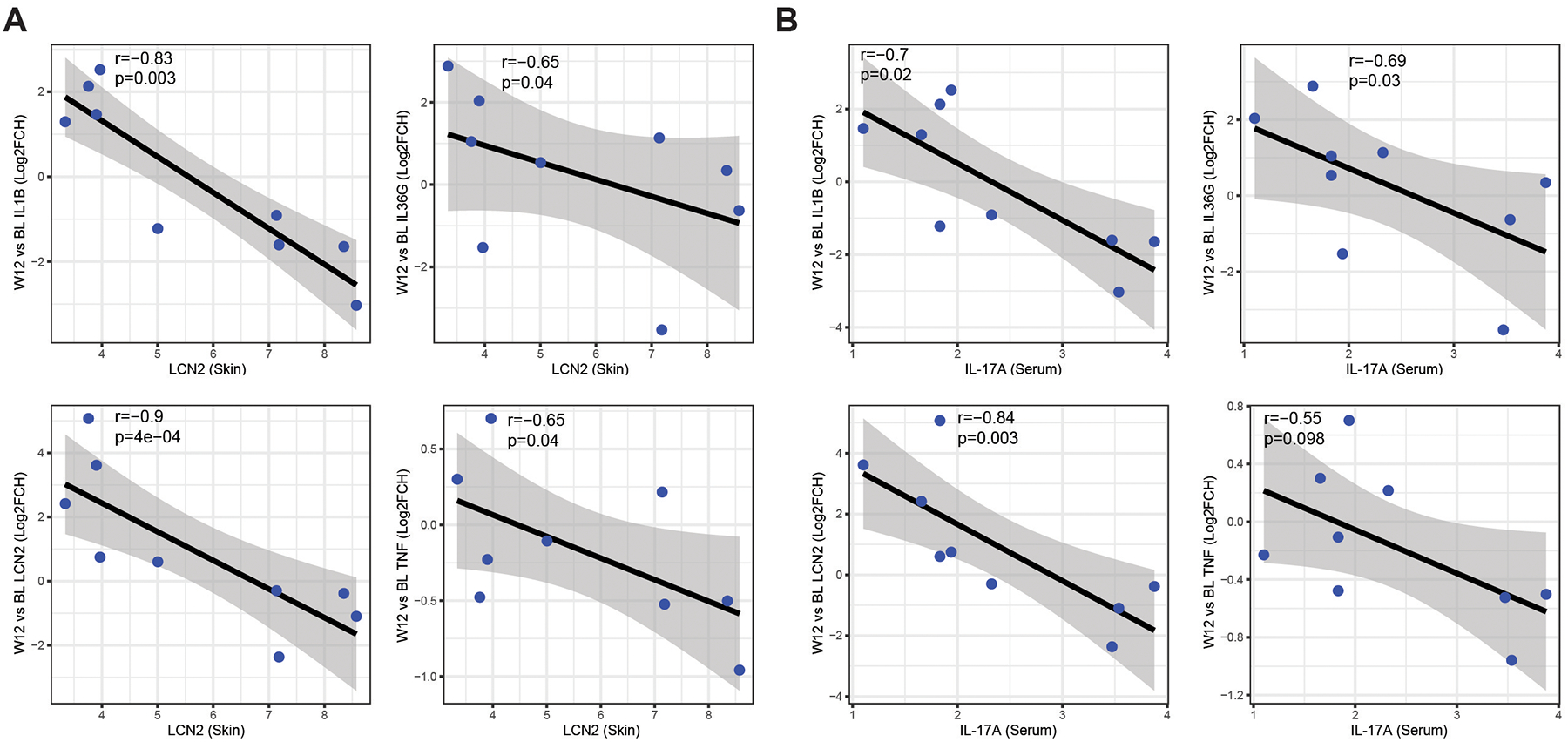
A) Expression of baseline LCN2 expression in lesional skin correlated with changes in inflammatory markers at week 12 in perilesional skin. B) expression of IL-17A in serum correlate with a decrease of inflammatory markers at week (W) 12 in perilesional skin. r is Spearman correlation. X axis is log2(Expression).
DISCUSSION
A pathogenic role for IL-17 and Th17 T-cells is suggested by preliminary studies conducted with secukinumab, bimekizumab, and brodalumab, which demonstrated clinical improvements20–23,26,34,48 24. IL-17 effects on epithelial cells in epidermis and tunnels may be particularly important in HS as there are marked upregulation of products including IL-17C, CXCL8/IL-8 and IL-36 isoforms that are produced by IL-17 activated epithelial cells12,14,49–52. However, in the context of the disease that displays connective tissue pathology and significant B cell infiltration, IL-17 may also have important effects on these cell types53–61. We identify several pathways significantly modulated by brodalumab in HS including an overall decrease of inflammatory response and psoriasis-related pathways. In addition to decreased neutrophil pathways in the skin, we also observed a reduction in granulocyte and neutrophil chemotaxis and migration pathways in the serum with treatment. Dermal IL-17 can lead to neutrophil migration towards affected areas and IL-17 pathway antagonists have been shown to be effective in patients with generalized pustular psoriasis, a disease that is also dominated by neutrophilic inflammation62 60.
Recent work has reported the attenuation of a B cell response following anti-TNF therapy60,61. Involvement of B cells, plasma cells and fibroblasts only recently became acknowledged as potentially important in HS pathogenesis5,53,60,61,63. Our data demonstrates a decrease in infiltration of naïve B cells following treatment with brodalumab. The multitude of pathways modulated by IL-17RA blockade suggests that IL-17RA may be a critical node in the multidimensional axis underlying HS pathogenesis. Furthermore, previous mechanistic studies of skin and/or serum following treatment with apremilast, infliximab and ustekinumab in HS were not able to identify a significant singular biomarker of treatment response9,10,64,65. Beyond interaction of IL-17 pathway with innate cytokines, inflammation in HS may also be contributed by B cells and complement activated cascades, making the identification of cohesive treatment biomarkers more difficult than it is in psoriasis which is driven by a dominant Th17 inflammatory axis. As such, a pathway approach, such as the one we have utilized, may have more power to determine disease associated or treatment associated changes in inflammation.
Further work is needed to determine whether innate cytokines interact with IL-17 to produce a feed forward inflammatory pathway as is the case in psoriasis, or whether these are independent inflammatory pathways that may be dominant in different subset of patients, leading to the question of whether targeted treatment based on HS subtypes or endotypes may be needed in HS. In our cohort, patients with high LCN2 in lesional skin had greater decreases in neutrophil-related cytokines (IL36G, IL1B) following treatment, suggesting that brodalumab likely targets the neutrophil-related component of HS in this HS subtype. Future larger scale studies are needed to link these observations with outcomes but reduction in the overall inflammation in skin would be expected to lead to a decreased clinical burden.
The decision of which site to biopsy is also critical for assessment of molecular response in therapeutic trials. Biopsying a target nodule may hasten its resolution unrelated to the use of a therapeutic agent10. Additionally, due to the cyclical nature of HS, the regression to the mean phenomenon must be considered at an individual level10,66,67. At a given time, a lesion may spontaneously improve, and thus biopsying the index or the lesional site may produce a false positive treatment result. Our biopsy methods are based on proposed lesional definitions and are the only suggested biopsy guidelines for therapeutic trials in HS. Biopsy of perilesional skin which displays molecular and cellular pathology comparable to the main inflammatory lesion, may be preferable and the results of this study show that better treatment responses can be assessed in sequential biopsies of the larger area of perilesional skin.
The limitations of our study include a small cohort size and a lack of a placebo-controlled group. Another limitation is that kinetics of IL-17RA saturation by brodalumab have not been established and it is possible that our posttreatment biopsies were performed at a timepoint when IL-17 receptor saturation was incomplete as patients tended to have an increase of clinical symptoms towards the end of a two-week treatment cycle, which is when biopsies were collected. Receptor mediated clearance of brodalumab in HS might be increased due to the high neutrophil production and relatively short lifespan of neutrophils, as neutrophils express high levels of IL-17RA34. Furthermore, the decision to biopsy active inflammatory nodules during brodalumab treatment may have limited the ability to define change as active lesions regressed at other sites.
Treatment with brodalumab modulates several pathways consistent with multi-axis disease pathogenesis. Our data demonstrates that neutrophil-related biomarkers can be used to identify patients that may have a better molecular response to brodalumab. Furthermore, this study establishes that perilesional skin may be used to better assess molecular response in therapeutic trials of HS. Given that perilesional skin is roughly ten times the area of a lesional nodule, this allows for a greater area for biopsying without the concern of a biopsy hasting resolution of an active inflammatory nodule.
Supplementary Material
Supplemental Figure S2: Perilesional skin has a decrease of immunoglobulin-related genes at week 12 relative to baseline A) The top downregulated genes based on fold change (FCH) at week 12 in perilesional skin compared to baseline perilesional skin. Genes in bold are those with a p value ≤0.05 B) CIBERSORT fraction of infiltration of naïve B cells into HS perilesional (PL) skin and nonlesional (NL) skin.
Supplemental Figure S1: Venn diagram of genes modulated by treatment with brodalumab at week 12 in perilesional skin. Immune-related differentially expressed genes (DEGs fold change ≥ |1.5| and false discovery rate ≤ 0.05) in perilesional skin at baseline and at week 12, both relative to nonlesional skin at baseline. Numbers designate the total number of DEGs.
Supplemental Figure S5: Brodalumab treatment effect at week 4 in HS perilesional skin. Ingenuity pathway analysis (IPA) of 399 down-modulated genes with FCH <−1.5 (excluding B- cell genes). Z-score > 0, p value <0.001. Color denotes Z-score with darker bars representing higher score.
Supplemental Figure S3: Treatment response at week 4 in skin. Means of percentage improvement at week 4 between perilesional and lesional skin. N (%) = number of genes in the hidradenitis suppurativa transcriptome shared with pathway (percentage of total gene in pathway).
Supplemental Figure S4: Differentially expressed proteins in serum A) Heatmap of differentially expressed proteins at Baseline, 4 weeks and 12 weeks using OLINK proteomics data. |FCH| > 1.5, uncorrected p value <0.05. B) Pearson correlation (r) for differentially expressed proteins. Stars denote significance with * p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001
What’s already known about this topic
Hidradenitis suppurativa (HS) is a chronic inflammatory disease with upregulation of several inflammation and keratinocyte proliferation related pathways. IL-17 is a known modulator of these pathways and has been implicated in the pathogenesis of HS. We have recently reported a clinical improvement in HS with brodalumab, a monoclonal antibody targeting the IL-17 receptor A.
What does this study add?:
This study details the molecular response to brodalumab using RNA-sequencing, high throughput proteomics and immunohistochemistry. This research identifies lipocalin-2 in skin and IL-17A in serum as potential predictive biomarkers for treatment response. This may be useful in guiding physicians in choosing the appropriate therapy for patients with moderate-to-severe HS.
What is the translational message:
IL-17RA blockade with brodalumab is an effective treatment for patients with moderate-to-severe HS. Assessing pathways that respond to treatment, rather than individual biomarkers, may be a superior approach to molecular characterization of treatment response in HS therapeutic studies. Brodalumab modulates neutrophil-related pathways in the skin and blood, and known psoriasis-pathways in the skin. We demonstrate that patients with high baseline expression of IL-17 regulated neutrophil-created Lipocalin-2 (LCN2) in the skin and IL-17A in the serum have a superior molecular response to treatment, allowing the possibility for physicians to select therapeutic treatment based on molecular profiles baseline.
Acknowledgements:
The authors would like to thank members of the Krueger laboratory for thoughtful discussions, staff at the Rockefeller University Hospital, and the Center for the Clinical and Translational Science at the Rockefeller University.
Funding:
K.N. and S.C.W. were supported by a MSTP grant from the National Institute of General Medical Sciences of the NIH under award number T32GM007739 to the Weill Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD-PhD Program. J.W.F. and J.G.K. were supported in part by grant #UL1TR001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. J.W.F. was supported by the Shapiro-Silverberg Fund for the Advancement of Translational Research and the Hidradenitis Suppurativa Foundation Danby Grant.
Conflicts of Interest:
J.G.K. has received research support (grants paid to the institution) from AbbVie, Amgen, BMS, Boehringer, EMD Serono, Innovaderm, Kineta, LEO Pharma, Novan, Novartis, Paraxel, Pfizer, Regeneron, and Vitae and personal fees from AbbVie, Acros, Allergan, Aurigne, BiogenIdec, Boehringer, Escalier, Janssen, Lilly, Novartis, Pfizer, Roche, and Valeant. All other authors have nothing to disclose.
Data Availability Statement:
All data has been uploaded to publicly available database (GEO#GSE189266) and is also available by written request to the corresponding author.
REFERENCES:
- 1.Mac Mahon J, Kirthi S, Byrne N et al. An Update on Health-Related Quality of Life and Patient-Reported Outcomes in Hidradenitis Suppurativa. Patient Relat Outcome Meas 2020; 11: 21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol 2010; 90: 264–8. [DOI] [PubMed] [Google Scholar]
- 3.Matusiak Ł, Bieniek A, Szepietowski JC. Hidradenitis suppurativa markedly decreases quality of life and professional activity. J Am Acad Dermatol 2010; 62: 706–8, 8.e1. [DOI] [PubMed] [Google Scholar]
- 4.Kimball AB, Okun MM, Williams DA et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med 2016; 375: 422–34. [DOI] [PubMed] [Google Scholar]
- 5.Frew JW, Marzano AV, Wolk K et al. A Systematic Review of Promising Therapeutic Targets in Hidradenitis Suppurativa: A Critical Evaluation of Mechanistic and Clinical Relevance. J Invest Dermatol 2020. [DOI] [PubMed] [Google Scholar]
- 6.Frew JW, Lowes MA, Goldfarb N et al. Global Harmonization of Morphological Definitions in Hidradenitis Suppurativa for a Proposed Glossary. JAMA Dermatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran B, Sweeney CM, Hughes R et al. Hidradenitis Suppurativa Is Characterized by Dysregulation of the Th17:Treg Cell Axis, Which Is Corrected by Anti-TNF Therapy. J Invest Dermatol 2017; 137: 2389–95. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher JM, Moran B, Petrasca A et al. IL-17 in inflammatory skin diseases psoriasis and hidradenitis suppurativa. Clin Exp Immunol 2020; 201: 121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blok JL, Li K, Brodmerkel C et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol 2016; 174: 839–46. [DOI] [PubMed] [Google Scholar]
- 10.Vossen A, van der Zee HH, Davelaar N et al. Apremilast for moderate hidradenitis suppurativa: no significant change in lesional skin inflammatory biomarkers. J Eur Acad Dermatol Venereol 2019; 33: 761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlapbach C, Hanni T, Yawalkar N et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol 2011; 65: 790–8. [DOI] [PubMed] [Google Scholar]
- 12.Kelly G, Hughes R, McGarry T et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol 2015; 173: 1431–9. [DOI] [PubMed] [Google Scholar]
- 13.Navrazhina K, Garcet S, Gonzalez J, Grand D, Frew JW, Krueger JG. In-Depth Analysis of the Hidradenitis Suppurativa Serum Proteome Identifies Distinct Inflammatory Subtypes. Journal of Investigative Dermatology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navrazhina K, Frew JW, Gilleaudeau P et al. Epithelialized Tunnels are a Source of Inflammation in Hidradenitis Suppurativa. J Allergy Clin Immunol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navrazhina K, Frew JW, Krueger JG. Interleukin 17C is elevated in lesional tissue of hidradenitis suppurativa. Br J Dermatol 2020; 182: 1045–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotz C, Boniotto M, Guguin A et al. Intrinsic Defect in Keratinocyte Function Leads to Inflammation in Hidradenitis Suppurativa. J Invest Dermatol 2016; 136: 1768–80. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman LK, Tomalin LE, Schultz G et al. Integrating the skin and blood transcriptomes and serum proteome in hidradenitis suppurativa reveals complement dysregulation and a plasma cell signature. PLoS One 2018; 13: e0203672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolk K, Warszawska K, Hoeflich C et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol 2011; 186: 1228–39. [DOI] [PubMed] [Google Scholar]
- 19.Wolk K, Join-Lambert O, Sabat R. Aetiology and pathogenesis of hidradenitis suppurativa. Br J Dermatol 2020; 183: 999–1010. [DOI] [PubMed] [Google Scholar]
- 20.Casseres RG, Prussick L, Zancanaro P et al. Secukinumab in the treatment of moderate to severe hidradenitis suppurativa: Results of an open-label trial. J Am Acad Dermatol 2020; 82: 1524–6. [DOI] [PubMed] [Google Scholar]
- 21.Ribero S, Ramondetta A, Fabbrocini G et al. Effectiveness of Secukinumab in the treatment of moderate-severe hidradenitis suppurativa: results from an Italian multicentric retrospective study in a real-life setting. J Eur Acad Dermatol Venereol 2021. [DOI] [PubMed] [Google Scholar]
- 22.Reguiaï Z, Fougerousse AC, Maccari F et al. Effectiveness of secukinumab in hidradenitis suppurativa: an open study (20 cases). J Eur Acad Dermatol Venereol 2020; 34: e750–e1. [DOI] [PubMed] [Google Scholar]
- 23.Prussick L, Rothstein B, Joshipura D et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol 2019; 181: 609–11. [DOI] [PubMed] [Google Scholar]
- 24.Glatt S, Jemec GBE, Forman S et al. Efficacy and Safety of Bimekizumab in Moderate to Severe Hidradenitis Suppurativa: A Phase 2, Double-blind, Placebo-Controlled Randomized Clinical Trial. JAMA Dermatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hymowitz SG, Filvaroff EH, Yin JP et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. Embo j 2001; 20: 5332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nirula A, Nilsen J, Klekotka P et al. Effect of IL-17 receptor A blockade with brodalumab in inflammatory diseases. Rheumatology (Oxford) 2016; 55: ii43–ii55. [DOI] [PubMed] [Google Scholar]
- 27.Chang SH, Reynolds JM, Pappu BP et al. Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity 2011; 35: 611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez-Carrozzi V, Sambandam A, Luis E et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol 2011; 12: 1159–66. [DOI] [PubMed] [Google Scholar]
- 29.Wright JF, Bennett F, Li B et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol 2008; 181: 2799–805. [DOI] [PubMed] [Google Scholar]
- 30.Kuestner RE, Taft DW, Haran A et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol 2007; 179: 5462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papp KA, Leonardi C, Menter A et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 2012; 366: 1181–9. [DOI] [PubMed] [Google Scholar]
- 32.Russell CB, Rand H, Bigler J et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol 2014; 192: 3828–36. [DOI] [PubMed] [Google Scholar]
- 33.Papp KA, Reich K, Paul C et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol 2016; 175: 273–86. [DOI] [PubMed] [Google Scholar]
- 34.Frew JW, Navrazhina K, Grand D et al. The effect of subcutaneous brodalumab on clinical disease activity in hidradenitis suppurativa: An open-label cohort study. J Am Acad Dermatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Health B. SILIQ-brodalumab injection. In. 2020.
- 36.Frew JW, Navrazhina K, Byrd AS et al. Defining lesional, perilesional and unaffected skin in hidradenitis suppurativa: proposed recommendations for clinical trials and translational research studies. Br J Dermatol 2019; 181: 1339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visvanathan S, Baum P, Vinisko R et al. Psoriatic skin molecular and histopathologic profiles after treatment with risankizumab versus ustekinumab. J Allergy Clin Immunol 2019; 143: 2158–69. [DOI] [PubMed] [Google Scholar]
- 38.Lind L, Ärnlöv J, Lindahl B et al. Use of a proximity extension assay proteomics chip to discover new biomarkers for human atherosclerosis. Atherosclerosis 2015; 242: 205–10. [DOI] [PubMed] [Google Scholar]
- 39.van der Zee HH, Laman JD, de Ruiter L et al. Adalimumab (antitumour necrosis factor-α) treatment of hidradenitis suppurativa ameliorates skin inflammation: an in situ and ex vivo study. Br J Dermatol 2012; 166: 298–305. [DOI] [PubMed] [Google Scholar]
- 40.Krueger JG, Wharton KA Jr., Schlitt T et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol 2019; 144: 750–63. [DOI] [PubMed] [Google Scholar]
- 41.Hawkes JE, Yan BY, Chan TC et al. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J Immunol 2018; 201: 1605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navrazhina K, Garcet S, Zheng X et al. High inflammation in hidradenitis suppurativa extends to perilesional skin and can be subdivided by lipocalin-2 expression. J Allergy Clin Immunol 2021. [DOI] [PubMed] [Google Scholar]
- 43.Navrazhina K, Garcet S, Gonzalez J et al. In-Depth Analysis of the Hidradenitis Suppurativa Serum Proteome Identifies Distinct Inflammatory Subtypes. J Invest Dermatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolk K, Wenzel J, Tsaousi A et al. Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. Br J Dermatol 2017; 177: 1385–93. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira MC, Whibley N, Mamo AJ et al. Interleukin-17-induced protein lipocalin 2 is dispensable for immunity to oral candidiasis. Infect Immun 2014; 82: 1030–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stallhofer J, Friedrich M, Konrad-Zerna A et al. Lipocalin-2 Is a Disease Activity Marker in Inflammatory Bowel Disease Regulated by IL-17A, IL-22, and TNF-α and Modulated by IL23R Genotype Status. Inflamm Bowel Dis 2015; 21: 2327–40. [DOI] [PubMed] [Google Scholar]
- 47.Shao S, Cao T, Jin L et al. Increased Lipocalin-2 Contributes to the Pathogenesis of Psoriasis by Modulating Neutrophil Chemotaxis and Cytokine Secretion. J Invest Dermatol 2016; 136: 1418–28. [DOI] [PubMed] [Google Scholar]
- 48.Goldburg SR, Strober BE, Payette MJ. Part 2. Current and emerging treatments for hidradenitis suppurativa. J Am Acad Dermatol 2019. [DOI] [PubMed] [Google Scholar]
- 49.Frew JW, Jiang CS, Singh N et al. Clinical response rates, placebo response rates, and significantly associated covariates are dependent on choice of outcome measure in hidradenitis suppurativa: A post hoc analysis of PIONEER 1 and 2 individual patient data. J Am Acad Dermatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marzano AV, Damiani G, Ceccherini I et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol 2017; 176: 1588–98. [DOI] [PubMed] [Google Scholar]
- 51.Thomi R, Kakeda M, Yawalkar N et al. Increased expression of the interleukin-36 cytokines in lesions of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2017; 31: 2091–6. [DOI] [PubMed] [Google Scholar]
- 52.Di Caprio R, Balato A, Caiazzo G et al. IL-36 cytokines are increased in acne and hidradenitis suppurativa. Arch Dermatol Res 2017; 309: 673–8. [DOI] [PubMed] [Google Scholar]
- 53.Frew JW, Navrazhina K, Marohn M et al. Contribution of fibroblasts to tunnel formation and inflammation in hidradenitis suppurativa/ acne inversa. Exp Dermatol 2019; 28: 886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Algood HM, Allen SS, Washington MK et al. Regulation of gastric B cell recruitment is dependent on IL-17 receptor A signaling in a model of chronic bacterial infection. J Immunol 2009; 183: 5837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halwani R, Al-Kufaidy R, Vazquez-Tello A et al. IL-17 Enhances Chemotaxis of Primary Human B Cells during Asthma. PLoS One 2014; 9: e114604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Chan CC, Yang M et al. A critical role of IL-17 in modulating the B-cell response during H5N1 influenza virus infection. Cell Mol Immunol 2011; 8: 462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schirmer C, Klein C, von Bergen M et al. Human fibroblasts support the expansion of IL-17-producing T cells via up-regulation of IL-23 production by dendritic cells. Blood 2010; 116: 1715–25. [DOI] [PubMed] [Google Scholar]
- 58.Kehlen A, Thiele K, Riemann D et al. Expression, modulation and signalling of IL-17 receptor in fibroblast-like synoviocytes of patients with rheumatoid arthritis. Clin Exp Immunol 2002; 127: 539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park MJ, Moon SJ, Lee EJ et al. IL-1-IL-17 Signaling Axis Contributes to Fibrosis and Inflammation in Two Different Murine Models of Systemic Sclerosis. Front Immunol 2018; 9: 1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gudjonsson JE, Tsoi LC, Ma F et al. Contribution of plasma cells and B-cells to hidradenitis suppurativa pathogenesis. JCI Insight 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lowe MM, Naik HB, Clancy S et al. Immunopathogenesis of hidradenitis suppurativa and response to anti-TNFα therapy. JCI Insight 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griffin GK, Newton G, Tarrio ML et al. IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol 2012; 188: 6287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grand D, Navrazhina K, Frew JW. Integrating complement into the molecular pathogenesis of Hidradenitis Suppurativa. Exp Dermatol 2020; 29: 86–92. [DOI] [PubMed] [Google Scholar]
- 64.Montaudié H, Seitz-Polski B, Cornille A et al. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol 2017; 76: 156–8. [DOI] [PubMed] [Google Scholar]
- 65.Lowe MM, Naik HB, Clancy S et al. Immunopathogenesis of hidradenitis suppurativa and response to anti-TNF-α therapy. JCI Insight 2020; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcet S, Nograles K, Correa da Rosa J et al. Synergistic cytokine effects as apremilast response predictors in patients with psoriasis. J Allergy Clin Immunol 2018; 142: 1010–3.e6. [DOI] [PubMed] [Google Scholar]
- 67.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol 2005; 34: 215–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S2: Perilesional skin has a decrease of immunoglobulin-related genes at week 12 relative to baseline A) The top downregulated genes based on fold change (FCH) at week 12 in perilesional skin compared to baseline perilesional skin. Genes in bold are those with a p value ≤0.05 B) CIBERSORT fraction of infiltration of naïve B cells into HS perilesional (PL) skin and nonlesional (NL) skin.
Supplemental Figure S1: Venn diagram of genes modulated by treatment with brodalumab at week 12 in perilesional skin. Immune-related differentially expressed genes (DEGs fold change ≥ |1.5| and false discovery rate ≤ 0.05) in perilesional skin at baseline and at week 12, both relative to nonlesional skin at baseline. Numbers designate the total number of DEGs.
Supplemental Figure S5: Brodalumab treatment effect at week 4 in HS perilesional skin. Ingenuity pathway analysis (IPA) of 399 down-modulated genes with FCH <−1.5 (excluding B- cell genes). Z-score > 0, p value <0.001. Color denotes Z-score with darker bars representing higher score.
Supplemental Figure S3: Treatment response at week 4 in skin. Means of percentage improvement at week 4 between perilesional and lesional skin. N (%) = number of genes in the hidradenitis suppurativa transcriptome shared with pathway (percentage of total gene in pathway).
Supplemental Figure S4: Differentially expressed proteins in serum A) Heatmap of differentially expressed proteins at Baseline, 4 weeks and 12 weeks using OLINK proteomics data. |FCH| > 1.5, uncorrected p value <0.05. B) Pearson correlation (r) for differentially expressed proteins. Stars denote significance with * p ≤ 0.05 ** p ≤ 0.01 *** p ≤ 0.001
Data Availability Statement
All data has been uploaded to publicly available database (GEO#GSE189266) and is also available by written request to the corresponding author.


