SUMMARY
Iron is the most abundant transition metal essential for numerous cellular processes. While most mammalian cells acquire iron through transferrin receptor, molecular players of iron utilization under iron-restriction are incompletely understood. To address this, we performed metabolism-focused CRISPRa gain-of-function screens, which revealed metabolic limitations under stress conditions. Iron restriction screens identified expected members of iron utilization pathways, but also SLCO2B1 a poorly characterized membrane carrier. SLCO2B1 expression is sufficient to increase intracellular iron, bypass the essentiality of transferrin receptor and enable proliferation under iron restriction. Mechanistically, SLCO2B1 mediates heme-analog import in cellular assays. Heme uptake by SLCO2B1 provides sufficient iron for proliferation through heme oxygenases. Notably, SLCO2B1 is predominantly expressed in microglia in the brain and primary Slco2b1−/− microglia mice exhibit strong defects in heme-analog import. Altogether, our work identifies SLCO2B1 as a microglia-enriched plasma membrane heme importer and provides a genetic platform to identify metabolic limitations under stress conditions.
Graphical Abstract
eTOC blurb
Unlu et al. perform metabolism scale CRISPRa screens to reveal metabolic limitations under stress conditions. Focusing on iron restriction, they identify SLCO2B1 as a plasma membrane heme carrier that increases cellular iron availability independently of TFRC. SLCO2B1 is enriched in microglia in the brain and required for heme-analog import.
INTRODUCTION
Iron is the most abundant transition metal and essential for almost all living species. A significant fraction (2%) of human proteins binds to iron in the form of heme (iron-protoporphyrin IX), iron-sulfur (Fe-S) clusters, and as free ion (Andreini et al., 2018; Kaplan and Ward, 2013). Owing to its unique redox characteristics, iron mediates a wide range of biological process including oxidative phosphorylation, DNA replication and antioxidant response. In mammals, iron deficiency causes anemia but is also associated with impaired neurological function and weakened immune response (Jiang et al., 2019; Yager and Hartfield, 2002). In contrast, excess cellular iron generates reactive oxygen species that damage cellular macromolecules, and is linked to aging, liver failure and neurodegeneration (Sato et al., 2022; Wang et al., 2017). Most mammalian cells take up iron in the form of transferrin, a serum glycoprotein that maintains it in soluble form and limits the generation of free radicals. However, iron also associates with other carriers in the serum such as ferritin, heme and albumin. These carriers are particularly critical under disease conditions where transferrin-bound iron levels are limiting (Leitner and Connor, 2012; Moos and Morgan, 1998). However, molecular players involved in iron utilization under iron-restricted conditions are incompletely understood.
To address this, we developed a metabolic scale CRISPR activation (CRISPRa) screening platform and performed gain-of-function genetic screens under iron-limiting conditions. These screens revealed multiple mechanisms that enable cell proliferation under iron restriction. Our work also identified SLCO2B1, a poorly characterized plasma membrane transporter, as a heme transporter that promotes cellular iron availability. SLCO2B1 is predominantly expressed in microglia in the brain and primary microglia from Slco2b1−/− mice exhibit a strong defect in heme analog import. Our results reveal metabolic limitations under iron restriction and identify a plasma membrane heme transporter in microglia.
RESULTS
Metabolic-scale gene activation screens identify metabolic processes limiting for cell proliferation under iron restriction
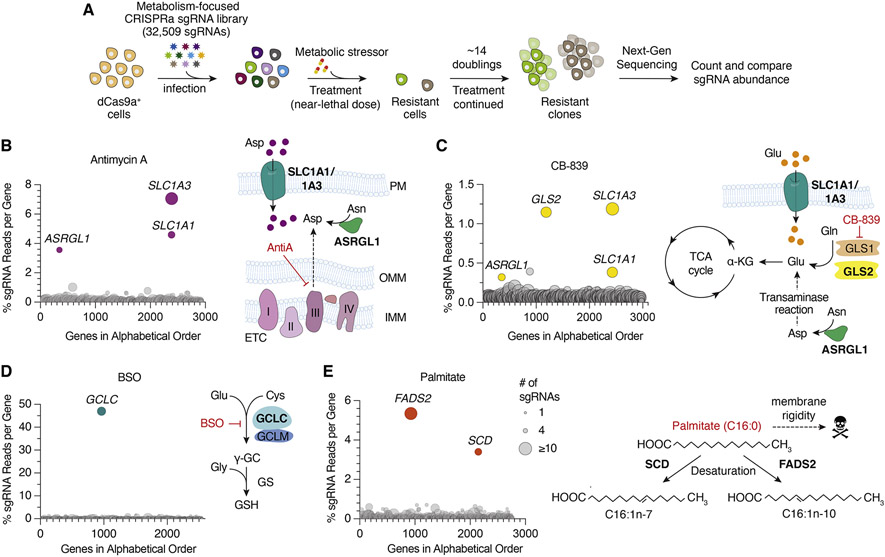
One approach to identify metabolic limitations under stress is to systematically induce the expression of small molecule transporters and enzymes and determine those that restore cell proliferation in response to a particular stress. Activation of target gene expression can be achieved with nuclease dead Cas9 (dCas9a) fused to a transcriptional activator (VP64-p65-Rta). Using this technology, we generated a metabolism focused sgRNA library consisting of 32,509 sgRNAs that target 2989 metabolic enzymes and small molecule transporters (Figure 1A, Table S1). To test the robustness of the approach, we first performed gain-of-function genetic screens in dCas9a expressing PaTu-8988t pancreatic cancer cells (Figure S1A) treated with near-lethal doses of several metabolic inhibitors. These small molecules include Antimycin A, an electron transport chain (ETC) inhibitor, CB-839, an inhibitor of glutaminolysis, buthionine sulfoximine (BSO), an inhibitor of glutathione synthesis, and palmitate, a saturated fatty acid that disrupts membrane lipid homeostasis at high levels. After selection with these agents, we harvested DNA from surviving cell populations and calculated the percentage of total sgRNA reads mapping to each gene. Genes with highest percentage sgRNA reads protect cells from the corresponding toxic agents and reveal potential metabolic limitations for cell proliferation under these stress conditions.
Figure 1. Metabolic-scale gene activation screens identify metabolic processes limiting for cell proliferation under stress conditions.
(A) Scheme describing metabolism-focused positive selection CRISPRa screens.
(B-E) CRISPRa screen results for (B) Antimycin A (10 nM), (C) CB-839 (6 μM), (D) BSO (2 mM) in Pa-Tu 8988t dCas9a cells and (E) Palmitate (200 μM) in Jurkat dCas9a cells. Data were plotted as genes in alphabetical order vs. percentage of sgRNAs reads per gene. Percent total sgRNA reads was calculated by summing up all sgRNA reads mapped to a gene and calculating the percentage of this sum to the number of sgRNA reads acquired from the entire population. Bubble size indicates number of detected sgRNAs post-screen.
Among the scoring genes that protect cells against ETC inhibition are SLC1A1 and SLC1A3, both of which are aspartate/glutamate plasma membrane transporters and increase aspartate import from the culture media. Indeed, previous work has determined that aspartate is a limiting metabolite for cancer cell proliferation under ETC inhibition (Birsoy et al., 2015) (Figures 1B and S1B). Interestingly, our screens also identified ASRGL1, a putative human L-asparaginase, which catalyzes the deamidation of asparagine to produce aspartate. While ASRGL1 has previously been shown not to display amidase activity at physiological levels of expression (Pavlova et al., 2018), our data suggest that ASRGL1 may synthesize meaningful levels of aspartate when overexpressed in human cells. Conferring resistance to CB-839, our screens identify GLS2, a paralog of GLS1, as a gene that can bypass GLS1 inhibition, supporting the selective on-target effect of CB-839 on GLS1 (Figures 1C, S1C). SLC1A1/3 and ASRGL1 also scored likely due to their ability increase the cellular levels of glutamate, a limiting metabolite for TCA cycle progression under glutaminase inhibition. In line with the fact that BSO is a competitive glutamate analog (Meister, 1995), the only scoring gene in the BSO screen was glutamate—cysteine ligase catalytic subunit (GCLC), which catalyzes the first enzymatic step in glutathione (GSH) synthesis (Figures 1D and S1D). Finally, expression of FADS2 (fatty acid desaturase) and SCD (stearoyl-CoA desaturase), the two enzymes involved in fatty acid desaturation, protect cells against lipid saturation stress upon palmitate treatment (Figures 1E and S1E). Additional hits conferring resistance to palmitate were FADS1, which introduces cis Δ5 double bond during polyunsaturated fatty acid (PUFA) synthesis (Cho et al., 1999); ACSL4 ,the major enzyme that produces long chain PUFA containing lipids, ADIPOR2, which acts as a sensor for membrane fluidity and SLCO4A1, an uncharacterized transporter (Devkota et al., 2017; Ruiz et al., 2019) (Figure S1F). Notably, SLCO4A1 has previously been shown to be coessential with enzymes in fatty acid metabolism, indicating its potential role in membrane lipid homeostasis (Aregger et al., 2020). These results highlight the utility of metabolism-scale gain of function screens to discover potential metabolic limitations under stress conditions.
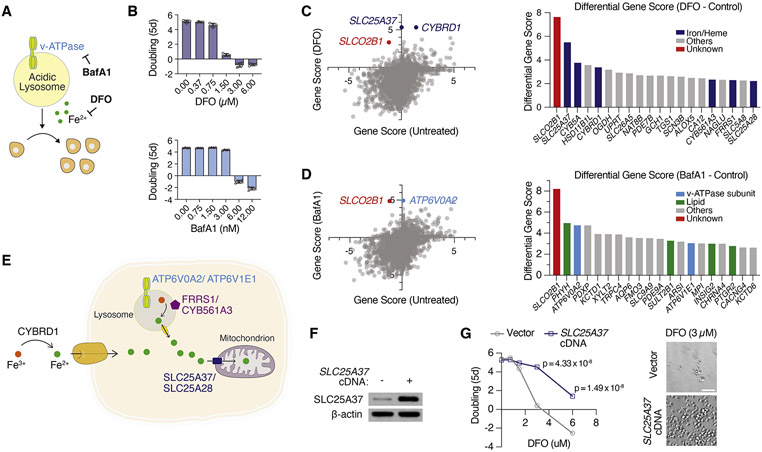
We next sought to use this platform as a discovery tool to identify cellular pathways that regulate cellular iron availability under iron restriction. While iron chelators are commonly used to deplete cellular iron, we recently showed that disrupting endosomal and lysosomal acidity also decreases cellular iron levels by hindering transferrin-mediated iron import (Weber et al., 2020). We therefore performed positive selection CRISPRa screens in Jurkat dCas9a cells (Figure S1A) and determined genes whose expression would overcome anti-proliferative effects of iron restriction induced by an iron chelator, deferoxamine mesylate (DFO, 2 μM), and an endo-lysosomal pH inhibitor, Bafilomycin A1 (BafA1, 5 nM) (Figures 2A and 2B). Scoring genes should identify metabolic limitations under iron depletion and reveal alternative pathways involved in iron acquisition. Consistent with the observation that DFO and BafA1 impair cell proliferation via distinct mechanisms, most of the scoring genes were unique to each condition. Among the scoring genes in DFO screens are SLC25A37 and SLC25A28, mitochondrial iron importers (Shaw et al., 2006). Additionally, plasma membrane and endosomal ferric reductases that reduce Fe3+ to Fe2+ also scored, including CYBRD1, FRRS1 and CYB561A3 (Figure 2C) (Vargas et al., 2003; Wang et al., 2021). In contrast, genes conferring resistance to BafA1 included v-ATPase subunits ATP6V0A2 and ATPV1E1 among the top hits. These subunits are adjacent to the BafA1 binding site on the v-ATPase, and their overexpression likely blocks inhibition of v-ATPase activity by BafA1 (Figure 2D and 2E).
Figure 2. CRISPRa screens reveal metabolic genes conferring resistance to iron depletion.
(A) BafA1 and DFO decreases cellular iron availability through different mechanisms.
(B) Dose-dependent effects of DFO and BafA1 on proliferation of Jurkat cells (Mean ± SEM, n = 5).
(C) Gene scores of untreated vs. DFO (2 μM)-treated Jurkat-dCas9a cells (left). Gene score is the median log2 fold change in the abundance of all sgRNAs targeting that gene during the screening period. Differential gene scores of top 20 genes providing resistance to DFO (right). Iron/heme-related genes are indicated in dark blue.
(D) Gene scores of untreated vs. BafA1 (5 nM)-treated Jurkat-dCas9a cells (left). Differential gene scores of top 20 genes providing resistance to BafA1 (right). Vacuolar ATPase subunits are indicated in light blue, lipid metabolism-related genes in green, other genes in gray.
(E) Scheme displaying selected scoring genes conferring resistance to iron-restriction.
(F) Immunoblot analysis of control and SLC25A37-overexpressing Jurkat cells. β-actin was used as loading control.
(G) Fold change in the number (log2) of control vector and SLC25A37-overexpressing cells after 5 days in the presence of indicated DFO concentrations (left). Representative bright-field micrographs of Jurkat cells after 5-day treatment with 3 μM DFO (right). Scale bar is 50 μM.
See also Figures S1-S3, Tables S9-S14
Iron plays essential roles in different compartments in cells. In addition to proteins containing heme or iron-sulfur (Fe-S) clusters, whose syntheses occur in the mitochondrial matrix, there are other key enzymes containing iron, but devoid of Fe-S clusters or heme. For example, di-iron center is present in a group of non-mitochondrial enzymes such as ribonucleotide reductases, oxygenases and fatty acid desaturases (Bollinger Jr et al., 1991; Ryle and Hausinger, 2002; Shen et al., 2020). Given that both mitochondrial iron transporters scored in our genetic screens, we hypothesized that iron availability in mitochondria, but not in the cytosol or other organelles, is the limiting process for cell proliferation under environmental iron restriction. Consistent with this hypothesis, overexpression of either mitochondrial iron transporter SLC25A37 or SLC25A28 was sufficient to sustain cell proliferation at lethal DFO concentrations (Figures 2F, 2G and S1G). These data suggest that mitochondrial iron availability is the limiting process for proliferation when cells are deprived of iron. Altogether, our screens identify distinct metabolic players involved in cell survival and proliferation under iron chelation and lysosomal pH inhibition. Strikingly, SLCO2B1, a poorly characterized small molecule transporter, was the only common hit upon DFO and BafA1-induced iron restriction (Figures 2C, 2D, S2A, S2B and S2C). We therefore focused our attention on it.
SLCO2B1 expression is sufficient to sustain cell proliferation under iron restriction
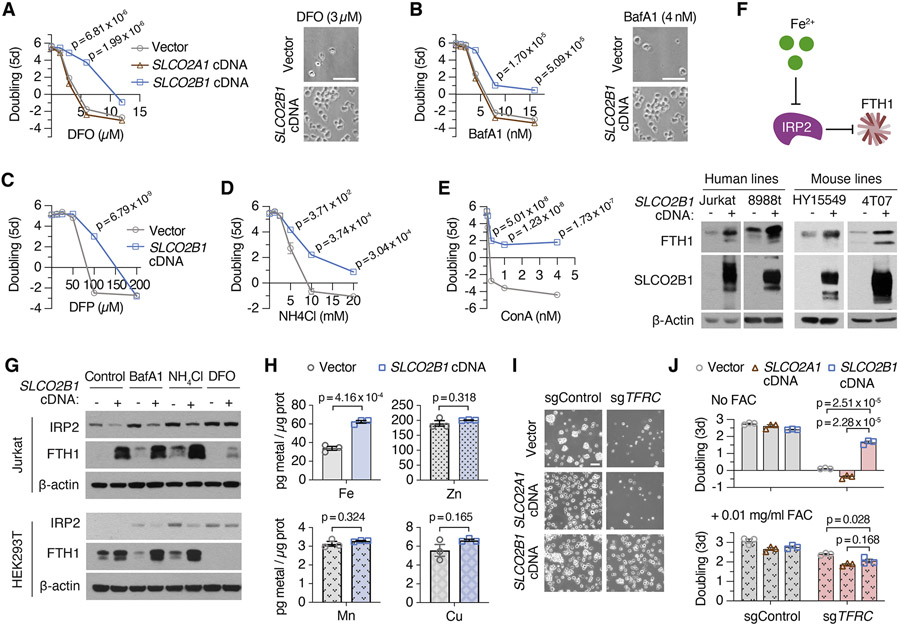
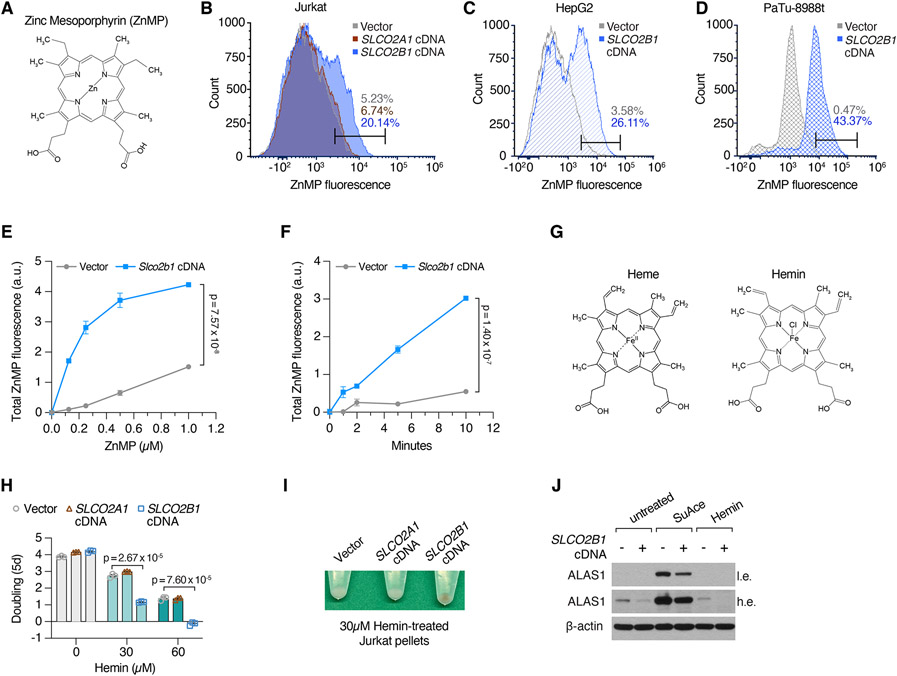
SLCO2B1 is a member of organic anion transporter family (König, 2011) and has previously been shown to transport a multitude of drugs and steroid hormone conjugates (Grube et al., 2006; Medwid et al., 2019). However, its precise physiological substrate and relevance to iron metabolism is unknown. To begin to study its function, we generated a Jurkat cell line that expresses SLCO2B1 cDNA (Figure S2D). Consistent with the screen results, cells expressing SLCO2B1 can proliferate even under lethal doses of DFO and BafA1, in contrast to those expressing a control vector, its closely related paralog SLCO2A1 or a short SLCO2B1 isoform (Figures 3A, 3B, S2E, S2F). This finding is generalizable to other cell lines, as SLCO2B1 expression in PaTu-8988t cells protected them from DFO and BafA1-induced toxicity (Figures S2G and S2H). Additionally, positive selection CRISPRa screens in a mouse pancreas cancer cell line identified Slco2b1 as the top protective gene against lysosomal pH dysfunction (Figures S2I, S2J, S2K, S2L and S2M; Table S2). Iron supplementation diminished the proliferation difference between control and SLCO2B1-expressing cells growing in otherwise lethal dose of BafA1 (Figure 2N). Furthermore, SLCO2B1-overexpressing cells displayed similar resistance to deferiprone (DFP), a lipid-soluble iron chelator, v-ATPase inhibitor concanamycin (ConA) and NH4Cl, which disrupts lysosomal acidity by acting as a lysosomotropic weak base (Klempner and Styrt, 1983) (Figures 3C, 3D and 3E). These findings reveal that SLCO2B1 expression is sufficient to sustain proliferation of mammalian cells under different modes of iron depletion.
Figure 3. SLCO2B1 expression is sufficient to promote iron availability and sustain cell proliferation under iron restriction.
(A) Fold change in the number (log2) of vector, SLCO2A1 and SLCO2B1-expressing cells after 5 days in the presence of indicated DFO concentrations (left). Representative bright-field micrographs of Jurkat cells after 5-day treatment with 3 μM DFO (right). Scale bar is 50 μm.
(B) Fold change in the number (log2) of vector, SLCO2A1 and SLCO2B1-expressing cells after 5 days in the presence of indicated BafA1 concentrations (left) and representative bright-field micrographs of Jurkat cells after 5-day treatment with 4 nM BafA1 (right). Scale bar is 50 μm.
(C-E) Fold change in the number (log2) of vector and SLCO2B1-expressing cells after 5 days in the presence of indicated (C) DFP, (D) NH4Cl and (E) ConA concentrations
(F) Schematic (top) showing IRP2-mediated regulation of ferritin heavy chain (FTH1). Increased iron levels inhibit IRP2, a negative regulator of FTH1 expression. Immunoblot analysis of FTH1 and SLCO2B1 in indicated human and mouse cell lines expressing vector or SLCO2B1 cDNA (bottom). β-actin was used as loading control.
(G) Immunoblot analysis of IRP2 and FTH1 in Jurkat and 293T cells expressing vector or SLCO2B1 cDNA, in the absence or presence of iron depleting agents BafA1 (3 nM in Jurkat; 10 nM in 293T), NH4Cl (1 mM in Jurkat; 5 mM in 293T) or DFO (3 μM in Jurkat; 100 μM in 293T).
(H) Abundance of indicated metals in Jurkat cells expressing vector or SLCO2B1 cDNA. Results were normalized to total amount of protein and reported as ‘pg metal per μg protein’.
(I) Representative bright-field micrographs of TFRC knockout Jurkat cells expressing vector, SLCO2A1 or SLCO2B1 cDNA. Scale bar is 50 μm.
(J) Fold change in the number (log2) of TFRC knockout Jurkat cells expressing a control vector, SLCO2A1 or SLCO2B1 cDNA without (top) or with 0.01 mg/ml ammonium ferric citrate (FAC) supplementation (bottom) after 3 days. (Mean ± SEM, n= 3, Student’s t-test, 95% confidence interval). See also Figure S3
SLCO2B1 promotes iron availability, independently of transferrin-mediated iron uptake in mammalian cells
We next sought to determine the role of SLCO2B1 in cellular iron metabolism. To address this in an unbiased way, we profiled cellular metabolites from Jurkat cells expressing SLCO2A1 (control) or SLCO2B1 cDNA by liquid chromatography-mass spectrometry (LC-MS). However, only few metabolites change in response to SLCO2B1 overexpression, suggesting that SLCO2B1 expression does not cause a strong change in the levels of cellular metabolites (Figures S3A and S3B). Cellular iron homeostasis is tightly controlled in cells by a post-translational mechanism mediated by IRP1/2 pathway (Sviderskiy et al., 2019). An increase in iron levels negatively regulate IRP2, enhancing mRNA stability of ferritin heavy chain 1 (FTH1), the major iron storage protein in mammalian cells. To test whether SLCO2B1 modulates iron homeostasis, we determined IRP2 and FTH1 protein levels in cells expressing SLCO2B1. Expression of SLCO2B1 caused a marked induction in the protein levels of FTH1 across several mammalian cell lines tested, under standard culture conditions (Figures 3F and S3C). We next asked whether SLCO2B1-expressing cells maintain higher levels of iron stores even under iron restriction. Indeed, expression of SLCO2B1 strongly blocked the increase in IRP2 and the decrease in FTH1 protein levels in response to treatment with lysosomal pH inhibitors (Figure 3G). Finally, to directly measure iron levels in these cells, we conducted inductively coupled plasma mass spectrometry (ICP/MS) measurements. Consistent with the increase in ferritin levels, iron levels in SLCO2B1-expressing cells were ~2-fold higher than those of the parental controls. Notably, we did not observe any significant change in the levels of other divalent metals (Cu, Mn and Zn), indicating a specific effect of SLCO2B1 on iron homeostasis (Figure 3H). These results suggest that SLC2OB1 regulates the iron response pathway by increasing cellular iron stores.
Under physiological conditions, TFRC-mediated iron uptake is the main route of iron acquisition (Richardson and Ponka, 1997). Indeed, TFRC is a universally essential gene for the proliferation of most cell lines (Tsherniak et al., 2017). To determine whether SLCO2B1 expression bypasses the requirement of TFRC-mediated iron uptake, we knocked out TFRC by CRISPR/Cas9 in Jurkat cells expressing SLCO2B1, its paralog SLCO2A1 or vector control (Figure S3D). Consistent with the essential function of TFRC for cell survival, parental Jurkat cells and those expressing SLC2OA1 died upon its depletion. Supplementation of culture media with excess free iron, i.e., ferric iron citrate (FAC), was sufficient to restore cell proliferation. In contrast, SLCO2B1 expression in Jurkat cells completely restored their survival and proliferation in response to TFRC loss even in the absence of iron supplementation (Figures 3I and 3J). These results strongly suggest that SLCO2B1 promotes iron availability and cell proliferation under iron restriction, independently of transferrin-mediated iron uptake.
Heme oxygenase is essential for SLCO2B1-induced resistance to iron restriction
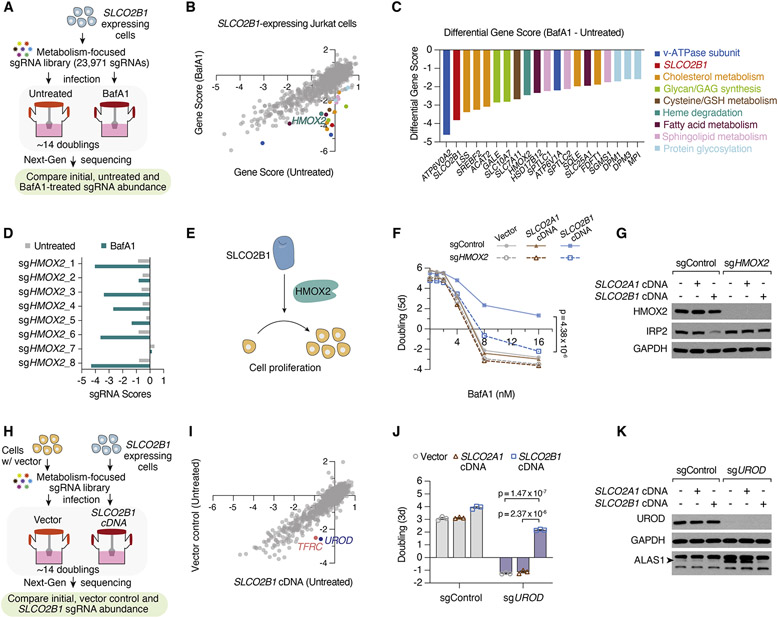
To understand how SLCO2B1 expression enables cell survival under iron restriction, we performed a negative selection CRISPR screen in parental Jurkat cells and those expressing SLCO2B1, treated with a normally lethal dose of BafA1. These screens should identify metabolic processes required for SLCO2B1-mediated rescue of iron restriction. Additionally, given that SLCO2B1-expressing cells tolerate high levels of BafA1 treatment, this provides a unique opportunity to determine metabolic processes that enable cell proliferation under severe lysosomal dysfunction (Figures 4A, 4B, S4A and S4B). Indeed, among the scoring genes differentially essential under lysosomal pH inhibition are those involved in cholesterol (LSS, SREBF2, ACAT2, SQLE, FDFT1) and sphingolipid (SPTLC1, SPTLC2 and SGMS1) biosynthesis. Cholesterol and sphingolipids can be synthesized de novo, but also acquired from extracellular lipoproteins through lysosomal enzymes (Goldstein and Brown, 2009; Levade et al., 1996). Similarly, cysteine import into lysosomes requires the lysosomal pH gradient. Likely due to the decrease in lysosomal cysteine storage, cells become dependent on cystine uptake mediated by SLC7A11 (Figures 4C and S4B) (Pisoni et al., 1990). Further work is needed to determine why several other processes scored as conditionally essential and how they may compensate for lysosomal dysfunction in mammalian cells.
Figure 4. Heme oxygenase is essential for SLCO2B1-mediated resistance to iron restriction.
(A) Schematic describing CRISPR loss of function screens in Jurkat cells expressing SLCO2B1 cDNA in the presence and absence of BafA1.
(B) Gene scores of untreated vs. BafA1-treated (4 nM) Jurkat cells expressing SLCO2B1 cDNA.
(C) Top 20 genes scoring as differentially essential upon BafA1 treatment in SLCO2B1-expressing cells compared to vector controls. Differential gene scores are plotted, and color matched with metabolic processes they are involved in, as listed on right.
(D) sgRNA scores of HMOX2-targeting sgRNAs in untreated and BafA1-treated Jurkat cells expressing SLCO2B1 cDNA.
(E) Schematic illustrating the proposed model for SLCO2B1-mediated rescue of cell proliferation under iron restriction.
(F) Fold change in the number (log2) of control and HMOX2 knockout Jurkat cells expressing a control vector, SLCO2A1 or SLCO2B1 cDNA, during 5-day incubation with or without BafA1 at indicated concentrations. (Mean ± SEM, n= 3, Student’s t-test, 95% confidence interval).
(G) Immunoblot analysis for HMOX2 and IRP2 in control and HMOX2 knockout Jurkat cells expressing vector, SLCO2A1 or SLCO2B1 cDNA. GAPDH was used as loading control.
(H) Schematic depicting CRISPR loss of function screen in Jurkat cells expressing a vector control compared to those expressing SLCO2B1 cDNA.
(I) Gene scores of Jurkat cells expressing a control vector or SLCO2B1 cDNA.
(J) Fold change in the number (log2) of control and UROD knockout Jurkat cells expressing vector, SLCO2A1 or SLCO2B1 cDNA after 3 days. Cells were seeded 7 days after infecting with UROD sgRNA vector. (Mean ± SEM, n= 3, Student’s t-test, 95% confidence interval).
(K) Immunoblot analysis for UROD and ALAS1 in Jurkat cells expressing vector, SLCO2A1 or SLCO2B1 cDNA infected with a control sgRNA or UROD sgRNA. GAPDH was used as loading control. See also Figure S4, Tables S15-S18
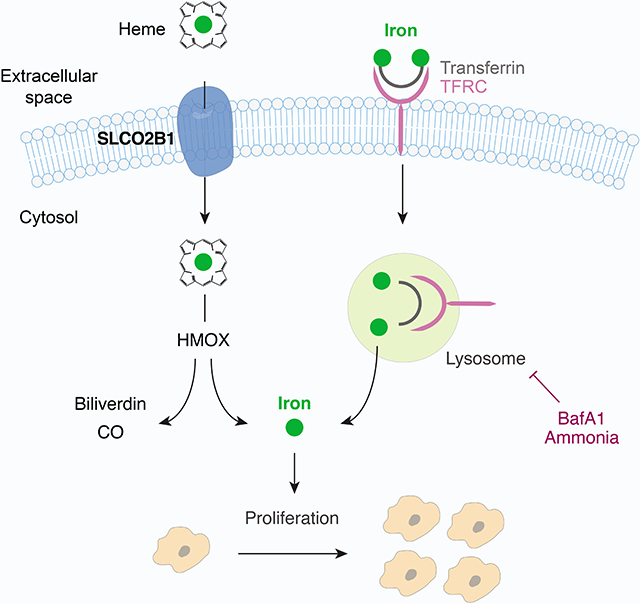
Interestingly, the only scoring gene relevant to iron metabolism and differentially essential in SLCO2B1 expressing cells was HMOX2, a heme oxygenase (HMOX) required for heme degradation (Figures 4B, 4C, 4D and S4A). HMOX enzymes break down the porphyrin ring of heme to produce biliverdin, carbon monoxide and iron. Notably, while most mammalian cells express two HMOX paralogs, HMOX2 is the predominantly expressed one in Jurkat cells (TPM of HMOX1 = 0.3, HMOX2 = 32.8, DepMap portal). To test whether HMOX2 is necessary for SLCO2B1-mediated resistance against iron restriction, we deleted HMOX2 in parental and SLCO2A1 or SLCO2B1-expressing Jurkat cells. Consistent with the screen results, HMOX2 loss completely abolished SLCO2B1-mediated resistance to BafA1 and DFO treatment (Figures 4E, 4F and S4C). Given the requirement of HMOX2 for SLCO2B1-mediated resistance, we reasoned that HMOX2 loss would block the increase in iron availability and the corresponding decrease in IRP2 protein abundance of SLCO2B1 expressing cells. In line with our model, HMOX2 loss restored IRP2 protein levels in SLCO2B1-expressing cells (Figure 4G). Collectively, these results suggest that HMOX2 acts downstream of SLCO2B1 to increase intracellular iron availability.
Given that heme degradation is required for SLC2OB1 function, we next sought to determine whether SLCO2B1 is involved in heme metabolism. Interestingly, when we compared gene essentialities between parental cells and those expressing SLCO2B1, we found that UROD (uroporphyrinogen decarboxylase), like TFRC, is essential only in parental cells and not in SLCO2B1 expressing counterparts (Figures 4H, 4I, S4E, S4F). UROD is a universally essential enzyme in mammalian cells and catalyzes the third step of heme biosynthesis in the cytosol. We therefore asked whether SLCO2B1 expression may bypass the dependence of mammalian cells on de novo heme synthesis. While loss of UROD blocked the proliferation of parental Jurkat cells, expression of SLCO2B1 completely eliminated the anti-proliferative effects of UROD depletion (Figure 4J). Protein levels of ALAS1, the rate limiting enzyme of heme synthesis, is negatively regulated by cellular heme levels. In line with this, expression of SLCO2B1, but not control cDNAs, reduced the increase in ALAS1 protein levels in response to UROD depletion (Figure 4K and S4G). Similarly, SLC2OB1 expression blocked the increase in ALAS1 levels in cells treated with succinyl acetone, the pharmacological inhibitor of heme synthesis (Figures S4D and S4H). Altogether, these results indicate that SLCO2B1 expression is sufficient to restore cell proliferation in cells deficient for heme synthesis enzyme, UROD.
SLCO2B1 expression is necessary and sufficient for the uptake of heme analogs
SLCO2B1-expressing cells are remarkably resistant to the effects of iron restriction and heme synthesis deficiency, suggesting that SLCO2B1 may be a potential heme importer. To first determine the localization of SLCO2B1, we expressed in PaTu-8988t cells a C-terminally eGFP-tagged version of SLCO2B1, which is comparably functional to restore cell viability upon BafA1 treatment (Figures S5A, S5B, S5C and S5D). Confocal microscopy analysis of the SLCO2B1-eGFP fusion protein showed a strong plasma membrane localization (Figure S5A). To quantitatively measure steady state heme uptake, we used Zinc mesoporphyrin (ZnMP), a fluorescent heme analog (Figure 5A). We treated cells with ZnMP and measured intracellular fluorescence by flow cytometry. ZnMP uptake was markedly higher in SLCO2B1-expressing cells across three human cell lines tested (Jurkat 24h uptake; HepG2 and PaTu-8988t 15 min uptake assays) compared to parental controls (Figures 5B, 5C and 5D). To formally prove the direct cellular uptake, we next performed a ZnMP uptake assay in a time- and dose-dependent manner. During a 15-min dose-dependent uptake assay, Slco2b1-expressing cells took up significantly more ZnMP at all doses tested (e.g. >5 fold at 0.5 μM) (Figures 5E, 5F and S5E). Finally, since heme is a highly redox-active molecule, excess uptake of heme should be more toxic to cells that import it more efficiently. We therefore assayed sensitivity of SLCO2B1-expression and control cells to Hemin, a hemelike porphyrin structure with a ferric chloride center (Figure 5G). Indeed, SLCO2B1 expressing Jurkat cells displayed slower proliferation rates than control and SLCO2A1-expressing cells, in response to hemin treatment (Figure 5H). Remarkably, upon hemin addition, SLCO2B1-expressing cell pellets, in contrast to controls, displayed a discernably brown color, characteristic of hemin accumulation and greater reduction of ALAS1 (Figure 5I and J). In parallel, SLCO2B1-overexpressing cells treated with succinyl acetone upregulated ALAS1 protein levels to a lesser degree than that of control cells, indicating higher heme availability. These results suggest that SLCO2B1 is sufficient for the transport of heme analogs.
Figure 5. SLCO2B1 expression is sufficient for the uptake of heme analogs.
(A) Chemical structure of Zinc Mesoporphyrin (ZnMP).
(B-D) Flow cytometry analysis of ZnMP uptake in (B) Jurkat cells expressing vector, SLCO2A1 or SLCO2B1 cDNA, treated with 5 μM ZnMP for 24 h (C) HepG2 cells expressing vector or SLCO2B1 cDNA treated with 1 μM ZnMP for 15 min (D) PaTu-8988t cells expressing vector or SLCO2B1 cDNA treated with 2 μM ZnMP for 15 min.
(E) Dose curve for ZnMP uptake in mouse HY15549 pancreas cells expressing vector or Slco2b1 cDNA treated with indicated doses of ZnMP for 15 min at room temperature. (Mean ± SEM, n= 3, Student’s t-test, 95% confidence interval).
(F) Time course for ZnMP uptake in mouse HY15549 pancreas cells expressing vector or Slco2b1 cDNA treated with 1 μM ZnMP for the indicated time points.
(G) Chemical structures of Heme B and Hemin.
(H) Fold change in the number (log2) of Jurkat cells expressing control vector, SLCO2A1 or SLCO2B1 cDNA, during 5-day incubation with or without Hemin treatment at indicated concentrations. (Mean ± SEM, n= 3, Student’s t-test, 95% confidence interval).
(I) Representative images of cell pellets from Hemin-treated Jurkat cells expressing vector, SLCO2A1 or SLCO2B1 cDNA. Cells were treated with 30 μM Hemin for 24 h prior to pelleting.
(J) Immunoblot analysis for ALAS1 in Succinyl Acetone (1mM) or Hemin (30 μM) treated Jurkat cells expressing vector or SLCO2B1 cDNA. β-actin was used as loading control.
See also Figure S5
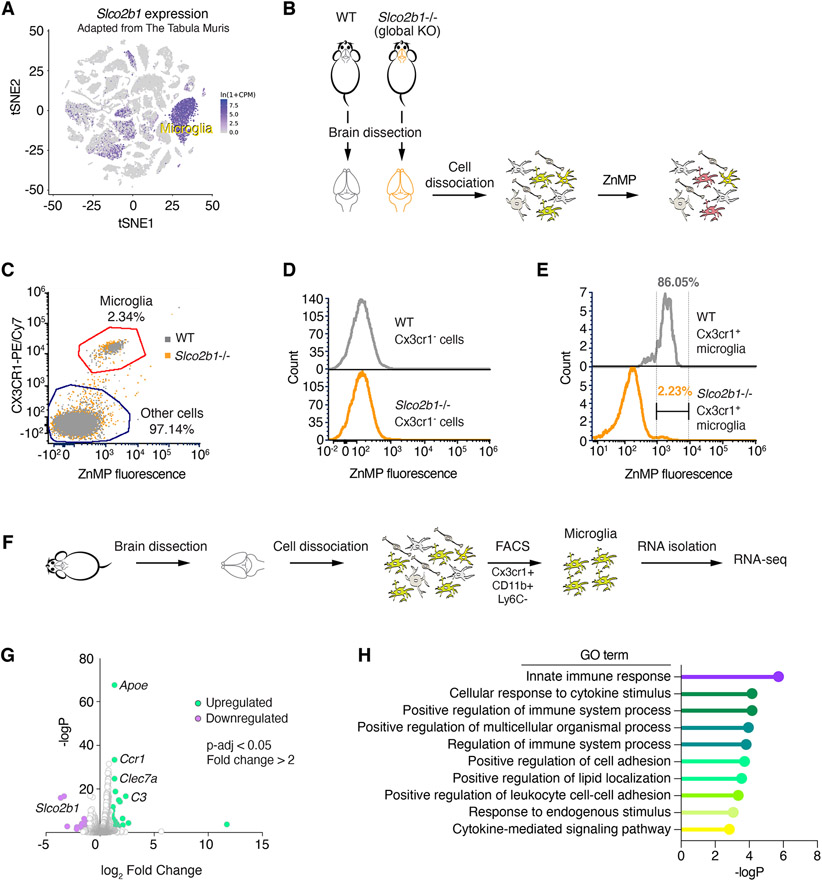
We next sought to test whether SLCO2B1 is necessary for heme analog import. Notably, most cultured mammalian cells we have tested did not exhibit appreciable levels of SLCO2B1 expression. We therefore analyzed a publicly available single cell transcriptome dataset (Tabula Muris Consortium, 2018) to identify cell types with highest Slco2b1 expression (Figure 6A). Strikingly, Slco2b1 expression displays a highly restricted expression pattern in microglial cells in brain. To study loss of function of Slco2b1, we therefore generated a full body Slco2b1-null mouse model (Figure S5F). These knockout mice are viable and enabled us to test the necessity of Slco2b1 for heme analog uptake in isolated microglial cells (Ledo et al., 2020) (Figure 6B). Cells dissociated from brain, other than microglia, did not display detectable ZnMP positivity (Figures 6C and 6D), suggesting that microglial cells could indeed be the predominant cell type with heme uptake activity in the brain. Remarkably, this ZnMP uptake activity in wild type microglia almost exclusively depends on Slco2b1 expression. Indeed, microglia from Slco2b1 knockout mice display a strong reduction in ZnMP import compared to those from wild type controls (i.e., 86% of wild type microglia, as opposed to 2.23% Slco2b1-null microglia, were ZnMP+) (Figure 6E). These results suggest that Slco2b1 is necessary for heme analog uptake in microglia. Finally, to determine whether Sloc2b1 loss leads to any physiological changes in microglia, we performed RNA-seq analysis on sorted microglia from adult Slco2b1−/− mice and their wild type littermates. Gene ontology (GO) enrichment analysis of upregulated genes in Slco2b1−/− microglia revealed inflammation-related GO terms ‘innate immune response’ and ‘cellular response to cytokine-stimulus’ signatures (Figure 6G, H). Notably, Apoe and Clec7a were amongst the most upregulated genes (Figure 6G; Table S3), which have been implicated in disease-associated microglia during Alzheimer progression and neuroinflammation (Krasemann et al., 2017; Lanfranco et al., 2021). Future studies will determine whether these phenotypes are linked to heme uptake deficiencies in microglia.
Figure 6. Slco2b1 is necessary for heme analog uptake in microglial cells.
(A) t-SNE plot for Slco2b1 expression across single cell transcriptome of mouse cell types. Adapted from Tabula Muris.
(B) Experimental strategy for the ZnMP uptake assay of primary cells derived from brains of wild type and Slco2b1 knockout mice.
(C) Flow cytometry analysis of ZnMP fluorescence and Cx3cr1-labeling (microglia marker) in cells isolated from wild-type and Slco2b1 knockout mouse brains.
(D) Flow cytometry analysis of ZnMP fluorescence in Cx3cr1− non-microglial cells.
(E) Flow cytometry analysis of ZnMP fluorescence in Cx3cr1+ microglia.
(F) Experimental strategy for sorting mouse microglia by FACS for RNA-seq.
(G) Volcano plot for differential gene expression analysis between WT and Slco2b1−/− mouse microglia. p-value adjusted <0.05. Upregulated genes (fold change > 2) are shown in green, downregulated (fold change < −2) in purple. See also Table S3.
(H) Gene ontology enrichment analysis for significantly upregulated genes. p < 0.05, hypergeometric test.
See also Figure 5
DISCUSSION
Proliferating cells commonly experience nutrient limitations due to environmental metabolic stress conditions. This is particularly relevant for tumor cells, where they frequently starve for nutrients due to hypoxia and poor vascularization. Previous work has shown that, under defined metabolic stressors, supplementation of a single metabolite has the potential to overcome metabolic bottlenecks (Birsoy et al., 2015; Garcia-Bermudez et al., 2018; Possemato et al., 2011). Here, we devised a metabolic-scale gain-of-function screening platform to identify these metabolic bottlenecks. This platform can be readily applied to study metabolic characteristics of diverse tumor models, and to determine limiting metabolic processes for cell growth in culture as well as in vivo. A major outcome of our study is the identification of SLCO2B1 as a plasma membrane transporter that mediates heme import. SLCO2B1-mediated heme uptake bypasses two essential metabolic processes in mammalian cells: TFRC-mediated iron uptake and heme biosynthesis. Similar to SLCO2B1, heme uptake by the heme-responsive paralogs, hrg-1 and hrg-4, has extensively been studied in the context of heme-auxotrophic C. elegans. Mammalian homolog of hrg-1 facilitates lysosomal export of heme in splenic macrophages (Rajagopal et al., 2008; White et al., 2013) and hrg-4 in worms lacks a mammalian homolog. Furthermore, while previous work proposed FLVCR2 as a plasma membrane heme transporter, its ectopic expression failed to rescue heme-deficiency (Chambers et al., 2021; Duffy et al., 2010). Notably, single cell transcriptomics data revealed that SLCO2B1 expression is highly enriched in microglia (Cao et al., 2020; Tabula Muris Consortium, 2018), indicating that SLCO2B1 might mediate microglial heme import. As free heme levels increase in disease conditions like hematoma and intracranial hemorrhage (Mracsko and Veltkamp, 2014; Vasconcellos et al., 2021), microglial heme uptake by SLCO2B1 could be a defense mechanism to protect neurons and astrocytes from oxidative heme damage. Furthermore, heme uptake may also be relevant in the context of neurodegenerative diseases, as induced HMOX1 expression and iron accumulation in microglial cells have been observed in Alzheimer’s and Parkinson’s disease (Fernández-Mendívil et al., 2021; Kenkhuis et al., 2021). Given that neuroinflammation markers, Apoe and Clec7a, are highly upregulated in Slco2b1−/− microglia, it is conceivable that Slco2b1 knockouts might be more susceptible to neurodegeneration and neuroinflammation when aged or challenged. Future studies should determine the precise physiological role of Slco2b1-mediated microglial heme uptake in disease and physiology.
Limitations of the Study
In this study, we conducted several gain-of-function screens using metabolism-scale CRISPRa libraries. While activation of gene expression by CRISPRa can identify genes with redundant functions in well-designed experiments followed by validation assays, it should be noted that overexpression could result in neomorphic phenotypes that may be physiologically irrelevant. Therefore, loss-of-function assays should be carried out to investigate relevance of the gene function in particular phenotypes. In addition, we hereby characterized heme as a substrate of SLCO2B1, while leaving open the possibility that it can be a broad-spectrum transporter with other physiological substrates. Indeed, steroid hormone conjugates, xenobiotics, and co-porphyrins were implicated as SLCO2B1 substrates in previous biochemical assays (Medwid et al., 2021). To precisely calculate the affinity of SLCO2B1 for heme and compare it to those of other transporters, liposome-based in vitro reconstituted uptake assays should be conducted in future work.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Kıvanç Birsoy (kbirsoy@rockefeller.edu)
Materials Availability
All unique reagents generated in this study are available from the lead contact upon request.
Data and code availability
Original western blot images have been deposited on Mendeley at https://doi.org/10.17632/sg3b3f25p4.1 and are publicly available as of the date of publication. The DOI is also listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| SLC25A37 | Invitrogen | Cat#PA5-26720; RRID:AB_2544220 |
| SLCO2B1 | Novus Biologicals | Cat#NBP1-80979; RRID:AB_11038565 |
| Fth1 | Cell Signaling Technology | Cat#3998S; RRID:AB_1903974 |
| IRP2 | Cell Signaling Technology | Cat#37135S; RRID:AB_2799110 |
| β-Actin | GeneTex | Cat#GTX109639; RRID:AB_1949572 |
| GAPDH | GeneTex | Cat#GTX627408; RRID:AB_11174761 |
| HMOX2 | Proteintech | Cat#14817-1-AP; RRID:AB_2118843 |
| UROD | Proteintech | Cat#15547-1-AP; RRID:AB_2213813 |
| ALAS1 | Proteintech | Cat#16200-1-AP; RRID:AB_2878227 |
| CRISPR-Cas9 | Novus Biologicals | Cat#NBP2-36440; Clone 7A9-3A3 |
| TFRC (CD71) | Cell Signaling Technology | Cat#13113S; RRID:AB_2715594 |
| GFP (for immunofluorescence) | Aves Labs | Cat#GFP-1010; RRID:AB_2307313 |
| GFP (for immunoblotting) | Proteintech | Cat#50430-2-AP; RRID:AB_11042881 |
| Goat anti-Chicken IgY (H+L), Alexa Fluor™ 488 | Thermo Fisher Scientific | Cat#A11039; RRID:AB_2534096 |
| Goat anti-Rabbit IgG (H+L), Alexa Fluor™ 555 | Thermo Fisher Scientific | Cat#A21428; RRID:AB_2535849 |
| PE/Cy7 anti-mouse CX3CR1 [Clone: SA011F11] | Biolegend | Cat#149015; RRID:AB_2565699 |
| Anti-CD11b Rat Monoclonal Antibody (Brilliant Violet® 711) [clone: M1/70 | Biolegend | Cat#101241; RRID:AB_11218791 |
| Anti-Ly-6C Rat Monoclonal Antibody (Brilliant Violet® 510) [clone: HK1.4] | Biolegend | Cat#128033; RRID:AB_2562351 |
| Bacterial and Virus Strains | ||
| NEB Stable Competent E. coli | New England Biolabs | C3040 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| RPMI 1640 Culture Media | GIBCO | 11875 |
| DMEM Culture Media | GIBCO | 11965 |
| Trypsin | GIBCO | 25200 |
| Fetal Bovine Serum | Sigma | 12306C |
| Bafilomycin A1 | Cayman Chemical | 11038 |
| Concanamycin A | Sigma-Aldrich | C9705 |
| Deferoxamine Mesylate | Sigma | D9533 |
| Deferiprone | Selleck Chemicals | S4067 |
| Antimycin A | Sigma-Aldrich | A8674 |
| CB-839 | Selleck Chemicals | S7655 |
| Palmitic Acid | Cayman Chemical | 10006627 |
| L-Buthionine-sulfoximine | Sigma | B2515 |
| DMSO | AlfaAesar | A13280-36 |
| HPLC Grade Water | Fisher Scientific | W6 |
| HPLC Grade Methanol | Fisher Scientific | A454-1 |
| Heavy labeled amino acid internal standards | Cambridge Isotopes | MSK-A2-1.2 |
| Ammonium Iron (III) Citrate (FAC) | Sigma | F5879 |
| Ammonium Chloride | Sigma | A9434 |
| Zn(II) Mesoporphyrin IX | Frontier Scientific | M40628 |
| Hemin | Sigma-Aldrich | H9039 |
| Succinylacetone | Cayman Chemical | 25501 |
| Ethanol | Fisher Scientific | 04-355-222 |
| BsmBI | New England Biolabs | R0580 |
| T4 DNA ligase | New England Biolabs | M0202 |
| ExTaq DNA Polymerase | Takara | RR001A |
| Hank's Buffered Salt Solution | GIBCO | 24020117 |
| XtremeGene9 | Roche | 6365779001 |
| Polybrene | Sigma | H9268 |
| Puromycin | Sigma | P8833 |
| Blasticidin | Invivogen | ant-bl-1 |
| Critical Commercial Assays | ||
| CellTiter-Glo® Luminescent Cell Viability Assay | Promega | G7571 |
| Deposited Data | ||
| Original western blot images | This work | https://doi.org/10.17632/sg3b3f25p4.1 |
| Experimental Models: Cell Lines | ||
| Jurkat | ATCC | TIB-152 |
| HepG2 | ATCC | HB-8065 |
| MDA-MB-231 | ATCC | CRM-HTB-26 |
| HEK293T | ATCC | CRL-1573 |
| PaTu-8988t | Dr. Monther Abu-Remaileh (Stanford University) | N/A |
| AK196 | Dr. Haoqiang Yang (MD Anderson Cancer Center) | N/A |
| HY15549 | Dr. Nabeel El-Bardeesy (Massachusetts General Hospital Cancer Center) | N/A |
| 4T1 | Dr. Sohail Tavazoie (Rockefeller University) | N/A |
| 4T07 | Dr. Sohail Tavazoie (Rockefeller University) | N/A |
| Experimental Models: Organisms/Strains | ||
| C57BL/6N-Slco2b1tm1a(KOMP)Wtsi/Mmucd | Mutant Mouse Resource and Research Center (MMRRC) | 049765-UCD |
| C57BL/6J | The Jackson Laboratory | 000664 |
| Oligonucleotides | ||
| DNA oligonucleotides and primers | This study | See Table S19 |
| Recombinant DNA | ||
| pLV-EF1a-IRES-Blast | Addgene | 85133 |
| plentiCRISPR puro v2 | Addgene | 52961 |
| lentiGuide Puro | Addgene | 52963 |
| CRISPRa dCas9-VPR vector with hEF1a promoter | Horizon Discovery | CAS11916 |
| pLV-EF1a-IRES-Blast_SLC25A37 | This study | N/A |
| pLV-EF1a-IRES-Blast_SLCO2B1 | This study | N/A |
| pLV-EF1a-IRES-Blast_SLCO2B1 (short isoform) | This study | N/A |
| pLV-EF1a-IRES-Blast_SLCO2B1-eGFP | This study | N/A |
| pLV-EF1a-IRES-Blast_SLCO2A1 | This study | N/A |
| pLV-EF1a-IRES-Blast_SLCO2A1-eGFP | This study | N/A |
| pLV-EF1a-IRES-Blast_Slco2b1 (mouse cDNA) | This study | N/A |
| plentiCRISPR puro v2_sgTFRC_1 | This study | N/A |
| plentiCRISPR puro v2_sgUROD_1 | This study | N/A |
| plentiCRISPR puro v2_sgUROD_2 | This study | N/A |
| plentiCRISPR puro v2_sgHMOX2_1 | This study | N/A |
| Human metabolism-focused CRISPRa sgRNA library | This study | N/A |
| Mouse metabolism-focused CRISPRa sgRNA library | This study | N/A |
| Metabolism-focused sgRNA library | Birsoy et al., 2015 | N/A |
| Software and Algorithms | ||
| XCaliburQual Browser 2.2 | ThermoFisher | https://www.thermofisher.com/order/catalog/product/OPTON-30965#/OPTON-30965; RRID: SCR_014593 |
| Skyline Daily | MacCoss Lab | https://skyline.ms/project/home/software/Skyline/begin.view; RRID: SCR_014080 |
| FCS Express 7 | DeNovo Software | https://denovosoftware.com/; RRID: SCR_016431 |
| Prism 9 | GraphPad | https://www.graphpad.com/scientific-software/prism/; RRID: SCR_002798 |
| Other | ||
| Z2 Coulter Counter | Beckman | Model Z2 |
| SpectraMax Microplate Reader | Molecular Devices | Model M5 |
| Primovert Microscpe | Carl Zeiss | 415510-1105-000 |
| FACSAriaII | BD Biosciences | 643181 |
| Multiphoton microscope with confocal modality | Nikon | A1R MP |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
All animal studies were performed according to a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at Rockefeller University. Animals were housed in ventilated caging on a standard light-dark cycle with food and water ad libitum. The mouse strain C57BL/6N-Slco2b1tm1a(KOMP)Wtsi/Mmucd (MMRRC line: 049765-UCD) was recovered from cryopreserved sperm obtained from Mutant Mouse Resource and Research Center (MMRRC) at University of California, Davis. C57BL/6N-Slco2b1tm1a(KOMP)Wtsi/Mmucd sperm was crossed to C57BL/6J wild-type strain (from The Jackson Laboratory) through in vitro fertilization at Rockefeller University Transgenic and Reproductive Technology Center. The progeny is maintained under standard conditions and identified through PCR amplification-based genotyping method provided by MMRRC. Homozygous mutant and homozygous wild type female animals of 5-8 weeks were used in microglia isolation experiments. Genotypes were revealed to blinded investigators post-analysis.
Cell Lines, Compounds and Constructs
Jurkat, HepG2, HEK293T and MDA-MB-231 cell lines were purchased from ATCC. Other cell lines were kindly provided by the following investigators: PaTu-8988t pancreatic adenocarcinoma cell line by Dr. Monther Abu-Remaileh (Stanford University, CA), the HY15549 mouse pancreatic cancer line by Nabeel El-Bardeesy (Massachusetts General Hospital Cancer Center, MA), AK196 mouse pancreatic cancer line by Haoqiang Yang (MD Anderson Cancer Center, TX), 4T1 and 4T07 mouse breast cancer cell lines by Dr. Sohail Tavazoie (Rockefeller University, NY). All cell lines were verified to be mycoplasma contamination-free and authenticated by STR profiling. Reagents, compounds, and antibodies used in this study are listed in Table S1.
Cell Culture Conditions
Unless otherwise indicated, all listed cell lines, except for HepG2, were cultured in RPMI-1640 media (GIBCO) containing 2mM glutamine, 10% fetal bovine serum (SAFC, Sigma Aldrich) and 1% penicillin and streptomycin (Invitrogen). HepG2 cells were cultured in DMEM (GIBCO) containing 4.5g/L glucose, 110mg/L pyruvate, 4mM glutamine, supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin. All cells were maintained at 37°C, 21% O2 and 5% CO2. For Figure S3, Jurkat cells were cultured in RPMI1640 supplemented with dialyzed FBS (GIBCO #26400-044).
METHOD DETAILS
Generation of overexpression and knockout constructs
Gene fragments for coding sequences of human SLCA25A37, SLCO2B1, SLCO2A1; and mouse Slco2b1 were purchased from Twist Biosciences, then cloned into pLV-EF1a-IRES-Blast vector (Addgene #85133) by Gibson assembly method. pLV-EF1a-IRES-Blast was used as a control vector control in overexpression experiments. Knockout cells were generated with CRISPR/Cas9 method. Forward and reverse oligos targeting TFRC, UROD and HMOX2 were annealed and ligated into BsmBI-linearized pLentiCRISPR v2 vector.
Cell proliferation assays
2,000 Jurkat cells or 500 PaTu-8988t or 500 mouse KPC cells/ per well were seeded, in triplicates, in 0.2 mL RPMI-1640 medium containing indicated treatments in 96-well plates. On the day of seeding and the final day of treatment (as indicated in corresponding figures), 40 μL of CellTiter-Glo reagent (Promega) was added, then, luminescence was measured on a SpectraMax M3 plate reader (Molecular Devices). Data are presented as cell doublings or the log2 fold change in luminescence on final treatment day compared to initial reading on the day of seeding.
Generation of knockout and overexpression cell lines
For generation of knockout cells, VSV-G and Delta-VPR lentiviral packaging vectors were simultaneously transfected into HEK293T cells along with plentiCRISPR v2 vector expressing Cas9 and the gene-targeting sgRNA, using XtremeGene9 transfection reagent (Roche). Similarly, for overexpression, pLV-EF1a-IRES-Blast vector containing the gene-of-interest was transfected along with lentiviral packaging vectors VSV-G and Delta-VPR. 60 h post-transfection, the supernatant was collected after passing through a 0.45 μm syringe filter. For transduction, 1 x 105 cells were plated in 6-well plates containing 4 μg/mL polybrene and virus, and then spin-infected by centrifugation at 2,200 rpm for 80 minutes. Mixed population knockouts were selected with puromycin; overexpression cells were selected with blasticidin. Knockout and/or overexpression efficiency was assessed via immunoblotting. Jurkat or PaTu-8988t cells expressing dCas9-VPR were generated following a similar lentiviral transduction strategy. Instead, Edit-R Lentiviral CRISPRa dCas9-VPR vector (Horizon Discovery) was used as the overexpression vector. Single cell clones overexpressing dCas9-VPR were generated by FACS sorting single cells on a BD FACSAriaII, 72-96 h post-infection into 96 well plates and grown for 2 weeks. Clones with strong overexpression were identified by Cas9 immunoblotting.
CRISPR/Cas9 genetic screens
For metabolic scale CRISPRa screens in human cell lines, a metabolism-focused sgRNA library was designed and screens were performed following established protocols (Birsoy et al., 2015; Wang et al., 2015) with the following details and modifications. To specifically focus on metabolic pathways, 2,989 genes encoding for metabolic enzymes and small molecule transporters, as described in Birsoy et al., 2015, were targeted with a total of 32,460 CRISPRa sgRNAs, whose designing approach was described previously (Horlbeck et al., 2016), and 49 non-targeting sgRNAs were included as controls. Sequences of sgRNAs included in human metabolism-focused CRISPRa library are provided in Table S1. Oligonucleotides containing sgRNA sequences were synthesized by Agilent Technologies, PCR amplified and cloned into lentiGuide-Puro vector (Addgene #52963). Briefly, amplicons were inserted into BsmBI-linearized lentiGuide-Puro vector by Gibson Assembly (NEB). Then, Gibson Assembly products were transformed into E. coli 10G SUPREME electrocompetent cells (Lucigen). This plasmid pool was used to produce lentivirus-containing supernatants in HEK293T cells. The titer of lentiviral supernatants was determined by infecting target cells at several amounts of virus in the presence of polybrene (4 μg/mL), counting the number of puromycin-resistant infected cells 3 days post-selection. For CRISPRa positive selection screens, 4 million target cells were infected at an MOI of ~0.7 and selected with puromycin (4 μg/mL) 72 h post-infection. An initial pool of 4 million cells was harvested for genomic DNA extraction. The remaining cells were cultured for 14 doublings under specified drug treatment or untreated control conditions. On the final day of screening, cells were harvested for genomic DNA extraction. sgRNA inserts were PCR amplified, purified, and sequenced on MiSeq platform (Illumina). Sequencing reads were aligned to the library of sgRNA sequences and the abundance of each sgRNA was tallied. Results were reported as percentage of total sgRNA reads acquired per gene, gene score or differential gene score. Percent total sgRNA reads was calculated by summing up all sgRNA reads mapped to a gene and calculating the percentage of this sum to the number of sgRNA reads acquired from the entire population. sgRNA scores, representing the log2 fold change of the normalized final read count of the sgRNA from the initial read count of the sgRNA, were calculated. Gene score is defined as the median log2 fold change of all sgRNAs targeting the gene in the abundance of all sgRNAs targeting that gene, between the initial and final population. The differential gene score refers to the difference between the gene scores of drug-treated and untreated control groups.
For metabolic scale CRISPRa screens in mouse AK196 pancreas cell line, a metabolism-focused sgRNA library was designed and screens were performed similarly, as described above. For these screens, 1,839 metabolic genes were targeted with a total of 17,031 CRISPRa sgRNAs. Sequences of sgRNAs included in mouse metabolism-focused CRISPRa library are provided in Table S2.
Metabolism-focused CRISPR knockout screens in human lines were performed following the screen protocol detailed above with the following modifications. 3 x 107 cells were infected with sgRNA library containing 23,971 sgRNAs targeting metabolic genes as reported by Zhu et al. (Zhu et al., 2019). Abundance of sgRNAs was quantified post-sequencing on NextSeq500 platform (Illumina). Full results of all CRISPR screens can be found in Supplemental Tables S4-S18.
Metabolite Profiling
For bulk metabolite profiling, 1 x106 Jurkat cells, in triplicate, were grown for 24 h under DFO-, BafA1-treatment or untreated conditions. Then, cells were rinsed in 0.9 % NaCl twice. Polar metabolites were extracted in 80 % methanol containing 15N and 13C fully-labeled amino acid standards (MSK-A2-1.2, Cambridge Isotope Laboratories, Inc). Extracts were shaken for 10 min with a vortexer, spun at 19,000 g to remove insoluble cell debris, nitrogen-dried and stored at −80°C until liquid chromatography-mass spectrometry analysis (LC-MS). Then, LC-MS analysis was conducted on a QExactive benchtop orbitrap mass spectrometer equipped with an Ion Max source and a HESI II probe coupled to a Dionex UltiMate 3000 UPLC system (Thermo Fisher Scientific). External mass calibration was performed using the standard calibration mixture every 7 days. Air dried polar samples were resuspended in 100 μL water and 2 μL were injected into a ZIC-pHILIC 150 × 2.1 mm (5 μm particle size) column (EMD Millipore). Chromatographic separation was achieved using the following conditions: Buffer A was 20 mM ammonium carbonate, 0.1% ammonium hydroxide; buffer B was acetonitrile. The column oven and autosampler tray were held at 25°C and 4°C, respectively. The chromatographic gradient was run at a flow rate of 0.150 mL/min as follows: 0–20 min: linear gradient from 80% to 20% B; 20–20.5 min: linear gradient from 20% to 80% B; 20.5–28 min: hold at 80% B. The mass spectrometer was operated in fullscan, polarity switching mode with the spray voltage set to 3.0 kV, the heated capillary held at 275°C, and the HESI probe held at 350°C. The sheath gas flow was set to 40 units, the auxiliary gas flow was set to 15 units, and the sweep gas flow was set to 1 unit. The MS data acquisition was performed in a range of 70–1000 m/z, with the resolution set at 70,000, the AGC target at 106, and the maximum injection time at 20 msec. Relative metabolite abundances were quantified using XCalibur QualBrowser 2.2 and Skyline Targeted Mass Spec Environment (MacCoss Lab) using a 5 ppm mass tolerance and a pooled-library of metabolite standards to verify metabolite identity. Relative metabolite levels were calculated by normalizing to total protein levels, as measured by Bicinchoninic Acid Assay (BCA).
Measurement of Metals by Inductively Couple Plasma / Mass Spectrometry (ICP/MS)
3 x107 Jurkat cells were seeded in triplicates and grown in standard culture conditions for 24 h. Cell pellets were collected and stored at −80°C until ICP/MS analysis. The cell pellet was resuspended in pure, distilled water of ≥80 μl. The cell pellet was lysed on ice, using 5 pulses with a cell disruptor/sonicator microtip. For each ICP/MS replicate, 30 to 50 μl of cell lysate was digested using a 5:1 mixture of nitric acid (OPTIMA grade, 70%, Fisher Scientific) and ultrapure hydrogen peroxide (ULTREX II, 30%, Fisher Scientific). 30 lysate, 500 uL of nitric acid and 100 uL hydrogen peroxide are added to 2 mL polypropylene tubes. This mixture was allowed to digest overnight at room temperature, heated at 95°C just until dry, and resuspended overnight in 1 ml of 2% nitric acid for analysis. The mixture was mixed well the following day to ensure resuspension, and 800 μl more 2% nitric acid is added along with 200 μl of 10X internal standard (prepared in 2% nitric acid). For ICP/MS run, an Agilent 7900 ICP/MS instrument was operated in helium (He) collision cell gas mode for all measurements. Elements were measured at the following isotopes: 56Fe, 55Mn, 63Cu, 66Zn. Calibration standards and samples were prepared in an acid matrix of 2% OPTIMA Grade Nitric Acid. Solutions of Agilent Multi-element Calibration Standard 2A were prepared to obtain an eight-point calibration curve. Agilent Germanium (or Scandium) Standard(s) were added to calibration standards, blanks, and samples. Standards were used to correct for potential sample matrix and/or nebulization effects.
Amounts of metals within a sample were normalized by total amount of protein, as determined by BCA assay (Thermo Fisher Scientific).
Immunoblotting
1 x 106 cells were washed in cold PBS and lysed in a buffer containing 10 mM Tris-HCl pH 7.4, 150 NaCl, 1 mM EDTA, 1% Triton X-100, 2% SDS, 0.1% CHAPS, and protease inhibitors (Milipore Sigma). Lysates were sonicated, centrifuged at 1,000 g, and supernatant was collcted as the protein lysate. Total protein quantified using BCA Protein Assay Kit (Thermo Fisher) with bovine serum albumin as a protein standard. Protein samples were resolved on 8% or 10%–20% SDS-PAGE gels (Novex, ThermoFisher) and analyzed by standard immunoblotting protocol. Briefly, PVDF membranes (Milipore) were incubated with primary antibodies at 4°C overnight. After washing off the primary antibodies in tris buffered saline/ 0.1% Tween-20 (TBS-T), secondary antibody incubation was performed at room temperature for 1h. Secondary antibodies including anti-mouse IgG–HRP linked (Cell Signaling, 7076) and anti-rabbit IgG–HRP linked (Cell Signaling, 7074), were used at 1:3,000 dilution. Blots were developed by ECL Chemiluminescent detection system (Perkin Elmer LLC) and film exposure. SRX-101A Film Processor (Konica Minolta) and Premium autoradiography Films (Thomas Scientific) were used for developing.
Immunocytochemistry and Confocal Microscopy
PaTu-8988t cells were seeded on coverslips and grown overnight at 37°C with 5% CO2. Next day, cells were fixed in 4 % PFA at room temperature for 15 min, permeabilized in 1% triton X-100 in PBA, and then, blocked in 1% bovine serum albumin (Sigma) for 30 min at room temperature. Primary antibody incubation was performed overnight at 4°C. Then, cells were rinsed in PBS twice and secondary antibody incubation was performed at room temperature for 30 min. After washing twice in PBS, DAPI, nuclear counterstain, incubation was performed at room temperature for 10 min, then cells were washed twice in PBS and mounted in ProLong Gold antifade mountant (Molecular Probes). Slides were imaged with Nikon A1R MP multiphoton microscope with confocal modality, using Nikon Plan Apo γ 60X/1.40 oil immersion objective.
Real-time quantitative PCR
Total RNA was isolated from mouse livers using TRIzol reagent (Thermo Fisher Scientific), following manufacturer’s manual. After DNase I treatment (New England Biolabs), 1 μg total RNA was used for cDNA synthesis with Superscript III RT kit (Invitrogen). qPCR was performed on a Thermo QuantStudio 6 Flex Real-Time PCR machine. The primer sequences were listed in Table S19. Gene expression levels were normalized to beta-actin using ΔΔCt method.
Zinc Mesoporphyrin (ZnMP) Uptake and Flow Cytometry Analysis
Adherent cells (HepG2, PaTu-8988t and KP pancreas) were trypsinized and washed with 1X Hank’s Balanced Salt Solution (HBSS) (Milipore Sigma) twice. 1 x105 cells were placed in a microfuge tube and incubated with the indicated concentration of ZnMP (Frontier Scientific) in 1x HBSS for 15 min at room temperature. Uptake was terminated by placing cells on ice. Then, cell pellets were collected, washed twice in 1X HBSS and resuspended in cold FACS buffer (DPBS + 2% FBS + 5mM EDTA). ZnMP uptake was assessed by Flow Cytometry (Attune NxT Flow Cytometer, Thermo Fisher). 10,000 events were recorded per each sample. ZnMP signal was detected by exciting with 561 nm laser, AlexaFluor 568 emission track was recorded as it overlaps with ZnMP emission. Data were analyzed and plotted with FCS Express 7 Research software, version 7.12 (De Novo Software, Inc).
For ZnMP uptake in Jurkat cells, 5μM ZnMP was diluted directly in culture media (RPMI 1640 + 10% FBS). Cells were treated with ZnMP overnight at standard culture conditions (37°C, 5% CO2). After that, Jurkat cells were washed twice in PBS and resuspended in FACS buffer. Flow cytometry and data analyses were carried out as described above.
For dose and time-dependent ZnMP uptake assays, mouse KP pancreas cells were used. Cells, in triplicate, were treated with 1:2 serial dilutions of ZnMP, between 0.125 μM and 1μM, including 0 μM control (equal volume DMSO), for 15 minutes at room temperature. For time course uptake assay, cells, in triplicate, were treated with 1 μM ZnMP for 0, 1, 2, 5 and 10 minutes at room temperature. Median fluorescence value of ZnMP+-gated population and number of gated events were multiplied to calculate total ZnMP fluorescence. Results were plotted in Prism 9 (GraphPad) as dose or time vs. total ZnMP fluorescence.
Mouse microglia isolation and ZnMP uptake assay
Mouse microglial cells were isolated from 8-10 week-old female mice as previously described (Ledo et al., 2020). Briefly, mice were sacrificed, whole brains were removed and placed in DPBS (Ca2+ and Mg2+ free) containing 5% FBS and 1mM HEPES pH7.4. Brain tissue was minced with scissors and incubated in 4,000 U/ml of collagenase D (Roche) at 37°C for 30 minutes. Collagenase digestion was stopped by adding 10 mM EDTA and incubating for additional 5 minutes at 37°C. Digested tissue was passed through 70-μm cell strainer, centrifuged at 2,000 rpm, washed in DPBS, and centrifuged in 38% Percoll gradient for 30 min. Cell pellet containing microglia fraction was washed and resuspended in cold FACS buffer (DPBS + 2% FBS + 5mM EDTA). Non-specific binding was blocked by incubation with Fc blocking antibody (BD Biosciences, Clone 2.4G2) for 15 min. Cells were washed in FACS buffer and stained with Cx3cr1 antibody (Clone: SA011F11) conjugated to PE/Cy7. After that, cells were washed twice in FACS buffer to remove unbound, excess antibody. 5 x105 cells were placed in a microfuge tube and incubated with 2 μM ZnMP (Frontier Scientific) in 1x HBSS for 15 min at room temperature. Uptake was terminated by placing the cells on ice. Then, cell pellets were collected, washed twice in 1X HBSS and resuspended in cold FACS buffer (DPBS + 2% FBS + 5mM EDTA). ZnMP uptake was assessed by Flow Cytometry (Attune NxT Flow Cytometer, Thermo Fisher). 10,000 events were recorded per each sample. ZnMP signal was detected by exciting with 561 nm laser, AlexaFluor 568 emission track was recorded. Cx3cr1-PE/Cy7 signal was detected by exciting with 488 nm laser and recording emission at 780 nm. Data were analyzed and plotted with FCS Express 7 Research software, version 7.12 (De Novo Software, Inc).
Sorting microglial cells with FACS and RNA-seq
Mouse microglial cells were isolated as described above. For FACS, cells were stained with Cx3cr1-PE/Cy7 (clone:SA011F11) , CD11b-Brilliant Violent 711 (clone: M1/70) and Ly6C-Brilliant Violet 510 (clone: HK1.4) and DAPI for viability. Next, Cx3cr1+ CD11b+ Ly6C− DAPI− population was sorted directly into TRIzol-LS with BD FACS Aria II. Then, total RNA was isolated following TRIzol-LS (Thermo Fisher Scientific) manufacturer’s manual. RNA concentrations were determined using Qubit 2.0 Fluorometer (Life Technologies); RNA intregrity was checked using Agilent TapeStation 4200 (Agilent Technologies). RNA sequencing library was prepared using NEBNext Ultra RNA library kit for Illumina (NEB) following manufacturer’s manual. The sequencing libraries were clustered on a single lane of a flowcell. Afterwards, the flowcell was loaded on the Illumina HiSeq instrument (4000 or equivalent) according to manufacturer’s guidelines. The samples were sequenced using a 2x150bp Paired End (PE) configuration. For RNA-seq analysis, sequence and transcript coordinates for mouse genome (mm 10 UCSC) and gene models were retrieved from the Bioconductor Bsgenome.Hsapiens.UCSC.mm10 (version 1.4.0) and TxDb.Hsapiens.UCSC.mm10.knownGene (version 3.4.0) Bioconductor libraries respectively. Transcript expressions were calculated using the Salmon quantification software (Patro et al., 2017) (version 0.8.2) and gene expression levels as transcripts per million (TPM). Normalization and rlog transformation of raw read counts in genes were performed using DESeq2 (Love et al., 2014) (version 1.20.0). Genes significantly differentially expressed between conditions were identified using DESeq2 with a Benjamini Hochberg adjusted p-value cutoff of 0.05. Gene Ontology term enrichment was obtained for all genes significantly differentially expressed between conditions (absolute logFC > 0, adjusted p-value < 0.05) using the fisher test in the topGO Bioconductor package and ranked using the elim algorithm and functional annotation from the org.Mm.eg.db Bioconductor package (version 3.10).
Quantification and statistical analysis
Statistical analysis was performed with built-in statistics tools in Prism9 (GraphPad Software). Statistical tests, error bars and confidence intervals can be found in the figure legends. p-values for comparisons between groups are indicated in figures.
Supplementary Material
Table S1. Human metabolism-focused CRISPRa sgRNA library, related to Figure 1
Table S2. Mouse metabolism-focused CRISPRa sgRNA library, related to Figure 2
Table S3. Differential gene expression analysis (Slco2b1−/− vs. WT mouse microglia), related to Figure 6
Table S4. Antimycin A CRISPRa screen results in PaTu-8988t cells, related to STAR Methods
Table S5. CB-839 CRISPRa screen results in PaTu-8988t cells, related to STAR Methods
Table S6. BSO CRISPRa screen results in PaTu-8988t cells, related to STAR Methods
Table S7. Palmitate CRISPRa screen results in Jurkat cells, related to STAR Methods
Table S8. Differential Gene Scores in Palmitate CRISPRa screen performed in Jurkat cells, related to STAR Methods
Table S9. Gene Scores from Jurkat CRISPRa screens for DFO, related to STAR Methods
Table S10. Differential Gene Scores in DFO CRISPRa screen performed in Jurkat cells, related to STAR Methods
Table S11. Gene Scores from Jurkat CRISPRa screens for BafA1, related to STAR Methods
Table S12. Differential Gene Scores in BafA1 CRISPRa screen performed in Jurkat cells, related to STAR Methods
Table S13. Gene Scores from CRISPRa screens for BafA1 in mouse AK196 pancreas cells, related to STAR Methods
Table S14. Gene Scores from CRISPRa screens for ConA in mouse AK196 pancreas cells, related to STAR Methods
Table S15. Gene Scores from CRISPR knockout screens for BafA1 in SLCO2B1-expressing Jurkat cells, related to STAR Methods
Table S16. Differential Gene Scores from CRISPR knockout screens for BafA1 in SLCO2B1-expressing Jurkat cells, related to STAR Methods
Table S17. Gene Scores from CRISPR knockout screens for BafA1 in parental Jurkat cells, related to STAR Methods
Table S18. Gene Scores from CRISPR knockout screens for differential essentiality of genes between control vector and SLCO2B1-expressing Jurkat cells, related to STAR Methods
Table S19. DNA nucleotides and primers, related to STAR Methods
Highlights.
CRISPRa screens identify metabolic limitations for growth under stress conditions
Expression of SLCO2B1 confers resistance to iron limitation
SLCO2B1-mediated heme uptake increases iron availability via heme oxygenase
SLCO2B1 is required for heme analog import in primary microglia
ACKNOWLEDGEMENTS
We thank all members of the Birsoy laboratory for helpful suggestions. G.U. is a Damon Runyon Fellow, supported by the Damon Runyon Cancer Research Foundation (DRG-2431-21), and by the grant UL1 TR001866 from the National Center for Advancing Translational Sciences (NCATS, National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. H.-W.Y is supported by a C. H. Li Memorial Scholar Fund Award at The Rockefeller University. K.B. is supported by the NIH/NCI (DP2 OD024174-01), NIH/NIDDK (R01 DK123323-01), Pershing Square Sohn Foundation and Mark Foundation Emerging Leader Award; and is a Searle and Pew-Stewart Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
K.B. is scientific advisor to Nanocare Pharmaceuticals and Barer Institute. Other authors declare no competing interests.
REFERENCES
- Andreini C, Putignano V, Rosato A, and Banci L (2018). The human iron-proteome. Metallomics : integrated biometal science 10. 10.1039/c8mt00146d. [DOI] [PubMed] [Google Scholar]
- Aregger M, Lawson KA, Billmann M, Costanzo M, Tong AHY, Chan K, Rahman M, Brown KR, Ross C, Usaj M, et al. (2020). Systematic mapping of genetic interactions for de novo fatty acid synthesis identifies C12orf49 as a regulator of lipid metabolism. Nature metabolism 2. 10.1038/s42255-020-0211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsoy K, Wang T, Chen W, Freinkman E, Abu-Remaileh M, and Sabatini DM (2015). An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162, 540–551. 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JM Jr, Edmondson DE, Huynh BH, Filley J, Norton JR, and Stubbe J (1991). Mechanism of assembly of the tyrosyl radical-dinuclear iron cluster cofactor of ribonucleotide reductase. Science (New York, N.Y.) 253. 10.1126/science.1650033. [DOI] [PubMed] [Google Scholar]
- Cao J, O'Day DR, Pliner HA, Kingsley PD, Deng M, Daza RM, Zager MA, Aldinger KA, Blecher-Gonen R, Zhang F, et al. (2020). A human cell atlas of fetal gene expression. Science (New York, N.Y.) 370. 10.1126/science.aba7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers IG, Willoughby MM, Hamza I, and Reddi AR (2021). One ring to bring them all and in the darkness bind them: The trafficking of heme without deliverers. Biochimica et biophysica acta. Molecular cell research 1868. 10.1016/j.bbamcr.2020.118881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HP, Nakamura M, and Clarke SD (1999). Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. The Journal of biological chemistry 274. 10.1074/jbc.274.52.37335. [DOI] [PubMed] [Google Scholar]
- Devkota R, Svensk E, Ruiz M, Ståhlman M, Borén J, and Pilon M (2017). The adiponectin receptor AdipoR2 and its Caenorhabditis elegans homolog PAQR-2 prevent membrane rigidification by exogenous saturated fatty acids. PLoS Genetics 13. 10.1371/journal.pgen.1007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SP, Shing J, Saraon P, Berger LC, Eiden MV, Wilde A, and Tailor CS (2010). The Fowler syndrome-associated protein FLVCR2 is an importer of heme. Molecular and cellular biology 30. 10.1128/MCB.00690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Mendívil C, Luengo E, Trigo-Alonso P, García-Magro N, Negredo P, and López MG (2021). Protective role of microglial HO-1 blockade in aging: Implication of iron metabolism. Redox biology 38. 10.1016/j.redox.2020.101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bermudez J, Baudrier L, La K, Zhu XG, Fidelin J, Sviderskiy VO, Papagiannakopoulos T, Molina H, Snuderl M, Lewis CA, et al. (2018). Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat Cell Biol 20, 775–781. 10.1038/s41556-018-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, and Brown MS (2009). The LDL receptor. Arteriosclerosis, thrombosis, and vascular biology 29. 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M, Köck K, Karner S, Reuther S, Ritter CA, Jedlitschky G, and Kroemer HK (2006). Modification of OATP2B1-mediated transport by steroid hormones. Molecular pharmacology 70. 10.1124/mol.106.026450. [DOI] [PubMed] [Google Scholar]
- Horlbeck MA, Gilbert LA, Villalta JE, Adamson B, Pak RA, Chen Y, Fields AP, Park CY, Corn JE, Kampmann M, and Weissman JS (2016). Compact and highly active next-generation libraries for CRISPR-mediated gene repression and activation. eLife 5. 10.7554/eLife.19760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Li C, Wu Q, An P, Huang L, Wang J, Chen C, Chen X, Zhang F, Ma L, et al. (2019). Iron-dependent histone 3 lysine 9 demethylation controls B cell proliferation and humoral immune responses. Nature communications 10. 10.1038/s41467-019-11002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J, and Ward DM (2013). The essential nature of iron usage and regulation. Current Biology 23. 10.1016/j.cub.2013.05.033. [DOI] [PubMed] [Google Scholar]
- Kenkhuis B, Somarakis A, de Haan L, Dzyubachyk O, IJsselsteijn ME, de Miranda NFCC, Lelieveldt BPF, Dijkstra J, van Roon-Mom WMC, Höllt T, and van der Weerd L (2021). Iron loading is a prominent feature of activated microglia in Alzheimer's disease patients. Acta Neuropathologica Communications 9. 10.1186/s40478-021-01126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempner MS, and Styrt B (1983). Alkalinizing the intralysosomal pH inhibits degranulation of human neutrophils. The Journal of clinical investigation 72. 10.1172/JCI111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O'Loughlin E, Xu Y, Fanek Z, et al. (2017). The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 47. 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König J (2011). Uptake transporters of the human OATP family: molecular characteristics, substrates, their role in drug-drug interactions, and functional consequences of polymorphisms. In Handbook of Experiemental Pharmacology, (Handb Exp Pharmacol). 10.1007/978-3-642-14541-4_1. [DOI] [PubMed] [Google Scholar]
- Lanfranco MF, Sepulveda J, Kopetsky G, and Rebeck GW (2021). Expression and secretion of apoE isoforms in astrocytes and microglia during inflammation. Glia 69. 10.1002/glia.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledo JH, Zhang R, Mesin L, Mourão-Sá D, Azevedo EP, Troyanskaya OG, Bustos V, and Greengard P (2020). Lack of a site-specific phosphorylation of Presenilin 1 disrupts microglial gene networks and progenitors during development. PloS one 15. 10.1371/journal.pone.0237773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner DF, and Connor JR (2012). Functional roles of transferrin in the brain. Biochimica et Biophysica Acta 1820. 10.1016/j.bbagen.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Levade T, Leruth M, Graber D, Moisand A, Vermeersch S, Salvayre R, and Courtoy PJ (1996). In situ assay of acid sphingomyelinase and ceramidase based on LDL-mediated lysosomal targeting of ceramide-labeled sphingomyelin. Journal of lipid research 37. [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medwid S, Li MMJ, Knauer MJ, Lin K, Mansell SE, Schmerk CL, Zhu C, Griffin KE, Yousif MD, Dresser GK, et al. (2019). Fexofenadine and Rosuvastatin Pharmacokinetics in Mice with Targeted Disruption of Organic Anion Transporting Polypeptide 2B1. Drug metabolism and disposition: the biological fate of chemicals 47. 10.1124/dmd.119.087619. [DOI] [PubMed] [Google Scholar]
- Medwid S, Price HR, Taylor DP, Mailloux J, Schwarz UI, Kim RB, and Tirona RG (2021). Organic Anion Transporting Polypeptide 2B1 (OATP2B1) Genetic Variants: In Vitro Functional Characterization and Association With Circulating Concentrations of Endogenous Substrates. Frontiers in pharmacology 12. 10.3389/fphar.2021.713567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A (1995). Mitochondrial changes associated with glutathione deficiency. Biochimica et biophysica acta 1271. 10.1016/0925-4439(95)00007-q. [DOI] [PubMed] [Google Scholar]
- Moos T, and Morgan EH (1998). Evidence for low molecular weight, non-transferrin-bound iron in rat brain and cerebrospinal fluid. Journal of Neuroscience Research 54. . [DOI] [PubMed] [Google Scholar]
- Mracsko E, and Veltkamp R (2014). Neuroinflammation after intracerebral hemorrhage. Frontiers in cellular neuroscience 8. 10.3389/fncel.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, and Kingsford C (2017). Salmon provides fast and bias-aware quantification of transcript expression. Nature Methods 14. 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova NN, Hui S, Ghergurovich JM, Fan J, Intlekofer AM, White RM, Rabinowitz JD, Thompson CB, and Zhang J (2018). As Extracellular Glutamine Levels Decline, Asparagine Becomes an Essential Amino Acid. Cell metabolism 27. 10.1016/j.cmet.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni RL, Acker TL, Lisowski KM, Lemons RM, and Thoene JG (1990). A cysteine-specific lysosomal transport system provides a major route for the delivery of thiol to human fibroblast lysosomes: possible role in supporting lysosomal proteolysis. The Journal of Cell Biology 110. 10.1083/jcb.110.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, et al. (2011). Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476. 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal A, Rao AU, Amigo J, Tian M, Upadhyay SK, Hall C, Uhm S, Mathew MK, Fleming MD, Paw BH, et al. (2008). Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature 453. 10.1038/nature06934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DR, and Ponka P (1997). The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochimica et biophysica acta 1331. 10.1016/s0304-4157(96)00014-7. [DOI] [PubMed] [Google Scholar]
- Ruiz M, Ståhlman M, Borén J, and Pilon M (2019). AdipoR1 and AdipoR2 maintain membrane fluidity in most human cell types and independently of adiponectin. Journal of lipid research 60. 10.1194/jlr.M092494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryle MJ, and Hausinger RP (2002). Non-heme iron oxygenases. Current opinion in chemical biology 6. 10.1016/s1367-5931(02)00302-2. [DOI] [PubMed] [Google Scholar]
- Sato T, Shapiro JS, Chang HC, Miller RA, and Ardehali H (2022). Aging is associated with increased brain iron through cortex-derived hepcidin expression. eLife 11. 10.7554/eLife.73456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GC, Cope JJ, Li L, Corson K, Hersey C, Ackermann GE, Gwynn B, Lambert AJ, Wingert RA, Traver D, et al. (2006). Mitoferrin is essential for erythroid iron assimilation. Nature 440. 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- Shen J, Wu G, Tsai AL, and Zhou M (2020). Structure and Mechanism of a Unique Diiron Center in Mammalian Stearoyl-CoA Desaturase. Journal of molecular biology 432. 10.1016/j.jmb.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sviderskiy VO, Terzi EM, and Possemato R (2019). Iron–Sulfur Cluster Metabolism Impacts Iron Homeostasis, Ferroptosis Sensitivity, and Human Disease. In Ferroptosis in Health and Disease., (Springer, Cham). 10.1007/978-3-030-26780-3_12. [DOI] [Google Scholar]
- Tabula Muris Consortium (2018). Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562. 10.1038/s41586-018-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsherniak A, Vazquez F, Montgomery PG, Weir BA, Kryukov G, Cowley GS, Gill S, Harrington WF, Pantel S, Krill-Burger JM, et al. (2017). Defining a Cancer Dependency Map. Cell 170. 10.1016/j.cell.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas JD, Herpers B, McKie AT, Gledhill S, McDonnell J, van den Heuvel M, Davies KE, and Ponting CP (2003). Stromal cell-derived receptor 2 and cytochrome b561 are functional ferric reductases. Biochimica et biophysica acta 1651. 10.1016/s1570-9639(03)00242-5. [DOI] [PubMed] [Google Scholar]
- Vasconcellos LRC, Martimiano L, Dantas DP, Fonseca FM, Mata-Santos H, Travassos L, Mendez-Otero R, Bozza MT, and Pimentel-Coelho PM (2021). Intracerebral Injection of Heme Induces Lipid Peroxidation, Neuroinflammation, and Sensorimotor Deficits. Stroke 52. 10.1161/STROKEAHA.120.031911. [DOI] [PubMed] [Google Scholar]
- Wang H, An P, Xie E, Wu Q, Fang X, Gao H, Zhang Z, Li Y, Wang X, Zhang J, et al. (2017). Characterization of ferroptosis in murine models of hemochromatosis. Hepatology (Baltimore, Md.) 66. 10.1002/hep.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, Lander ES, and Sabatini DM (2015). Identification and characterization of essential genes in the human genome. Science 350. 10.1126/science.aac7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Guo R, Trudeau SJ, Wolinsky E, Ast T, Liang JH, Jiang C, Ma Y, Teng M, Mootha VK, and Gewurz BE (2021). CYB561A3 is the key lysosomal iron reductase required for Burkitt B-cell growth and survival. Blood 138. 10.1182/blood.2021011079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RA, Yen FS, Nicholson SPV, Alwaseem H, Bayraktar EC, Alam M, Timson RC, La K, Abu-Remaileh M, Molina H, and Birsoy K (2020). Maintaining Iron Homeostasis Is the Key Role of Lysosomal Acidity for Cell Proliferation. Mol Cell. 10.1016/j.molcel.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, Yuan X, Schmidt PJ, Bresciani E, Samuel TK, Campagna D, Hall C, Bishop K, Calicchio ML, Lapierre A, et al. (2013). HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell metabolism 17. 10.1016/j.cmet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager JY, and Hartfield DS (2002). Neurologic manifestations of iron deficiency in childhood. Pediatric neurology 27. 10.1016/s0887-8994(02)00417-4. [DOI] [PubMed] [Google Scholar]
- Zhu XG, Nicholson Puthenveedu S, Shen Y, La K, Ozlu C, Wang T, Klompstra D, Gultekin Y, Chi J, Fidelin J, et al. (2019). CHP1 Regulates Compartmentalized Glycerolipid Synthesis by Activating GPAT4. Mol Cell 74, 45–58.e47. 10.1016/j.molcel.2019.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Human metabolism-focused CRISPRa sgRNA library, related to Figure 1
Table S2. Mouse metabolism-focused CRISPRa sgRNA library, related to Figure 2
Table S3. Differential gene expression analysis (Slco2b1−/− vs. WT mouse microglia), related to Figure 6
Table S4. Antimycin A CRISPRa screen results in PaTu-8988t cells, related to STAR Methods
Table S5. CB-839 CRISPRa screen results in PaTu-8988t cells, related to STAR Methods
Table S6. BSO CRISPRa screen results in PaTu-8988t cells, related to STAR Methods
Table S7. Palmitate CRISPRa screen results in Jurkat cells, related to STAR Methods
Table S8. Differential Gene Scores in Palmitate CRISPRa screen performed in Jurkat cells, related to STAR Methods
Table S9. Gene Scores from Jurkat CRISPRa screens for DFO, related to STAR Methods
Table S10. Differential Gene Scores in DFO CRISPRa screen performed in Jurkat cells, related to STAR Methods
Table S11. Gene Scores from Jurkat CRISPRa screens for BafA1, related to STAR Methods
Table S12. Differential Gene Scores in BafA1 CRISPRa screen performed in Jurkat cells, related to STAR Methods
Table S13. Gene Scores from CRISPRa screens for BafA1 in mouse AK196 pancreas cells, related to STAR Methods
Table S14. Gene Scores from CRISPRa screens for ConA in mouse AK196 pancreas cells, related to STAR Methods
Table S15. Gene Scores from CRISPR knockout screens for BafA1 in SLCO2B1-expressing Jurkat cells, related to STAR Methods
Table S16. Differential Gene Scores from CRISPR knockout screens for BafA1 in SLCO2B1-expressing Jurkat cells, related to STAR Methods
Table S17. Gene Scores from CRISPR knockout screens for BafA1 in parental Jurkat cells, related to STAR Methods
Table S18. Gene Scores from CRISPR knockout screens for differential essentiality of genes between control vector and SLCO2B1-expressing Jurkat cells, related to STAR Methods
Table S19. DNA nucleotides and primers, related to STAR Methods
Data Availability Statement
Original western blot images have been deposited on Mendeley at https://doi.org/10.17632/sg3b3f25p4.1 and are publicly available as of the date of publication. The DOI is also listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| SLC25A37 | Invitrogen | Cat#PA5-26720; RRID:AB_2544220 |
| SLCO2B1 | Novus Biologicals | Cat#NBP1-80979; RRID:AB_11038565 |
| Fth1 | Cell Signaling Technology | Cat#3998S; RRID:AB_1903974 |
| IRP2 | Cell Signaling Technology | Cat#37135S; RRID:AB_2799110 |
| β-Actin | GeneTex | Cat#GTX109639; RRID:AB_1949572 |
| GAPDH | GeneTex | Cat#GTX627408; RRID:AB_11174761 |
| HMOX2 | Proteintech | Cat#14817-1-AP; RRID:AB_2118843 |
| UROD | Proteintech | Cat#15547-1-AP; RRID:AB_2213813 |
| ALAS1 | Proteintech | Cat#16200-1-AP; RRID:AB_2878227 |
| CRISPR-Cas9 | Novus Biologicals | Cat#NBP2-36440; Clone 7A9-3A3 |
| TFRC (CD71) | Cell Signaling Technology | Cat#13113S; RRID:AB_2715594 |
| GFP (for immunofluorescence) | Aves Labs | Cat#GFP-1010; RRID:AB_2307313 |
| GFP (for immunoblotting) | Proteintech | Cat#50430-2-AP; RRID:AB_11042881 |
| Goat anti-Chicken IgY (H+L), Alexa Fluor™ 488 | Thermo Fisher Scientific | Cat#A11039; RRID:AB_2534096 |
| Goat anti-Rabbit IgG (H+L), Alexa Fluor™ 555 | Thermo Fisher Scientific | Cat#A21428; RRID:AB_2535849 |
| PE/Cy7 anti-mouse CX3CR1 [Clone: SA011F11] | Biolegend | Cat#149015; RRID:AB_2565699 |
| Anti-CD11b Rat Monoclonal Antibody (Brilliant Violet® 711) [clone: M1/70 | Biolegend | Cat#101241; RRID:AB_11218791 |
| Anti-Ly-6C Rat Monoclonal Antibody (Brilliant Violet® 510) [clone: HK1.4] | Biolegend | Cat#128033; RRID:AB_2562351 |
| Bacterial and Virus Strains | ||
| NEB Stable Competent E. coli | New England Biolabs | C3040 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| RPMI 1640 Culture Media | GIBCO | 11875 |
| DMEM Culture Media | GIBCO | 11965 |
| Trypsin | GIBCO | 25200 |
| Fetal Bovine Serum | Sigma | 12306C |
| Bafilomycin A1 | Cayman Chemical | 11038 |
| Concanamycin A | Sigma-Aldrich | C9705 |
| Deferoxamine Mesylate | Sigma | D9533 |
| Deferiprone | Selleck Chemicals | S4067 |
| Antimycin A | Sigma-Aldrich | A8674 |
| CB-839 | Selleck Chemicals | S7655 |
| Palmitic Acid | Cayman Chemical | 10006627 |
| L-Buthionine-sulfoximine | Sigma | B2515 |
| DMSO | AlfaAesar | A13280-36 |
| HPLC Grade Water | Fisher Scientific | W6 |
| HPLC Grade Methanol | Fisher Scientific | A454-1 |
| Heavy labeled amino acid internal standards | Cambridge Isotopes | MSK-A2-1.2 |
| Ammonium Iron (III) Citrate (FAC) | Sigma | F5879 |
| Ammonium Chloride | Sigma | A9434 |
| Zn(II) Mesoporphyrin IX | Frontier Scientific | M40628 |
| Hemin | Sigma-Aldrich | H9039 |
| Succinylacetone | Cayman Chemical | 25501 |
| Ethanol | Fisher Scientific | 04-355-222 |
| BsmBI | New England Biolabs | R0580 |
| T4 DNA ligase | New England Biolabs | M0202 |
| ExTaq DNA Polymerase | Takara | RR001A |
| Hank's Buffered Salt Solution | GIBCO | 24020117 |
| XtremeGene9 | Roche | 6365779001 |
| Polybrene | Sigma | H9268 |
| Puromycin | Sigma | P8833 |
| Blasticidin | Invivogen | ant-bl-1 |
| Critical Commercial Assays | ||
| CellTiter-Glo® Luminescent Cell Viability Assay | Promega | G7571 |
| Deposited Data | ||
| Original western blot images | This work | https://doi.org/10.17632/sg3b3f25p4.1 |
| Experimental Models: Cell Lines | ||
| Jurkat | ATCC | TIB-152 |
| HepG2 | ATCC | HB-8065 |
| MDA-MB-231 | ATCC | CRM-HTB-26 |
| HEK293T | ATCC | CRL-1573 |
| PaTu-8988t | Dr. Monther Abu-Remaileh (Stanford University) | N/A |
| AK196 | Dr. Haoqiang Yang (MD Anderson Cancer Center) | N/A |
| HY15549 | Dr. Nabeel El-Bardeesy (Massachusetts General Hospital Cancer Center) | N/A |
| 4T1 | Dr. Sohail Tavazoie (Rockefeller University) | N/A |
| 4T07 | Dr. Sohail Tavazoie (Rockefeller University) | N/A |
| Experimental Models: Organisms/Strains | ||
| C57BL/6N-Slco2b1tm1a(KOMP)Wtsi/Mmucd | Mutant Mouse Resource and Research Center (MMRRC) | 049765-UCD |
| C57BL/6J | The Jackson Laboratory | 000664 |
| Oligonucleotides | ||
| DNA oligonucleotides and primers | This study | See Table S19 |
| Recombinant DNA | ||
| pLV-EF1a-IRES-Blast | Addgene | 85133 |
| plentiCRISPR puro v2 | Addgene | 52961 |
| lentiGuide Puro | Addgene | 52963 |
| CRISPRa dCas9-VPR vector with hEF1a promoter | Horizon Discovery | CAS11916 |
| pLV-EF1a-IRES-Blast_SLC25A37 | This study | N/A |
| pLV-EF1a-IRES-Blast_SLCO2B1 | This study | N/A |
| pLV-EF1a-IRES-Blast_SLCO2B1 (short isoform) | This study | N/A |
| pLV-EF1a-IRES-Blast_SLCO2B1-eGFP | This study | N/A |
| pLV-EF1a-IRES-Blast_SLCO2A1 | This study | N/A |
| pLV-EF1a-IRES-Blast_SLCO2A1-eGFP | This study | N/A |
| pLV-EF1a-IRES-Blast_Slco2b1 (mouse cDNA) | This study | N/A |
| plentiCRISPR puro v2_sgTFRC_1 | This study | N/A |
| plentiCRISPR puro v2_sgUROD_1 | This study | N/A |
| plentiCRISPR puro v2_sgUROD_2 | This study | N/A |
| plentiCRISPR puro v2_sgHMOX2_1 | This study | N/A |
| Human metabolism-focused CRISPRa sgRNA library | This study | N/A |
| Mouse metabolism-focused CRISPRa sgRNA library | This study | N/A |
| Metabolism-focused sgRNA library | Birsoy et al., 2015 | N/A |
| Software and Algorithms | ||
| XCaliburQual Browser 2.2 | ThermoFisher | https://www.thermofisher.com/order/catalog/product/OPTON-30965#/OPTON-30965; RRID: SCR_014593 |
| Skyline Daily | MacCoss Lab | https://skyline.ms/project/home/software/Skyline/begin.view; RRID: SCR_014080 |
| FCS Express 7 | DeNovo Software | https://denovosoftware.com/; RRID: SCR_016431 |
| Prism 9 | GraphPad | https://www.graphpad.com/scientific-software/prism/; RRID: SCR_002798 |
| Other | ||
| Z2 Coulter Counter | Beckman | Model Z2 |
| SpectraMax Microplate Reader | Molecular Devices | Model M5 |
| Primovert Microscpe | Carl Zeiss | 415510-1105-000 |
| FACSAriaII | BD Biosciences | 643181 |
| Multiphoton microscope with confocal modality | Nikon | A1R MP |