Abstract
Manganese (Mn), although important for multiple cellular processes, has posed environmental health concerns due to its neurotoxic effects. In recent years, there have been extensive studies on the mechanism of Mn-induced neuropathology, as well as the sex-dependent vulnerability to its neurotoxic effects. Nonetheless, cellular mechanisms influenced by sex differences in susceptibility to Mn have yet to be adequately characterized. Since oxidative stress is a key mechanism of Mn neurotoxicity, here, we have probed Hsp70 and Nrf2 proteins to investigate the sex-dependent changes following exposure to Mn. Male and female rats were administered intraperitoneal injections of MnCl2 (10mg/kg and 25mg/kg) 48 hourly for a total of 8 injections (15 days). We evaluated changes in body weight, as well as Mn accumulation, Nrf2 and Hsp70 expression across four brain regions; striatum, cortex, hippocampus and cerebellum in both sexes. Our results showed sex-specific changes in body-weight, specifically in males but not in females. Additionally, we noted sex-dependent accumulation of Mn in the brain, as well as in expression levels of Nrf2 and Hsp70 proteins. These findings revealed sex-dependent susceptibility to Mn-induced neurotoxicity corresponding to differential Mn accumulation, and expression of Hsp70 and Nrf2 across several brain regions.
Keywords: Manganese, brain, oxidative stress, male, female
Introduction
Manganese (Mn) is an essential trace metal for many physiological processes in the human body. It acts as a cofactor for a variety of enzymes, and it mediates/regulates synthesis, bone metabolism, brain function amongst others. However, in excessive quantity, Mn can accumulate in the brain leading to its dysfunction and a parkinsonian-like syndrome, called manganism (Miah et al. 2020). Exposure to Mn mainly occurs through the air (Kornblith et al. 2018), food, and drinking water (Luo et al. 2020). Following exposure, Mn accumulates in different brain regions, mainly the basal ganglia (which includes the striatum), hippocampus, and cerebral cortex (Akingbade et al. 2021; Cheng et al. 2018; Cordova et al. 2012; Lao et al. 2017; Ou et al. 2017). Previous studies found that chronic exposure to Mn led to adverse dose-dependent effects on neurobehavioral function in rats, with overt morphological changes and neurodegeneration in different brain regions including the hippocampus, striatum, cortex, and cerebellum (Akingbade et al. 2021; Caito and Aschner 2015; Ma et al. 2020; Sidoryk-Wegrzynowicz and Aschner 2013; Stanwood et al. 2009).
Oxidative stress plays an important role in Mn-induced toxicity in neurons (Akingbade et al. 2021; Peres et al. 2016) and glia cells (Gorojod et al. 2017; Ke et al. 2019). Oxidative stress and disrupted redox status are key events in Mn neurotoxicity and likely associated with Mn-induced mitochondrial dysfunction (Akingbade et al. 2021; Miah et al. 2020). Heat shock proteins (HSPs) including Hsp70 are molecular chaperones that regulate protein synthesis as well as preserving structural integrity of existing proteins. In pathological conditions, they exhibit several functions, including structural renovation of denatured proteins as well as attenuation of protein aggregation (Powers et al. 2010; Sõti et al. 2005). Oxidative stress caused by metal exposure can further result in protein aggregation. In addition, metals are able to trigger perturbed protein interactions. These protein dysfunction can consequently lead to HSPs upregulation (Avila et al. 2016). Particularly, Mn overexposure has been reported to increase Hsp70 levels in non-nervous as well as brain tissues in experimental models (Cheng et al. 2018; Moyano et al. 2020; Zhu et al. 2016; Zhu et al. 2013). Similarly, Nrf2, a key regulator of the endogenous antioxidant response to oxidative stress can be modulated by metal exposures. Nrf2 triggers the transcription of endogenous antioxidant enzymes such as glutathione, catalase, superoxide dismutase amongst others, through its interaction with the antioxidant responsive element (ARE) (Dong et al. 2017; Jeong et al. 2006; Wang et al. 2019). Several studies have shown that Mn-induced oxidative stress can significantly upregulate the expression of Nrf2 proteins in cells and that Nrf2 pathway modulate Mn-induced neuronal damage (Li et al. 2011a; Li et al. 2011b; Moyano et al. 2020).
Sex-dependent disparities in susceptibility to heavy metals, such as Mn, have received some attention in several studies. A cohort study in Denmark associated Mn-exposed female children with attention deficit hyperactivity disorder (ADHD) to a greater extent than males (Schullehner et al. 2020). Elsewhere, Broberg et al. (2019) reported sex-dependent sensitivity to Mn exposure on neurobehavioral outcomes in children. Further, Oulhote et al. (2014a) reported that females have significantly higher blood Mn levels than males. Evidence from other studies indicate that these differences might be a result of slight variation in genetics (Broberg et al. 2019) and metabolism across sexes (Bouchard et al. 2018; Dion et al. 2018). However, few reports exist on sex-dependent responses to Mn neurotoxicity. Furthermore, while the role of Nrf2 and Hsp70 in Mn neurotoxicity have been reported, there appears to be lack of attention on the influence of sex on Hsp70 and Nrf2 expression and function in Mn neurotoxicity. Hence, here we evaluated sex-specific changes to brain Hsp70 and Nrf2 expression, as well as variations in Mn accumulation across specific brain regions in response to Mn exposure.
Methods
Animals
Ten-week-old Sprague Dawley rats with average weight of 343±3.77 g for males and 211±3.14 g for females obtained from Charles Rivers Laboratories, USA, were used for the study. All animal experimental protocols were performed in strict accordance to the guidelines of the National Institute of Health for the care and use of laboratory animals and approved by the IACUC at the Albert Einstein College of Medicine (#20171008). Animals were kept under 12-hour light-dark cycle with free access to regular food and water.
Male and female rats (n = 6/sex) were randomly assigned to receive intraperitoneal (i.p.) injections of either saline vehicle (as controls), or 10 mg/kg or 25 mg/kg MnCl2 (MnCl2 was dissolved in saline), every 48 hours for 15 days, for a total of 8 injections. Doses and route of administration are based on our prior report (Morcillo et al. 2021). These doses are relevant to human exposure scenarios as Mn toxicity has been shown in individuals who have ingested water containing high levels of Mn at dose ≥10 mg (Keen et al. 2013). Additionally, this dosing paradigm of Mn injections has been shown to permit accumulation of detectable Mn levels in the brain whilst reducing its effects on other organs (Santos et al. 2012a; Santos et al. 2012b). Furthermore, administration of MnCl2 by i.p. produces steady-state blood concentrations of about 1000 ng Mn/100 ml and is similar to levels obtained via oral gavage and intratracheal routes (Roels et al. 1997). Body weight were monitored twice weekly. At day 16, rats were euthanized via isoflurane inhalation, brains were rapidly excised and the striatum, cortex, hippocampus and cerebellum were isolated for subsequent analysis.
Mn bioavailability by inductively coupled plasma – mass spectrometry
Quantification of Mn concentration in the striatum, cortex, hippocampus and cerebellum was determined via inductively coupled plasma – mass spectrometry (ICP-MS). Brain tissues were processed via a MARS 6 microwave digestion system (CEM GmBH, Germany), using a 15 min ramp to 200 °C and kept for 20 min at 200 °C. Samples were then diluted to final concentrations of 3.25% HNO3 and 1 μg/L Rh with ultrapure H2O. ICP-MS was performed on the Agilent 8800 ICP-QQQ (Agilent Technologies Deutschland GmbH & Co. KG, Germany). The following parameters were used for ICP-MS analysis: 1550 W plasma Rf power, Ni-cones, MicroMist nebulizer at 1.08 L Ar/min, and Scott-type spray chamber. The following ratios of mass-to-charge and gas modes were used: (Q1 ➔ Q2): He-mode, Mn (55 ➔ 55) and Rh (103 ➔ 103) (internal standard). Results were validated via the certified reference material ERM-BB 422 (fish muscle). Alongside Mn concentration, we also measured levels of other essential metals including iron (Fe), copper (Cu), and zinc (Zn).
Western blotting
Isolated brain regions were homogenized in cold lysis buffer of 2 ml volume per 500 mg tissue. Lysis buffer contains 970 μl RIPA buffer (Sigma Aldrich, USA; #R0278), supplemented with 10 μl protease inhibitor cocktail (ThermoFischer Scientific, USA; #1861278), 10 μl each of phosphatase inhibitor cocktails 2 (Sigma Aldrich, USA; P5726) and 3 (Sigma Aldrich, USA; #P0044). Homogenized tissues were centrifuged at 10000 rpm for 10 min at 4 °C. The supernatant was collected and protein content was quantified using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific, USA; #23227). Lysates were heated at 100 oC for 5 minutes in 1:1 of Laemmli buffer solution containing 950 μl 2X Laemmli sample buffer (Bio-Rad, USA; #1610737) + 50 μl β-mercaptothanol (Bio-Rad, USA; #1610710). After heating, samples were centrifuged for 1 min, and cooled on ice for 5 min. Then, 10 μg of protein were resolved on 4–20 % sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE, Bio-Rad, USA; #4561096) and transferred to a nitrocellulose membrane. Blocking was performed in Tris buffer saline-Tween-20 (TBS-T) buffer containing 5 % bovine serum albumin (BSA, Sigma Aldrich, USA; #A3059) for 1 h at room temperature. The blots were incubated in primary antibodies diluted in 5% BSA in TBS-T buffer overnight at 4 °C. Primary antibodies used were rabbit anti-Hsp70 (Cell Signaling Technology, USA; #4872), rabbit anti-nrf2 (Cell Signaling Technology, USA; #12721) and mouse anti-β-actin (Sigma Aldrich, USA; A1978). Next, membranes were incubated in appropriate HRP-conjugated secondary antibody, and visualized by chemiluminescent method using SuperSignal™ West Pico Plus Kit (ThermoFischer Scientific, USA; #34579). The densitometric values were quantified using ImageJ software (https://imagej.nih.gov/ij/), and normalized using β-actin as loading control.
Statistics
Data were analyzed on GraphPad Prism 8 software (GraphPad Inc, USA) by two-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc tests. Values of p < 0.05 were considered statistically significant.
Results
MnCl2 treatment caused a dose-dependent weight loss in male, but not in female rats
Two-way ANOVA revealed significant interaction [p < 0.001; F(2,30) = 16.74] between sex and treatment on body-weight. Similarly, there was significant sex effect [p < 0.001; F(1,30) =36.78], and treatment effect [p < 0.001; F(2,30) = 15.49]. Further, post-hoc analysis revealed significantly increased body weight-only in male rats following 10 mg/kg (p < 0.05) and 25 mg/kg (p < 0.001) MnCl2 treatment compared with control. In addition, body-weight was significantly higher (p < 0.001) in male than in female rats for control and the 10 mg/kg MnCl2 groups (Fig. 1).
Fig. 1.
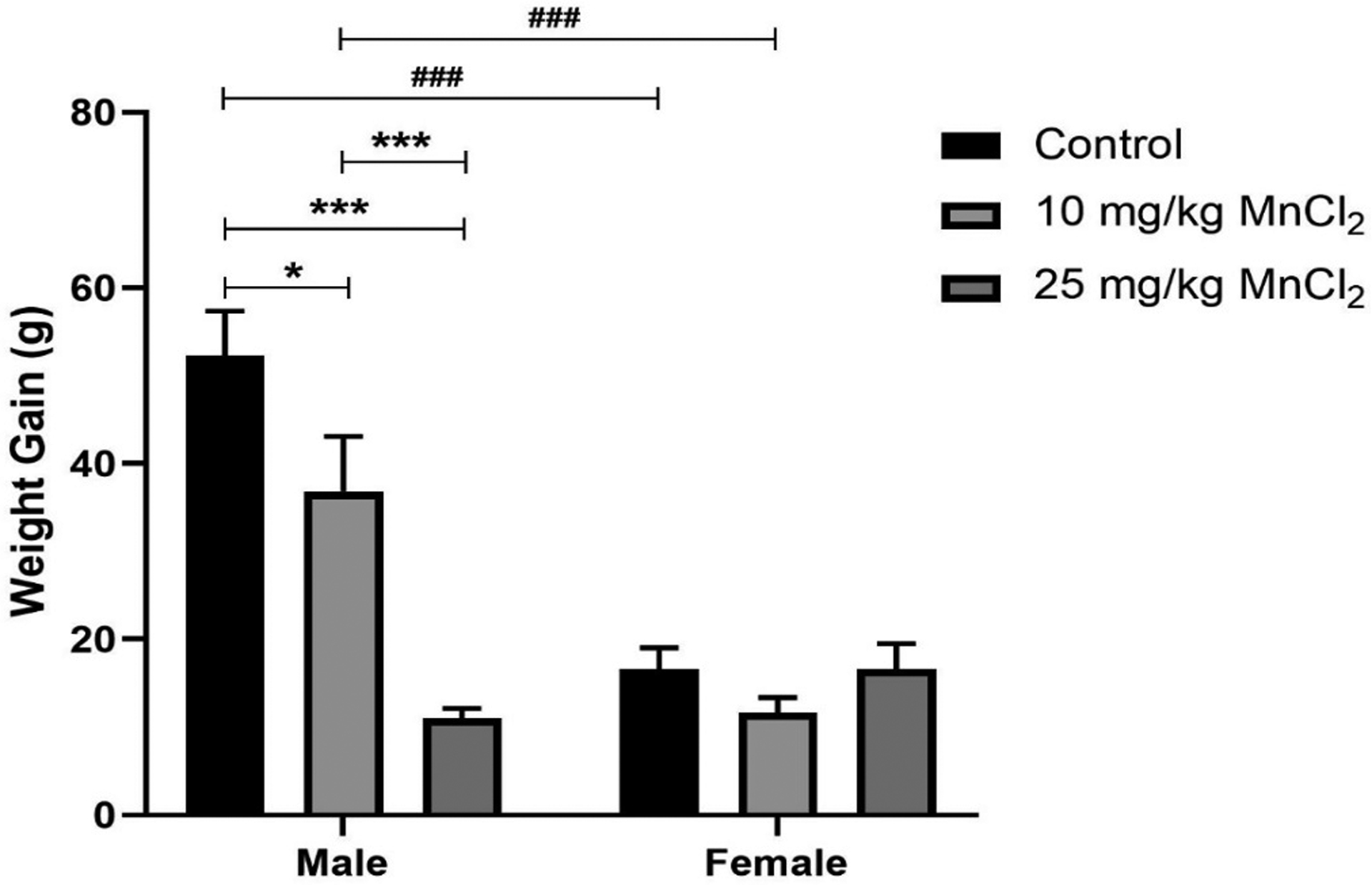
Effect of MnCl2 treatment on the body weight gain of male and female rats. There was a dose-dependent decrease in the weight gain of male rats but not in female rats. Data were analyzed by two-way ANOVA followed by Bonferroni’s post-test. *p < 0.05, **p < 0.01, ***p < 0.001. ###p < 0.001 between male and female of same treatment.
Mn quantification in the brain of Mn treated male and female rats
MnCl2 treatment increased Mn levels in the striatum of male and female rats:
Two-way ANOVA analysis revealed no significant interaction [p = 0.5100; F(2, 30) = 0.69] between sex and Mn treatment in the striatum. However, analysis revealed significant sex [p < 0.01; F(1, 30) = 11.30] and treatment [p < 0.001; F(2, 30) = 73.59] effect. Bonferroni’s post hoc test revealed significantly increased Mn levels in the striatum of both male and female rats after exposure to 10 mg/kg (male: p < 0.001; female: p < 0.001) and 25 mg/kg (male/female: p < 0.001) of MnCl2, compared with the respective controls. Similarly, Mn levels in the striatum after 25 mg/kg MnCl2 was significantly higher compared to 10 mg/kg treated rats in both male and female rats. Additionally, at 10 mg/kg, Mn levels in the striatum were significantly higher (p < 0.05) in male compared to female rats at an analogous dose. For levels of the other metals measured in the striatum, Fe levels showed no significant changes on interaction nor treatment effects but showed significant sex effect [p < 0.001; F(1, 30) = 18.52]. Here, Bonferroni’s test confirmed significant (p < 0.05) Fe increase in males compared to females at 25 mg/kg Mn treatment. No significant effects were seen for Cu levels while Zn showed significant interaction [p < 0.05; F(1, 30) = 0.52] but not significant sex nor treatment effects (Fig. 2).
Fig. 2.
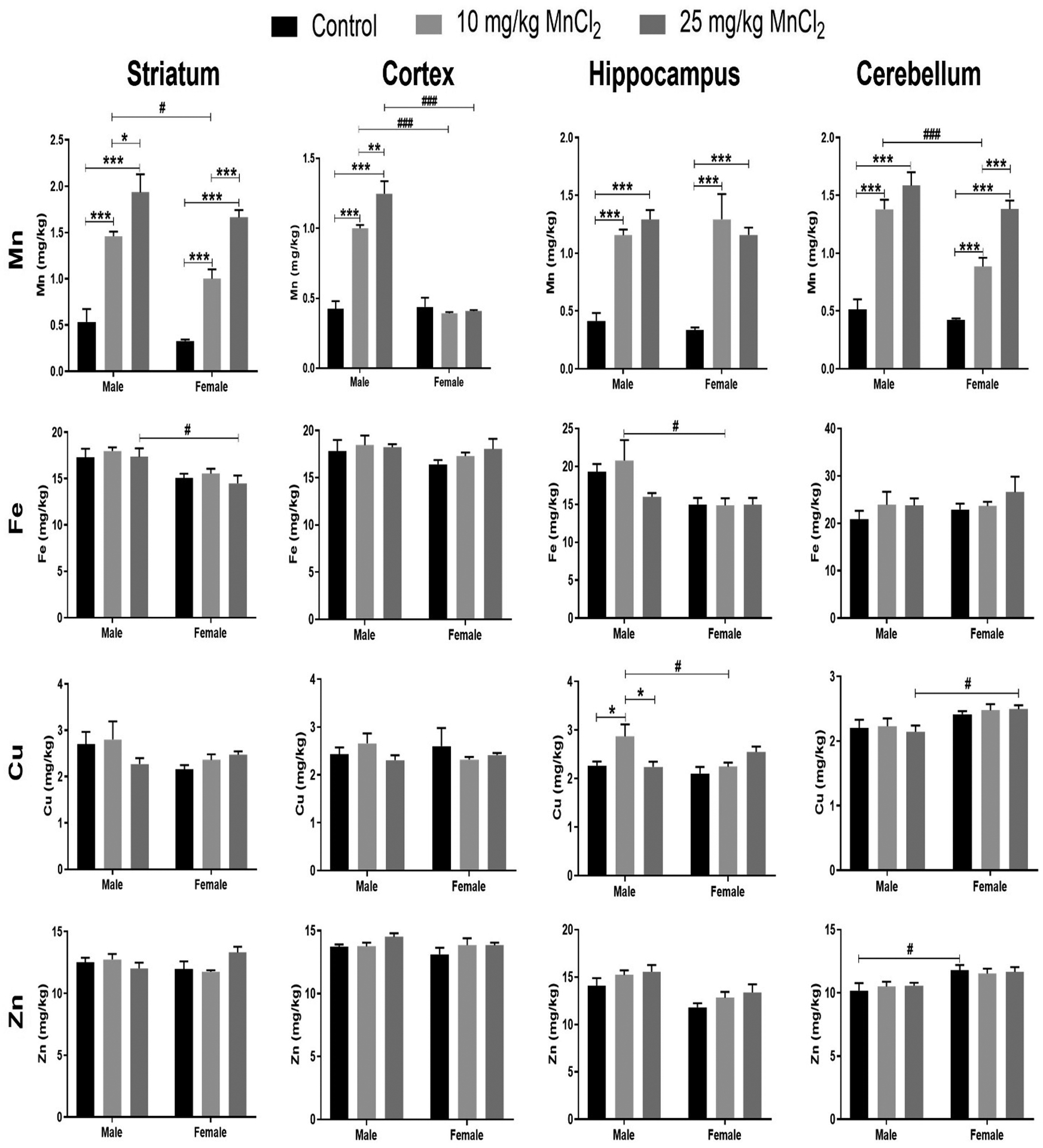
Effect of MnCl2 treatment on levels of Mn and other metals in the striatum, cortex, hippocampus and cerebellum of male and female rats. Data were analyzed by two-way ANOVA followed by Bonferroni’s post-test. *p < 0.05, **p < 0.01, ***p < 0.001. #p < 0.05, ###p < 0.001 between male and female of same treatment.
MnCl2 treatment increased Mn levels in the cortex of male rats but not in female rats:
Two-way ANOVA revealed significant interaction [p < 0.001; F(2, 30) = 36.37] between sex and treatment on cortical Mn levels. Similarly, there was significant sex effect [p < 0.001; F(1, 30) = 127.56], and treatment effect [p < 0.001; F(2, 30) =30.43]. Multiple comparison with Bonferroni’s test showed significantly increased (p < 0.001) Mn levels in the cortex of male rats following 10 mg/kg and 25 mg/kg MnCl2 treatment compared with respective controls. No significant differences were observed in female rats. Consequently, MnCl2 treatment at 10 mg/kg and 25 mg/kg caused significantly increased (p < 0.001) Mn levels in the cortex of males compared to females. For levels of the other metals measured in the cortex, Fe, Cu, and Zn, no significant interaction, sex nor treatment effect was seen (Fig. 2).
MnCl2 treatment increased Mn levels in the hippocampus of male and female rats:
Two-way ANOVA analysis showed no significant interaction [p = 0.4071; F(2, 30) = 0.93] between sex and treatment on Mn accumulation in the hippocampus. Additionally, there was no significant sex effect [p = 0.7702; F(1, 30) = 0.09] on hippocampal Mn accumulation, however, a significant treatment effect [p < 0.001; F(2, 30) = 43.79] was observed. Post hoc analysis revealed significantly increased (p < 0.001) Mn levels in both male and female rats following 10 mg/kg and 25 mg/kg MnCl2 treatment compared with respective controls. For levels of the other metals measured in the hippocampus, Fe levels showed no significant changes on interaction, nor treatment effects but showed significant sex effect [p < 0.01; F(1, 30) = 11.45]. Here, Bonferroni’s test confirmed significant Fe increase in males compared to females at 10 mg/kg Mn treatment. Cu levels showed significant interaction [p < 0.01; F(2, 30) = 5.58] and treatment effect [p < 0.05; F(2, 30) = 3.59] but no significant sex effects. Bonferroni’s test showed significant (p < 0.05) Cu increase at 10 mg/kg compared to control and 25 mg/kg treatment in males, and also significant increase (p < 0.05) in Cu levels in males compared to females at 10 mg/kg. For Zn levels, no significant interaction nor treatment effect is seen however significant sex effect [p < 0.001; F(1, 30) = 17.25] is observed. However, further Bonferroni’s test did not confirm significant sex effect for any treatment groups (Fig. 2).
MnCl2 treatment increased Mn levels in the cerebellum of male and female rats:
Two-way ANOVA analysis showed significant interaction [p < 0.05; F(2, 30) = 3.44] between sex and Mn treatment in the cerebellum. In addition, ANOVA analysis shows significant sex [p < 0.001; F(1, 30) = 16.04], and treatment [p < 0.001; F(2, 30) = 82.85] effect. Multiple comparison using Bonferroni’s test revealed significantly increased Mn levels after exposure to 10 mg/kg (male: p < 0.001; female: p < 0.01) and 25 mg/kg (male/female: p<0.001) MnCl2, compared with respective controls. Furthermore, female rats treated with 25 mg/kg MnCl2 showed significantly higher (p < 0.001) Mn levels in the cerebellum compared to 10 mg/kg treatment. Additionally, at 10 mg/kg, Mn levels significantly increased (p < 0.001) in the cerebellum of male compared to female rats.
For levels of the other metals measured in the cerebellum, Fe levels showed no significant interaction, sex nor treatment effects. Cu levels showed no significant changes on interaction nor treatment effects but showed significant sex effect [p < 0.01; F(1, 30) = 12.00]. Here, Bonferroni’s test confirmed significant (p < 0.05) Cu increase in females compared to males at 25 mg/kg Mn treatment. Similarly, Zn showed only significant sex effect [p < 0.001; F(1, 30) = 13.52]. Bonferroni’s test confirmed significant (p < 0.05) Cu increase in females compared to males in the control groups (Fig. 2).
Hsp70 expression in the brain of MnCl2 treated male and female rats
Striatal Hsp70 expression was increased in male and female rats after MnCl2 treatment:
Two-way ANOVA revealed no significant interaction [p = 0.8137; F(2, 30) = 0.21] between sex and treatment on striatal Hsp70 expression. In contrast, there was a significant sex [p < 0.05; F(1, 30) = 5.09], and treatment [p < 0.001; F(2, 30) = 11.13] effect. Post hoc analysis with Bonferroni’s test revealed significantly increased Hsp70 expression in males following MnCl2 treatment at 10 mg/kg (p < 0.05) and 25 mg/kg (p < 0.001) compared to controls. However, in females, Hsp70 expression was significantly increased (p < 0.05) only at 25 mg/kg (Fig. 3).
Figure 3:
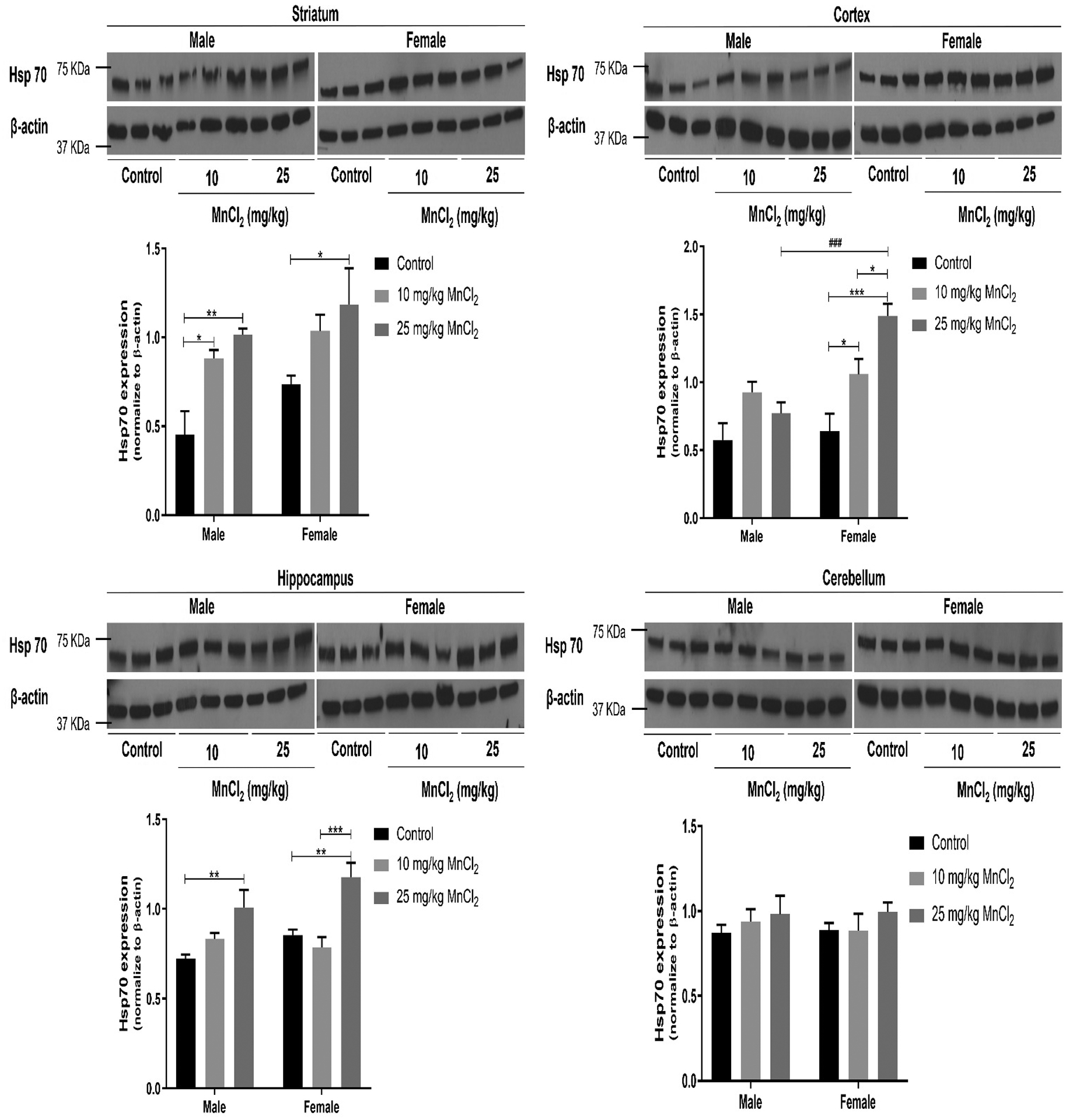
Effect of MnCl2 treatment on Hsp70 expression in the striatum, cortex, hippocampus and cerebellum of male and female rats. Data were analyzed by two-way ANOVA followed by Bonferroni’s post-test. *p < 0.05, **p < 0.01, ***p < 0.001. ###p < 0.001 between male and female of same treatment.
MnCl2 treatment increased cortical Hsp70 expression in female rats but not males:
Two-way ANOVA revealed significant interaction [p < 0.01; F(2, 30) = 5.95] between sex and treatment on cortical Hsp70 expression. Similarly, there was significant sex effect [p < 0.01; F(1, 30) = 12.95], and treatment effect [p<0.001; F(2, 30) = 13.56]. Bonferroni’s post hoc analysis showed significantly increased Hsp70 expression only in cortex of female rats following 10 mg/kg (p < 0.05) and 25 mg/kg (p < 0.001) MnCl2 treatment compared to respective control. In addition, at 25 mg/kg MnCl2 treatment, cortical Hsp70 expression was significantly higher (p < 0.001) in females compared to males (Fig. 3).
MnCl2 treatment increased hippocampal Hsp70 expression in male and female rats:
Two-way ANOVA showed no significant interaction [p = 0.1763; F(2, 30) = 1.84] between sex and treatment. While there was significant treatment effect [p < 0.001; F(2, 30) = 15.82], there was no significant sex effect [p = 0.0971; F (1, 30) = 2.93]. Post hoc analysis showed significantly increased (p < 0.01) hippocampal Hsp70 expression in both males and females following 25 mg/kg MnCl2 treatment. Additionally, in female rats only, 25 mg/kg MnCl2 treatment showed significantly higher (p < 0.001) hippocampal Hsp70 expression compared to 10 mg/kg treatment (Fig. 3).
MnCl2 treatment did not alter cerebellar Hsp70 expression in male and female rats:
Two-way ANOVA of cerebellar Hsp70 expression showed no significant interaction [p = 0.87; F(2, 29) = 0.14] between sex and treatment. Similarly, there was no significant sex effect [p = 0.8889; F(1, 29) = 0.02] and treatment effect [p = 0.3238; F(2, 29) = 1.17]. Bonferroni’s post hoc analysis revealed no significant differences in Hsp70 expression across sex and treatments groups (Fig. 3).
Nrf2 expression in the brain of MnCl2 treated male and female rats
MnCl2 treatment increased striatal Nrf2 expression in male and female rats:
Two-way ANOVA of striatal Nrf2 expression showed no significant interaction [p = 0.8285; F(2, 27) = 0.19] between sex and treatment. In addition, no significant sex effect [p = 0.0798; F(1, 27) = 3.31] was observed, however, there was a significant treatment effect (p < 0.001; F(2, 27) = 13.05). Bonferroni’s post hoc analysis in males, revealed significantly increased Nrf2 expression following 10 mg/kg (p < 0.05) and 25 mg/kg MnCl2 (p < 0.001) treatment compared to control. On the other hand, in female rats, only at 25 mg/kg MnCl2 treatment a significant increase (p < 0.05) was ascertained in Nrf2 expression compared to controls (Fig. 4).
Figure 4:
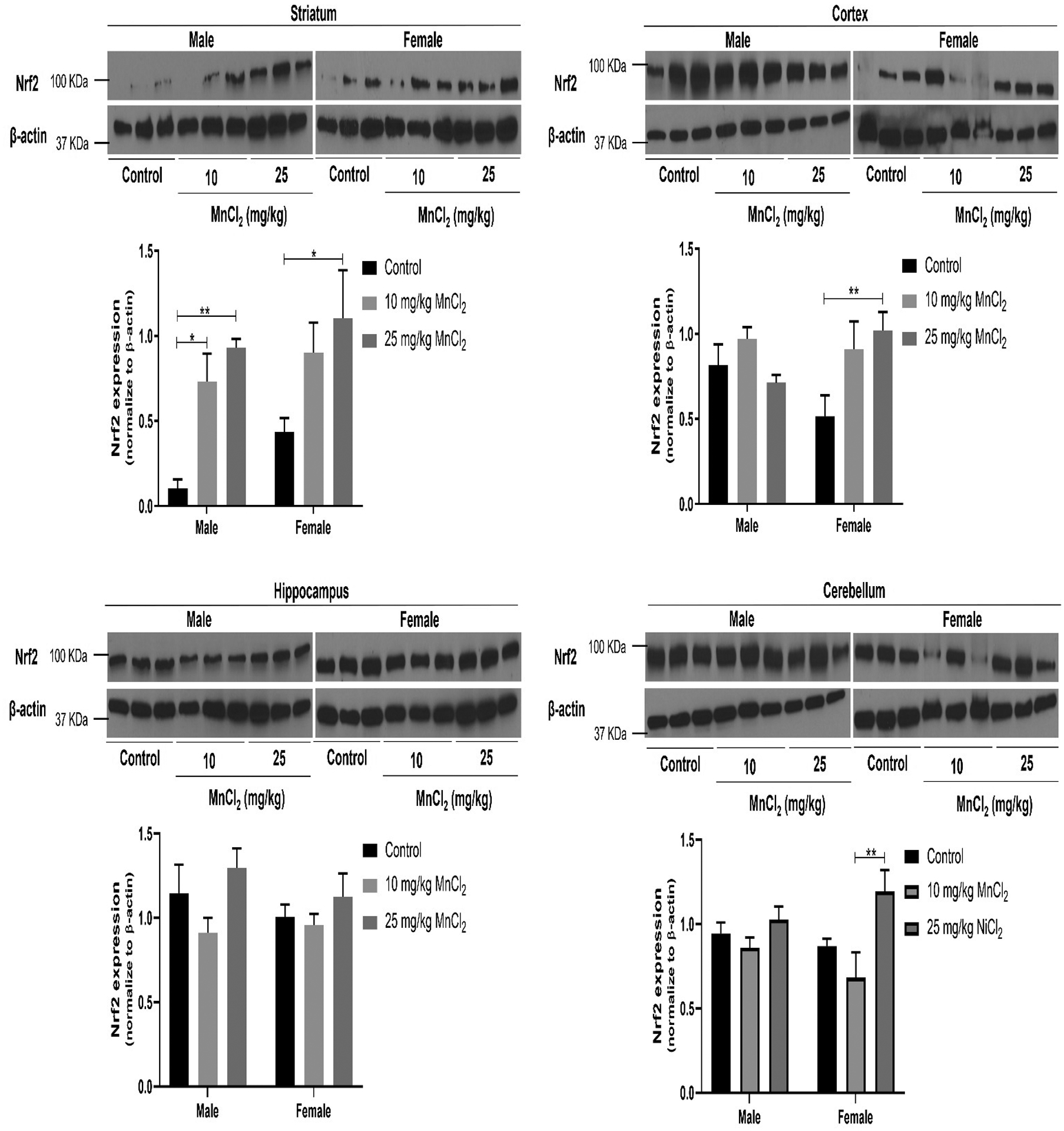
Effect of MnCl2 treatment on Nrf2 expression in the striatum, cortex, hippocampus and cerebellum of male and female rats. Data were analyzed by two-way ANOVA followed by Bonferroni’s post-test. *p < 0.05, **p < 0.01.
MnCl2 treatment increased cortical Nrf2 expression in female rats only:
Two-way ANOVA of cortical Nrf2 expression revealed significant interaction [p < 0.05; F(2, 30) = 3.78] between sex and treatment. In contrast, there was no significant sex [p = 0.8395; F(1, 30) = 0.04], and treatment effect [p = 0.0520; F(2, 30) = 3.27]. Bonferroni’s post hoc analysis revealed significantly higher (p < 0.01) Nrf2 expression in females after 25 mg/kg of MnCl2 compared to respective control.
Hippocampal Nrf2 expression is not significantly changed after MnCl2 treatment in male and female rats:
Two-way ANOVA of hippocampal Nrf2 expression showed no significant interaction [p = 0.5960; F(2, 29) = 0.53] between sex and treatment. Similarly, no significant sex [p = 0.3553; F 1, 29) = 0.88] or treatment [p = 0.0720; F(2, 29) = 2.88] effects were noted. Bonferroni’s post hoc analysis revealed no significant difference in Hsp70 expressions across sex and treatment groups (Fig. 4).
MnCl2 treatment increased cerebellar Nrf2 expression in female rats:
Two-way ANOVA of cerebellar Nrf2 expression revealed no significant interaction [p = 0.1875; F(2, 30) = 1.77] between sex and treatment. Additionally, no significant sex effect [p = 0.7165; F(1, 30) = 0.13], however, a significant treatment effect [p < 0.01; F(2, 30) = 6.61] is observed. Bonferroni’s post hoc analysis revealed no significant difference between the MnCl2 treated groups compared to respective control. However, MnCl2 treatment at 25 mg/kg in female rats led to significantly higher (p < 0.01) Nrf2 expression compared to the group treated with 10mg/kg MnCl2.
Discussion
There is evidence that sex can influence susceptibility to Mn-induced neurotoxicity (Bailey et al. 2019; Dorman et al. 2004; Llop et al. 2013; Vorhees et al. 2014; Zheng et al. 2000). In this study, we demonstrate sex- and structure-specific changes in brain Nrf2 and Hsp70 protein expression levels following Mn exposure.
We observed sex-specific changes in body weight gain following Mn exposure. Mn significantly decreased body weight gain in male rats with increased dosing, but this effect was absent in females. In our results, we see that though males gained weight, Mn treatment significantly reduced the weight gain compared to male control. However, compared to females, it is apparent that since the males already higher body weight (at the same age), hence the weight gain is higher than that of the females. Consistent with our results Kim et al. (2012) previously reported no significant change in body-weight following Mn exposure in female rats, while Schmitz et al. (2019) reported significant decrease weight gain in male rats following Mn exposure, with no significant effect in female rats. In contrast, Zhang et al. (2003) reported significant decrease and increase in the weight of male and female rats, respectively, following Mn exposure. Loss of body-weight has been previously reported in several neuropathologies, both in experimental animal models (Apland et al. 2017; Shih et al. 2019; Wang et al. 2020) and humans (Bachmann and Trenkwalder 2006; Barrett-Connor et al. 1998; Buchman et al. 2005; Djousse et al. 2002). Furthermore, it has been shown that reduced body-weight alters the responsiveness and status of the redox system (García-Sánchez et al. 2020; Milagro et al. 2006). Increased oxidative stress and inflammatory responses, established consequences of Mn neurotoxicity (Miah et al. 2020), have been linked to weight loss by inhibition of appetite and induction of muscle loss. It is thus possible, that these changes could contribute to the weight-loss in males as corroborated in an earlier study (Schmitz et al. 2019). Furthermore, Schmitz et al. (2019), as previously noted that differences between male and female body weights following Mn exposure may be due to association between induction of inflammatory responses and accumulation and distribution of visceral fat in males.
We also examined potential sex-influenced variations in Mn deposition across different brain regions - striatum, cortex, hippocampus, and cerebellum, following Mn exposure. Mn exposure has previously associated with pathologic changes in the several brain regions; the striatum is particularly susceptible to the deleterious effect of Mn (Zhao et al. 2019). Similarly, Mn accumulates in the hippocampus, cerebellum (Hernández et al. 2020; Nkpaa et al. 2022), and cortex (Guilarte 2010; Guilarte et al. 2008). Although it well established that Mn deposition in the brain is region-specific owing to differential expression of important Mn transporters – DMT-1, transferrin, across specific brain regions (Nyarko-Danquah et al. 2020), sex-specific distribution and metabolism of Mn have also been recorded both in humans (Bauer et al. 2017; Wahlberg et al. 2018; Zhou et al. 2020) and experimental models (Madison et al. 2011; Vorhees et al. 2014). Similarly, we observed differences in Mn levels in male vs. female cortex, with elevated Mn levels in males and absent of significant change in females. While brain Mn levels increased in both sexes, we failed to note sex-dependent effects in striatal, hippocampal or cerebellar Mn levels. Our finding on striatal Mn accumulation show the lack-of sex-dependent Mn accumulation corroborates an earlier report by Madison et al. (2011), though their study employed a mouse model. Guilarte et al. (2006) demonstrated increased Mn accumulation in the frontal cortex of male Cynomolgus macaques. Finally, Mn levels increased in the hippocampus and cerebellum of exposed male and female rats after Mn exposure (Yamagata et al. 2017). Additionally, we measured levels of other essential metals included Fe, Cu, and Zn, after Mn exposure. We observed no overt and consistent influence on Mn administration on these metals. However, our results do show that Mn triggers sex-dependent response in levels of Fe in the striatum and hippocampus, with Mn administration triggering Fe increase in males compared to females. Our results have not fully supported Anderson and colleagues who have previously shown that Mn supplementation resulted in lowered Fe levels in brains of both male and female rats (Anderson et al. 2007). It is worth noting that a recent report found no significant changes to Fe, Cu and Zn levels in striatum and cortex after treatment with Mn, albeit in mice (Foster et al. 2017).
Since increased Mn level is associated with oxidative stress (Akingbade et al. 2021; Alarifi et al. 2017; Huang et al. 2021). We further explored sex-dependent oxidative response following Mn accumulation via Hsp70 and Nrf2 modulation. Oxidative stress is a key event in many brain pathologies (Erukainure et al. 2019; Ijomone et al. 2018). Reactive oxygen species (ROS)-mediated oxidative stress following Mn exposure is known to activate different cellular signalling pathways involving the activation of the stress-responsive Hsp70 (Cheng et al. 2018; Liu et al. 2021) and antioxidant modulator, Nrf2 (Bahar et al. 2017; Costa-Silva et al. 2018).
The brain is the main target of Mn exposure, with excess Mn accumulating predominantly in the basal ganglia, particularly in the striatum (caudate nucleus, putamen and nucleus accumbens) (Peres et al. 2016). Hence, the striatum is most prone to Mn-induced neurostructural and neurochemical perturbations. This is supported by our present data, showing consistently dose-dependent Mn accumulation in the striatum in both male and female animals. In addition, we show upregulation of striatal Hsp70 and Nrf2 for both doses used in males, but only in higher dose in females. Since we detected no difference in striatal Mn accumulation between sexes, the inability of Mn to elicit cellular stress via Hsp70 and Nrf2 expression at a lower dose in the female brain suggests that Mn may interact with sex-specific hormones (Oulhote et al. 2014b), and that a higher Mn dose may be required to push striatal cells to an oxidative state and altered redox status in females.
In the cortex, upregulated Hsp70 and Nrf2 were observed in females only. Surprisingly, the increase in cortical Nrf2 and Hsp70 in the female cortex were not accompanied by elevated Mn levels. Conversely, males failed to exhibit cortical Hsp70 and Nrf2 changes despite significant Mn accumulation. The sex discrepancy observed in this study could be linked to the presence or absence of the hormones, such as estrogen and β-estradiol. Thakur and Sharma (2007) reported a higher level of ERα (estrogen receptor alpha) mRNA in the cortex of adult females than male mice. These ERα are activated by β-estradiol, which protected SHSY5Y neuroblastoma cells from cobalt and mercury-induced oxidative stress by increasing the GSH production (Olivieri et al. 2002). Similarly, β-estradiol afford neuroprotection to Mn-induced toxicity in rat cortical primary neuronal and astroglial cultures via the attenuation of oxidative stress and activation of the MAPK/ERK and PI3K/Akt signaling pathway (Lee et al. 2009). Interestingly, administration of β-estradiol in male mice offer neuroprotection in experimental model of PD (Ramirez et al. 2003). The neuroprotective effect of β-estradiol is largely attributed to its interaction with nuclear estrogen receptors. Notably, β-estradiol can modulate gene expression via the regulation of signaling pathways, such as the ERK (Jover-Mengual et al. 2007) and PI3K/Akt (Dominguez et al. 2007). Through the aforementioned mechanism, β-estradiol can protect against Aβ-induced neurotoxicity by increasing the levels of Hsp70 protein (Zhang et al. 2004). Hence, the upregulated Hsp70 and Nrf2 may be a protective feedback response to oxidative stress in the female cortex. A plausible explanation for the lack of changes in Hsp70 and Nrf2 levels in male cortex despite significant Mn accumulation could be that estrogen, through the regulation of antioxidant genes, contributes to important antioxidant defense with lower oxidative damage and higher antioxidant activation in females compared to the male counterpart as shown by Razmara et al. (2007). However, this fails to explain the insignificant change in cortical Mn levels in females and further study is needed to elucidate this observation. Nonetheless, the lack of cellular response in male cortex could suggest an ability to withstand greater levels of Mn deposit compared to female rats.
Hsp70-mediated oxidative response was accompanied by Mn accumulation in the hippocampus of in males and female rats. This observation is consistent with the findings in (Cheng et al. 2018; Moyano et al. 2020). Conversely, Nrf2 levels remained unchanged, suggesting selective activation of the Hsp70 system to combat neuronal oxidative injury in the hippocampus. Further, the similar oxidative responses in both sexes suggest that the neurotoxic impact of Mn in the hippocampus is non-sex-specific. In the cerebellum, male and female rats did not show significant alterations to Hsp70-mediated oxidative response. However, a significant increase in the activities of Nrf2 was observed in female rats, only after exposure to a higher concentration of Mn. Thus, the sex-specific difference in the cerebellum was observed only in the Nrf2 system. As mentioned above, several sex-specific hormones can play significant roles in these observed variations during oxidative response.
In conclusion, our findings support sex-dependent vulnerability to Mn exposure. We quantified Nrf2 and Hsp70 proteins on the antioxidant system in rats, showing sex-dependent susceptibility to Mn-induced neurotoxicity, particularly in the cortex and cerebellum. Nonetheless, it is still essential to identify the regulators that mediates these differential responses, to better target potential therapeutic modalities.
Funding Information:
OMI was supported by the International Brain Research Organization (IBRO)-International Society for Neurochemistry (ISN) Research Fellowship 2017. MA was supported by National Institute of Health (NIH) R01 ES10563, R01 ES07331 and R01 ES020852. JB and TS were supported by the DFG Research Unit TraceAge (FOR 2558).
Footnotes
Conflict of Interests: The authors declare no conflict of interests.
Data Availability Statement:
The data that supports the findings of this study are available from the corresponding author, upon appropriate request.
References
- Akingbade GT, Ijomone OM, Imam A, Aschner M, Ajao MS (2021) d-Ribose-l-Cysteine Improves Glutathione Levels, Neuronal and Mitochondrial Ultrastructural Damage, Caspase-3 and GFAP Expressions Following Manganese-Induced Neurotoxicity Neurotoxicity Research:1–13 [DOI] [PubMed] [Google Scholar]
- Alarifi S, Ali D, Alkahtani S (2017) Oxidative stress-induced DNA damage by manganese dioxide nanoparticles in human neuronal cells BioMed research international 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JG, Cooney PT, Erikson KM (2007) Brain manganese accumulation is inversely related to γ-amino butyric acid uptake in male and female rats Toxicological Sciences 95:188–195 [DOI] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Prager EM, Olsen CH, Braga MF (2017) Susceptibility to soman toxicity and efficacy of LY293558 against soman-induced seizures and neuropathology in 10-month-old male rats Neurotoxicity research 32:694–706 [DOI] [PubMed] [Google Scholar]
- Avila DS, Benedetto A, Au C, Bornhorst J, Aschner M (2016) Involvement of heat shock proteins on Mn-induced toxicity in Caenorhabditis elegans BMC Pharmacology and Toxicology 17:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann CG, Trenkwalder C (2006) Body weight in patients with Parkinson’s disease Movement disorders: official journal of the Movement Disorder Society 21:1824–1830 [DOI] [PubMed] [Google Scholar]
- Bahar E, Kim J-Y, Yoon H (2017) Quercetin attenuates manganese-induced neuroinflammation by alleviating oxidative stress through regulation of apoptosis, iNOS/NF-κB and HO-1/Nrf2 pathways International journal of molecular sciences 18:1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RA et al. (2019) Effects of Preweaning Manganese in Combination with Adult Striatal Dopamine Lesions on Monoamines, BDNF, TrkB, and Cognitive Function in Sprague–Dawley Rats Neurotoxicity research 35:606–620 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Edelstein S, Corey-Bloom J, Wiederholt W (1998) Weight loss precedes dementia in community-dwelling older adults Age & nutrition (Paris) 9 [PubMed] [Google Scholar]
- Bauer JA et al. (2017) Manganese in teeth and neurobehavior: Sex-specific windows of susceptibility Environment international 108:299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Surette C, Cormier P, Foucher D (2018) Low level exposure to manganese from drinking water and cognition in school-age children Neurotoxicology 64:110–117 [DOI] [PubMed] [Google Scholar]
- Broberg K et al. (2019) Manganese transporter genetics and sex modify the association between environmental manganese exposure and neurobehavioral outcomes in children Environment international 130:104908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA (2005) Change in body mass index and risk of incident Alzheimer disease Neurology 65:892–897 [DOI] [PubMed] [Google Scholar]
- Caito S, Aschner M (2015) Neurotoxicity of metals Handbook of clinical neurology 131:169–189 [DOI] [PubMed] [Google Scholar]
- Cheng H et al. (2018) PI3K/Akt signaling pathway and Hsp70 activate in hippocampus of rats with chronic manganese sulfate exposure Journal of Trace Elements in Medicine and Biology 50:332–338 [DOI] [PubMed] [Google Scholar]
- Cordova FM et al. (2012) In vivo manganese exposure modulates Erk, Akt and Darpp-32 in the striatum of developing rats, and impairs their motor function PloS one 7:e33057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva DG et al. (2018) Mancozeb exposure results in manganese accumulation and Nrf2-related antioxidant responses in the brain of common carp Cyprinus carpio Environmental Science and Pollution Research 25:15529–15540 [DOI] [PubMed] [Google Scholar]
- Dion L-A, Saint-Amour D, Sauvé S, Barbeau B, Mergler D, Bouchard MF (2018) Changes in water manganese levels and longitudinal assessment of intellectual function in children exposed through drinking water Neurotoxicology 64:118–125 [DOI] [PubMed] [Google Scholar]
- Djousse L, Knowlton B, Cupples L, Marder K, Shoulson I, Myers R (2002) Weight loss in early stage of Huntington’s disease Neurology 59:1325–1330 [DOI] [PubMed] [Google Scholar]
- Dominguez R, Liu R, Baudry M (2007) 17-β-estradiol-mediated activation of extracellular-signal regulated kinase, phosphatidylinositol 3-kinase/protein kinase B-Akt and N-methyl-d-aspartate receptor phosphorylation in cortical synaptoneurosomes Journal of neurochemistry 101:232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F et al. (2017) Quercetin ameliorates learning and memory via the Nrf2-ARE signaling pathway in d-galactose-induced neurotoxicity in mice Biochemical and biophysical research communications 491:636–641 [DOI] [PubMed] [Google Scholar]
- Dorman DC, McManus BE, Marshall MW, James RA, Struve MF (2004) Old age and gender influence the pharmacokinetics of inhaled manganese sulfate and manganese phosphate in rats Toxicology and applied pharmacology 197:113–124 [DOI] [PubMed] [Google Scholar]
- Erukainure OL, Ijomone OM, Sanni O, Aschner M, Islam MS (2019) Type 2 diabetes induced oxidative brain injury involves altered cerebellar neuronal integrity and elemental distribution, and exacerbated Nrf2 expression: therapeutic potential of raffia palm (Raphia hookeri) wine Metabolic brain disease 34:1385–1399 [DOI] [PubMed] [Google Scholar]
- Foster ML, Bartnikas TB, Maresca-Fichter HC, Mercadante C, Dash M, Miller C, Dorman DC (2017) Interactions of manganese with iron, zinc, and copper in neonatal C57BL/6J and parkin mice following developmental oral manganese exposure Data in brief 15:908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sánchez A et al. (2020) The effect of visceral abdominal fat volume on oxidative stress and proinflammatory cytokines in subjects with normal weight, overweight and obesity Diabetes, metabolic syndrome and obesity: targets and therapy 13:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorojod RM, Alaimo A, Alcon SP, Saravia F, Kotler ML (2017) Interplay between lysosomal, mitochondrial and death receptor pathways during manganese-induced apoptosis in glial cells Archives of toxicology 91:3065–3078 [DOI] [PubMed] [Google Scholar]
- Guilarte TR (2010) APLP1, Alzheimer’s-like pathology and neurodegeneration in the frontal cortex of manganese-exposed non-human primates Neurotoxicology 31:572–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS (2008) Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates Journal of neurochemistry 105:1948–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL, Degaonkar M, Chen M-K, Barker PB, Syversen T, Schneider JS (2006) Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: a 1H-MRS and MRI study Toxicological sciences 94:351–358 [DOI] [PubMed] [Google Scholar]
- Hernández RB et al. (2020) Manganese-induced neurotoxicity in cerebellar granule neurons due to perturbation of cell network pathways with potential implications for neurodegenerative disorders Metallomics 12:1656–1678 [DOI] [PubMed] [Google Scholar]
- Huang Y, Wen Q, Huang J, Luo M, Xiao Y, Mo R, Wang J (2021) Manganese (II) chloride leads to dopaminergic neurotoxicity by promoting mitophagy through BNIP3-mediated oxidative stress in SH-SY5Y cells Cellular & Molecular Biology Letters 26:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijomone OM, Okori SO, Ijomone OK, Ebokaiwe AP (2018) Sub-acute nickel exposure impairs behavior, alters neuronal microarchitecture, and induces oxidative stress in rats’ brain Drug and chemical toxicology 41:377–384 [DOI] [PubMed] [Google Scholar]
- Jeong W-S, Jun M, Kong A-NT (2006) Nrf2: a potential molecular target for cancer chemoprevention by natural compounds Antioxidants & redox signaling 8:99–106 [DOI] [PubMed] [Google Scholar]
- Jover-Mengual T, Zukin RS, Etgen AM (2007) MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia Endocrinology 148:1131–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke T, Sidoryk-Wegrzynowicz M, Pajarillo E, Rizor A, Soares FAA, Lee E, Aschner M (2019) Role of astrocytes in manganese neurotoxicity revisited Neurochemical research 44:2449–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen CL, Ensunsa JL, Lönnerdal B, Zidenberg-Cherr S (2013) Manganese. In: Caballero B (ed) Encyclopedia of Human Nutrition (Third Edition). Academic Press, Waltham, pp 148–154. doi: 10.1016/B978-0-12-375083-9.00182-3 [DOI] [Google Scholar]
- Kim SI et al. (2012) Effect of manganese exposure on the reproductive organs in immature female rats Development & reproduction 16:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblith E, Casey SL, Lobdell DT, Colledge MA, Bowler RM (2018) Environmental exposure to manganese in air: Tremor, motor and cognitive symptom profiles Neurotoxicology 64:152–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao Y et al. (2017) Mapping the basal ganglia alterations in children chronically exposed to manganese Scientific reports 7:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E-SY, Yin Z, Milatovic D, Jiang H, Aschner M (2009) Estrogen and tamoxifen protect against Mn-induced toxicity in rat cortical primary cultures of neurons and astrocytes Toxicological Sciences 110:156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wu S, Shi N, Lian S, Lin W (2011a) Nrf2/HO-1 pathway activation by manganese is associated with reactive oxygen species and ubiquitin–proteasome pathway, not MAPKs signaling Journal of Applied Toxicology 31:690–697 [DOI] [PubMed] [Google Scholar]
- Li H, Wu S, Shi N, Lin W, You J, Zhou W (2011b) NF-E2-related factor 2 activation in PC12 cells: its protective role in manganese-induced damage Archives of toxicology 85:901–910 [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu M, Cui J, Du Y, Teng X, Zhang Z (2021) Heat shock proteins took part in oxidative stress-mediated inflammatory injury via NF-κB pathway in excess manganese-treated chicken livers Ecotoxicology and Environmental Safety 226:112833. [DOI] [PubMed] [Google Scholar]
- Llop S, Lopez-Espinosa M-J, Rebagliato M, Ballester F (2013) Gender differences in the neurotoxicity of metals in children Toxicology 311:3–12 [DOI] [PubMed] [Google Scholar]
- Luo X, Ren B, Hursthouse AS, Jiang F, Deng R-j(2020) Potentially toxic elements (PTEs) in crops, soil, and water near Xiangtan manganese mine, China: potential risk to health in the foodchain Environmental geochemistry and health 42:1965–1976 [DOI] [PubMed] [Google Scholar]
- Ma Z et al. (2020) Alpha-synuclein is involved in manganese-induced spatial memory and synaptic plasticity impairments via TrkB/Akt/Fyn-mediated phosphorylation of NMDA receptors Cell death & disease 11:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison JL, Wegrzynowicz M, Aschner M, Bowman AB (2011) Gender and manganese exposure interactions on mouse striatal neuron morphology Neurotoxicology 32:896–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miah MR et al. (2020) The effects of manganese overexposure on brain health Neurochemistry international 135:104688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milagro FI, Campión J, Martínez JA (2006) Weight gain induced by high-fat feeding involves increased liver oxidative stress Obesity 14:1118–1123 [DOI] [PubMed] [Google Scholar]
- Morcillo P et al. (2021) Defective Mitochondrial Dynamics Underlie Manganese-Induced Neurotoxicity Molecular Neurobiology 58:3270–3289 doi: 10.1007/s12035-021-02341-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano P et al. (2020) Manganese increases Aβ and Tau protein levels through proteasome 20S and heat shock proteins 90 and 70 alteration, leading to SN56 cholinergic cell death following single and repeated treatment Ecotoxicology and Environmental Safety 203:110975. [DOI] [PubMed] [Google Scholar]
- Nkpaa KW et al. (2022) Selenium abates manganese–induced striatal and hippocampal toxicity via abrogation of neurobehavioral deficits, biometal accumulation, oxidative stress, inflammation, and caspase-3 activation in rats Psychopharmacology 239:399–412 [DOI] [PubMed] [Google Scholar]
- Nyarko-Danquah I, Pajarillo E, Digman A, Soliman KF, Aschner M, Lee E (2020) Manganese accumulation in the brain via various transporters and its neurotoxicity mechanisms Molecules 25:5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri G, Novakovic M, Savaskan E, Meier F, Baysang G, Brockhaus M, Müller-Spahn F (2002) The effects of β-estradiol on SHSY5Y neuroblastoma cells during heavy metal induced oxidative stress, neurotoxicity and β-amyloid secretion Neuroscience 113:849–855 [DOI] [PubMed] [Google Scholar]
- Ou C-Y et al. (2017) Sodium P-aminosalicylic acid improved manganese-induced learning and memory dysfunction via restoring the ultrastructural alterations and γ-aminobutyric acid metabolism imbalance in the basal ganglia Biological trace element research 176:143–153 [DOI] [PubMed] [Google Scholar]
- Oulhote Y et al. (2014a) Neurobehavioral function in school-age children exposed to manganese in drinking water Environmental health perspectives 122:1343–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Mergler D, Bouchard MF (2014b) Sex-and age-differences in blood manganese levels in the US general population: national health and nutrition examination survey 2011–2012 Environmental Health 13:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres TV, Schettinger MRC, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M (2016) Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies BMC Pharmacology and Toxicology 17:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MV, Jones K, Barillari C, Westwood I, Montfort RLv, Workman P (2010) Targeting HSP70: the second potentially druggable heat shock protein and molecular chaperone? Cell cycle 9:1542–1550 [DOI] [PubMed] [Google Scholar]
- Ramirez AD, Liu X, Menniti FS (2003) Repeated estradiol treatment prevents MPTP-induced dopamine depletion in male mice Neuroendocrinology 77:223–231 [DOI] [PubMed] [Google Scholar]
- Razmara A, Duckles SP, Krause DN, Procaccio V (2007) Estrogen suppresses brain mitochondrial oxidative stress in female and male rats Brain research 1176:71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels H, Meiers G, Delos M, Ortega I, Lauwerys R, Buchet J-P, Lison D (1997) Influence of the route of administration and the chemical form (MnCl2, MnO2) on the absorption and cerebral distribution of manganese in rats Archives of toxicology 71:223–230 [DOI] [PubMed] [Google Scholar]
- Santos D, Batoreu M, Almeida I, Ramos R, Sidoryk-Wegrzynowicz M, Aschner M, Marreilha dos Santos A (2012a) Manganese alters rat brain amino acids levels Biological trace element research 150:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D, Milatovic D, Andrade V, Batoreu MC, Aschner M, Dos Santos AM (2012b) The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain Toxicology 292:90–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz CRR et al. (2019) Sex differences in subacute manganese intoxication: oxidative parameters and metal deposition in peripheral organs of adult Wistar rats Regulatory Toxicology and Pharmacology 104:98–107 [DOI] [PubMed] [Google Scholar]
- Schullehner J, Thygesen M, Kristiansen SM, Hansen B, Pedersen CB, Dalsgaard S (2020) Exposure to manganese in drinking water during childhood and association with attention-deficit hyperactivity disorder: A nationwide cohort study Environmental health perspectives 128:097004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T-M, Koenig JA, Acon Chen C (2019) Comparative effects of scopolamine and phencynonate on organophosphorus nerve agent-induced seizure activity, neuropathology and lethality Toxicology mechanisms and methods 29:322–333 [DOI] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Aschner M (2013) Manganese toxicity in the central nervous system: the glutamine/glutamate-γ-aminobutyric acid cycle Journal of internal medicine 273:466–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sõti C, Nagy E, Giricz Z, Vígh L, Csermely P, Ferdinandy P (2005) Heat shock proteins as emerging therapeutic targets British journal of pharmacology 146:769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD et al. (2009) Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia Journal of neurochemistry 110:378–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur M, Sharma P (2007) Transcription of estrogen receptor α and β in mouse cerebral cortex: effect of age, sex, 17β-estradiol and testosterone Neurochemistry international 50:314–321 [DOI] [PubMed] [Google Scholar]
- Vorhees CV et al. (2014) Effects of developmental manganese, stress, and the combination of both on monoamines, growth, and corticosterone Toxicology reports 1:1046–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg K et al. (2018) Polymorphisms in manganese transporters show developmental stage and sex specific associations with manganese concentrations in primary teeth Neurotoxicology 64:103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J et al. (2019) Melatonin receptor activation provides cerebral protection after traumatic brain injury by mitigating oxidative stress and inflammation via the Nrf2 signaling pathway Free radical biology and medicine 131:345–355 [DOI] [PubMed] [Google Scholar]
- Wang S, Tatman M, Monteiro MJ (2020) Overexpression of UBQLN1 reduces neuropathology in the P497S UBQLN2 mouse model of ALS/FTD Acta neuropathologica communications 8:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata A et al. (2017) Gender influence on manganese induced depression-like behavior and Mn and Fe deposition in different regions of CNS and excretory organs in intraperitoneally exposed rats Toxicology 376:137–145 [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhou Z, Fu J (2003) Effect of manganese chloride exposure on liver and brain mitochondria function in rats Environmental Research 93:149–157 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A (2004) Estrogen and androgen protection of human neurons against intracellular amyloid β1–42 toxicity through heat shock protein 70 Journal of Neuroscience 24:5315–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X et al. (2019) Manganese induces neuroinflammation via NF-κB/ROS NLRP3 pathway in rat brain striatum and HAPI cells Molecular & Cellular Toxicology 15:173–183 [Google Scholar]
- Zheng W, Kim H, Zhao Q (2000) Comparative toxicokinetics of manganese chloride and methylcyclopentadienyl manganese tricarbonyl (MMT) in Sprague-Dawley rats Toxicological Sciences 54:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T et al. (2020) Sex-specific differences in cognitive abilities associated with childhood cadmium and manganese exposures in school-age children: a prospective cohort study Biological trace element research 193:89–99 [DOI] [PubMed] [Google Scholar]
- Zhu Y-W et al. (2016) Effect of dietary manganese on antioxidant status and expressions of heat shock proteins and factors in tissues of laying broiler breeders under normal and high environmental temperatures British Journal of Nutrition 116:1851–1860 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Lu X, Wu D, Cai S, Li S, Teng X (2013) The effect of manganese-induced cytotoxicity on mRNA expressions of HSP27, HSP40, HSP60, HSP70 and HSP90 in chicken spleen lymphocytes in vitro Biological trace element research 156:144–152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author, upon appropriate request.


