Abstract
The ATP-binding cassette (ABC) transporter superfamily comprises membrane proteins that efflux various substrates across extra- and intra-cellular membranes. Mutations in ABC genes cause 21 human disorders or phenotypes with Mendelian inheritance, including cystic fibrosis, adrenoleukodystrophy, retinal degeneration, cholesterol, and bile transport defects. To provide tools to study the function of human ABC transporters we compiled data from multiple genomics databases. We analyzed ABC gene conservation within human populations and across vertebrates surveyed phenotypes of ABC gene mutations in mice. Most mouse ABC gene disruption mutations have a phenotype that mimics human disease, indicating they are applicable models. Interestingly several ABCA family genes, whose human function is unknown, have cholesterol level phenotypes in the mouse. Genome-wide association studies confirm and extend ABC traits and suggest several new functions to investigate. Whole exome sequencing of tumors from diverse cancer types demonstrates that mutations in ABC genes are not common in cancer, but specific genes are overexpressed in select tumor types. Finally, an analysis of the frequency of loss-of-function mutations demonstrates that many human ABC genes are essential with a low level of variants, while others have a higher level of genetic diversity.
Keywords: ATP-binding cassette transporter, human disease, evolution, lipid transport
Introduction
ABC transporters are intricate molecular systems that characterize the vectorial transport of various substrates across biological membranes. They are present in all extant species ranging from prokaryotes to humans (Childs & Ling, 1994; Jones & George, 2004) (Srikant, 2020) and comprise the largest family of transmembrane proteins. These transmembrane proteins bind ATP and utilize the energy to drive the active transport of diverse molecules across all cell membranes against the concentration gradient (Dean & Annilo, 2005; Higgins, 1992; KM Moitra, 2012). Characterization of this important class of transporters, which contain one of the largest and ancient protein subfamilies that transport a myriad of substrates from sugars, amino acids, proteins to metal ions, will yield invaluable insights into the molecular basis and physiology of human diseases (Higgins, 1992).
Organization of ABC Genes and Proteins
The classification of proteins as ABC transporters is based on the sequence and organization of ATP-binding domain(s), also known as nucleotide-binding domains (NBDs) or nucleotide-binding folds. Characteristic motifs, the Walker A motif and Walker B motif separated by ~90–120 amino acids, are found in the nucleotide-binding fold of all ATP-binding proteins. In addition, the ABC genes contain an additional distinctive component referred to as the signature motif or the C-loop situated upstream of the Walker B site (Hyde et al., 1990).
The typical ABC protein contains two NBDs and two transmembrane domains (TMDs). The TM domains generally have 6–12 membrane-spanning alpha-helices, determining substrate specificity. While the topology of the transmembrane domains is complex (Tusnady, Sarkadi, Simon, & Varadi, 2006), structural data has allowed the distinct transmembrane folds to be classified (C. Thomas et al., 2020). The eukaryotic ABC genes can be full transporters containing two TMs and two NBDs, or half transporters (Hyde et al., 1990; C. Thomas & Tampe, 2020). To create a functional transport molecule, half transporters need to form either homodimers or heterodimers. Specific mutations in ABC genes can contribute to several human genetic disorders, including cystic fibrosis, neurological disease, cholesterol/bile transport defects, retinal degeneration, anemia, and differential drug response (Dean, Hamon, & Chimini, 2001).
In bacteria, ABC transporters tend to be unidirectional. Most of them are importers, which import essential molecules involved in bacterial metabolism such as vitamins, metal ions, and sugars. However, several MDR-like transporters (primarily involved in drug resistance) and other ABC ATPases involved in cellular processes like DNA repair or other regulatory functions have also been identified (Lubelski, Konings, & Driessen, 2007). Eukaryotic ABC transporters are mainly engaged in the shuttling of hydrophobic compounds either within the cell as part of a metabolic process or outside the cell for transport to other organs or secretion from the body.
ABC genes exhibit evolutionary conservation from bacteria to humans, and multiple gene duplication and deletion events in the ABC genes point to the fact that gene evolution is still ongoing. Phylogenetic analysis of the NBDs has been used to classify prominent ABC gene families across prokaryotic and eukaryotic organisms (Srikant, 2020). ABC genes are dispersed widely in eukaryotic genomes. In humans, the ABC transporter superfamily contains 48 genes, divided into seven subfamilies ranging from A to G based on similarity in gene structure, order of the domains, and sequence homology in the NBD and TM domains (Dean, Rzhetsky, & Allikmets, 2001). To date, mutations in 21 of the 48 human ABC genes cause a Mendelian disease or an inherited phenotype (Table 1)
Table 1.
Mendelian disease and phenotypes of ABC genes
| Symbol | Other name | Location | Mendelian disease | OMIM | Frequency |
|---|---|---|---|---|---|
| ABCA1 | 09q31.1 | Tangier Disease | MIM# 205400 | Rarea | |
| HDL deficiency, type 2 | MIM# 604091 | Rare | |||
| ABCA2 | 09q34.3 | ||||
| ABCA3 | 16p13.3 | Neonatal respiratory failure | MIM# 610921 | Rare | |
| ABCA4 | ABCR | 01p21.3 | Stargardt Disease | MIM# 248200 | 1:10,000 |
| Cone-rod dystrophy 3 | MIM# 604116 | Rare | |||
| Retinitis pigmentosa 19 | MIM# 601718 | Rare | |||
| ABCA5 | 17q24.3 | Hypertrichosis | MIM# 135400 | Rarea | |
| ABCA6 | 17q24.3 | ||||
| ABCA7 | ABCX | 19p13.3 | |||
| ABCA8 | 17q24.3 | ||||
| ABCA9 | 17q24.3 | ||||
| ABCA10 | 17q24.3 | ||||
| ABCA12 | 02q34 | Ichthyosis, autosomal recessive | MIM# 242500, MIM# 601277 | 1:200,000 | |
| ABCA13 | 07p12.3 | ||||
| ABCB1 | PGP | 07q21.12 | |||
| TAP1 | ABCB2 | 06p21 | Bare lymphocyte syndrome | MIM# 604571 | Rareb |
| TAP2 | ABCB3 | 06p21 | Bare lymphocyte syndrome | MIM# 604571 | |
| ABCB4 | MDR3 | 07q21.12 | Cholestasis, intrahepatic | MIM# 171060 | 1:10,000 |
| ABCB5 | 07p21.1 | ||||
| ABCB6 | MTABC3 | 02q35 | Dyschromatosis universalis | MIM# 605452 | Rare |
| Microphthalmia, isolated, with coloboma | MIM# 614497 | Rare | |||
| Lan Blood group | MIM# 605452 | 1:1500–1:50,000c | |||
| ABCB7 | ABC7 | Xq21–22 | Anemia, sideroblastic, with ataxia | MIM# 301310 | Rare |
| ABCB8 | MABC1 | 07q36.1 | |||
| ABCB9 | TAPL | 12q24.31 | |||
| ABCB10 | MTABC2 | 01q42.13 | |||
| ABCB11 | BSEP, SPGP | 02q24.3 | Hepatic cholestasis | MIM# 605479, MIM# 601847 | 1:100,000 |
| ABCC1 | MRP1 | 16p13.12 | |||
| ABCC2 | MRP2, CMOAT | 10q24.2 | Dubin-Johnson syndrome | MIM# 237500 | 1:1300d |
| ABCC3 | MRP3 | 17q21.33 | |||
| ABCC4 | MRP4 | 13q32.1 | Pel-negative blood group | ||
| ABCC5 | MRP5 | 03q27.1 | |||
| ABCC6 | MRP6 | 16p13.12 | Pseudoxanthoma elasticum | MIM# 264800 | 1:25,000 |
| Arterial calcification in infancy | MIM# 614473 | Rare | |||
| CFTR | ABCC7 | 07q31.31 | Cystic fibrosis | MIM# 219700 | 1:2500 |
| CBAVD | MIM# 277180 | 1:2000 | |||
| ABCC8 | SUR1 | 11p15.1 | Diabetes mellitus, permanent neonatal | MIM# 606176, MIM# 610374 | 1:400,000 |
| Hyperinsulinemic hypoglycemia | MIM# 256450, MIM# 240800 | Rare | |||
| ABCC9 | SUR2 | 12p12.1 | Hypertrichotic osteochondrodysplasia | MIM# 239850 | Rare |
| Cardiomyopathy, dilated, 10 | MIM# 608569 | Rare | |||
| ABCC10 | MRP7 | 06p21.1 | |||
| ABCC11 | MRP8 | 16q12.1 | Earwax, (wet/dry), colostrum secretion, odor | MIM# 117800 | up to 95%e |
| ABCC12 | MRP9 | 16q12.1 | |||
| ABCD1 | ALDP | Xq28 | Adrenoleukodystrophy | MIM# 300100 | 1:20,000 |
| ABCD2 | ALPR | 12q11 | |||
| ABCD3 | PMP70 | 01p22.1 | Bile acid synthesis defect, congenital, 5 | MIM# 616278 | Very raref |
| ABCD4 | P70R | 14q24.3 | Methylmalonic aciduria and homocystinuria, cblJ type | MIM# 603214 | Rare |
| ABCE1 | RNASELI | 04q31.31 | |||
| ABCF1 | ABC50 | 06p21.1 | |||
| ABCF2 | 07q36.1 | ||||
| ABCF3 | 03q27.1 | ||||
| ABCG1 | ABC8 | 21q22.3 | |||
| ABCG2 | BCRP | 04q22 | Junior blood group system, Gout | MIM# 614490 | up to 1:60g |
| ABCG4 | WHITE2 | 11q23 | |||
| ABCG5 | 02p21 | Sitosterolemia | MIM# 210250 | Rarea | |
| ABCG8 | 02p21 | Sitosterolemia | MIM# 210250 |
50–100 cases reported;
, 30 cases reported;
, 1 in 50,000 in Japanese populations, 1 in 20,000 in Caucasians, and 1 in 1,500 in black people from South Africa;
, Iranian and Moroccan Jews living in Israel, with 1 in 1,300 individuals;
, dry ear wax type is 80 to 95% among East Asians, 0 to 3% European and African populations frequencies;
, one family reported;
, 1 in 60 to 1/3800 in Japan.
Human ABC Gene Subfamilies and Disease Associations: An Overview
ABCA (ABC1)
The human ABCA subfamily contains 12 full transporters. These transporters separate into two subgroups based primarily on phylogenetic analysis and intron structure (Arnould et al., 2001). The first group of transporters contains seven genes distributed among six chromosomes (ABCA1, ABCA2, ABCA3, ABCA4, ABCA7, ABCA12, and ABCA13), whereas the second group comprises five genes (ABCA5, ABCA6, ABCA8, ABCA9, and ABCA10) on chromosome 17q24.3 (Broccardo et al., 2001). Several ABCA sub-family genes are associated with human disease. The ABCA1 protein is definitively involved in cholesterol transport disorders and HDL biosynthesis defects. Tangier Disease (TD), a rare genetic disorder of lipoprotein metabolism, is caused by mutations in the ABCA1 gene (Remaley et al., 1999; Rust et al., 1999). This disease presents with deficient levels of HDL cholesterol and apoprotein A-I causing a myriad of symptoms, including orange-yellow tonsils, anemia, thrombocytopenia, peripheral neuropathy, hepatosplenomegaly, lymphadenopathy, and corneal opacity. Tangier Disease can also be associated with an increased risk of coronary artery disease (Maranghi et al., 2019). The ABCA4 protein performs critical steps in the visual cycle by transporting vitamin A derivatives in the outer segments of rod photoreceptor cells. Pathogenic variants in ABCA4 cause Stargardt disease (STGD1), a common early-onset maculopathy.
ABCB (TAP)
The human genome contains three full transporters of the ABCB family. The intensively studied multidrug resistance protein, P-glycoprotein, ABCB1/PGP is a full transporter playing an essential role in xenobiotic removal, regulation of the blood: brain and other tissue barriers and is amplified in many chemotherapy drug-resistant cell lines (Borst, 1999; Childs & Ling, 1994; Gottesman, Fojo, & Bates, 2002; Hartz & Bauer, 2011; Roninson et al., 1986). The ABCB4 protein plays a significant role in transporting phosphatidylcholine into bile in the liver (de Vree et al., 1998; Groen et al., 2011) (Robey et al., 2018). Moreover, the ABCB11 protein, also known as the bile salt export pump, BSEP, transports bile salts from the liver (Strautnieks et al., 1998; R. Wang et al., 2001). In addition, germline ABCB11 mutations are associated with pediatric hepatocellular cancer (Iannelli et al., 2014; Knisely et al., 2006).
The half transporters in this subfamily include ABCB2 (TAP1) and ABCB3 (TAP2) that need to form a functional heterodimer to transport peptides into the ER providing the antigens presented by class I HLA molecules. Specific Tap1/Tap2 polymorphisms are associated with ankylosing spondylitis (Qian et al., 2017), celiac disease (Powis et al., 1993), Graves’ disease (Rau et al., 1997), and other autoimmune diseases (de la Salle et al., 1994). ABCB9, a half transporter and the closest homolog of the TAPs, has been localized to lysosomes, and several other transporters including ABCB6 have been shown to localize to the endolysosomal system (Szakacs & Abele, 2020) (Helias et al., 2012) (Kiss et al., 2012). The other four half transporters, ABCB6, ABCB7, ABCB8, and ABCB10, localize to the mitochondria, where they function in iron metabolism and the transport of Fe/S protein precursors (Dean, 2002) (Schaedler et al., 2015). ABCB10 is also associated with protection from oxidative stress, and it is essential for erythropoiesis and recovery from cardiac ischemia-reperfusion (Liesa, Qiu, & Shirihai, 2012).
ABCC (CFTR/MRP)
Twelve full transporters make up the ABCC subfamily, and they have divergent functional roles that include ion transport, cell-surface receptor, and toxin secretion activities. These transporters are responsible for human diseases, including cystic fibrosis (ABCC7) and pseudoxanthoma elasticum (ABCC6). The ABCC1/MRP1 gene encodes a multidrug resistance protein (S. P. C. Cole et al., 1992). ABCC1 and the cytokine cysteinyl leukotriene C(4) (S. P. Cole & Deeley, 1998). The cystic fibrosis transmembrane receptor (CFTR/ABCC7) protein functions as a chloride channel that plays a role in exocrine secretion. Different genetic mutations in the CFTR gene cause cystic fibrosis (Quinton, 1999; Rommens et al., 1989). Cystic fibrosis is an (autosomal recessive) disease that may affect the lungs and the digestive system. Mutations in the cystic fibrosis (CF) gene (ABCC7) result in the production of a thick, sticky mucous that can clog up the lungs and sometimes lead to life-threatening infections. In addition, CF can result in obstruction of the pancreas that has the effect of preventing certain enzymes from breaking down and absorbing food into the body (http://www.cff.org). The CF protein has a regulatory domain, otherwise called the R domain, located between NBD1 and TMD2 and contains several potential sites for phosphorylation or binding of cAMP-dependent PKA or PKC. Kinase-mediated phosphorylation of the cytoplasmic R domain or binding of PKA is required to transmit the signal from the NBDs to the channel gate (Mihalyi, Iordanov, Torocsik, & Csanady, 2020). Hence this domain is essential for the functioning of the transporter.
Pseudoxanthoma elasticum (PXE) is an autosomal recessive Mendelian disease that affects multiple body systems caused by mutations in ABCC6 (Bergen et al., 2000). Its main characteristic feature is the mineralization of the soft connective tissue that primarily affects the skin, eyes, and arterial blood vessels. PXE has high phenotypic variability, likely modulated by variants in several modifier genes (K. Moitra et al., 2017). Analysis of gene defects controlling mineralization has led to functional insight and therapeutic strategies (Li, Jiang, Pfendner, Varadi, & Uitto, 2009; Shimada et al., 2021). The ABCC8 and ABCC9 proteins bind sulfonylurea and regulate potassium channels involved in modulating insulin secretion. The additional nine MRP-related genes in this superfamily have diverse functions. ABCC1, ABCC2, and ABCC3 transport drugs conjugated to glutathione and other organic anions. The N-terminal domain (TMD0) is absent in ABCC4, ABCC5, ABCC11, and ABCC12, so these proteins are smaller than the other MRP1-like gene products (Bakos et al., 2000). The remaining ABCC subfamily proteins, ABCC4 and ABCC5, confer resistance to nucleosides, including 9-(2-phosphonylmethoxyethyl) adenine (PMEA) and purine analogs. A recent study found an MRP4 variant (rs3751333) significantly associated with hepatitis B viral DNA level suppression in a cohort of chronic hepatitis B patients treated with entecavir. This result suggests that Han Chinese patients with the rs3751333 GG genotype may respond better to entecavir treatment (Yuan et al., 2016). ABCC4 inhibition, when coupled with phosphodiesterase inhibition in human platelets, convincingly impaired the process of platelet aggregation. The clinical implications of this finding shed light on a crucial relationship between ABCC4 transporter function and phosphodiesterases. The data suggests that the cAMP-directed activity of antithrombotic agents can reduce the occurrence of blood clots (Cheepala et al., 2015).
ABCD
The ABCD subfamily comprises four human genes, and ABCD1, ABCD2, ABCD3 encode proteins located in the peroxisome. The ABCD proteins are all half-transporters and, consequently, function as homo- or heterodimers, and two of these (ABCD1, ABCD2) take part in the regulation of very-long-chain fatty acid transport (Dean, 2002).
The mammalian peroxisomal ABC transporters are the adrenoleukodystrophy protein (ALDP/ABCD1), ALDP-related protein (ALDRP/ABCD2), and a 70-kDa peroxisomal membrane protein (PMP70/ABCD3). X-linked adrenoleukodystrophy (X-ALD) is associated with variants in ABCD1 (Mosser et al., 1993). X-ALD is characterized by the accumulation of very long-chain fatty acids in the peroxisome that is an outcome of impaired beta-oxidation. Patients with X-ALD display progressive demyelination of the neurons in the central nervous system, testicular malfunction, and adrenal insufficiency (Smith et al., 1999). ABCD2 may be involved in the metabolic transport of VLCFA’s (Morita & Imanaka, 2012). The function of ABCD3 consists of the transportation of branched-chain acyl-CoA into peroxisomes. ABCD4 resides in the mitochondria and the lysosomes. In the lysosomes, ABCD4 is involved in transporting vitamin B12 from lysosomes into the cytosol. Mutations in ABCD4 cause an inborn error of vitamin B12 metabolism, resulting in the lysosomes failing to release cobalamin resulting in symptoms mimicking cobalamin deficiency (Kawaguchi & Morita, 2016).
ABCE (OABP) and ABCF (GCN20)
The ABCE and ABCF subfamilies consist of gene products with no TM domain and are not involved in membrane transport functions. They have only ATP-binding domains and no TM domain. The ABCE subfamily has a single member – the oligo-adenylate-binding protein (OABP). This molecule recognizes oligo-adenylate and is produced in response to infection by certain viruses. For example, it interacts with HIV-1 proteins Vif and Gag and the HIV-2 protein GAG. (Dooher & Lingappa, 2004; Zimmerman et al., 2002).
In the ABCF gene family, each gene contains a pair of NBDs. The best-characterized member is the S. cerevisiae GCN20 gene product that mediates the activation of the eIF-2a kinase (Marton, Vazquez de Aldana, Qiu, Chakraburtty, & Hinnebusch, 1997). The human homolog is named ABCF1 and is associated with the ribosome. It appears to have a similar functional role (Tyzack, Wang, Belsham, & Proud, 2000). ABCF1 was also identified as a new retinal pigment epithelium (RPE) phagocytotic ligand by functional screening assays, where it extrinsically promoted phagocytosis of shed photoreceptor cells by the RPE. This function is essential in the visual cycle because RPE cells are specialized phagocytes that maintain retinal homeostasis and prevent retinal degeneration (Guo et al., 2015). Additional roles of ABCF1 include regulating innate immune response and its role as a risk gene for autoimmune pancreatitis and arthritis. Abcf1 expression also plays a role in the development of mouse embryos, and its expression in adult animals correlates with actively proliferating and differentiating cell types (Wilcox et al., 2017).
ABCG
The ABCG subfamily comprises six reverse-configured half transporters with a unique structural organization. The NBD is situated at the N-terminal half of the transporter, followed by the TMD. The white locus of Drosophila is one of the most extensively studied ABC proteins. The white protein (with brown and scarlet) transports precursors of eye pigments (guanine and tryptophan) in the eye cells of the fly (H. Chen et al., 1996). In addition, it can potentially transport biogenic amines, 5-hydroxytryptamine (5-HT), and dopamine that are essential in Drosophila for olfactory learning and memory (Myers, 2017).
The ABCG1 protein in mammals is involved in cholesterol transport regulation (Klucken et al., 2000), and the gene is on chromosome 21q22.3 in humans. ABCG1 is a significant player in cholesterol efflux from macrophages to extracellular lipid acceptors that include high-density lipoprotein (HDL) and phosphatidylcholine (PC) vesicles (N. Wang, Lan, Chen, Matsuura, & Tall, 2004). In addition, ABCG1 may have a role in T-cell proliferation and provide a protective role for apoptosis in macrophages (Bensinger et al., 2008; Wojcik, Skaflen, Srinivasan, & Hedrick, 2008).
Other notable ABCG genes are ABCG2, which serves as a drug-resistance gene, and ABCG5 and ABCG8, which encode sterols transporters in the intestine and liver. The excretion of sterols in the liver and intestines is facilitated by the ABCG5/ABCG8 heterodimer (G5G8). Specific mutations in G5G8 cause sitosterolemia, a genetic disease characterized by the accumulation of plant sterols and cholesterol, leading to premature atherosclerosis (Lee et al., 2016). A genome-wide association study (GWAS) revealed that a single nucleotide polymorphism, p.Asp19His (NG_008883.1:g.4712C>G) in ABCG8, is a susceptibility factor for human gallstone disease (Buch et al., 2007). Abcg3 is only found in rodents and has an unknown function. The last member of this family, ABCG4, is expressed in the brain, spinal cord, heart, and thymus in humans and the retina. ABCG4 facilitates the efflux of cellular cholesterol to high-density lipoproteins (N. Wang et al., 2004). Thus, the dual processes of ‘gene birth’ and ‘gene death’ are involved in the evolution of ABCG genes. By studying the evolution of these transporters in other vertebrate species, we can facilitate developing animal models for functional and clinical studies (K. Moitra, Silverton, Limpert, Im, & Dean, 2011).
Materials and Methods
Vertebrate ABC genes
Each human or other vertebrate ABC gene was queried in the ENSEMBL database (ensemble.org). Full-length, partial, and putative missing genes were recorded for 62 vertebrate ABC genes across 64 vertebrate species (12 primates, five rodents, 21 other mammals, three marsupials, five birds, 13 fish, two reptiles, and one amphibian). Details are in Supp. Table S1.
Functional data for mice and other organisms
The phenotype of mouse ABC gene alleles was queried in the Mouse Genome Informatics data base (http://www.informatics.jax.org). The gene symbol, phenotype, and reference were recorded.
Human genome-wide association loci and rare germline and somatic variation
Genome-wide associations in or near Human ABC genes were queried in the GWAS Catalog (https://www.ebi.ac.uk/gwas). The gene, phenotype, associated SNP, P-value, allele frequency, and reference were recorded for associations greater than the accepted genome-wide threshold of 5 × 10−8.
Rare human variants were obtained from the gnomAD database (https://gnomad.broadinstitute.org/). The gene, number of synonymous, non-synonymous, loss-of-function variants were recorded and adjusted for the length of the gene in amino acids.
The variation observed in tumor samples was obtained from the COSMIC database (https://cancer.sanger.ac.uk/cosmic). The number of synonymous, non-synonymous, loss-of-function variants were recorded as well as the tissue type that is most frequently mutated. In addition, the tumor type with the highest percentage of samples displaying common copy number gain and with the highest percentage of samples with the gene overexpressed were extracted from the COSMIC database.
Results and Discussion
Conservation and gene birth and death across vertebrate species
To comprehensively understand the evolutionary history of the ABC transporter superfamily in vertebrates, we interrogated 62 vertebrate ABC genes across 64 vertebrate species (12 primates, five rodents, 21 other mammals, three marsupials, five birds, 13 fish, two reptiles, and one amphibian). Each gene was examined in the gene tree of the human or representative species in the ENSEMBL database. We noted the appearance of a full-length or partial gene as well as potential missing or duplicated genes. We compared these species against species with formal analyses of the ABC superfamily (human, mouse, zebrafish, and lamprey) (Dean, Rzhetsky, et al., 2001). There are high coverage genomes for 13 species that are likely to provide an accurate gene count (human, chimp, macaque, mouse, rat, dog, opossum, chicken, Xenopus, zebrafish, and fugu). This result provides at least one index species for most of the major orders of primates, rodents, carnivores, marsupials, birds, amphibians, and fish. However, as many of the remaining species have low-coverage draft genome assemblies, many missing genes are not likely to be gene loss events (Milinkovitch, Helaers, Depiereux, Tzika, & Gabaldon, 2010).
The number of ABC genes in primates is very stable. The ABCA10 gene is missing from the orangutan, gibbon, and marmoset genomes; ABCA10 is part of a cluster of five ABCA5-related genes that are duplicated head-to-tail on human chromosome 17. The gene loss event converting ABCC13 into a pseudogene (Annilo & Dean, 2004) appears to be confined to the great apes, as ABCC13 is intact in all other primates. The bushbaby (Otolemur garnettii) genome seems to have an additional TAP2/ABCB3 gene. The predicted amino acid sequences show that the two bushbaby TAP2 genes are in the same sequence contig. Their amino acid sequences have diverged, consistent with gene duplication. TAP1 and TAP2 play essential roles in antigen presentation, and duplication of TAP2 also occurs in many fish genomes. This result is of potential interest for the study of the evolution of immunogenetics of primates. In total, all primates contain between 48 and 50 ABC genes.
Rodents have many gene gain and loss events affecting the A, B, and G subfamilies. The ABCA5-like cluster contains from three to five genes, and a cluster of Abca14, Abca15, Abca16, and Abca17 genes (Ban, Sasaki, Sakai, Ueda, & Inagaki, 2005; Z. Q. Chen, Annilo, Shulenin, & Dean, 2004) is present only in the mouse, rat, and squirrel genomes, not in the guinea pig or kangaroo rat. The well-described duplication of the Abcb1 gene in the mouse and rat genomes is also found in the guinea pig but not in other rodents. The loss of the ABCC11 gene from the mouse genome extends to all rodents, but ABCC11 is present in the Lagomorphs (rabbit, pika), indicating that this gene loss is specific to rodents. Abcg3 is a gene first discovered in the mouse genome closely related to ABCG2, a well-described efflux transporter (Mickley et al., 2001). Abcg3 is only found in rodents, but the rat genome is predicted to have two Abcg3 genes, and the hamster 4–6 copies.
Further examination of additional rodent genomes shows an Abcg3 gene present in the prairie vole and up to four copies in the deer mouse genome. The function of Abcg3 is unknown but proposed to be an efflux pump due to its close sequence homology with ABCG2. However, it is exclusively expressed in the spleen and thymus in the mouse, suggesting it has a role in the immune response (Mickley et al., 2001). In addition, the presence of multiple Abcg3 gene birth events in the rodent lineage suggests that it has an unknown vital function.
There are no other apparent ABC gene death or birth events within other mammalian genomes, and for those mammals with complete genome assemblies, there are 44–54 ABC genes annotated. However, it is difficult to accurately determine the gene counts in the ABCA5 and ABCA14 gene clusters. These clusters contain from 3 to 5 genes in most mammals and pseudogene fragments (Annilo, Chen, Shulenin, & Dean, 2003). Examination of the assemblies in these regions in species with apparently missing genes shows gaps in the assembly. More complete genomes, including long-range sequencing or assembly methods, are needed to resolve these areas. However, we did not search for species for new ABC genes, and there may be yet undiscovered gene birth events.
There has been no previous formal analysis of the ABC gene family for birds, amphibians, or marsupials. The opossum is the index marsupial species with a 7.3x genome coverage and contains 37 predicted full-length and ten partial ABC genes for 47 genes. The opossum appears to be missing ABCA15, 16 and 17, ABCB5 and ABCB13. These same genes were absent from the genomes of other marsupials, the Tasmanian devil, and the wallaby. The frog, Xenopus tropicalis, is an amphibian index species with 37 full and four partially predicted genes. There are two predicted Xenopus ABCB5 genes on separate contigs. An alignment of these sequences shows considerable diversity in well-aligned regions, suggesting that this is an actual duplication. The anole lizard is the one reptile species with a high-density genome assembly (Alfoldi et al., 2011). There are 38 complete and four partial gene annotations for 42 ABC transporters. The lizard and other reptile genomes (snake, turtles, tortoises, tegu lizard, and tuatara) duplicate the ABCG2 gene.
The chicken is the index bird species and has multiple apparent ABC gene loss events, with the genome lacking ABCB12 and ABCB13, ABCD1, and ABCF1. As ABCD1 and ABCF1 are very conserved genes, this is unexpected. ABCD1 and ABCD2 are closely related, and a single ABCD1/2 gene is found in in vertebrates. However, fish have both ABCD1 and ABCD2 orthologs, suggesting that birds lost the ABCD1 gene. In the human genome, the ABCD1 gene is on the X chromosome, and mutations in ABCD1 are responsible for the severe, often lethal, X-linked recessive disease, adrenoleukodystrophy. ABCD1 is expressed in the peroxisome and adrenoleukodystrophy is a demyelinating disorder, but the functional effect of ABCD1 defects in the disease is not clear.
There have been detailed analyses published of the ABC gene superfamily in zebrafish, carp, catfish, and lamprey (S. Liu, Li, & Liu, 2013; X. Liu et al., 2016; Ren et al., 2015). These studies all document multiple gene birth events in fish, such as duplications of ABCA1, ABCA4, ABCB3, ABCB6, ABCB11, ABCC5, ABCC6, ABCG2, and ABCG4. Only the ABCC6 genes have been studied in detail, with the Abcc6a gene shown to be essential and Abcc6b expressed in the developing kidney (Li et al., 2010). As fish underwent a whole-genome duplication, the number of genes that have been retained and now carry out new functions is complex. Some duplications are confined to specific species, such as a duplicated ABCF2 in zebrafish, catfish, and a few other species (S. Liu et al., 2013). Many other examples of lineage-specific duplications and losses in specific fish lineages have been described, and it will require highly accurate genome assemblies to understand the complexity (X. Liu et al., 2016). For example, there are four ABCG2-related genes in the zebrafish, and other fish species have complex combinations of these genes, including additional duplications. As a representative of a more primitive fish species, the lamprey has few of the gene duplications seen in jawed fish and has only 34 predicted ABC genes.
In conclusion, the availability of many vertebrate genome assemblies allows a more detailed analysis of the evolution of ABC transporters. There have been dynamic changes in the gene number in each of the seven common subfamilies, with the most dramatic changes in the A, B, and G subfamilies. Because ABC proteins can carry out a wide variety of transport functions, it is likely that individual lineages of species, and even specific animals, would develop specific transporters for highly specialized functions, probably due to environmental pressure. It is also apparent within the phylogenetic trees of individual genes that considerable diversification has taken place. As even a single amino acid change can alter the substrate specificity of an ABC transporter, the true diversity of substrates is enormous. One of the most diversified sets of genes is the multispecific transporters ABCB1/PGP and ABCG2. This finding is consistent with an essential role for these pumps in xenobiotic elimination and maintaining tissue barriers in the brain, intestine, and placenta. ABCB1 has independently duplicated in several species such as certain rodents, the cow, and fish. Even more dramatic are the duplications of ABCG2 that have taken place in fish species. As fish live in highly diverse aquatic environments, they are exposed internally and externally to an aqueous environment. Therefore, it is not surprising that they need to excrete many environmental toxins and protect internal organs from xenobiotics. For some gene clusters, particularly the ABCA5 and ABCA14 clusters, these genes are challenging to assemble, as the genes are large and closely related. Therefore, the complete annotation will require complete draft genome assemblies.
One of the most intriguing ABC gene subfamilies is the ABCH family. Initially identified in Drosophila and Dictyostelium, ABCH genes are half transporters, with an N-terminal NBD, the same structure as the ABCG genes. Invertebrates, the ABCH genes are only found in fish. There is a single ABCH1 in most fish species and the coelacanth, but the gene is missing from lamprey and other fish species (Jeong et al., 2015). A function in lipid transport has been described for an ABCH gene (LmABCH-9C) in the locust, Locusta migratoria (Yu et al., 2017). Still, to date, there is no functional information on this gene group in vertebrates.
Functional data in the mouse and other model organisms
As nearly all human ABC genes have a one-to-one ortholog in the mouse genome, murine knockouts and other modified alleles represent an excellent source of functional information in animal models for ABC gene diseases. Mutant alleles have been generated for all murine ABC genes allowing for phenotypic screens. Specific phenotypes are reported for 46 of the 53 murine ABC genes (Table 2). The reported phenotypes for mouse ABC genes are highly diverse. They include abnormal lipid, cholesterol, and glucose levels, development of specific organ systems such as the eye, adrenal gland, lung, liver, thyroid, male reproductive tract, heart, spleen, thymus, arteries, lymphocytes, and brain. In mice, disruption (homozygous knockout) of the Abca3, Abcb7, Abcb10, Abce1, and Abcf1 genes results in embryonic lethality, and Cftr/Abcc7, Abcc9, and the trac allele in Abcg5 are associated with premature lethality (Chase et al., 2010).
Table 2.
Mouse gene disruptions and phenotypes of ABC genes
| Symbol | Phenotype | Comment | Reference |
|---|---|---|---|
| Abca1 | HDL deficiency, foam cell accumulation | PMID: 10760292 | |
| HDL cholesterol levels | PMID: 18974039 | ||
| Abca2 | Multiple behavioral/neurological phenotypes | ||
| Decreased body weight | |||
| Abca3 | Abnormal lung development, morphology | ||
| Abca4 | Abnormal dark adaptation, photoreceptor morphology and degeneration | ||
| Vertebral fusion, respiratory quotient | |||
| Abca5 | Abnormal liver morphology and physiology | Lysosomal disease-like symptoms. | PMID: 15870284 |
| Tremors, cardiomyopathy, absent thyroid gland, decreased thyroid activity | |||
| Abca6 | Decreased circulating serum albumin, decreased total protein and increased hematocrit | jax.org/reference/J:211773 | |
| Abca7 | Decreased HDL cholesterol, adipose tissue, kidney size | ||
| Abca8a | nd | 2 null alleles, no reported phenotype | |
| Abca8b | Decreased cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride levels | PMID:28882873 | |
| Abnormal eye morphology | PMID:28882873 | ||
| Abca9 | Abnormal behavioral response to light | Expression in embryo mesenchyme and CNS | jax.org/reference/J:211773 |
| Abca12 | Reddish, scaly, abnormal, tight skin | jax.org/reference/J:161652 | |
| Dehydration, no suckling reflex | jax.org/reference/J:262458 | ||
| Surfactant deficiency, lamellar body, alveolar collapse | jax.org/reference/J:139048 | ||
| Abca13 | Abnormal male genitalia morphology, decrease blood urea nitrogen level | jax.org/reference/J:211773 | |
| Abca14 | Decreased total body fat amount | Expression only in reproductive tract | jax.org/reference/J:211773 |
| Abca15 | nd | Expression only in reproductive tract | |
| Abca16 | nd | Expression only in reproductive tract | |
| Abca17 | Decreased circulating potassium level | jax.org/reference/J:211773 | |
| Abcb1a | Abnormal intestinal epithelium and ulcers, colitis | ||
| Xenobiotic sensitivity | |||
| Hippocampal neuronal degeneration, tremors | |||
| Abnormal immunoglobulin and T cell levels | |||
| Abcb1b | Abnormal xenobiotic sensitivity | ||
| Tap1/Abcb2 | Abnormal T cell levels, antigen presentation | ||
| Tap2/Abcb3 | Abnormal T cell levels, antigen presentation | ||
| Abcb4 | Abnormal bile secretion, bile salt level, gallstones | ||
| Decreased bone mineral density and morphology, calcium level | jax.org/reference/J:199949 | ||
| Liver cirrhosis, | |||
| Abcb5 | Abnormal cornea and retina morphology | jax.org/reference/J:213708 | |
| Abcb6 | Abnormal erythropoiesis, mitochondrial physiology | ||
| Abcb7 | Prenatal, postnatal lethality, hemorrhage, liver morphology | ||
| Abcb8 | Abnormal heart morphology, weight, cardiac output, cardiomyopathy, heart iron levels | ||
| Abcb9 | Small adrenal glands | jax.org/reference/J:211773 | |
| Abcb10 | Embryonic lethal, oxidative stress, abnormal mitochondrial physiology | ||
| Abcb11 | Abnormal bile secretion, bile salt level, enlarged liver, cholestasis | ||
| Abcc1 | Increased sensitivity to xenobiotics | ||
| Decreased inflammatory response, mast cell physiology | |||
| Abcc2 | Abnormal liver weight and physiology, bile secretion and composition | ||
| Increased sensitivity to xenobiotics | |||
| Abcc3 | Abnormal bile salt and bilirubin levels, bile salt homeostasis | ||
| Abnormal xenobiotic pharmacology | |||
| Abcc4 | Abnormal intestinal morphology, mucosa morphology and inflammation | ||
| Small spleen, thymus cortex hypoplasia | |||
| Abnormal blood-brain barrier function | |||
| Abcc5 | Decreased circulating glucose level | ||
| Decreased circulating eosinophils, leukocytes, and lymphocytes | |||
| Limb grasping | |||
| Abcc6 | Calcified arteries and retina, calcified skin, decreased HDL levels | ||
| Abnormal vibrissa follicle morphology | |||
| Cftr/Abcc7 | Abnormal intestinal development, lacrimal gland atrophy, pancreatic atrophy, | ||
| Postnatal lethality | |||
| Impaired fertilization, azoospermia, decreased litter size and sexual maturation | |||
| Abcc8 | Hypoglycemia, abnormal insulin secretion, circulating glucose | ||
| Abcc9 | Premature death | jax.org/reference/J:216539 | |
| Hypoglycemia, increased insulin sensitivity | jax.org/reference/J:71840 | ||
| Hypertension | jax.org/reference/J:78066 | ||
| Abcc10 | Decreased and abnormal spleen, decreased leukocytes and bone marrow cells, and body weight | ||
| Thymus cortex hypoplasia | |||
| Abcc12 | nd | ||
| Abcd1 | Abnormal myelination, brain cell morphology, astrocytosis, axon degeneration, impaired coordination | ||
| Cataract | |||
| Increased fatty acid, levels, lipid homeostasis | |||
| Abnormal adrenal cortex morphology | |||
| Abcd2 | Neuronal and axon degeneration, ataxia, hyperactivity, tremors, microglial cell morphology, posture, coordination | ||
| Abcd3 | Enlarged liver, abnormal bile composition | ||
| Abcd4 | Abnormal startle reflex, response to tactile stimulation, new environment | ||
| Abce1 | Compete embryonic lethal | ||
| Abcf1 | Embryonic lethality prior to jax.organogenesis | jax.org/reference/J:245222 | |
| Abcf2 | nd | ||
| Abcf3 | nd | ||
| Abcg1 | Abnormal blood cell levels, macrophage physiology, cytokine secretion | ||
| Hypoglycemia | |||
| Hyperactivity, increased energy expenditure | |||
| Abcg2 | Increased blood and urine uric acid, | ||
| Abnormal mitochondria morphology and oxidative phoshorylation, porphyria | |||
| Abcg3 | nd | ||
| Abcg4 | Abnormal lipid level | ||
| Abcg5 | Premature death at 4–6 months | trac allele | jax.org/reference/J:157223 |
| Abnormal lipid levels, bile secretion | knockout | jax.org/reference/J:87209 | |
| Male and female infertility | |||
| Abcg8 | Decreased cholesterol levels, circulating triglyceride levels | ||
Available phenotype data from the Mouse Genome Informatics Database (MGI) http://www.informatics.jax.org is shown. nd-not determined.
Many mouse ABC gene alleles recapitulate features of human Mendelian disorders or known human ABC gene function. ABCA1 mutations in humans cause Tangier disease characterized by the defective formation of HDL particles, and Abca1−/− mice also have HDL deficiency. ABCA3 transports lipids into the lung’s lamellar bodies, and mutations cause severe neonatal lung surfactant deficiency; Abca3−/− mice have abnormal lung development and morphology. ABCA4 flips retinoid-lipid complexes in photoreceptor disks (Molday, Zhong, & Quazi, 2009). ABCA4 mutations cause Stargardt disease and related maculopathies, characterized by excessive lipofuscin (A2E) accumulation (Allikmets et al., 1997). Abca4−/− mice also accumulate lipofuscin/A2E, have abnormal dark adaptation, and thinning of the outer nuclear layer (Mata et al., 2001). ABCA12 is expressed in the lamellar granules of the skin, and mutations cause two forms of recessive congenital ichthyosis (Kelsell et al., 2005; Lefevre et al., 2003). Abca12−/− mice also have abnormal scaly skin. Interestingly, Abca12−/− mice also have lung surfactant deficiency, lamellar body abnormalities, and alveolar collapse, demonstrating an essential function in lipid transport in the mouse lung.
ABCB1 encodes P-glycoprotein, an efflux transporter found overexpressed in many chemotherapy multidrug-resistant tumor cell lines and plays a vital role in eliminating xenobiotics. ABCB1−/− collie dogs have sensitivity to ivermectin toxicity (Mealey, Bentjen, Gay, & Cantor, 2001), and Abcb1a/Abcb1b−/− mice have an abnormal distribution of compounds in the brain (Mason, Pariante, & Thomas, 2008). The TAP1 and TAP2 proteins (ABCB2 and ABCB3) together form a transporter for peptides subsequently loaded onto class I HLA molecules, and mutations in either gene cause immunodeficiency in humans, abnormal T cell levels, and antigen presentation in the mouse (de la Salle et al., 1994; Van Kaer, Ashton-Rickardt, Ploegh, & Tonegawa, 1992). ABCB4 transports phospholipids in bile and protein defects lead to recessive intrahepatic cholestasis in both humans and mice. Abcb4−/− mice also have extensive bone developmental abnormalities (Dixon et al., 2000; Hochrath et al., 2013).
ABCC2 transports organic anions, including bile salts, and recessive mutations cause Dubin-Johnson syndrome, a hereditary hyperbilirubinemia (Wada et al., 1998). A rat model, the TR rat, and Abcc2−/− mice have abnormal liver weight and physiology, bile secretion, and composition (Vlaming et al., 2006). ABCC6 mutations cause pseudoxanthoma elasticum, a calcification disorder, and ABCC6 facilitates the release of nucleoside triphosphates, the source of pyrophosphate, and inhibitor of calcification (Bergen et al., 2000; Jansen et al., 2013; Le Saux et al., 2000; K. Moitra et al., 2017). Mouse Abcc6−/− mice have abnormal skin calcification and morphology (Gorgels et al., 2005). CFTR/ABCC7 encodes a chloride ion channel that plays a crucial role in exocrine secretion in the lung, intestine, pancreas, vas deferens, and skin, and recessive mutations cause cystic fibrosis (Anguiano et al., 1992; Quinton, 1999). Multiple Cftr mutations on different mouse backgrounds recapitulate nearly all the features of the human disease (O’Neal et al., 1993; Zeiher et al., 1995). ABCC8 and ABCC9 encode the sulfonylurea receptors SUR and SUR2, and mutations in ABCC8 (Chutkow et al., 2001) in humans cause non-insulin-dependent diabetes (P. M. Thomas et al., 1995). Knockouts of either Abcc8 or Abcc9 cause hypoglycemia and insulin abnormalities, and Abcc9−/− mice also die prematurely (Chutkow et al., 2001; Seghers, Nakazaki, DeMayo, Aguilar-Bryan, & Bryan, 2000).
ABCD1 mutations in humans cause the X-linked adrenoleukodystrophy; Abcd1−/− mice display abnormal myelination, brain cell morphology, astrocytosis, axon degeneration, and impaired coordination. Interestingly, Abcd2−/− mice manifest neuronal and axon degeneration, ataxia, hyperactivity, tremors, abnormal microglial cell morphology, posture, and coordination, suggesting that this protein also functions in the brain (Ferrer et al., 2005). A family with a bile acid synthesis defect and peroxisomal abnormalities displayed mutations in ABCD3(?). In addition, Abcd3−/− mice display increased liver size, abnormal peroxisomes, and a deficit in mature C24 bile acids (Ferdinandusse et al., 2015). ABCG2 is a uric acid transporter, and common variants in the ABCG2 gene are associated with gout, a disorder of blood uric acid levels (Dehghan et al., 2008). Abcg2−/− mice display sensitivity to phytotoxins and increased blood and urine uric acid (Ichida et al., 2012; Jonker et al., 2002). The ABCG5 and ABCG8 genes encode half-transporters that function as a heterodimer and transport phytosterols. Mutations in these genes cause recessive sitosterolemia in humans and abnormal blood lipid and cholesterol levels in the mouse (Chase et al., 2010; Klett et al., 2004).
Many mouse ABC gene mutations are associated with phenotypes likely caused by a defect in cellular transport or provide clues to human gene function. For example, the human ABCA2 gene is highly expressed in the brain and closely related to the known lipid transporters ABCA1, ABCA3, and ABCA4. Disruption of the mouse Abca2 gene leads to multiple behavioral and neurological phenotypes and decreased body weight (Sakai et al., 2007). ABCA7 is also a lipid transporter, and mouse Abca7 disruption mice exhibit reduced HDL cholesterol, adipose tissue, and kidney size (Kim et al., 2005). There is an ABCA5-related gene cluster in all mammals, but the function of these genes is unknown. Abca5−/− mice display abnormal liver morphology and physiology and develop an adult lethal dilated cardiomyopathy-like heart phenotype. The protein locates primarily in the lysosomes (Kubo et al., 2005). Abca6 knockout mice display decreased circulating serum albumin, decreased total protein, and increased hematocrit. Abca8b−/− mice show diminished cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride levels, and humans with ABCA8 mutations and low HDL cholesterol have been described (Trigueros-Motos et al., 2017). Expression of the Abca9 gene is principally in the brain (Piehler, Kaminski, Wenzel, Langmann, & Schmitz, 2002).
The human ABCG1 and ABCG4 genes are evolutionarily related to each other and the sterol transporters ABCG5 and ABCG8, but their function in humans is unknown. The mouse Abcg1 knockout results in the accumulation of neutral lipids and phospholipids in hepatocytes and macrophages and plays a role in loading cholesterol onto HDL particles (Kennedy et al., 2005). Loss of Abcg1 can affect the immune function of macrophages (Wojcik et al., 2008). ABCG4 is highly expressed in the brain, and the Abcg4−/− mouse has abnormal sterol efflux in the brain (Annilo et al., 2001; N. Wang et al., 2008).
Several ABCB family half transporters proteins localize to the mitochondria, and their functions were elucidated from mouse models. In Abcb6−/− mice, the gene is expressed in the mitochondria and transport coproporphyrin III into the mitochondria (Ulrich et al., 2012). The ABCB8 protein localizes to the mitochondria, and Abcb8 disruption leads to defects in mitochondrial iron export, cytosolic Fe/S protein levels, and cardiomyopathy (Ichikawa et al., 2012). In addition, disruption of Abcb10 leads to a lack of heme biosynthesis and erythropoiesis (Yamamoto et al., 2014).
In summary, for nearly all the human ABC genes causing Mendelian disorders, the mouse knockout strains recapitulate one or more phenotypes and serve as valuable models for further studies and development of therapeutics. There are, however, differences in the mouse and human phenotypes. For example, individuals with the PEL-negative blood group phenotype have deletions in the ABCC4 gene and platelet aggregation (Azouzi et al., 2020), a phenotype not observed in the mouse. We have not carried out a detailed analysis of human and mouse ABC gene tissue expression patterns. It should also be pointed out that some mouse knockouts may not produce a phenotype similar to the human gene due to compensation by other transporters. Finally, the disruption of mouse ABC genes of unknown function has ascribed multiple cellular transport properties to several proteins, leading to extensive new knowledge on the full spectrum of ABC transporter efflux properties.
GWAS loci in or near ABC genes
Genome-wide association studies (GWAS) entail the genotyping of large numbers of single nucleotide polymorphisms (SNP) and individuals with (cases) and without (controls) for a specific genetic disease or phenotype. By applying conservative statistical correction and replication in additional datasets, many loci in the human genome are associated with a wide range of conditions. Furthermore, most GWAS loci are in non-coding regions of the genome and often affect the expression of nearby genes (Visscher et al., 2017). To identify common variants of genome-side significance in or near ABC genes that may provide insight into their function, we searched the GWAS catalog, as well as the publicly available data from the UK Biobank study (Table 3).
Table 3.
Genome-wide association loci near human ABC genes
| Symbol | Location | GWAS Phenotype | p-Value | SNP | Allele freq. | OR/effect | Notes | Reference |
|---|---|---|---|---|---|---|---|---|
| ABCA1 | 09q31.1 | HDL-C | 3E-27 | rs1883025 | 0.25 | 2.2mg/dL decrease | Multiple studies | |
| Glaucoma | 2E-19 | rs2472493 | 0.44 | 1.31 | Multiple studies | |||
| ABCA4 | 01p21.3 | Oral Facial Clefts | 3E10–12 | rs560426 | NR | 1.4 | ||
| ABCA7 | 19p13.3 | Alzheimer’s Disease | 2E-09 | rs115550680 | 0.07 | 1.79 | Multiple SNPs and studies | |
| ABCA8 | 17q24.3 | HDL-C | 2E-12 | rs4148008 | 0.33 | 0.028 U decrease | Two studies | |
| ABCB1 | 07q21.12 | Antisocial behavior | 6E-17 | rs4728702 | http://europepmc.org/abstract/MED/25918995 | |||
| ABCB5 | 07p21.1 | Prostate cancer | 5E-13 | rs12155172 | 0.23 | 1.11 | http://europepmc.org/articles/PMC3832790 | |
| ABCC1 | 16p13.12 | Carnitine levels | 1E-25 | rs2062541 | 0.61 | 0.02 U decrease | http://europepmc.org/articles/PMC4064254#SD2 | |
| ABCC4 | 13q32.1 | N-acetylcarnosine levels | 8E-23 | rs8002180 | 0.72 | 0.024 U decrease | http://europepmc.org/articles/PMC4064254#SD2 | |
| Irinotecan response | 2E-07 | rs16950650 | NR | 30 | http://europepmc.org/abstract/MED/23478653 | |||
| ABCC5 | 03q27.1 | Primary angle closure glaucoma | 7E-09 | rs1401999 | 0.41 | 1.13 | Also with orbit depth of the eye | http://europepmc.org/articles/PMC3945113 |
| ABCC9 | 12p12.1 | Hippocampal sclerosis of aging | 1E-09 | rs704178 | 0.49 | 2.13 | http://europepmc.org/articles/PMC4113197 | |
| ABCC11 | 16q12.1 | Fasting plasma glucose | 2E-36 | rs13387347 | NR | 0.11 U increase | G6PC2 gene at this locus | http://europepmc.org/articles/PMC4274808 |
| ABCF1 | 06p21.1 | Bone mineral density | 7E-09 | rs7812088 | 0.13 | Beta 0.04 | Both femoral neck and lumbar spine | http://europepmc.org/articles/PMC3338864 |
| ABCF2 | 07q36.1 | N-Glycosylation of IgG | 2E-10 | rs1122979 | 0.88 | 0.31 U decrease | SMARCD3 gene at the locus | http://europepmc.org/articles/PMC3561084 |
| ABCG2 | 04q22 | Gout, urate levels | 1E-75 | rs2199936 | 0.11 | 18umol/L increase | http://europepmc.org/abstract/MED/24513273 | |
| Statin induces LDL change | 2E-15 | rs1481012 | 0.1 | 5.1% decrease | http://circgenetics.ahajournals.org/content/5/2/257.full | |||
| ABCG5 | 02p21 | Gallstone disease | 1E-14 | rs11887534 | 0.1 | 2.2 | http://www.nature.com/ng/journal/v39/n8/full/ng2101.html | |
| LDL-cholesterol | 4E-72 | rs4299376 | 0.31 | 0.081 unit increase | Several studies, also total cholesterol | http://europepmc.org/abstract/MED/24097068 | ||
| ABCG8 | 02p21 | Gallstone disease | http://www.nature.com/ng/journal/v39/n8/full/ng2101.html | |||||
| LDL-cholesterol | Several studies, also total cholesterol |
GWAS loci with an association above the genome-wide threshold of 5 × 10–8 are shown from the GWAS Catalog, https://www.ebi.ac.uk/gwas. Phenotypes in bold are confirmed from functional studies or consistent with known gene function. NR, not reported
There are several GWAS loci associated with levels of known substrates of ABC transporter genes. The ABCA1 gene is the cause of the recessive Tangier disease, a disorder of cholesterol transport to ApoA1 molecules. An SNP in an intron of ABCA1, rs1883025, is highly associated (<10−50) in multiple studies with HDL and total cholesterol levels and to a lesser extent with triglyceride levels and metabolic syndrome (Spracklen et al., 2017; Willer et al., 2013). However, neither this SNP nor the closely linked rs2575876 are eQTLs for ABCA1, and both have low regulomeDB scores, so the specific mechanism of action of this locus is unclear. Several studies link the ABCB4 gene (a bile acid transporter) to gallstone disease and gallbladder cancer, as well as cholesterol levels (Ferkingstad et al., 2018; Mhatre et al., 2017). The lead SNP for the gallstone association of ABCB4, rs4148808, is in a large LD block in the gene’s promoter, with multiple predicted protein binding sites. Several GWAS studies for gout and uric acid levels identified associations in the ABCG2 gene in diverse populations (Dehghan et al., 2008; Woodward et al., 2009). The association peaks are inside the ABCG2 gene and contain common functional missense and stop-codon variants (Q141K, Q126X) (Ichida, 2009; Matsuo et al., 2009; Woodward et al., 2009). The ABCG5 and ABCG8 genes are closely linked in a head-to-head arrangement. The rs6756629 SNP in an ABCG8 intron is associated with cholesterol levels and gallstone disease. This SNP is in a block of polymorphisms that includes variants with high regulomeDB scores for adipose tissues.
Several additional GWAS loci near ABC genes involve plausible substrates. For example, the rs2062541 SNP in the ABCC1 gene is associated with blood carnitine levels (Shin et al., 2014) and N-acetylcarnosine levels. Response to the irinotecan chemotherapy drug is associated with loci near ABCC4 (Han et al., 2014). A locus in the ABCA6 gene is associated with LDL cholesterol levels and a locus in ABCA8 with HDL cholesterol (Surakka et al., 2015; Willer et al., 2013). Therefore, several phenotypes associated with variants in and near multiple ABC genes could be the basis for further functional follow-up studies.
Several associations linked to ABC genes may provide novel insight into human phenotypes. Multiple SNPs near the ABCA1 gene have been linked to primary open angle glaucoma in independent studies (Y. Chen et al., 2014), but a functional role for ABCA1 in glaucoma has not been established. A consistent association to prostate cancer is found about 350kb 3’ to the ABCB5 gene; however, the associated SNPs are in LINC01162 gene (Eeles et al., 2009). Functional analysis would need to be performed to show that the SNPs in these loci indeed regulate that ABC transporter and not another gene in the region.
The spectrum of rare variation in humans
Through interrogation of the gnomAD database (Karczewski, 2019) of publicly available exome and whole genome sequence, the number of synonymous (SYN), non-synonymous (NS), and loss-of-function (LOF) variants were tabulated (Figure 1, Supp Table S2). In addition, we determined the number of individuals homozygous for LOF variants and adjusted for gene size. Therefore, this data can provide information on how essential a gene is by lower-than-expected levels of LOF mutations, rate of LOF variants per gene. The ratio of NS/SYN variants provides an estimate of the proportion of variants in the gene that potentially alter function. The frequency of NS and LOF variants per 1000AA varies widely by gene, with half transporters displaying 0–67 LOF/1000AA (mean 27); and full transporters 18–56 (mean 32). For comparison, a set of conserved housekeeping genes (DHFR, GAPDH, ACTB, RPL19, LDHA) shows 9.7 LOF/1000 and tumor suppressor genes (PTEN, RB1, TP53, PTCH1, VHL) 8.1 (Supp. Table S2). To compare the LOF/1000 AA values within the ABC gene family we calculated Z scores for each gene. The essential, X-linked genes (ABCD1, ABCB7) and the essential ABCA3 and ABCE1 genes all have Z scores < −1. That is, they are greater than 1 standard deviation below the mean. Interestingly, ABCC7/CFTR and ABCG2 have Z scores of 1.8 and 2.5, indicating that they have an excess of loss of function mutations.
Figure 1. Rate of loss-of-function mutations.
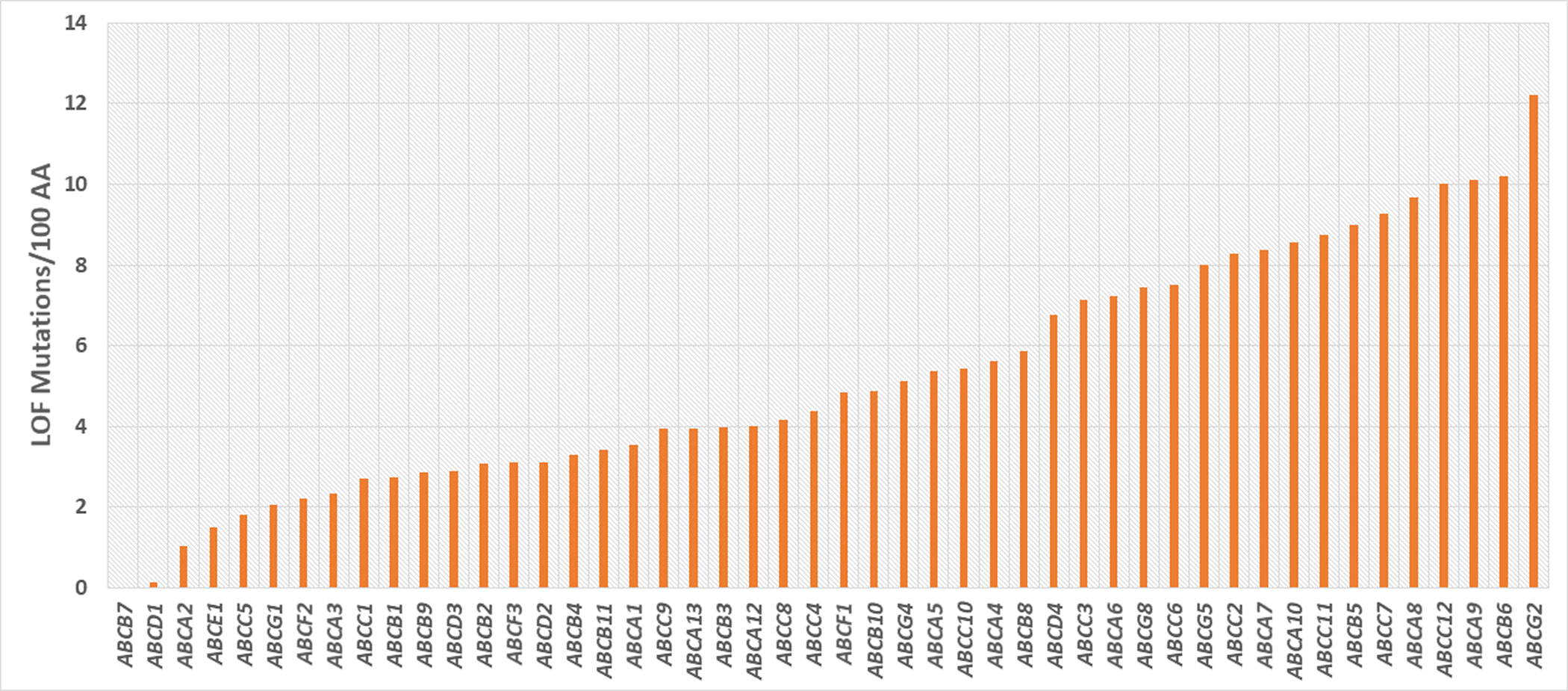
The number of loss-of-function mutations in the gnomAD database of exome and whole-genome sequences is shown. The values are adjusted for gene size. The ABCB7 and ABCD1 genes are on the X-chromosome.
Interestingly the ABCA2, ABCB1, ABCC5, and ABCG1 also have low levels of LOF variants, suggesting that they do not tolerate loss-of-function. This result is particularly surprising for ABCB1 where knockout alleles in the mouse, rat, and dog are viable (Robey et al., 2018). Genes with a higher rate of NS and LOF variants include ABCC7/CFTR and ABCG2. Several groups have proposed a heterozygous advantage for LOF alleles of CFTR to account for the high frequency of cystic fibrosis mutations in multiple populations (Angelicheva et al., 1994; Bosch et al., 2017; Prince, 1998). Furthermore, the increased blood uric acid levels in ABCG2 mutant carriers may also have a selective advantage against infectious diseases.
Analysis of specific predicted LOF mutations showed that all the cytoplasmic ABC genes (ABCE1, ABCF1, ABCF2, and ABCF3) have no reported homozygotes for LOF mutations, suggesting that they are essential. Similarly, almost all the half transporters (except for ABCB6 and ABCG2) also have no homozygous LOF individuals (Supp. Table S2). In contrast, nearly half (46%) of all ABC full-transporters have at least 1 LOF allele reported to be homozygous in at least one individual (Figure 2). Interestingly some predicted LOF variants are frequent, including in some specific populations. For example, several genes in the ABCA5 gene cluster (ABCA6, ABCA8, ABCA10) have common LOF alleles in Latin American and African populations. ABCA7, ABCA13, ABCB5, ABCC11, and ABCC12 also have specific common predicted LOF variants. Some of the predicted LOF alleles are in splice sites, and it may be that they can produce a functional protein by alternative splicing.
Figure 2. Genes with individuals homozygous for loss-of-function mutations.
The number of full-transporters (FT) with at least one individual with loss-of-function mutations is shown to that of other genes (half-transporters and cytoplasmic). * P-value less than 0.05 (Actual P-value 0.012).
Somatic mutations in cancer
Many ABC genes are efflux transporters involved in the resistance to multiple chemotherapy drugs. However, there are no ABC transporters amongst the 299 documented frequently mutated genes in cancer (Bailey et al., 2018). Therefore, to determine the potential role of somatic mutations in ABC genes in cancer, we tabulated the number and class of modifications along with the rate of gene amplification, overexpression, or gene fusion (Suppl. Table S3).
Nearly all ABC genes have low levels of somatic mutations and the only observed gene fusions involve ABCB1 (Christie et al., 2019) (Huff, Lee, Robey, & Fojo, 2006). The most frequently mutated ABC gene in cancer is ABCA13, but this is a large gene with low expression and late replication timing. However, neither ABCA13 nor any other ABC gene passes the criteria for a significantly mutated gene in cancer. Common recurrent missense mutations are found in many oncogenes and are usually associated with a gain of function. There are a few common recurrent mutations such as p.R1476Q in ABCA5(c.4427G>A), p.S422F (c.1265C>T) in ABCC7/CFTR, and p.S606P (C1816T>C) and p.G608D (C1823G>A) in ABCD1. The ABCD1 mutations are found primarily in lung cancer and the conserved LSGG motif in the ATP-binding domain. This data suggests that ABCD1 may play a role in a subset of lung malignancies.
Several ABC genes have frequent copy number gains (>10%) in specific cancers. The amplifications include ABCB10 in liver cancer, ABCC4 in colon cancer, ABCC9 in testicular cancer, and ABCC5 and ABCF3 in cervical cancer. The role of these events in these cancers is unknown. Independent confirmation of ABCF3 overexpressed in cervical cancer has been performed (Choi et al., 2007). ABCC5 and ABCF3 are in the region of chromosome 3q that includes the PIK3CA oncogene, frequently mutated and present in 3 or more copies in cervical tumors (Lou et al., 2015). In addition, there is an elevated expression of many ABC genes >30% of specific tumors. For example, ABCA7 and ABCC8 expressed are high in 33% of adrenal tumors, ABCC5 in 45% of esophageal cancers, and ABCF3 in 45% of cervical cancers. Specific ABC genes have been demonstrated to be over-expressed in select cancers. For example, ABCC5 is overexpressed and a driver of subtypes of medulloblastoma (Wijaya et al., 2020) and several efflux transporters are overexpressed, associated with poor outcome, and drug resistance in acute myelogenous leukemia (Barghout et al., 2021; B. Liu et al., 2018) Whether these over-expressed genes contribute to drug resistance or other phenotypes of these cancers remains undetermined.
Conclusions
Analysis of the presence or absence of a gene in the ABC transporter superfamily across vertebrates demonstrates a tremendous diversity in potential efflux functions. Nearly all the ABC genes have been disrupted in the mouse, and most display a phenotype, often including lipid and cholesterol transport, blood chemistry, developmental or neurological abnormalities, or lethality. Several polymorphisms in or near human ABC genes are genome-wide associated with either known important human traits or new phenotypes. For example, there is an association of polymorphisms in the ABCA5 gene cluster with HDL- and LDL-cholesterol levels providing strong evidence that one or more genes in this locus are essential for cholesterol homeostasis. Somatic mutations in ABC transporters do not seem to be a significant mechanism of carcinogenesis; however, several genes are amplified or overexpressed in specific tumor types, suggesting future research areas. Finally, several ABC genes have deficient levels of loss-of-function mutation and are likely to be essential genes. In contrast, at least three genes (CFTR, ABCG2, and ABCG8) have an apparent excess of non-synonymous or loss-of-function mutations. Future genomic, genetic, and functional studies of these transporters across vertebrate species will likely yield important new insights into biology and disease.
Supplementary Material
Acknowledgements
We thank colleagues at the FEBS ABC Special Meeting for helpful discussions. Supported, in part, by the NIH/NEI grants R01EY028203, R01EY028954, R01EY029315, P30EY019007 (Core grant), Foundation Fighting Blindness award PPA-1218-0751-COLU, and the Unrestricted funds from the Research to Prevent Blindness (RPB) to the Department of Ophthalmology, Columbia University, New York, NY, USA and funded in part by the Intramural Program of the NIH.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Data Availability Statement
All data used in the manuscript are from publicly available sources.
References
- Alfoldi J, Di Palma F, Grabherr M, Williams C, Kong L, Mauceli E, … Lindblad-Toh K (2011). The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature, 477(7366), 587–591. doi: 10.1038/nature10390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, … Lupski JR (1997). A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nature Genetics, 15, 236–246. [DOI] [PubMed] [Google Scholar]
- Angelicheva D, Boteva K, Jordanova A, Savov A, Kufardjieva A, Tolun A, … et al. (1994). Cystic fibrosis patients from the Black Sea region: the 1677delTA mutation. Hum Mutat, 3(4), 353–357. [DOI] [PubMed] [Google Scholar]
- Anguiano A, Oates RD, Amos JA, Dean M, Gerrard B, Stewart C, … Milunsky A (1992). Congenital bilateral absence of the vas deferens. A primarily genital form of cystic fibrosis. JAMA, 267(13), 1794–1797. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.ncbi.nlm.nih.gov/htbin-post/Omim/getmim%3ffield=medline_uid&search=1545465 [PubMed] [Google Scholar]
- Annilo T, Chen ZQ, Shulenin S, & Dean M (2003). Evolutionary analysis of a cluster of ATP-binding cassette (ABC) genes. Mamm Genome, 14(1), 20. Retrieved from file:///C|/Documents%20and%20Settings/dean/Local%20Settings/Application%20Data/Quosa/Data/My%20Citations/12532264.qpw [DOI] [PubMed] [Google Scholar]
- Annilo T, & Dean M (2004). Degeneration of an ATP-binding cassette transporter gene, ABCC13, in different mammalian lineages. Genomics, 84(1), 34–46. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15203202 [DOI] [PubMed] [Google Scholar]
- Annilo T, Tammur J, Hutchinson A, Rzhetsky A, Dean M, & Allikmets R (2001). Human and mouse orthologs of a new ATP-binding cassette gene, ABCG4. Cytogenet Cell Genet, 94(3–4), 196–201. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.karger.com/journals/ccg/ccg_jh.htm [DOI] [PubMed] [Google Scholar]
- Arnould I, Schriml L, Prades C, Lachtermacher-Triunfol M, Schneider T, Maintoux C, … Dean M (2001). Identification and characterization of a cluster of five new ATP-Binding Cassette transporter genes on human chromosome 17q24: A new sub-group within the ABCA sub-family. GeneScreen, 1, 157–164. [Google Scholar]
- Azouzi S, Mikdar M, Hermand P, Gautier EF, Salnot V, Willemetz A, … Peyrard T (2020). Lack of the multidrug transporter MRP4/ABCC4 defines the PEL-negative blood group and impairs platelet aggregation. Blood, 135(6), 441–448. doi: 10.1182/blood.2019002320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, … Ding L (2018). Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell, 173(2), 371–385 e318. doi: 10.1016/j.cell.2018.02.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakos E, Evers R, Calenda G, Tusnady GE, Szakacs G, Varadi A, & Sarkadi B (2000). Characterization of the amino-terminal regions in the human multidrug resistance protein (MRP1). J Cell Sci, 113(Pt), 4451–4461. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.biologists.com/JCS/113/24/jcs2013.html [DOI] [PubMed] [Google Scholar]
- Ban N, Sasaki M, Sakai H, Ueda K, & Inagaki N (2005). Cloning of ABCA17, a novel rodent sperm-specific ABC (ATP-binding cassette) transporter that regulates intracellular lipid metabolism. Biochem J, 389(Pt 2), 577–585. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15810880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghout SH, Aman A, Nouri K, Blatman Z, Arevalo K, Thomas GE, … Schimmer AD (2021). A genome-wide CRISPR/Cas9 screen in acute myeloid leukemia cells identifies regulators of TAK-243 sensitivity. JCI Insight, 6(5). doi: 10.1172/jci.insight.141518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, … Tontonoz P (2008). LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell, 134(1), 97–111. doi: 10.1016/j.cell.2008.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, … de Jong PT (2000). Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet, 25(2), 228–231. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.ncbi.nlm.nih.gov/htbin-post/Omim/getmim%3ffield=medline_uid&search=10835643 [DOI] [PubMed] [Google Scholar]
- Borst P (1999). Multidrug resistance: a solvable problem? Ann Oncol, 10 Suppl 4, 162–164. [PubMed] [Google Scholar]
- Bosch L, Bosch B, De Boeck K, Nawrot T, Meyts I, Vanneste D, … da Silva Filho L (2017). Cystic fibrosis carriership and tuberculosis: hints toward an evolutionary selective advantage based on data from the Brazilian territory. BMC Infect Dis, 17(1), 340. doi: 10.1186/s12879-017-2448-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broccardo C, Osorio J, Luciani MF, Schriml L, Prades C, Shulenin S, … Chimini G (2001). Comparative analysis of promoter structure and genomic organization of human and mouse ABCA7, a novel ABCA transporter. Biochim. Biophys. ACTA, 92, 264–270. [DOI] [PubMed] [Google Scholar]
- Buch S, Schafmayer C, Volzke H, Becker C, Franke A, von Eller-Eberstein H, … Hampe J (2007). A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat Genet, 39(8), 995–999. doi: 10.1038/ng2101 [DOI] [PubMed] [Google Scholar]
- Chase TH, Lyons BL, Bronson RT, Foreman O, Donahue LR, Burzenski LM, … Shultz LD (2010). The mouse mutation “thrombocytopenia and cardiomyopathy” (trac) disrupts Abcg5: a spontaneous single gene model for human hereditary phytosterolemia/sitosterolemia. Blood, 115(6), 1267–1276. doi: 10.1182/blood-2009-05-219808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheepala SB, Pitre A, Fukuda Y, Takenaka K, Zhang Y, Wang Y, … Schuetz JD (2015). The ABCC4 membrane transporter modulates platelet aggregation. Blood, 126(20), 2307–2319. doi: 10.1182/blood-2014-08-595942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Rossier C, Lalioti MD, Lynn A, Chakravarti A, Perrin G, & Antonarakis SE (1996). Cloning of the cDNA for a human homologue of the Drosophila white gene and mapping to chromosome 21q22.3. Am J Hum Genet, 59(1), 66–75. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.ncbi.nlm.nih.gov/htbin-post/Omim/getmim%3ffield=medline_uid&search=8659545 [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lin Y, Vithana EN, Jia L, Zuo X, Wong TY, … Yang Z (2014). Common variants near ABCA1 and in PMM2 are associated with primary open-angle glaucoma. Nat Genet, 46(10), 1115–1119. doi: 10.1038/ng.3078 [DOI] [PubMed] [Google Scholar]
- Chen ZQ, Annilo T, Shulenin S, & Dean M (2004). Three ATP-binding cassette transporter genes, Abca14, Abca15, and Abca16, form a cluster on mouse Chromosome 7F3. Mamm Genome, 15(5), 335–343. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15170222 [DOI] [PubMed] [Google Scholar]
- Childs S, & Ling V (1994). The MDR superfamily of genes and its biological implications. Important Adv Oncol, 21–36. [PubMed] [Google Scholar]
- Choi YW, Bae SM, Kim YW, Lee HN, Kim YW, Park TC, … Ahn WS (2007). Gene expression profiles in squamous cell cervical carcinoma using array-based comparative genomic hybridization analysis. Int J Gynecol Cancer, 17(3), 687–696. doi: 10.1111/j.1525-1438.2007.00834.x [DOI] [PubMed] [Google Scholar]
- Christie EL, Pattnaik S, Beach J, Copeland A, Rashoo N, Fereday S, … Bowtell DDL (2019). Multiple ABCB1 transcriptional fusions in drug resistant high-grade serous ovarian and breast cancer. Nat Commun, 10(1), 1295. doi: 10.1038/s41467-019-09312-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, & Burant CF (2001). Disruption of Sur2-containing K(ATP) channels enhances insulin- stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci U S A, 98(20), 11760–11764. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.pnas.org/cgi/content/full/98/20/11760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SP, & Deeley RG (1998). Multidrug resistance mediated by the ATP-binding cassette transporter protein MRP. Bioessays, 20(11), 931–940. [DOI] [PubMed] [Google Scholar]
- Cole SPC, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, … Deeley RG (1992). Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science (Washington, DC), 258, 1650–1654. [DOI] [PubMed] [Google Scholar]
- de la Salle H, Hanau D, Fricker D, Urlacher A, Kelly A, Salamero J, … et al. (1994). Homozygous human TAP peptide transporter mutation in HLA class I deficiency. Science, 265(5169), 237–241. [DOI] [PubMed] [Google Scholar]
- de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, … Hadchouel M (1998). Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci U S A, 95(1), 282–287. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.ncbi.nlm.nih.gov/htbin-post/Omim/getmim%3ffield=medline_uid&search=9419367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M (2002). The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Bethesda, MD: National Center for Biotechnology Information (US). [Google Scholar]
- Dean M, & Annilo T (2005). Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet, 6, 123–142. doi: 10.1146/annurev.genom.6.080604.162122 [DOI] [PubMed] [Google Scholar]
- Dean M, Hamon Y, & Chimini G (2001). The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res, 42(7), 1007–1017. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.jlr.org/cgi/content/abstract/42/7/1007 [PubMed] [Google Scholar]
- Dean M, Rzhetsky A, & Allikmets R (2001). The human ATP-binding cassette (ABC) transporter superfamily. Genome Res, 11(7), 1156–1166. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.genome.org/cgi/content/abstract/11/7/1156 [DOI] [PubMed] [Google Scholar]
- Dehghan A, Kottgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, … Fox CS (2008). Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet, 372(9654), 1953–1961. doi: 10.1016/S0140-6736(08)61343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon PH, Weerasekera N, Linton KJ, Donaldson O, Chambers J, Egginton E, … Williamson C (2000). Heterozygous MDR3 missense mutation associated with intrahepatic cholestasis of pregnancy: evidence for a defect in protein trafficking. Hum Mol Genet, 9(8), 1209–1217. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.ncbi.nlm.nih.gov/htbin-post/Omim/getmim%3ffield=medline_uid&search=10767346 [DOI] [PubMed] [Google Scholar]
- http://www.oup.co.uk/hmg/Volume_09/Issue_08/ddd138_gml.abs.html .
- Dooher JE, & Lingappa JR (2004). Conservation of a stepwise, energy-sensitive pathway involving HP68 for assembly of primate lentivirus capsids in cells. J Virol, 78(4), 1645–1656. doi: 10.1128/jvi.78.4.1645-1656.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, … Easton DF (2009). Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet, 41(10), 1116–1121. doi: 10.1038/ng.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinandusse S, Jimenez-Sanchez G, Koster J, Denis S, Van Roermund CW, Silva-Zolezzi I, … Valle D (2015). A novel bile acid biosynthesis defect due to a deficiency of peroxisomal ABCD3. Hum Mol Genet, 24(2), 361–370. doi: 10.1093/hmg/ddu448 [DOI] [PubMed] [Google Scholar]
- Ferkingstad E, Oddsson A, Gretarsdottir S, Benonisdottir S, Thorleifsson G, Deaton AM, … Stefansson K (2018). Genome-wide association meta-analysis yields 20 loci associated with gallstone disease. Nat Commun, 9(1), 5101. doi: 10.1038/s41467-018-07460-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Kapfhammer JP, Hindelang C, Kemp S, Troffer-Charlier N, Broccoli V, … Pujol A (2005). Inactivation of the peroxisomal ABCD2 transporter in the mouse leads to late-onset ataxia involving mitochondria, Golgi and endoplasmic reticulum damage. Hum Mol Genet, 14(23), 3565–3577. doi: 10.1093/hmg/ddi384 [DOI] [PubMed] [Google Scholar]
- Gorgels TG, Hu X, Scheffer GL, van der Wal AC, Toonstra J, de Jong PT, … Bergen AA (2005). Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet, 14(13), 1763–1773. doi: 10.1093/hmg/ddi183 [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, & Bates SE (2002). Multidrug resistance in cancer: role of ATP-dependent transporters. Nature Rev Cancer, 2(1), 48–58. [DOI] [PubMed] [Google Scholar]
- Groen A, Romero MR, Kunne C, Hoosdally SJ, Dixon PH, Wooding C, … Oude Elferink RP (2011). Complementary functions of the flippase ATP8B1 and the floppase ABCB4 in maintaining canalicular membrane integrity. Gastroenterology, 141(5), 1927–1937 e1921–1924. doi: 10.1053/j.gastro.2011.07.042 [DOI] [PubMed] [Google Scholar]
- Guo F, Ding Y, Caberoy N, Alvarado G, Wang F, Chen R, & Li W (2015). ABCF1 extrinsically regulates retinal pigment epithelial cell phagocytosis. Mol Biol Cell, 26(12), 2311–2320. doi: 10.1091/mbc.E14-09-1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JY, Lee YS, Shin ES, Hwang JA, Nam S, Hong SH, … Lee JS (2014). A genome-wide association study of survival in small-cell lung cancer patients treated with irinotecan plus cisplatin chemotherapy. Pharmacogenomics J, 14(1), 20–27. doi: 10.1038/tpj.2013.7 [DOI] [PubMed] [Google Scholar]
- Hartz AM, & Bauer B (2011). ABC transporters in the CNS - an inventory. Curr Pharm Biotechnol, 12(4), 656–673. doi: 10.2174/138920111795164020 [DOI] [PubMed] [Google Scholar]
- Helias V, Saison C, Ballif BA, Peyrard T, Takahashi J, Takahashi H, … Arnaud L (2012). ABCB6 is dispensable for erythropoiesis and specifies the new blood group system Langereis. Nat Genet, 44(2), 170–173. doi: 10.1038/ng.1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CF (1992). ABC transporters: From micro-organisms to man. Annu. Rev. Cell. Biol, 8, 67–113. [DOI] [PubMed] [Google Scholar]
- Hochrath K, Ehnert S, Ackert-Bicknell CL, Lau Y, Schmid A, Krawczyk M, … Nussler AK (2013). Modeling hepatic osteodystrophy in Abcb4 deficient mice. Bone, 55(2), 501–511. doi: 10.1016/j.bone.2013.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff LM, Lee JS, Robey RW, & Fojo T (2006). Characterization of gene rearrangements leading to activation of MDR-1. J Biol Chem, 281(48), 36501–36509. doi: 10.1074/jbc.M602998200 [DOI] [PubMed] [Google Scholar]
- Hyde SC, Emsley P, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, … Higgins CF (1990). Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature, 346(6282), 362–365. [DOI] [PubMed] [Google Scholar]
- Iannelli F, Collino A, Sinha S, Radaelli E, Nicoli P, D’Antiga L, … Ciccarelli FD (2014). Massive gene amplification drives paediatric hepatocellular carcinoma caused by bile salt export pump deficiency. Nat Commun, 5, 3850. doi: 10.1038/ncomms4850 [DOI] [PubMed] [Google Scholar]
- Ichida K (2009). What lies behind serum urate concentration? Insights from genetic and genomic studies. Genome Med, 1(12), 118. doi: 10.1186/gm118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida K, Matsuo H, Takada T, Nakayama A, Murakami K, Shimizu T, … Suzuki H (2012). Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun, 3, 764. doi: 10.1038/ncomms1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa Y, Bayeva M, Ghanefar M, Potini V, Sun L, Mutharasan RK, … Ardehali H (2012). Disruption of ATP-binding cassette B8 in mice leads to cardiomyopathy through a decrease in mitochondrial iron export. Proc Natl Acad Sci U S A, 109(11), 4152–4157. doi: 10.1073/pnas.1119338109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RS, Kucukosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IE, … van de Wetering K (2013). ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Natl Acad Sci U S A, 110(50), 20206–20211. doi: 10.1073/pnas.1319582110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong CB, Kim BM, Kang HM, Choi IY, Rhee JS, & Lee JS (2015). Marine medaka ATP-binding cassette (ABC) superfamily and new insight into teleost Abch nomenclature. Sci Rep, 5, 15409. doi: 10.1038/srep15409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PM, & George AM (2004). The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol Life Sci, 61(6), 682–699. doi: 10.1007/s00018-003-3336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, … Schinkel AH (2002). The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A, 99(24), 15649–15654. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12429862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski KJ et al. (2019). Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv. doi: 10.1101/531210 [DOI] [Google Scholar]
- Kawaguchi K, & Morita M (2016). ABC Transporter Subfamily D: Distinct Differences in Behavior between ABCD1–3 and ABCD4 in Subcellular Localization, Function, and Human Disease. Biomed Res Int, 2016, 6786245. doi: 10.1155/2016/6786245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsell DP, Norgett EE, Unsworth H, Teh MT, Cullup T, Mein CA, … O’Toole EA (2005). Mutations in ABCA12 underlie the severe congenital skin disease harlequin ichthyosis. Am J Hum Genet, 76(5), 794–803. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15756637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, … Edwards PA (2005). ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab, 1(2), 121–131. doi: 10.1016/j.cmet.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Kim WS, Fitzgerald ML, Kang K, Okuhira K, Bell SA, Manning JJ, … Freeman MW (2005). Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J Biol Chem, 280(5), 3989–3995. doi: 10.1074/jbc.M412602200 [DOI] [PubMed] [Google Scholar]
- Kiss K, Brozik A, Kucsma N, Toth A, Gera M, Berry L, … Szakacs G (2012). Shifting the paradigm: the putative mitochondrial protein ABCB6 resides in the lysosomes of cells and in the plasma membrane of erythrocytes. PLoS One, 7(5), e37378. doi: 10.1371/journal.pone.0037378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klett EL, Lu K, Kosters A, Vink E, Lee MH, Altenburg M, … Patel SB (2004). A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med, 2, 5. doi: 10.1186/1741-7015-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken J, Buchler C, Orso E, Kaminski WE, Porsch-Ozcurumez M, Liebisch G, … Schmitz G (2000). ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc Natl Acad Sci U S A, 97(2), 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, … Thompson RJ (2006). Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology, 44(2), 478–486. doi: 10.1002/hep.21287 [DOI] [PubMed] [Google Scholar]
- Kubo Y, Sekiya S, Ohigashi M, Takenaka C, Tamura K, Nada S, … Yamaguchi A (2005). ABCA5 resides in lysosomes, and ABCA5 knockout mice develop lysosomal disease-like symptoms. Mol Cell Biol, 25(10), 4138–4149. doi: 10.1128/MCB.25.10.4138-4149.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, … Boyd CD (2000). Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet, 25(2), 223–227. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.ncbi.nlm.nih.gov/htbin-post/Omim/getmim%3ffield=medline_uid&search=10835642 [DOI] [PubMed] [Google Scholar]
- Lee JY, Kinch LN, Borek DM, Wang J, Wang J, Urbatsch IL, … Rosenbaum DM (2016). Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature, 533(7604), 561–564. doi: 10.1038/nature17666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre C, Audebert S, Jobard F, Bouadjar B, Lakhdar H, Boughdene-Stambouli O, … Fischer J (2003). Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum Mol Genet, 12(18), 2369–2378. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12915478 [DOI] [PubMed] [Google Scholar]
- Li Q, Jiang Q, Pfendner E, Varadi A, & Uitto J (2009). Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol, 18(1), 1–11. doi: 10.1111/j.1600-0625.2008.00795.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sadowski S, Frank M, Chai C, Varadi A, Ho SY, … Uitto J (2010). The abcc6a gene expression is required for normal zebrafish development. J Invest Dermatol, 130(11), 2561–2568. doi: 10.1038/jid.2010.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesa M, Qiu W, & Shirihai OS (2012). Mitochondrial ABC transporters function: the role of ABCB10 (ABC-me) as a novel player in cellular handling of reactive oxygen species. Biochim Biophys Acta, 1823(10), 1945–1957. doi: 10.1016/j.bbamcr.2012.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Li LJ, Gong X, Zhang W, Zhang H, & Zhao L (2018). Co-expression of ATP binding cassette transporters is associated with poor prognosis in acute myeloid leukemia. Oncol Lett, 15(5), 6671–6677. doi: 10.3892/ol.2018.8095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Li Q, & Liu Z (2013). Genome-wide identification, characterization and phylogenetic analysis of 50 catfish ATP-binding cassette (ABC) transporter genes. PLoS One, 8(5), e63895. doi: 10.1371/journal.pone.0063895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li S, Peng W, Feng S, Feng J, Mahboob S, … Xu P (2016). Genome-Wide Identification, Characterization and Phylogenetic Analysis of ATP-Binding Cassette (ABC) Transporter Genes in Common Carp (Cyprinus carpio). PLoS One, 11(4), e0153246. doi: 10.1371/journal.pone.0153246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Villagran G, Boland JF, Im KM, Polo S, Zhou W, … Dean M (2015). Genome Analysis of Latin American Cervical Cancer: Frequent Activation of the PIK3CA Pathway. Clin Cancer Res, 21(23), 5360–5370. doi: 10.1158/1078-0432.CCR-14-1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubelski J, Konings WN, & Driessen AJ (2007). Distribution and physiology of ABC-type transporters contributing to multidrug resistance in bacteria. Microbiol Mol Biol Rev, 71(3), 463–476. doi: 10.1128/MMBR.00001-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranghi M, Truglio G, Gallo A, Grieco E, Verrienti A, Montali A, … Lucarelli M (2019). A novel splicing mutation in the ABCA1 gene, causing Tangier disease and familial HDL deficiency in a large family. Biochem Biophys Res Commun, 508(2), 487–493. doi: 10.1016/j.bbrc.2018.11.064 [DOI] [PubMed] [Google Scholar]
- Marton MJ, Vazquez de Aldana CR, Qiu H, Chakraburtty K, & Hinnebusch AG (1997). Evidence that GCN1 and GCN20, translational regulators of GCN4, function on elongating ribosomes in activation of eIF2alpha kinase GCN2. Mol Cell Biol, 17(8), 4474–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BL, Pariante CM, & Thomas SA (2008). A revised role for P-glycoprotein in the brain distribution of dexamethasone, cortisol, and corticosterone in wild-type and ABCB1A/B-deficient mice. Endocrinology, 149(10), 5244–5253. doi: 10.1210/en.2008-0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Tzekov RT, Liu X, Weng J, Birch DG, & Travis GH (2001). Delayed dark-adaptation and lipofuscin accumulation in abcr+/− mice: implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Vis Sci, 42(8), 1685–1690. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.iovs.org/cgi/content/abstract/42/8/1685 [PubMed] [Google Scholar]
- Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Ikebuchi Y, … Shinomiya N (2009). Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med, 1(5), 5ra11. doi: 10.1126/scitranslmed.3000237 [DOI] [PubMed] [Google Scholar]
- Mealey KL, Bentjen SA, Gay JM, & Cantor GH (2001). Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics, 11(8), 727–733. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.ncbi.nlm.nih.gov/htbin-post/Omim/getmim%3ffield=medline_uid&search=11692082 [DOI] [PubMed] [Google Scholar]
- Mhatre S, Wang Z, Nagrani R, Badwe R, Chiplunkar S, Mittal B, … Rajaraman P (2017). Common genetic variation and risk of gallbladder cancer in India: a case-control genome-wide association study. Lancet Oncol, 18(4), 535–544. doi: 10.1016/S1470-2045(17)30167-5 [DOI] [PubMed] [Google Scholar]
- Mickley L, Jain P, Miyake K, Schriml LM, Rao K, Fojo T, … Dean M (2001). An ATP-binding cassette gene (ABCG3) closely related to the multidrug transporter ABCG2 (MXR/ABCP) has an unusual ATP-binding domain. Mammalian Genome, 12, 86–88. [DOI] [PubMed] [Google Scholar]
- Mihalyi C, Iordanov I, Torocsik B, & Csanady L (2020). Simple binding of protein kinase A prior to phosphorylation allows CFTR anion channels to be opened by nucleotides. Proc Natl Acad Sci U S A, 117(35), 21740–21746. doi: 10.1073/pnas.2007910117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milinkovitch MC, Helaers R, Depiereux E, Tzika AC, & Gabaldon T (2010). 2x genomes--depth does matter. Genome Biol, 11(2), R16. doi: 10.1186/gb-2010-11-2-r16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra K (2012). ABC transporters in human disease (Vol. 1): Morgan & Claypool Life Sciences. [Google Scholar]
- Moitra K, Garcia S, Jaldin M, Etoundi C, Cooper D, Roland A, … Dean M (2017). ABCC6 and Pseudoxanthoma Elasticum: The Face of a Rare Disease from Genetics to Advocacy. Int J Mol Sci, 18(7). doi: 10.3390/ijms18071488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra K, Silverton L, Limpert K, Im K, & Dean M (2011). Moving out: from sterol transport to drug resistance - the ABCG subfamily of efflux pumps. Drug Metabol Drug Interact, 26(3), 105–111. doi: 10.1515/DMDI.2011.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, Zhong M, & Quazi F (2009). The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim Biophys Acta, 1791(7), 573–583. doi: 10.1016/j.bbalip.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, & Imanaka T (2012). Peroxisomal ABC transporters: structure, function and role in disease. Biochim Biophys Acta, 1822(9), 1387–1396. doi: 10.1016/j.bbadis.2012.02.009 [DOI] [PubMed] [Google Scholar]
- Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser H, … Aubourg P (1993). Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature, 361(6414), 726–730. [DOI] [PubMed] [Google Scholar]
- Myers JL (2017). Mutants of Drosophila Melanogaster’s ABC Transporter, White, Have Altered Cholesterol-Mediated Acquisition of Memory that is Moderated with 5-HT. Universuty of Houston, Retrieved from https://uh-ir.tdl.org/handle/10657/2885 [Google Scholar]
- O’Neal WK, Hasty P, McCray PB Jr., Casey B, Rivera-Perez J, Welsh MJ, … Bradley A (1993). A severe phenotype in mice with a duplication of exon 3 in the cystic fibrosis locus. Hum Mol Genet, 2(10), 1561–1569. doi: 10.1093/hmg/2.10.1561 [DOI] [PubMed] [Google Scholar]
- Piehler A, Kaminski WE, Wenzel JJ, Langmann T, & Schmitz G (2002). Molecular structure of a novel cholesterol-responsive A subclass ABC transporter, ABCA9. Biochem Biophys Res Commun, 295(2), 408–416. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.idealibrary.com/links/citation/0006-291X/295/408 [DOI] [PubMed] [Google Scholar]
- Powis SH, Rosenberg WM, Hall M, Mockridge I, Tonks S, Ivinson A, … et al. (1993). TAP1 and TAP2 polymorphism in coeliac disease. Immunogenetics, 38(5), 345–350. doi: 10.1007/bf00210476 [DOI] [PubMed] [Google Scholar]
- Prince A (1998). The CFTR advantage--capitalizing on a quirk of fate. Nat Med, 4(6), 663–664. doi: 10.1038/nm0698-663 [DOI] [PubMed] [Google Scholar]
- Qian Y, Wang G, Xue F, Chen L, Wang Y, Tang L, & Yang H (2017). Genetic association between TAP1 and TAP2 polymorphisms and ankylosing spondylitis: a systematic review and meta-analysis. Inflamm Res, 66(8), 653–661. doi: 10.1007/s00011-017-1047-1 [DOI] [PubMed] [Google Scholar]
- Quinton PM (1999). Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev, 79(1 Suppl), S3–S22. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://physrev.physiology.org/cgi/content/full/79/1/S3 [DOI] [PubMed] [Google Scholar]
- http://physrev.physiology.org/cgi/content/full/79/1/S3 .
- Rau H, Nicolay A, Usadel KH, Finke R, Donner H, Walfish PG, & Badenhoop K (1997). Polymorphisms of TAP1 and TAP2 genes in Graves’ disease. Tissue Antigens, 49(1), 16–22. doi: 10.1111/j.1399-0039.1997.tb02704.x [DOI] [PubMed] [Google Scholar]
- Remaley AT, Rust S, Rosier M, Knapper C, Naudin L, Broccardo C, … Brewer HB Jr. (1999). Human ATP-binding cassette transporter 1 (ABC1): genomic organization and identification of the genetic defect in the original Tangier disease kindred. Proc Natl Acad Sci U S A, 96(22), 12685–12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Chung-Davidson YW, Yeh CY, Scott C, Brown T, & Li W (2015). Genome-wide analysis of the ATP-binding cassette (ABC) transporter gene family in sea lamprey and Japanese lamprey. BMC Genomics, 16, 436. doi: 10.1186/s12864-015-1677-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, & Gottesman MM (2018). Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer, 18(7), 452–464. doi: 10.1038/s41568-018-0005-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, … et al. (1989). Identification of the cystic fibrosis gene: chromosome walking and jumping. Science, 245(4922), 1059–1065. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.ncbi.nlm.nih.gov/htbin-post/Omim/getmim%3ffield=medline_uid&search=2772657 [DOI] [PubMed] [Google Scholar]
- Roninson IB, Chin JE, Choi K, Gros P, Housman DE, Fojo A, … Pastan I (1986). Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc. Natl. Acad. Sci. USA, 83, 4538–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, … Assmann G (1999). Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet, 22(4), 352–355. [DOI] [PubMed] [Google Scholar]
- Sakai H, Tanaka Y, Tanaka M, Ban N, Yamada K, Matsumura Y, … Inagaki N (2007). ABCA2 deficiency results in abnormal sphingolipid metabolism in mouse brain. J Biol Chem, 282(27), 19692–19699. doi: 10.1074/jbc.M611056200 [DOI] [PubMed] [Google Scholar]
- Schaedler TA, Faust B, Shintre CA, Carpenter EP, Srinivasan V, van Veen HW, & Balk J (2015). Structures and functions of mitochondrial ABC transporters. Biochem Soc Trans, 43(5), 943–951. doi: 10.1042/BST20150118 [DOI] [PubMed] [Google Scholar]
- Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, & Bryan J (2000). Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem, 275(13), 9270–9277. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.jbc.org/cgi/content/abstract/275/13/9270 [DOI] [PubMed] [Google Scholar]
- Shimada BK, Pomozi V, Zoll J, Kuo S, Martin L, & Le Saux O (2021). ABCC6, Pyrophosphate and Ectopic Calcification: Therapeutic Solutions. Int J Mol Sci, 22(9). doi: 10.3390/ijms22094555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, … Soranzo N (2014). An atlas of genetic influences on human blood metabolites. Nat Genet, 46(6), 543–550. doi: 10.1038/ng.2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KD, Kemp S, Braiterman LT, Lu JF, Wei HM, Geraghty M, … Watkins PA (1999). X-linked adrenoleukodystrophy: genes, mutations, and phenotypes. Neurochem Res, 24(4), 521–535. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10227685 [DOI] [PubMed] [Google Scholar]
- Spracklen CN, Chen P, Kim YJ, Wang X, Cai H, Li S, … Sim X (2017). Association analyses of East Asian individuals and trans-ancestry analyses with European individuals reveal new loci associated with cholesterol and triglyceride levels. Hum Mol Genet, 26(9), 1770–1784. doi: 10.1093/hmg/ddx062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikant S (2020). Evolutionary history of ATP-binding cassette proteins. FEBS Lett, 594(23), 3882–3897. doi: 10.1002/1873-3468.13985 [DOI] [PubMed] [Google Scholar]
- Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, … Thompson RJ (1998). A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet, 20(3), 233–238. doi: 10.1038/3034 [DOI] [PubMed] [Google Scholar]
- Surakka I, Horikoshi M, Magi R, Sarin AP, Mahajan A, Lagou V, … Consortium, E. (2015). The impact of low-frequency and rare variants on lipid levels. Nat Genet, 47(6), 589–597. doi: 10.1038/ng.3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakacs G, & Abele R (2020). An inventory of lysosomal ABC transporters. FEBS Lett, 594(23), 3965–3985. doi: 10.1002/1873-3468.13967 [DOI] [PubMed] [Google Scholar]
- Thomas C, Aller SG, Beis K, Carpenter EP, Chang G, Chen L, … Tampe R (2020). Structural and functional diversity calls for a new classification of ABC transporters. FEBS Lett, 594(23), 3767–3775. doi: 10.1002/1873-3468.13935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, & Tampe R (2020). Structural and Mechanistic Principles of ABC Transporters. Annu Rev Biochem, 89, 605–636. doi: 10.1146/annurev-biochem-011520-105201 [DOI] [PubMed] [Google Scholar]
- Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, Rabl W, … Bryan J (1995). Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science, 268(5209), 426–429. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.ncbi.nlm.nih.gov/htbin-post/Omim/getmim%3ffield=medline_uid&search=7716548 [DOI] [PubMed] [Google Scholar]
- Trigueros-Motos L, van Capelleveen JC, Torta F, Castano D, Zhang LH, Chai EC, … Singaraja RR (2017). ABCA8 Regulates Cholesterol Efflux and High-Density Lipoprotein Cholesterol Levels. Arterioscler Thromb Vasc Biol, 37(11), 2147–2155. doi: 10.1161/ATVBAHA.117.309574 [DOI] [PubMed] [Google Scholar]
- Tusnady GE, Sarkadi B, Simon I, & Varadi A (2006). Membrane topology of human ABC proteins. FEBS Lett, 580(4), 1017–1022. doi: 10.1016/j.febslet.2005.11.040 [DOI] [PubMed] [Google Scholar]
- Tyzack JK, Wang X, Belsham GJ, & Proud CG (2000). ABC50 interacts with eukaryotic initiation factor 2 and associates with the ribosome in an ATP-dependent manner. J Biol Chem, 275(44), 34131–34139. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.jbc.org/cgi/content/full/275/44/34131 [DOI] [PubMed] [Google Scholar]
- Ulrich DL, Lynch J, Wang Y, Fukuda Y, Nachagari D, Du G, … Schuetz JD (2012). ATP-dependent mitochondrial porphyrin importer ABCB6 protects against phenylhydrazine toxicity. J Biol Chem, 287(16), 12679–12690. doi: 10.1074/jbc.M111.336180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kaer L, Ashton-Rickardt PG, Ploegh HL, & Tonegawa S (1992). TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4–8+ T cells. Cell, 71(7), 1205–1214. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, & Yang J (2017). 10 Years of GWAS Discovery: Biology, Function, and Translation. Am J Hum Genet, 101(1), 5–22. doi: 10.1016/j.ajhg.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaming ML, Mohrmann K, Wagenaar E, de Waart DR, Elferink RP, Lagas JS, … Schinkel AH (2006). Carcinogen and anticancer drug transport by Mrp2 in vivo: studies using Mrp2 (Abcc2) knockout mice. J Pharmacol Exp Ther, 318(1), 319–327. doi: 10.1124/jpet.106.101774 [DOI] [PubMed] [Google Scholar]
- Wada M, Toh S, Taniguchi K, Nakamura T, Uchiumi T, Kohno K, … Kuwano M (1998). Mutations in the canilicular multispecific organic anion transporter (cMOAT) gene, a novel ABC transporter, in patients with hyperbilirubinemia II/Dubin-Johnson syndrome. Hum Mol Genet, 7(2), 203–207. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.ncbi.nlm.nih.gov/htbin-post/Omim/getmim%3ffield=medline_uid&search=9425227 [DOI] [PubMed] [Google Scholar]
- http://www.oup.co.uk/hmg/Volume_07/Issue_02/ddb032_gml.abs.html .
- Wang N, Lan D, Chen W, Matsuura F, & Tall AR (2004). ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A, 101(26), 9774–9779. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15210959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Yvan-Charvet L, Lutjohann D, Mulder M, Vanmierlo T, Kim TW, & Tall AR (2008). ATP-binding cassette transporters G1 and G4 mediate cholesterol and desmosterol efflux to HDL and regulate sterol accumulation in the brain. FASEB J, 22(4), 1073–1082. doi: 10.1096/fj.07-9944com [DOI] [PubMed] [Google Scholar]
- Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, … Ling V (2001). Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A, 98(4), 2011–2016. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.ncbi.nlm.nih.gov/htbin-post/Omim/getmim%3ffield=medline_uid&search=11172067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijaya J, Vo BT, Liu J, Xu B, Wu G, Wang Y, … Schuetz JD (2020). An ABC Transporter Drives Medulloblastoma Pathogenesis by Regulating Sonic Hedgehog Signaling. Cancer Res, 80(7), 1524–1537. doi: 10.1158/0008-5472.CAN-19-2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox SM, Arora H, Munro L, Xin J, Fenninger F, Johnson LA, … Jefferies WA (2017). The role of the innate immune response regulatory gene ABCF1 in mammalian embryogenesis and development. PLoS One, 12(5), e0175918. doi: 10.1371/journal.pone.0175918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, … Global Lipids Genetics, C. (2013). Discovery and refinement of loci associated with lipid levels. Nat Genet, 45(11), 1274–1283. doi: 10.1038/ng.2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik AJ, Skaflen MD, Srinivasan S, & Hedrick CC (2008). A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol, 180(6), 4273–4282. doi: 10.4049/jimmunol.180.6.4273 [DOI] [PubMed] [Google Scholar]
- Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, & Kottgen M (2009). Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A, 106(25), 10338–10342. doi: 10.1073/pnas.0901249106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Arimura H, Fukushige T, Minami K, Nishizawa Y, Tanimoto A, … Furukawa T (2014). Abcb10 role in heme biosynthesis in vivo: Abcb10 knockout in mice causes anemia with protoporphyrin IX and iron accumulation. Mol Cell Biol, 34(6), 1077–1084. doi: 10.1128/MCB.00865-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Wang Y, Zhao X, Liu X, Ma E, Moussian B, & Zhang J (2017). The ABC transporter ABCH-9C is needed for cuticle barrier construction in Locusta migratoria. Insect Biochem Mol Biol, 87, 90–99. doi: 10.1016/j.ibmb.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Yuan M, Wang W, Chen H, Lu J, He M, Liu C, … Wang L (2016). ABCC4, ABCC5 and SLC28A1 polymorphisms: host genome on responses of chronic hepatitis B patients with entecavir treatment. Antivir Ther, 21(8), 689–696. doi: 10.3851/IMP3063 [DOI] [PubMed] [Google Scholar]
- Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, McCray PB Jr., … Thomas KR (1995). A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest, 96(4), 2051–2064. doi: 10.1172/JCI118253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman C, Klein KC, Kiser PK, Singh AR, Firestein BL, Riba SC, & Lingappa JR (2002). Identification of a host protein essential for assembly of immature HIV-1 capsids. Nature, 415(6867), 88–92. doi: 10.1038/415088a [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in the manuscript are from publicly available sources.


