Abstract
Background:
Blepharospasm is one of the most common subtypes of dystonia, and often spreads to other body regions. Despite published guidelines, the approach to diagnosis and classification of affected body regions varies among clinicians.
Objective:
To delineate the clinical features used by movement disorder specialists in the diagnosis and classification of blepharospasm according to body regions affected, and to develop recommendations for a more consistent approach.
Methods:
Cross-sectional data for subjects diagnosed with all types of isolated dystonia were acquired from the Dystonia Coalition, an international, multicenter collaborative research network. Data were evaluated to determine how examinations recorded by movement disorder specialists were used to classify blepharospasm as focal, segmental, or multifocal.
Results:
Among all 3222 participants with isolated dystonia, 210 (6.5%) had a diagnosis of focal blepharospasm. Among these 210 participants, 34 (16.2%) had dystonia outside of upper face region. Factors such as dystonia severity across different body regions and number of body regions affected influenced the classification of blepharospasm as focal, segmental, or multifocal.
Conclusions:
Although focal blepharospasm is the second most common type of dystonia, a high percentage of individuals given this diagnosis had dystonia outside of the eye/upper face region. These findings are not consistent with existing guidelines for the diagnosis and classification of focal blepharospasm, and point to the need for more specific guidelines for more consistent application of existing recommendations for diagnosis and classification.
Keywords: Blepharospasm, Craniofacial dystonia, Meige syndrome
1. Introduction
Blepharospasm (BSP) is among the most common of the focal dystonias. Overall, half of all BSP subjects experience spread over a period of five years.1–8 The most common site of spread is the oromandibular region, but other regions may also be involved. This spread has an enormous impact on functional disability and treatment strategies.8, 9 Spread also has an important impact on studies of the biology of BSP, because those with and without spread may be more likely to have abnormal imaging or genetic results.10
According to existing recommendations for the classification of the dystonias into relevant subgroups, one of the most important features involves describing the regions of the body affected.11, 12 Focal dystonia refers to involvement of only one body region, segmental dystonia refers to involvement of at least two contiguous body regions, multifocal dystonia refers to involvement of multiple non-contiguous body regions, and generalized dystonia refers to involvement of the trunk and at least two other body regions. Although this aspect of the published guidelines for classifying dystonia seems very straightforward, recent studies have suggested they are not consistently applied.13–16 The lack of consistency may reflect disagreement with these guidelines or different interpretations of designations as focal, segmental, and multifocal.
In view of the importance of spread in BSP, the current study focussed on how clinical specialists in movement disorders describe this spread, and particularly the use of existing guidelines for classifying BSP according to body regions affected as focal, segmental, or multifocal. The goal was not to describe the temporal or anatomical aspects of spread, but rather to examine how the spread is described after it has occurred. Diagnostic criteria for BSP have been proposed, with key features being bilateral spasms of the orbicularis oculi and nearby muscles of the upper face often with excessive blinking.17, 18 However, these diagnostic criteria do not address the classification of BSP according to other body regions that are frequently also affected with dystonia. Data collected by the Dystonia Coalition were evaluated to reveal differences in classification of BSP by movement disorder specialists and used to generate empirical recommendations for more consistent approach to classification according to body region.
2. Methods
For these analyses, data were obtained from the Dystonia Coalition Natural History Project, and included 3222 subjects recruited by 58 different movement disorder specialists working at 41 differrent sites located in North America, Europe, and Australia. A description of the Dystonia Coalition, and some clinical details regarding the cohort studied have been published.7, 19 Adults >17 years of age with all types of isolated (primary) dystonia were included. Subjects were excluded if they had evidence for acquired dystonia, such as neuroleptic exposure, brain lesions, or other causes. They were also excluded if dystonia was related to another disorder, such as Parkinson’s disease. Subjects undergoing treatment with botulinum toxin were recruited only after symptoms returned, usually at least 3 months after their last treatment. Written informed consent was obtained at the recruiting site for all subjects, according to the Declaration of Helsinki and The Common Rule.
All subjects were evaluated by a movement disorder specialist using the same protocol, and all data were entered into a central database. The Dystonia Coalition Natural History Project is ongoing, and the current study included data collected between 19 January 2011 and 14 July 2020. For subjects with more than one evaluation, the current study used only the data from the most recent visit. Analysis of de-identified data was approved by the Emory University Human Subjects Review Board.
Although the Dystonia Coalition includes all types of isolated dystonias, the current study focused on BSP. Participants with BSP were identified using three independent methods. First, the examination protocol included a checklist that listed all body regions and asked whether dystonia was present or not. Second, the examination required the investigator to assess severity in each body region using the Global Dystonia Rating Scale (GDRS), a semiquantitative Likert-like scale rated from 0 (no dystonia) to 10 (extreme dystonia).17, 20 For both the exam checklist and the GDRS, the upper and lower face are separate items. Third, the examination required the movement disorder specialist to give an overal diagnosis using published guidelines for classifying dystonia as focal, segmental, multifocal, or generalized.11 The availability of these three different types of data enabled a comparison of how the diagnosis and classification compared to exam features on a case-by-case basis. Summary statistics are provided as average values ± standard deviations, and groups were compared via Student’s t-tests.
3. Results
3.1. Cohort studied
After elimination of 16 participants with incomplete data, information was available for 3222 unique individuals with isolated dystonia (Table 1). Clinical details regarding these cases have been presented elsewhere.8 Among these 3222 individuals, 214 (6.6%) had a diagnosis of focal BSP. Among these 214 participants, 210 (98.1%) had involvement of the eye region on the examination checklist, confirming the diagnosis of BSP. The remaining 4 participants were excluded due to insufficient data recorded in the exam checklist or GDRS to confirm the diagnosis, leaving 210 focal BSP participants for further analyses.
Table 1.
Descriptive statistics for subjects studied.
| Exam Checklist Showing Upper Face Only (N=146) | GDRS Scores Non-Zero for Upper Face only (N=161) | Diagnosis = Focal Dystonia, Upper Face (N=214) | |
|---|---|---|---|
| Gender | |||
| Females | 96 (65.6%) | 112 (70.0%) | 144 (67.3%) |
| Males | 50 (34.2%) | 49 (30.0%) | 70 (32.7%) |
| Age | |||
| At recruitment | 59±0.8 (36–86) | 59±1.0 (36–87) | 59.0±1.0 (28–87) |
| At dystonia onset | 56.0±2.2 (3–82) | 56.0±2.6 (3–82) | 56±2.6 (3–82) |
| Duration of illness | 11.3±9.1 (0–60) | 11.2±9.4 (0–60) | 11.7±10.3 (0–61) |
| Race | |||
| White | 128 (88.9) | 142 (88.2%) | 190(88.8%) |
| Black | 8 (5.6%) | 8 (5.0%) | 9 (4.2%) |
| Asian | 3 (2.1%) | 5 (3.1%) | 5 (2.3%) |
| Other | 4 (2.8%) | 5 (3.1%) | 9 (4.2%) |
| Unknown | 1 (0.7%) | 1 (0.6%) | 1 (0.5%) |
Among 3222 total subjects with isolated dystonia, 3 different sources of information suggested different numbers of participants with isolated focal blepharospasm (exam=146, GDRS=161, diagnosis=214). Age, age of onset, and disease duration are reported as mean ± SD (range).
3.2. Discrepancies in use of terms focal, segmental, and multifocal
Among the 210 subjects diagnosed with focal BSP, 34 (16.2%) had examination checklists that included the upper face, as well as at least one other body region (Table 2). The diagnoses for these 34 individuals conflict with current guidelines, which state that only one body region should be involved in focal dystonia.11 The most common region affected in these 34 participants was the lower face (N=26). This finding implies that some movement disorder specialists consider lower face involvement to be compatible with a diagnosis of focal BSP.
Table 2.
Regions involved among 210 subjects diagnosed with focal BSP.
| Regions affected | Number of participants (%) | |
|---|---|---|
| Based on exam checklist | Based on GDRS scores | |
| Focal BSP with only upper face involvement | 172 (81.9) | 152 (72.4) |
| Focal BSP with upper face and at least one other body region | 34 (16.2) | 52 (24.8) |
| UF+LF | 26 (12.4) | 37 (17.6) |
| UF+NE | 21 (10.0) | 27 (12.9) |
| UF+L | 6 (2.9) | 6 (2.9) |
| UF+SH/PA | 7 (3.3) | 4 (1.9) |
| UF+H/DA/EL | 1 (0.5) | 5 (2.4) |
| UF+UL/PE | 1 (0.5) | 1 (0.5) |
| UF+FT/DL | 1 (0.5) | 1 (0.5) |
| UF+TR | 1 (0.5) | 2 (1.0) |
| UF+J+T | 0 (0.0) | 3 (1.4) |
UF: upper face, LF: lower face, NE: neck, L: larynx, SH: shoulder, PA: proximal arm, H: hand, DA: distal arm, EL: elbow, UL: upper leg, PE: pelvis, FT: foot, DL: distal leg, TR: trunk, J: jaw, T: toungue
To assess this hypothesis, the examination checklist was reviewed for all 3222 participants where the only regions affected were both upper face and lower face, regardless of the diagnosis applied. Among 71 participants meeting these criteria, 3 were excluded because involvement of both regions could not be confirmed with GDRS scores, and 3 participants were excluded because of data entry errors. Of the remaining 65 participants, 56 (86.1%) had a diagnosis of focal dystonia. The Dystonia Coalition database permitted participants of focal dystonia that included BSP to be described in different ways including focal dystonia of the upper face, focal cranial dystonia involving the upper and lower face, or segmental dystonia. Among these 65 participants with upper and lower facial involvement, 46 (70.8%) were given a diagnosis of focal cranial dystonia of the upper and lower face, 10 (15.4%) were given a diagnosis of focal dystonia with involvement of the upper face, 9 (13.8%) were given a diagnosis of segmental dystonia (Table 3). Although errors in entering the diagnosis given cannot be entirely excluded, these results imply that involvement of the upper and/or lower face is used variably by movement disorder specialists when classifying dystonia as focal or segmental.
Table 3.
Anatomical classification vs body regions affected.
| Body region affected per exam (N=3222) | Focal (%) | Focal Cranial (upper&lower face)(%) | Segmental (%) | Multifocal (%) |
|---|---|---|---|---|
| UF+LF (N=65) | 15.4 | 70.8 | 13.8 | 0.0 |
| UF+LF+NE (N=70) | 30.0 | 0.0 | 60.0 | 10.0 |
| UF+LF+J+NE (N=16) | 18.8 | 0.0 | 75.0 | 1.0 |
| UF+NE (N=63) | 42.9 | 0.0 | 47.6 | 9.5 |
Abbreviations: UF: upper face, LF: lower face, NE: neck, J: jaw
The examination checklist also had 77 participants where upper face, lower face, and neck were involved, a combination that should be considered segmental dystonia according to current guidelines.11 Among these participants, 7 were excluded because GDRS scores were zero for one of the regions. Among the remaining 70 participants, 42 (60.0%) were given a diagnosis of segmental dystonia, 21 (30.0%) were given a diagnosis of focal dystonia, and 7 (10.0%) were given a diagnosis of multifocal dystonia (Table 3). The examination checklist also included 16 participants that included upper face, lower face, jaw, and neck. Of these 16 participants, 12 (75%) were given a diagnosis of segmental dystonia, 3 (18.8%) were given a diagnosis of focal dystonia (2 upper and lower face, 1 OMD), and 1 (6.3%) was given a diagnosis of multifocal dystonia (Table 3). These results again reveal significant variations in the application of guidelines among movement disorder specialists for using the classification terms of focal, segmental, or multifocal.
Among the 210 subjects diagnosed with focal BSP, the second most common region affected after lower face was the neck (N=21). According to current guidelines, this combination should be considered multifocal dystonia if only upper face and neck are involved, but segmental dystonia if the lower face and/or oromandibular regions are involved.11 To delineate the diagnostic labels given to subjects with upper face and neck, we first examined examination checklists for all 3222 participants to identify participants where only the upper face and neck were involved. Among 67 participants meeting these criteria, 4 participants were excluded because they could not be confirmed via GDRS scores. Of the remaining 63 participants, 30 (47.6%) were given a diagnosis of segmental dystonia, 27 (42.9%) were given a diagnosis of focal dystonia (22 with focal CD and 5 with focal BSP), and 6 (9.5%) were given a diagnosis of multifocal dystonia. These results provide further evidence for varied use of the terms focal, segmental, and multifocal (Table 3).
To address the hypothesis that these discrepancies might reflect differences in opinion or different interpretations of published guidelines, all authors of this manuscript were asked to classify individuals with varied combinations of dystonia in the upper face, lower face, jaw, tongue, and neck. There was full agreement when only one body region was involved, but different labels were applied for various combinations (Table 4).
Table 4.
Survey of authors regarding anatomical classification.
| BODY REGION | FOCAL | SEGMENTAL | MULTIFOCAL |
|---|---|---|---|
| UF | 12 | 0 | 0 |
| LF | 12 | 0 | 0 |
| UF+LF | 2 | 10 | 0 |
| UF+NE | 0 | 2 | 10 |
| LF+NE | 0 | 12 | 0 |
| UF+LF+NE | 0 | 12 | 0 |
| J | 12 | 0 | 0 |
| T | 12 | 0 | 0 |
| J+T | 8 | 4 | 0 |
| UF+J | 2 | 7 | 3 |
| LF+J | 6 | 6 | 0 |
| UF+T | 3 | 6 | 3 |
| LF+T | 8 | 4 | 0 |
| UF+LF+J | 2 | 10 | 0 |
| UF+LF+T | 2 | 10 | 0 |
| UF+LF+J+T | 2 | 10 | 0 |
| UF+LF+J+NE | 0 | 12 | 0 |
| UF+LF+T+NE | 0 | 12 | 0 |
| UF+LF+J+T+NE | 0 | 12 | 0 |
Abbreviations: UF: upper face, LF: lower face, NE: neck, J: jaw, T: tongue
Most authors preferred the term “segmental” when upper and lower face were involved, but some preferred the term “focal”. In contrast, most authors preferred to group all oromandibular regions (lower face, jaw, and tongue) together as focal dystonia, rather than segmental dystonia. These findings confirm that differences in diagnosis and classification of BSP at least partly reflect differences of opinion or different interpretations of published guidelines.
3.3. The influence of severity on use of terms focal, segmental, and multifocal
One possible explanation for varied use of terminology is that movement disorder specialists may be considering the severity of dystonia in different body regions when making assessments, and ignoring areas where severity is minimal. To test this hypothesis, GDRS scores were compared for subjects who had both upper and lower face involvement on exam checklist. For subjects diagnosed with focal dystonia of the upper face, GDRS scores were significantly higher (p<0.002) for upper face (5.2±2.2) compared to lower face (2.1±1.4). However, for participants given a diagnosis of segmental dystonia, GDRS scores for upper face (4.4±2.1) and lower face (3.6±1.4) were not signifantly different (p>0.3) (Figure 1A). These results imply that some movement disorder specialists provide a diagnosis of focal dystonia when one body region is more severely affected, and a diagnosis of segmental dystonia when the severity of dystonia is more similar across contiguous body regions.
Figure 1.
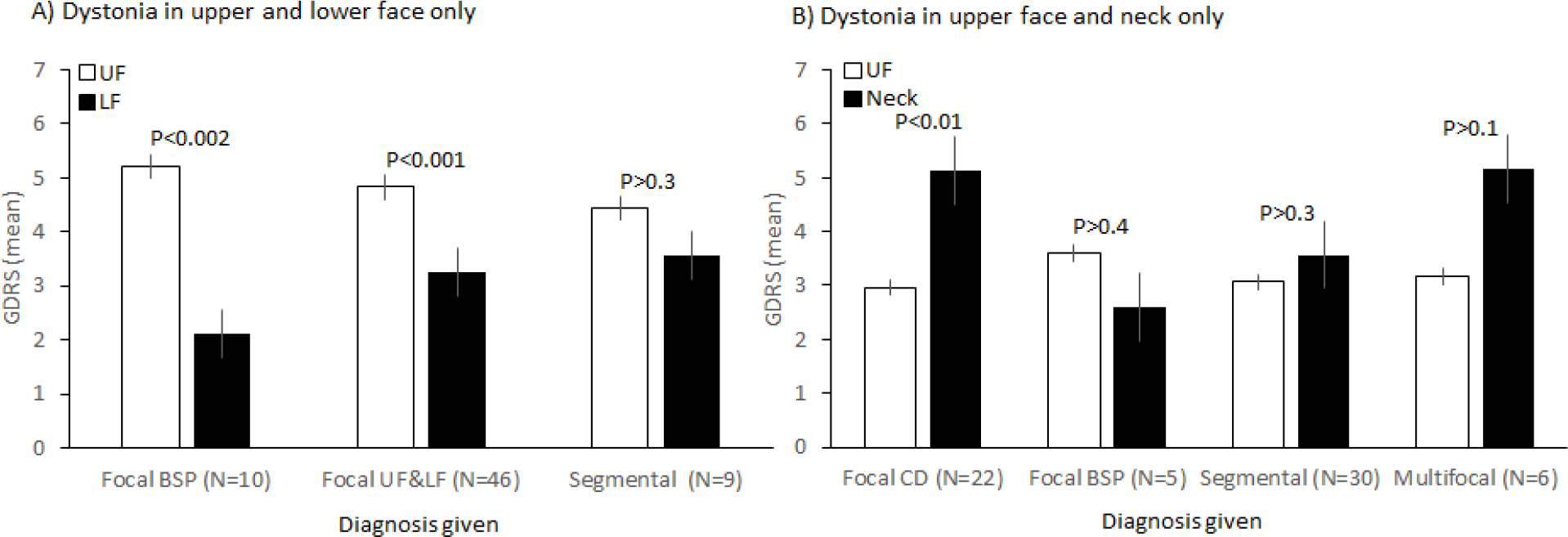
Impact of Severity on Classification of BSP. A) Global dystonia rating scale (GDRS) scores for subjects with dystonia of upper face (UF) and lower face (LF) only. B) GDRS scores for subjects with dystonia of upper face and neck only.
To explore this hypothesis further, GDRS scores were also evaluated for 63 subjects who had only upper face and neck involvement. For participants diagnosed with focal CD, GDRS scores were significantly higher (p<0.001) for neck (5.1±2.0) compared to upper face (3.0±1.8). For participants diagnosed with focal BSP, GDRS scores trended higher for face (3.6±2.1) compared with neck (2.6±1.1), but the difference was not significant (p>0.4). GDRS scores were similar for face and neck for individuals diagnosed with segmental or multifocal dystonia (Figure 1B). These results again imply that the relative severity of dystonia in different body regions was influencing the application of labels of focal, segmental, or multifocal dystonia.
4. Discussion
Most epidemiological studies indicate that BSP is the second most common of the adult-onset focal dystonias.21 BSP spreads from the orbicularis oculi and nearby muscles of the upper face to other regions in approximately half of all affected individuals.1–8 The most common sites for spread involve the oromandibular region (lower face, jaw, and tongue), although further spread to the neck and other regions may also occur. In keeping with these observations, many prior studies addressing BSP include high proportions of subjects with dystonia outside of the upper face region. Guidelines have been published for the diagnosis of BSP,17, 18 and for classifying patterns that may be focal, segmental, or multifocal.11, 12 The most important finding from the current study is that the published guidelines are not consistently applied, even by highly trained specialists in movement disorders.
There are several potential reasons for the inconsistent use of classification guidelines for BSP including data entry errors, lack of expertise with these guidelines, a preference to focus on the most severely affected region or the region being treated, differences in opinion, and differences in the interpretation of published guidelines. Among these possibilities, data entry errors are not likely to be a major contributor, because many could be detected and excluded by searching for discrepancies in 3 independent but similar sources of information regarding the diagnosis. Lack of familiarity with diagnostic guidelines also seems unlikely because participants were recruited by experienced movement disorder specialists. Assessment of GDRS scores suggested that diagnostic labels were at least partly influenced by the severity of dystonia in specific regions, even though severity is not a factor in published guidelines for classification. For example an investigator may give a diagnosis of focal BSP because the GDRS score for upper face was 8, when the lower face or neck may have a score of only 2. The results also revealed differences in opinion or interpretation of diagnostic guidelines. For example, some investigators view the common combination of blepharospasm and oromandibular dystonia to be a focal cranial dystonia, while others prefer to view it as a segmental dystonia affecting two separate but contiguous regions of the face. Regardless of the many reasons that may account for the variations in the application of diagnostic guidelines in BSP, the results indicate a need for more specific guidance, similar to recent studies for cervical dystonia.16
We propose that the diagnosis of focal BSP be based on dystonic spasms of muscles in the upper face around the eyes only. This recommendation is in keeping with existing guidelines for the diagnosis of BSP,17 its classification according to body regions affected,11 and the opinion of the majority of the investigators in this study (Table 4). In keeping with the distribution of body regions in the GDRS and Burke-Fahn-Marsden dystonia rating scales, the term segmental dystonia should be used when BSP is combined with oromandibular dystonia, defined by involvement of the lower face, jaw, or tongue. Individuals with BSP often have cervical dystonia too. The combinaton of BSP plus cervical dystonia should also be labeled segmental dystonia if there also is oromandibular involvement, or multifocal dystonia if the oromandibular region is not affected. A summary of recommendations for the most commonly encountered combinations involving BSP is provided in Table 5.
Table 5.
Recommendations for classification of blepharospasm according to affected body regions. Oromandibular region includes lower face, jaw, and tongue.
| Diagnosis | Body regions involved |
|---|---|
| Focal blepharospasm | UF only |
| Segmental dystonia with blepharospasm | UF and OMD region |
| UF, OMD region, and NE | |
| UF, OMD, NE and SH | |
| Multifocal dystonia with blepharospasm | UF and L |
| UF and NE, excluding OMD region | |
| Generalized dystonia with blepharospasm | UF and TR and at least one other body region |
UF: upper face, OMD: oromandibular, NE: neck, SH: shoulder, L: larynx, TR: trunk
The combination of BSP with involvement of the oromandibular and/or neck regions is sometimes labelled “Meige syndrome”. In keeping with prior recommendations, the terms “segmental craniofacial dystonia” or “segmental craniocervical dystonia” are preferrable.22 The reasons for this recommendation is summarized in detail in prior publications and include 1) Meige was not the first to describe this combination, 2) the majority of participants reported by Meige had focal BSP without involvement of other regions, 3) the eponym lacks specificity because it is variably applied in the literature to oromandibular dystonia, with or without BSP or neck involvement, 4) it is too easily confused with the closely related eponym (Meig syndrome) that refers to a tumor syndrome.
Precise and consistent diagnostic terminology for BSP is important, because spread to the lower face is quite common, and involvement of the lower face has a disproportionately high impact on quality of life,9 due to negative impacts on eating, drinking, and speaking. Precise and consistent descriptions of the common patterns of BSP are also important for clinical trials that target upper face only, because untreated areas may impact outcome measures, especially those relating to patient-reported outcomes or global clinical impression. Results from the current study may facilitate a more consistent approach to the diagnosis of BSP.
Highlights:
Blepharospasm (BSP) is one of the most common subtypes of dystonia
Current guidelines for diagnosis and classification are ambiguous
These ambiguities are interpreted differently by movement disorder specialists
Here we provide more specific recommendations for diagnosis and classification
Acknowledgements
This work was supported by grants to The Dystonia Coalition (NS065701, TR001456, NS116025) which is part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported by the Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), and the National Institute of Neurological Diseases and Stroke (NINDS).
Footnotes
Disclosures
AB has no additional disclosures to report.
AW has received funding from the Else Kröner-Fresenius foundation (EKFS, 2018_A55) and the German Research Foundation (DFG, WE5919/2-1).
CK serves as a medical advisor to Centogene for genetic testing reports in the field of movement disorders and dystonia, excluding Parkinson’s disease and is a member of the scientific advisory board of Retromer Therapeutics. She also received an honorarium from Lundbeck for reviewing a grant application.
DB has no additional disclosures to report.
GD has no additional disclosures to report.
GF has no additional disclosures to report.
GKB has no additional disclosures to report.
HAJ has active or recent grant support from the US government (National Institutes of Health), private philanthropic organizations (Cure Dystonia Now), and industry (Revance Therapeutics, Inc.). Dr. Jinnah has also served on advisory boards or as a consultant for Addex, Allergan, CoA Therapeutics, Cavion Therapeutics, EnePharmaceuticals, Ipsen, Retrophin, Revance, and Takaha Pharmaceuticals. He has received honoraria or stipends for lectures or administrative work from the International Parkinson’s Disease and Movement Disorders Society. Dr. Jinnah serves on the Scientific Advisory Boards for several private foundations including the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now, the Dystonia Medical Research Foundation, the Tourette Association of America, and Tyler’s Hope for a Cure. He also is principle investigator for the Dystonia Coalition, which has received the majority of its support through the NIH (grants NS116025, NS065701 from the National Institutes of Neurological Disorders and Stroke and TR001456 from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences). The Dystonia Coalition has received additional material or administrative support from industry sponsors (Allergan Inc. and Merz Pharmaceuticals) as well as private foundations (The Benign Essential Blepharospasm Foundation, Cure Dystonia Now, The Dystonia Medical Research Foundation, and The National Spasmodic Dysphonia Association).
JF has no additional disclosures to report.
JSP was supported by NIH (NINDS/NIA) NS075321, the American Parkinson Disease Association (APDA), the Greater St. Louis Chapter of the APDA, the Barnes Jewish Hospital Foundation (Elliot Stein Family Fund), the Oertli Fund, the Murphy Fund, the Paula and Rodger Riney Fund, and N Grant Williams Fund.
MH is an inventor of patents held by NIH for an immunotoxin for the treatment of focal movement disorders and the H-coil for magnetic stimulation; in relation to the latter, he has received license fee payments from the NIH (from Brainsway). He is on the Medical Advisory Boards of CALA Health and Brainsway (both unpaid positions). He is on the Editorial Board of approximately 15 journals and receives royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, Springer, Wiley, Wolters Kluwer, and Elsevier. He has research grants from Medtronic, Inc. for a study of DBS for dystonia and CALA Health for studies of a device to suppress tremor. MH is supported by the NINDS Intramural Program.
TB is an employee of the University Hospital Schleswig Holstein. He received speaker and consultant fees from Pelzerhaken Children’s Centre, Allergan/Abbvie, Ipsen Pharma and Merz Pharmaceuticals. He has received research funding from: German Research Foundation (FG 2698 and SFB 936) and Ipsen Pharma. He was supported with exhibition ultrasound equipment on loan from Cannon and ESAOTE.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiss EM, Hershey T, Karimi M, et al. Relative risk of spread of symptoms among the focal onset primary dystonias. Mov Disord 2006;21:1175–1181. [DOI] [PubMed] [Google Scholar]
- 2.Svetel M, Pekmezovic T, Jovic J, et al. Spread of primary dystonia in relation to initially affected region. J Neurol 2007;254:879–883. [DOI] [PubMed] [Google Scholar]
- 3.Abbruzzese G, Berardelli A, Girlanda P, et al. Long-term assessment of the risk of spread in primary late-onset focal dystonia. J Neurol Neurosurg Psychiatry 2008;79:392–396. [DOI] [PubMed] [Google Scholar]
- 4.Martino D, Berardelli A, Abbruzzese G, et al. Age at onset and symptom spread in primary adult-onset blepharospasm and cervical dystonia. Mov Disord 2012;27:1447–1450. [DOI] [PubMed] [Google Scholar]
- 5.Svetel M, Pekmezovic T, Tomic A, Kresojevic N, Kostic VS. The spread of primary late-onset focal dystonia in a long-term follow up study. Clin Neurol Neurosurg 2015;132:41–43. [DOI] [PubMed] [Google Scholar]
- 6.Williams L, McGovern E, Kimmich O, et al. Epidemiological, clinical and genetic aspects of adult onset isolated focal dystonia in Ireland. Eur J Neurol 2016;24:73–81. [DOI] [PubMed] [Google Scholar]
- 7.Berman BD, Groth CL, Sillau SH, et al. Risk of spread in adult-onset isolated focal dystonia: a prospective international cohort study. J Neurol Neurosurg Psychiatry 2020;91:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scorr L, Cho HJ, Kilic-Berkmen G, et al. Clinical Features and Evolution of Blepharospasm: A Multicenter International Cohort and Systematic Literature Review. Dystonia 2022;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scorr LM, Factor SA, Perez-Parra S, et al. Oromandibular dystonia: A clinical examination of 2,020 cases. Frontiers in Neurology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zech M, Jech R, Boesch S, et al. Monogenic variants in dystonia: an exome-wide sequencing study. Lancet Neurol 2020;19:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: A consensus update. Mov Disord 2013;28:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jinnah HA, Albanese A. The new classification for the dystonias: Why was it needed and how was it accomplished? Mov Disord Clin Pract 2014;1:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasikumar S, Albanese A, Krauss JK, Fasano A. Implementation of the current dystonia classification from 2013 to 2018. Mov Disord Clin Pract 2019;6:250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumsden DE, Gimeno H, Lin JP. Classification of dystonia in childhood. Parkinsonism Relat Disord 2016;33:138–141. [DOI] [PubMed] [Google Scholar]
- 15.van Egmond ME, Contarino MF, Lugtenberg CHA, et al. Variable Interpretation of the dystonia consensus classification items compromises its solidity. Mov Disord 2019;34:317–320. [DOI] [PubMed] [Google Scholar]
- 16.Kilic-Berkmen G, Pirio Richardson S, Perlmutter JS, et al. Current guidelines for classifying and diagnosing cervical dystonia: Empirical evidence and recommendations. Movement Disorders Clinical Practice 2021;9:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Defazio G, Hallett M, Jinnah HA, Berardelli A. Development and validation of a clinical guideline for diagnosing blepharospasm. Neurology 2013;81:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Defazio G, Jinnah HA, Berardelli A, et al. Diagnostic criteria for blepharospasm: A multicenter international study. Parkinsonism Relat Disord 2021;91:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilic-Berkmen G, Wright LJ, Perlmutter JS, et al. The Dystonia Coalition: A multicenter network for clinical and translational studies. Frontiers in Neurology 2021;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T. Rating scales for dystonia: a multicenter trial. Mov Disord 2003;18:303–312. [DOI] [PubMed] [Google Scholar]
- 21.Ortiz R, Scheperjans F, Mertsalmi T, Pekkonen E. The prevalence of adult-onset isolated dystonia in Finland 2007–2016. PLoS One 2018;13:e0207729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeDoux MS. Meige syndrome: what’s in a name? Parkinsonism Relat Disord 2009;15:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]


