Abstract
An aminotransferase which catalyzes the final step in methionine recycling from methylthioadenosine, the conversion of α-ketomethiobutyrate to methionine, has been purified from Klebsiella pneumoniae and characterized. The enzyme was found to be a homodimer of 45-kDa subunits, and it catalyzed methionine formation primarily using aromatic amino acids and glutamate as the amino donors. Histidine, leucine, asparagine, and arginine were also functional amino donors but to a lesser extent. The N-terminal amino acid sequence of the enzyme was determined and found to be almost identical to the N-terminal sequence of both the Escherichia coli and Salmonella typhimurium tyrosine aminotransferases (tyrB gene products). The structural gene for the tyrosine aminotransferase was cloned from K. pneumoniae and expressed in E. coli. The deduced amino acid sequence displayed 83, 80, 38, and 34% identity to the tyrosine aminotransferases from E. coli, S. typhimurium, Paracoccus denitrificans, and Rhizobium meliloti, respectively, but it showed less than 13% identity to any characterized eukaryotic tyrosine aminotransferase. Structural motifs around key invariant residues placed the K. pneumoniae enzyme within the Ia subfamily of aminotransferases. Kinetic analysis of the aminotransferase showed that reactions of an aromatic amino acid with α-ketomethiobutyrate and of glutamate with α-ketomethiobutyrate proceed as favorably as the well-known reactions of tyrosine with α-ketoglutarate and tyrosine with oxaloacetate normally associated with tyrosine aminotransferases. The aminotransferase was inhibited by the aminooxy compounds canaline and carboxymethoxylamine but not by substrate analogues, such as nitrotyrosine or nitrophenylalanine.
The amino acid methionine (Met) is required for a number of important cellular functions, including the initiation of protein synthesis, the methylation of rRNA and xenobiotics, and the biosynthesis of cysteine, phospholipids, and polyamines. The last function is especially important in rapidly growing cells, such as most parasites, bacteria, and cancer cells, which synthesize large amounts of polyamines (30). The production of spermidine from putrescine and of spermine from spermidine consumes Met (in the form of decarboxylated S-adenosylmethionine) in a one-to-one stoichiometry, with methylthioadenosine as a by-product. As the availability of Met is often limiting, a unique pathway to recycle the amino acid from methylthioadenosine exists (Fig. 1). This pathway has been partially characterized for a number of organisms, including rat liver (2, 3, 46), plants (44), yeast (29) and protozoal parasites (19, 38, 40). However, the only organism for which all the steps have been delineated is the gram-negative bacterium Klebsiella pneumoniae, where a series of unusual enzymes have been found to be responsible for the conversion of methylthioribose-phosphate to α-ketomethiobutyrate (KMTB) (17, 32, 43, 45, 46). To date, these enzymes have not been well studied in any other system.
FIG. 1.
The Met regeneration pathway. The cycle functions to recycle Met (top of figure) for use as an aminopropyl donor in polyamine synthesis. The enzymes involved are as follows: 1, S-adenosylmethionine synthetase; 2, S-adenosylmethionine decarboxylase; 3, spermidine/spermine synthetase; 4, methylthioadenosine phosphorylase; 4a, methylthioadenosine nucleosidase; 4b, methylthioribose kinase; 5, undetermined isomerase; 6, undetermined dehydratase; 7a and 7b, bifunctional E-1 enolase-phosphatase; and 8a, E-2 dioxygenase. The reaction labelled 8 occurs nonenzymatically in K. pneumoniae and via the E-3 dioxygenase in rat liver (46). The reaction catalyzed by 4 occurs in eukaryotes, and those catalyzed by 4a and 4b occur in prokaryotes. The final step, labelled KMAT (KMTB to Met aminotransferase), is the subject of the present study.
The final step of this Met recycling pathway, the transaminative conversion of KMTB to Met (KMAT activity), was originally discovered in rat liver by Backlund et al. (2), but it has not been thoroughly characterized in any system. In the original study, rat liver homogenates were found to produce Met from KMTB using glutamine or asparagine as the amino donor, and glutamate and aspartate were found to be ineffective donors. No other amino acids were examined as potential amino donors. These results were consistent with the findings of Cooper and Meister (9), who found that purified rat liver or kidney glutamine aminotransferase could utilize KMTB as a substrate. Again, a wider examination of KMTB amino donor specificity was not conducted. These early studies have led to the impression that glutamine aminotransferase was responsible for Met recycling in all organisms and that glutamine and asparagine were the primary amino donors. Indeed, even in K. pneumoniae the final step has been characterized no further than citation of the rat liver data (10).
In previous studies examining Met recycling in the trypanosomatids Crithidia fasciculata and Trypanosoma brucei brucei, aromatic amino acids were found to be the preferred amino donors for KMTB (4). Purification of this activity from C. fasciculata uncovered three distinct aminotransferases catalyzing the reaction, none of which effectively used glutamine or asparagine, and one of which displayed a peptide sequence and activities consistent with a cytosolic aspartate aminotransferase (ASAT) (5). Due to the lack of comparative information available on the conversion of KMTB to Met in other organisms, we are interested in characterizing KMTB transamination in bacteria and mammals. Using K. pneumoniae as a model system, due to the large amount of existing information on the other steps in the Met recycling pathway available in this organism, we have found that the aromatic amino acids and glutamate are the most effective amino donors. The aminotransferase responsible for this activity has been purified, cloned, and expressed as a recombinant enzyme and has been found to be a tyrosine aminotransferase (TyrAT).
MATERIALS AND METHODS
Bacteria and culture conditions.
K. pneumoniae ATCC 13883 (type strain) was obtained from the Public Health Laboratory Service (London, United Kingdom) and was grown in a defined minimal medium (20) consisting of 25 mM NH4Cl, 35 mM glucose, 1.5 mM KCl, 0.4 mM MgSO4, 0.045 mM NaCl, 0.025 mM FeSO4, 0.025 μg of thiamine per ml, 66.6 mM sodium phosphate (pH 7.4), and Ca, Co, Mn, BO3, Zn, Cu, and Mo as micronutrients. All cell cultures were grown at 37°C with agitation for 18 h before harvesting by centrifugation.
Enzyme preparation and purification.
Pelleted cells were resuspended in 2 to 3 volumes of isolation buffer (25 mM potassium phosphate [pH 7.8], 120 mM KCl, 1 mM dithiothreitol [DTT], 2.5 mM α-ketoglutarate, 0.2 mM pyridoxal phosphate) and disrupted by sonication on ice or by passage through a French Press (American Instrument Co., Silver Spring, Md.) at 1,500 lb/in2. The homogenate was then centrifuged at 25,000 × g for 30 min at 4°C, and the resulting supernatant was dialyzed against 25 mM potassium phosphate (pH 7.4)–1 mM DTT–1 mM EDTA. The dialysate was either used directly to assay KMAT activity by using a variety of amino acid amino donors, as outlined below, or used as the starting material for enzyme purification.
All purification steps were performed at 4°C by using a Beckman model 510 biocompatible high-performance liquid chromatograph (HPLC) (Beckman Instruments, High Wycombe, United Kingdom) equipped with an ultraviolet/visible spectrophotometric detector. The crude, dialyzed supernatant was loaded onto a 2.6 cm by 13 cm DEAE-Sepharose-FF (Pharmacia, St. Albans, United Kingdom) column which had been previously equilibrated with 10 mM potassium phosphate (pH 7.4)–1 mM DTT–1 mM EDTA (buffer A). Following a wash with buffer A, protein was eluted with a linear gradient to buffer B (buffer A plus 1.75 M KCl). The column eluate was monitored by measuring absorbance at 280 nm, and fractions were assayed as described below.
The active fractions from the DEAE column were pooled, dialyzed against buffer A, and applied to either a 1.6 cm by 13 cm Reactive Red 120-Sepharose-FF (Pharmacia) column or a 1.6 cm by 11 cm hydroxylapatite (Merck, Poole, United Kingdom) column. For the former, the column was equilibrated with buffer A and was eluted with a linear gradient to buffer C (buffer A plus 250 mM KCl) followed by a step gradient to buffer B. For the latter, the column was equilibrated with buffer A and eluted with a linear gradient to buffer D (same as buffer A, except that 750 mM (pH 7.4) rather than 10 mM potassium phosphate was used).
The active fractions were pooled and applied to a 1.0 cm by 10 cm Mono-Q column (Pharmacia) which had been equilibrated with buffer A. Proteins were eluted with a linear gradient to 50% buffer B. The active fractions were pooled and concentrated to less than 2.0 ml by using 30-kDa-cutoff centrifugal filters (Flowgen, Lichfield, United Kingdom) before being loaded onto a 1.6 cm by 54 cm Sephacryl S200 (Pharmacia) column. The column was equilibrated and eluted with buffer A and was calibrated by using a mixture of blue dextran, aldehyde dehydrogenase, and carbonic anhydrase. The active fractions were pooled and concentrated to 1.0 ml, and an equal volume of buffer A–4 M ammonium sulfate was added. This sample was then applied to a 4.2 mm by 100 mm Poros phenylethyl column (Perseptive Biosystems, Warrington, United Kingdom) equilibrated with buffer E (buffer A plus 2 M ammonium sulfate). Proteins were then eluted with a linear gradient to buffer A.
The active fractions were pooled, concentrated to 0.3 ml, and utilized for native gel electrophoresis carried out by a modified method of Geleherter et al. (18). Proteins were separated at 4°C on 5% acrylamide gels (without sodium dodecyl sulfate) with a 2.8% acrylamide stacking region by using 5 mM Tris–38 mM glycine–2 mM α-ketoglutarate–0.1 mM pyridoxal phosphate as the tank buffer. Upon completion of electrophoresis, half of the gel was stained with Coomassie brilliant blue R-250, and the other half was stained for KMAT activity. For the latter stain, the gel was soaked in 3 mM potassium ferricyanide for 3 min at room temperature, rinsed in distilled water, and stained in the dark at 37°C with 100 mM potassium phosphate (pH 7.4)–10 mM iodotyrosine–5 mM KMTB–40 μM pyridoxal phosphate–5 μg of phenazine methosulfonate/ml–0.2 mg of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide/ml. After development of the dark purple bands, the gel was washed in distilled water and fixed with 5% acetic acid.
To determine the N-terminal amino acid sequence of the active protein, the native gel electrophoresis was repeated, the product was electroblotted onto polyvinylidene difluoride membrane (Bio-Rad, Hemel Hempstead, United Kingdom) and stained with Amido Black, and the band was excised. N-terminal amino acid sequencing was performed by the MRC Protein Phosphorylation Unit, University of Dundee, Dundee, United Kingdom.
Enzyme assays.
All column fractions were screened for KMAT activity by a modified method of Diamondstone (12). Test fraction (5 or 10 μl) was added to 1.0 ml of 10 mM potassium phosphate (pH 7.4)–2 mM tyrosine–0.5 mM KMTB–50 μM pyridoxal phosphate. The sample was then mixed and incubated for 30 min at 37°C before the addition of 70 μl of 10 M NaOH and further incubation at 37°C for 30 min. The absorbance of the p-hydroxybenzaldehyde generated was measured at 331 nm.
For quantitative measurement of KMAT activity, Met production was determined by HPLC. A volume of enzyme sample (1 to 10 μl) was added to 100 μl of reaction mixture containing 10 mM phosphate buffer (pH 7.4)–2 mM amino acid–1 mM KMTB–50 μM pyridoxal phosphate and incubated for 30 min at 37°C. Reaction mixtures contained either individual amino acids (2 mM) or a mixture of certain amino acids (ADEFGHIKNQRSTWY) at 2 mM each. At the end of the incubation, the samples were frozen at −20°C until assayed. After thawing of the samples, 1 μl of 100 mM norleucine was added as an internal standard. Ten microliters of sample was mixed with 50 μl of 0.4 M borate (pH 10.5) and then with 10 μl of 10 mg of o-phthalaldehyde/ml–12 μl of 3-mercaptopropionate/ml–0.4 M borate (pH 10.5). Seven microliters of this mixture was then injected onto a 2.1 mm by 200 mm Amino-Quant column (Hewlett-Packard, Stockport, United Kingdom) run on a Beckman HPLC system consisting of a model 126 binary pump, 166 photodiode array ultraviolet/visible light spectrophotometer, 507e autosampler, and System Gold operating software. The column was run at ambient temperature with an initial flow rate of 0.45 ml/min, and 2.72 mg of sodium acetate/ml–0.018% (vol/vol) triethylamine–0.3% (vol/vol) tetrahydrofuran (pH 7.2) was used as solvent A and 2.72 mg of sodium acetate/ml–40% (vol/vol) methanol–40% (vol/vol) acetonitrile (pH 7.2) was used as solvent B. Elution was effected with a linear solvent gradient of 0 to 60% solvent B over 17 min followed by 60 to 100% solvent B over 1 min and 100% solvent B for a further 6 min; a flow rate of 0.45 ml/min was used for the first 18 min followed by 0.8 ml/min for the next 6 min. Met eluted at 14.2 min, and norleucine eluted at 16.2 min. Detection was by spectrophotometric detection at 338 nm. Peak area ratios of Met to norleucine were utilized to calculate the amount of Met in a given sample.
Alanine aminotransferase activity was assayed by HPLC for the production of alanine from pyruvate, and ASAT activity was assayed by measuring the production of aspartate from oxaloacetate. The enzyme source (1 to 10 μl) was added to 100 μl of reaction mixture containing 10 mM phosphate buffer (pH 7.4)–2 mM glutamate–1 mM pyruvate or oxaloacetate–50 μM pyridoxal phosphate and incubated for 30 min at 37°C. The samples were then treated as described above for the HPLC detection of KMAT activity. Aspartate eluted at 2.0 min and alanine eluted at 10.3 min on the chromatographic system. HPLC detection of TyrAT activity was accomplished by incubating samples as described above except that the reaction mixture contained 2 mM tyrosine and 1 mM α-ketoglutarate and glutamate production was determined.
For inhibition studies, 5 μl of purified enzyme was preincubated with 100 μM or 1 mM malic acid, carboxymethoxylamine, canaline, nitrophenylalanine, nitrotyrosine, or serine-O-sulfate at 37°C for 5 min before the addition of 100 μl of reaction mixture containing 10 mM potassium phosphate (pH 7.4)–1 mM KMTB–50 μM pyridoxal phosphate–2 mM each amino acid (ADEFGHIKNQRSTWY). The samples were incubated at 37°C for 30 min and analyzed by HPLC for Met production as described above.
Cloning and expression of K. pneumoniae tyrosine aminotransferase.
The tyrosine aminotransferase gene was amplified from K. pneumoniae genomic DNA by using GCCATATGATGTTTCAAAAAGTTGACGCCTAC and CGGATCCTTACATCACCGCAGCAAACGCCTT as the 5′ sense and 3′ antisense primers (incorporating NdeI and BamHI restriction sites, respectively). Amplified product of the expected length was purified from a 1% agarose gel and ligated into the PCRScript vector (Stratagene, Cambridge, United Kingdom) and transformed into Escherichia coli XL1-Blue MRF′ Kan cells (Stratagene). Plasmid DNA was isolated from positive clones, the insert was sequenced by using the ABI cycle-sequencing kit (ABI, Warrington, United Kingdom), and the NdeI-BamHI fragment containing the tyrosine aminotransferase gene was subcloned into the pET-16b expression vector, which contains a sequence for an N-terminal poly-His tag (Novagen, Cambridge, United Kingdom). After confirmation of ligation, the plasmid was transformed into E. coli BL21 (DE3)pLysS cells (Stratagene).
The transformed cells were grown in liquid Luria-Bertani medium supplemented with ampicillin and chloramphenicol and were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 27°C for 6 h. The cells were then pelleted, resuspended in 10 mM HEPES (pH 7.4) (buffer F), and disrupted by sonication on ice. After centrifugation, the supernatant was loaded onto a 1.6 cm by 10 cm iminodiacetate Sepharose-FF (Pharmacia) column which had been charged with NiSO4 and was equilibrated with buffer F. The column was then washed with buffer F plus 80 mM imidazole, and the recombinant protein was eluted with buffer F plus 800 mM imidazole. The eluted aminotransferase was concentrated and dialyzed against buffer F before use.
Nucleotide sequence accession number.
The TyrAT sequence has been deposited with GenBank under the accession no. AF074934.
RESULTS
KMAT activity in K. pneumoniae homogenates.
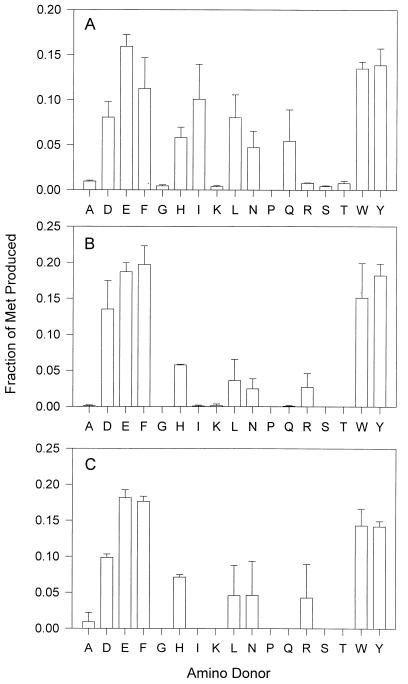
In order to study the amino donor range for the conversion of KMTB to Met, K. pneumoniae homogenates were incubated with 1 mM KMTB, a single amino acid at 2 mM, and 50 μM pyridoxal phosphate. HPLC analysis then allowed quantitation of the Met produced. A wide range of amino acids were found to be effective donors, with Asp, Glu, Phe, His, Ile, Leu, Asn, Gln, Trp, and Tyr all producing >0.5 nmol of Met/min/mg of protein (Fig. 2A). As with C. fasciculata and T. brucei brucei homogenates (4, 5), Glu, Phe, Trp, and Tyr were the best amino donors, with >1.5 nmol of Met/min/mg of protein produced.
FIG. 2.
The amino donor range for KMAT activity in K. pneumoniae. The enzyme source was incubated with 1 mM KMTB, 2 mM amino acid, and 50 μM pyridoxal phosphate for 30 min at 37°C before the Met produced was quantified by HPLC. Met production for each amino acid is shown as a relative percentage of activity for all amino acids. The enzyme sources were supernatants of cellular homogenates collected after centrifugation at 25,000 × g (A), purified aminotransferase from supernatants collected after centrifugation at 25,000 × g (B), and recombinant TyrAT (C).
Purification and characterization of an aminotransferase catalyzing KMAT activity.
Due to the ease of utilizing a modified Diamondstone reaction when assaying large numbers of fractions, tyrosine was used as the amino donor for KMAT activity in the initial screening during protein purification. Fractions containing KMAT activity with tyrosine were pooled and then assayed by HPLC by using a mixture of amino acids as amino donors (Table 1). From the first, DEAE-Sepharose, column, only one peak of activity was found (eluting with 0.5 M KCL) which utilized tyrosine for Met regeneration. The enzyme was then passaged over a Red-120 Sepharose column, where it was not retained, and a Mono-Q column, where it eluted with 0.2 M KCl. After further separation by size exclusion, the enzyme was brought to 2 M of ammonium sulfate, loaded onto a phenylethyl column, and eluted at 0.2 M ammonium sulfate. At this point, the enzyme had been enriched over 700-fold for KMAT activity, and it gave a 45,000-Da band on a sodium dodecyl sulfate-polyacrylamide gel and a 90,000-Da peak on an S200-Sephacryl column (data not shown). Like most aminotransferases, the purified enzyme is a homodimer.
TABLE 1.
The purification of an aminotransferase catalyzing Met regeneration in K. pneumoniaea
| Step | Total protein (mg) | Protein yield (%) | Sp act (nmol/min/mg) | Purification (fold) | Total activity (nmol/ml) | Activity yield (%) |
|---|---|---|---|---|---|---|
| Start | 5,043 | 100 | 3.14 | 1.0 | 15,835 | 100 |
| DEAE | 1,087 | 21.55 | 10.05 | 3.2 | 10,924 | 68.99 |
| Red-120 | 286 | 5.67 | 32.15 | 10.2 | 9,195 | 58.07 |
| Mono-Q | 103 | 2.04 | 70.77 | 22.5 | 7,289 | 46.03 |
| S200 | 4.10 | 0.08 | 610.20 | 194.3 | 797 | 5.03 |
| Phenyl | 0.34 | 0.01 | 2,269.31 | 722.7 | 762 | 4.82 |
Enzyme activity was determined by HPLC analysis after incubation with 2 mM each amino acid (ADEFGHIKLNQRSTWY) and 1 mM KMTB, as described in Materials and Methods.
The purified aminotransferase was found to be responsible for all of the KMAT activities observed in the bacterial homogenates with the exception of that generated with Ile and Gln and part of that generated with Asn and Leu (Fig. 2B). Again, Glu, Phe, Trp, and Tyr were the preferred amino donors, catalyzing the formation of >1,100 nmol/min/mg of protein. The purified enzyme was also able to catalyze tyrosine to α-ketoglutarate aminotransfer but was unable to effectively utilize systems containing glutamate and oxaloacetate, glutamate and pyruvate, or alanine and α-ketoglutarate (data not shown).
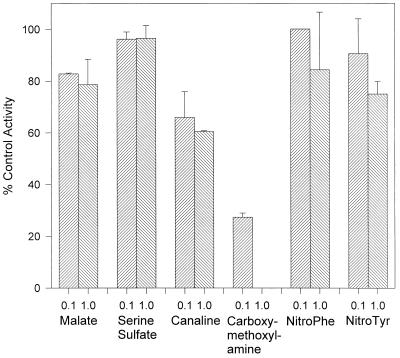
A series of potential aminotransferase inhibitors (47) were screened against the purified aminotransferase in order to determine their effects on KMAT activity. Using a mixture of amino acids (ADEFGHIKLNQRSTWY) at 2 mM each, 1 mM KMTB, and 50 μM pyridoxal phosphate as the substrate, each inhibitor was tested at 0.1 or 1.0 mM (Fig. 3). Serine-O-sulfate and nitrophenylalanine were found to have no inhibitory activity at either concentration, while malate and nitrotyrosine gave approximately 20% inhibition at the higher concentration. Only canaline and carboxymethoxylamine, both compounds which interact with the functional aldehyde on the pyridoxal phosphate cofactor, had any appreciable effect. At 100 μM, these two compounds inhibited KMAT activity by 35 and 70%, respectively. Addition of 1.0 mM carboxymethoxylamine led to a complete inhibition of KMAT activity. These results suggest that KMAT activity can be inhibited but that agents which bind to the prosthetic group, such as the aminooxy compounds, are more likely to be potent inhibitors.
FIG. 3.
The inhibition of Met production by the purified aminotransferase. The purified enzyme from K. pneumoniae was preincubated for 5 min with a single inhibitor at 0.1 mM (left column) or 1.0 mM (right column) before the addition of a reaction mixture containing 1 mM KMTB, certain amino acids (ADEFGHIKLNQRSTWY) at 2 mM each as amino donors, and 50 μM pyridoxal phosphate. After incubation at 37°C for 30 min, Met production was quantified by HPLC analysis. All inhibition is presented relative to a control value of 100%, which was determined by preincubating the enzyme with distilled water prior to adding the reagent mixture.
Amino-terminal sequence of the purified aminotransferase.
The purified aminotransferase was separated by native polyacrylamide gel electrophoresis and transblotted, and the active band was excised and subjected to automated Edman degradation. The amino-terminal sequence was found to be MFQKVDAYAGDPILXLMERFXE. This sequence is 86% identical to the N terminus of the E. coli TyrAT (tyrB gene product) (28), 81% identical to the Salmonella typhimurium TyrAT (33), 36% identical to the Paracoccus denitrificans TyrAT (34), and 36% identical to the Rhizobium meliloti TyrAT (36), but it is less than 18% identical to any eukaryotic TyrAT (6, 21, 22, 37) (Fig. 4A). Therefore, the purified K. pneumoniae aminotransferase, which catalyzes KMAT activity, appears to be a TyrAT.
FIG. 4.
Alignment of the K. pneumoniae TyrAT with prokaryotic and eukaryotic analogues. In all cases, the boxed residues are identical to those found in the K. pneumoniae sequence. (A) A direct comparison of the N-terminal amino acid sequence obtained from the purified K. pneumoniae KMAT with known TyrATs. (B) Clustal alignment of the deduced amino acid sequence from the product of the K. pneumoniae tyrB gene with other known TyrATs. Regions around key residues which are conserved across the class I aminotransferase family are shown. In the top sequence, N-194, P-195, and G-197 have been marked with asterisks, in the middle sequence D-222 and Y-225 have been marked, and in the bottom sequence, K-258, R-266, and G-268 have been marked. K-258 (marked with a double asterisk) is the pyridoxal phosphate binding site. All conserved residues are numbered according to the nomenclature of Mehta et al. (31). Alignment was performed using the Megalign program of the DNAStar package (DNAStar, Madison, Wis.).
Cloning of the K. pneumoniae TyrAT.
Given the high identity of the N terminus of the K. pneumoniae enzyme to the E. coli tyrB gene product, oligonucleotide primers were designed which matched the 5′ and 3′ ends of the coding sequence for the E. coli enzyme. These primers, when utilized in PCR on K. pneumoniae genomic DNA, successfully amplified the expected 1,200-bp product (data not shown), which was cloned and sequenced.
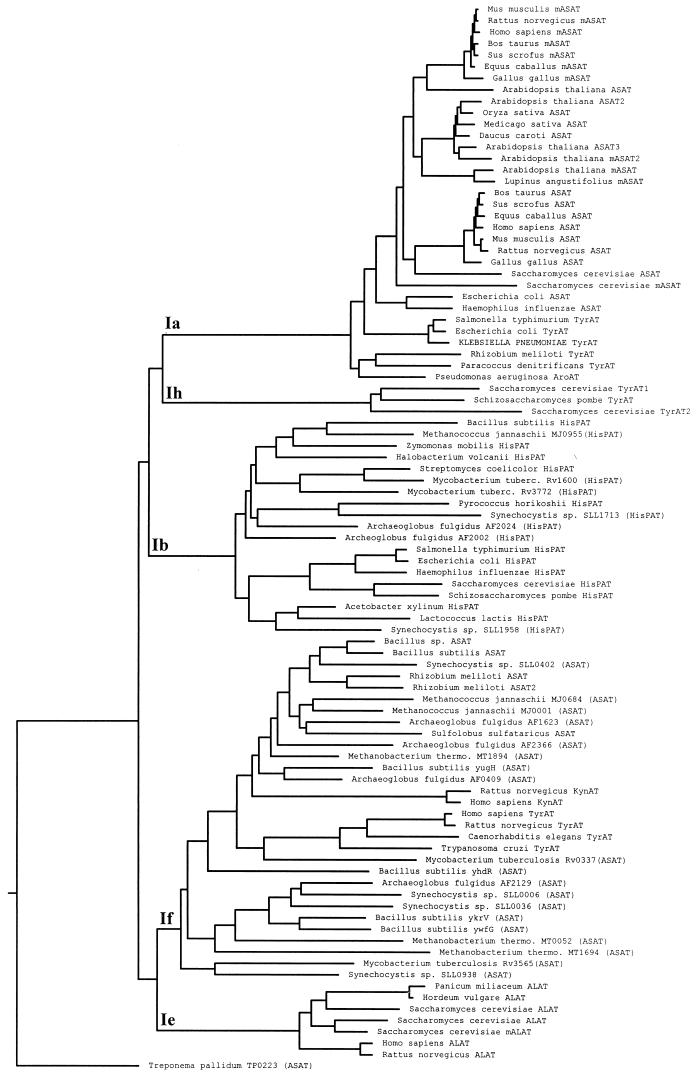
The amino acid sequence of the predicted product was found to have close identity to the TyrATs from E. coli and S. typhimurium (83 and 80% identity, respectively), when compared by clustal analysis. In addition, the sequence identities to other known TyrATs were 38% to P. denitrificans, 34% to R. meliloti, 13% to Caenorhabditis elegans, 12% to Trypanosoma cruzi, 12% to Saccharomyces cerevisiae ARO8, 13% to S. cerevisiae ARO9, 12% to Schizosaccharomyces pombe ARO8, 12% to rat, and 12% to human. Aside from residues completely conserved in class I aminotransferases (23, 31), there was little sequence identity between the prokaryotic and eukaryotic TyrATs. The presence of the motifs LLHXCXHNPTGXDXXXXXW, PXXDXAYQGFXXGXXXD, and SKXXXLYXERXG around key invariant residues (Fig. 4B) confirmed that the K. pneumoniae TyrAT belongs to the Ia subfamily of aminotransferases (23). Clustal analysis of many class I aminotransferases showed, as previously suggested (23), that all bacterial TyrATs sequenced to date (including the K. pneumoniae enzyme) are related to eukaryotic and gram-negative bacterial ASATs in subfamily Ia (Fig. 5). The yeast TyrATs (ARO8 and ARO9 gene products) formed a unique Ih subfamily (22), while all other eukaryotic TyrATs sequenced to date are related to gram-positive bacterial and archaeal ASATs in subfamily If (23).
FIG. 5.
The class I family of aminotransferases. The dendrogram was constructed based on the clustal analysis of most aminotransferase members of the class I family, with nonaminotransferase members (subfamilies Ic and Id) and single member subfamilies (subfamily Ig) omitted. The K. pneumoniae TyrAT is shown capitalized, and subfamily groupings are presented according to the Iraqui et al. (22) revision of the nomenclature of Jensen and Gu (23). Enzyme abbreviations are as follows: ASAT, cytosolic aspartate aminotransferase; mASAT, mitochondrial aspartate aminotransferase; chASAT, chloroplast aspartate aminotransferase; TyrAT, tyrosine aminotransferase; ALAT, alanine aminotransferase; KynAT, kynurenine aminotransferase; and HisPAT, histidinol-phosphate aminotransferase. Putative aminotransferases identified from published whole genome sequences (7, 8, 11, 14–16, 24, 25, 27, 39, 42) are shown with the appropriate identification code and with the annotation given in parentheses.
Properties of the recombinant tyrosine aminotransferase.
The full-length coding sequence of the K. pneumoniae TyrAT was subcloned into an E. coli expression vector, and the resulting recombinant protein was purified. This enzyme was then utilized in the single amino acid screen for Met regeneration and was found to produce Met from KMTB with the same amino donor preference as was seen with the purified native enzyme (Fig. 2C). This result confirmed that a single aminotransferase was responsible for all the activity in the purified material and also demonstrated that the recombinant material was fully active.
A number of substrates were examined with the recombinant enzyme in order to determine the kinetic parameters of the K. pneumoniae TyrAT (Table 2). While glutamate was found to be capable of producing the highest maximum initial velocity (Vmax) for Met production from KMTB, it was found to be 5- to 10-fold poorer in terms of substrate specificity when compared with tyrosine, tryptophan, and phenylalanine. Examination of other substrate combinations showed that the “classic” TyrAT substrate combinations of tyrosine with α-ketoglutarate and tyrosine with oxaloacetate were not significantly more active than the combination of tyrosine and KMTB, while that of tyrosine and pyruvate was a poor substrate combination. These results demonstrated that the K. pneumoniae enzyme is capable of performing methionine regeneration equally as well as the classic TyrAT reactions.
TABLE 2.
Kinetic parameters for the K. pneumoniae recombinant TyrATa
| Substrate | Cosubstrate | Apparent Km (mM) | Apparent Vmax (μmol/min/mg) |
|---|---|---|---|
| Tyrosine | 10 mM KMTB | 2.01 ± 0.13 | 3.25 ± 0.09 |
| Phenylalanine | 10 mM KMTB | 2.01 ± 0.46 | 1.80 ± 0.14 |
| Tryptophan | 10 mM KMTB | 1.42 ± 0.20 | 2.63 ± 0.11 |
| Glutamate | 10 mM KMTB | 11.93 ± 0.75 | 7.65 ± 0.29 |
| KMTB | 5 mM tyrosine | 2.46 ± 0.27 | 3.29 ± 0.13 |
| α-Ketoglutarate | 5 mM tyrosine | 3.00 ± 0.24 | 5.21 ± 0.15 |
| Oxaloacetate | 5 mM tyrosine | 6.00 ± 0.91 | 7.39 ± 0.52 |
| Pyruvate | 5 mM tyrosine | 20.13 ± 8.21 | 0.09 ± 0.03 |
Amino acid production was determined by incubations of mixtures containing 10 mM phosphate buffer (pH 7.4), 50 μM pyridoxal phosphate, cosubstrate (at the indicated concentration), and 0.01 to 10 mM substrate and subsequent HPLC analysis as described in Materials and Methods. Kinetic values were determined by nonlinear least-squares fitting to the Michaelis-Menton equation by using the Scientist software package (MicroMath, Salt Lake City, Utah) and are shown with the standard deviations.
DISCUSSION
K. pneumoniae is a common cause of urinary tract, respiratory tract, and bloodstream infections and has been found, like many other common bacterial pathogens, to be increasingly resistant to antibiotics. The recent SENTRY study on bloodstream infections found that the percentage of K. pneumoniae isolates that were resistant was 87% for penicillins, 14% for cephalosporins, 4% for penems, 5% for aminoglycosides, 4% for fluoroquinolones, 15% for tetracycline, and 15% for trimethoprim-sulfamethoxazole (35). In addition, hospital outbreaks of multiple-drug-resistant K. pneumoniae have been reported (26). As with other pathogens, the spread of resistance in K. pneumoniae requires the discovery and development of novel drug targets. In the past, work on the inhibition of methylthioribose kinase (enzyme 4b in Fig. 1) has shown that interference with the Met recycling pathway led to K. pneumoniae cell death (20). Similar studies have also demonstrated that inhibition of methylthioadenosine phosphorylase (enzyme 4 in Fig. 1) has a similar effect on the causative organisms of malaria and African trypanosomiasis (1, 40). Many of the enzymes in the Met recycling pathway have been poorly characterized and could constitute additional points for chemotherapeutic intervention in the cycle.
We have found that the final step in the pathway, the conversion of KMTB to Met, is catalyzed in K. pneumoniae by a TyrAT (TyrB homologue) and that this enzyme can be inhibited by aminooxy compounds (such as canaline or carboxymethoxylamine) which interact with the pyridoxal phosphate cofactor. Analogues of the substrate, such as nitrotyrosine or nitrophenylalanine, were found to be poor inhibitors. However, it is interesting that nitrotyrosine does not act as a substrate for the K. pneumoniae TyrAT, as was seen with the aminotransferase which catalyzes KMAT using tyrosine in C. fasciculata (5). In addition, while carboxymethoxylamine was equally efficient in inhibiting the K. pneumoniae and C. fasciculata aminotransferases, canaline was 20% less effective in inhibiting the K. pneumoniae enzyme than in inhibiting the C. fasciculata enzyme under identical conditions. This result suggests that the structure of the compound carrying the aminooxy group may play a significant role in obtaining access to the pyridoxal phosphate and thus may provide scope for the design of more-specific inhibitors of this class.
The primary structure of the K. pneumoniae TyrAT was found to be almost identical to those of the homologous enzymes from E. coli and S. typhimurium. While it is tempting to assume that TyrAT performs the same function in Met recycling in these latter two organisms, it has been previously suggested that E. coli cannot recycle methylthioadenosine due to a lack of methylthioribose kinase (10). We have expressed the E. coli TyrAT and have found that it is capable of catalyzing KMAT to the same extent and by using the same amino group donors as the K. pneumoniae enzyme (data not shown). Thus, E. coli retains the ability to produce Met should it encounter or produce KMTB. The status of Met recycling in S. typhimurium or, indeed, other bacteria is completely unknown. However, it is interesting that obvious homologues to the tyrB gene are absent from the genomes of Haemophilus influenzae, Mycoplasma genitalium, Methanococcus jannaschii, Synechocystis sp., Helicobacter pylori, Archaeoglobus fulgidus, Borrelia burgdorferi, Methanobacterium thermoautotrophicum, Bacillus subtilis, Aquifex aeolicus, Treponema pallidum, and Mycobacterium tuberculosis (7, 8, 11, 13–16, 24, 25, 27, 39, 42).
The observation that the purified and recombinant TyrAT is unable to catalyze KMAT to the same extent as seen in K. pneumoniae cell homogenates when using Ile, Gln, Asn, or Leu as an amino donor clearly suggests that another aminotransferase(s) is responsible for this activity. While the relative amounts of individual amino acids free to act as amino donors in K. pneumoniae are unknown, and probably vary considerably, the range of KMAT activity catalyzed by TyrAT suggests that it is the main enzyme for this reaction. Certainly, the kinetic parameters determined for the TyrAT show that KMAT reactions proceed equally as well as the reaction involving tyrosine and α-ketoglutarate and that involving tyrosine and oxaloacetate. The structures of the amino donors other than TyrAT suggested that the K. pneumoniae homologue of the E. coli ilvE gene product (branched-chain amino acid aminotransferase) could be involved. However, we have expressed the E. coli enzyme and have found no detectable activity for systems containing isoleucine and KMTB, leucine and KMTB, or glutamine and KMTB (data not shown).
Our previous work with the trypanosomatid C. fasciculata had disclosed that aromatic amino acids and glutamate were also favored as amino donors in that system (5). However, unlike in K. pneumoniae, the aminotransferase that utilized aromatic amino acids and glutamate for catalyzing the reaction of KMAT in C. fasciculata had a peptide sequence consistent with an ASAT. Since that time, we have successfully cloned approximately 40% of the gene for this C. fasciculata aminotransferase, which clearly has close homology to eukaryotic ASATs and not to TyrATs (data not shown). This difference in enzyme specificity for Met recycling is intriguing. As mentioned above, eukaryotic and gram-negative bacterial ASATs and bacterial TyrATs have a common ancestor and form the Ia subfamily of aminotransferases (Fig. 5 and reference 23). Jensen and Gu (23) have suggested that a “gradient” exists within the Ia subfamily with respect to specificity for reactions of aspartate with α-ketoglutarate and of tyrosine with α-ketoglutarate, with higher eukaryote ASATs specific for the former reaction, lower eukaryotic and gram-negative bacterial ASATs catalyzing both reactions but with a preference for the former, and the bacterial TyrATs catalyzing both reactions almost equally. Our work with Met recycling may well support this idea, since a bacterial TyrAT and a lower eukaryotic ASAT are able to catalyze the reaction resulting in KMAT activity with aromatic amino acids and glutamate as the amino donor but mammalian ASAT is unable to catalyze the reaction with any amino donor (5). As mammalian tissues contain substances that can clearly catalyze Met recycling (data not shown; reference 46), it appears that subfamily Ia aminotransferases are definitely not involved in this reaction in the host. Indeed, it appears unlikely that any class I aminotransferases are involved in mammalian Met metabolism. The exact aminotransferase(s) performing Met recycling in host tissues is unclear and is the focus of our current investigations.
ACKNOWLEDGMENTS
We thank Nick Morrice for his assistance in N-terminal sequencing, Emmanuel Tetaud for assistance in the cloning and expression of the tyrB gene, and Alan H. Fairlamb for helpful discussions.
This work was funded by the Wellcome Trust (B.J.B.).
REFERENCES
- 1.Bacchi C J, Sufrin J R, Nathan H C, Spiess A J, Hannan T, Garafolo J, Alecia K, Katz L, Yarlett N. 5′-Alkyl-substituted analogs of 5′-methylthioadenosine as trypanocides. Antimicrob Agents Chemother. 1991;35:1315–1320. doi: 10.1128/aac.35.7.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backlund P S, Chang C P, Smith R A. Identification of 2-keto-4-methylthiobutyrate as an intermediate compound in methionine synthesis from 5′-methylthioadenosine. J Biol Chem. 1982;257:4196–4202. [PubMed] [Google Scholar]
- 3.Backlund P S, Smith R A. Methionine synthesis from 5′-methylthioadenosine in rat liver. J Biol Chem. 1980;256:1533–1535. [PubMed] [Google Scholar]
- 4.Berger B J, Dai W W, Wang H, Stark R E, Cerami A. Aromatic amino acid transamination and methionine recycling in trypanosomatids. Proc Natl Acad Sci USA. 1996;93:4126–4130. doi: 10.1073/pnas.93.9.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger B J, Dai W W, Wilson J. Methionine formation from α-ketomethiobutyrate in the trypanosomatid Crithidia fasciculata. FEMS Microbiol Lett. 1998;165:305–312. doi: 10.1111/j.1574-6968.1998.tb13162.x. [DOI] [PubMed] [Google Scholar]
- 6.Bontempi E J, Bua J, Aslund L, Porcel B, Segura E L, Henriksson J, Orn A, Pettersson U, Ruiz A M. Isolation and characterization of a gene from Trypanosoma cruzi encoding a 46-kilodalton protein with homology to human and rat tyrosine aminotransferase. Mol Biochem Parasitol. 1993;59:253–262. doi: 10.1016/0166-6851(93)90223-k. [DOI] [PubMed] [Google Scholar]
- 7.Bult C J, White O, Olsen G J, Zhou L X, Fleishmann R D, et al. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 8.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 9.Cooper A J L, Meister A. Isolation and properties of highly purified glutamine aminotransferase. Biochemistry. 1972;11:661–671. doi: 10.1021/bi00755a001. [DOI] [PubMed] [Google Scholar]
- 10.Cornell K A, Winter R W, Tower P A, Riscoe M K. Affinity purification of 5-methylthioadenosine kinase and 5-methylthioribose/S-adenosylmethionine nucleosidase from Klebsiella pneumoniae. Biochem J. 1996;317:285–290. doi: 10.1042/bj3170285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deckert G, Warren P V, Gaasterland T, Young W G, Lenox A L, Graham D E, Overbeek R, Snead M A, Keller M, Aujay M, Huber R, Feldman R A, Short J M, Olsen G J, Swansen R V. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature. 1998;392:353–358. doi: 10.1038/32831. [DOI] [PubMed] [Google Scholar]
- 12.Diamondstone T I. Assay of tyrosine aminotransferase by conversion of p-hydroxyphenylpyruvate to p-hydroxybenzaldehyde. Anal Biochem. 1966;16:395–401. [Google Scholar]
- 13.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 14.Fraser C M, Casjens S, Wang W M, Sutton G G, Clayton R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 15.Fraser C M, Gocayne J D, White O, Adams M D, Clayton R A, et al. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 16.Fraser C M, Norris S J, Weinstock G M, White O, Sutton G G, et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 17.Furfine E S, Abeles R H. Intermediates in the conversion of 5′-methylthioadenosine to methionine in Klebsiella pneumoniae. J Biol Chem. 1988;263:9596–9606. [PubMed] [Google Scholar]
- 18.Geleherter T D, Emanuel J R, Spencer C J. Induction of tyrosine aminotransferase by dexamethasone, insulin, and serum: characterization of the induced enzyme. J Biol Chem. 1972;247:6197–6203. [PubMed] [Google Scholar]
- 19.Ghoda L Y, Savarese T M, Northrup C H, Parks R E, Garafolo J, Katz L, Ellenbogen B B, Bacchi C J. Substrate specificities of 5′-deoxy-5′-methylthioadenosine phosphorylase from Trypanosoma brucei brucei and mammalian cells. Mol Biochem Parasitol. 1988;27:109–118. doi: 10.1016/0166-6851(88)90030-8. [DOI] [PubMed] [Google Scholar]
- 20.Gianotti A J, Tower P A, Sheley J H, Conte P A, Spiro C, Ferro A J, Fitchen J H, Riscoe M K. Selective killing of Klebsiella pneumoniae by 5-trifluoromethylthioribose. Chemotherapeutic exploitation of the enzyme 5-methylthioribose kinase. J Biol Chem. 1990;265:831–837. [PubMed] [Google Scholar]
- 21.Grange T, Guenet C, Dietrich J B, Chasserot S, Fromont M, Befort N, Jami J, Beck G, Pictet R. Complete complementary DNA of rat tyrosine aminotransferase messenger RNA. Deduction of the primary structure of the enzyme. J Mol Biol. 1985;184:347–350. doi: 10.1016/0022-2836(85)90386-9. [DOI] [PubMed] [Google Scholar]
- 22.Iraqui I, Vissers S, Cartiaux M, Urrestarazu A. Characterisation of Saccharomyces cerevisiae ARO8 and ARO9 genes encoding aromatic aminotransferases I and II reveals a new aminotransferase subfamily. Mol Gen Genet. 1998;257:238–248. doi: 10.1007/s004380050644. [DOI] [PubMed] [Google Scholar]
- 23.Jensen R A, Gu W. Evolutionary recruitment of biochemically specialized subdivisions of family I within the protein superfamily of aminotransferases. J Bacteriol. 1996;178:2161–2171. doi: 10.1128/jb.178.8.2161-2171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko T, Matsubayashi T, Sugita M, Sugiura M. Physical and gene maps of the unicellular cyanobacterium Synechocystis sp. strain PCC6301 genome. Plant Mol Biol. 1996;31:193–201. doi: 10.1007/BF00020621. [DOI] [PubMed] [Google Scholar]
- 25.Klenk H P, Clayton R A, Tomb J F, White O, Nelson K E, et al. The complete sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature. 1997;390:364. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 26.Kumarasinghe G, Chow C, Koh B L, Chiang K L, Liew H Y, Ti T Y. Antimicrobial resistance problem in a university hospital. Pathology. 1995;27:67–70. doi: 10.1080/00313029500169502. [DOI] [PubMed] [Google Scholar]
- 27.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 28.Kuramitsu S, Inoue K, Ogawa T, Ogawa H, Kagamiyama H. Aromatic amino acid aminotransferase of Escherichia coli: nucleotide sequence of the tyrB gene. Biochem Biophys Res Commun. 1985;133:134–139. doi: 10.1016/0006-291x(85)91851-0. [DOI] [PubMed] [Google Scholar]
- 29.Marchitto K S, Ferro A J. The metabolism of 5-methylthioadenosine and 5-methylthioribose-1-phosphate in Saccharomyces cerevisiae. J Gen Microbiol. 1985;131:2153–2164. doi: 10.1099/00221287-131-9-2153. [DOI] [PubMed] [Google Scholar]
- 30.Marston L J, Pegg A E. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 31.Mehta P K, Hale T I, Christen P. Aminotransferases: demonstration of homology and division into evolutionary subgroups. Eur J Biochem. 1993;214:549–561. doi: 10.1111/j.1432-1033.1993.tb17953.x. [DOI] [PubMed] [Google Scholar]
- 32.Myers R W, Abeles R H. Purification and characterization of an enzyme involved in oxidative carbon-carbon bond-cleavage reactions in the methionine salvage pathway of Klebsiella pneumoniae. J Biol Chem. 1993;268:24785–24791. [PubMed] [Google Scholar]
- 33.Nakai Y, Hayashi H, Kagamiyama H. Cloning and characterization of the tyrB gene from Salmonella typhimurium. Biochim Biophys Acta. 1996;1308:189–192. doi: 10.1016/0167-4781(96)00113-3. [DOI] [PubMed] [Google Scholar]
- 34.Oue S, Okamoto A, Nakai Y, Nakahira M, Shibatani T, Hayashi H, Kagamiyama H. Paracoccus denitrificans aromatic amino acid aminotransferase: a model enzyme for the study of dual substrate recognition mechanism. J Biochem. 1997;121:161–171. doi: 10.1093/oxfordjournals.jbchem.a021561. [DOI] [PubMed] [Google Scholar]
- 35.Pfaller M A, Jones R N, Doern G V, Kugler K the SENTRY Participants Group. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997) Antimicrob Agents Chemother. 1998;42:1762–1770. doi: 10.1128/aac.42.7.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rastogi V K, Watson R J. Aspartate aminotransferase activity is required for aspartate catabolism and symbiotic nitrogen fixation in Rhizobium meliloti. J Bacteriol. 1991;173:2879–2887. doi: 10.1128/jb.173.9.2879-2887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rettenheimer R, Natt E, Zentgraf H, Scherer G. Isolation and characterization of the human tyrosine aminotransferase gene. Nucleic Acids Res. 1990;18:3853–3861. doi: 10.1093/nar/18.13.3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riscoe M K, Tower P A, Peyton D H, Ferro A J, Fitchen J H. Methionine recycling as a target for antiprotozoal drug development. In: Coombs G, North M, editors. Biochemical protozoology. London, United Kingdom: Taylor and Francis; 1991. pp. 450–457. [Google Scholar]
- 39.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H, Dubois J, et al. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genetics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sufrin J R, Meshnik S R, Spiess A J, Garafolo-Hannon J, Pan X Q, Bacchi C J. Methionine recycling pathways and antimalarial drug design. Antimicrob Agents Chemother. 1995;39:2511–2515. doi: 10.1128/aac.39.11.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The C. elegans Sequencing Consortium. Genome sequencing of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 42.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 43.Trackman P C, Abeles R H. Methionine synthesis from 5′-S-methylthioadenosine. Resolution of enzyme activities and identification of 1-phospho-5-S-methlthioribulose. J Biol Chem. 1983;258:6717–6720. [PubMed] [Google Scholar]
- 44.Wang S Y, Adams D O, Lieberman M. Recycling of 5′-methylthioadenosine-ribose carbon atoms into methionine in tomato tissue in relation to ethylene production. Plant Physiol (Rockville) 1982;70:117–121. doi: 10.1104/pp.70.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wray J W, Abeles R H. A bacterial enzyme that catalyzes formation of carbon monoxide. J Biol Chem. 1993;268:21466–21469. [PubMed] [Google Scholar]
- 46.Wray J W, Abeles R H. The methionine salvage pathway in Klebsiella pneumoniae and rat liver. Identification and characterization of two novel dioxygenases. J Biol Chem. 1995;270:3147–3152. doi: 10.1074/jbc.270.7.3147. [DOI] [PubMed] [Google Scholar]
- 47.Zollner H. Handbook of enzyme inhibitors. 2nd ed. New York, N.Y: VCH Publishers; 1993. [Google Scholar]