Abstract
Adenomatous Polyposis Coli (APC) protein is mostly known as a tumor suppressor that regulates Wnt signaling, but is also an important cytoskeletal protein. Mutations in the APC gene are linked to colorectal cancer and various neurological disorders and intellectual disabilities. Cytoskeletal functions of APC appear to have significant contributions to both types of these disorders. As a cytoskeletal protein, APC can regulate both actin and microtubule cytoskeletons, which together form the main machinery for cell migration. As APC is a multifunctional protein with numerous interaction partners, the complete picture of how APC regulates cell motility is still unavailable. However, some molecular mechanisms begin to emerge. Here, we review available information about roles of APC in cell migration and propose a model explaining how microtubules, using APC as an intermediate, can initiate leading edge protrusion in response to external signals by stimulating Arp2/3 complex-dependent nucleation of branched actin filament networks via a series of intermediate events.
INTRODUCTION
The Adenomatous Polyposis Coli (APC) gene was first identified in 1991 (Groden et al., 1991), when mutations of this gene were found to be associated with familial adenomatous polyposis (FAP), an inherited condition characterized by the presence of multiple colorectal polyps. If not treated, the FAP patients often develop colorectal cancer. These findings led to a concept that APC functions as a tumor suppressor (Zhang and Shay, 2017). In addition, APC mutations have been also linked to various neurological disorders and intellectual disabilities (Gonzalez et al., 2015; Mohn et al., 2014; Onouchi et al., 2014).
The APC gene encodes a 312 kDa, multi-domain protein that has multiple interacting partners and diverse cellular functions. In humans, APC has highest expression levels in the brain, but is also expressed in multiple other tissues including high levels of expression in the gastrointestinal tract (https://www.proteinatlas.org/) (Bhat et al., 1994). The APC gene undergoes alternative splicing that primarily affects the N-terminal region of the APC protein. The APC gene produces a conventional ubiquitously expressed APC isoform with 2843 amino acids (aa) and several splice variants that are usually expressed in differentiated tissues (Carson et al., 2004; Liou et al., 2004; Santoro and Groden, 1997; Thliveris et al., 1994). Human genome also encodes a brain-specific APC paralog, APC2, which shares some, but not all functions with APC and plays important roles in nervous system development (Shintani et al., 2012; Yamanaka et al., 2002).
The tumor suppressor activity of APC largely relies on its roles in the canonical Wnt/β-catenin signaling pathway, which regulates organogenesis, embryonic development, tissue homeostasis and tumorigenesis (Schaefer and Peifer, 2019). In steady-state conditions, when Wnt signaling is not activated, APC, together with scaffolding protein Axin, serine/threonine kinases GSK3β and CK1, and β-catenin, forms a destruction complex that phosphorylates β-catenin, thus promoting its ubiquitination and degradation. Activation of the Wnt pathway disrupts the destruction complex, leading to β-catenin accumulation in the cytoplasm, and its translocation into the nucleus, where β-catenin interacts with transcription factors to activate expression of Wnt target genes. The FAP-linked APC mutations typically produce a truncated APC protein unable to assemble a functional destruction complex, which leads to constitutive activation of the Wnt pathway, abnormal target gene transcription, and cancerous lesions in the colon.
In addition to regulating Wnt signaling, APC has been subsequently recognized to be a microtubule-binding protein (Nathke et al., 1996), and a multifunctional regulator of the cytoskeleton that is able to interact with and regulate all three major components of the cytoskeleton – microtubules, actin filaments and intermediate filaments (Dogterom and Koenderink, 2019; Nelson and Nathke, 2013). Through its ability to regulate the cytoskeleton, APC plays important roles in various aspects of cell behavior, such as cell migration, adhesion, polarity, division, and morphogenesis. In this review, we focus on the cytoskeletal functions of APC, especially those that affect directional cell motility through regulation and coordination of actin and microtubule dynamics. Additional APC functions are discussed in other recent reviews (Hajka et al., 2021; Lesko et al., 2014; Li et al., 2021; Narayan and Sharma, 2015; Schaefer and Peifer, 2019; van der Wal and van Amerongen, 2020; Zhang and Shay, 2017).
APC DOMAINS
Here, we will primarily discuss the conventional human APC isoform (2843 aa), but comment on other APC variants and APC2, as needed. The amino acid sequence of APC can roughly be divided into three major regions (Figure 1): (1) the N-terminal segment (subsequently referred to as APC-N), which contains protein-protein interaction and self-assembly domains, (2) the middle domain (APC-M), which is involved in the assembly of the β-catenin destruction complex, and (3) the C-terminal region (APC-C), which contains microtubule- and actin-interacting sites. Compared with APC, APC2 contains well-conserved N-terminal and middle domains, but has a different C-terminal region (Nakagawa et al., 1998; van Es et al., 1999).
Figure 1.
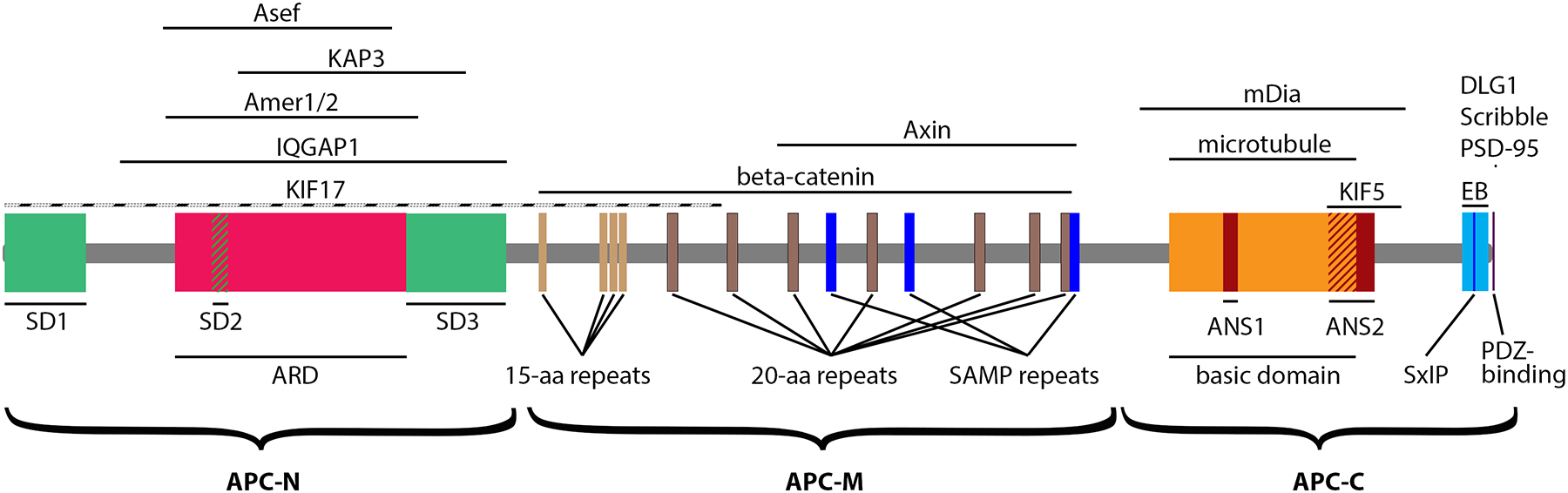
APC domains and interaction partners. Domain names are shown underneath the APC diagram. APC regions that interact with proteins shown above the APC diagram are shown as black lines. KIF17-interacting regions was determined approximately and is shown by a dashed line.
The APC N-terminus (APC-N)
In the conventional human APC isoform, APC-N comprises ~1,000 aa that includes three self-association domains (SD1–3) and an Armadillo repeat domain (ARD).
Self-association domains (SDs)
SD1 of APC was originally mapped to the first 55 aa of APC, when an isolated N-terminal peptide of APC (aa 2–55) was found to form an α-helical coiled-coil, suggesting that it could be responsible for dimerization (Joslyn et al., 1993). However, subsequently characterized biochemical and crystal structure features of the peptide dimer suggested that within the full length APC, this coiled coil likely extends beyond the first 55 aa (Day and Alber, 2000). Furthermore, since the neighboring region of APC (aa 46–256) that lacks most of SD1, is also able to dimerize (Joslyn et al., 1993), possibly, using the heptad repeats at aa 125–156 (Day and Alber, 2000), SD1 likely extends at least till residue 156. Additional APC self-association regions have been mapped to aa 396–426 (SD2) and aa 768–958 (SD3) (Kunttas-Tatli et al., 2014; Li et al., 2008). A shorter splice isoform of APC that is enriched in the brain and the heart lacks the first 45 aa of SD1, and possibly also SD2, which could render this isoform largely monomeric (Kunttas-Tatli et al., 2014), although this possibility has not been specifically addressed. Despite having several SDs within APC-N plus a dimerizarion motif in the APC-C, purified recombinant full-length mouse APC behaved predominantly as a dimer in vitro (Baumann et al., 2020).
Armadillo repeat domain (ARD)
The ARD of APC is located between SD2 and SD3, and is involved in multiple protein-protein interactions of APC (Nelson and Nathke, 2013). The Armadillo repeat was first characterized for Drosophila Armadillo (β-catenin) (Riggleman et al., 1989) and refers to a 42-aa motif consisting of three α-helices (Huber et al., 1997). Initial studies have identified seven tandem Armadillo repeats in APC (aa 453–767) (Peifer et al., 1994), but a subsequent crystal structure analysis showed that the Armadillo repeats fold together with a preceding region (aa 326–442), forming a common right-handed solenoid (Zhang et al., 2012). Therefore, the proper ARD boundaries should be aa 326–767. However, since the pre-ARD region completely encompasses SD2, it remains to be understood whether SD2 remains dimerization competent in the context of the extended ARD. As a typical protein-protein interaction domain, ARD of APC interacts with multiple proteins via the groove of the solenoid. The interaction partners of ARD include Asef1 and Asef2 (Kawasaki et al., 2000), IQGAP1 (Watanabe et al., 2004), Amer1 and Amer2 (Grohmann et al., 2007), KAP3 (Jimbo et al., 2002), and vimentin (Sakamoto et al., 2013; Wang et al., 2009) among others (Nelson and Nathke, 2013).
Asef1 (APC-stimulated guanine nucleotide exchange factor) and its paralog Asef2 are Cdc42-activating GEFs (Kawasaki et al., 2007; Kawasaki et al., 2000). The Asef1/2 sequences consist of an APC-Binding Region (ABR) that can bind ARD of APC, the SH3 domain, and the DH-PH tandem domain responsible for GEF activity. Asef1/2 can acquire an autoinhibited conformation, in which the ABR-SH3 domains bind the DH-PH domain, thus blocking its interaction with the GTPase (Hamann et al., 2007; Zhang et al., 2012). Binding of ARD of APC to ABR of Asef1/2 disrupts the autoinhibition and releases the GEF activity of Asef1/2. Although the initial study showed that Asef activates Rac1 (Kawasaki et al., 2000), subsequent analyses demonstrated that Asef1/2 activate Cdc42, but not Rac (Gotthardt and Ahmadian, 2007; Hamann et al., 2007).
IQGAPs are cytoskeletal scaffolding and regulatory proteins that have dozens of binding partners (Abel et al., 2015; Watanabe et al., 2015), including the small GTPases Cdc42 and Rac, APC, other microtubule plus end-tracking proteins (+TIPs), actin filaments, and the stimulators of actin polymerization N-WASP and formin mDia1, as well as itself. IQGAP1 is the best studied member of this family. It is ubiquitously expressed and acts both downstream of active Rac and Cdc42, which stimulate the binding of IQGAP1 to APC and actin filaments, and upstream of these GTPases (Mosaddeghzadeh et al., 2021). Thus, IQGAP1 binding to GTP-Cdc42 stabilizes the active Cdc42 state by inhibiting the GTP hydrolysis. Binding of IQGAP1 to Rac1, on the other hand, appears to involve both positive and negative regulation (Jacquemet and Humphries, 2013).
Amer1 is a membrane-binding protein that contains two binding sites for interaction with phosphoinositides PI(4,5)P2, PI(4)P and PIP3 (Grohmann et al., 2007). Its paralog Amer2 has been shown to be a +TIP (Jiang et al., 2012; Pfister et al., 2012).
KAP3 is a cargo-binding subunit of the heterotrimeric kinesin-2 (KIF3A-KIF3B-KAP3) that was also reported to bind ARD (Jimbo et al., 2002), although another study was not able to detect the KAP3-APC interaction by coimmunoprecipitation from cell lysates (Jaulin and Kreitzer, 2010). However, it detected coimmunoprecipitation of homodimeric kinesin-2 (KIF17) with an N-terminal half of APC, although whether this interaction is mediated by the ARD was not determined.
Vimentin is a structural subunit of intermediate filaments in mesenchymal cells. Intermediate filaments, a third major component of the cytoskeleton, mainly function to provide mechanical integrity and resilience to cells and tissues, but they are also involved in regulation of cell motility and adhesion (Dutour-Provenzano and Etienne-Manneville, 2021). Distribution of vimentin intermediate filaments throughout the cytoplasm strictly depends on microtubules (Goldman, 1971). ARD of APC has been shown to directly bind vimentin intermediate filaments, promote vimentin polymerization in vitro and contribute to the distribution of intermediate filaments along microtubules (Sakamoto et al., 2013).
The APC middle domain (APC-M)
APC-M (aa ~1000–2100) is mainly responsible for APC participation in the β-catenin destruction complex. APC-M is an intrinsically disordered region of APC that binds β-catenin through two types of short imperfect amino acid repeats (McCartney and Nathke, 2008; Minde et al., 2011): four 15-aa repeats in the N-terminal part of the APC-M (aa 1020–1034; 1136–1150; 1155–1169; 1173–1187) and seven 20-aa repeats spread throughout the rest of APC-M (aa 1265–1284; 1378–1397; 1494–1513; 1646–1664; 1850–1869; 1957–1976; 2015–2034). Three additional short sequences interlaced with the 20-aa repeats – the SAMP repeats (aa 1568–1587; 1718–1736; 2033–2051) – bind to Axin, a scaffolding protein within the destruction complex. The middle region of APC2 lacks the 15-aa repeats and has only five 20-aa repeats and two SAMP repeats (Nakagawa et al., 1998; van Es et al., 1999).
The APC C-terminus (APC-C)
APC-C (aa 2130–2843) is the main cytoskeleton interacting domain of APC. It contains three subdomains important for the cytoskeletal functions of APC: the basic domain (aa 2223–2579) that interacts with both actin and microtubules, the EB-binding domain (aa 2782–2831) that enables APC to act as a +TIP, and the PDZ-binding domain (aa 2841–2843). In APC2, the C-terminal region is not conserved and lacks the PDZ-binding motif, but still binds microtubules, actin, and EB proteins (Kahn et al., 2018).
Basic domain
The ability of APC to bind microtubules was discovered when full length APC was ectopically expressed in epithelial cells (Munemitsu et al., 1994). The same study also demonstrated that such localization was mediated by APC-C, and that APC-C can also nucleate and bundle microtubules in vitro. Subsequent data showed that the direct APC-microtubule interaction, bundling and nucleation of microtubules were mediated by the APC basic domain (2219–2580) within APC-C (Deka et al., 1998). An APC-C region overlapping with the end of the basic domain and extending further in the C-terminal direction (aa 2539–2680) has been shown to directly bind kinesin-1 (KIF5) (Ruane et al., 2016). The distinct basic domain in APC2 is still able to bind microtubules (Kahn et al., 2018).
The basic domain, together with additional C-terminal residues, also can nucleate and bundle actin filaments in vitro in a manner that is mutually exclusive with microtubule binding (Moseley et al., 2007; Okada et al., 2010). Two regions, ANS1 (aa 2326–2353) and ANS2 (aa 2526–2612), are critical for actin nucleation. They are responsible for interaction with actin monomers and dimerization, respectively (Juanes et al., 2017; Okada et al., 2010). The basic domain can also link APC to actin filaments indirectly through a formin family protein mDia1, which is an actin nucleator and elongator on its own right (Schonichen and Geyer, 2010). mDia1 can interact with the APC region (aa 2167–2674) encompassing the basic domain (Wen et al., 2004). Although details of APC-mDia1 interaction remain unclear, mDia1 strongly potentiates actin nucleation mediated by the APC basic domain (Breitsprecher et al., 2012; Okada et al., 2010). In the proposed model, the APC basic domain brings together several actin monomers to promote nucleation, whereas mDia1 binds the newly formed barbed (fast-growing) end of the actin filament to accelerate its elongation, thereby producing long unbranched actin filaments. The actin-nucleating activity of the APC basic domain was proposed to be inhibited by a +TIP, EB1 (Juanes et al., 2020). However, since in vitro inhibition of actin nucleation was observed at a very large molar excess of EB1 over APC-C, physiological relevance of this mechanism remains to be further assessed.
EB-binding domain
In addition to direct interaction of APC with microtubules through the basic domain, APC-C can also bind microtubules indirectly using the EB-binding region (Slep et al., 2005). The EB family of +TIPs (EB1–3) can directly recognize the GTP cap of growing microtubules and recruit additional +TIPs that contain either SxIP or Cap-Gly motifs (Akhmanova and Steinmetz, 2015; Galjart, 2010). APC interacts with EB1 (Su et al., 1995) through an SxIP sequence (2803-SQIP-2806) at the C-terminus of APC (Honnappa et al., 2009). An additional SxIP sequence within the basic domain (2537-SRLP-2540) can also contribute to APC-EB1 interaction (Serre et al., 2019). Through binding to EB proteins, APC becomes a part of a large +TIP network at growing microtubule tips, where individual +TIPs are involved in multiple intra- and intermolecular interactions (Gupta et al., 2014).
PDZ-binding motif
A canonical PDZ-binding domain of APC comprises the last three residues of its sequence (TSV). This motif has been shown to interact with two epithelial basolateral polarity proteins, DLG1 (Matsumine et al., 1996) and Scribble (Takizawa et al., 2006), and with a postsynaptic scaffolding protein of excitatory synapses, PSD-95 (Yanai et al., 2000). The crystal structures of the C-terminal peptides of APC bound to the PDZ domains of DLG1 (Zhang et al., 2011) or Scribble (How et al., 2019) suggested that additional residues upstream of the canonical TSV sequence enhanced the interaction. An APC-C fragment containing both the EB- and PDZ-binding regions, but not the basic domain, was found to bind the intermediate chain of cytoplasmic dynein (Gao et al., 2017), the minus end-directed microtubule motor.
APC REGULATION
APC in cells undergoes phosphorylation (Li and Nathke, 2005; Mimori-Kiyosue et al., 2000a), although the exact phosphorylation pattern and significance of individual sites are still poorly understood. Among them, the roles of APC phosphorylation by GSK3β are best characterized. GSK3β-mediated phosphorylation of APC at the 20-aa repeats in APC-M enhances β-catenin binding (Minde et al., 2011), but reduces microtubule binding in vitro and in cells (Etienne-Manneville and Hall, 2003; Zumbrunn et al., 2001), suggesting a mechanism that balances the APC functions between the destruction complex and the cytoskeleton. Consistent with this idea, APC was isolated from cells as two distinct complexes that interacted with microtubules and β-catenin in a mutually exclusive manner (Penman et al., 2005). However, the GSK3β-mediated phosphorylation of APC-M represents a rather indirect way to inhibit APC-microtubule association. It remains unclear whether GSK3β can regulate this interaction more directly, for example, by phosphorylating APC-C.
Multiple consensus phosphorylation sites for the p34cdc2, PKA, CKII and PKC kinases have been identified in APC-C and proposed to regulate the APC-EB interaction (Askham et al., 2000; Trzepacz et al., 1997), which offers a more direct way to regulate APC-microtubule binding by phosphorylation. Consistent with this idea, in vitro phosphorylation by p34cdc2 of the C-terminal region of APC (aa 2560–2943), downstream of the basic domain, inhibited its interaction with EB1 (Nakamura et al., 2001). Interestingly, in vitro dephosphorylation of APC-C increased its ability to bundle actin filaments (Moseley et al., 2007), suggesting that the phosphorylation state of APC-C can balance its binding to either actin or microtubules.
APC phosphorylation was also found to render APC-N more resistant to proteolytic degradation, suggesting that APC phosphorylation affects either APC-N conformation or its interactions with other proteins (Li and Nathke, 2005). Additionally, phosphorylation of S948 in APC-N that allows for binding of 14-3-3 protein was found to enhance APC self-association through SD3 (Li et al., 2008).
APC AND MICROTUBULES
Microtubules are highly dynamic cytoskeleton components. They are intrinsically polar, with a fast-growing plus end and a slow-growing minus end. In cells, microtubule minus ends are usually stabilized, while plus ends undergo constant shrinkage and elongation. The most essential functions of microtubules in cells are intracellular transport, cell division and directional cell migration (Dogterom and Koenderink, 2019). Because of their diverse functions, microtubule dynamics are highly regulated by various microtubule-associated proteins, including APC.
APC colocalizes with microtubules in cells
Overexpressed APC was initially found to localize along the entire length of microtubules (Munemitsu et al., 1994). However, endogenous APC in various cell types was found to localize only at the microtubule tips (Mogensen et al., 2002; Nathke et al., 1996; Rosin-Arbesfeld et al., 2001). It is generally considered that such localization is mediated via EB interaction. This idea is supported by partial and/or transient colocalization of EB1 and APC at APC-positive microtubule tips, although many EB1-positive microtubule tips lacked APC (Kita et al., 2006; Mimori-Kiyosue et al., 2000b; Wen et al., 2004). Also, experiments with exogenous expression of APC fragments demonstrated that an isolated APC basic domain localized along the length of microtubules, whereas the EB1-binding region, either alone or in combination with the basic domain, concentrated at microtubule tips and colocalized with EB1 (Askham et al., 2000; Rosin-Arbesfeld et al., 2001; Zumbrunn et al., 2001). Although the microtubule lattice-binding ability of the APC basic domain is not sufficient for tip localization, it can help stabilize both APC and EB1 at microtubule tips (Serre et al., 2019). The reported inability of EB1 knockdown to affect APC tip localization (Kita et al., 2006) could potentially result from compensation by other EB proteins or from incomplete EB1 knockdown. The lattice-binding ability of the basic domain can also explain existence of a fraction of APC-positive microtubule tips lacking EB proteins (Kita et al., 2006; Koester et al., 2007). Indeed, live cell imaging showed that when a microtubule stops growing, APC can remain at the microtubule tips for seconds after EB proteins dissociate (Kita et al., 2006).
Another mechanism by which APC can localize to microtubule tips is by serving as a kinesin cargo. Both full length APC and an APC mutant lacking the C-terminus were observed to move along microtubules toward the plus end in A6 Xenopus epithelial cells in an ATP-dependent manner (Mimori-Kiyosue et al., 2000a), consistent with the plus end-directed motor activity of kinesins. The translocation of the APC-C-deleted construct likely depended either on heterotrimeric (KIF3A/3B/KAP3) or homodimeric (KIF17) kinesin-2, both of which can bind APC-N and facilitate APC accumulation at the cell periphery (Jaulin and Kreitzer, 2010; Jimbo et al., 2002). The C-terminus-dependent interaction of APC with KIF5 is another potential mechanism, but it seems to contribute mostly to APC stabilization at the microtubule tips, rather than to its delivery (Ruane et al., 2016).
APC forms clusters around microtubule tips
Compared with other +TIPs, APC association with microtubule tips has two distinctive features (Barth et al., 2002; Etienne-Manneville and Hall, 2003; Langford et al., 2006; Nathke et al., 1996). First, both endogenous and ectopically expressed APC are typically present only at a subset of microtubule tips; specifically, at those penetrating into actively extending membrane protrusions. Second, APC forms relatively large clusters at these sites, which only partially colocalize with microtubule tips and can be positioned microns away, indicating that APC clusters are not equivalent to bundled microtubule tips. Additionally, when growing APC-positive microtubules switch to shortening, the tip-associated APC can be left behind and remain stationary until being dispersed or picked up by another microtubule (Kita et al., 2006; Mimori-Kiyosue et al., 2000a). Nonetheless, these clusters still depended on microtubule integrity and gradually dissipated after nocodazole-induced microtubule disassembly (Langford et al., 2006; Mimori-Kiyosue et al., 2000a; Nathke et al., 1996). The microtubule-associated APC clusters were also separate from the APC-containing destruction complexes (Lupo et al., 2016; Penman et al., 2005; Schaefer and Peifer, 2019). The mechanism of APC clustering remains unclear, but it seems to depend on APC-N, as APC constructs lacking ARD failed to incorporate into cortical clusters of endogenous APC at the microtubule tips (Barth et al., 2002). The APC clustering likely involves contribution of the ARD interaction partners. For example, IQGAP1 – an oligomerizing scaffolding protein – can organize large multiprotein complexes and link them to the actin cytoskeleton, whereas Amer1/2 might link such complexes to the plasma membrane. In cultured hippocampal neurons, APC2 clustering at microtubule tips was proposed to be driven by an interaction between the APC2 C-terminus and dynein (Kahn et al., 2018).
Together, the APC localization and clustering at microtubule tips in actively extending cell protrusions of different cell types likely depends on a combination of different mechanisms, including interactions between the APC basic domain and microtubule lattice, between the APC C-terminus with EB proteins, and between APC and kinesin family members (Figure 2). However, how APC binds to selective microtubule tips specifically at the protruding region of the cell but not elsewhere remains an open question. The mechanism of APC clustering also remains to be clarified.
Figure 2.
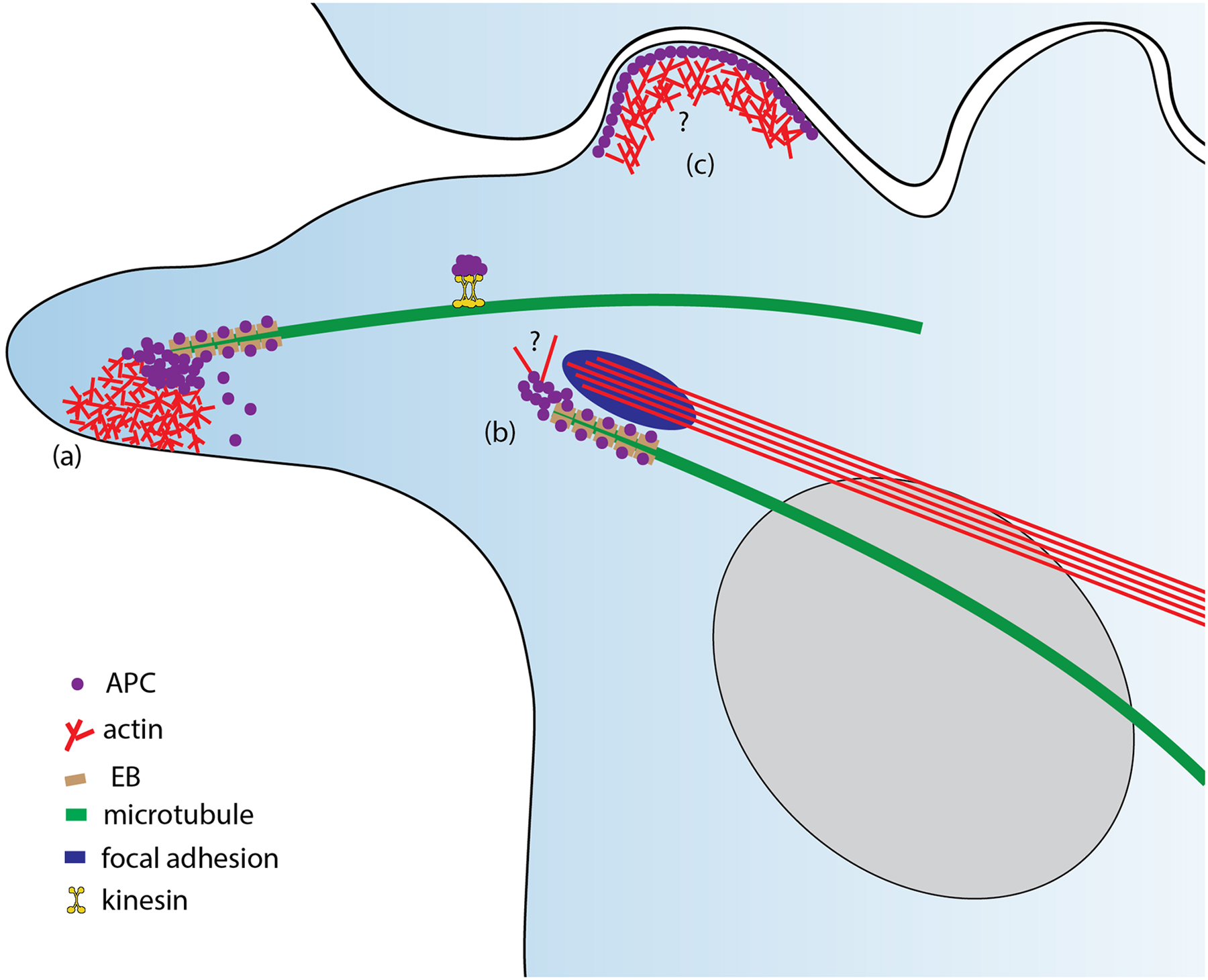
Localization and functions of APC in cells. (a) APC clusters at microtubule tips initiate branched actin assembly at the cell leading edge for membrane protrusion; (b) APC clusters assembled in the vicinity of focal adhesions nucleate linear actin filaments to facilitate focal adhesion turnover by as yet unknown mechanism; (c) APC can localize to cell-cell junctions, but its roles at this location remain unclear. APC can accumulate at microtubule tips through interaction with EB family +TIPs or be delivered by kinesin family motors.
APC stabilizes microtubules
The multimodal association of APC with microtubules raises a question of whether APC affects microtubule dynamics. Collectively, available information from in vitro and in vivo assays leads to a conclusion that APC promotes microtubule assembly and stabilization.
The microtubule-stabilizing potential of APC could already be inferred based on the original in vitro analyses showing that APC-C can nucleate and bundle microtubules (Munemitsu et al., 1994). Subsequent studies demonstrated that the APC fragment comprising both APC-M and APC-C slowed down microtubule depolymerization upon dilution in vitro (Zumbrunn et al., 2001). Furthermore, the EB-binding region of APC-C in combination with EB1 induced tubulin polymerization in vitro and microtubule elongation in permeabilized cells, whereas these proteins had no effect in either assay when added individually (Nakamura et al., 2001). A TIRF microscopy analysis of dynamic microtubules assembled in vitro showed that EB3 and the entire APC-C segment prolonged residence of each other at microtubule tips, which led to faster microtubule elongation, but also to increased catastrophe frequency, which were attributed to the activities of APC and EB3, respectively (Serre et al., 2019).
Consistent with in vitro studies, APC was also found to stabilize microtubules in cells. Thus, overexpression of full length APC or APC-C in Ptk2 cells protected microtubules from depolymerization by nocodazole treatment, whereas deletion of the basic domain from either APC or APC-C significantly reduced this protective effect (Zumbrunn et al., 2001). Overexpressed APC also increased the formation of detyrosinated (stable) microtubules in cells (Wen et al., 2004). Conversely, downregulation of APC reduced the amount of acetylated (stable) microtubules in cells and tissues and made microtubules less resistant to nocodazole treatment (Eom et al., 2014; Hughes et al., 2002; Kroboth et al., 2007; Yokota et al., 2009). Detailed analysis of microtubule dynamics in MDCK cells showed that microtubules possessing endogenous APC at their tips (visualized by an introduced fluorescently labeled non-perturbing APC antibody) spent more time in growth and less time in shortening, as well as exhibited reduced frequencies of catastrophes and rescues, thus leading to overall microtubule stabilization (Kita et al., 2006). A discrepancy with the in vitro study detecting greater catastrophe frequency despite the presence of APC (Serre et al., 2019) can be attributed to accumulation of many more +TIPs at microtubule ends in cells, as compared with the reconstitution system, which can collectively prevent microtubule catastrophes (Gupta et al., 2014). An increase in microtubule acetylation and protection against nocodazole-driven depolymerization was also observed upon overexpression of APC2, while APC2 knockdown in chicken retinal neurons decreased tubulin acetylation and detyrosination (Shintani et al., 2009).
In addition to stabilization of preexisting cytoplasmic microtubules, APC can also facilitate microtubule nucleation at the centrosome. Analysis of mitotic spindle assembly in a cell-free system revealed that APC depletion from Xenopus egg cytoplasmic extracts reduced the size of microtubule asters nucleated at the centrosomes. This phenotype could be rescued by either full length APC or by the APC-M+APC-C fragment, but not by the latter fragment lacking the basic domain (Dikovskaya et al., 2004). Similarly, APC knockdown in U2OS cells, which endogenously express full length APC, impaired the formation of the centrosome-associated microtubule asters induced by the nocodazole treatment and its subsequent washout, while expression of full length APC in colon cancer SW480 cells, which endogenously express only a C-terminally truncated APC (aa 1–1337), stimulated astral microtubule growth (Lui et al., 2016). These effects are likely explained by the APC-dependent stabilization of initial microtubule oligomers nucleated by the γ-tubulin ring complex at the centrosome.
In summary, APC most likely exerts microtubule stabilization effects by binding to the microtubule lattice through the basic domain with contribution from the APC-EB1 interaction.
APC AND THE ACTIN CYTOSKELETON
The actin cytoskeleton is the major force-generating system in cells, which can produce pushing, pulling and resistance forces and also initiate and strengthen cell-cell and cell-matrix adhesions (Svitkina, 2018). Pushing force for protrusion of lamellipodia at the leading edge of migrating cells is predominantly generated by polymerizing branched actin filaments, which are nucleated by the Arp2/3 complex following its activation by nucleation-promoting factors. Pulling or contractile forces are produced by myosin II moving along actin filaments. For adhesion, initial interactions of adhesion receptors (cadherins for cell-cell junctions and integrins for cell-matrix adhesions) with their ligands are stimulated by actin-dependent protrusions, whereas adhesion stabilization requires actin-myosin II-dependent contractility. Although APC was initially viewed exclusively as a microtubule-associated protein in the context of its cytoskeletal functions, accumulating evidence increasingly points to important roles of APC for the actin cytoskeleton as well (Figure 2).
APC colocalizes with actin cytoskeleton in cells
The initial studies that addressed mechanisms of APC localization using pharmacological inhibitors observed that APC clusters in cell protrusions were sensitive to microtubule inhibitors, such as nocodazole, but not to actin polymerization inhibitors (Nathke et al., 1996; Rosin-Arbesfeld et al., 2001; Zhou et al., 2004). More detailed analyses, however, revealed a pool of APC that colocalized with actin at cell-cell junctions in endothelial and epithelial cells in an actin-dependent, but microtubule-independent manner (Harris and Nelson, 2010; Kawasaki et al., 2013; Langford et al., 2006; Reinacher-Schick and Gumbiner, 2001). The microtubule- and actin-dependent pools of APC in cells were dynamically interchangeable, as nocodazole-mediated disruption of microtubules shifted APC from the microtubule-associated clusters to the plasma membrane-associated cortical actin (Rosin-Arbesfeld et al., 2001).
Besides cell-cell junctions, APC localization was also detected in the vicinity of focal adhesions (Iizuka-Kogo et al., 2005; Matsui et al., 2008; Matsumoto et al., 2010; Rosin-Arbesfeld et al., 2001), the cell-matrix interaction sites, which are typically linked to the ends of contractile actin-myosin II bundles, called stress fibers. The APC clusters were juxtaposed to focal adhesions without colocalizing with them. They appeared to be the same as the APC clusters associated with microtubule tips. This correlation suggests that APC can mediate a cross-talk between microtubules and contractile actin bundles associated with focal adhesions.
As it has been noted early on, the microtubule-associated APC clusters are specifically found in actively advancing cell protrusions (Koester et al., 2007; Nathke et al., 1996). APC was also found to colocalize with actin filaments at the leading edge of various cell mammalian types, including fibroblasts (Watanabe et al., 2004), neurons (Caro-Gonzalez et al., 2012; Shimomura et al., 2005), and Schwann cells (Elbaz et al., 2016), as well as in Drosophila S2 cells (Zhou et al., 2011). Given that membrane protrusion at the cell leading edge is normally driven by actin polymerization in an Arp2/3 complex-dependent manner, these observations suggest that APC also mediates a cross-talk between microtubules and protrusive branched actin networks. This possibility has been supported by electron microscopy data showing that APC localizes at the interface between microtubules and branched actin filaments in neuronal growth cones (Efimova et al., 2020).
Mechanisms of APC-actin association
Several mechanisms have been proposed to mediate the APC interaction with the actin cytoskeleton. Given that β-catenin can interact with both APC and cadherin, it was initially thought that APC was localized at cell-cell junctions via the cadherin/β-catenin/α-catenin complex that can interact with actin filaments. However, β-catenin binding to APC and E-cadherin was found to be mutually exclusive, thus ruling out this model (Hulsken et al., 1994; Penman et al., 2005; Rubinfeld et al., 1995). Furthermore, the N-terminal fragment of APC truncated right after the ARD, and thus lacking the β-catenin-binding APC-M, was sufficient for junctional localization of APC in an actin-dependent manner (Langford et al., 2006). This result suggested a role of the ARD interaction partners, such as IQGAP1 that can directly bind actin filaments (Watanabe et al., 2004), in the APC-actin interaction. Asef1/2 also have a potential to indirectly link APC to the actin cytoskeleton, specifically to branched actin networks, because Asef-mediated activation of Cdc42 can stimulate nucleating activity of the Arp2/3 complex through intermediate activation of the Arp2/3 complex activator N-WASP (Rohatgi et al., 1999). This mechanism can explain the localization of APC at the microtubule-branched actin interface (Efimova et al., 2020).
Given that the actin cytoskeleton typically functions in tight association with the plasma membrane, APC localization at the actin-containing cortical structures can be stabilized by APC-membrane links. One such link can be provided by the membrane-binding protein AMER1, which interacts with APC-N and facilitates cortical localization of APC (Grohmann et al., 2007). In the context of full length protein, APC can also be anchored to the plasma membrane by the polarity protein DLG, which binds the C-terminal PDZ-binding motif of APC. This interaction was found to play a role for APC localization at cell protrusions (Etienne-Manneville et al., 2005; Iizuka-Kogo et al., 2005). The APC-membrane anchorage also may be mediated by an ERM (ezrin-radixin-moesin) family protein ezrin, which can interact with membrane components, actin and DLG1 (Aguera-Gonzalez et al., 2017; Lasserre et al., 2010), although it is not clear whether Dlg can simultaneously bind ezrin and APC.
The APC-membrane linkage in the vicinity of focal adhesions can be mediated by APC interactions with focal adhesion components. One candidate is a Wnt-signaling component, Dvl, which was reported to bind both the ARD of APC and the focal adhesion components, paxillin and FAK (Matsumoto et al., 2010). Another candidate is DDEF that also can bind paxillin and FAK, as well as the first SAMP repeat of APC, although this interaction appeared to negatively regulate APC recruitment to focal adhesions (Matsui et al., 2008). The observed accumulation of APC next to focal adhesions resembles the localization of the cortical microtubule stabilization complex (CMSC), which traps microtubule tips at the focal adhesion periphery through a series of protein-protein interactions (Meiring et al., 2019). However, APC has not been formally shown to be a part of this complex.
Regulation of actin structures by APC
Consistent with the APC localization in actively advancing protrusions, downregulation of APC impaired protrusion formation in epithelial cells (Watanabe et al., 2004), fibroblasts (Kroboth et al., 2007), neurons (Chen et al., 2011) and T cells (Juzans et al., 2020), whereas APC overexpression in fibroblasts promoted or stabilized cell protrusions (Kroboth et al., 2007; Mimori-Kiyosue et al., 2007). APC was also found to be necessary for induction of lamellipodia by growth factors in HeLa cells (Kawasaki et al., 2009). Even more impressively, local laser-mediated inactivation of APC at one side of the neuronal growth cone led to growth cone turning toward the other side (Koester et al., 2007). APC knockdown in primary hippocampal neurons decreased the overall F-actin levels in cells, whereas the detailed analysis of actin cytoskeleton architecture by platinum replica electron microscopy revealed that APC deficiency specifically abrogated branched actin filament networks in growth cones, but spared long unbranched actin filaments (Efimova et al., 2020). A decrease in total F-actin levels after APC depletion was also observed in U2OS cells (Juanes et al., 2017). This phenotype was attributed to the ability of APC to directly nucleate actin filaments, as F-actin levels were not rescued by an actin nucleation-deficient APC mutant. In some cells, downregulation of APC had no effect on protrusions (Zaoui et al., 2010) or even increased lamellipodia formation (Elbaz et al., 2016).
APC is also implicated in focal adhesion dynamics, although the reported results are not fully consistent with each other, possibly, reflecting cell type variations. In HeLa cells, APC knockdown inhibited focal adhesion formation (Matsumoto et al., 2010). In contrast, APC knockdown in Xenopus epithelial A6 cells increased focal adhesion size and strengthened cell-substrate attachment, while APC overexpression in these cells increased the number of focal adhesions, although no effects on their size was reported (Matsui et al., 2008; Mimori-Kiyosue et al., 2007). Consistent with the latter results, APC knockdown decreased the number but increased the size of focal adhesions in U2OS cells, which resulted from an impaired focal adhesion turnover (Juanes et al., 2017; Juanes et al., 2019). A positive role of APC in focal adhesion turnover could potentially be linked to its ability to somehow promote longitudinal splitting of focal adhesions (Young and Higgs, 2018). A role of APC in restricting the formation of invadopodia was reported for breast cancer cells (Chanez et al., 2021).
The roles of APC in cell-cell adhesion are poorly understood despite the reported localization of APC to intercellular junctions. A most conclusive study conducted using Drosophila ovary showed that a dAPC loss-of-function mutation in flies led to the formation of large gaps between nurse cells and reduced formation of adherens junctions between epithelial cells in the ovary (Hamada and Bienz, 2002). In mammalian cells, overexpression of APC led to impaired formation of a polarized epithelial monolayer, even though it did not disrupt cell-cell junctions (Mimori-Kiyosue et al., 2007). APC is also important for epithelial cell polarity and proper formation of junctional complexes in other systems, although these functions can involve both cytoskeletal and transcriptional APC activities (Lesko et al., 2014).
ROLES OF APC IN CELL MIGRATION
The ability of cells to migrate as a whole, move their subcellular components, and interact with their neighbors and the extracellular matrix is essential for many normal and pathological aspects of physiology of multicellular organisms. Microtubules and the actin cytoskeleton are the major cellular machinery executing these complex tasks. Toward this end, they carry out their individual functions but also are engaged in multifaceted cooperation. Most proteins that are currently considered as candidates to mediate such cooperation belong to the +TIP family. Among them, APC seems to be a lead candidate due to its ability to interact and regulate both cytoskeleton subsets and influence cell motility, polarity and adhesion.
The individual and cooperative functions of actin and microtubules are most clearly exhibited, and best understood during directional cell migration, which is essential for multiple aspects of normal development and disease. The precise navigation during cell migration is especially critical for neurons, which extend neurites over enormous distances and find exact targets to form functional neural circuitry during development and after injury. In this context, the high expression levels of APC in the brain and existence of the neuron-specific APC2 paralog support the idea of APC proteins being at the heart of cell polarity and navigation. Accordingly, much information about roles of APC in regulating cell migration comes from studies in neurons.
Phenotypic links between APC and cell migration
The role of APC in cell migration could already be gleaned from the FAP phenotypes, which are characterized by aberrant migration patterns of epithelial cells in colonic polyps (Moss et al., 1996) and the intestine of APC mutant mice (Mahmoud et al., 1997; Moser et al., 1995; Oshima et al., 1997; Sansom et al., 2004; Wong et al., 1996). Studies with cultured cells strongly support the notion that cytoskeletal functions of APC play key roles in regulating cell migration. Initial insights into these roles came from a correlation of APC localization with actively protruding cell regions in various epithelial cells and fibroblasts (Nathke et al., 1996; Neufeld and White, 1997), as well as in astrocytes (Etienne-Manneville and Hall, 2003). In addition, cell lines originating from the ApcMin/+ mouse model of FAP, which carry one APC allele truncated at codon 850, exhibited impaired migration in culture (Fenton et al., 2002; Forest et al., 2003). Aberrant migration of T cells isolated from FAP patients was also recently reported (Mastrogiovanni et al., 2022). In developing neurons, APC is particularly enriched at the tip of the axon, which exhibits the fastest growth rate relative to other neurites (Shimomura et al., 2005; Votin et al., 2005). Moreover, APC enrichment at the future axon tip preceded axon specification (Votin et al., 2005), whereas local enrichment of APC within an individual growth cone predicted future direction of growth cone movement (Koester et al., 2007). These observations suggested a causative relationship between APC enrichment and axon growth.
Functional experiments largely support a causative role of APC in cell migration. Thus, APC depletion led to slower cell migration in several cell lines, either due to impaired protrusion formation (Kroboth et al., 2007; Pfister et al., 2012; Watanabe et al., 2004) or abnormal cell-matrix adhesion turnover (Juanes et al., 2017), or both (Mastrogiovanni et al., 2022). In various neuronal cell types, APC deficiency retarded neurite extension (Dobashi et al., 2000; Eom et al., 2014; Kroboth et al., 2007; Namekata et al., 2012), or impaired neuron polarity leading to multidirectional neurite outgrowth, formation of ectopic branches and inability of neurons to respond to guidance cues without affecting the elongation speed per se (Chen et al., 2011; Eom et al., 2014; Yokota et al., 2009). APC also plays important roles in establishing apicobasal polarity of epithelial cells (Lesko et al., 2014; Lesko et al., 2015), polar apical extrusion of dying epithelial cells from an epithelial monolayer (Marshall et al., 2011) or T cell polarity (Mastrogiovanni et al., 2022). APC2 also plays an important role in neuronal navigation, because knockout of this paralog abrogated migratory response of cerebellar granule cells in cultured explants to guidance cues, although APC2 knockout had no effect on migration of these cells in basal conditions (Shintani et al., 2012).
In addition to downregulation approaches, APC functions were investigated using manipulations of its upstream regulators, mainly GSK3β. GSK3β is constitutively active in cells unless it is inhibited by phosphorylation at Ser9 in response to many diverse signaling pathways (reviewed in (Hajka et al., 2021)). As GSK3β-mediated APC phosphorylation inhibits APC-microtubule association (Zumbrunn et al., 2001), manipulations of GSK3β activity or expression are frequently used to investigate roles of APC in cells. However, since GSK3β phosphorylates multiple targets, including several +TIPs, the results of such studies are not strictly conclusive regarding the roles of APC.
An elegant model linking APC to cell polarity has been proposed for directional migration of astrocytes in culture, which was studied using a combination of imaging and inhibitory approaches, including manipulation of GSK3β (Etienne-Manneville and Hall, 2003). According to this model, local activation of Cdc42 at the cell leading edge activates the Par3/Par6/aPKC polarity complex, which then phosphorylates and inhibits GSK3β, thus allowing APC to bind and anchor microtubule plus ends, thereby inducing reorientation of the centrosome toward the cell front, a readout of cell polarization. However, how Cdc42 becomes activated in a polarized manner was not addressed in this study. Local APC accumulation in a GSK3β inactivation-dependent manner was also reported to occur in cultured neurons (Asada and Sanada, 2010; Kim et al., 2006; Shi et al., 2004; Zhou et al., 2004). Neuron polarity in these studies was impaired by either global activation or inactivation of GSK3β, as well as by interference with various signaling pathways triggering local inactivation of GSK3β, suggesting a requirement for a proper level and/or localization of GSK3β activity.
Collectively, the phenotypes of APC downregulation and GSK3β manipulation suggest that APC contributes to cell migration and neurite extension by facilitating local leading edge protrusion not only through proper interpretation of guidance cues and establishment of cell polarity, but also through regulation of cell-matrix adhesions, and possibly, cell-cell junctions.
Molecular mechanisms of APC functions in cell migration
Understanding molecular mechanisms underlying cytoskeletal functions of APC is a daunting task due to diverse activities of APC and its numerous interaction partners. The experimental approaches that have been used to reach this goal include the use of various APC mutants, mostly truncations, and addressing the roles of the APC interaction partners.
Functions of APC domains
Domain-based analyses of APC-dependent mechanisms of cell motility suggest that virtually all functional determinants of APC play a role in at least some aspects of cell motility.
APC-C
The microtubule-binding regions of APC-C (basic and EB-binding domains) were found to be necessary for centrosome reorientation (i.e. cell polarization) in directionally migrating astrocytes (Etienne-Manneville and Hall, 2003) and axon specification in differentiating neurons (Shi et al., 2004), as they were impaired by overexpression of C-terminally truncated APC proteins. The dominant negative effects of such constructs were explained by sequestration of the endogenous full length APC away from microtubules through the APC-N-mediated dimerization. However, a similar approach did not elicit a dominant negative effect in PtK2 cells, but instead increased cell elongation and protrusion formation (Kroboth et al., 2007). These distinct effects could result from different overexpression levels relative to endogenous APC.
Overexpressing the isolated APC-C fragment retarded axon elongation in neurons containing endogenous APC (Zhou et al., 2004), but partially rescued the aberrant branching and protrusion phenotype in APC-deficient neurons (Chen et al., 2011). Together with dominant negative effects of the C-terminally truncated APC proteins, these results suggest that APC interaction with microtubules is necessary but not sufficient for APC-mediated cell polarity. A partial rescue of cell polarity by APC-C could be explained by its interaction with the polarity protein Dlg1, which can partially anchor microtubules to the plasma membrane and has been shown to contribute to astrocyte polarization (Etienne-Manneville et al., 2005). However, expression of APC-C, with or without the Dlg1-binding motif, was sufficient to normalize the orientation of epithelial cell extrusion disrupted by expression of C-terminally truncated APC (Marshall et al., 2011), suggesting that microtubule stabilization by APC-C was sufficient for restoring cell polarity in this system.
APC-N
APC-N has significant contributions to cell migration. An early indication of this role was the observed decrease in cell motility after APC knockdown in the colon cancer cells that expressed only C-terminally truncated APC mutants and lacked full length APC (Kawasaki et al., 2003). On the other hand, expression of APC-N in the APC-deficient neurons rescued excessive axon branching (Chen et al., 2011). These data suggested that although APC-N overexpression could impair normal phenotype through a dominant negative effect, it can mitigate APC deficiency by providing some missing functionality. A convincing evidence for an important role of the APC-N in directional protrusion was obtained using chromophore-assisted laser inactivation (CALI) of APC domains in the context of full length APC protein in growth cones of chick retinal neurons. In these experiments, local application of CALI to one half of the growth cone triggered growth cone turning away from the treated side, if the chromophore was attached to the APC-N-targeting antibody, but toward the illuminated side if APC-M was targeted (Koester et al., 2007). These results suggested that APC-M negatively regulates APC-N, while APC-N is needed for protrusion.
Given existence of several functional subdomains within APC-N, additional studies were aimed to reveal their individual roles for cell migration. A positive role of the APC-N-mediated self-association of APC was uncovered using over-expression of a SD3-containing APC construct, which disrupted APC dimerization, or by inhibiting the 14-3-3 protein, which facilitated the SD3-mediated self-interaction (Li et al., 2008). However, the APC fragment containing both SD1 and SD2, but lacking ARD, was insufficient for rescuing the APC deficiency in cultured cortical neurons (Chen et al., 2011), suggesting that APC oligomerization facilitates activities of other APC domains rather than performs its own functions. The ARD appears to be a key functional module within APC-N for regulation of cell motility. In APC-deficient neurons, expression of ARD was able to partially rescue excessive axon branching (Chen et al., 2011). These key roles of ARD appear to be mediated by its multiple interaction partners (discussed below).
APC-M
The degree of involvement of APC-M, which interacts with the destruction complex components, in cytoskeletal functions of APC is less clear. In general, manipulation of APC expression can change the β-catenin levels, and therefore the cell transcription profile, which could induce the observed phenotypes. Although this mechanism was indeed found to be at play in some cases (Duraikannu et al., 2018; Lang et al., 2013; Rusan et al., 2008; Sansom et al., 2004), other studies showed that the APC-dependent phenotypes were β-catenin independent (Chen et al., 2011; Harris and Nelson, 2010; Lang et al., 2013; Lesko et al., 2015; Lesko and Prosperi, 2017; Nelson et al., 2012; Odenwald et al., 2013; Yokota et al., 2009).
Notably, β-catenin can collaborate with APC not only as a transcriptional regulator, but also as a cytoskeletal protein. In particular, β-catenin was found to partially colocalize with APC clusters in cell protrusions, which were thought to contain a separate APC pool than that in destruction complexes (Faux et al., 2010; Odenwald et al., 2013; Sharma et al., 2006; Votin et al., 2005). On a functional side, expression of a degradation-resistant β-catenin mutant in PC-12 cells inhibited neurite outgrowth in PC-12 cells, whereas HeLa cells acquired an unusually elongated bipolar shape after β-catenin knockdown (Votin et al., 2005). These results suggest that an interaction of β-catenin with APC-M has an inhibitory effect on protrusion-promoting functions of APC. Other studies, however, showed that β-catenin colocalizing with APC clusters improved APC clustering in protrusions of NIH 3T3 cells (Sharma et al., 2006) and stimulated protrusion formation in MDCK cells (Odenwald et al., 2013). These conflicting results suggest that the roles of the β-catenin/APC cooperation or competition for cell migration remain to be further explored.
Roles of APC interaction partners
Among multiple interaction partners of APC, most significant mechanistic insights have been obtained through investigation of roles of Asef, IQGAPs, mDia and microtubule motors.
Asef1/2
Functional interrogation of the roles of Asef-APC interaction suggests that this interaction is important for membrane protrusion. Overexpression of constitutively active Asef1 or Asef2 lacking their autoinhibitory ABR, or coexpression of full length Asef with ARD of APC, stimulated cell migration and formation of lamellipodia (Kawasaki et al., 2007; Kawasaki et al., 2000; Mitin et al., 2007). Similarly, overexpression of Asef1 (Lee et al., 2021) or Asef2 (Evans et al., 2015) in neurons enhanced the formation of dendritic spines – postsynaptic protrusions induced by Arp2/3 complex-dependent actin polymerization. Asef also colocalized with APC at cell-cell junctions in epithelial cells (Kawasaki et al., 2013) and could enhance cell-cell junctions in endothelial cells (Tian et al., 2015). As such junctions are supported in part by branched actin networks (Efimova and Svitkina, 2018), this finding is also consistent with the idea that Asef stimulates branched actin assembly. However, Asef overactivation disrupted cell-cell junctions in epithelial cells indicating a need for its proper regulation (Kawasaki et al., 2003). Disruption of Asef-APC interaction in cells using either the ABR of Asef, or peptides mimicking the Asef-binding pocket in APC, or an N507K APC mutant unable to bind Asef, inhibited lamellipodia formation and cell migration (Jiang et al., 2017; Mitin et al., 2007; Yan et al., 2019).
Importantly, coexpression of full length APC with full length Asef did not enhance lamellipodia formation in MDCK cells beyond the level induced by full length Asef alone, suggesting that APC first needs to be “activated” in order to activate Asef (Kawasaki et al., 2003). The autoinhibition of APC that prevents Asef binding was proposed to be mediated by an interaction between the ARD of APC and sequences within APC-M (Kim et al., 2018). According to this mechanism, the C-terminally truncated APC proteins in colon cancer cells would constitutively activate Asef to promote cell migration, which is likely a part of the cancer promoting mechanism in FAP. How this autoinhibition can be disrupted in the full length APC by normal signaling remains unknown.
IQGAP
IQGAP1 functions are tightly linked to positive regulation of various actin-based protrusions, such as lamellipodia, neuronal growth cones and dendritic spines. Depletion of either IQGAP1 or APC inhibited lamellipodia and polarized migration of Vero cells, although effects of IQGAP1 depletion were stronger (Watanabe et al., 2004). IQGAP1 has multiple ways to stimulate APC functions in protrusion. As an actin-binding protein, IQGAP1 can link APC to actin filaments, which together with its PIP2-binding ability (Choi et al., 2013), can help anchor APC at the plasma membrane-associated actin cortex (Watanabe et al., 2004). IQGAP1 can also promote actin polymerization at microtubule ends by recruiting and stabilizing active Rac and Cdc42, because IQGAP1 can bind ARD of APC simultaneously with GTP-bound forms of Cdc42 and/or Rac (Watanabe et al., 2004). Asef and IQGAP1 also do not obviously compete for binding to ARD, as the ARD carrying the N507K mutation that disrupts Asef binding can still displace IQGAP1 from clusters of endogenous APC (Watanabe et al., 2004). Additionally, Asef and IQGAP1 can be recovered in the same complex by immunoprecipitation (Tian et al., 2015). Thus, APC can integrate both Asef- and IQGAP1-dependent pathways of actin polymerization. In addition, IQGAP1 can directly stabilize actin filaments (Hoeprich et al., 2022) and function as a coactivator of mDia1 (Chen et al., 2020) and N-WASP (Bensenor et al., 2007; Wallrabe et al., 2013) to employ yet additional actin assembly pathways.
mDia
Formins mDia1, mDia2 and mDia3 can nucleate and elongate actin filaments using their FH1-FH2 domain module (Schonichen and Geyer, 2010). These formins can stabilize microtubules using different determinants in the same domains (Bartolini et al., 2008). An initial idea that APC can cooperate with mDia1 for cell motility was suggested by a loss of APC accumulation at the leading edge in mDia1-depleted cells and its enhancement after expression of constitutively active mDia1 (Yamana et al., 2006). Similar observations were subsequently made in mDia1-deficient T cells (Dong et al., 2013). Discovery of direct interaction of mDia proteins with APC suggested a molecular basis for their cooperation (Cheng et al., 2011; Wen et al., 2004). There are two potential mechanisms by which mDia1 can cooperate with APC. First, a combination of microtubule-stabilizing activities of both proteins can make stabilization more efficient if they function as a complex (Wen et al., 2004). Also, mDia1 was proposed to promote GSK3β inactivation through aPKC (Eng et al., 2006), which opens another route to microtubule stabilization. Second, an ability of APC and mDia1 to cooperate for efficient assembly of unbranched actin filaments in vitro (Breitsprecher et al., 2012; Okada et al., 2010) can also contribute to actin-based protrusion in cells. However, the actin-nucleating activity of APC was found to be more essential for proper F-actin levels in cells and focal adhesion turnover with no reported effects on leading edge protrusion (Juanes et al., 2017; Juanes et al., 2019).
Kinesins
APC is able to interact with kinesin-2 family motors (KIF3 and KIF17) via APC-N and with kinesin-1 (KIF5) through APC-C. An interaction of APC with kinesin-2 was reported to facilitate APC localization to microtubule tips (Jaulin and Kreitzer, 2010; Murawala et al., 2009). Accordingly, KIF3A depletion in MDCK cells prevented formation of peripheral APC clusters, and impaired cell migration and polarization (Boehlke et al., 2013; Jaulin and Kreitzer, 2010). Besides delivering APC, kinesin-2 also cotransports the Par3/Par6/aPKC polarity complex (Shi et al., 2004), which is important for cell polarity. Binding of kinesin-1 to APC-C was found to be important for APC localization to cell protrusions in fibroblasts and to axon tips in neurons (Ruane et al., 2016). An ability of APC to act as an RNA-binding protein (Preitner et al., 2014) adds another aspect to its numerous functions. The kinesin-2 heterotrimer (KIF3A-KIF3B-KAP3) (Baumann et al., 2020) and the kinesin-3 member KIF1C (Pichon et al., 2021) can transport multiple APC-bound mRNAs, which encode proteins important for protrusion (Chrisafis et al., 2020; Wang et al., 2017).
INTEGRATED MODEL OF APC FUNCTIONS IN CELL MOTILITY
A conventional model of cell migration involves two main components: (1) protrusion and adhesion of the cell front and (2) retraction and deadhesion of the cell rear. During these events, the actin cytoskeleton generates necessary forces, whereas microtubules can regulate the distribution of these forces. A large knowledge gap in the cytoskeleton field is how microtubules accomplish this task. Available data put APC at the core of this mechanism, as APC can regulate both microtubules and the actin cytoskeleton at the same locality in response to upstream signals (Figure 3).
Figure 3.
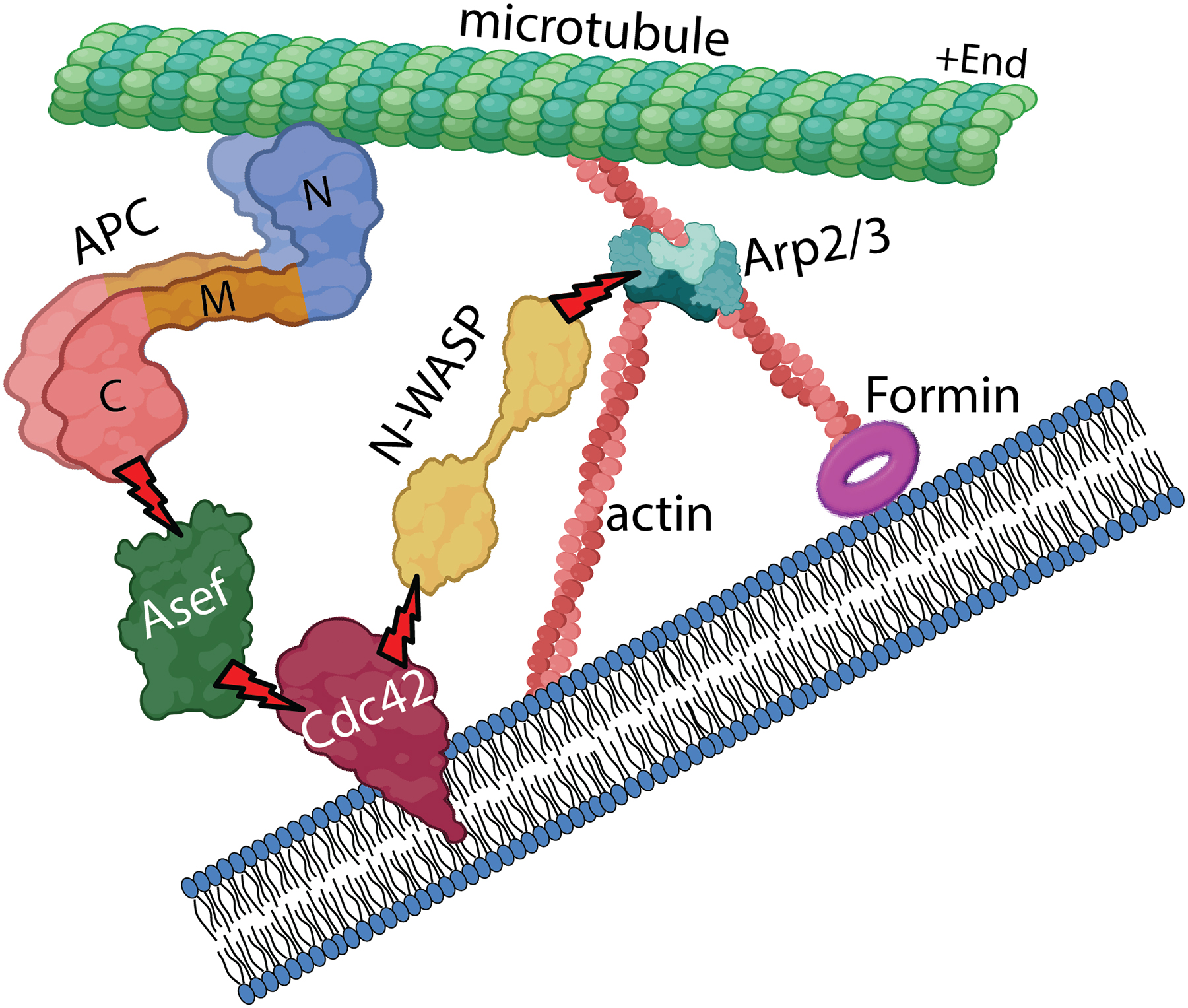
Model for the role of APC in protrusion. APC associates with the plus end of a dynamic microtubule using the APC-C region. When the microtubule encounters the cell edge, an interaction of APC-N with Asef triggers the APC – Asef – Cdc42 – N-WASP – Arp2/3 complex pathway. Activation of the Arp2/3 complex by this pathway leads to the assembly of branched actin filament network, which pushes the plasma membrane. Elongation of some actin barbed ends can be further accelerated by formins. The end result of this mechanism is a local plasma membrane protrusion driven by actin polymerization at the site of the microtubule-plasma membrane encounter. The figure is created with BioRender.com.
APC-mediated regulation of microtubules for cell migration
On the microtubule side, APC mechanistically contributes to microtubule stabilization and anchors microtubules at the site of (future) membrane protrusion. A key question is what good this stabilization does for the protrusion.
Microtubules have been long known to regulate directional cell migration and neuron navigation (Cammarata et al., 2016; Etienne-Manneville, 2013; Pacheco and Gallo, 2016; Vasiliev et al., 1970), but the underlying mechanisms are complex and still not fully understood. Microtubules are best known to support intracellular transport, which enables them to deliver necessary cargo to proper sites. This mechanism in principle can benefit from microtubule stabilization at the site of future protrusion. Indeed, stabilized microtubules often are enriched at the cell front (Dogterom and Koenderink, 2019; Gundersen and Bulinski, 1988) and can promote outgrowth of neuronal processes (Buck and Zheng, 2002; Witte and Bradke, 2008). However, abundant data support the critical need in dynamic microtubules, rather than just stable microtubule tracks, for virtually all migratory processes (Kaverina and Straube, 2011). These conflicting ideas can be reconciled by assuming that the microtubules regulating directional cell migration have stable “trunks” and dynamic tips.
APC is well positioned to reach the goal of generating such “stably dynamic” microtubules by locally retaining microtubule plus ends without blocking their dynamic instability. First, APC can form large but dynamic clusters through multivalent interactions between APC and its interaction partners that have dimerization or oligomerization capabilities including APC itself. Multimolecular clusters of other +TIPs have been recently proposed to exhibit properties of biomolecular condensates and promote microtubule polymerization (Wu et al., 2021). The APC clusters likely also have such function. Second, a special property of the APC clusters is that they are attached to the plasma membrane by their various membrane-binding components, such as Amers, IQGAPs and polarity proteins. This membrane anchorage can facilitate microtubule retention at the plasma membrane, as has been shown for Amer2 (Pfister et al., 2012). The interaction between APC and cortical actin can also contribute to the anchorage of APC clusters. As a result, APC clusters can exhibit their “liquid” nature within a relatively restricted locality. Third, APC binds to the microtubule lattice, thus leaving the actual plus end free to undergo short-range dynamic instability within the limits of the cluster, because the high concentration of +TIPs there would quickly rescue a shortening microtubule tip before it escapes the cluster. Finally, since APC binds microtubules when GSK3β is locally inhibited by upstream signaling, the formation of APC clusters can occur in response to various inputs, thereby defining cell polarity.
Roles of stably dynamic microtubules in cell adhesion
Assuming that APC clusters can recruit and transiently retain stably dynamic microtubules at a specific site, the next question is how these microtubules regulate cell migration. A stable part of the microtubule can serve as a track to bring important cargo. Such mechanism can be indeed at play for the microtubule-stimulated focal adhesion disassembly (Kaverina et al., 1999) through delivery of cargo that degrades focal adhesion from outside, such as using metalloprotease secretion (Stehbens et al., 2014), or from inside by delivering autophagosomes (Kenific et al., 2016) or endocytic machinery (Ezratty et al., 2005). However, even for this mechanism microtubule dynamics was found to be important (Kaverina et al., 2002), suggesting the involvement of +TIPs including APC as a candidate. In this context, the autophagosome delivery to focal adhesions was found to be regulated by APC, specifically, by the ability of APC to nucleate actin filaments (Juanes et al., 2019). However, exact roles of the APC-nucleated actin filaments in this process remain a puzzle.
Roles of stably dynamic microtubules in membrane protrusion
Mechanistic experiments strongly point to the idea that APC can stimulate membrane protrusion. In contrast to focal adhesion turnover, no microtubule-delivered cargo that would be able to induce protrusion has been identified so far. Therefore, a more likely possibility is that the protrusion initiation depends on dynamic microtubule tips, which in turn suggests that protrusions are induced by signaling triggered by +TIPs. This signaling undoubtedly should lead to actin polymerization – the actual driving force for membrane protrusion. Given that branched actin networks are the most powerful and relevant cellular machinery for protrusion, the +TIP-mediated signaling is expected to eventually activate the Arp2/3 complex. Several striking experimental observations in neurons strongly support this possibility. Specifically, when “pioneer” microtubules approach the growth cone periphery or enter a dendritic spine, they induce growth cone advance or turning (Buck and Zheng, 2002; Dent et al., 2003) and spine expansion (Gu et al., 2008; Hu et al., 2011; Hu et al., 2008; Jaworski et al., 2009), respectively. Both of these events are driven by the assembly of branched actin networks (Korobova and Svitkina, 2008; Korobova and Svitkina, 2010) suggesting that microtubules were able to induce Arp2/3 complex activation in these situations.
Given these considerations, APC again takes a center stage as a functional linker between microtubule dynamics and actin assembly. This function of APC clearly depends on its N-terminus, most likely through its ability to stimulate the Asef – Cdc42 – N-WASP – Arp2/3 signaling pathway (Figure 3). As a Cdc42 GEF that can be activated by ARD of APC, Asef1/2 can induce protrusion through Cdc42 and its downstream effectors, one of which is a nucleation-promoting factor N-WASP (Rohatgi et al., 1999). Upon binding GTP-Cdc42, N-WASP can activate the Arp2/3 complex, which then nucleates branched actin filaments to exert pushing force onto the plasma membrane, where Cdc42 is localized upon activation (Osmani et al., 2010). This cascade can be triggered when an APC-positive pioneer microtubule hits the plasma membrane, where APC can encounter its downstream effectors. This model is strongly supported by experimental findings showing that APC localizes at the expected site – an interface between microtubule tips and branched actin networks – and is critically required for branched actin network formation in neuronal growth cones. Moreover, when dynamic APC-positive microtubule tips hit the plasma membrane, a local actin-rich protrusion is formed at that site within seconds (Efimova et al., 2020).
Another Cdc42 effector, the Par3-Par6-aPKC polarity complex (Joberty et al., 2000), can cooperate with the APC – Asef – Cdc42 – N-WASP – Arp2/3 complex pathway to amplify the initial protrusive event. The PAR complex can inhibit GSK3β activity, thus promoting further APC clustering (Etienne-Manneville and Hall, 2003), and induce activation of Rac1 – a more potent driver of lamellipodial protrusion – through Rac GEF Tiam1 (Nishimura et al., 2005).
In the context of membrane protrusion, a role of APC-C-dependent nucleation of unbranched actin filaments in cooperation with mDia1 awaits further elucidation. Given that full activation of the Arp2/3 complex requires its binding to the side of a pre-existing “mother” actin filament (Machesky et al., 1999), the APC-mDia1 actin nucleation pathway is well-positioned to generate mother filaments for the first round of branched nucleation when pre-existing actin filaments are scarce. This exciting idea, however, is not consistent with the actual experimental data showing that APC knockdown in neurons does not have any negative impact on linear actin filaments (Efimova et al., 2020).
CONCLUSIONS
APC has emerged as a key regulator of the microtubule-actin crosstalk during cell protrusion, polarization and adhesion dynamics. APC accomplishes these functions by integrating localization and activities of its various interaction partners. The key outcomes produced by APC-dependent cytoskeletal machinery include “dynamic stabilization” of microtubules at specific locations, recruitment of polarity proteins to impose cell polarity, initiation of downstream signaling events leading to microtubule-associated actin polymerization to induce protrusion, and actin nucleation to stimulate focal adhesion disassembly. Among these functions, microtubule-dependent membrane protrusion has long been most enigmatic. At present, however, it seems to be a best defined molecular mechanism among other functions of microtubules in cell migration.
By focusing here on APC functions in cell migration, we propose an APC-centered model of microtubule-dependent actin-driven membrane protrusion in motile cells. This model integrates the APC-dependent regulation of microtubule dynamics and the APC-dependent initiation of branched actin network assembly. It explains how microtubules can regulate directional migration and achieve high precision of protrusive activity in response to external signals, which is especially important for neurons. This mechanism is consistent with numerous observations of normal cell behavior, as well as phenotypes induced by perturbations of APC functions. However, many postulates and predictions of this model remain to be experimentally tested.
Cell-cell and cell-matrix adhesion are also important factors for cell migration. APC plays important roles for their proper organization and dynamics. However, available data are not yet sufficient to come up with as explicit molecular model that would explain how exactly APC-mediated actin assembly promotes focal adhesion turnover.
Most APC mutations found in colorectal cancer patients result in expression of C-terminally truncated APC proteins with an intact N-terminal region. These mutations can disrupt not only Wnt signaling, but also cytoskeletal functions of APC. First, due to a loss of functions provided by the microtubule-binding C-terminus, truncated APC cannot localize properly to impose cell polarity. Second, by having an incomplete middle domain, the intact N-terminus in cancer-related APC mutants is no longer inhibited through an intramolecular interaction, which leads to uncontrolled cell protrusion and migration. Whereas aberrant Wnt signaling resulting from APC mutations clearly makes important contribution to colorectal cancer, the APC-related neurological disorders may be more heavily dependent on disorganization of cytoskeletal functions of APC. In this context it will be important to advance our knowledge of specific functions of the neuron-specific APC2, as well as APC splice isoforms.
Besides cell migration and adhesion, APC plays important roles in other cytoskeleton-driven cellular activities, which we largely left out of the scope of this review. They include cell division (Fodde et al., 2001; Green and Kaplan, 2003), intermediate filament dynamics (Dutour-Provenzano and Etienne-Manneville, 2021), mRNA and organelle trafficking (Brocardo et al., 2008; Mili et al., 2008; Preitner et al., 2014; Qian et al., 2010; Yasuda et al., 2013), as well as non-cytoskeletal activities in the nucleus (Neufeld and White, 1997) or as a part of the destruction complex (Schaefer and Peifer, 2019). How APC handles all these diverse and intertwined activities is a fascinating topic for future research.
Highlights:
APC regulates both actin and microtubule cytoskeleton
APC stabilizes microtubules while maintaining its dynamics at the plus end
APC stimulates actin-based protrusion by activating APC-Cdc42-N-WASP-Arp2/3 complex pathway
APC promotes focal adhesion dynamics by yet to be understood mechanism
ACKNOWLEDGEMENTS
This work is supported by NIH grant R35 GM 140832 to TS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abel AM, Schuldt KM, Rajasekaran K, Hwang D, Riese MJ, Rao S, Thakar MS, and Malarkannan S. 2015. IQGAP1: Insights into the function of a molecular puppeteer. Mol Immunol 65:336–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguera-Gonzalez S, Burton OT, Vazquez-Chavez E, Cuche C, Herit F, Bouchet J, Lasserre R, Del Rio-Iniguez I, Di Bartolo V, and Alcover A. 2017. Adenomatous polyposis coli defines Treg differentiation and anti-inflammatory function through microtubule-mediated NFAT localization. Cell Rep 21:181–194. [DOI] [PubMed] [Google Scholar]
- Akhmanova A, and Steinmetz MO. 2015. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol 16:711–726. [DOI] [PubMed] [Google Scholar]
- Asada N, and Sanada K. 2010. LKB1-mediated spatial control of GSK3beta and adenomatous polyposis coli contributes to centrosomal forward movement and neuronal migration in the developing neocortex. J Neurosci 30:8852–8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askham JM, Moncur P, Markham AF, and Morrison EE. 2000. Regulation and function of the interaction between the APC tumour suppressor protein and EB1. Oncogene 19:1950–1958. [DOI] [PubMed] [Google Scholar]
- Barth AI, Siemers KA, and Nelson WJ. 2002. Dissecting interactions between EB1, microtubules and APC in cortical clusters at the plasma membrane. J Cell Sci 115:1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini F, Moseley JB, Schmoranzer J, Cassimeris L, Goode BL, and Gundersen GG. 2008. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J Cell Biol 181:523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann S, Komissarov A, Gili M, Ruprecht V, Wieser S, and Maurer SP. 2020. A reconstituted mammalian APC-kinesin complex selectively transports defined packages of axonal mRNAs. Sci Adv 6:eaaz1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensenor LB, Kan HM, Wang N, Wallrabe H, Davidson LA, Cai Y, Schafer DA, and Bloom GS. 2007. IQGAP1 regulates cell motility by linking growth factor signaling to actin assembly. J Cell Sci 120:658–669. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Baraban JM, Johnson RC, Eipper BA, and Mains RE. 1994. High levels of expression of the tumor suppressor gene APC during development of the rat central nervous system. J Neurosci 14:3059–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehlke C, Kotsis F, Buchholz B, Powelske C, Eckardt KU, Walz G, Nitschke R, and Kuehn EW. 2013. Kif3a guides microtubular dynamics, migration and lumen formation of MDCK cells. PLoS One 8:e62165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D, Jaiswal R, Bombardier JP, Gould CJ, Gelles J, and Goode BL. 2012. Rocket launcher mechanism of collaborative actin assembly defined by single-molecule imaging. Science. 336:1164–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocardo M, Lei Y, Tighe A, Taylor SS, Mok MT, and Henderson BR. 2008. Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: regulation of Bcl-2 and implications for cell survival. J Biol Chem 283:5950–5959. [DOI] [PubMed] [Google Scholar]
- Buck KB, and Zheng JQ. 2002. Growth cone turning induced by direct local modification of microtubule dynamics. J Neurosci 22:9358–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarata GM, Bearce EA, and Lowery LA. 2016. Cytoskeletal social networking in the growth cone: How +TIPs mediate microtubule-actin cross-linking to drive axon outgrowth and guidance. Cytoskeleton (Hoboken). 73:461–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro-Gonzalez HY, Nejsum LN, Siemers KA, Shaler TA, Nelson WJ, and Barth AI. 2012. Mitogen-activated protein kinase (MAPK/ERK) regulates adenomatous polyposis coli during growth-factor-induced cell extension. J Cell Sci 125:1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DJ, Santoro IM, and Groden J. 2004. Isoforms of the APC tumor suppressor and their ability to inhibit cell growth and tumorigenicity. Oncogene. 23:7144–7148. [DOI] [PubMed] [Google Scholar]
- Chanez B, Ostacolo K, Badache A, and Thuault S. 2021. EB1 restricts breast cancer cell invadopodia formation and matrix proteolysis via FAK. Cells. 10:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Arora PD, Lai CC, Copeland JW, Moraes TF, McCulloch CA, Lavoie BD, and Wilde A. 2020. The scaffold-protein IQGAP1 enhances and spatially restricts the actin-nucleating activity of Diaphanous-related formin 1 (DIAPH1). J Biol Chem 295:3134–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tian X, Kim WY, and Snider WD. 2011. Adenomatous polyposis coli regulates axon arborization and cytoskeleton organization via its N-terminus. PLoS One 6:e24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Zhang J, Ahmad S, Rozier L, Yu H, Deng H, and Mao Y. 2011. Aurora B regulates formin mDia3 in achieving metaphase chromosome alignment. Dev Cell 20:342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Thapa N, Hedman AC, Li Z, Sacks DB, and Anderson RA. 2013. IQGAP1 is a novel phosphatidylinositol 4,5 bisphosphate effector in regulation of directional cell migration. EMBO J 32:2617–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisafis G, Wang T, Moissoglu K, Gasparski AN, Ng Y, Weigert R, Lockett SJ, and Mili S. 2020. Collective cancer cell invasion requires RNA accumulation at the invasive front. Proc Natl Acad Sci U S A 117:27423–27434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, and Alber T. 2000. Crystal structure of the amino-terminal coiled-coil domain of the APC tumor suppressor. J Mol Biol 301:147–156. [DOI] [PubMed] [Google Scholar]
- Deka J, Kuhlmann J, and Muller O. 1998. A domain within the tumor suppressor protein APC shows very similar biochemical properties as the microtubule-associated protein tau. Eur J Biochem 253:591–597. [DOI] [PubMed] [Google Scholar]
- Dent EW, Tang F, and Kalil K. 2003. Axon guidance by growth cones and branches: common cytoskeletal and signaling mechanisms. Neuroscientist. 9:343–353. [DOI] [PubMed] [Google Scholar]
- Dikovskaya D, Newton IP, and Nathke IS. 2004. The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts. Mol Biol Cell 15:2978–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobashi Y, Katayama K, Kawai M, Akiyama T, and Kameya T. 2000. APC protein is required for initiation of neuronal differentiation in rat pheochromocytoma PC12 cells. Biochem Biophys Res Commun 279:685–691. [DOI] [PubMed] [Google Scholar]
- Dogterom M, and Koenderink GH. 2019. Actin-microtubule crosstalk in cell biology. Nat Rev Mol Cell Biol 20:38–54. [DOI] [PubMed] [Google Scholar]
- Dong B, Zhang SS, Gao W, Su H, Chen J, Jin F, Bhargava A, Chen X, Jorgensen L, Alberts AS, Zhang J, and Siminovitch KA. 2013. Mammalian Diaphanous-related formin 1 regulates GSK3beta-dependent microtubule dynamics required for T cell migratory polarization. PLoS One. 8:e80500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraikannu A, Martinez JA, Chandrasekhar A, and Zochodne DW. 2018. Expression and manipulation of the APC-beta-catenin pathway during peripheral neuron regeneration. Sci Rep 8:13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutour-Provenzano G, and Etienne-Manneville S. 2021. Intermediate filaments. Curr Biol 31:R522–R529. [DOI] [PubMed] [Google Scholar]
- Efimova N, and Svitkina TM. 2018. Branched actin networks push against each other at adherens junctions to maintain cell-cell adhesion. J Cell Biol 217:1827–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova N, Yang C, Chia JX, Li N, Lengner CJ, Neufeld KL, and Svitkina TM. 2020. Branched actin networks are assembled on microtubules by adenomatous polyposis coli for targeted membrane protrusion. J Cell Biol 219:e20200309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz B, Traka M, Kunjamma RB, Dukala D, Brosius Lutz A, Anton ES, Barres BA, Soliven B, and Popko B. 2016. Adenomatous polyposis coli regulates radial axonal sorting and myelination in the PNS. Development. 143:2356–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng CH, Huckaba TM, and Gundersen GG. 2006. The formin mDia regulates GSK3beta through novel PKCs to promote microtubule stabilization but not MTOC reorientation in migrating fibroblasts. Mol Biol Cell 17:5004–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom TY, Stanco A, Guo J, Wilkins G, Deslauriers D, Yan J, Monckton C, Blair J, Oon E, Perez A, Salas E, Oh A, Ghukasyan V, Snider WD, Rubenstein JL, and Anton ES. 2014. Differential regulation of microtubule severing by APC underlies distinct patterns of projection neuron and interneuron migration. Dev Cell 31:677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S 2013. Microtubules in cell migration. Annu Rev Cell Dev Biol 29:471–499. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, and Hall A. 2003. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 421:753–756. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, and Hall A. 2005. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol 170:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JC, Robinson CM, Shi M, and Webb DJ. 2015. The guanine nucleotide exchange factor (GEF) Asef2 promotes dendritic spine formation via Rac activation and spinophilin-dependent targeting. J Biol Chem 290:10295–10308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty EJ, Partridge MA, and Gundersen GG. 2005. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol 7:581–590. [DOI] [PubMed] [Google Scholar]
- Faux MC, Coates JL, Kershaw NJ, Layton MJ, and Burgess AW. 2010. Independent interactions of phosphorylated beta-catenin with E-cadherin at cell-cell contacts and APC at cell protrusions. PLoS One. 5:e14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton JI, Wolff MS, Orth MW, and Hord NG. 2002. Membrane-type matrix metalloproteinases mediate curcumin-induced cell migration in non-tumorigenic colon epithelial cells differing in Apc genotype. Carcinogenesis. 23:1065–1070. [DOI] [PubMed] [Google Scholar]
- Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, van Es JH, Breukel C, Wiegant J, Giles RH, and Clevers H. 2001. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol 3:433–438. [DOI] [PubMed] [Google Scholar]
- Forest V, Clement M, Pierre F, Meflah K, and Menanteau J. 2003. Butyrate restores motile function and actin cytoskeletal network integrity in apc mutated mouse colon epithelial cells. Nutr Cancer 45:84–92. [DOI] [PubMed] [Google Scholar]
- Galjart N 2010. Plus-end-tracking proteins and their interactions at microtubule ends. Curr Biol 20:R528–537. [DOI] [PubMed] [Google Scholar]
- Gao FJ, Shi L, Hines T, Hebbar S, Neufeld KL, and Smith DS. 2017. Insulin signaling regulates a functional interaction between adenomatous polyposis coli and cytoplasmic dynein. Mol Biol Cell 28:587–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman RD 1971. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol 51:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez L, Alvarez J, Weinstein E, and Korenis P. 2015. Familial adenomatous polyposis in an adolescent with coexisting schizophrenia: treatment strategies and implications. Mol Genet Genomic Med 3:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthardt K, and Ahmadian MR. 2007. Asef is a Cdc42-specific guanine nucleotide exchange factor. Biol Chem 388:67–71. [DOI] [PubMed] [Google Scholar]
- Green RA, and Kaplan KB. 2003. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J Cell Biol 163:949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hughes JP, Warrington J, McPherson J, Wasmuth J, Le Paslier D, Abderrahim H, Cohen D, Leppert M, and White R. 1991. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 66:589–600. [DOI] [PubMed] [Google Scholar]
- Grohmann A, Tanneberger K, Alzner A, Schneikert J, and Behrens J. 2007. AMER1 regulates the distribution of the tumor suppressor APC between microtubules and the plasma membrane. J Cell Sci 120:3738–3747. [DOI] [PubMed] [Google Scholar]
- Gu J, Firestein BL, and Zheng JQ. 2008. Microtubules in dendritic spine development. J Neurosci 28:12120–12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, and Bulinski JC. 1988. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci U S A 85:5946–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta KK, Alberico EO, Nathke IS, and Goodson HV. 2014. Promoting microtubule assembly: A hypothesis for the functional significance of the +TIP network. Bioessays. 36:818–826. [DOI] [PubMed] [Google Scholar]
- Hajka D, Budziak B, Pietras L, Duda P, McCubrey JA, and Gizak A. 2021. GSK3 as a regulator of cytoskeleton architecture: Consequences for health and disease. Cells. 10:2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada F, and Bienz M. 2002. A Drosophila APC tumour suppressor homologue functions in cellular adhesion. Nat Cell Biol 4:208–213. [DOI] [PubMed] [Google Scholar]
- Hamann MJ, Lubking CM, Luchini DN, and Billadeau DD. 2007. Asef2 functions as a Cdc42 exchange factor and is stimulated by the release of an autoinhibitory module from a concealed C-terminal activation element. Mol Cell Biol 27:1380–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ES, and Nelson WJ. 2010. Adenomatous polyposis coli regulates endothelial cell migration independent of roles in beta-catenin signaling and cell-cell adhesion. Mol Biol Cell 21:2611–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeprich GJ, Sinclair AN, Shekhar S, and Goode BL. 2022. Single-molecule imaging of IQGAP1 regulating actin filament dynamics. Mol Biol Cell 33:ar2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, Grigoriev I, van Rijssel FJ, Buey RM, Lawera A, Jelesarov I, Winkler FK, Wuthrich K, Akhmanova A, and Steinmetz MO. 2009. An EB1-binding motif acts as a microtubule tip localization signal. Cell. 138:366–376. [DOI] [PubMed] [Google Scholar]
- How JY, Caria S, Humbert PO, and Kvansakul M. 2019. Crystal structure of the human Scribble PDZ1 domain bound to the PDZ-binding motif of APC. FEBS Lett 593:533–542. [DOI] [PubMed] [Google Scholar]
- Hu X, Ballo L, Pietila L, Viesselmann C, Ballweg J, Lumbard D, Stevenson M, Merriam E, and Dent EW. 2011. BDNF-induced increase of PSD-95 in dendritic spines requires dynamic microtubule invasions. J Neurosci 31:15597–15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Viesselmann C, Nam S, Merriam E, and Dent EW. 2008. Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci 28:13094–13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AH, Nelson WJ, and Weis WI. 1997. Three-dimensional structure of the armadillo repeat region of beta-catenin. Cell. 90:871–882. [DOI] [PubMed] [Google Scholar]
- Hughes SA, Carothers AM, Hunt DH, Moran AE, Mueller JD, and Bertagnolli MM. 2002. Adenomatous polyposis coli truncation alters cytoskeletal structure and microtubule stability in early intestinal tumorigenesis. J Gastrointest Surg 6:868–874; discussion 875. [DOI] [PubMed] [Google Scholar]
- Hulsken J, Birchmeier W, and Behrens J. 1994. E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. J Cell Biol 127:2061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka-Kogo A, Shimomura A, and Senda T. 2005. Colocalization of APC and DLG at the tips of cellular protrusions in cultured epithelial cells and its dependency on cytoskeletons. Histochem Cell Biol 123:67–73. [DOI] [PubMed] [Google Scholar]
- Jacquemet G, and Humphries MJ. 2013. IQGAP1 is a key node within the small GTPase network. Small GTPases. 4:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaulin F, and Kreitzer G. 2010. KIF17 stabilizes microtubules and contributes to epithelial morphogenesis by acting at MT plus ends with EB1 and APC. J Cell Biol 190:443–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J, Krugers H, Defilippi P, Akhmanova A, and Hoogenraad CC. 2009. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 61:85–100. [DOI] [PubMed] [Google Scholar]
- Jiang H, Deng R, Yang X, Shang J, Lu S, Zhao Y, Song K, Liu X, Zhang Q, Chen Y, Chinn YE, Wu G, Li J, Chen G, Yu J, and Zhang J. 2017. Peptidomimetic inhibitors of APC-Asef interaction block colorectal cancer migration. Nat Chem Biol 13:994–1001. [DOI] [PubMed] [Google Scholar]
- Jiang K, Toedt G, Montenegro Gouveia S, Davey NE, Hua S, van der Vaart B, Grigoriev I, Larsen J, Pedersen LB, Bezstarosti K, Lince-Faria M, Demmers J, Steinmetz MO, Gibson TJ, and Akhmanova A. 2012. A proteome-wide screen for mammalian SxIP motif-containing microtubule plus-end tracking proteins. Curr Biol 22:1800–1807. [DOI] [PubMed] [Google Scholar]
- Jimbo T, Kawasaki Y, Koyama R, Sato R, Takada S, Haraguchi K, and Akiyama T. 2002. Identification of a link between the tumour suppressor APC and the kinesin superfamily. Nat Cell Biol 4:323–327. [DOI] [PubMed] [Google Scholar]
- Joberty G, Petersen C, Gao L, and Macara IG. 2000. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2:531–539. [DOI] [PubMed] [Google Scholar]
- Joslyn G, Richardson DS, White R, and Alber T. 1993. Dimer formation by an N-terminal coiled coil in the APC protein. Proc Natl Acad Sci U S A. 90:11109–11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juanes MA, Bouguenina H, Eskin JA, Jaiswal R, Badache A, and Goode BL. 2017. Adenomatous polyposis coli nucleates actin assembly to drive cell migration and microtubule-induced focal adhesion turnover. J Cell Biol 216:2859–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juanes MA, Fees CP, Hoeprich GJ, Jaiswal R, and Goode BL. 2020. EB1 directly regulates APC-mediated actin nucleation. Curr Biol 30:4763–4772 e4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juanes MA, Isnardon D, Badache A, Brasselet S, Mavrakis M, and Goode BL. 2019. The role of APC-mediated actin assembly in microtubule capture and focal adhesion turnover. J Cell Biol 218:3415–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juzans M, Cuche C, Rose T, Mastrogiovanni M, Bochet P, Di Bartolo V, and Alcover A. 2020. Adenomatous polyposis coli modulates actin and microtubule cytoskeleton at the immunological synapse to tune CTL functions. Immunohorizons. 4:363–381. [DOI] [PubMed] [Google Scholar]
- Kahn OI, Schatzle P, van de Willige D, Tas RP, Lindhout FW, Portegies S, Kapitein LC, and Hoogenraad CC. 2018. APC2 controls dendrite development by promoting microtubule dynamics. Nat Commun 9:2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, and Small JV. 1999. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol 146:1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, and Small JV. 2002. Regulation of substrate adhesion dynamics during cell motility. Int J Biochem Cell Biol 34:746–761. [DOI] [PubMed] [Google Scholar]
- Kaverina I, and Straube A. 2011. Regulation of cell migration by dynamic microtubules. Semin Cell Dev Biol 22:968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Furukawa S, Sato R, and Akiyama T. 2013. Differences in the localization of the adenomatous polyposis coli-Asef/Asef2 complex between adenomatous polyposis coli wild-type and mutant cells. Cancer Sci 104:1135–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Sagara M, Shibata Y, Shirouzu M, Yokoyama S, and Akiyama T. 2007. Identification and characterization of Asef2, a guanine-nucleotide exchange factor specific for Rac1 and Cdc42. Oncogene. 26:7620–7267. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Sato R, and Akiyama T. 2003. Mutated APC and Asef are involved in the migration of colorectal tumour cells. Nat Cell Biol 5:211–215. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Senda T, Ishidate T, Koyama R, Morishita T, Iwayama Y, Higuchi O, and Akiyama T. 2000. Asef, a link between the tumor suppressor APC and G-protein signaling. Science. 289:1194–1197. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Tsuji S, Sagara M, Echizen K, Shibata Y, and Akiyama T. 2009. Adenomatous polyposis coli and Asef function downstream of hepatocyte growth factor and phosphatidylinositol 3-kinase. J Biol Chem 284:22436–22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenific CM, Wittmann T, and Debnath J. 2016. Autophagy in adhesion and migration. J Cell Sci 129:3685–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SB, Zhang L, Yoon J, Lee J, Min J, Li W, Grishin NV, Moon YA, Wright WE, and Shay JW. 2018. Truncated Adenomatous polyposis coli mutation induces Asef-activated Golgi fragmentation. Mol Cell Biol 38:e00135–00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Zhou FQ, Zhou J, Yokota Y, Wang YM, Yoshimura T, Kaibuchi K, Woodgett JR, Anton ES, and Snider WD. 2006. Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron. 52:981–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K, Wittmann T, Nathke IS, and Waterman-Storer CM. 2006. Adenomatous polyposis coli on microtubule plus ends in cell extensions can promote microtubule net growth with or without EB1. Mol Biol Cell 17:2331–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester MP, Muller O, and Pollerberg GE. 2007. Adenomatous polyposis coli is differentially distributed in growth cones and modulates their steering. J Neurosci 27:12590–12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, and Svitkina T. 2008. Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell 19:1561–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, and Svitkina T. 2010. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell 21:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroboth K, Newton IP, Kita K, Dikovskaya D, Zumbrunn J, Waterman-Storer CM, and Nathke IS. 2007. Lack of adenomatous polyposis coli protein correlates with a decrease in cell migration and overall changes in microtubule stability. Mol Biol Cell 18:910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunttas-Tatli E, Roberts DM, and McCartney BM. 2014. Self-association of the APC tumor suppressor is required for the assembly, stability, and activity of the Wnt signaling destruction complex. Mol Biol Cell 25:3424–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J, Maeda Y, Bannerman P, Xu J, Horiuchi M, Pleasure D, and Guo F. 2013. Adenomatous polyposis coli regulates oligodendroglial development. J Neurosci 33:3113–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford KJ, Askham JM, Lee T, Adams M, and Morrison EE. 2006. Examination of actin and microtubule dependent APC localisations in living mammalian cells. BMC Cell Biol 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasserre R, Charrin S, Cuche C, Danckaert A, Thoulouze MI, de Chaumont F, Duong T, Perrault N, Varin-Blank N, Olivo-Marin JC, Etienne-Manneville S, Arpin M, Di Bartolo V, and Alcover A. 2010. Ezrin tunes T-cell activation by controlling Dlg1 and microtubule positioning at the immunological synapse. EMBO J 29:2301–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Yoo KS, Park YS, and Kim HK. 2021. Activity of Arhgef4 is modulated through Staufen1 in neurons. Neurosci Lett 756:135962. [DOI] [PubMed] [Google Scholar]
- Lesko AC, Goss KH, and Prosperi JR. 2014. Exploiting APC function as a novel cancer therapy. Curr Drug Targets. 15:90–102. [DOI] [PubMed] [Google Scholar]
- Lesko AC, Goss KH, Yang FF, Schwertner A, Hulur I, Onel K, and Prosperi JR. 2015. The APC tumor suppressor is required for epithelial cell polarization and three-dimensional morphogenesis. Biochim Biophys Acta 1854:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesko AC, and Prosperi JR. 2017. Epithelial Membrane Protein 2 and beta1 integrin signaling regulate APC-mediated processes. Exp Cell Res 350:190–198. [DOI] [PubMed] [Google Scholar]
- Li L, Yu J, and Ji SJ. 2021. Axonal mRNA localization and translation: local events with broad roles. Cell Mol Life Sci 78:7379–7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Kroboth K, Newton IP, and Nathke IS. 2008. Novel self-association of the APC molecule affects APC clusters and cell migration. J Cell Sci 121:1916–1925. [DOI] [PubMed] [Google Scholar]
- Li Z, and Nathke IS. 2005. Tumor-associated NH2-terminal fragments are the most stable part of the adenomatous polyposis coli protein and can be regulated by interactions with COOH-terminal domains. Cancer Res 65:5195–5204. [DOI] [PubMed] [Google Scholar]
- Liou GI, Samuel S, Matragoon S, Goss KH, Santoro I, Groden J, Hunt RC, Wang F, Miller SS, Caldwell RB, Rustgi AK, Singh H, and Marcus DM. 2004. Alternative splicing of the APC gene in the neural retina and retinal pigment epithelium. Mol Vis 10:383–391. [PubMed] [Google Scholar]
- Lui C, Ashton C, Sharma M, Brocardo MG, and Henderson BR. 2016. APC functions at the centrosome to stimulate microtubule growth. Int J Biochem Cell Biol 70:39–47. [DOI] [PubMed] [Google Scholar]
- Lupo B, Vialard J, Sassi F, Angibaud P, Puliafito A, Pupo E, Lanzetti L, Comoglio PM, Bertotti A, and Trusolino L. 2016. Tankyrase inhibition impairs directional migration and invasion of lung cancer cells by affecting microtubule dynamics and polarity signals. BMC Biol 14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, Hall ME, and Pollard TD. 1999. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci U S A. 96:3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud NN, Boolbol SK, Bilinski RT, Martucci C, Chadburn A, and Bertagnolli MM. 1997. Apc gene mutation is associated with a dominant-negative effect upon intestinal cell migration. Cancer Res 57:5045–5050. [PubMed] [Google Scholar]
- Marshall TW, Lloyd IE, Delalande JM, Nathke I, and Rosenblatt J. 2011. The tumor suppressor adenomatous polyposis coli controls the direction in which a cell extrudes from an epithelium. Mol Biol Cell 22:3962–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiovanni M, Vargas P, Rose T, Cuche C, Esposito E, Juzans M, Laude H, Schneider A, Bernard M, Goyard S, Renaudat C, Ungeheuer MN, Delon J, Alcover A, and Di Bartolo V. 2022. The tumor suppressor adenomatous polyposis coli regulates T lymphocyte migration. Sci Adv 8:eabl5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui C, Kaieda S, Ikegami T, and Mimori-Kiyosue Y. 2008. Identification of a link between the SAMP repeats of adenomatous polyposis coli tumor suppressor and the Src homology 3 domain of DDEF. J Biol Chem 283:33006–33020. [DOI] [PubMed] [Google Scholar]
- Matsumine A, Ogai A, Senda T, Okumura N, Satoh K, Baeg GH, Kawahara T, Kobayashi S, Okada M, Toyoshima K, and Akiyama T. 1996. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science. 272:1020–1023. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Fumoto K, Okamoto T, Kaibuchi K, and Kikuchi A. 2010. Binding of APC and dishevelled mediates Wnt5a-regulated focal adhesion dynamics in migrating cells. Embo J 29:1192–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney BM, and Nathke IS. 2008. Cell regulation by the Apc protein Apc as master regulator of epithelia. Curr Opin Cell Biol 20:186–193. [DOI] [PubMed] [Google Scholar]
- Meiring JCM, Shneyer BI, and Akhmanova A. 2019. Generation and regulation of microtubule network asymmetry to drive cell polarity. Curr Opin Cell Biol 62:86–95. [DOI] [PubMed] [Google Scholar]
- Mili S, Moissoglu K, and Macara IG. 2008. Genome-wide screen reveals APC-associated RNAs enriched in cell protrusions. Nature. 453:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Matsui C, Sasaki H, and Tsukita S. 2007. Adenomatous polyposis coli (APC) protein regulates epithelial cell migration and morphogenesis via PDZ domain-based interactions with plasma membranes. Genes Cells. 12:219–233. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, and Tsukita S. 2000a. Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J Cell Biol 148:505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, and Tsukita S. 2000b. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol 10:865–868. [DOI] [PubMed] [Google Scholar]
- Minde DP, Anvarian Z, Rudiger SG, and Maurice MM. 2011. Messing up disorder: how do missense mutations in the tumor suppressor protein APC lead to cancer? Mol Cancer. 10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitin N, Betts L, Yohe ME, Der CJ, Sondek J, and Rossman KL. 2007. Release of autoinhibition of ASEF by APC leads to CDC42 activation and tumor suppression. Nat Struct Mol Biol 14:814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen MM, Tucker JB, Mackie JB, Prescott AR, and Nathke IS. 2002. The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells. J Cell Biol 157:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn JL, Alexander J, Pirone A, Palka CD, Lee SY, Mebane L, Haydon PG, and Jacob MH. 2014. Adenomatous polyposis coli protein deletion leads to cognitive and autism-like disabilities. Mol Psychiatry. 19:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosaddeghzadeh N, Nouri K, Krumbach OHF, Amin E, Dvorsky R, and Ahmadian MR. 2021. Selectivity determinants of RHO GTPase binding to IQGAPs. Int J Mol Sci 22:12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley JB, Bartolini F, Okada K, Wen Y, Gundersen GG, and Goode BL. 2007. Regulated binding of adenomatous polyposis coli protein to actin. J Biol Chem 282:12661–12668. [DOI] [PubMed] [Google Scholar]
- Moser AR, Shoemaker AR, Connelly CS, Clipson L, Gould KA, Luongo C, Dove WF, Siggers PH, and Gardner RL. 1995. Homozygosity for the Min allele of Apc results in disruption of mouse development prior to gastrulation. Dev Dyn 203:422–433. [DOI] [PubMed] [Google Scholar]
- Moss SF, Liu TC, Petrotos A, Hsu TM, Gold LI, and Holt PR. 1996. Inward growth of colonic adenomatous polyps. Gastroenterology. 111:1425–1432. [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Souza B, Muller O, Albert I, Rubinfeld B, and Polakis P. 1994. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res 54:3676–3681. [PubMed] [Google Scholar]
- Murawala P, Tripathi MM, Vyas P, Salunke A, and Joseph J. 2009. Nup358 interacts with APC and plays a role in cell polarization. J Cell Sci 122:3113–3122. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Murata Y, Koyama K, Fujiyama A, Miyoshi Y, Monden M, Akiyama T, and Nakamura Y. 1998. Identification of a brain-specific APC homologue, APCL, and its interaction with beta-catenin. Cancer Res 58:5176–5181. [PubMed] [Google Scholar]
- Nakamura M, Zhou XZ, and Lu KP. 2001. Critical role for the EB1 and APC interaction in the regulation of microtubule polymerization. Curr Biol 11:1062–1067. [DOI] [PubMed] [Google Scholar]
- Namekata K, Harada C, Guo X, Kimura A, Kittaka D, Watanabe H, and Harada T. 2012. Dock3 stimulates axonal outgrowth via GSK-3beta-mediated microtubule assembly. J Neurosci 32:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S, and Sharma R. 2015. Molecular mechanism of adenomatous polyposis coli-induced blockade of base excision repair pathway in colorectal carcinogenesis. Life Sci 139:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathke IS, Adams CL, Polakis P, Sellin JH, and Nelson WJ. 1996. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol 134:165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S, and Nathke IS. 2013. Interactions and functions of the adenomatous polyposis coli (APC) protein at a glance. J Cell Sci 126:873–877. [DOI] [PubMed] [Google Scholar]
- Nelson SA, Li Z, Newton IP, Fraser D, Milne RE, Martin DM, Schiffmann D, Yang X, Dormann D, Weijer CJ, Appleton PL, and Nathke IS. 2012. Tumorigenic fragments of APC cause dominant defects in directional cell migration in multiple model systems. Dis Model Mech 5:940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KL, and White RL. 1997. Nuclear and cytoplasmic localizations of the adenomatous polyposis coli protein. Proc Natl Acad Sci U S A. 94:3034–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, and Kaibuchi K. 2005. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol 7:270–277. [DOI] [PubMed] [Google Scholar]
- Odenwald MA, Prosperi JR, and Goss KH. 2013. APC/beta-catenin-rich complexes at membrane protrusions regulate mammary tumor cell migration and mesenchymal morphology. BMC Cancer. 13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Bartolini F, Deaconescu AM, Moseley JB, Dogic Z, Grigorieff N, Gundersen GG, and Goode BL. 2010. Adenomatous polyposis coli protein nucleates actin assembly and synergizes with the formin mDia1. J Cell Biol 189:1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onouchi T, Kobayashi K, Sakai K, Shimomura A, Smits R, Sumi-Ichinose C, Kurosumi M, Takao K, Nomura R, Iizuka-Kogo A, Suzuki H, Kondo K, Akiyama T, Miyakawa T, Fodde R, and Senda T. 2014. Targeted deletion of the C-terminus of the mouse adenomatous polyposis coli tumor suppressor results in neurologic phenotypes related to schizophrenia. Mol Brain 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H, Oshima M, Kobayashi M, Tsutsumi M, and Taketo MM. 1997. Morphological and molecular processes of polyp formation in Apc(delta716) knockout mice. Cancer Res 57:1644–1649. [PubMed] [Google Scholar]
- Osmani N, Peglion F, Chavrier P, and Etienne-Manneville S. 2010. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol 191:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco A, and Gallo G. 2016. Actin filament-microtubule interactions in axon initiation and branching. Brain Res Bull 126:300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Berg S, and Reynolds AB. 1994. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 76:789–791. [DOI] [PubMed] [Google Scholar]
- Penman GA, Leung L, and Nathke IS. 2005. The adenomatous polyposis coli protein (APC) exists in two distinct soluble complexes with different functions. J Cell Sci 118:4741–4750. [DOI] [PubMed] [Google Scholar]
- Pfister AS, Hadjihannas MV, Rohrig W, Schambony A, and Behrens J. 2012. Amer2 protein interacts with EB1 protein and adenomatous polyposis coli (APC) and controls microtubule stability and cell migration. J Biol Chem 287:35333–35340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon X, Moissoglu K, Coleno E, Wang T, Imbert A, Robert MC, Peter M, Chouaib R, Walter T, Mueller F, Zibara K, Bertrand E, and Mili S. 2021. The kinesin KIF1C transports APC-dependent mRNAs to cell protrusions. RNA. 27:1528–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner N, Quan J, Nowakowski DW, Hancock ML, Shi J, Tcherkezian J, Young-Pearse TL, and Flanagan JG. 2014. APC is an RNA-binding protein, and its interactome provides a link to neural development and microtubule assembly. Cell. 158:368–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Perchiniak EM, Sun K, and Groden J. 2010. The mitochondrial protein hTID-1 partners with the caspase-cleaved adenomatous polyposis cell tumor suppressor to facilitate apoptosis. Gastroenterology. 138:1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinacher-Schick A, and Gumbiner BM. 2001. Apical membrane localization of the adenomatous polyposis coli tumor suppressor protein and subcellular distribution of the beta-catenin destruction complex in polarized epithelial cells. J Cell Biol 152:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggleman B, Wieschaus E, and Schedl P. 1989. Molecular analysis of the armadillo locus: uniformly distributed transcripts and a protein with novel internal repeats are associated with a Drosophila segment polarity gene. Genes Dev. 3:96–113. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, and Kirschner MW. 1999. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 97:221–231. [DOI] [PubMed] [Google Scholar]
- Rosin-Arbesfeld R, Ihrke G, and Bienz M. 2001. Actin-dependent membrane association of the APC tumour suppressor in polarized mammalian epithelial cells. EMBO J 20:5929–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruane PT, Gumy LF, Bola B, Anderson B, Wozniak MJ, Hoogenraad CC, and Allan VJ. 2016. Tumour suppressor Adenomatous polyposis coli (APC) localisation is regulated by both Kinesin-1 and Kinesin-2. Sci Rep 6:27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Munemitsu S, and Polakis P. 1995. The APC protein and E-cadherin form similar but independent complexes with alpha-catenin, beta-catenin, and plakoglobin. J Biol Chem 270:5549–5555. [DOI] [PubMed] [Google Scholar]
- Rusan NM, Akong K, and Peifer M. 2008. Putting the model to the test: are APC proteins essential for neuronal polarity, axon outgrowth, and axon targeting? J Cell Biol 183:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y, Boeda B, and Etienne-Manneville S. 2013. APC binds intermediate filaments and is required for their reorganization during cell migration. J Cell Biol 200:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, Clarke AR, and Winton DJ. 2004. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 18:1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro IM, and Groden J. 1997. Alternative splicing of the APC gene and its association with terminal differentiation. Cancer Res 57:488–494. [PubMed] [Google Scholar]
- Schaefer KN, and Peifer M. 2019. Wnt/beta-catenin signaling regulation and a role for biomolecular condensates. Dev Cell 48:429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonichen A, and Geyer M. 2010. Fifteen formins for an actin filament: A molecular view on the regulation of human formins. Biochim Biophys Acta 1803:152–163. [DOI] [PubMed] [Google Scholar]
- Serre L, Stoppin-Mellet V, and Arnal I. 2019. Adenomatous polyposis coli as a scaffold for microtubule end-binding proteins. J Mol Biol 431:1993–2005. [DOI] [PubMed] [Google Scholar]
- Sharma M, Leung L, Brocardo M, Henderson J, Flegg C, and Henderson BR. 2006. Membrane localization of adenomatous polyposis coli protein at cellular protrusions: targeting sequences and regulation by beta-catenin. J Biol Chem 281:17140–17149. [DOI] [PubMed] [Google Scholar]
- Shi SH, Cheng T, Jan LY, and Jan YN. 2004. APC and GSK-3beta are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol 14:2025–2032. [DOI] [PubMed] [Google Scholar]
- Shimomura A, Kohu K, Akiyama T, and Senda T. 2005. Subcellular localization of the tumor suppressor protein APC in developing cultured neurons. Neurosci Lett 375:81–86. [DOI] [PubMed] [Google Scholar]
- Shintani T, Ihara M, Tani S, Sakuraba J, Sakuta H, and Noda M. 2009. APC2 plays an essential role in axonal projections through the regulation of microtubule stability. J Neurosci 29:11628–11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T, Takeuchi Y, Fujikawa A, and Noda M. 2012. Directional neuronal migration is impaired in mice lacking adenomatous polyposis coli 2. J Neurosci 32:6468–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slep KC, Rogers SL, Elliott SL, Ohkura H, Kolodziej PA, and Vale RD. 2005. Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the microtubule plus end. J Cell Biol 168:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehbens SJ, Paszek M, Pemble H, Ettinger A, Gierke S, and Wittmann T. 2014. CLASPs link focal-adhesion-associated microtubule capture to localized exocytosis and adhesion site turnover. Nat Cell Biol 16:561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LK, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, Trent J, Vogelstein B, and Kinzler KW. 1995. APC binds to the novel protein EB1. Cancer Res 55:2972–2977. [PubMed] [Google Scholar]
- Svitkina T 2018. The actin cytoskeleton and actin-based motility. Cold Spring Harb Perspect Biol 10:a018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa S, Nagasaka K, Nakagawa S, Yano T, Nakagawa K, Yasugi T, Takeuchi T, Kanda T, Huibregtse JM, Akiyama T, and Taketani Y. 2006. Human scribble, a novel tumor suppressor identified as a target of high-risk HPV E6 for ubiquitin-mediated degradation, interacts with adenomatous polyposis coli. Genes Cells. 11:453–464. [DOI] [PubMed] [Google Scholar]
- Thliveris A, Samowitz W, Matsunami N, Groden J, and White R. 1994. Demonstration of promoter activity and alternative splicing in the region 5’ to exon 1 of the APC gene. Cancer Res 54:2991–2995. [PubMed] [Google Scholar]
- Tian Y, Gawlak G, Shah AS, Higginbotham K, Tian X, Kawasaki Y, Akiyama T, Sacks DB, and Birukova AA. 2015. Hepatocyte growth factor-induced Asef-IQGAP1 complex controls cytoskeletal remodeling and endothelial barrier. J Biol Chem 290:4097–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzepacz C, Lowy AM, Kordich JJ, and Groden J. 1997. Phosphorylation of the tumor suppressor adenomatous polyposis coli (APC) by the cyclin-dependent kinase p34. J Biol Chem 272:21681–21684. [DOI] [PubMed] [Google Scholar]
- van der Wal T, and van Amerongen R. 2020. Walking the tight wire between cell adhesion and WNT signalling: a balancing act for beta-catenin. Open Biol 10:200267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Kirkpatrick C, van de Wetering M, Molenaar M, Miles A, Kuipers J, Destree O, Peifer M, and Clevers H. 1999. Identification of APC2, a homologue of the adenomatous polyposis coli tumour suppressor. Curr Biol 9:105–108. [DOI] [PubMed] [Google Scholar]
- Vasiliev JM, Gelfand IM, Domnina LV, Ivanova OY, Komm SG, and Olshevskaja LV. 1970. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol 24:625–640. [PubMed] [Google Scholar]
- Votin V, Nelson WJ, and Barth AI. 2005. Neurite outgrowth involves adenomatous polyposis coli protein and beta-catenin. J Cell Sci 118:5699–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrabe H, Cai Y, Sun Y, Periasamy A, Luzes R, Fang X, Kan HM, Cameron LC, Schafer DA, and Bloom GS. 2013. IQGAP1 interactome analysis by in vitro reconstitution and live cell 3-color FRET microscopy. Cytoskeleton (Hoboken). 70:819–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Hamilla S, Cam M, Aranda-Espinoza H, and Mili S. 2017. Extracellular matrix stiffness and cell contractility control RNA localization to promote cell migration. Nat Commun 8:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Azuma Y, Friedman DB, Coffey RJ, and Neufeld KL. 2009. Novel association of APC with intermediate filaments identified using a new versatile APC antibody. BMC Cell Biol 10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Wang S, and Kaibuchi K. 2015. IQGAPs as key regulators of actin-cytoskeleton dynamics. Cell Struct Funct 40:69–77. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, and Kaibuchi K. 2004. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell 7:871–883. [DOI] [PubMed] [Google Scholar]
- Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, and Gundersen GG. 2004. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol 6:820–830. [DOI] [PubMed] [Google Scholar]
- Witte H, and Bradke F. 2008. The role of the cytoskeleton during neuronal polarization. Curr Opin Neurobiol 18:479–487. [DOI] [PubMed] [Google Scholar]
- Wong MH, Hermiston ML, Syder AJ, and Gordon JI. 1996. Forced expression of the tumor suppressor adenomatosis polyposis coli protein induces disordered cell migration in the intestinal epithelium. Proc Natl Acad Sci U S A. 93:9588–9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YO, Bryant AT, Nelson NT, Madey AG, Fernandes GF, and Goodson HV. 2021. Overexpression of the microtubule-binding protein CLIP-170 induces a +TIP network superstructure consistent with a biomolecular condensate. PLoS One. 16:e0260401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamana N, Arakawa Y, Nishino T, Kurokawa K, Tanji M, Itoh RE, Monypenny J, Ishizaki T, Bito H, Nozaki K, Hashimoto N, Matsuda M, and Narumiya S. 2006. The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol Cell Biol 26:6844–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H, Hashimoto N, Koyama K, Nakagawa H, Nakamura Y, and Noguchi K. 2002. Expression of Apc2 during mouse development. Brain Res Gene Expr Patterns. 1:107–114. [DOI] [PubMed] [Google Scholar]
- Yan XQ, Wang ZC, Qi PF, Li G, and Zhu HL. 2019. Design, synthesis and biological evaluation of 2-H pyrazole derivatives containing morpholine moieties as highly potent small molecule inhibitors of APC-Asef interaction. Eur J Med Chem 177:425–447. [DOI] [PubMed] [Google Scholar]
- Yanai H, Satoh K, Matsumine A, and Akiyama T. 2000. The colorectal tumour suppressor APC is present in the NMDA-receptor-PSD-95 complex in the brain. Genes Cells. 5:815–822. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Zhang H, Loiselle D, Haystead T, Macara IG, and Mili S. 2013. The RNA-binding protein Fus directs translation of localized mRNAs in APC-RNP granules. J Cell Biol 203:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Kim WY, Chen Y, Wang X, Stanco A, Komuro Y, Snider W, and Anton ES. 2009. The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron. 61:42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LE, and Higgs HN. 2018. Focal adhesions undergo longitudinal splitting into fixed-width units. Curr Biol 28:2033–2045 e2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaoui K, Benseddik K, Daou P, Salaun D, and Badache A. 2010. ErbB2 receptor controls microtubule capture by recruiting ACF7 to the plasma membrane of migrating cells. Proc Natl Acad Sci U S A. 107:18517–18522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, and Shay JW. 2017. Multiple roles of APC and its therapeutic implications in colorectal cancer. J Natl Cancer Inst 109:djw332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chen L, Gao L, Lin K, Zhu L, Lu Y, Shi X, Gao Y, Zhou J, Xu P, Zhang J, and Wu G. 2012. Structural basis for the recognition of Asef by adenomatous polyposis coli. Cell Res 22:372–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li H, Chen L, Lu X, Zhang J, Xu P, Lin K, and Wu G. 2011. Molecular basis for the recognition of adenomatous polyposis coli by the Discs Large 1 protein. PLoS One. 6:e23507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FQ, Zhou J, Dedhar S, Wu YH, and Snider WD. 2004. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 42:897–912. [DOI] [PubMed] [Google Scholar]
- Zhou MN, Kunttas-Tatli E, Zimmerman S, Zhouzheng F, and McCartney BM. 2011. Cortical localization of APC2 plays a role in actin organization but not in Wnt signaling in Drosophila. J Cell Sci 124:1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbrunn J, Kinoshita K, Hyman AA, and Nathke IS. 2001. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr Biol 11:44–49. [DOI] [PubMed] [Google Scholar]


