Abstract
Neuropathic pain in rodents can be driven by ectopic spontaneous activity (SA) generated by sensory neurons in dorsal root ganglia (DRG). The recent demonstration that SA in dissociated human DRG neurons is associated with reported neuropathic pain in patients enables detailed comparison of pain-linked electrophysiological alterations driving SA in human DRG neurons to alterations that distinguish SA in nociceptors from SA in low-threshold mechanoreceptors (LTMRs) in rodent neuropathy models. Analysis of recordings from dissociated somata of patient-derived DRG neurons showed that SA and corresponding pain in both sexes were significantly associated with the three functional electrophysiological alterations sufficient to generate SA in the absence of extrinsic depolarizing inputs. These include enhancement of depolarizing spontaneous fluctuations of membrane potential (DSFs), which were analyzed quantitatively for the first time in human DRG neurons. The functional alterations were indistinguishable from SA-driving alterations reported for nociceptors in rodent chronic pain models. Irregular, low-frequency DSFs in human DRG neurons closely resemble DSFs described in rodent nociceptors while differing substantially from the high-frequency sinusoidal oscillations described in rodent LTMRs. These findings suggest that conserved physiological mechanisms of SA in human nociceptor somata can drive neuropathic pain despite documented cellular differences between human and rodent DRG neurons.
Perspective:
Electrophysiological alterations in human sensory neurons associated with patient-reported neuropathic pain include all three of the functional alterations that logically can promote spontaneous activity. The similarity of distinctively altered spontaneous depolarizations in human DRG neurons and rodent nociceptors suggests that spontaneously active human nociceptors can persistently promote neuropathic pain in patients.
Keywords: Spontaneous pain, Neuropathic pain, Ectopic activity, Hyperexcitability, Depolarizing spontaneous fluctuation
Introduction
Most people with neuropathic pain experience spontaneous pain27. Both spontaneous and evoked neuropathic pain result from dysregulation of neuronal activity within central and peripheral somatosensory pathways17,27. Alterations in primary afferent neurons are prominent in neuropathic conditions and offer promise as therapeutic targets that may minimize central side-effects of treatment21,60,69. Neuropathic pain has been linked to a persistent conversion of electrically silent sensory neurons into a state of spontaneous activity (SA), observed both in low-threshold mechanoreceptor neurons (LTMRs)21,24,72,80 and in primary nociceptors. Because activation of nociceptors produces conscious pain in humans51, sufficient SA in nociceptors would be expected to promote spontaneous pain. Nociceptor SA recorded microneurographically from nociceptors of patients correlates with reported neuropathic pain38,57,67 and phantom limb pain56. Experimental blockade of ongoing activity (from both nociceptors and LTMRs) in a neuropathic nerve34 or in a DRG that had innervated an amputated limb73 dramatically reduced patients’ ongoing pain. Persistent SA recorded from probable nociceptors in vivo or in vitro also occurs in diverse animal models of neuropathic pain8,10,12,22,23,70,71,75,82. Nociceptor SA can originate in peripheral terminals13,78, and ectopically along axons at sites of damage or inflammation11,30, or in cell somata within a DRG8.
The most extensive mechanistic analysis of SA40,48,64 has been on somal SA induced acutely by axonal injury in large Aβ DRG neurons, most of which in rodents are LTMRs26. In contrast, little is known about how electrophysiological alterations in nociceptors produce SA. Spontaneous generation of action potentials (APs) requires that membrane potential exceed AP threshold. Assuming that AP generation is critical for DRG neuron function, only three intrinsic functional alterations directly related to membrane potential can lead to spontaneous generation of APs in a previously silent neuron: depolarization of resting membrane potential (RMP), lowering (hyperpolarization) of AP threshold, and enhancement of DSFs to transiently exceed AP threshold. A rat model of chronic neuropathic pain (spinal cord injury, SCI) revealed that all three of these functional electrophysiological alterations were associated with SA in dissociated neurons exhibiting properties of primary nociceptors such as capsaicin sensitivity58. The same SA-driving alterations were found in dissociated mouse DRG neurons after SCI9 or cisplatin treatment41. SA and slow, irregular DSFs described in somata of probable nociceptors in these neuropathy models showed striking differences from the SA and rapid, subthreshold sinusoidal oscillations of membrane potential investigated in Aβ neurons (largely LTMRs).
SA in dissociated DRG neurons was associated recently with self-reported neuropathic pain in human patients caused by tumors compressing a spinal nerve or DRG55. While some similarities in the electrophysiological alterations in human and rodent sensory neurons were noted54,55, major questions remained about how closely electrophysiological alterations supporting pain-linked SA in human DRG neurons parallel those reported in rodent DRG neurons, and whether the human alterations are more like those described in LTMRs or nociceptors of rodents. These questions are important because, while rodent sensory neurons have provided nearly all the existing mechanistic information about SA generation in DRG neurons, emerging findings of cellular differences between rodent and human DRG neurons raise the possibility of divergent SA mechanisms across these taxa15,33,54,65,66,68,84. Here we show that multiple hyperexcitable alterations in DRG neuron somata linked to SA and neuropathic pain in human patients more closely parallel those previously described in primary nociceptors than in LTMRs of rodents.
Methods
General design and premise
The experimental design derived from the availability of electrophysiological recordings from dissociated DRG neurons harvested from patients with self-reported pain in dermatomes corresponding to receptive fields of many of the recorded neurons. Using dissociated neuronal somata trades off the inevitable cellular complications of surgical isolation and culturing against the unique advantages of whole-cell patch recording to reveal details of electrophysiological alterations that might be correlated with human pain. A major premise was that useful information about mechanisms driving ongoing human pain could be extracted from neuronal somata removed from their natural environment and damaged by experimental procedures needed to gain access for recording. Encouraging this premise were a previous report that somal generation of neuropathy-induced SA was preserved after recording from a DRG neuron in vivo and then after it was isolated52, and a report showing a remarkable parallel in the pattern and discharge frequency of SA generated in the somata of probable nociceptors in vivo and after dissociation8. Our design centered on planned comparisons of electrically silent neurons versus neurons exhibiting SA, and of neurons from DRGs corresponding to dermatomes without reported pain versus those with pain. The current study design differed from previous designs54,55 by 1) including quantitative analysis of DSF incidence and patterns plus automated measurement of RMP, 2) applying more rigorous electrophysiological criteria for accepting sampled neurons for analysis, 3) increasing the number of patients providing DRG neurons to sample, and 4) explicitly comparing detailed properties of the sampled human DRG neurons to corresponding properties of probable LTMRs and probable nociceptors described in rodents.
Study approval
Written informed consent for participation, including use of tissue samples, was obtained from each patient prior to inclusion in the study. The protocol was reviewed and approved by the M.D. Anderson Institutional Review Boards and all experiments conform to relevant guidelines and regulations.
Clinical data collection
Clinical data were collected as described previously55. Data from 27 patients are included in this analysis, 16 from the study by North et al.55 and 11 added later. All patients (19 males and 8 females, ranging in age from 41 to 71) received treatment at MD Anderson Cancer Center for malignant tumors involving the spine. Data came from retrospective review of medical records plus medical history and symptoms reported at the time of study enrollment.55 Preoperative MRI was evaluated for radiographic evidence of spinal cord or nerve root compression. Evidence of compression was found in all but one of the patients accepted for intensive neurophysiological analysis, and axial spine pain was present in all of these patients. Therefore, the pain analyses here did not include compression or axial pain as analytic factors, focusing exclusively on radicular neuropathic pain determined for the dermatomes associated with each DRG31. Pain was deemed present within the peripheral receptive field of a DRG taken from an included patient if symptoms of spontaneous pain, paresthesia, dysesthesia, hyperalgesia, or allodynia were documented in an ipsilateral distribution within two classically defined dermatomes of the harvested ganglion. The most common complaint, occurring in a majority of the patients, was spontaneous pain. Neuropathic pain was considered absent from that distribution if the patient had no history of relevant symptoms in those dermatomes or if the ganglion came from a patient having only contralateral symptoms. Although some patients had a history of chemotherapy, the peripheral fields of the DRG investigated were distant from dermatomes affected by length-dependent neuropathy.
Human DRG neuron preparation
DRG neuron somata were prepared and recorded electrophysiologically as described43,46,55, based on earlier studies7,19. Briefly, Spinal roots were ligated proximal to the DRG, the spinal root sharply cut both proximal and distal to the DRG, and excised DRG were transferred immediately into sterile balanced salt solution (~4°C) containing nutrients. DRG were transported to the laboratory on ice in a sterile, sealed 50-mL centrifuge tube. In the laboratory, each ganglion was carefully dissected from the surrounding connective tissue and sectioned into three to four parts. One or two sections of DRG were further cut into several 1–2 mm pieces and prepared for recording by digestion in 2 mL of (w/v, final concentration) 0.1% trypsin (Sigma, T9201), 0.1% collagenase Sigma, C1764), and 0.01% DNase (Sigma, D5025) diluted in DMEM/F12. The pieces were transferred to a 37°C rotator to shake with speed 124–128/min. Every 20 min, the pieces were allowed to settle and the supernatant containing dissociated cells was collected and transferred to F12/DMEM with trypsin inhibitor. Supernatant was replaced with 2 mL fresh digestion solution. The tissue was returned to the 37°C rotator and this process was repeated until tissue fragments were well digested. Dissociated cells were centrifuged at 180 g for 5 minutes, supernatant removed, and gently resuspended in culture medium with DMEM/F12 supplemented with 10% horse serum, 2 mM glutamine and 25 ng/ mL hNGF. Cells were plated onto poly-lysine-coated cover slips and cultured at 37°C with 5% CO2 for 24–48 hours prior to electrophysiological experiments.
Whole-cell patch recording from dissociated DRG neurons
Whole-cell patch recordings from dissociated somata were made at room temperature within 24–48 hours of plating using a Multiclamp 700B amplifier and Digidata 1550 interface controlled by pClamp 11 software (Molecular Devices, San Jose, CA). Borosilicate glass pipettes (World Precision Instruments; Sarasota, Fl) were pulled on a P-97 micropipette puller (Sutter Instrument; Novato, CA). DRG neurons were perfused with extracellular solution containing (in mM) 117 NaCl, 3.6 KCl, 25 NaHCO3, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, and 30.5 Glucose (adjusted to pH 7.3 with NaOH) at 34°C and constantly bubbled with O2. The recording electrode was filled with a solution containing 135 mM K-gluconate, 5 mM KCl, 5 mM Mg-ATP, 0.5 mM Na2GTP, 5 mM HEPES, 2 mM MgCl2, 5 mM EGTA, and 0.5 mM CaCl2 adjusted to pH 7.4 with KOH. Similar pipette solutions have been used in various studies of DRG neurons, including human DRG neurons19, but the low Cl− concentration may result in a more negative Cl− equilibrium potential and potentially more negative membrane potentials and lower excitability than normal for DRG neurons62,76.
Series resistance was compensated to above 70%. Whole cell mode was established under voltage clamp, during which membrane capacitance was measured. The recording mode was switched to current clamp. To determine rheobase (the minimum current required to initiate an AP) and the properties of single APs (Fig 1), a series of incremental 300–400 ms depolarizing current steps (10 to 100 pA) were injected at 5-s intervals until AP threshold was reached. AP voltage threshold was estimated from the largest subthreshold depolarization during the depolarizing steps at or below rheobase (e.g.,28,58). This usually occurred at the beginning of the step (Fig 1) but could also occur during a DSF later in the step, as observed previously in dissociated rodent nociceptors58. As shown in Figure 1, the first AP at rheobase was used to measure AP amplitude (relative to AP voltage threshold), AP overshoot, AP rise time (from the point where AP threshold was reached), AP fall time (from the AP peak back to the threshold value), AP duration at half amplitude, and afterhyperpolarization (AHP) amplitude (relative to AP threshold).
Figure 1.
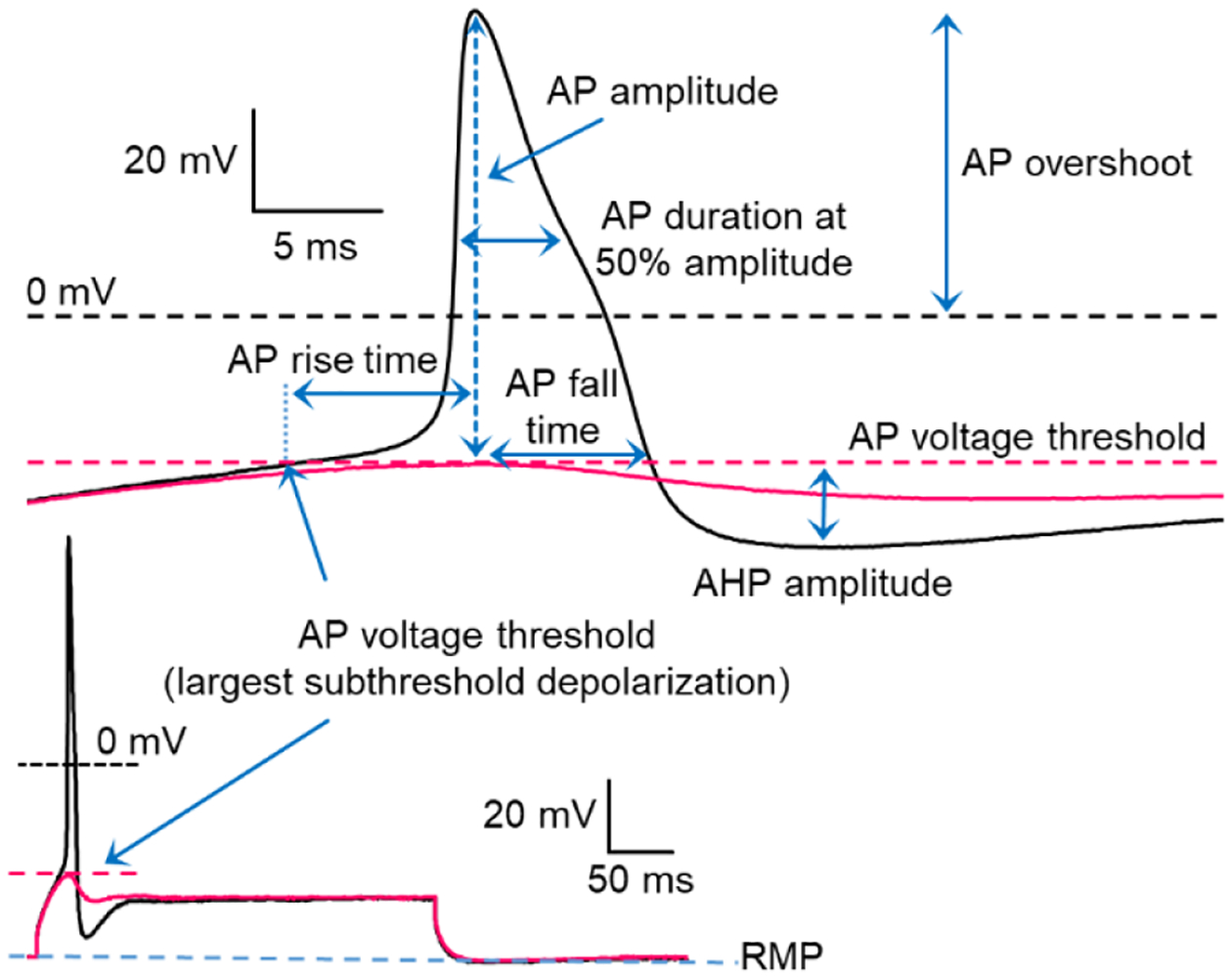
Representative action potential (AP) evoked at rheobase depicting AP measurements used in this study. Rheobase was defined as the minimal current required to evoke an AP during an incrementing series of 300–400 ms pulses (see lower traces). AP voltage threshold (dashed red line) was defined as the largest subthreshold depolarization recorded at rheobase (solid black trace) or during (solid red trace) the last subthreshold depolarizing step. AP rise time, AP amplitude, AP fall time, and afterhyperpolarization (AHP) were measured relative to AP threshold. AP duration at half the AP amplitude captured part of the shoulder (note inflection) typically seen in the falling phase of the AP in the tested neurons.
Following the rheobase tests, spontaneous fluctuations of membrane potential and any SA were recorded for 2 minutes. A 30-second segment starting at or shortly after 60 seconds into this period was selected for intensive electrophysiological analysis. SA was defined as the spontaneous occurrence during this segment of one or more rapidly depolarizing spikes that reached 0 mV or above. This segment also was used to measure DSFs, as previously described58. DSFs were quantified with the electrophysiological analysis program, FIBSI.py14, written with the Anaconda v2019.7.0.0 (Anaconda, Inc, Austin, TX) distribution of Python v3.5.2, and the matplotlib.pyplot and NumPy libraries. Briefly, FIBSI.py reads time and voltage coordinate data extracted from pClamp and calculates slow changes in RMP using a sliding median function (with a 1-second window for these analyses). This sliding median was used to compute the mean RMP during the segment. Using the sliding median as the reference, FIBSI.py quantifies all depolarizing spontaneous fluctuations (DSFs) and hyperpolarizing spontaneous fluctuations (HSFs) exceeding user-defined minima (1.5 mV amplitude and 10 ms duration in this study). Output includes time and voltage coordinates for each spontaneous fluctuation. Amplitudes of DSFs suprathreshold for triggering APs were calculated conservatively as the difference between RMP (sliding median) at the computed start of a suprathreshold DSF and the amplitude of the largest DSF measured during the entire 30-second sample.
Inclusion criteria for all 133 neurons from 27 patients used for intensive analysis were the following: known donor sex, known source DRG level and side, reported presence or absence of neuropathic pain in the dermatomes corresponding to the excised DRG, SA status determined during a ≥ 3-minute observation period, negative AP voltage threshold, positive AP overshoot, and a ≥ 30-second recording period with sampling rate of 1–10 KHz (usually 10 KHz) for DSF analysis randomly selected during the SA observation period. In addition, a measured RMP ≤ −40 mV 2 min after beginning current clamp was demanded for continued testing of a neuron, while a RMP ≤ −35 mV measured during the later DSF analysis segment was required for inclusion in the final dataset (as explained in the next section, the actual RMPs were up to ~15 mV more negative than the measured values). Inclusion decisions for patients and DRG neurons were made by an investigator blinded to the pain association and SA status of the neurons. For 10 of 27 patients, the neurosurgeon performing the surgery and harvest of DRGs (R.Y.N.) also conducted the electrophysiological recordings, making blind data collection impossible in these cases. However, no significant differences were found in the data collected by this investigator and the other electrophysiologist (Y.L.), who was fully blinded. Furthermore, possible unconscious bias in some of the electrophysiological measurements was avoided by using the automated FIBSI program.
Uncertainties in membrane potential measurements
Whole-cell patch experiments involve uncertainties in the measurement of membrane potential because of the liquid junction potential at the pipette tip, which is zeroed out prior to breaking into a cell. The magnitude of the liquid junction potential changes with time after break-in as the contents of the pipette and cell meet and mix, and the mixing will take longer for cells with larger volumes. Given sufficient time for equilibration after break-in, the liquid junction potential during recording can be estimated approximately5, which would be ~15 mV with our solutions. However, we chose to trade off the significant times likely to be needed for pipette equilibration in recordings from relatively large human DRG neurons against the need to sample sufficiently large numbers of these neurons (which represent a rare opportunity because they came from patients with documented pain conditions) in order to detect statistically significant differences in electrophysiological properties across the neuronal groups of interest. Thus, we tested electrophysiological properties rapidly (within ~5 minutes of break-in), making liquid junction potential estimates highly uncertain, and we report the uncorrected values for all membrane potential measurements, as done in the preceding study55 and another study on human DRG neurons using similar intracellular and extracellular solutions19. This means that the actual membrane potentials were up to 15 mV more negative than the values reported in this paper, and that the RMP inclusion cutoffs may have been closer to −55 mV (testing criterion early in the recordings) and −50 mV (DSF analysis criterion later in the recording) than the measured −40 and −35 mV criterion values. While the absolute membrane potential values are uncertain, this uncertainty should not affect the differences found between groups (i.e., neurons with SA versus without SA, and neurons associated with pain versus without pain) in RMP, AP threshold, AP overshoot, and other measured or derived membrane potentials.
Characterization of DSF irregularity
Following the quantitative DSF analysis with the automated FIBSI program, inter-event intervals (IEI) between the DSF peak-to-peak time points in each neuron were measured to determine whether groups of neurons (i.e., with SA and without SA, having dermatomes with and without pain) exhibited preferred IEI values. To identify the dominant frequencies of voltage fluctuations in neurons, the voltage time series of each neuron was normalized to its sliding median (an optional output of the FIBSI program that eliminates linear trends) and analyzed using the periodogram function (TSA: Time Series Analysis package v1.3) in R statistical software v4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Finally, autocorrelations of each neuron with up to a 5 s lag were used to assess randomness (cyclic periods of high and low correlation would suggest that non-random components exist within the signal). The normalized time series of each neuron was analyzed using the acf (autocorrelation) function in R. Default settings for the periodogram and acf functions were used.
Statistical analysis
Normality was assessed using the D’Agostino & Pearson omnibus and Shapiro-Wilk tests. Normally distributed data are presented as means ± standard error of the mean (SEM), and other data as medians with interquartile range. Incidence of SA or DSFs (% of neurons sampled) is also reported as the absolute number of neurons with SA or DSFs over the sample total. Comparisons between groups were performed using an unpaired t-test with Welch’s correction or Mann-Whitney U test, and incidence measures were compared using Fisher’s exact test. Statistical significance was set at P < 0.05 and all reported values are two-tailed.
Data Availability
The neurophysiological data can be shared on request. The FIBSI.py source code and a detailed tutorial for using our Frequency-Independent Biology Signal Identification analysis program are available on a Github repository titled “FIBSI Project” by user rmcassidy (https://github.com/rmcassidy/FIBSI_program).
Results
Spontaneous activity in dissociated human DRG neurons is irregular and more likely in smaller neurons
All neurons sampled after dissociation appeared clear under bright-field illumination, they sometimes exhibited perinuclear brown pigment, they had a smooth membrane surface, and they often had small adherent cells, which probably included satellite glial cells (Fig 2A). Nearly all neurons sampled exhibited neurites, sometimes with complex branching (Fig 2B). Diameters of the recorded neuronal somata ranged from 23 to 65 μm, corresponding to previously described small to medium-sized human DRG neurons19. Within this range, neurons with SA had significantly smaller soma diameters than neurons without SA (Fig. 2C; mean diameter, 35.8 versus 43.2 μm, respectively; P < 0.0001, unpaired t test). Membrane capacitance, which has been weakly correlated with soma diameter in dissociated human DRG neurons19, was also found to be significantly lower in neurons with SA than those without SA (Fig 2D; median capacitance, 88 versus 164 pF, respectively; P < 0.0001, Mann-Whitney U test). The association of SA with smaller DRG neuron somata cannot be explained by faster equilibration of Cl− between smaller cells and the pipette because a more rapid equilibration could potentially reduce excitability more rapidly (by more rapidly hyperpolarizing ECl and thereby enhancing inward diffusion of Cl− through any open Cl− channels in the soma membrane) but not increase excitability. In 26 of 27 of the small to medium-sized neurons exhibiting SA, the discharge pattern was clearly irregular and low in frequency, as illustrated in Figure 2E. Only 1 of these 26 neurons exhibited bursts (Fig. 2F), which were prolonged (sometimes >20 s) and composed of irregular discharge at <2Hz, in marked contrast to the high frequency (10–250 Hz), regular discharge in the brief bursts often seen in rodent LTMRs under neuropathic conditions2,36,79. Because we did not sample DRG neurons >65 μm in diameter, the possibility remains that larger neurons, which may be more likely to be LTMRs, exhibit high frequency bursting like that in rodent LTMRs. The 27th neuron with SA had a regular (tonic) pattern of discharge, firing constantly at the highest rate observed in our samples (4.3 Hz, not shown). The irregular, low-frequency discharge we found in 97% of the neurons with SA38,67 is typical of patterns recorded both in vivo and in vitro from rodent nociceptors in neuropathic and postsurgical models4,8,23,70,83. As reported earlier for human DRG neurons with small- to medium-sized diameters19, most of the DRG neurons sampled (88%, 117/133 neurons) exhibited a distinct inflection or shoulder that prolonged the falling phase of the AP (Fig 1). In rodent DRG neurons, such inflections and long AP durations are associated with nociceptors (e.g.,29,42,53).
Figure 2.
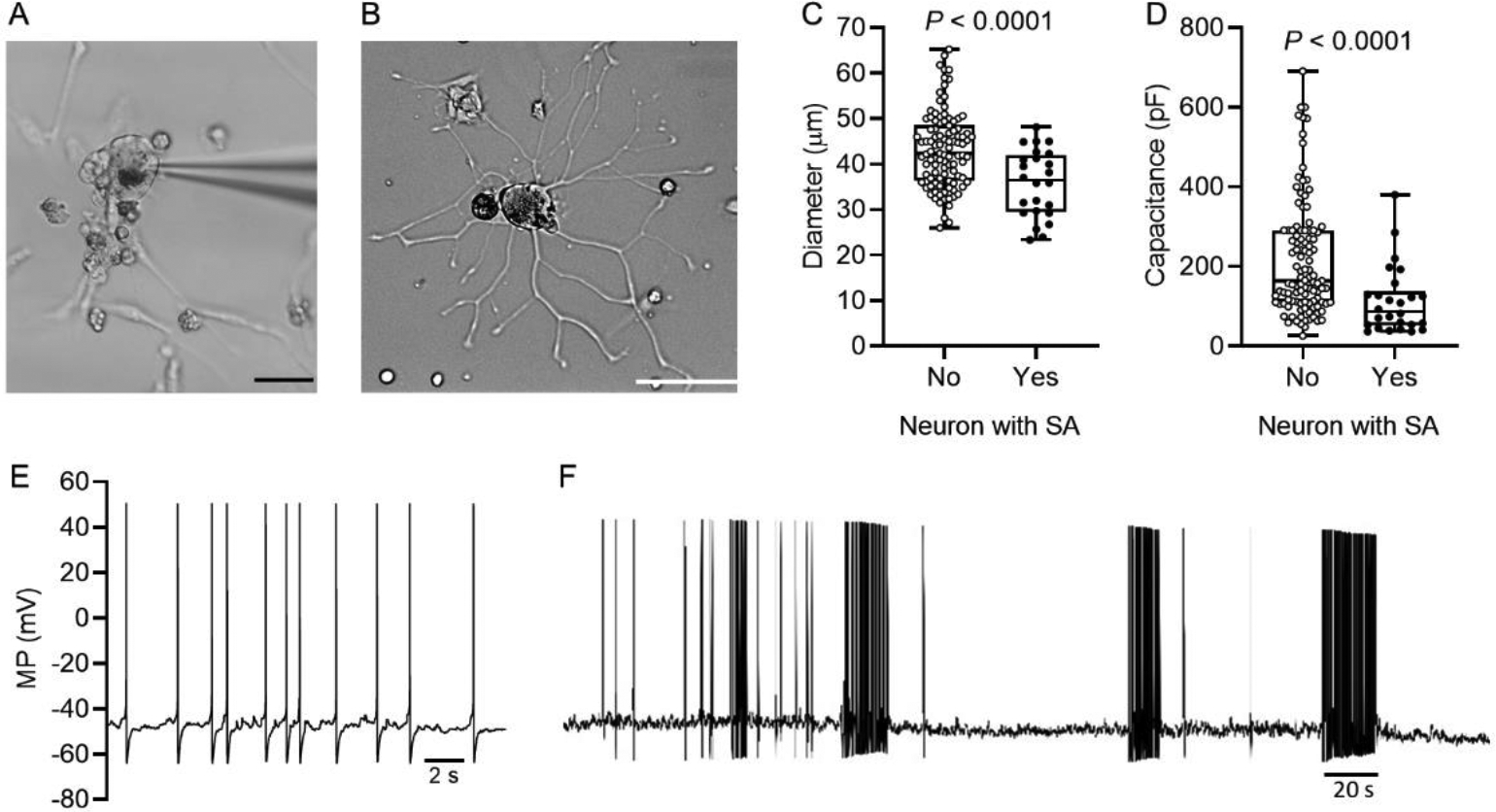
Spontaneous activity (SA) occurs preferentially in smaller dissociated human DRG neurons. (A) Human DRG neuron during whole cell patch recording one day after dissociation, showing the patch pipette, neurites, probable satellite glial cells (and possibly other cell types), and cellular debris. Calibration bar is 50 μm. (B) Example of a recorded neuron with extensive growth of neurites by the second day after dissociation. Calibration bar is 50 μm. (C) Neurons with SA had smaller soma diameter (unpaired t test) as well as lower membrane capacitance (D) (Mann-Whitney U test) compared with other neurons sampled. (E) SA recorded from a dissociated human DRG neuron illustrating the low-frequency, irregular discharge pattern and irregular, subthreshold DSFs between APs. (F) The only sampled neuron to show a bursting pattern during SA. The pattern of bursting and burst durations were irregular throughout the recording (6 min shown).
Spontaneous activity is associated with neuropathic pain but not with donor sex
Before proceeding with intensive electrophysiological analysis, we asked whether expanding our set of potential donors and making our inclusion criteria more restrictive than used previously altered the significant association that had been found earlier between SA and neuropathic pain55. Most patients (22 of 27) reported pain in a dermatome corresponding to at least one of the excised DRGs. Similar to the prior results, the proportion of neurons with SA during the 30-second period of intensive electrophysiological analysis was significantly greater when the source DRG corresponded to dermatomes reported by the donor to exhibit neuropathic pain (26 of 103 neurons sampled, 25.2%) than for neurons without corresponding dermatomal pain (1 of 30 neurons sampled, 3.2%) (Fig 3A; P = 0.0085 Fisher’s exact test). The discharge frequency was low, ranging from 0 to 4.3 Hz and, and was higher in neurons with corresponding dermatomal pain (Fig 3B; P = 0.0224, Welch’s unpaired t test). While the overall mean firing rate of neurons corresponding to painful dermatomes was 0.15 Hz (including the silent neurons, Fig 3B), it was 0.96 Hz for the subset of neurons exhibiting SA. In contrast, the firing rate was 0.11 Hz for the single SA neuron without a pain association. No significant differences were found in the incidence of SA in neurons taken from male versus female donors (Fig 3C), or in the median discharge frequency of those neurons that exhibited SA from male (0.30 Hz) versus female (0.10 Hz) donors. This similarity suggests that alterations promoting nociceptor SA under our in vitro conditions and associated with neuropathic pain in vivo might be clinically important in both sexes.
Figure 3.
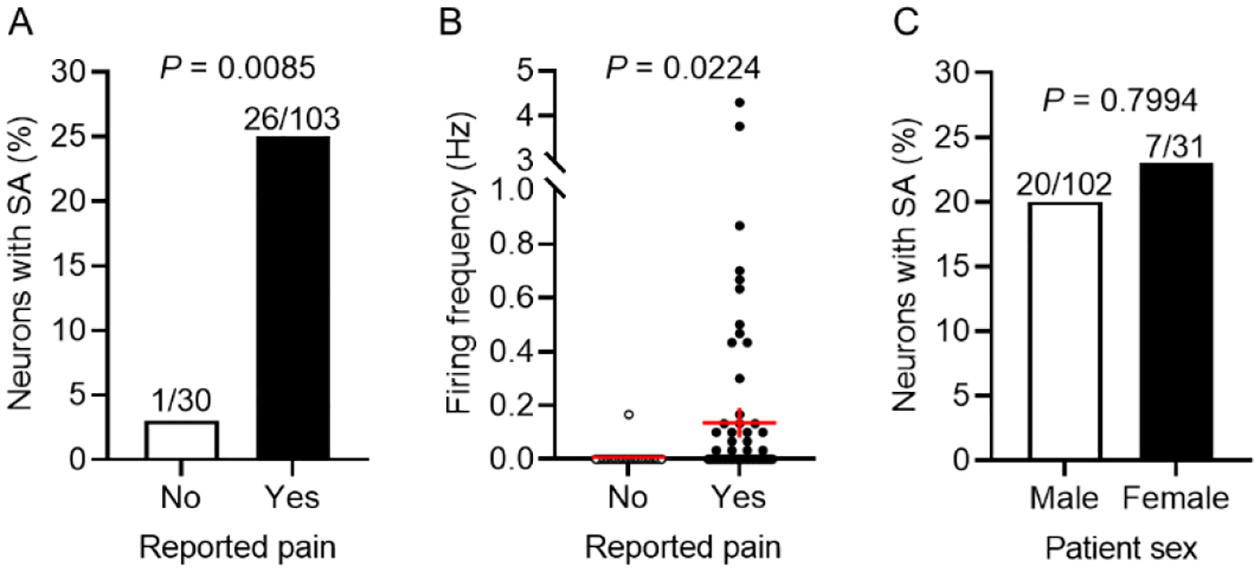
SA is associated with neuropathic pain but not with patient sex. (A) The incidence of neurons with SA was higher for neurons from DRG associated with painful dermatomes compared with other neurons (Fisher’s exact test). (B) Although discharge frequencies were low, they were higher during SA in neurons from DRG associated with painful dermatomes than in other neurons (Welch’s unpaired t test). Red lines indicate means ± SEM. (C) The incidence of neurons with SA did not differ significantly for neurons from male and female patients (Fisher’s exact test).
Spontaneous activity is associated with multiple electrophysiological alterations that promote action potential generation and function
What are the functional electrophysiological alterations underlying the observed SA? In terms of membrane potential, SA can be generated or initiated in isolated sensory neuron somata by any or all of only three potentially independent alterations: depolarization of RMP to reach AP threshold, hyperpolarization of AP threshold to reach RMP, and enhancement of transient DSFs that bridge the gap between RMP and AP threshold58. At higher SA frequencies, properties related to the aftereffects of each AP (including AHP, any depolarizing afterpotentials, and inactivation properties of channels contributing to each AP) can also contribute to SA, but SA with the low discharge frequencies observed in this study and in rodent nociceptors (usually <1 Hz) should show little dependence on AP aftereffects. Previously, some of us55 had shown that AP threshold was significantly more hyperpolarized in human DRG neurons associated with dermatomal pain than in neurons without a pain association, but no significant difference was found in RMP. Large DSFs were noted only in SA neurons, not silent neurons, but the DSFs were not quantified. More recently, these data were combined with other published data45,46 to reveal apparently more depolarized RMP in SA neurons associated with pain, and apparent restriction of large DSFs to SA neurons54. However, neither these publications nor others reported statistically significant associations of RMP, AP threshold, or DSF amplitude with SA in human DRG neurons. Using rigorous neuronal inclusion criteria (see Methods) and quantification of DSFs, we now show that all three of these electrophysiological alterations are significantly associated with SA in human DRG neurons isolated from patients with neuropathic pain. First, RMP was depolarized in neurons with SA compared with those without SA (Fig 4A; median RMP, −46.4 versus −59.0 mV, respectively; P < 0.0001, Mann-Whitney U test). Second, the voltage threshold for AP generation was hyperpolarized in neurons with SA versus those without SA (Fig 4B; median threshold, −37.2 versus −28.2 mV, respectively; P < 0.0001, Mann-Whitney U test).
Figure 4.
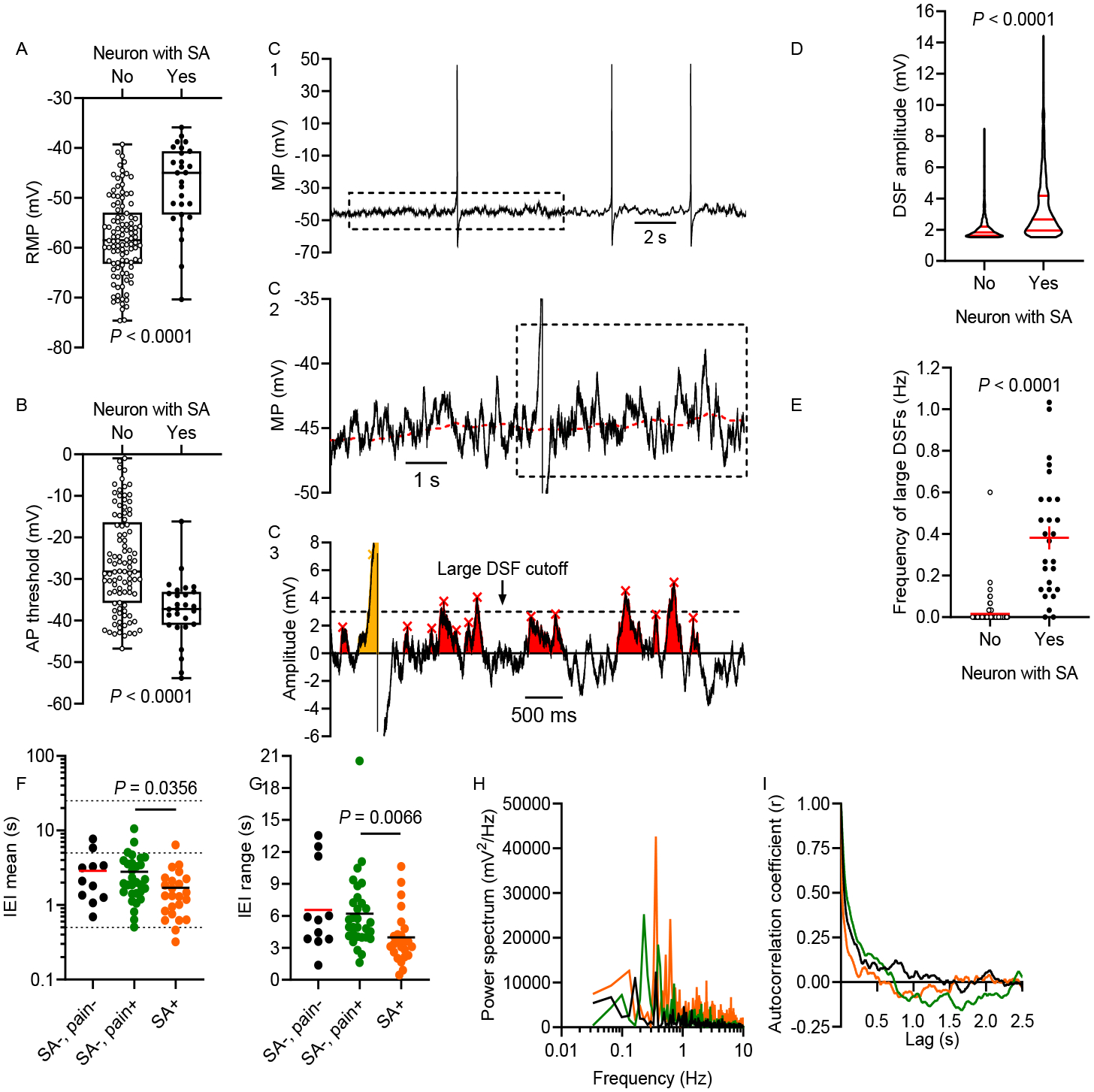
SA is associated with all three of the functional electrophysiological alterations that can initiate APs. (A) RMP was depolarized in neurons with SA compared with other neurons (Mann-Whitney U test). (B) AP threshold was reduced (hyperpolarized) in neurons with SA (unpaired t test). RMP and AP threshold measurements were not corrected for the liquid junction potential, so actual values might be up to 15 mV more negative (see Methods). (C) Examples of DSFs during SA. 30-s recordings (C1) were fit using a running median (red dashed line in C2) for RMP. Residual data were used to analyze subthreshold (red) and suprathreshold (yellow) DSFs as shown in C3. Large DSFs are defined as those depolarized ≥ 3 mV relative to the median RMP. (D) Amplitudes of all DSFs ≥ 1.5 mV were larger in neurons with SA (Mann-Whitney U test). (E) The frequency of large DSFs (≥ 3.0 mV) was higher in neurons with SA (Welch’s unpaired t test). (F) Mean interevent intervals (IEIs) between DSFs ≥ 1.5 mV across neurons without SA or pain association (SA−, pain−), without SA but with associated pain (SA−, pain+), and with SA and a pain association (SA+, pain+). Horizontal lines indicate 0.5, 5, and 25 s on the y-axis. (G) Large range of IEIs within individual neurons representing each group (same group colors as in panel F). (H) Distributions of frequencies within the 30-s recordings found by Fourier analysis for each neuron in panel G, revealing low dominant frequencies. (I) Autocorrelation analysis for the neurons in panels G-H, showing little temporal correlation between the occurrence of each presumptive DSF and subsequent DSFs. No prominent peaks were found at lag times greater than those plotted.
The third alteration was an enhancement of DSFs, which have not been quantified previously in human DRG neurons. Our DSF analysis program enabled automated measurement of DSFs relative to the sliding median value computed for RMP as shown in Figure 4C. DSFs >1.5 mV (red) were common in most of the sampled DRG neurons, and large DSFs (defined as those >3 mV) sometimes triggered APs (see the yellow DSF in Figure 4C). The amplitude of DSFs was larger in neurons with SA than those without SA (Fig 4D; median amplitude, 2.7 versus 1.8 mV, respectively; P < 0.0001, Mann-Whitney U test). Furthermore, the frequency of large DSFs (Fig 4C) was higher in neurons with SA versus those without SA (Fig 4E; mean frequency of large DSFs, 0.38 versus 0.02 Hz, respectively; P < 0.0001, Welch’s unpaired t test). An important question was whether the DSFs observed in the small and medium-sized human DRG neurons in the present study exhibit properties more similar to previously described fluctuations in rodent LTMRs or to those in rodent nociceptors. In rodent large-diameter LTMRs, spontaneous subthreshold fluctuations of membrane potential exhibit regular, high-frequency, sinusoidal oscillations1,2,48,52,79, whereas rodent small-to-medium diameter nociceptors exhibit stochastic, low-frequency patterns of irregular DSFs.58 Simple plots of the intervals between DSFs >1.5 mV revealed a large range of intervals across neurons, with the mean interval being ~2 s for neurons without SA and a tendency for the intervals to be somewhat lower in neurons with SA (Fig 4F). The range of intervals within individual neurons was also large (Fig 4G). A Fourier analysis similarly revealed a large range of frequency peaks, with the highest peaks <1 Hz, which presumably correspond to DSFs. Representative neurons exhibited dominant frequencies of ~0.2–0.3 Hz (Fig 4H). Autocorrelation analysis on data from the same neurons revealed few or no correlations at lag periods where we would expect autoregressive patterns due to any regular oscillations in membrane potential at the dominant DSF frequencies implicated by our Fourier analysis (or by the much higher dominant frequencies reported for sinusoidal oscillations in probable LTMRs), indicating very little temporal correlation between the occurrence of each DSF and subsequent DSFs (Fig 4I). These analyses confirm that the DSFs in the small and medium-sized human DRG neurons sampled in this study exhibit ~100x lower frequency and much less regularity in pattern of occurrence than the subthreshold oscillations analyzed in rat LTMRs1,2,48.
No statistically significant sex differences were found among silent (SA−) or spontaneously active (SA+) DRG neurons in the functional electrophysiological alterations: median RMP (male vs female: SA+, −47.1 vs −45.8 mV; SA−, −59.0 vs −59.0), median AP threshold (male vs female: SA+, −36.7 vs −37.7 mV; SA−, −26.2 vs −23.3), and median frequency of large DSFs (male vs female: SA+, 0.32 vs 0.47 Hz; SA−, 0.00 vs 0.00 Hz). Thus, all three of the functional electrophysiological properties related to membrane potential that might promote SA were altered in the hyperactive direction in dissociated human DRG neuron somata, and these effects were the same in neurons from male and female donors. Intrinsic changes in RMP, AP threshold, and irregular DSFs could by themselves drive low-frequency, irregular SA generated in the soma and/or peripheral terminals (promoting spontaneous pain)58. In addition, they might enhance excitatory effects of extrinsic influences such as cross-excitation through satellite glial cells, or excitation by humoral factors (e.g., cytokines, amines, damage-associated molecular patterns [DAMPs]) that access the DRGs and peripheral terminals to further drive SA20,37,74.
Other electrophysiological properties that were altered in neurons with SA also demonstrate a multi-faceted, hyperexcitable neuronal state, as summarized in Table 1. The minimum current (rheobase) required to generate an AP was lower in neurons with SA than those without SA, as was the amplitude of the afterhyperpolarization (AHP) following each AP. Multiple properties of individual APs (Fig 1) also were altered in neurons with SA. The AP rise time was significantly prolonged. AP amplitude (relative to AP threshold) was unaltered, but AP overshoot was significantly reduced. AP fall time and duration at half amplitude were significantly prolonged. Both the decrease in rheobase and the prolonged AP rise time are likely to represent, in part, effects of the hyperpolarized AP threshold, which should enable smaller depolarizing currents to slowly reach threshold with less accommodation than occurs with larger depolarizations. The prolonged AP duration/fall time, if it also occurs in vivo at sites of neurotransmitter release in the spinal cord and/or peripheral terminals, might enhance neurotransmitter release35. The decrease in AP overshoot was likely a result of the large depolarization of RMP (mean change 12.6 mV) causing steady-state inactivation of some of the voltage-gated Na+ channels in SA neurons59. This modest decrease in overshoot, combined with the lack of a significant alteration in somal AP amplitude (which falls within the large range of AP amplitudes reported for different types of DRG neuron in diverse species) (e.g.,26), seems unlikely to have significant functional consequences.58 If these SA-promoting alterations in the soma also occur in spike initiation zones within a nociceptor’s peripheral terminals, they and the decreases observed in rheobase and AHP amplitude might also enhance bursts of activity and consequent pain evoked by sensory generator potentials.
Table 1.
Action potential properties of human DRG neurons with (SA+) and without (SA−) spontaneous activity
| + | 27 | 20 | 89 | 194 |
| + | 21 | 88.3 | 87.9 | 12.9 |
| + | 21 | 52.0 | 50.6 | 11.8 |
| + | 21 | 56.8 | 68.0 | 46.7 |
| + | 21 | 10.7 | 12.2 | 6.8 |
| + | 21 | 6.6 | 7.1 | 2.8 |
| + | 21 | −10.9 | −11.7 | 5.1 |
AP, action potential; AHP, afterhyperpolarization potential; MW: Mann-Whitney U test; SD, standard deviation; t, the t ratio – the difference between sample means divided by standard error of the difference; U, the U value in the Mann Whitney test – the smaller of two calculations counting the number of times that an observation in group A is larger than B, or number of times that an observation in group B is larger than A. Membrane potential measurements are not corrected for the liquid junction potential, so actual values might be up to 15 mV more negative (see Methods).
Each functional electrophysiological alteration is associated with neuropathic pain
Given our finding that SA in human DRG neuron somata is associated with all three of the functional electrophysiological alterations that might generate low-frequency SA, and evidence that this SA is associated with neuropathic pain55 (see also Figure 3A and6), we tested the prediction that each of the functional SA-driving alterations is also significantly associated with patient-reported pain. Although only one of these alterations (reduced AP threshold) was found to be significantly altered in the previous study55, we wondered whether a larger patient sample size and more stringent inclusion criteria would demonstrate additional hyperexcitable alterations associated with reported pain. Indeed, RMP was depolarized in neurons isolated from DRG corresponding to painful regions of the body compared with those without corresponding pain (Fig 5A; median RMP, −56.4 versus −59.8 mV, respectively, P = 0.0099 Mann-Whitney U test); AP threshold was hyperpolarized in the pain-associated neurons relative to those without pain association (Fig 5B; median threshold, −33.1 versus −22.4 mV, respectively; P < 0.0001, Mann-Whitney U test); and DSF amplitude was larger in the pain-associated neurons than those without pain association (Fig 5C; median amplitude, 2.2 versus 1.9 mV, respectively; P < 0.0001, Mann-Whitney U test). As previously reported55, rheobase was lower in the pain-associated neurons than those without pain association, while no difference in AP amplitude was found (Table 2). In contrast to initial findings55, pain-associated DRG neurons exhibited significantly lower AHP amplitudes and AP overshoot, longer AP rise and fall times, and longer AP durations (Table 2, see also Fig 1). Thus, multiple electrophysiological alterations were significantly associated with neuropathic pain, including all three of the functional electrophysiological alterations of membrane potential that are in principle sufficient to generate low-frequency SA.
Figure 5.
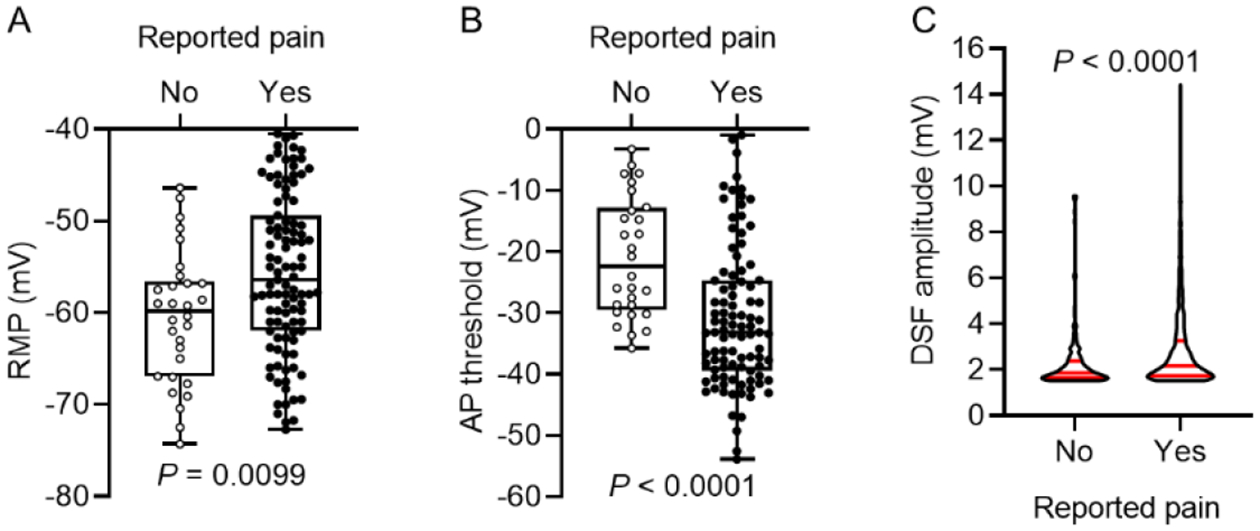
All three of the functional electrophysiological alterations that might produce SA are associated with patient-reported pain. Neurons from DRGs associated with dermatomal pain had depolarized RMP (A) (unpaired t test), hyperpolarized AP threshold (B) (Mann-Whitney U test), and increased DSF amplitude (C) (Mann-Whitney test) compared with other neurons. RMP and AP threshold measurements were not corrected for the liquid junction potential, so actual values might be up to 15 mV more negative (see Methods).
Table 2.
Action potential properties of DRG neurons corresponding to dermatomes with (Pain+) and without (Pain−) pain
| + | 103 | 180 | 490 | 718 |
| + | 87 | 88.5 | 87.0 | 11.4 |
| + | 87 | 59.7 | 58.8 | 14.4 |
| + | 87 | 17.9 | 40.7 | 51.7 |
| + | 87 | 6.4 | 7.7 | 5.4 |
| + | 87 | 4.2 | 4.7 | 2.6 |
| + | 87 | −14.1 | −14.2 | 5.6 |
AP, action potential; AHP, afterhyperpolarization potential; MW: Mann-Whitney U test; SD, standard deviation; t, the t ratio – the difference between sample means divided by standard error of the difference; U, the U value in the Mann Whitney test – the smaller of two calculations counting the number of times that an observation in group A is larger than B, or number of times that an observation in group B is larger than A. Membrane potential measurements are not corrected for the liquid junction potential, so actual values might be up to 15 mV more negative (see Methods).
Discussion
A necessary step towards linking molecular mechanisms to neuronal hyperactivity that drives neuropathic pain is to define the critical electrophysiological alterations underlying SA. The importance of this step was highlighted recently by demonstrations of unexpected differences in pain-related features of human and rodent DRG neurons15,33,54,65,66,68,84. For example, use-dependent inactivation of tetrodotoxin-resistant currents is nearly absent in human DRG neurons, which may promote their sustained activity84. Such differences raise the possibility that critical cellular mechanisms promoting neuropathic pain have diverged across mammalian species. As most mechanistic information about neuropathic pain has come from rodents, such divergence might explain some of the failure to translate promising preclinical treatments to clinical application (e.g.,54). At the functional electrophysiological level, however, we now demonstrate that the alterations underlying pain-associated SA in DRG neurons are the same in humans (male and female) and rodents. Moreover, these alterations more closely parallel those previously reported for primary nociceptors than LTMRs of rodents.
SA in human DRG neurons is associated with the functional hyperexcitable alterations that drive SA in rodent DRG neurons
In terms of membrane potential, the only electrophysiological alterations sufficient to generate or initiate SA in the absence of transient depolarizing inputs such as sensory generator potentials or preceding APs are a prolonged depolarization of RMP, lowering of AP threshold, and enhancement of DSFs, all of which correlated with SA in human DRG neurons. While earlier papers54,55 reported associations of SA and reduced AP threshold (plus lowered rheobase) with patient-reported pain, they did not document statistically significant associations of SA with specific excitability properties. Moreover, unlike the current study, the initial study55 did not measure DSFs and it failed to find a significant association between pain and depolarized RMP. The earlier lack of an RMP association may be explained by the smaller patient sample and less stringent neuronal inclusion criteria. We have now excluded neurons with unusually positive AP thresholds, which may represent the initiation of APs in a small neurite (see Fig 2B) when the recorded soma is relatively inexcitable (perhaps because of somal damage). Larger depolarizations in such somata would reflect the larger current injections needed to reach threshold in excitable membrane outside the soma.
The same functional SA-driving alterations and lowered rheobase have been shown in dissociated DRG neurons in rodent neuropathy models, including a rat spinal cord injury (SCI) model9,58, a mouse SCI model9, and a mouse chemoneuropathy model41. While the incidence of DRG neurons with pain-associated SA was somewhat lower here than reported for rodent nociceptors9,41,58, our recording conditions may have reduced excitability and SA. Specifically, the lower Cl− concentration in the recording pipette would increase any background hyperpolarizing Cl− currents, thereby reducing excitability62,76. Nevertheless, qualitatively identical findings across species and conditions indicate that all three functional electrophysiological alterations for driving SA in DRG neurons may often be recruited to promote ongoing pain.
SA and DSF properties in human DRG neurons resemble those described in rodent nociceptors rather than LTMRs
Mechanisms of SA in DRG neurons have been studied most extensively in large A-fiber neurons in rodents, a large majority of which are LTMRs21,26. Normally, SA in probable LTMRs and nociceptors is not generated in the soma. After axotomizing injury (spinal nerve ligation, SNL), SA developed in ~35% of rat A-fiber neurons sampled 1 day after the injury, and then declined to control values within about a week49. This period of SA generation in previously axotomized A-fiber neurons is much briefer than SA in rat nociceptors after SCI (most of which, like the human DRG neurons described here, are unlikely to be axotomized in vivo, and exhibit SA for months8). SA in rodent LTMRs often manifests as either regular tonic firing or periodic bursts of regularly spaced APs, in which reported discharge rates are relatively high: 10–70 Hz in vivo2,36, 60–250 Hz in excised ganglia1, and 75–130 Hz in dissociated neurons48. In contrast, the SA we observe in human DRG neurons is low frequency (usually <1 Hz) and is almost always irregular without regular bursts. This parallels the SA exhibited by dissociated nociceptors in rodent neuropathy models8,9,41,44–46,58,70,77.
In LTMRs with uninflected APs, repetitive discharge is driven by high-frequency, sinusoidal oscillations of membrane potential, which show a marked voltage dependence and are rarely observed at RMP1,2,49,64. Indeed, experimental depolarization into the range of −40 to −20 mV is typically used to reveal the oscillations and generate discharge. In contrast, the DSFs we find associated with SA in human DRG neurons occur in neurons with inflected APs, they are prominent across the full range of RMPs in our samples (−35 to −74 mV without correction for the liquid junction potential), their frequency is low, their patterns of occurrence and individual waveforms are highly irregular, and the waveforms are more prolonged than the individual oscillations in rodent LTMRs. These DSF properties are the same in rat nociceptors9,41,44,50,58.
In rodent nociceptors, the complex mechanisms underlying enhanced DSFs remain undefined, but are under investigation. Both an increase in background inward currents at RMP, including Nav1.8 current81, and an increase in membrane resistance8 (suggesting reduced K+ conductance) have been implicated. In rodent LTMRs and nociceptors, published SA-related mechanistic investigations have focused largely on changes in ion conductances that promote sinusoidal oscillations at depolarized potentials1,16,25,40,48 or that produce a switch in AP initiation dynamics that enables repetitive firing and sinusoidal oscillations during prolonged extrinsic depolarization61,64. Two of the SA-associated hyperexcitable alterations described here have been reported in probable LTMRs after dissociation: depolarized RMP and decreased rheobase following axotomizing SNL48. However, neither alterations of RMP nor AP voltage threshold have received much attention as mechanisms of SA in LTMRs (e.g.,21,40,64).
Four lines of evidence suggest that many of the sampled human neurons were nociceptors. First, the SA patterns and DSF properties in hyperactive human DRG neurons appear identical to those reported in hyperactive rodent nociceptors. Second, as in rat nociceptors (e.g.,26,29,39,53), SA was largely expressed in human neurons having inflected APs, whereas APs in rat LTMRs with SA have uninflected APs.1 Third, SA occurred more often in human DRG neurons with smaller somata, paralleling observations that rodent nociceptors usually have smaller somata (e.g.,29,42), although this correlation may be weaker in humans84. Fourth, substantial fractions of human DRG neurons with small- to-medium-sized somata express classical markers for nociceptors, including TrkA, TRPV1, TRPA1, CGRP, substance P, Nav1.7, Nav1.8, and Nav1.9 (reviewed by32,54).
Clinical and biological implications of hyperexcitable alterations underlying SA in human DRG neurons
Our demonstration that pain-associated SA in human DRG neurons is linked to multiple electrophysiological alterations that can generate SA extends prior evidence for multiple mechanisms promoting hyperactivity in rodent DRG neurons. In probable LTMRs, degeneracy of SA mechanisms (i.e., multiple different mechanisms) was shown by the sufficiency of different ion conductance alterations to enable sinusoidal oscillations of membrane potential and repetitive firing during sustained depolarization64. A clinical implication of multiplicity and degeneracy of hyperactivity mechanisms both in LTMRs and nociceptors is that effective pharmacological targeting of one or a few of the different mechanisms may not block hyperactivity to alleviate neuropathic pain63,64,74.
An interesting biological question is why nociceptors would display multiple mechanisms for generating SA in their somata under neuropathic conditions. One possibility is that somal SA results pathologically from dysregulated control of complex hyperexcitability mechanisms that normally function at injury sites to produce protective sensitization during healing (with somal dysregulation potentially amplified by dissociation). A complementary possibility is that persistent nociceptor SA in the soma is a physiological specialization that promoted survival during evolution, and which also can be recruited in some disease states. Strong evolutionary selection pressures for a function often result in multiple underlying mechanisms3. One proposed function for persistent SA in nociceptors and some LTMRs is to promote painful hypervigilance long after injury severe enough to produce substantial nerve damage74. Severe injury such as amputation can cause lasting physical impairment that increases vulnerability to attack by predators or competitors. Injury-induced hypervigilance associated with nociceptor SA has been shown to increase survival in squid18, and persistent hypervigilance to predator cues has also been demonstrated in neuropathic mice47. Thus, degenerate mechanisms could function to enable SA in nociceptors that promotes hypervigilance when severe injury persistently increases an animal’s risk of being assaulted74. A corollary is that ectopic generation of persistent SA within anatomically protected DRGs could have been adaptive when deep tissue injury disconnected DRGs from peripheral tissue74. This adaptive possibility encourages human microneurography studies utilizing appropriate nerve blocks (e.g.,73) to determine whether ganglionic generation of pain-linked C-fiber SA occurs in vivo in some neuropathic conditions. Such conditions, including the tumor-induced compression of spinal nerve roots in this study, may sometimes mimic SA-triggering features of traumatic nerve injury. Therapeutically, an important question is how conserved the molecular mechanisms are that underlie the shared electrophysiological alterations that can persistently drive somally generated SA in rodent and human nociceptors.
Highlights.
Spontaneous activity in dissociated human sensory neurons correlates with reported pain
Multiple features promote irregular ongoing discharge in sensory neuron somata
Irregular spontaneous depolarizing fluctuations pattern low-frequency spontaneous activity
Hyperexcitable alterations closely resemble alterations reported in rodent nociceptors
Human nociceptor somata have electrophysiological specializations that may promote ongoing pain
Acknowledgements
The authors thank Sai Cheruvu for assistance with DSF analyses, Dr. Alexis Bavencoffe for useful comments and guidance on DSF analyses, and Dr. Carmen Dessauer for stimulating discussions about mechanisms important for spontaneous activity.
This work was supported by grants from the National Institutes of Health: NS111521 (E.T.W.), and NS091759 (Carmen W. Dessauer and E.T.W.), NS111929, CA200263 (P.M.D.), as well as the Fondren Chair in Cellular Signaling (E.T.W.), H.E.B. Professorship in Cancer Research (P.M.D.), Thompson Family Foundation Initiative (P.M.D.), and a Zilkha Family Fellowship (M.A.O.).
Disclosures:
This work was supported by grants from the National Institutes of Health: NS111521 (E.T.W.), NS091759 (Carmen W. Dessauer and E.T.W.), NS111929, CA200263 (P.M.D.), as well as the Fondren Chair in Cellular Signaling (E.T.W.), H.E.B. Professorship in Cancer Research (P.M.D.), Thompson Family Foundation Initiative (P.M.D.), and a Zilkha Family Fellowship (M.A.O.). The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
References
- 1.Amir R, Michaelis M, Devor M: Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci 19:8589–96, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amir R, Michaelis M, Devor M: Burst discharge in primary sensory neurons: triggered by subthreshold oscillations, maintained by depolarizing afterpotentials. J Neurosci 22:1187–98, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews PW, Gangestad SW, Matthews D: Adaptationism--how to carry out an exaptationist program. Behav Brain Sci 25:489–504; discussion 504, 2002. 10.1017/s0140525x02000092 [DOI] [PubMed] [Google Scholar]
- 4.Banik RK, Brennan TJ: Spontaneous discharge and increased heat sensitivity of rat C-fiber nociceptors are present in vitro after plantar incision. Pain 112:204–13, 2004. 10.1016/j.pain.2004.08.026 [DOI] [PubMed] [Google Scholar]
- 5.Barry PH: JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 51:107–16, 1994. 10.1016/0165-0270(94)90031-0 [DOI] [PubMed] [Google Scholar]
- 6.Baumann TK, Martenson ME. Spontaneous action potential discharge in cultured dorsal root ganglion neurons from patients with neuropathic pain. In Devor M, Rowbotham MC, Wiesenfeld-Hallin Z (eds.) 16. Progress in Pain Research and Management. 2000. pp. 101–8. [Google Scholar]
- 7.Baumann TK, Burchiel KJ, Ingram SL, Martenson ME: Responses of adult human dorsal root ganglion neurons in culture to capsaicin and low pH. Pain 65:31–8, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, Grill RJ, Carlton SM, Walters ET: Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci 30:14870–82, 2010. 10.1523/JNEUROSCI.2428-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkey SC, Herrera JJ, Odem MA, Rahman S, Cheruvu SS, Cheng X, Walters ET, Dessauer CW, Bavencoffe AG: EPAC1 and EPAC2 promote nociceptor hyperactivity associated with chronic pain after spinal cord injury. Neurobiol Pain 7:100040, 2020. 10.1016/j.ynpai.2019.100040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernal L, Lopez-Garcia JA, Roza C: Spontaneous activity in C-fibres after partial damage to the saphenous nerve in mice: Effects of retigabine. Eur J Pain 20:1335–45, 2016. 10.1002/ejp.858 [DOI] [PubMed] [Google Scholar]
- 11.Bove GM, Delany SP, Hobson L, Cruz GE, Harris MY, Amin M, Chapelle SL, Barbe MF: Manual therapy prevents onset of nociceptor activity, sensorimotor dysfunction, and neural fibrosis induced by a volitional repetitive task. Pain 160:632–44, 2019. 10.1097/j.pain.0000000000001443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burchiel KJ, Russell LC, Lee RP, Sima AA: Spontaneous activity of primary afferent neurons in diabetic BB/Wistar rats. A possible mechanism of chronic diabetic neuropathic pain. Diabetes 34:1210–3, 1985. 10.2337/diab.34.11.1210 [DOI] [PubMed] [Google Scholar]
- 13.Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE: Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 147:265–76, 2009. 10.1016/j.pain.2009.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassidy RM, Bavencoffe A, Lopez ER, Cheruvu SS, Walters ET, Uribe RA, Krachler AM, Odem MA: Frequency-independent biological signal identification (FIBSI): A free program that simplifies intensive analysis of non-stationary time series data. bioRxiv 2020.05.29.1230422020. [Google Scholar]
- 15.Chang W, Berta T, Kim YH, Lee S, Lee SY, Ji RR: Expression and Role of Voltage-Gated Sodium Channels in Human Dorsal Root Ganglion Neurons with Special Focus on Nav1.7, Species Differences, and Regulation by Paclitaxel. Neurosci Bull 2017. 10.1007/s12264-017-0132-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi JS, Waxman SG: Physiological interactions between Na(v)1.7 and Na(v)1.8 sodium channels: a computer simulation study. J Neurophysiol 106:3173–84, 2011. 10.1152/jn.00100.2011 [DOI] [PubMed] [Google Scholar]
- 17.Costigan M, Scholz J, Woolf CJ: Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32:1–32, 2009. 10.1146/annurev.neuro.051508.135531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crook RJ, Dickson K, Hanlon RT, Walters ET: Nociceptive sensitization reduces predation risk. Curr Biol 24:1121–5, 2014. 10.1016/j.cub.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 19.Davidson S, Copits BA, Zhang J, Page G, Ghetti A, Gereau RW: Human sensory neurons: Membrane properties and sensitization by inflammatory mediators. Pain 155:1861–70, 2014. 10.1016/j.pain.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devor M: Unexplained peculiarities of the dorsal root ganglion. Pain Suppl 6:S27–35, 1999. 10.1016/S0304-3959(99)00135-9 [DOI] [PubMed] [Google Scholar]
- 21.Devor M: Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 196:115–28, 2009. 10.1007/s00221-009-1724-6 [DOI] [PubMed] [Google Scholar]
- 22.Djouhri L, Fang X, Koutsikou S, Lawson SN: Partial nerve injury induces electrophysiological changes in conducting (uninjured) nociceptive and nonnociceptive DRG neurons: Possible relationships to aspects of peripheral neuropathic pain and paresthesias. Pain 153:1824–36, 2012. 10.1016/j.pain.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN: Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci 26:1281–92, 2006. 10.1523/JNEUROSCI.3388-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djouhri L, Zeidan A, Abd El-Aleem SA, Smith T: Cutaneous Aβ-Non-nociceptive, but Not C-Nociceptive, Dorsal Root Ganglion Neurons Exhibit Spontaneous Activity in the Streptozotocin Rat Model of Painful Diabetic Neuropathy in vivo. Front Neurosci 14:530, 2020. 10.3389/fnins.2020.00530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estacion M, Dib-Hajj SD, Benke PJ, Te Morsche RH, Eastman EM, Macala LJ, Drenth JP, Waxman SG: NaV1.7 gain-of-function mutations as a continuum: A1632E displays physiological changes associated with erythromelalgia and paroxysmal extreme pain disorder mutations and produces symptoms of both disorders. J Neurosci 28:11079–88, 2008. 10.1523/JNEUROSCI.3443-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang X, McMullan S, Lawson SN, Djouhri L: Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol 565:927–43, 2005. 10.1113/jphysiol.2005.086199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finnerup NB, Kuner R, Jensen TS: Neuropathic pain: From mechanisms to treatment. Physiol Rev 2020. 10.1152/physrev.00045.2019 [DOI] [PubMed] [Google Scholar]
- 28.Flake NM, Lancaster E, Weinreich D, Gold MS: Absence of an association between axotomy-induced changes in sodium currents and excitability in DRG neurons from the adult rat. Pain 109:471–80, 2004. 10.1016/j.pain.2004.02.024 [DOI] [PubMed] [Google Scholar]
- 29.Gold MS, Dastmalchi S, Levine JD: Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience 71:265–75, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Gorodetskaya N, Constantin C, Janig W: Ectopic activity in cutaneous regenerating afferent nerve fibers following nerve lesion in the rat. Eur J Neurosci 18:2487–97, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA, Iannetti GD, Jensen TS, Kauppila T, Nurmikko TJ, Rice AS, Rowbotham M, Serra J, Sommer C, Smith BH, Treede RD: NeuPSIG guidelines on neuropathic pain assessment. Pain 152:14–27, 2011. 10.1016/j.pain.2010.07.031 [DOI] [PubMed] [Google Scholar]
- 32.Haberberger RV, Barry C, Dominguez N, Matusica D: Human Dorsal Root Ganglia. Front Cell Neurosci 13:271, 2019. 10.3389/fncel.2019.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han C, Estacion M, Huang J, Vasylyev D, Zhao P, Dib-Hajj SD, Waxman SG: Human Na(v)1.8: enhanced persistent and ramp currents contribute to distinct firing properties of human DRG neurons. J Neurophysiol 113:3172–85, 2015. 10.1152/jn.00113.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haroutounian S, Nikolajsen L, Bendtsen TF, Finnerup NB, Kristensen AD, Hasselstrom JB, Jensen TS: Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain 155:1272–9, 2014. 10.1016/j.pain.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 35.Hochner B, Klein M, Schacher S, Kandel ER: Action-potential duration and the modulation of transmitter release from the sensory neurons of Aplysia in presynaptic facilitation and behavioral sensitization. Proc Natl Acad Sci U S A 83:8410–4, 1986. 10.1073/pnas.83.21.8410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kajander KC, Bennett GJ: Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J Neurophysiol 68:734–44, 1992. 10.1152/jn.1992.68.3.734 [DOI] [PubMed] [Google Scholar]
- 37.Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C, Saijilafu, Young L, He S, LaVinka PC, Zhou F, Bergles D, Hanani M, Guan Y, Spray DC, Dong X: Coupled Activation of Primary Sensory Neurons Contributes to Chronic Pain. Neuron 91:1085–96, 2016. 10.1016/j.neuron.2016.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleggetveit IP, Namer B, Schmidt R, Helås T, Rückel M, Ørstavik K, Schmelz M, Jørum E: High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain 153:2040–7, 2012. 10.1016/j.pain.2012.05.017 [DOI] [PubMed] [Google Scholar]
- 39.Koerber HR, Druzinsky RE, Mendell LM: Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol 60:1584–96, 1988. 10.1152/jn.1988.60.5.1584 [DOI] [PubMed] [Google Scholar]
- 40.Kovalsky Y, Amir R, Devor M: Simulation in sensory neurons reveals a key role for delayed Na+ current in subthreshold oscillations and ectopic discharge: implications for neuropathic pain. J Neurophysiol 102:1430–42, 2009. 10.1152/jn.00005.2009 [DOI] [PubMed] [Google Scholar]
- 41.Laumet G, Bavencoffe A, Edralin JD, Huo XJ, Walters ET, Dantzer R, Heijnen CJ, Kavelaars A: Interleukin-10 resolves pain hypersensitivity induced by cisplatin by reversing sensory neuron hyperexcitability. Pain 2020. 10.1097/j.pain.0000000000001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawson SN, Fang X, Djouhri L: Nociceptor subtypes and their incidence in rat lumbar dorsal root ganglia (DRGs): focussing on C-polymodal nociceptors, Aβ-nociceptors, moderate pressure receptors and their receptive field depths. Curr Opin Physiol 11:125–46, 2019. 10.1016/j.cophys.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Adamek P, Zhang H, Tatsui CE, Rhines LD, Mrozkova P, Li Q, Kosturakis AK, Cassidy RM, Harrison DS, Cata JP, Sapire K, Zhang H, Kennamer-Chapman RM, Jawad AB, Ghetti A, Yan J, Palecek J, Dougherty PM: The Cancer Chemotherapeutic Paclitaxel Increases Human and Rodent Sensory Neuron Responses to TRPV1 by Activation of TLR4. J Neurosci 35:13487–500, 2015. 10.1523/JNEUROSCI.1956-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Marri T, North RY, Rhodes HR, Uhelski ML, Tatsui CE, Rhines LD, Rao G, Corrales G, Abercrombie TJ, Johansson CA, Dougherty PM: Chemotherapy induced peripheral neuropathy in a dish: dorsal root ganglion cells treated in vitro with paclitaxel show biochemical and physiological responses parallel to that seen in vivo. Pain 2020. 10.1097/j.pain.0000000000002005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, North RY, Rhines LD, Tatsui CE, Rao G, Edwards DD, Cassidy RM, Harrison DS, Johansson CA, Zhang H, Dougherty PM: DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain. J Neurosci 38:1124–36, 2018. 10.1523/JNEUROSCI.0899-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Tatsui CE, Rhines LD, North RY, Harrison DS, Cassidy RM, Johansson CA, Kosturakis AK, Edwards DD, Zhang H, Dougherty PM: Dorsal root ganglion neurons become hyperexcitable and increase expression of voltage-gated T-type calcium channels (Cav3.2) in paclitaxel-induced peripheral neuropathy. Pain 158:417–29, 2017. 10.1097/j.pain.0000000000000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lister KC, Bouchard SM, Markova T, Aternali A, Denecli P, Pimentel SD, Majeed M, Austin JS, de C Williams AC, Mogil JS: Chronic pain produces hypervigilance to predator odor in mice. Curr Biol 30:R866–7, 2020. 10.1016/j.cub.2020.06.025 [DOI] [PubMed] [Google Scholar]
- 48.Liu CN, Devor M, Waxman SG, Kocsis JD: Subthreshold oscillations induced by spinal nerve injury in dissociated muscle and cutaneous afferents of mouse DRG. J Neurophysiol 87:2009–17, 2002. 10.1152/jn.00705.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu CN, Michaelis M, Amir R, Devor M: Spinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: relation to neuropathic pain. J Neurophysiol 84:205–15, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Lopez ER, Carbajal AG, Tian JB, Bavencoffe A, Zhu MX, Dessauer CW, Walters ET: Serotonin enhances depolarizing spontaneous fluctuations, excitability, and ongoing activity in isolated rat DRG neurons via 5-HT4 receptors and cAMP-dependent mechanisms. Neuropharmacology 184:108408, 2021. 10.1016/j.neuropharm.2020.108408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lundberg LE, Jørum E, Holm E, Torebjörk HE: Intra-neural electrical stimulation of cutaneous nociceptive fibres in humans: effects of different pulse patterns on magnitude of pain. Acta Physiol Scand 146:41–8, 1992. 10.1111/j.1748-1716.1992.tb09391.x [DOI] [PubMed] [Google Scholar]
- 52.Ma C, LaMotte RH: Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. J Neurosci 27:14059–68, 2007. 10.1523/JNEUROSCI.3699-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH: Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol 89:1588–602, 2003. 10.1152/jn.00855.2002 [DOI] [PubMed] [Google Scholar]
- 54.Middleton SJ, Barry AM, Comini M, Li Y, Ray PR, Shiers S, Themistocleous AC, Uhelski ML, Yang X, Dougherty PM, Price TJ, Bennett DL: Studying human nociceptors: from fundamentals to clinic. Brain 144:1312–35, 2021. 10.1093/brain/awab048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.North RY, Li Y, Ray P, Rhines LD, Tatsui CE, Rao G, Johansson CA, Zhang H, Kim YH, Zhang B, Dussor G, Kim TH, Price TJ, Dougherty PM: Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain 142:1215–26, 2019. 10.1093/brain/awz063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nyström B, Hagbarth KE: Microelectrode recordings from transected nerves in amputees with phantom limb pain. Neurosci Lett 27:211–6, 1981. [DOI] [PubMed] [Google Scholar]
- 57.Ochoa JL, Campero M, Serra J, Bostock H: Hyperexcitable polymodal and insensitive nociceptors in painful human neuropathy. Muscle Nerve 32:459–72, 2005. 10.1002/mus.20367 [DOI] [PubMed] [Google Scholar]
- 58.Odem MA, Bavencoffe AG, Cassidy RM, Lopez ER, Tian J, Dessauer CW, Walters ET: Isolated nociceptors reveal multiple specializations for generating irregular ongoing activity associated with ongoing pain. Pain 159:2347–62, 2018. 10.1097/j.pain.0000000000001341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patrick Harty T, Waxman SG: Inactivation properties of sodium channel Nav1.8 maintain action potential amplitude in small DRG neurons in the context of depolarization. Mol Pain 3:12, 2007. 10.1186/1744-8069-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raja SN, Ringkamp M, Guan Y, Campbell JN: John J. Bonica Award Lecture: Peripheral neuronal hyperexcitability: the “low-hanging” target for safe therapeutic strategies in neuropathic pain. Pain 161 Suppl 1:S14–26, 2020. 10.1097/j.pain.0000000000001838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ratté S, Lankarany M, Rho YA, Patterson A, Prescott SA: Subthreshold membrane currents confer distinct tuning properties that enable neurons to encode the integral or derivative of their input. Front Cell Neurosci 8:452, 2014. 10.3389/fncel.2014.00452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ratté S, Prescott SA: ClC-2 channels regulate neuronal excitability, not intracellular chloride levels. J Neurosci 31:15838–43, 2011. 10.1523/JNEUROSCI.2748-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ratté S, Prescott SA: Afferent hyperexcitability in neuropathic pain and the inconvenient truth about its degeneracy. Curr Opin Neurobiol 36:31–7, 2016. 10.1016/j.conb.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 64.Ratté S, Zhu Y, Lee KY, Prescott SA: Criticality and degeneracy in injury-induced changes in primary afferent excitability and the implications for neuropathic pain. Elife 3:e02370, 2014. 10.7554/eLife.02370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rostock C, Schrenk-Siemens K, Pohle J, Siemens J: Human vs. Mouse Nociceptors - Similarities and Differences. Neuroscience 387:13–27, 2018. 10.1016/j.neuroscience.2017.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwaid AG, Krasowka-Zoladek A, Chi A, Cornella-Taracido I: Comparison of the Rat and Human Dorsal Root Ganglion Proteome. Sci Rep 8:13469, 2018. 10.1038/s41598-018-31189-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serra J, Bostock H, Sola R, Aleu J, Garcia E, Cokic B, Navarro X, Quiles C: Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain 153:42–55, 2012. 10.1016/j.pain.2011.08.015 [DOI] [PubMed] [Google Scholar]
- 68.Shiers S, Klein RM, Price TJ: Quantitative differences in neuronal subpopulations between mouse and human dorsal root ganglia demonstrated with RNAscope in situ hybridization. Pain 161:2410–24, 2020. 10.1097/j.pain.0000000000001973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith ESJ: Advances in understanding nociception and neuropathic pain. J Neurol 265:231–8, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Study RE, Kral MG: Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain 65:235–42, 1996. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki Y, Sato J, Kawanishi M, Mizumura K: Lowered response threshold and increased responsiveness to mechanical stimulation of cutaneous nociceptive fibers in streptozotocin-diabetic rat skin in vitro--correlates of mechanical allodynia and hyperalgesia observed in the early stage of diabetes. Neurosci Res 43:171–8, 2002. [DOI] [PubMed] [Google Scholar]
- 72.Tode J, Kirillova-Woytke I, Rausch VH, Baron R, Jänig W: Mechano- and thermosensitivity of injured muscle afferents 20 to 80 days after nerve injury. J Neurophysiol 119:1889–901, 2018. 10.1152/jn.00894.2017 [DOI] [PubMed] [Google Scholar]
- 73.Vaso A, Adahan HM, Gjika A, Zahaj S, Zhurda T, Vyshka G, Devor M: Peripheral nervous system origin of phantom limb pain. Pain 155:1384–91, 2014. 10.1016/j.pain.2014.04.018 [DOI] [PubMed] [Google Scholar]
- 74.Walters ET: Adaptive mechanisms driving maladaptive pain: how chronic ongoing activity in primary nociceptors can enhance evolutionary fitness after severe injury. Philos Trans R Soc Lond B Biol Sci 374:20190277, 2019. 10.1098/rstb.2019.0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang T, Hurwitz O, Shimada SG, Qu L, Fu K, Zhang P, Ma C, LaMotte RH: Chronic Compression of the Dorsal Root Ganglion Enhances Mechanically Evoked Pain Behavior and the Activity of Cutaneous Nociceptors in Mice. PLoS One 10:e0137512, 2015. 10.1371/journal.pone.0137512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilke BU, Kummer KK, Leitner MG, Kress M: Chloride - The Underrated Ion in Nociceptors. Front Neurosci 14:287, 2020. 10.3389/fnins.2020.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Z, Yang Q, Crook RJ, O’Neil RG, Walters ET: TRPV1 channels make major contributions to behavioral hypersensitivity and spontaneous activity in nociceptors after spinal cord injury. Pain 154:2130–41, 2013. 10.1016/j.pain.2013.06.040 [DOI] [PubMed] [Google Scholar]
- 78.Xiao WH, Bennett GJ: Persistent low-frequency spontaneous discharge in A-fiber and C-fiber primary afferent neurons during an inflammatory pain condition. Anesthesiology 107:813–21, 2007. 10.1097/01.anes.0000286983.33184.9c [DOI] [PubMed] [Google Scholar]
- 79.Xie W, Strong JA, Kim D, Shahrestani S, Zhang JM: Bursting activity in myelinated sensory neurons plays a key role in pain behavior induced by localized inflammation of the rat sensory ganglion. Neuroscience 206:212–23, 2012. 10.1016/j.neuroscience.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie W, Strong JA, Zhang JM: Local knockdown of the NaV1.6 sodium channel reduces pain behaviors, sensory neuron excitability, and sympathetic sprouting in rat models of neuropathic pain. Neuroscience 291:317–30, 2015. 10.1016/j.neuroscience.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Q, Wu Z, Hadden JK, Odem MA, Zuo Y, Crook RJ, Frost JA, Walters ET: Persistent pain after spinal cord injury is maintained by primary afferent activity. J Neurosci 34:10765–9, 2014. 10.1523/JNEUROSCI.5316-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H, Dougherty PM: Enhanced excitability of primary sensory neurons and altered gene expression of neuronal ion channels in dorsal root ganglion in paclitaxel-induced peripheral neuropathy. Anesthesiology 120:1463–75, 2014. 10.1097/ALN.0000000000000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang JM, Song XJ, LaMotte RH: An in vitro study of ectopic discharge generation and adrenergic sensitivity in the intact, nerve-injured rat dorsal root ganglion. Pain 72:51–7, 1997. [DOI] [PubMed] [Google Scholar]
- 84.Zhang X, Priest BT, Belfer I, Gold MS: Voltage-gated Na+ currents in human dorsal root ganglion neurons. Elife 6:e23235, 2017. 10.7554/eLife.23235 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The neurophysiological data can be shared on request. The FIBSI.py source code and a detailed tutorial for using our Frequency-Independent Biology Signal Identification analysis program are available on a Github repository titled “FIBSI Project” by user rmcassidy (https://github.com/rmcassidy/FIBSI_program).


