Abstract
Background:
Use of haploidentical donor hematopoietic cell transplantation (haploHCT) has expanded but recent reports raise concern for increased rates of infectious complications. The incidence and risk factors for invasive fungal disease (IFD) after haploHCT have not been well elucidated.
Objective:
The objective of this study is to evaluate the incidence and risk factors for IFD after haploHCT. The identification of key risk factors will permit targeted prevention measures and may explain elevated risk for other infectious complications after haploHCT.
Study Design:
We performed a single-center retrospective study of all adults undergoing haploHCT between May 2011 and May 2021 (n=205). The 30-day and one-year cumulative incidence of proven or probable IFD and one-year non-relapse mortality (NRM) were assessed. Secondary analysis evaluated risk factors for invasive yeast infection (IYI) using univariate and multivariable Cox regression models.
Results:
Twenty-nine patients (14%) developed IFD following haploHCT. Nineteen (9.3%) developed IYI in the first year, 13 of which occurred early with a 30-day cumulative incidence of 6.3% (95% CI 2.9 – 9.6%) and increased NRM in patients with IYI (53.9% versus 10.9%). The majority of yeast isolates (17/20; 85%) were fluconazole susceptible. The incidence of IYI in the first 30 days after haploHCT was 10% among the 110 (54%) patients who developed cytokine release syndrome (CRS) and 21% among the 29 (14%) who received tocilizumab. On multivariable analysis, AML (HR 6.24; 1.66 – 23.37; p=0.007) and CRS (HR 4.65; 1.00 – 21.58; p=0.049) were associated with an increased risk of early IYI after haploHCT.
Conclusion:
CRS after haploHCT is common and is associated with increased risk of early IYI. The identification of CRS as a risk factor for IYI raises questions about its potential association with other infections after haploHCT. Recognition of key risk factors for infection may permit individualized strategies for prevention and intervention and minimize potential side effects.
Keywords: Invasive fungal disease, Candida, Cytokine release syndrome, Haploidentical transplantation, Post-transplant cyclophosphamide
Summary:
Invasive fungal disease (IFD) is a morbid complication of hematopoietic cell transplantation (HCT). Here we identified cytokine release syndrome (CRS) as a key risk factor for early yeast infection after haploHCT. CRS may contribute to risk for other infections after haploHCT.
INTRODUCTION:
Hematopoietic cell transplantation (HCT) remains an important potentially curative therapy for a variety of hematologic disorders, but is limited by significant toxicities as well as challenges in finding an appropriate donor match 1. Matched donor accessibility varies geographically and across racial and ethnic groups, and other strategies for matching such as haploidentical donor HCT (haploHCT) have arisen in that setting 2. While this strategy expands a patient’s potential donor pool, alternative strategies for T-cell depletion and modulation generally have to be adopted to mitigate an increased risk of GVHD 3,4. This in turn may contribute to a greater risk of infectious complications.
Use of haploHCT has increased as novel GVHD prophylaxis strategies such as post-transplant cyclophosphamide (PTCy) have been developed 5–7. However, recent studies have identified an increased incidence of viral infections including cytomegalovirus (CMV), non-CMV herpesvirus, and respiratory viral infections following haploHCT 8–10. Invasive fungal disease (IFD) is one of the most morbid infectious complications of HCT with estimated mortality ranging as high as 80% 11,12. Several studies have evaluated the incidence and risk factors for IFD following haploHCT, but given the wide variation in epidemiology and management between centers, it remains important to identify variables that may universally predict IFD and permit targeted prevention or intervention 13–15. Furthermore, unique risk factors for IFD after haploHCT may provide insight on the increased incidence of other infections observed in patients with haploidentical donors.
Antifungal medications may be used as prophylactic or preemptive treatment to reduce the risk of IFD, however these medications have a variety of adverse effects and can lead to toxicity, particularly in combination with GVHD prophylaxis regimens, as well as contribute to the development of fungal resistance 16–19. Breakthrough fungal infections have also been widely reported with incidence ranging from 3 to 13% in published literature 20–24. Given these factors, current guidelines suggest the use of prophylactic therapies only when the institutional incidence of invasive mold infection (IMI) is ≥6% and invasive yeast infection (IYI) is ≥10% 25,26. At our center, rates of IFD after HCT have historically been low, ranging from 1-3%, leading to an institutional decision not to institute routine antifungal prophylaxis for non-umbilical cord blood HCT, and instead to pursue a strategy of preemptive treatment based on symptoms, emerging risk factors, and monitoring of fungal markers 27–31.
There are several unique aspects to haploHCT that could predispose patients to infections including IFD. First, patients receive additional immunosuppressive therapies for the prevention of GVHD such as PTCy, and the effect of these therapies on immune reconstitution is still not fully understood 32. In addition, between infusion of cells and the administration of cyclophosphamide, patients can develop cytokine release syndrome (CRS), a unique syndrome characterized by fever, vascular leak, hypotension, organ dysfunction, and elevated inflammatory markers 33–35. Previous studies have described delayed neutrophil recovery, reduced overall survival, and increased transplant-related mortality in patients undergoing haploHCT who developed severe CRS 33. Notably, infectious complications were not reported in several of these studies and may have been a driver of poor outcomes. Furthermore, patients may receive tocilizumab, an anti-IL6 monoclonal antibody with additional immunosuppressive effects, for prevention and treatment of severe CRS 33,35. While several studies did not show an increased risk of infections in patients who received tocilizumab for CRS related to CAR T-cell therapy or for treatment of COVID-19 infection, further data are needed to elucidate the role of this immune modulator following HCT 36,37. Finally, increased rates of graft failure or GVHD in the haploHCT population may lead to an elevated risk of late onset infections. Developing a clear understanding of the risk factors that may predispose patients to IFD after haploHCT may inform more individualized strategies for prevention and point to potential drivers of other infections after haploHCT.
MATERIALS AND METHODS:
Study Design and Patients:
We performed a single-center retrospective study of sequential adults (age ≥ 18) undergoing haploHCT between May 2011 and May 2021 at Brigham and Women’s Hospital and Dana-Farber Cancer Institute (Boston, MA). This study was approved by the Mass General Brigham Human Research Office and Institutional Review Board. Patients were identified through the Dana-Farber Cancer Institute HCT database. Patients with a prior diagnosis of IFD who were receiving active treatment at the time of transplantation were excluded. Patients with a history of IFD who had completed treatment prior to transplantation and were deemed not to have an ongoing active infection were included.
Transplantation Procedures:
Patients received peripheral blood (PB; n=100) or bone marrow (BM; n=105) hematopoietic cell grafts from HLA-haploidentical donors with either myeloablative (MAC; n=51; 25%) or reduced intensity conditioning (RIC; n=154; 75%) regimens. The majority of patients (68%) received a conditioning regimen of fludarabine, cyclophosphamide and low dose TBI, while the remaining patients received a variety of other conditioning regiments as outlined in the Supplementary Data. All patients received GVHD prophylaxis that consisted of post-transplant cyclophosphamide (PTCy) 50 mg/kg on days +3 and +4 and tacrolimus (TAC) plus mycophenolate mofetil (MMF), except for 3 patients who received no additional GVHD prophylaxis per an experimental conditioning protocol and 4 patients who developed toxicity to tacrolimus and were switched to sirolimus. Unless contraindicated all patients received herpesvirus prophylaxis with acyclovir, valacyclovir, or famciclovir from day −1 to day +365 as well as Pneumocystis jirovecii (PJP) prophylaxis with trimethoprim-sulfamethoxazole (TMP/SMX) or atovaquone from day +30 to day +365. No routine antifungal prophylaxis was administered given low rates of invasive fungal disease in our center historically. Patients were followed with preemptive monitoring for CMV until letermovir was approved. Subsequently patients who received PTCy that were CMV seropositive received prophylaxis with letermovir. Patients were additionally treated according to a preemptive febrile neutropenia protocol where empiric anti-Pseudomonal gram-negative antibacterials including cefepime or ceftazidime were initiated at first onset of febrile neutropenia. Empiric antifungal therapy with micafungin was initiated for persistent or recurrent fevers >4 days after start of treatment with empiric antibacterials or for recurrent fevers after defervescence while on empiric antibacterials. Patient outcomes were followed for one year after HCT.
Data Collection:
Data collected from the electronic medical record included baseline characteristics such as age, sex, underlying disease, history of prior HCT, conditioning regimen and intensity, graft source (PB or BM), and GVHD prophylaxis. Data were also collected on exposure to potential mediators of IFD including development of CRS, administration of tocilizumab, diagnosis of acute GVHD, and administration of corticosteroids ≥ 1mg/kg for treatment of aGVHD.
IFD was identified between day of HCT (d0) to one year after transplantation. Proven or probable IFD was defined according to the revised European Organization for Research and Treatment of Cancer and the Mycoses Study Group (EORTC/MSG) criteria 38,39. Only proven and probable infections were included. The date of diagnosis was recorded as the day of first positive microbiologic testing. CRS was defined according to the consensus criteria 40. Treatment with tocilizumab was considered for patients with ≥2 CRS or grade 1 CRS with comorbidities that placed them for adverse outcomes related to CRS.
Statistical Analysis:
One-year and 30-day Cumulative Incidence Functions (CIF) were calculated based on time to IYI using dates of infection-free death, disease relapse, and repeat hematopoietic cell transplantation as censoring dates. Emphasis was placed on the evaluation of early infections within the first 30 days after transplantation as this represents a high-risk period of pre-engraftment where antifungal prophylaxis may be optimally targeted. One-year non-relapse mortality (NRM) was calculated for patients with IYI and patients without IYI using date of disease relapse and repeat HCT as censoring dates. A secondary analysis was performed to evaluate risk factors for IYI in the first 30 days using univariate and multivariable Cox regression models. The end point was time to diagnosis of IYI. Dates of relapsed disease, repeat HCT, or IYI-free death were used as censoring dates. Candidate risk factors included age >55, male sex, underlying disease, history of HCT, graft source, and conditioning intensity. We also included post-transplantation risk factors for IYI including diagnosis of CRS and administration of tocilizumab. No cases of acute GVHD occurred prior to IYI in the first 30 days after HCT, so this variable was not included in our risk factor analysis.
RESULTS:
We identified all patients who had undergone haploHCT (n=205) at our center between May 2011 and May 2021. The baseline characteristics of these patients are described in Table 1. Among this cohort, the median age was 57 years (range 19 – 77) and 59% were male. Acute myeloid leukemia (AML) was the most common underlying diagnosis. Twenty-nine patients (14%) had undergone prior allogeneic or autologous HCT. A total of 105 patients (51%) received BM grafts, and 100 patients received PB stem cell grafts (49%). More patients received RIC conditioning regimens than MAC regimens (75% versus 25%).
Table 1.
Baseline Characteristics and Transplant Outcomes
| Baseline Characteristics | No IFD n=176 | IFD n=29 | All Patients, No. (%) (n = 205) |
|---|---|---|---|
| Demographics | |||
| Age, median (range) | 57 (19 – 76) | 57 (21 – 77) | 57 (19 – 77) |
| Male sex, no. (%) | 102 (58) | 19 (66) | 121 (59) |
| Underlying Disease | |||
| AML, no. (%) | 64 (36) | 17 (59) | 81 (40) |
| MDS/MPN, no. (%) | 42 (24) | 4 (14) | 46 (22) |
| NHL, no. (%) | 21 (12) | 3 (10) | 24 (12) |
| ALL, no. (%) | 17 (10) | 4 (14) | 21 (10) |
| HL, no. (%) | 8 (5) | 1 (3) | 9 (4) |
| Other acute leukemia | 9 (5) | 0 (0) | 9 (4) |
| SAA, no. (%) | 4 (2) | 1 (3) | 5 (2) |
| Hemoglobinopathy, no. (%) | 4 (2) | 0 (0) | 4 (2) |
| CML, no. (%) | 3 (2) | 0 (0) | 3 (1) |
| HLH, no. (%) | 2 (1) | 0 (0) | 2 (1) |
| Autoimmune, no. (%) | 1 (0.6) | 0 (0) | 1 (0.5) |
| Prior HCT | |||
| Allogeneic | 12 (7) | 5 (17) | 17 (8) |
| Autologous | 11 (6) | 1 (3) | 12 (6) |
| Graft | |||
| Bone Marrow | 93 (53) | 12 (41) | 105 (51) |
| Peripheral blood | 83 (47) | 17 (59) | 100 (49) |
| Conditioning Intensity | |||
| Myeloablative | 41 (23) | 10 (34) | 51 (25) |
| RIC / NMA | 135 (77) | 19 (66) | 154 (75) |
| Transplant Outcomes | No IFD n=176 | IFD n=29 | All Patients, No. (%) (n = 205) |
| Acute GVHD | 92 (52) | 13 (45) | 105 (51) |
| GVHD Grade | |||
| Grade-I | 50 (54) | 10 (77) | 60 (57) |
| Grade II | 34 (37) | 3 (23) | 37 (35) |
| Grade III | 8 (9) | 0 (0) | 8 (8) |
| Tx ≥ 1 mg/kg Corticosteroids | 53 (58) | 8 (62) | 61 (58) |
| CRS Diagnosis | 92 (52) | 18 (62) | 110 (54) |
| Tocilizumab | 20 (11) | 9 (31) | 29 (14) |
Acute GVHD (Grade II-IV) was observed in 45/205 (22%) patients following transplantation, of which 37 (18%) were grade II and 8 (4%) were grade III. Sixty one of the 105 patients with acute GVHD (58%) were treated with ≥ 1 mg/kg of corticosteroids. Over half of the patients (110; 54%) developed CRS following infusion of cells, and 29 (14%) required administration of tocilizumab for more severe CRS. More patients who developed CRS received PB grafts than BM grafts (70% versus 30%). The median time to tocilizumab administration after infusion was 3 days (range 1 – 6).
Nineteen patients (9%) developed invasive yeast infections, of which 18 cases were proven and 1 was probable as shown in Table 2. The median time to onset of IYI was 28 days and 13 cases (72%) were diagnosed prior to day + 30. The time to infection for those diagnosed after day +30 ranged from day +41 to day +303. Twenty-nine patients (14%) had a diagnosis of proven or probable IFD following haploHCT. The one-year cumulative incidence of any IFD was 13.2% (95% CI 8.5% - 17.8%). Eight patients (4%) developed invasive mold infections following haploHCT, the majority of which were probable invasive aspergillosis (6 cases, 75%). The mold infections occurred much later in the transplant course with a median time to onset of 76 days and only one mold infection prior to day +30. One case of IMI contributed to the patient’s death on day + 204. There was one fatal case of probable Pneumocystis pneumonia that occurred at day +198 and one fatal case of probable disseminated coccidioidomycosis that occurred at day +96.
Table 2.
Early versus Late Invasive Fungal Disease after Haploidentical HCT
| Invasive Fungal Disease | Patients, No. (%) (n = 205) | |
|---|---|---|
| Early (Day 0 – 30) | Late (Day 30 – 365) | |
| All Invasive Fungal Disease, n (%) | 14 (7) | 15 (7) |
| Median time to any IFD | 28 days | |
| Proven Infection | 20 | |
| Probable infection | 9 | |
| Invasive Yeast Infections, n (%) | 13 (6) | 6 (3) |
| Median time to Invasive Yeast Infection | 16 days | |
| Proven Infection | 18 | |
| Probable Infection | 1 | |
| Invasive Mold Infections, n (%) | 1 (0.5) | 7 (3) |
| Median time to Invasive Mold Infection | 76 days | |
| Proven Infection | 2 | |
| Probable Infection | 6 | |
Invasive yeast infections in the first 30 days and 1 year after haploHCT occurred in 13 and 19 patients with a cumulative incidence of 6.3% (95% CI 2.9 – 9.6) and 9.3% (95% CI 5.2 – 13.2). Patients with CRS had a higher cumulative incidence of IYI in the first 30 days (10% versus 2.1%; p=0.02) as shown in Figure 1. Characteristics of the IYIs are described in Table 3. These infections were most commonly bloodstream (13; 68%) or disseminated infections (6; 32%). Three patients (20%) with candidemia or disseminated candidiasis were receiving total parenteral nutrition at the time of diagnosis. The disseminated infections had multiorgan involvement including hepatic (n=4), splenic (n=2), pulmonary (n=4), and central nervous system (n=4).
Figure 1.
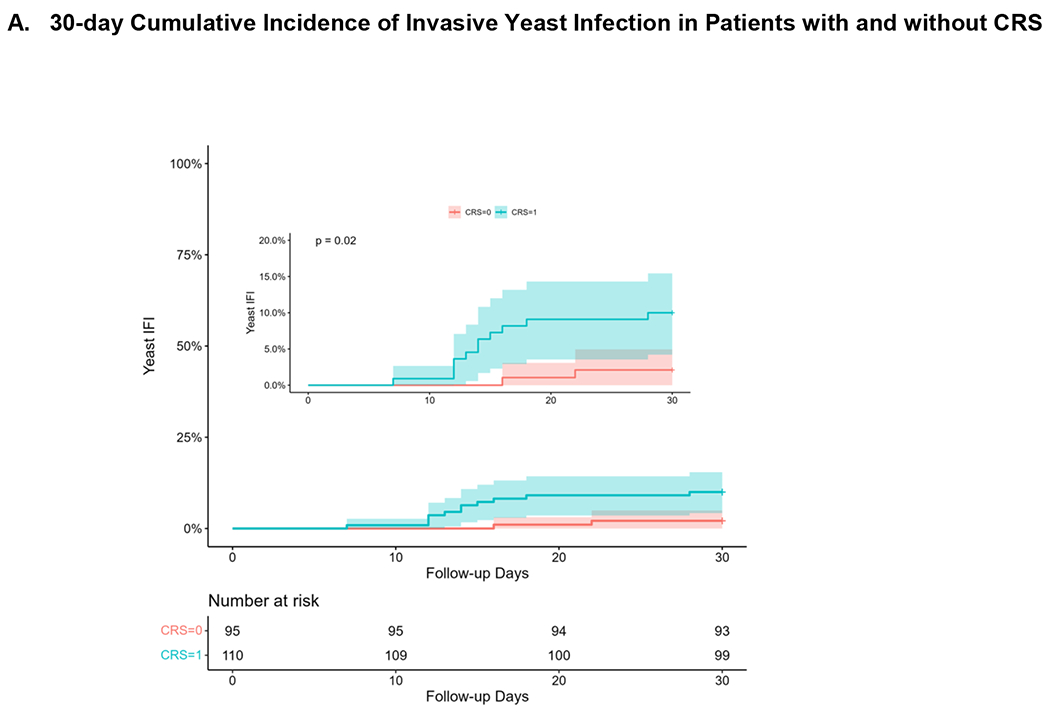
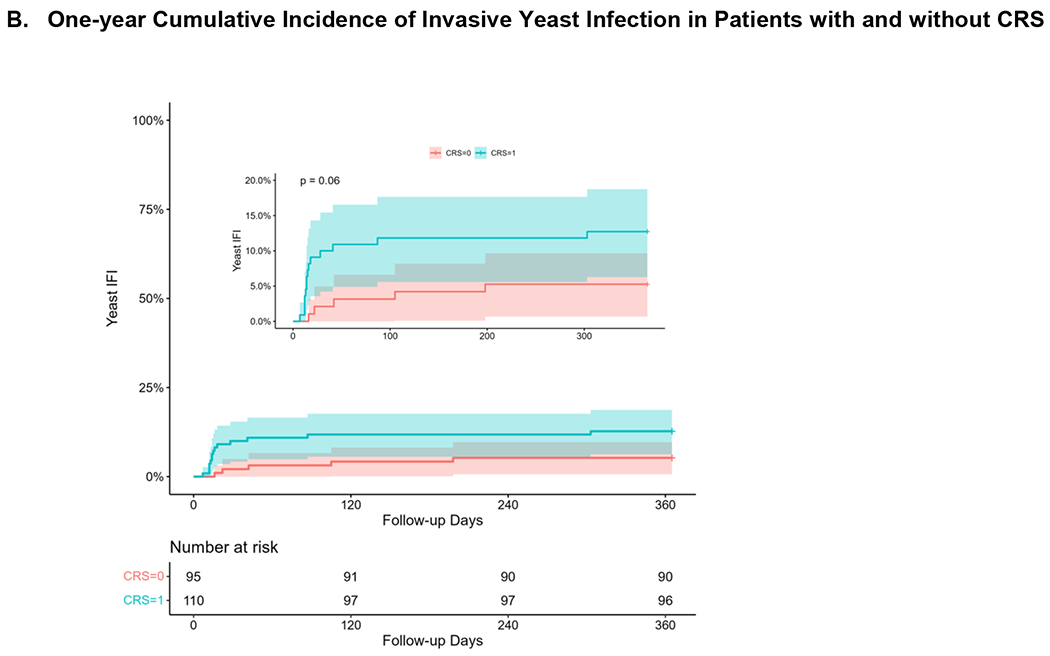
Cumulative Incidence of Invasive Yeast Infection in Patients with and without CRS at A) 30 days after transplantation and B) 1 year after transplantation.
Table 3.
Characteristics of Invasive Yeast Infections after Haploidentical HCT
| Yeast Infection Site | Patients, No. (n = 205) |
|---|---|
| Bloodstream Infection | 13 |
| Disseminated / Visceral Infection | 6 |
| Hepatic Involvement | 4 |
| Splenic Involvement | 2 |
| Pulmonary Involvement | 4 |
| Central Nervous System Involvement | 4 |
| Intraabdominal Infection | 2 |
| Other Site of Infection | 2 |
| Yeast Species Isolated | Isolates, No. |
| Candida albicans | 7 |
| Candida parapsilosis | 4* |
| Candida dubliniensis | 4 |
| Candida krusei | 2* |
| Candida tropicalis | 2 |
| Saccharomyces cerevisiae | 1 |
| Not identified | 1 |
Two patients had two isolates grow at the same time
Candida albicans was most frequently isolated yeast species (n=7). Other species included Candida parapsilosis (n=4), Candida dubliniensis (n=4), Candida krusei (n=2), Candida tropicalis (n=2), and Saccharomyces cerevisiae (n=1). Two of the patients grew more than one isolate on the same day. All of the isolates were fluconazole-susceptible except for 2 cases of Candida krusei and one case of Saccharomyces cerevisiae where fluconazole susceptibilities were not reported. Three of the infections were breakthrough infections in patients receiving empiric micafungin for the treatment of febrile neutropenia (for 12, 13, and 17 days prior to diagnosis). Patients with IYIs had a higher NRM of 10/19 or 53.9% (95% CI 24.0 – 72.1) at one year compared with those without IYIs with NRM of 20/186 or 10.9% (95% CI 6.1 – 15.4; Figure 2). Six patients without evidence of disease relapse who died within the first year had active IYI at the time of death and three deaths were attributed directly to IYI. Of the 19 patients with IYI in the first year, 12 were treated with prolonged antifungal therapy until time of death, 4 patients were treated for greater than 6 months, and 3 patients were treated for less than 6 months.
Figure 2.
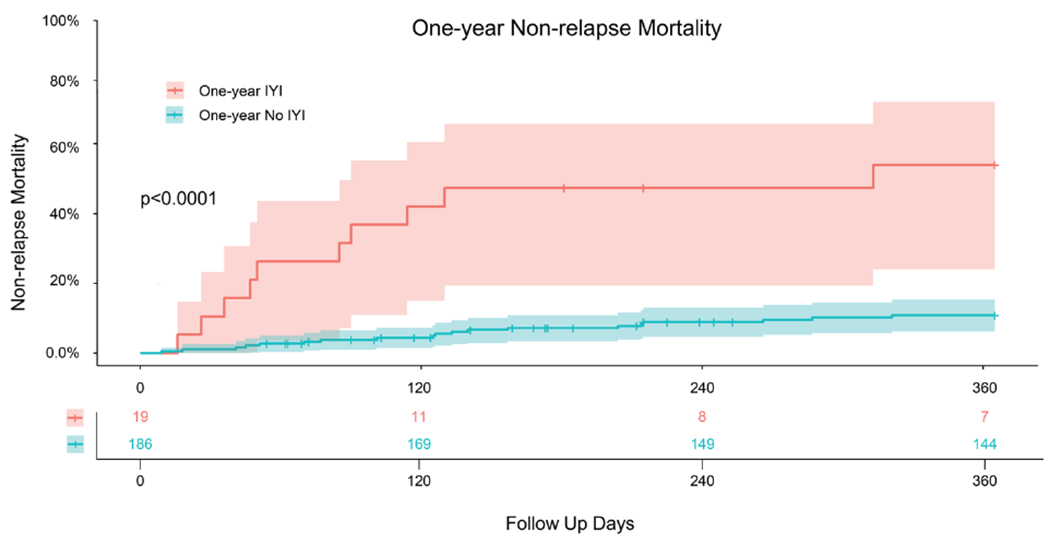
One-year Non-relapse Mortality in Patients with Invasive Yeast Infections
Risk factors for IYI in the first 30 days after haploHCT are shown in Table 4. Patients with underlying AML, as well as those with a diagnosis of CRS or administration of tocilizumab following cell infusion had an increased risk of early IYI on univariate analysis. Age, sex, other underlying diseases, history of HCT, graft source, and conditioning intensity were not associated with an increased risk of IYIs. On multivariable analysis, underlying AML (HR 6.24; 95% CI 1.66 – 23.37; p=0.007) and diagnosis of CRS (HR 4.65; 95% CI 1.00 – 21.58; p=0.049) were associated with an increased risk of yeast infection in the first 30 days after haploHCT. CRS was observed in 53.7% (110/205) of the patients and 10.0% (11/110) developed an IYI within the first 30 days. Approximately one fourth of patients (26.4%; 29/110) who developed CRS were treated with tocilizumab and 20.7% of those (6/29) developed an early IYI. Among the patients who developed IYI, 84.6% (11/13) had a diagnosis of CRS and 46.2% (6/13) had received tocilizumab treatment. The diagnosis of CRS and tocilizumab use were strongly colinear and both showed significant association with invasive yeast infections in separate multivariable regression models.
Table 4.
Risk Factors for Invasive Yeast Infection from D0 to D30 after Haploidentical HCT.
| Variable | No Early IYI n = 192 | Early IYI n = 13 | Univariate HR (95% CI) | Univariate P-value | Multivariable HR (95% CI) | Multivariable P-value |
|---|---|---|---|---|---|---|
| Age >55 | 100 | 8 | 1.02 (0.98 – 1.06) | 0.334 | 1.01 (0.97 – 1.04) | 0.748 |
| Male Sex | 112 | 8 | 1.56 (0.48 – 5.05) | 0.462 | 1.38 (0.42 – 4.57) | 0.597 |
| Underlying Disease | ||||||
| AML | 71 | 10 | 5.29 (1.46 – 19.24) | 0.005 | 6.24 (1.66 – 23.37) | 0.007 |
| MDS/MPN | 45 | 1 | 0.28 (0.04 – 2.19) | 0.227 | - | - |
| ALL | 20 | 1 | 0.73 (0.09 – 5.58) | 0.758 | - | - |
| HL | 8 | 1 | 1.87 (0.24 – 14.38) | 0.548 | - | - |
| Prior HCT | 25 | 4 | 2.85 (0.88 – 9.27) | 0.081 | 3.00 (0.89 – 10.14) | 0.077 |
| Graft source | ||||||
| Peripheral Blood | 91 | 9 | 1.43 (0.75 – 7.90) | 0.139 | - | - |
| Bone Marrow | 101 | 4 | 1.00 | - | - | - |
| Conditioning | ||||||
| MAC | 47 | 4 | 1.37 (0.42 – 4.45) | 0.600 | - | - |
| RIC | 145 | 9 | 1.00 | - | - | |
| CRS Diagnosis | 99 | 11 | 4.99 (1.11 – 22.53) | 0.036 | 4.65 (1.00 – 21.58) | 0.049 |
| Tocilizumab | 23 | 6 | 5.61 (1.88 – 16.69) | 0.002 | - | - |
DISCUSSION:
Invasive yeast infections were the most frequent cause of IFD in this cohort of patients who underwent haploidentical HCT and occurred early after transplantation. In our center, where anti-yeast prophylaxis is not routinely used due to institutional rates below the 10% recommended threshold for prophylaxis, the most common yeast isolate was Candida albicans, and almost all organisms isolated (17/20; 85%) were fluconazole-susceptible 31. History of AML and diagnosis of CRS were independently associated with an increased risk of invasive yeast infection in the first 30 days after haploHCT on multivariable analysis, indicating a higher-risk group where antifungal prophylaxis may be optimally targeted.
The use of haploHCT has greatly expanded globally following the development of PTCy for GVHD prophylaxis. Initial trials involving PTCy in haploHCT showed a low overall incidence of IFD 5. However, more recent studies have demonstrated a significant increase in viral infections following haploHCT raising concern that the risk for other infections such as IFD may be increased as well 8–10. The incidence and characteristics of IFD after HCT varies widely between centers leading to challenges in reporting and comparing data between institutions 11,41. We found a rate of early IYI amongst patients undergoing haploHCT of 10% in those who developed CRS and 21% in those who were treated with tocilizumab. Of note, we found increased NRM (53.9% versus 10.9%) in patients who developed IYI as compared to those without IYI. While IYI may contribute directly to increased NRM, it is possible that this is a marker of those at higher risk for complications as they also have more severe CRS, another marker of increased NRM 33. Taken together, these data suggest that patients undergoing haploHCT who develop these complications may require additional care strategies.
In many centers, the use of routine antifungal prophylaxis has led to shifts in the epidemiology of fungal organisms with increased numbers of resistant yeast infections and invasive mold infections as well as prevalence of breakthrough infections 11,21–23,41–43. Fungal dysbiosis driven by antifungal agent use (prophylactic or treatment-related) may negatively affect outcomes of HCT 44,45. Given these factors, current guidelines suggest the use of prophylactic therapies only when the institutional incidence of invasive mold infection is ≥6% and IYI is ≥10% 25,26. In our center, a strategy of preemptive treatment based on clinical findings, emerging risk factors such as high grade GVHD, or abnormal fungal markers is used in lieu of routine prophylaxis given historically low rates of IFD ranging from 1-3% where the risks of prophylaxis clearly outweigh any benefits. In this study, we demonstrate a low incidence of resistant yeast or mold infections, reinforcing our aim to target antifungal therapy only to patients with the highest risk in order to reduce drug-related toxicity and drug-drug interactions, as well as potential selection for more resistant organisms. The identification of broadly applicable risk factors or predictors of early IFD such as CRS or use of tocilizumab may permit individualized strategies for prophylaxis.
A clear understanding of the risk factors for the development of early IFD, may permit optimal targeting of antifungal prophylaxis to reduce adverse effects and maximize benefit. On multivariable analysis, AML was an independent risk factor for IYI in the first 30 days after haploHCT. Patients’ transplant outcomes may be affected by immune dysregulation related to underlying disease or prior treatment regimens 12,46. In particular, patients with AML may receive multiple courses of cytotoxic chemotherapy with prolonged cytopenias preceding transplantation and placing them at higher risk for IFD following HCT 31,47,48. Exposure to antimicrobials during these periods of neutropenia may also predispose patients to an increased risk of post-transplant infectious complications 49,50.
CRS is a unique inflammatory syndrome characterized by fever, vascular leak, and organ dysfunction following haploHCT that frequently requires treatment with immunosuppressive agents such as tocilizumab 33,34,40. In this analysis, we showed CRS to be an independent risk factor for invasive yeast infections in the first 30 days after transplant. While 6% of all patients developed IYIs in the first 30 days after cell infusion, 10% of those who developed CRS and 21% of those who received tocilizumab for severe CRS went on to develop early IYI between day 0 to day +30. Only 2 patients (2%) of those without CRS developed early IYI. Using the development of CRS as a potential trigger for pre-emptive antifungal therapy would capture 85% of these early IYI cases and spare the non-CRS population of patients with exceedingly low risk of early IYI from superfluous antifungal exposure. While our overall rates of IYI and IMI still did not meet the recommended threshold for routine antifungal prophylaxis, given the morbidity of these early IYIs, we would favor implementing targeted prophylaxis to the apparent high risk group of patients who develop CRS and had an elevated incidence rate of 10% 25,26.
The development of severe CRS has been shown to be associated with delayed neutrophil engraftment and increased transplant related mortality 33,34. Severe CRS may drive changes in immune reconstitution that lead to an increased risk of early IFD as well as possibly contributing to the increased risk for other infections that have previously been described 8,9,51. While tocilizumab has not been generally found to increase the risk of IFD in other settings, in this analysis, the use of tocilizumab may serve as a marker for severe CRS rather than incurring direct increase in risk of IFD. Tocilizumab may also be administered more readily to sicker patients with higher risk comorbidities as a preemptive measure. Further data are needed to evaluate the impact of CRS and tocilizumab administration on the development of other infectious complications and mortality after haploHCT.
This study is limited by retrospective single-center data and its applicability may be limited in centers where incidence of IFD is higher and routine antifungal prophylaxis is broadly used. However, in the face of rising antifungal resistance, stewardship remains a critical component of prophylaxis and preemptive treatment strategies 52,53. Differences in climate or geographic fungal epidemiology between institutions may lead to varying risks of IFD.54 Consideration of environmental and population-level risk factors as well as individual host risk factors may aid in determining the optimal antifungal prophylaxis strategy employed in each institution. Identification of unique host risk factors for IFD such as CRS may aid in these stewardship efforts and inform risk for other infectious complications following haploidentical HCT.
Here, we report a unique risk factor for IFD after haploidentical transplantation. CRS is a complication specific to haploidentical HCT and may contribute to an increased risk for infections in general following haploHCT. Further studies are needed to evaluate the impact of CRS on immune reconstitution and the development of other infectious complications. Based on our findings, with an elevated incidence of early IYI amongst patients who developed CRS and in particular those with severe CRS requiring tocilizumab administration, we favor targeted prophylaxis of this high-risk group of transplant recipients, maximizing the benefit while minimizing potential toxicities.
Highlights:
The rise of antifungal resistance represents a major threat for patients undergoing HCT
Improved understanding of population and individual host risk factors for IFD is critical
CRS after haploidentical HCT is a unique risk factor that may predispose patients to IFD
Assessment of institutional IFD incidence and host risk permits targeted strategies for antifungal prophylaxis
Individualized approaches to prophylaxis can maximize benefit and promote antifungal stewardship
Acknowledgments
We would like to acknowledge the enormous impact of Dr. Francisco M. Marty on the care of the patients in this study.
Funding
This work was supported in part by the National Center for Advancing Translational Sciences (NCATS) UL1 RR025758 [to Harvard].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests:
JSL: None
RMS: None
MMA: None
DWK: None
GZ: None
JHA: None
JK: None
SN: None
CSC: None
RR: None
NCI: None
VTH: None
MG: None
RJS: None
LRB: None
References:
- 1.Copelan EA. Hematopoietic Stem-Cell Transplantation. N Engl J Med. 2006;354(17):1813–1826. doi: 10.1056/NEJMRA052638 [DOI] [PubMed] [Google Scholar]
- 2.Gragert L, Eapen M, Williams E, et al. HLA Match Likelihoods for Hematopoietic Stem-Cell Grafts in the U.S. Registry. N Engl J Med. 2014;371(4):339–348. doi: 10.1056/nejmsa1311707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powles RL, Kay HEM, Clink HM, et al. Mismatched Family Donors For Bone-Marrow Transplantation As Treatment For Acute Leukemia. Lancet. 1983;321(8325):612–615. doi: 10.1016/S0140-6736(83)91793-2 [DOI] [PubMed] [Google Scholar]
- 4.Beatty PG, Clift RA, Mickelson EM, et al. Marrow Transplantation from Related Donors Other Than HLA-Identical Siblings. N Engl J Med. 1985;313(13):765–771. doi: 10.1056/nejm198509263131301 [DOI] [PubMed] [Google Scholar]
- 5.Luznik L, O’Donnell PV., Symons HJ, et al. HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luznik L, Bolaños-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–3230. doi: 10.1182/blood-2009-11-251595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13(1):10–24. doi: 10.1038/nrclinonc.2015.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldsmith SR, Fuchs EJ, Bashey A, et al. Incidence and Impact of Cytomegalovirus Infection in Haploidentical and Matched-Related Donors Receiving Post-Transplant Cyclophosphamide (PTCy): A CIBMTR Analysis. Biol Blood Marrow Transplant. 2020;26(3):S69–S70. doi: 10.1016/j.bbmt.2019.12.217 [DOI] [Google Scholar]
- 9.Mulroney CM, Bilal Abid M, Bashey A, et al. Incidence and impact of community respiratory viral infections in post-transplant cyclophosphamide-based graft-versus-host disease prophylaxis and haploidentical stem cell transplantation. Br J Haematol. 2021;194(1):145–157. doi: 10.1111/bjh.17563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh AK, Fuchs EJ, Bashey A, et al. Incidence and Impact of Non-CMV Herpes Viral Infection in Haploidentical and Matched Sibling Donors Receiving Post-Transplant Cyclophosphamide (PTCy): A CIBMTR Analysis. Biol Blood Marrow Transplant. 2020;26(3):S331–S332. doi: 10.1016/j.bbmt.2019.12.358 [DOI] [Google Scholar]
- 11.Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: Analysis of multicenter prospective antifungal therapy (PATH) alliance registry. Clin Infect Dis. 2009;48(3):265–273. doi: 10.1086/595846 [DOI] [PubMed] [Google Scholar]
- 12.Kontoyiennis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: Overview of the transplant- associated infection surveillance network (TRANSNET) database. Clin Infect Dis. 2010;50(8):1091–1100. doi: 10.1086/651263 [DOI] [PubMed] [Google Scholar]
- 13.Slade M, Goldsmith S, Romee R, et al. Epidemiology of infections following haploidentical peripheral blood hematopoietic cell transplantation. Transpl Infect Dis. 2017;19(1). doi: 10.1111/tid.12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fayard A, Daguenet E, Blaise D, et al. Evaluation of infectious complications after haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide following reduced-intensity and myeloablative conditioning: a study on behalf of the Francophone Society of Stem Cell. Bone Marrow Transplant. 2019;54(10):1586–1594. doi: 10.1038/s41409-019-0475-7 [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Xu L, Liu D, et al. Incidence of invasive fungal disease after unmanipulated haploidentical stem cell transplantation was significantly higher than that after HLA-matched sibling transplantation. Clin Microbiol Infect. 2013;19(11):1029–1034. doi: 10.1111/1469-0691.12120 [DOI] [PubMed] [Google Scholar]
- 16.Goodman JL, Winston DJ, Greenfield RA, et al. A Controlled Trial of Fluconazole to Prevent Fungal Infections in Patients Undergoing Bone Marrow Transplantation. N Engl J Med. 1992;326(13):845–851. doi: 10.1056/nejm199203263261301 [DOI] [PubMed] [Google Scholar]
- 17.Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116(24):5111–5118. doi: 10.1182/blood-2010-02-268151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marr KA, Seidel K, Slavin MA, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000;96(6):2055–2061. doi: 10.1182/BLOOD.V96.6.2055 [DOI] [PubMed] [Google Scholar]
- 19.Wingard JR, Merz WG, Rinaldi MG, Johnson TR, Karp JE, Saral R. Increase in Candida krusei Infection among Patients with Bone Marrow Transplantation and Neutropenia Treated Prophylactically with Fluconazole. N Engl J Med. 1991;325(18):1274–1277. doi: 10.1056/nejm199110313251803 [DOI] [PubMed] [Google Scholar]
- 20.Marty FM, Cosimi LA, Baden LR. Breakthrough Zygomycosis after Voriconazole Treatment in Recipients of Hematopoietic Stem-Cell Transplants. N Engl J Med. 2004;350(9):950–952. doi: 10.1056/nejm200402263500923 [DOI] [PubMed] [Google Scholar]
- 21.Jenks JD, Cornely OA, Chen SCA, Thompson GR, Hoenigl M. Breakthrough invasive fungal infections: Who is at risk? Mycoses. 2020;63(10):1021–1032. doi: 10.1111/myc.13148 [DOI] [PubMed] [Google Scholar]
- 22.Álvarez-Uría A, Guinea JV, Escribano P, et al. Invasive Scedosporium and Lomentosora infections in the era of antifungal prophylaxis: A 20-year experience from a single centre in Spain. Mycoses. 2020;63(11):1195–1202. doi: 10.1111/myc.13154 [DOI] [PubMed] [Google Scholar]
- 23.Chen XC, Xu J, Wu DP. Clinical Characteristics and Outcomes of Breakthrough Candidemia in 71 Hematologic Malignancy Patients and/or Allogeneic Hematopoietic Stem Cell Transplant Recipients: A Single-center Retrospective Study from China, 2011-2018. Clin Infect Dis. 2020;71(Suppl 4):S394–S399. doi: 10.1093/cid/ciaa1523 [DOI] [PubMed] [Google Scholar]
- 24.Imhof A, Balajee SA, Fredricks DN, England JA, Marr KA. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin Infect Dis. 2004;39(5):743–746. doi: 10.1086/423274 [DOI] [PubMed] [Google Scholar]
- 25.Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J Clin Oncol. 2018;36(30):3043–3054. doi: 10.1200/JCO.18.00374 [DOI] [PubMed] [Google Scholar]
- 26.De Pauw BE, Donnelly JP. Prophylaxis and Aspergillosis — Has the Principle Been Proven? N Engl J Med. 2007;356(4):409–411. doi: 10.1056/nejme068266 [DOI] [PubMed] [Google Scholar]
- 27.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or Fluconazole for Prophylaxis in Severe Graft-versus-Host Disease. N Engl J Med. 2007;356(4):335–347. doi: 10.1056/nejmoa061098 [DOI] [PubMed] [Google Scholar]
- 28.Koo S, Baden LR, Marty FM. Post-diagnostic kinetics of the (1 → 3)-β-d-glucan assay in invasive aspergillosis, invasive candidiasis and Pneumocystis jirovecii pneumonia. Clin Microbiol Infect. 2012;18(5). doi: 10.1111/j.1469-0691.2012.03777.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo S, Bryar JM, Baden LR, Marty FM. Prognostic features of galactomannan antigenemia in galactomannan-positive invasive aspergillosis. J Clin Microbiol. 2010;48(4):1255–1260. doi: 10.1128/JCM.02281-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marty FM, Lee SJ, Fahey MM, et al. Infliximab use in patients with severe graft-versus-host disease and other emerging risk factors of non-Candida invasive fungal infections in allogeneic hematopoietic stem cell transplant recipients: A cohort study. Blood. 2003;102(8):2768–2776. doi: 10.1182/blood-2003-01-0267 [DOI] [PubMed] [Google Scholar]
- 31.Hammond SP, Marty FM, Bryar JM, DeAngelo DJ, Baden LR. Invasive fungal disease in patients treated for newly diagnosed acute leukemia. Am J Hematol. 2010;85(9):695–699. doi: 10.1002/ajh.21776 [DOI] [PubMed] [Google Scholar]
- 32.Rambaldi B, Kim HT, Reynolds C, et al. Impaired T- And NK-cell reconstitution after haploidentical HCT with posttransplant cyclophosphamide. Blood Adv. 2021;5(2):352–364. doi: 10.1182/bloodadvances.2020003005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abboud R, Keller J, Slade M, et al. Severe Cytokine-Release Syndrome after T Cell–Replete Peripheral Blood Haploidentical Donor Transplantation Is Associated with Poor Survival and Anti–IL-6 Therapy Is Safe and Well Tolerated. Biol Blood Marrow Transplant. 2016;22(10):1851–1860. doi: 10.1016/j.bbmt.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abboud R, Wan F, Mariotti J, et al. Cytokine release syndrome after haploidentical hematopoietic cell transplantation: an international multicenter analysis. Bone Marrow Transplant. 2021;56(11):2763–2770. doi: 10.1038/s41409-021-01403-w [DOI] [PubMed] [Google Scholar]
- 35.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frigault MJ, Nikiforow S, Mansour MK, et al. Tocilizumab not associated with increased infection risk after CAR T-cell therapy: Implications for COVID-19? Blood. 2020;136(1):137–139. doi: 10.1182/BLOOD.2020006216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/nejmoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. doi: 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peter Donnelly J, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the european organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758 [DOI] [PubMed] [Google Scholar]
- 41.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective Surveillance for Invasive Fungal Infections in Hematopoietic Stem Cell Transplant Recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–1100. doi: 10.1086/651263 [DOI] [PubMed] [Google Scholar]
- 42.Van Burik JAH, Leisenring W, Myerson D, et al. The effect of prophylactic fluconazole on the clinical spectrum of fungal diseases in bone marrow transplant recipients with special attention to hepatic candidiasis. An autopsy study of 355 patients. Medicine (Baltimore). 1998;77(4):246–254. doi: 10.1097/00005792-199807000-00003 [DOI] [PubMed] [Google Scholar]
- 43.Singh N Trends in the epidemiology of opportunistic fungal infections: Predisposing factors and the impact of antimicrobial use practices. Clin Infect Dis. 2001;33(10):1692–1696. doi: 10.1086/323895 [DOI] [PubMed] [Google Scholar]
- 44.Rolling T, Zhai B, Gjonbalaj M, et al. Haematopoietic cell transplantation outcomes are linked to intestinal mycobiota dynamics and an expansion of Candida parapsilosis complex species. Nat Microbiol 2021. November 2021:1–11. doi: 10.1038/s41564-021-00989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mann PA, McNicholas PM, Chau AS, et al. Impact of antifungal prophylaxis on colonization and azole susceptibility of Candida species. Antimicrob Agents Chemother. 2009;53(12):5026–5034. doi: 10.1128/AAC.01031-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: Results of the SEIFEM B-2004 study - Sorveglianza Epidemiologica Infezioni Fungine nelle Emopatie Maligne. Clin Infect Dis. 2007;45(9):1161–1170. doi: 10.1086/522189 [DOI] [PubMed] [Google Scholar]
- 47.Maertens JA, Girmenia C, Brüggemann RJ, et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: Summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother. 2018;73(12):3221–3230. doi: 10.1093/jac/dky286 [DOI] [PubMed] [Google Scholar]
- 48.Patel HP, Perissinotti AJ, Patel TS, Bixby DL, Marshall VD, Marini BL. Incidence and Risk Factors for Breakthrough Invasive Mold Infections in Acute Myeloid Leukemia Patients Receiving Remission Induction Chemotherapy. Open Forum Infect Dis. 2019;6(5). doi: 10.1093/OFID/OFZ176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin MY, Carmeli Y, Zumsteg J, et al. Prior antimicrobial therapy and risk for hospital-acquired Candida glabrata and Candida krusei fungemia: a case-case-control study. Antimicrob Agents Chemother. 2005;49(11):4555–4560. doi: 10.1128/AAC.49.11.4555-4560.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben-Ami R, Olshtain-Pops K, Krieger M, et al. Antibiotic exposure as a risk factor for fluconazole-resistant Candida bloodstream infection. Antimicrob Agents Chemother. 2012;56(5):2518–2523. doi: 10.1128/AAC.05947-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh A, Dandoy CE, Chen M, et al. Post-Transplantation Cyclophosphamide Is Associated with an Increase in Non-Cytomegalovirus Herpesvirus Infections in Patients with Acute Leukemia and Myelodysplastic Syndrome. Transplant Cell Ther. 2021;0(0). doi: 10.1016/j.jtct.2021.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisher MC, Alastruey-Izquierdo A, Berman J, et al. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol 2022. March 2022:1–15. doi: 10.1038/s41579-022-00720-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kontoyiannis DP. Antifungal resistance: An emerging reality and a global challenge. J Infect Dis. 2017;216(suppl_3):S431–S435. doi: 10.1093/infdis/jix179 [DOI] [PubMed] [Google Scholar]
- 54.Friedman DZP, Schwartz IS. Emerging fungal infections: New patients, new patterns, and new pathogens. J Fungi. 2019;5(3). doi: 10.3390/jof5030067 [DOI] [PMC free article] [PubMed] [Google Scholar]


