Abstract
Background
Guidelines recommend transfusion of red blood cells (RBC's) when a hospitalized patient's hemoglobin (Hb) drops below a restrictive transfusion threshold, either at 7 or 8 g. Hospitals have implemented transfusion policies to encourage compliance with guidelines and reduce variation in transfusion practice. However, variation in transfusion practice remains. The purpose of this study was to examine whether there is variation in the receipt of transfusion by patient race.
Methods
Hospitalized general medicine patients with anemia (Hb < 10 g/dL) were eligible. Chi‐squared tests were used to compare the percent of patients receiving a transfusion by race overall and within strata of their nadir Hb. Linear regression was used to test the association between a patient's race, their nadir Hb, receipt of an RBC transfusion, and the number of units transfused.
Results
Four thousand nine hundred and fifty‐one patients consented, including 1363 (28%) who received a transfusion. 71% of patients were African American, 25% were White, and 4% were Other Race. Overall African Americans were less likely to be transfused compared to Whites (25% vs. 30%, p < .01), and within Hb strata below a Nadir Hb of 9 g/dL (Hb 8.0–8.9 g/dL 1% vs. 7%, p < .01; 7.0–7.9 g/dL 15% vs. 28%, p < .01; <7 g/dL 80% vs. 86%, p < .01). African Americans also received fewer units of RBC's (β = −.17, p < .01) overall and at lower Hb levels (β = .14, p < .01) compared to Whites.
Discussion
The Hb level at which patients are transfused at and the total number of RBC units received during hospitalization differ by patient race.
Keywords: hematology—red cells, RBC transfusion, transfusion practices
Short abstract
Abbreviations
- CCI
charlson comorbidity index
- EHR
electronic health record
- GI
gastroinestinal
- Hb
hemoglobin
- RBC's
red blood cells
- SC
sickle cell anemia
- UCMC
university of chicago medical center
1. INTRODUCTION
The two most recent red blood cell (RBC) transfusion guidelines from the Association for the Advancement of Blood and Biotherapies (AABB), recommend transfusion when a hospitalized patient's hemoglobin (Hb) drops below a restrictive transfusion threshold, either at 7 or 8 g/dL. 1 , 2 These guidelines are supported by a body of clinical trial evidence, 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 and clinicians have responded by largely adopting restrictive transfusion practices and only transfusing patients when their Hb drops below a restrictive threshold. Reducing the use of transfusion to comply with restrictive transfusion guidelines has also resulted in a preference for single unit transfusions, compared to transfusing ≥2 units at a time. 11 , 12 Together these practices have now become standard of care for clinicians when treating anemia in hospitalized patients. 13 , 14 , 15 Since transfusion is a common inpatient procedure, 16 , 17 hospitals have utilized the electronic health record to implement these transfusion policies in an attempt to increase compliance with guidelines and standardize transfusion practice across providers. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 Moreover, improving compliance with and reducing variation in the use of transfusion outside of restrictive transfusion practices has become a hospital quality metric. 26 , 28 , 31 , 32 , 33
However, despite the widespread acceptance and adoption of restrictive transfusion practices, variation in transfusion practice does exist, 34 , 35 , 36 , 37 , 38 , 39 including variation in transfusion practice by patients' race. 40 , 41 , 42 , 43 , 44 , 45 Variation in transfusion practice by race is concerning because it may represent a healthcare disparity, since it is not supported by empiric data or guidelines. Previous studies in adult surgical patients where restrictive transfusion practices would be standard of care have found that African Americans receive more perioperative transfusions when undergoing major surgery (CABG, Hip replacement) than do Whites, 40 and are less likely to receive an autologous transfusion (rather than allogenic) when undergoing orthopedic surgery than are Whites. 41 However, the data from these studies do not address whether there is variation outside of restrictive transfusion practices by race, and these studies were conducted before the widespread implementation of uniform restrictive transfusion policies enacted to discourage practice variation. Moreover, these studies were limited to narrow surgical patient populations and their findings may not be generalizable, since the largest volume of inpatient transfusions occurs in general medicine patients. 46 As a result, whether there is variation in the use of transfusion by race and outside of standard of care restrictive transfusion practices remains unknown.
Therefore, the purpose of this study was to examine transfusion practices in hospitalized general medicine patients, and test for differences in the Hb level at which patients are transfused at and the total number of RBC units received by patients' race.
2. METHODS
2.1. Study design and eligibility
The data for this study comes from an ongoing (2018‐) prospective observational study of quality of life and symptoms in hospitalized general medicine patients with anemia at the University of Chicago Medical Center (UCMC). Any hospitalized adult (≥18 years old) general medicine patient with a Hb < 10 g/dL at any point during their hospitalization was eligible for study participation, and all participating subjects provided written informed consent. At UCMC the general medicine services do not care for or include surgical, trauma, pediatric, obstetrics/gynecology, cardiology, or oncology patients, and as such those patients were not eligible for this study. Patients with sickle cell anemia were eligible for study participation, but excluded from this analysis because they have different transfusion practices and guideline recommendations than other hospitalized general medicine patients. 47 Patient's identifying as Jehovah Witness (2%) were included in the sample because some of them received transfusion (4%), not all of them identified as African American (3%), and a sensitivity analysis removing them did not change any results. This study was approved by the University of Chicago Institutional Review Board.
2.2. Patient demographic data
Hospital administrative data (including: electronic health record [EHR] and diagnosis codes) was used to determine patients' age, sex, race/ethnicity, length of stay (LOS), receipt of red blood cell transfusion, Hb levels, and comorbidities. Race and ethnicity were self‐reported and optional for all patients, with the available racial and ethnic categories matching those defined by the NIH (Race: American Indian or Alaskan Native, Asian/Mideast Indian, Black/African American, More than One Race, Native Hawaiian/Other Pacific Islander, Patient Declined to Identify Race, Unknown, and White; Ethnicity: Hispanic or Latino, Not Hispanic or Latino, Patient Declined to Identify Ethnicity, and Unknown). A Charlson Comorbidity Index (CCI) score was calculated for all patients using International Classification of Disease 10 codes. Health Care Utilization Project diagnosis categories were used to identify patients with sickle cell anemia (SC) and/or gastrointestinal (GI) bleeding, since these diagnoses are not included as part of the Charlson Comorbidity Index. Given the low number of non‐African Americans and non‐Whites cared for at our institution and enrolled in this study (<5%), racial categories were condensed and analyzed as a categorical variable including African American, White, and Other.
2.3. Restrictive transfusion policies
The EHR at UCMC utilizes a transfusion specific computerized provider order entry form, and the ordering form highlights a Hb <7 g/dL as a restrictive transfusion threshold.
2.4. Statistical analysis
Descriptive statistics were used to characterize the demographic and clinical characteristics of study participants, including patients' Hb levels during hospitalization, whether they received a transfusion, and how many units of RBC were transfused. After reviewing the descriptive data on the number of RBC units transfused in all study participants, a decision was made to limit all analyses to the 95% of patients in the study who received ≤7 units of RBC transfusion (0–7) during hospitalization. This was to account for and minimize any spurious statistical effect stemming from the 5% of patients with a very large number of transfused RBC units.
Chi‐squared tests were used to compare the percent of patients receiving or not receiving a transfusion by race overall during their hospitalization, and within strata of their nadir Hb during hospitalization (Hb 10‐9 g/dL, 8.9–8.0 g/dL, 7.9–7.0 g/dL, <7 g/dL). Among patients receiving a transfusion, Kruskal–Wallis tests were used to compare the average number of RBC units received during hospitalization by race overall during their hospitalization, and within strata of their nadir Hb during hospitalization.
A linear regression model was specified to test the association between a patient's race, their nadir Hb, whether they received an RBC transfusion, and the number of units transfused. The primary dependent variable in this model was the total number of RBC's units transfused during hospitalization, and the independent variables of interest included patient's race, nadir Hb level during hospitalization, and an interaction between patient's race and nadir Hb level during hospitalization. For ease of interpretation nadir Hb was mean centered (7.9 g/dL) in the model, so that the intercept for patient's race could be interpreted at the mean nadir Hb rather than at a Hb = 0 g/dL. The model controlled for patient's age, gender, ethnicity, and Charlson Comorbidity Index score. We also performed a stratified analysis using this model in patients with GI bleeding and without GI bleeding. Last, ethnicity was removed in the final reported models because it was collinear with race, and removing it did not affect the size or direction of the coefficients for the other independent variables.
Statistical analysis was performed using the Stata statistical software, version 17, StataCorp, College Station, TX.
3. RESULTS
3.1. Patient characteristics
A total of 5028 patients provided consent for study participation and did not have a diagnosis of sickle cell anemia. Of these, 77 (5%) patients were transfused ≥8 units of RBC's during their hospitalization and excluded from analysis, leaving 4951 patients in the analytic sample. In the analytic sample 1363 (28%) patients received at least 1 unit of RBC transfusion. The overall average age of the sample 60 years old, 58% were female, and 94% were not Hispanic or Latino. The average admission Hb was 9.7 (±1.9), and the average nadir Hb was 7.9 (±1.3). Overall 9% (n = 443) of the patients in the sample had a diagnosis of GI bleeding.
The racial breakdown of the sample was 71% (n = 3519) African American, 24% (n = 1168) White, and 5% (n = 264) Other. The average age of African Americans and Whites was slightly older than patients of Other race. There were fewer White females and females of Other race than African Americans females, and a higher percentage of patients of Other race were also Hispanic or Latino, than either African Americans or Whites in the sample. There were no differences in the average admission Hb (p = .49) or Nadir Hb (p = .69) between patients of African American, White, and Other race. There were however a higher percentage of White and Other race admitted for GI bleeding than African Americans (p < .01). All patient characteristics, both overall and by race, are reported in Table 1.
TABLE 1.
Patient characteristics
| All patients (n = 4951) | African American (n = 3519) | White (n = 1168) | Other (n = 264) | |
|---|---|---|---|---|
| Age (years) mean, (SD) | 60 (16) | 60 (17) | 61 (16) | 56 (16) |
| Female n, (%) | 2847 (58) | 2179 (62) | 526 (45) | 142 (54) |
| Ethnicity | ||||
| Not Hispanic or Latino | 4647 (94) | 3497 (99) | 1049 (90) | 101 (38) |
| Hispanic or Latino | 304 (6) | 22 (1) | 119 (10) | 163 (62) |
| Length of stay (days) median (IQR) | 6.1 (3.8–11) | 6.0 (3.7–10) | 7.1 (4.0–13) | 5.9 (3.7–12) |
| Charlson comorbidity index n, (%) | ||||
| 0 | 529 (11) | 358 (10) | 142 (12) | 29 (11) |
| 1–2 | 1366 (28) | 991 (28) | 302 (26) | 73 (28) |
| 3–4 | 1096 (22) | 812 (23) | 231 (20) | 53 (20) |
| >5 | 2960 (39) | 1358 (39) | 493 (42) | 109 (41) |
| Hemoglobin (Hb) mean, (SD) | ||||
| Admission Hb g/dL | 9.7 (1.9) | 9.7 (2.0) | 9.6 (1.8) | 9.7 (1.9) |
| Nadir Hb g/dL | 7.9 (1.3) | 7.9 (1.3) | 7.9 (1.2) | 7.9 (1.3) |
| GI bleeding n, (%) | 443 (9) | 285 (8) | 124 (11) | 34 (12) |
3.2. Transfusion percentages by race and nadir Hb
Figure 1 reports the percentage of patients transfused overall and within each Hb strata. Overall 25% (n = 861) of African Americans in the study received a transfusion, compared to 30% (n = 352) of Whites and 28% (n = 73) of Other patients (p < .01). For patients with a nadir Hb <7 g/dL, for which restrictive transfusion guidelines would suggest any patient be transfused, 80% of African Americans, 86% of Whites, and 92% of Other patients received a transfusion (p < .01). Similarly, for patients with a nadir Hb between 7.0 and 7.9 g/dL which is within restrictive transfusion threshold ranges and for which a transfusion would be considered consistent with transfusion guidelines, 15% of African Americans, 28% of Whites, and 12% of Other patients received a transfusion (p < .01). For patients with a nadir Hb between 8.0 and 8.9 g/dL which is above restrictive transfusion threshold, 1% of African Americans, 7% of Whites, and 10% of Other patients received a transfusion (p < .01). Last, consistent with restrictive transfusion guidelines, almost no patients of any race received a transfusion with a nadir Hb between 9 and 10 g/dL (African American 1%, White 1%, Other Race 0%, p = .39).
FIGURE 1.
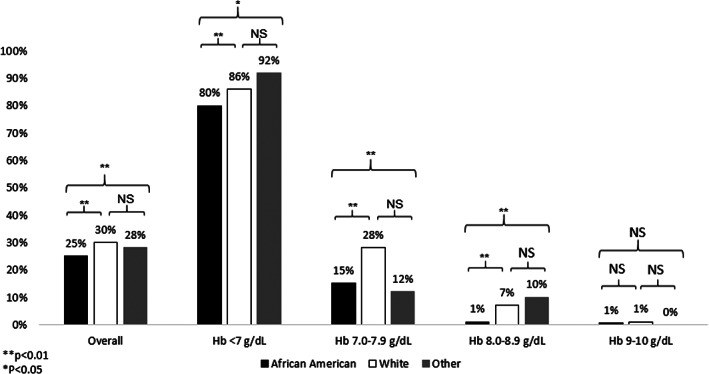
Percent of patients transfused by race and nadir Hb level
3.3. Association between race, nadir Hb and transfusion
Despite differences in the percentage of patients transfused by race, among patients that were transfused in unadjusted analysis there were not differences in the number of units transfused by patient race either overall or within any Hb strata (Table 2).
TABLE 2.
Mean (SD) number of RBC's transfused by hemoglobin level and race
| All patients (n = 1286) | African American (n = 861) | White (n = 352) | Other (n = 73) | p‐value a | |
|---|---|---|---|---|---|
| Overall | 2.1 (1.4) | 2.1 (1.3) | 2.2 (1.5) | 2.2 (1.4) | .43 |
| Hb 10–9 g/dL | 1.3 (0.49) | 1.5 (0.6) | 1 (0) | No Data | .18 |
| Hb 8.9–8.0 g/dL | 1.8 (1.3) | 1.4 (0.5) | 2.1 (1.6) | 1.5 (0.5) | .58 |
| Hb 7.9–7.0 g/dL | 1.5 (0.96) | 1.6 (1.1) | 1.4 (0.6) | 1.8 (1.6) | .96 |
| Hb <7.0 g/dL | 2.2 (1.4) | 2.1 (1.4) | 2.5 (1.6) | 2.1 (1.1) | .06 |
Note: Sample includes only patients receiving a transfusion and is restricted to patients with seven or less transfusions during hospitalization.
p‐values represent Kruskal–Wallis test comparing the average number of RBC units transfused across Hb level (rows) by racial group.
In the adjusted regression models, African Americans received less units of RBC's overall compared to Whites (β = −.17, p < .01) at any nadir Hb level, and the amount of transfusion varied across nadir Hb levels compared to Whites (African American × Nadir Hb, β = .14, p < .01). The interaction term between African American and Nadir Hb can also be interpreted as African Americans receiving less transfusion at decreasing and lower Nadir Hb values compared to Whites. Although patients of Other race also received less transfusion overall (β = −.08, p < .19) and at decreasing and lower nadir Hb levels compared to Whites (β = .08, p < .09), the effects were not statistically significant (Table 3).
TABLE 3.
Association between race and units of RBC transfusion during hospitalization
| All patients (n = 4951) | Excluding patients with GIBL (n = 4508) | Only patients with GIBL (n = 443) | |
|---|---|---|---|
| African American a | −0.17 (−0.23, −0.11)** | −0.13 (−0.19, −0.07)** | −0.28 (−0.60, 0.03) |
| Other a | −0.08 (−0.21, 0.04) | −0.03 (−0.15, 0.10) | −0.33 (−0.85, 0.18) |
| Nadir Hb b | −0.68 (−0.72, −0.64)** | −0.60 (−0.64, −0.55)** | −0.88 (−1.1, −0.70)** |
| African American × Nadir Hb a | 0.14 (0.09, 0.19)** | 0.09 (0.04, 0.14)** | 0.26 (0.05, 0.46)* |
| Other × Nadir Hb a | 0.08 (−0.14, 0.18) | 0.02 (−0.07, 0.13) | 0.30 (−0.03, 0.64) |
Referent category is white. Columns report estimated β coefficient and (95% CI).
Nadir Hb variable is mean centered. Models control for age, gender, and Charlson comorbidity index score and are limited to patients who received seven or less units of RBC's during hospitalization. **p < .01, *p < .05.
In the model that included only patients with GI bleeding, the differences in the number of units transfused by race were larger than in the overall model. Both African Americans (β = −.28, p = .08) and patients of Other race (β = −.33, p = .21) received less units of RBC's compared to Whites, but the effect of these lower order variables were not statistically significant. However, African Americans received fewer RBC's units compared to Whites across lower levels of nadir Hb (β = .26, p = .01), and this interaction effect was statistically significant. In the stratified model excluding patients with GI bleeding, the effects where the same as in the overall model and the model with only GI bleeding patients, but the difference in the amount of RBC units African Americans received compared to Whites was smaller (Table 3).
4. DISCUSSION
These data suggest that there are differences by race in the Hb level at which hospitalized patients are transfused and the total number of RBC units they receive. African Americans were less likely to be transfused overall and within each decreasing Hb strata during hospitalization below 9 g/dL compared to Whites. African Americans also received fewer units of RBC transfusion overall during hospitalization and fewer RBC units at lower Hb levels compared to Whites. This effect was greater in patients with GI bleeding.
These observed differences in the receipt of transfusion by race do meet the definition of a healthcare disparity. 48 However, when interpreted in the context of restrictive transfusion guidelines, it is less clear whether these differences represent worse care for any single racial group compared to others. Restrictive transfusion guidelines have been informed by clinical trial data showing that transfusion at liberal (or higher) Hb thresholds does not improve mortality compared to transfusion at restrictive Hb thresholds (7–8 g/dL). Since transfusion does include some risk of a transfusion reaction and other adverse events, 49 any transfusion above restrictive threshold levels is perhaps not only unnecessary, but also potentially harmful. As such, the greater use of transfusion observed in patients of White and Other race with a Hb between 8.0 and 8.9 g/dL is discordant with guidelines, and would be considered unnecessary over‐transfusion. Additionally, although the use of transfusion in patients with a Hb between 7 and 8 g/dL is consistent with restrictive transfusion guidelines, for most stable hospitalized patients there is stronger data to support and our electronic health record encourages a <7 g/dL Hb threshold. Therefore, the significantly higher percentage of Whites receiving transfusion between a Hb 7.0–7.9 g/dL could be considered harmful over‐transfusion, rather than under‐transfusion of African Americans and patients of Other race. Similarly, the greater number of RBC's units that Whites received compared to African Americans or Other patients may actually be over‐transfusion, if the extra units of RBC's were transfused when the patients Hb was already above the restrictive transfusion threshold of 7 g/dL, including in GI bleeding patients. 8 However, in the Hb <7 g/dL group where guidelines would suggest all patients receive transfusion, 20% of African Americans did not receive transfusion. While a non‐trivial amount of White and Other patients also were not transfused within this Hb strata, there is no obvious explanation for why African Americans should have the lowest rate of transfusion among the racial groups examined. Not receiving transfusion with a Hb <7 g/dL during hospitalization is guideline discordant, and African Americans were the racial group most affected by under‐transfusion. Regardless, while some patients in this study were over‐transfused (whites), and some were under‐transfused (African Americans), the differences detected in transfusion by race represent poor adherence to transfusion standards, and are concerning because they are not supported by empiric data or guidelines.
These data and the differences in transfusion practice by race raise several issues with respect to the quality of care and the use of transfusion in hospitalized patients with anemia. First, despite the widespread acceptance of restrictive transfusion practices, clinicians still transfuse outside of guideline recommendations. Although it has been suggested that the EHR can be a tool for standardizing clinical processes, our data suggest that even with concise practice guidelines embedded into the EHR to promote guideline adherence and practice uniformity, variation in practice still exists. Moreover, even if overall adherence to restrictive transfusion practices is high, variation in care by patient and provider level factors still occur and can result in disparities in care. Since both improved quality of care and reducing healthcare disparities are national priorities, health systems should be attentive to and ensure that variation in transfusion practice is not occurring by patient level factors not supported by empiric data, such as patient's race. Second, better understanding and data on why clinicians may still vary in their use of transfusion, particularly outside of restrictive transfusion practices may be useful. For example, there is a body of observational data suggesting that transfusion at higher Hb levels may alleviate patients' symptoms of anemia, 50 , 51 , 52 , 53 , 54 , 55 and the clinical transfusion trials to date have not adequately studied the effect of restrictive transfusion on patient‐reported outcomes like symptoms. 56 , 57 If clinicians are incorporating individual clinical factors, such as the severity of a patients' symptoms and patient preference, into the decision to transfuse outside of restrictive transfusion guidelines, requiring global adherence to uniform restrictive transfusion may not be optimal care for all patients. Therefore, more specific data on the clinical factors that clinicians consider when transfusing a patient may help identify important scientific and clinical questions to study, or targets for further standardizing clinical processes.
5. LIMITATIONS
This study has several limitations. First, it is an observational study from a single academic medical center, and the results may not be generalizable to other institutions. Second, while we measured and adjusted for potentially confounding variables that could influence transfusion decisions, there may be additional confounding variables that we did not consider and control for. Third, we did not have provider level data to compare to patient level transfusion data. Providers recommend to and order transfusion for hospitalized patients, and so provider level data would improve understanding of whether transfusion was offered but declined, and could help explain some variation by race in the receipt of transfusion for hospitalized patients. Fourth, although shortages of RBC's have not resulted in any limits on transfusion at our institution, we do not have allo‐immunization data on individual patients in our study that could account for some patients not receiving transfusion due to a lack of available matched RBC's. Fifth, we had a limited number of non‐African American non‐White racial groups in our study, and as a result in our model we could not compare the transfusion patterns in specific other racial groups, or account for the possibility of intersectionality with ethnicity and/or other sociodemographic factors that may influence transfusion patterns.
6. CONCLUSION
The Hb level at which patients are transfused at and the total number of RBC units received during hospitalization differ by patient race, with African Americans being less likely to be transfused overall and receiving less RBC units during hospitalization than Whites. These differences in transfusion practices by race represent poor adherence to transfusion standards, and further work needs to be done understand the cause of these differences and their impact on patients.
FUNDING INFORMATION
Dr. Prochaska is supported by an NHLBI K23 award (NHLBI K23HL140132). Dr. Meltzer is supported by an NIA P30 award (NIA P30AG066619).
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Prochaska M, Salcedo J, Berry G, Meltzer D. Racial differences in red blood cell transfusion in hospitalized patients with anemia. Transfusion. 2022;62(8):1519–1526. 10.1111/trf.16935
Funding information National Heart, Lung, and Blood Institute, Grant/Award Number: K23HL140132; National Institute on Aging, Grant/Award Number: P30AG066619
REFERENCES
- 1. Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316(19):2025–35. [DOI] [PubMed] [Google Scholar]
- 2. Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157(1):49–58. [DOI] [PubMed] [Google Scholar]
- 3. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in critical care investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–17. [DOI] [PubMed] [Google Scholar]
- 4. Lacroix J, Hébert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. Random Control Trial. 2007;356:1609–19. 10.1056/NEJMoa066240 [DOI] [PubMed] [Google Scholar]
- 5. Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–67. [DOI] [PubMed] [Google Scholar]
- 6. Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holst LB, Haase N, Wetterslev J, Wernerman J, Åneman A, Guttormsen AB, et al. Transfusion requirements in septic shock (TRISS) trial – comparing the effects and safety of liberal versus restrictive red blood cell transfusion in septic shock patients in the ICU: protocol for a randomised controlled trial. Trials. 2013;14:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez‐Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21. [DOI] [PubMed] [Google Scholar]
- 9. Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372(11):997–1008. [DOI] [PubMed] [Google Scholar]
- 10. DeZern AE, Williams K, Zahurak M, et al. Red blood cell transfusion triggers in acute leukemia: a randomized pilot study. Transfusion. 2016;56(7):1750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heyes J, Kelly PA, Monaghan K, Lawn M, Dhesi A, Mijovic A. A single unit transfusion policy reduces red cell transfusions in general medical in‐patients. QJM. 2017;110(11):735–9. [DOI] [PubMed] [Google Scholar]
- 12. AABB‐Choosing‐Wisely‐List.pdf. s. https://www.choosingwisely.org/wp‐content/uploads/2015/02/AABB‐Choosing‐Wisely‐List.pdf. Published 2021. Accessed.
- 13. Carson JL, Stanworth SJ, Alexander JH, Roubinian N, Fergusson DA, Triulzi DJ, et al. Clinical trials evaluating red blood cell transfusion thresholds: an updated systematic review and with additional focus on patients with cardiovascular disease. Am Heart J. 2018;200:96–101. [DOI] [PubMed] [Google Scholar]
- 14. Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;10(10):CD002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta‐analysis and trial sequential analysis. BMJ. 2015;350:h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfuntner A, Wier LM, Stocks C. Most frequent procedures performed in U.S. Hospitals, 2010. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 17. Pathak R, Bhatt VR, Karmacharya P, Aryal MR, Alweis R. Trends in blood‐product transfusion among inpatients in the United States from 2002 to 2011: data from the Nationwide Inpatient Sample. J Hosp Med. 2014;9(12):800–1. [DOI] [PubMed] [Google Scholar]
- 18. Thakkar RN, Lee KH, Ness PM, et al. Relative impact of a patient blood management program on utilization of all three major blood components. Transfusion. 2016;56(9):2212–20. [DOI] [PubMed] [Google Scholar]
- 19. Zuckerberg GS, Scott AV, Wasey JO, Wick EC, Pawlik TM, Ness PM, et al. Efficacy of education followed by computerized provider order entry with clinician decision support to reduce red blood cell utilization. Transfusion. 2015;55(7):1628–36. [DOI] [PubMed] [Google Scholar]
- 20. Goodnough LT, Shieh L, Hadhazy E, Cheng N, Khari P, Maggio P. Improved blood utilization using real‐time clinical decision support. Transfusion. 2014;54(5):1358–65. [DOI] [PubMed] [Google Scholar]
- 21. McKinney ZJ, Peters JM, Gorlin JB, Perry EH. Improving red blood cell orders, utilization, and management with point‐of‐care clinical decision support. Transfusion. 2015;55(9):2086–94. [DOI] [PubMed] [Google Scholar]
- 22. Butler CE, Noel S, Hibbs SP, Miles D, Staves J, Mohaghegh P, et al. Implementation of a clinical decision support system improves compliance with restrictive transfusion policies in hematology patients. Transfusion. 2015;55(8):1964–71. [DOI] [PubMed] [Google Scholar]
- 23. Hibbs SP, Nielsen ND, Brunskill S, Doree C, Yazer MH, Kaufman RM, et al. The impact of electronic decision support on transfusion practice: a systematic review. Transfus Med Rev. 2015;29(1):14–23. [DOI] [PubMed] [Google Scholar]
- 24. Jenkins I, Doucet JJ, Clay B, Kopko P, Fipps D, Hemmen E, et al. Transfusing wisely: clinical decision support improves blood transfusion practices. Jt Comm J Qual Patient Saf. 2017;43(8):389–95. [DOI] [PubMed] [Google Scholar]
- 25. Derzon JH, Clarke N, Alford A, Gross I, Shander A, Thurer R. Restrictive transfusion strategy and clinical decision support practices for reducing RBC transfusion overuse. Am J Clin Pathol. 2019;152(5):544–57. [DOI] [PubMed] [Google Scholar]
- 26. Saag HS, Lajam CM, Jones S, Lakomkin N, Bosco JA III, Wallack R, et al. Reducing liberal red blood cell transfusions at an academic medical center. Transfusion. 2017;57(4):959–64. [DOI] [PubMed] [Google Scholar]
- 27. Sadana D, Pratzer A, Scher LJ, Saag HS, Adler N, Volpicelli FM, et al. Promoting high‐value practice by reducing unnecessary transfusions with a patient blood management program. JAMA Intern Med. 2018;178(1):116–22. [DOI] [PubMed] [Google Scholar]
- 28. Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, Dunbar NM. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion. 2014;54(10 Pt 2):2640–5. [DOI] [PubMed] [Google Scholar]
- 29. Goodnough LT, Shah N. The next chapter in patient blood management: real‐time clinical decision support. Am J Clin Pathol. 2014;142(6):741–7. [DOI] [PubMed] [Google Scholar]
- 30. Sim EY, Tan DJA, Abdullah HR. The use of computerized physician order entry with clinical decision support reduces practice variance in ordering preoperative investigations: a retrospective cohort study. Int J Med Inform. 2017;108:29–35. [DOI] [PubMed] [Google Scholar]
- 31. Rothschild JM, McGurk S, Honour M, Lu L, McClendon AA, Srivastava P, et al. Assessment of education and computerized decision support interventions for improving transfusion practice. Transfusion. 2007;47(2):228–39. [DOI] [PubMed] [Google Scholar]
- 32. Hohmuth B, Ozawa S, Ashton M, Melseth RL. Patient‐centered blood management. J Hosp Med. 2014;9(1):60–5. [DOI] [PubMed] [Google Scholar]
- 33. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: impact of an education program and a clinical guideline on transfusion practice. J Hosp Med. 2014;9(12):745–9. [DOI] [PubMed] [Google Scholar]
- 34. Abdelsattar ZM, Hendren S, Wong SL, Campbell DA Jr, Henke P. Variation in transfusion practices and the effect on outcomes after noncardiac surgery. Ann Surg. 2015;262(1):1–6. [DOI] [PubMed] [Google Scholar]
- 35. Aquina CT, Blumberg N, Probst CP, et al. Large variation in blood transfusion use after colorectal resection: a call to action. Dis Colon Rectum. 2016;59(5):411–8. [DOI] [PubMed] [Google Scholar]
- 36. Bennett‐Guerrero E, Zhao Y, O'Brien SM, Ferguson TB, Peterson ED, Gammie JS, et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304(14):1568–75. [DOI] [PubMed] [Google Scholar]
- 37. Goodnough LT, Johnston MF, Toy PT. The variability of transfusion practice in coronary artery bypass surgery. Transfusion Medicine Academic Award Group. JAMA. 1991;265(1):86–90. [PubMed] [Google Scholar]
- 38. O'Malley SM, Sanders JO, Nelson SE, Rubery PT, O'Malley NT, Aquina CT. Significant variation in blood transfusion practice persists following adolescent idiopathic scoliosis surgery. Spine (Phila Pa 1976). 2021;46(22):1588–97. [DOI] [PubMed] [Google Scholar]
- 39. Pine AB, Lee EJ, Sekeres M, Steensma DP, Zelterman D, Prebet T, et al. Wide variations in blood product transfusion practices among providers who care for patients with acute leukemia in the United States. Transfusion. 2017;57(2):289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qian F, Eaton MP, Lustik SJ, Hohmann SF, Diachun CB, Pasternak R, et al. Racial disparities in the use of blood transfusion in major surgery. BMC Health Serv Res. 2014;14(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Menendez ME, Ring D. Minorities are less likely to receive autologous blood transfusion for major elective orthopaedic surgery. Clin Orthop Relat Res. 2014;472(11):3559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Davis E, Amdur R, Ahmadzia H. Associations of anaemia and race with peripartum transfusion in three United States datasets. Blood Transfus. 2021, 10.2450/2021.0217-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Main EK, Chang SC, Dhurjati R, Cape V, Profit J, Gould JB. Reduction in racial disparities in severe maternal morbidity from hemorrhage in a large‐scale quality improvement collaborative. Am J Obstet Gynecol. 2020;223(1):123 e121–123 e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maher KM, Owusu‐Akyaw K, Zhou J, Cooter M, Ross AK, Lark RK, et al. Analysis of the impact of race on blood transfusion in pediatric scoliosis surgery. Paediatr Anaesth. 2018;28(4):352–60. [DOI] [PubMed] [Google Scholar]
- 45. Schwab ME, Schmidt CN, Gonzalez‐Velez JM, Bakhtary S. Racial/ethnic disparities among women receiving intrauterine transfusions for alloimmunization at a single fetal treatment center. Transfusion. 2021;61(7):2019–24. [DOI] [PubMed] [Google Scholar]
- 46. Jones JM, Sapiano MRP, Savinkina AA, Haass KA, Baker ML, Henry RA, et al. Slowing decline in blood collection and transfusion in the United States – 2017. Transfusion. 2020;60(Suppl 2):S1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chou ST, Alsawas M, Fasano RM, Field JJ, Hendrickson JE, Howard J, et al. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood Adv. 2020;4(2):327–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Disparities. https://www.ahrq.gov/topics/disparities.html. Published 2021. Accessed.
- 49. Savinkina AA, Haass KA, Sapiano MRP, Henry RA, Berger JJ, Basavaraju SV, et al. Transfusion‐associated adverse events and implementation of blood safety measures – findings from the 2017 National Blood Collection and Utilization Survey. Transfusion. 2020;60(Suppl 2):S10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prochaska MT, Newcomb R, Jiang D, Meltzer DO. The effect of red‐blood‐cell transfusion on fatigue in hospitalized patients with anaemia. Vox Sang. 2018;113(7):669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chan KL, Mak WMV, Tam YH, Lee HKK. Change in patient‐reported outcomes in single‐unit transfusion comparable with double‐unit transfusion. Blood. 2016;128(22):2636–6. [Google Scholar]
- 52. Chan KLL, Mak WMV, Tam YH, Lee KKH. Factors affecting patient‐reported outcomes after red blood cell transfusion in medical patients. Transfusion. 2018;58(1):158–67. [DOI] [PubMed] [Google Scholar]
- 53. Chen LJA, Moeller KD, Wagman LD. Effect of red blood cell transfusions on patient‐reported outcomes in an ambulatory oncology population. J Clin Oncol. 2016;34(26):78–8. [Google Scholar]
- 54. Mercadante S, Ferrera P, Villari P, David F, Giarratano A, Riina S. Effects of red blood cell transfusion on anemia‐related symptoms in patients with cancer. J Palliat Med. 2009;12(1):60–3. [DOI] [PubMed] [Google Scholar]
- 55. Bruhn R, Karafin MS, Hilton JF, et al. Early and sustained improvement in fatigue‐related quality of life following red blood cell transfusion in outpatients. Qual Life Res. 2020;29:2737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lin Y, Buckstein R. Outpatient transfusions: time to study what matters to patients. Transfusion. 2019;59(6):1887–90. [DOI] [PubMed] [Google Scholar]
- 57. Yazer MH, Triulzi DJ. AABB red blood cell transfusion guidelines: something for almost everyone. JAMA. 2016;316(19):1984–5. [DOI] [PubMed] [Google Scholar]


