Abstract
Recently, our lab has shown that humans with genetic mutations resulting in high affinity haemoglobin (HAH) demonstrate better maintained aerobic capacity and peak power output during hypoxic exercise versus normoxic exercise compared to humans with normal affinity haemoglobin. However, the influence of HAH on tissue oxygenation within exercising muscle during normoxia and hypoxia is unknown. Therefore, we examined near-infrared spectroscopy (NIRS)-derived oxygenation profiles of the vastus lateralis during graded cycling exercise in normoxia and hypoxia among humans with HAH (n=5) and controls with normal affinity haemoglobin (n=12). The HAH group elicited a blunted increase of deoxygenated haemoglobin+myoglobin during hypoxic exercise compared to the control group (P=0.03), suggesting reduced fractional oxygen extraction in the HAH group. In addition, the HAH group maintained a higher level of muscle tissue oxygen saturation during normoxic exercise (HAH, 75±4% vs. controls, 65±3%, P=0.049) and there were no differences between groups in muscle tissue oxygen saturation during hypoxic exercise (HAH, 68±3% vs. controls, 68±2%, P=0.943). Overall, our results suggest that humans with HAH may demonstrate divergent patterns of fractional oxygen extraction during hypoxic exercise and elevated muscle tissue oxygenation during normoxic exercise compared to controls.
Keywords: Oxygen transport, Haemoglobin-oxygen affinity, Near-infrared spectroscopy, Muscle tissue saturation, Aerobic capacity
INTRODUCTION
Humans with increased haemoglobin-oxygen binding affinity (i.e., high affinity haemoglobin (HAH)) due to a genetic mutation in haemoglobin structure have abnormal systemic oxygen transport and uptake responses during normoxic and hypoxic exercise compared to humans with normal affinity haemoglobin. For instance, peak oxygen uptake () and peak power output (PPO) are better maintained among humans with HAH during hypoxic versus normoxic exercise compared to healthy controls (Dominelli et al., 2020; Hebbel et al., 1978).
The physiological manifestations of HAH largely depend on the degree of haemoglobin-oxygen binding affinity. The P50 (partial pressure of oxygen at which haemoglobin is 50% saturated with oxygen) is a commonly used metric of haemoglobin-oxygen binding affinity. Typical P50 values in humans range from 24 to 30 mmHg, whereas P50 values in humans with HAH range from 11 to 24 mmHg (Dominelli et al., 2020; Hebbel et al., 1978; Jensen et al., 1975; Mangin, 2017). Oxygen extraction and tissue oxygenation within skeletal muscle are determined by the balance of local oxygen delivery and consumption. Therefore, a greater haemoglobin-oxygen binding affinity may limit oxygen extraction and compromise tissue oxygenation for a given muscle blood flow. When oxygen delivery is chronically compromised, a compensatory increase in red blood cell production results in an elevated haemoglobin concentration and haematocrit (Jelkmann, 2011, 2013). This is corroborated by the strong inverse relation between P50 and haemoglobin concentration described in humans with variant haemoglobin structure (Shepherd et al., 2019). Therefore, the elevated haemoglobin concentration observed among humans with HAH could be a compensation for reduced peripheral oxygen off-loading at rest (Charache et al., 1966; Mangin, 2017; Webb et al., 2022).
The influence of HAH on muscle oxygen extraction and tissue oxygenation during exercise remains unclear. Some evidence suggests that oxygen extraction may be compromised in humans with HAH during both normoxic and hypoxic exercise (Dominelli et al., 2020; Santbergen & van der Heul, 2014; Wranne et al., 1983). Humans with HAH demonstrate a greater reliance on anaerobic metabolism during normoxic and hypoxic exercise (Dominelli et al., 2020; Santbergen & van der Heul, 2014). In addition, muscle tissue hypoxia has been reported during normoxic exercise in humans with HAH (Wranne et al., 1983). Despite proposed differences in muscle tissue oxygenation, some data suggest that the heart rate and responses during normoxic and hypoxic exercise are not different between humans with HAH and healthy controls (Dominelli et al., 2020). Therefore, to better understand possible abnormalities in muscle tissue oxygenation during exercise in humans with HAH compared to humans with normal affinity haemoglobin, we examined NIRS-derived muscle oxygenation profiles during graded cycling exercise in both normoxia and hypoxia. We hypothesized that fractional oxygen extraction, estimated by the concentration of deoxygenated haemoglobin and myoglobin (Deoxy[Hb+Mb]), would be lower during both normoxic and hypoxic exercise in humans with HAH compared to controls.
METHODS
Ethical Approval.
Experimental procedures were approved by the Institutional Review Board (IRB#18-001080) at the Mayo Clinic and conformed to the standards set by the Declaration of Helsinki, except registration in a public repository. All participants provided written informed consent prior to participation in the study.
Participants.
Five participants with HAH (Hb Malmö) were recruited from a Mayo Clinic database along with 12 control participants with normal affinity haemoglobin. Participants represent a subset of participants previously examined (Dominelli et al., 2020). Mutations resulting in HAH are rare in the general population and these analyses of NIRS-derived data are limited to five participants with HAH. None of the participants reported cardiovascular, respiratory, musculoskeletal or other ailments and all were nonsmokers.
Oxyhaemoglobin Dissociation Curves.
Heparinized venous blood was collected from each participant to determine P50 via a standardized protocol. Briefly, venous blood samples were deoxygenated with nitrogen. After deoxygenation, compressed air was used to reoxygenate haemoglobin using standardized ex vivo environmental conditions (pH=7.6 and temperature=37°C) using a laboratory-developed protocol (Winslow et al., 1977). Haemoglobin oxygen saturation and partial pressure of oxygen were measured by dual wavelength spectrophotometry and a Clark electrode, respectively, during the reoxygenation cycle (Hemox Analyzer, TCS Medical Products, Huntington Valley, Pennsylvania, U.S.A).
Graded Exercise Testing.
Participants completed the study on a single day. All cycling trials were completed on an upright electromagnetically-braked cycle ergometer (Corival CPET, Lode, Groninge, Netherlands). Prior to initiation of exercise, participants were familiarized with the cycling protocol and breathed appropriate gas mixtures for >5 minutes to allow for equilibration. During exercise, participants wore a mouthpiece connected to a two-way non-rebreathing valve (Series 7200, Hans Rudolph, Shawnee, KS) that was attached to a balloon reservoir on the inspiration port. Participants completed two graded exercise tests to exhaustion while breathing: 1) normoxic gas (fraction of inspired oxygen=0.21), and 2) normobaric hypoxic gas (fraction of inspired oxygen=0.15). The order of inspirate was randomized and participants were blinded to experimental condition. Graded exercise tests were separated by a minimum of 45 minutes. The initial workload and increments were tailored for each participant due to differences in age, sex, and fitness. The initial workload of each graded exercise test was between 20 and 120 W. Workload was increased every three minutes in increments ranging from 10-50 W per stage. The graded exercise tests were designed to have each participant exercise for 15-21 minutes (5-7 stages) and were the same in normoxia and hypoxia.
Near-infrared Spectroscopy (NIRS).
Frequency domain, multi-distance NIRS (Oxiplex TS, ISS Inc., Champaign, IL) was used to non-invasively estimate the concentration of oxygenated (Oxy[Hb+Mb]), and deoxygenated (Deoxy[Hb+Mb]) haemoglobin+myoglobin. The NIRS probe was placed on the surface of the right vastus lateralis 15 cm proximal and 5 cm lateral to the proximal border of the patella. The NIRS probe was secured with an elastic strap and black self-adhesive wrap to prevent movement and obstruct ambient light. The position of the NIRS probe was marked with indelible ink prior to removing the probe after the first exercise test. The NIRS probe was then removed during the rest period between graded exercise tests. Careful consideration was taken to place the NIRS probe in the same position prior to beginning the second graded exercise test. The NIRS system used in this study incorporates one detector-fiber bundle and eight light-emitting diodes operating at wavelengths of 690 and 830 nm (four per wavelength). The source-detector separation distances were 2.5, 3.0, 3.5, and 4.0 cm. Oxy[Hb+Mb] and Deoxy[Hb+Mb] were calculated according to the respective absorption and scattering coefficients at each wavelength. Total haemoglobin+myoglobin concentration (Total[Hb+Mb]) was calculated as the sum of Oxy[Hb+Mb] and Deoxy[Hb+Mb]. Tissue oxygen saturation was calculated as the ratio of Oxy[Hb+Mb] to Total[Hb+Mb] multiplied by 100%. NIRS-derived data were sampled at 5 Hz and analog signals were transferred to the data acquisition device (PowerLab 16/30, ADInstruments, Colorado Springs, CO) via an analog output interface (Output Interface Module, ISS Inc., Champaign, IL). NIRS-derived data were multiplied by a factor of 4 to convert the haemoglobin-corrected concentrations into units of heme (Barstow, 2019). Of particular importance for this investigation, the Deoxy[Hb+Mb] signal provides an estimate of fractional oxygen extraction within the microvasculature of exercising muscle (DeLorey et al., 2003; Ferreira et al., 2005; Grassi & Quaresima, 2016; Hammer et al., 2018).
Data and Statistical Analysis.
Two-tailed Student’s unpaired t-tests were used to examine between group differences in: 1) participant characteristics (Table 1), and 2) changes between normoxia and hypoxia for PPO and (described below). Changes between normoxia and hypoxia for both PPO and were calculated as the quotient of hypoxic value subtracted from normoxic value (numerator) relative to the normoxic value (denominator). The percent of normoxic and hypoxic PPO corresponding to 100 W for participants within each graded exercise test was also compared between groups using two-tailed Student’s unpaired t-tests (Table 2).
Table 1.
Participant Characteristics
| HAH (n=5) | Controls (n=12) | P-value | |
|---|---|---|---|
| Sex | 1M:4F | 5M:7F | |
| Age (years) | 35 ± 12 | 40 ± 13 | 0.472 |
| BMI (kg·m−2) | 27 ± 5 | 27 ± 4 | 0.999 |
| Height (cm) | 168 ± 8 | 172 ± 11 | 0.476 |
| Weight (kg) | 77 ± 22 | 80 ± 14 | 0.738 |
| P50 (mmHg) | 16 ± 1 | 26 ± 1 | <0.0001 |
| Haemoglobin (g·dL−1) | 18.9 ± 2.1 | 14.0 ± 1.4 | <0.0001 |
| Haematocrit (%) | 55 ± 6 | 41 ± 4 | <0.0001 |
| Normoxic (L·min−1) | 2.4 ± 1.0 | 2.5 ± 0.5 | 0.783 |
| Normoxic (mL·kg−1·min−1) | 31.5 ± 6.0 | 32.1 ± 7.3 | 0.874 |
| Normoxic PPO (W) | 136 ± 38 | 159 ± 45 | 0.340 |
| Hypoxic (L·min−1) | 2.4 ± 0.8 | 2.2 ± 0.4 | 0.495 |
| Hypoxic (mL·kg−1·min−1) | 30.9 ± 4.9 | 28.6 ± 6.6 | 0.496 |
| Hypoxic PPO (W) | 130 ± 28 | 141 ± 42 | 0.568 |
Abbreviations: HAH, high affinity haemoglobin; M, male; F, female BMI, body mass index; P50, partial pressure of oxygen at which haemoglobin is 50% saturated; , peak oxygen uptake, PPO, peak power output. Values displayed as mean ± SD.
Table 2.
NIRS values during rest and exercise at ~100 W
| Intensity | P-Values | |||||
|---|---|---|---|---|---|---|
| Rest | ~100 W | Group | Intensity | Interaction | ||
| Normoxia | ||||||
| HAH (n = 5) | Deoxy[Hb+Mb] (μM) | 31.0 ± 20.8 | 47.7 ± 33.3 | 0.412 | 0.012 | 0.215 |
| Oxy[Hb+Mb] (μM) | 134.6 ± 75.3 | 136.4 ± 76.3 | 0.531 | 0.456 | 0.209 | |
| Total[Hb+Mb] (μM) | 165.6 ± 96.0 | 184.1 ± 108.0 | 0.856 | 0.155 | 0.141 | |
| Tissue Oxygen Saturation (%) | 82.4 ± 3.7 | 75.6 ± 8.0 | 0.014 | < 0.001 | 0.149 | |
| PO (% Normoxic PPO) | 77.7 ± 19.8 | 0.418 | ||||
| PO binned to ~100 W (W) | 93 ± 5 | |||||
| Controls (n = 10) | Deoxy[Hb+Mb] (μM) | 49.9 ± 24.7 | 56.2 ± 37.0 | |||
| Oxy[Hb+Mb] (μM) | 122.4 ± 58.5 | 109.1 ± 62.6 | ||||
| Total[Hb+Mb] (μM) | 165.6 ± 80.7 | 165.3 ± 95.5 | ||||
| Tissue Oxygen Saturation (%) | 69.7 ± 7.1 | 68.7 ± 10.3 | ||||
| PO (% Normoxic PPO) | 66.6 ± 14.4 | |||||
| PO binned to ~100 W (W) | 95 ± 10 | |||||
| Hypoxia | ||||||
| HAH (n = 5) | Deoxy[Hb+Mb] (μM) | 47.9 ± 24.8 | 51.5 ± 24.6 | 0.807 | 0.032 | 0.181 |
| Oxy[Hb+Mb] (μM) | 125.3 ± 69.4 | 119.2 ± 69.5 | 0.989 | 0.320 | 0.619 | |
| Total[Hb+Mb] (μM) | 173.2 ± 93.2 | 170.7 ± 94.4 | 0.929 | 0.471 | 0.280 | |
| Tissue Oxygen Saturation (%) | 71.8 ± 2.5 | 68.7 ± 8.0 | 0.766 | < 0.001 | 0.235 | |
| PO (% Hypoxic PPO) | 79.4 ± 16.7 | 0.848 | ||||
| PO binned to ~100 W (W) | 93 ± 5 | |||||
| Controls (n = 11) | Deoxy[Hb+Mb] (μM) | 47.9 ± 22.8 | 60.4 ± 34.4 | |||
| Oxy[Hb+Mb] (μM) | 145.7 ± 113.3 | 121.7 ± 64.7 | ||||
| Total[Hb+Mb] (μM) | 200.0 ± 127.5 | 182.1 ± 94.4 | ||||
| Tissue Oxygen Saturation (%) | 73.5 ± 7.3 | 66.4 ± 8.0 | ||||
| PO (% Hypoxic PPO) | 75.8 ± 17.9 | |||||
| PO binned to ~100 W (W) | 101 ± 11 | |||||
The percent of PPO corresponding to ~100 W for individuals were compared between groups during both inspirates. Abbreviations: HAH, high affinity haemoglobin, Deoxy[Hb+MB], concentration of deoxygenated haemoglobin+myoglobin; Oxy[Hb+Mb], concentration of oxygenated haemoglobin+myoglobin; Total[Hb+Mb], concentration of total haemoglobin-myoglobin; PPO, peak power output; PO; power output. Values displayed as mean ± SD.
Due to interindividual differences in exercise protocols, the power outputs achieved during the graded exercise tests were compared via two-way repeated measures analysis of variance (ANOVA) to determine the effects of group [HAH, Controls] and stage of the graded exercise test [Stage 1, Stage 2, Stage 3, Stage 4, Stage 5, Stage 6]. Additionally, NIRS-derived data were compared at an absolute workload binned to ~100 W (individual values ranging from 80 W to 120 W, overall average 96±7 W). The power outputs binned to ~100 W were examined using a Univariate ANOVA model to determine the effects of group [HAH, Controls] and inspirate [Normoxia, Hypoxia]. NIRS-derived data at rest and ~100 W were compared via two-way repeated measures ANOVAs to determine the effects of group [HAH, Controls] and intensity [Rest, ~100 W] during normoxia and hypoxia separately (Table 2). Univariate ANOVAs were used to determine the effects of group and inspirate on resting and ~100 W NIRS-derived data. If significance was detected, post hoc analyses (Univariate ANOVA) were used to test for differences.
NIRS-derived data, heart rate, and oxygen uptake () were determined at rest and during the final 30 seconds of each exercise stage. These data were binned for each participant to the nearest 20, 40, 60, 80, and 100% of PPO in normoxia, for both graded exercise tests. Because of missing data for eight control participants at 20% of normoxic PPO, these data (NIRS-derived data, heart rate and ) were analyzed using linear mixed models with fixed effects during normoxia and hypoxia, separately. Linear mixed models were used to determine the effects of group [HAH, Controls] and intensity [20%, 40%, 60%, 80%, and 100% normoxic PPO], and the interaction between group and intensity. Additional linear mixed models were used to determine the effects of group [HAH, Controls] and inspirate [Normoxia, Hypoxia] on heart rate and during the graded exercise tests. All models included a random intercept for each participant to account for within-subject correlations during repeated measures. A Satterthwaite adjustment was used compute the degrees of freedom.
Before statistical comparisons, homogeneity of variance was assessed using Levene’s statistic. Significance was set at P<0.05 and data are presented as mean ± SD throughout. Reported P-values are two-sided and adjusted for multiplicity as appropriate. Statistical analyses were performed using SPSS® version 28.
RESULTS
Participant Characteristics
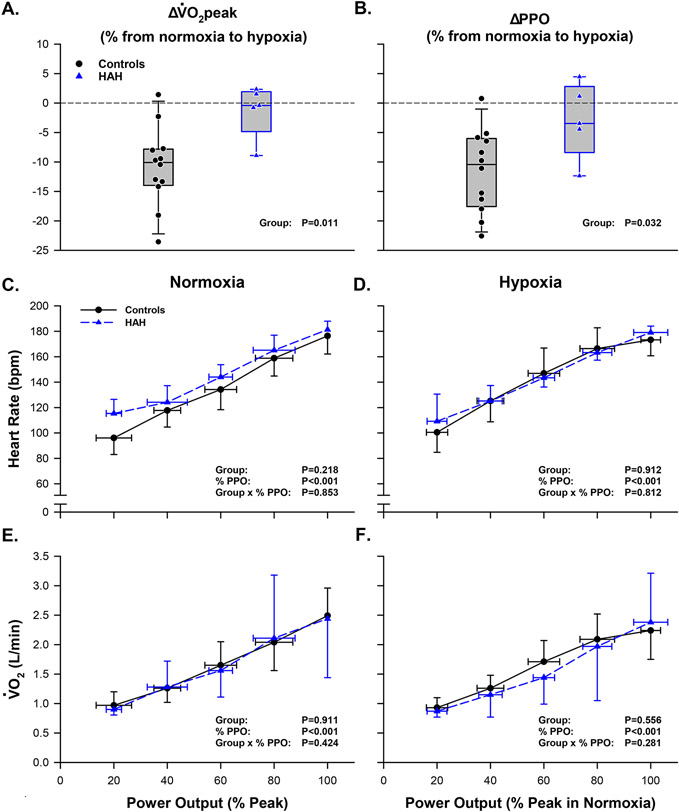
Participant characteristics and results from the graded exercise tests are shown in Table 1. Age, body mass index, height, and weight were not different between groups. As expected, the HAH group had a lower P50 than the control group (P<0.001). In addition, haemoglobin concentration and haematocrit were 35% and 34% greater, respectively, in the HAH group than the control group (P<0.001 for both). Relative , absolute , and PPO were not different between groups in neither normoxia nor hypoxia (Table 1). The reduction in and PPO from normoxia to hypoxia was attenuated in the HAH group compared to the control group (Figure 2. Panels A & B).
Figure 2: Normoxic and hypoxic graded exercise tests in humans with HAH and healthy controls.
(A, B) Box plots depicting the percent change in VȮ2peak and PPO from normoxia to hypoxia. (C, E) Heart rate and during the normoxic graded exercise test. (D, F) Heart rate and during the hypoxic graded exercise test. Abbreviations: HAH; high affinity hemoglobin, ; peak oxygen uptake; PPO; peak power output, ; oxygen uptake, bpm; beats per minute, L/min, liters per minute. Heart rate and data are presented as mean ± SD.
Graded Exercise Tests
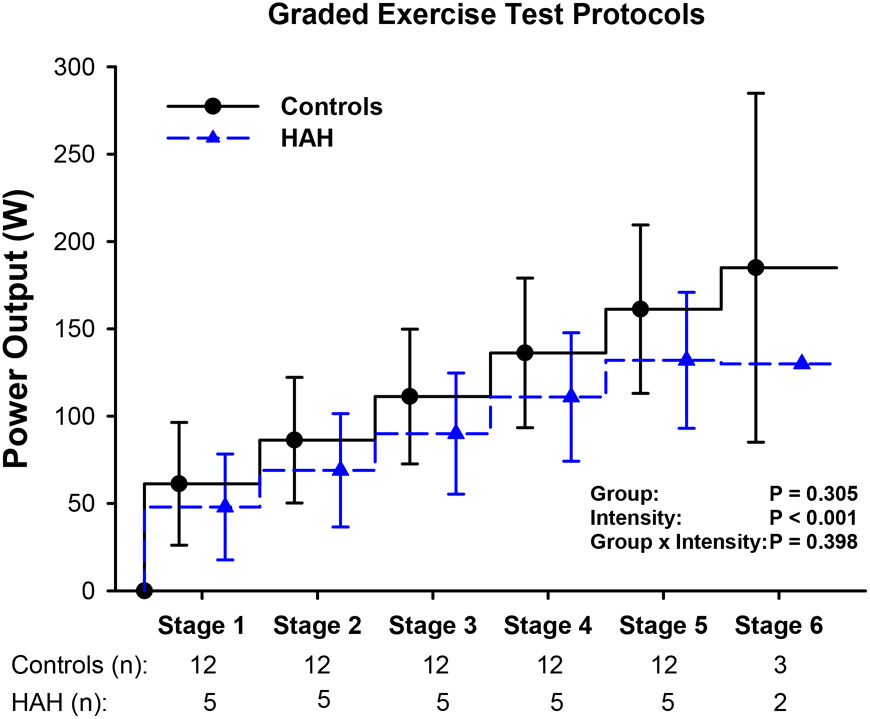
The power outputs achieved during the graded exercise tests were not different between groups (P=0.305) (Figure 1). Additionally, heart rate and were not different between groups during the normoxic (P>0.218) or hypoxic (P>0.556) graded exercise tests (Figure 2). There was no significant effect of inspirate on heart rate or during graded exercise tests in the HAH group (P>0.535) or control group (P>0.098).
Figure 1: Power outputs achieved during graded exercise tests in humans with HAH and healthy controls.
Stepwise plots depicting the power output achieved at each stage of the graded exercise test. The number of participants reaching the stage are denoted by ‘n’ below the x-axis. Abbreviations: HAH; high affinity hemoglobin, W; watts. Data are presented as mean ± SD.
Resting and ~100 W NIRS Data
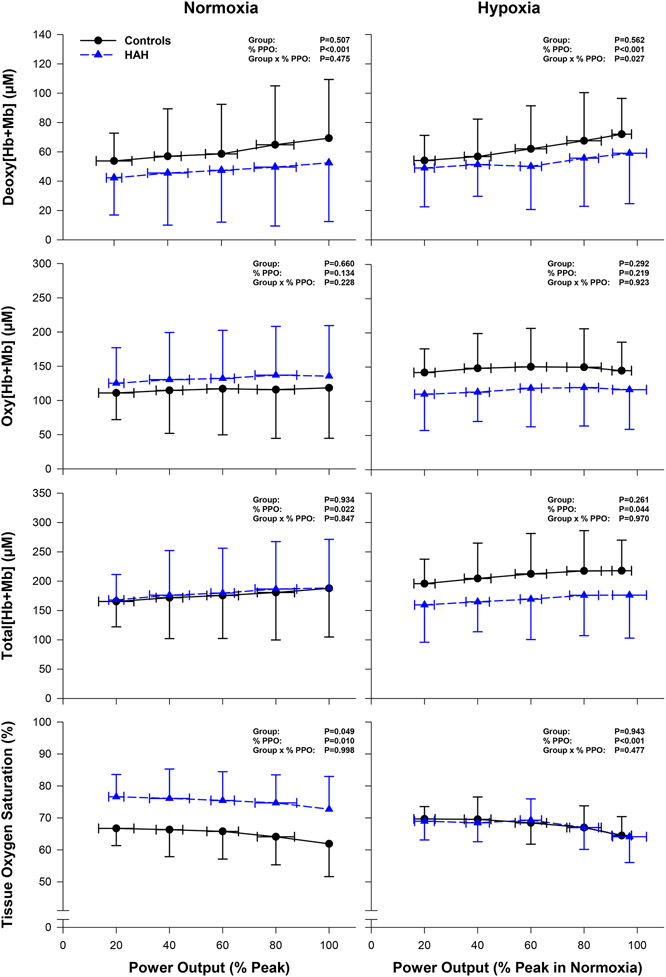
The power outputs binned to ~100 W were not different between groups (P=0.172) or inspirates (P=0.352), and there was no group by inspirate interaction (P=0.382) (Table 2). There was a main effect of intensity on Deoxy[Hb+Mb] during normoxia (P=0.012) and hypoxia (P=0.032) (Table 2). In addition, there was a group effect on tissue oxygen saturation during normoxia (P=0.014). Resting tissue oxygen saturation was lower during hypoxia compared to normoxia in the HAH group (P=0.003) but not for the control group (P=0.208). Deoxy[Hb+Mb] at ~100 W was not different between inspirates in the HAH group (P=0.859) or control group (P=0.779). Additionally at ~100 W, Oxy[Hb+Mb], Total[Hb+Mb], and tissue oxygen saturation were not different between inspirates for either group (P>0.184).
Normoxic Graded Exercise Test
Deoxy[Hb+Mb] increased with exercise intensity for both groups (P<0.001). Deoxy[Hb+Mb] was not different between groups throughout normoxic exercise (P=0.507). In addition, Oxy[Hb+Mb] and Total[Hb+Mb] were not different between groups during exercise (P>0.660). Participants with HAH demonstrated a higher tissue oxygen saturation compared to the control group during normoxic exercise (HAH: 75.1±4.1% vs. controls: 65.0±2.6%, P=0.049). There were no significant interactions between group and exercise intensity for Deoxy[Hb+Mb], Oxy[Hb+Hb], Total[Hb+Mb], or tissue oxygen saturation during normoxic exercise (Figure 3).
Figure 3: Near-infrared spectroscopy measurements of the vastus lateralis during graded exercise tests in normoxia and hypoxia.
The HAH group is denoted with blue triangles and dashed lines. The control group is denoted by black circles and solid lines. Abbreviations: Deoxy[Hb+MB], concentration of deoxygenated haemoglobin+myoglobin; Oxy[Hb+Mb], concentration of oxygenated haemoglobin+myoglobin; Total[Hb+Mb], concentration of total haemoglobin-myoglobin; PPO, peak power output. Data are presented as mean ± SD.
Hypoxic Graded Exercise Test
There was a significant interaction between group and intensity for Deoxy[Hb+Mb] during hypoxic exercise (P=0.027). However, Oxy[Hb+Mb], Total[Hb+Mb], and tissue oxygen saturation were not different between groups (P>0.261). There were no significant interactions between group and exercise intensity for Oxy[Hb+Hb], Total[Hb+Mb], or tissue oxygen saturation during hypoxic exercise (Figure 3).
DISCUSSION
High affinity haemoglobin and fractional oxygen extraction
Contrary to our hypothesis, our data support the notion that skeletal muscle fractional oxygen extraction was not different during normoxic exercise in humans with HAH compared to those with normal affinity haemoglobin (no difference in Deoxy[Hb+Mb] between groups). However, our results did indicate that the rise in fractional oxygen extraction during hypoxic exercise is blunted in humans with HAH compared to those with normal affinity haemoglobin (Figure 3), despite remarkably similar oxygen uptake between groups (Figure 2. Panels D & F). Compared to normoxia, humans with normal affinity haemoglobin have a lower during hypoxia despite increases in oxygen extraction within exercising muscle (Mazzeo, 2008). In contrast, our results demonstrate that humans with HAH can better maintain and PPO during hypoxic exercise compared to normoxic exercise (Dominelli et al., 2020) despite a blunted rise in fractional oxygen extraction. In theory, limitations in oxygen extraction during hypoxic exercise may compromise oxygen uptake in exercise muscle and decrease . Past work has shown that a greater arterial saturation during hypoxia correlates with better maintenance of (Dominelli et al., 2020). Combined with these previous studies, our data suggests that preservation of arterial saturation in humans with HAH during hypoxic exercise may outweigh limitations posed by compromised oxygen extraction (Figure 3), resulting in better maintenance of during hypoxia.
At matched absolute power outputs (~100 W), muscle energetic demands are likely similar between groups and inspirates (Figure 2). At ~100 W, Deoxy[Hb+Mb], indicative of oxygen delivery to utilization ratio (QO2 to muscle ), is not different between normoxia and hypoxia in the HAH or control group. These null findings suggest that greater oxygen delivery may compensate for limited oxygen availability during hypoxic exercise. Further research is needed to examine how compensations in oxygen delivery in the HAH group facilitate the better maintenance of and PPO observed during hypoxic exercise.
High affinity haemoglobin and muscle tissue oxygen saturation
An elevated haematocrit, as observed among humans with HAH, may lead to higher blood viscosity (Çınar et al., 1999) which is postulated to limit capillary blood flow and cause muscle tissue hypoxia during exercise (Mangin, 2017; Wranne et al., 1983). Contrary to these postulations, our results suggest that muscle tissue oxygen saturation is elevated in humans with HAH during normoxic exercise and similar to humans with normal affinity haemoglobin during hypoxic exercise (Table 2, Figure 3). Previous investigations suggest that muscle oxygenation may be compromised in humans with HAH (Bakker et al., 1976; Charache et al., 1966; Malmberg et al., 1979). Wranne and colleagues found that muscle oxygen tension was lower in two humans with HAH compared to normal reference values (Wranne et al., 1983). However, those with HAH had greater muscle tissue oxygen saturation at a given workload compared to humans with normal haemoglobin affinity.
In agreement with Wranne et al., our results suggest that humans with HAH have higher muscle tissue oxygen saturations during normoxic exercise, perhaps sequelae of the high affinity of haemoglobin for oxygen. If muscle oxygen utilization is not different between groups, as suggested by the and heart rate responses during exercise (Figure 2) (Dominelli et al., 2020), and humans with HAH retain a left-shifted oxygen dissociation curve in the microvasculature; muscle oxygen tension may be lower in the HAH group compared to controls during normoxic exercise. Due to this left-shifted nature of the oxygen dissociation curve in humans with HAH, a lower muscle oxygen tension may elicit a greater muscle tissue oxygen saturation compared to controls as observed (Figure 3). A lower muscle oxygen tension in humans with HAH would be consistent with reduced oxygen extraction, as observed by the attenuated rise in [DeoxyHb+Mb] during hypoxic exercise (Figure 3). This proposed mechanism is consistent with the findings of Wranne et al., where measurements of interstitial muscle oxygen tension via surgically placed oxygen electrodes were lower at rest during normoxia in humans with HAH compared to controls. However, it is unknown whether humans with HAH retain a left-shifted oxygen dissociation curve at the level of the microvasculature. Transient modulation of haemoglobin-oxygen binding affinity has been demonstrated in humans with Hb Malmö (Boyer et al., 1972; Webb et al., 2022) and some evidence indicates that the concentration of these modulatory factors produced during exercise (i.e. pronounced proton and lactate production) and may differ between humans with HAH and those with normal affinity haemoglobin (Dominelli et al., 2020).
However, our data do not clearly delineate differences in muscle oxygen tensions between humans with HAH and those with normal haemoglobin affinity due to potential differences in muscle perfusive properties and microvascular changes in haemoglobin-oxygen binding affinity. Yet, our results are consistent with animal studies in which muscle oxygenation was observed to be near normal values within a wide range of haematocrits and alterations in haemoglobin-oxygen affinity (Baer et al., 1987; Frietsch et al., 2007; Gaehtgens et al., 1979; Holzman et al., 1986; Lucas et al., 2019). More research is needed to understand how oxygen tension may differ along the oxygen transport chain between humans with HAH and those with normal haemoglobin affinity, particularly within the microvasculature and exercising muscle.
Muscle tissue oxygen saturation differs at rest between normoxia and hypoxia in humans with HAH, whereas it remains similar between inspirates in humans with normal affinity haemoglobin. Muscle tissue oxygen saturation at rest changed from 82±4% during normoxia to 72±3% during hypoxia in humans with HAH. For comparison, muscle tissue oxygen saturation remains relatively constant at 70±7% during normoxia and 74±7% during hypoxia in those with normal affinity haemoglobin. This divergence in muscle tissue oxygen saturation between groups during normoxia versus hypoxia could be associated with differences in muscle perfusive properties. During hypoxia, arterial saturation is reduced and muscle blood flow increases proportionally to maintain similar oxygen delivery to normoxic conditions (Joyner & Casey, 2014). Increases in arterial blood flow to muscle may explain the preserved muscle tissue oxygen saturation observed in humans with normal affinity haemoglobin during normoxia compared to hypoxia (Table 2). However, muscle tissue oxygen saturation decreases during transition from normoxia to hypoxia in humans with HAH (Table 2). It is possible that decreases in arterial saturation during hypoxia are not drastic enough to warrant a compensatory increase in blood flow in humans with HAH, which may help to maintain muscle tissue oxygen saturation levels near normoxic values. The influence of HAH on the regulation of muscle blood flow, particularly during hypoxic exercise, is not currently understood and warrants further investigation.
Limitations
Several limitations of these analyses warrant discussion. As demonstrated in Figure 1, individual graded exercise tests performed were different between participants. Due to inter-individual differences in workloads, we have elected to examine HR, , and NIRS-derived data primarily as a function of relative workload rather than absolute workload. To mitigate this limitation, we also present NIRS-derived data binned to an absolute workload of ~100 W (Table 2). However, not all participants performed cycling exercise at exactly 100 W. Therefore, we have binned similarly achieved power outputs (96±7 W) and presented these analyses as ~100 W (Table 2). Lastly, it is worth highlighting that mutations resulting in HAH are exceedingly rare (Mangin, 2017). Thus, analyses are limited to a relatively small HAH cohort of five participants.
Conclusion
Our results suggest that muscle oxygenation is augmented during rest and exercise in humans with HAH compared to those with normal affinity haemoglobin. Specifically, our findings suggest that fractional oxygen extraction in humans with HAH does not increase during hypoxic exercise to the same extent observed in humans with normal affinity haemoglobin. In addition, we propose that muscle tissue oxygen saturation is not compromised during either normoxic or hypoxic exercise in humans with HAH. Further research is warranted to better understand the influence of HAH on muscle tissue oxygenation during normoxia and hypoxia and how these changes may influence blood flow regulation and exercise capacity.
Supplementary Material
New Findings:
What is the central question of this study?
Do humans with high affinity haemoglobin (HAH) demonstrate attenuated skeletal muscle deoxygenation during normoxic and hypoxic exercise?
What is the main finding and its importance?
Examination of NIRS-derived muscle oxygenation profiles suggests that fractional oxygen extraction is blunted during hypoxic exercise in humans with HAH compared to controls. However, muscle tissue oxygen saturation levels were higher in humans with HAH during exercise in normoxia compared to controls. These alterations in fractional oxygen extraction in humans with HAH may influence blood flow regulation and exercise capacity during hypoxia.
Acknowledgements
We would like to acknowledge the contribution of the Human Integrative Physiology Laboratory and the Clinical Research and Trials Unit at the Mayo Clinic. We would like to thank Shelly Roberts, Meyer Nancy, Pamela Engrav, Andrew Miller, and Christopher Johnson for their continued assistance throughout the project.
Funding
This project was supported by the National Institute of Health R-35-HL139854 and the Mayo Foundation (MJJ). KLW was supported by NIH-T32-HL105355-09 and the Mayo Clinic Graduate School of Biomedical Sciences. CCW was supported by NIH-T32-DK-007352-39. PBD was supported by a post-doctoral fellowship from the Natural Sciences and Engineering Research Council of Canada. SMH was supported by NIH-T32-HL07111. SEB was supported by NIH-F32-HL131151. JWS was supported by NIH-F32-HL154320.
Abbreviations:
- HAH
High affinity haemoglobin
- P50
Partial pressure of oxygen at which haemoglobin is 50% saturated
- NIRS
Near-infrared spectroscopy
- Deoxy[Hb+Mb]
Concentration of deoxygenated haemoglobin+myoglobin
- Oxy[Hb+Mb]
Concentration of oxygenated haemoglobin+myoglobin
- Total[Hb+Mb]
Concentration of total haemoglobin+myoglobin
Peak oxygen uptake
- PPO
Peak power output
Footnotes
Competing Interests
None declared.
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
REFERENCES
- Baer RW, Vlahakes GJ, Uhlig PN, & Hoffman JI (1987). Maximum myocardial oxygen transport during anemia and polycythemia in dogs. American Journal of Physiology-Heart and Circulatory Physiology, 252(6), H1086–H1095. 10.1152/ajpheart.1987.252.6.H1086 [DOI] [PubMed] [Google Scholar]
- Bakker JC, Gortmaker GC, & Offerijns FGJ (1976). The influence of the position of the oxygen dissociation curve on oxygen-dependent functions of the isolated perfused rat liver. Pflügers Archiv., 366, 45–52. 10.1007/BF01063456 [DOI] [PubMed] [Google Scholar]
- Barstow TJ (2019). Understanding near infrared spectroscopy and its application to skeletal muscle research. Journal of Applied Physiology, 126(5), 1360–1376. 10.1152/japplphysiol.00166.2018 [DOI] [PubMed] [Google Scholar]
- Boyer SH, Charache S, Fairbanks VF, Maldonado JE, Noyes A, & Gayle EE (1972). Hemoglobin Malmö β-97 (FG-4) Histidine→Glutamine: A Cause of Polycythemia. Journal of Clinical Investigation, 51(3), 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S, Weatherall DJ, & Clegg JB (1966). Polycythemia associated with a hemoglobinopathy. Journal of Clinical Investigation, 45(6), 813–822. 10.1172/JCI105397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çınar Y, Demir G, Paç M, & Çınar AB (1999). Effect of hematocrit on blood pressure via hyperviscosity. American Journal of Hypertension, 12(7), 739–743. 10.1016/S0895-7061(99)00011-4 [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM, & Paterson DH (2003). Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. Journal of Applied Physiology, 95(1), 113–120. 10.1152/japplphysiol.00956.2002 [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Wiggins CC, Baker SE, Shepherd JA, Roberts SK, Roy TK, Curry TB, Hoyer JD, Oliveira JL, & Joyner MJ (2020). Influence of high affinity haemoglobin on the response to normoxic and hypoxic exercise. The Journal of Physiology, JP279161. 10.1113/JP279161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LF, Harper AJ, Townsend DK, Lutjemeier BJ, & Barstow TJ (2005). Kinetics of estimated human muscle capillary blood flow during recovery from exercise. Experimental Physiology, 90(5), 715–726. 10.1113/expphysiol.2005.030189 [DOI] [PubMed] [Google Scholar]
- Frietsch T, Maurer MH, Vogel J, Gassmann M, Kuschinsky W, & Waschke KF (2007). Reduced Cerebral Blood Flow but Elevated Cerebral Glucose Metabolic Rate in Erythropoietin Overexpressing Transgenic Mice with Excessive Erythrocytosis. Journal of Cerebral Blood Flow & Metabolism, 27(3), 469–476. 10.1038/sj.jcbfm.9600360 [DOI] [PubMed] [Google Scholar]
- Gaehtgens P, Kreutz F, & Albrecht KH (1979). Optimal hematocrit for canine skeletal muscle during rhythmic isotonic exercise. European Journal of Applied Physiology and Occupational Physiology, 41(1), 27–39. 10.1007/BF00424466 [DOI] [PubMed] [Google Scholar]
- Grassi B, & Quaresima V (2016). Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: A review from an exercise physiology perspective. Journal of Biomedical Optics, 21(9), 091313. 10.1117/1.JBO.21.9.091313 [DOI] [PubMed] [Google Scholar]
- Hammer SM, Alexander AM, Didier KD, Smith JR, Caldwell JT, Sutterfield SL, Ade CJ, & Barstow TJ (2018). The noninvasive simultaneous measurement of tissue oxygenation and microvascular hemodynamics during incremental handgrip exercise. Journal of Applied Physiology (Bethesda, Md.: 1985), 124(3), 604–614. 10.1152/japplphysiol.00815.2017 [DOI] [PubMed] [Google Scholar]
- Hebbel RP, Eaton JW, Kronenberg RS, Zanjani ED, Moore LG, & Berger EM (1978). Human llamas: Adaptation to altitude in subjects with high hemoglobin oxygen affinity. Journal of Clinical Investigation, 62(3), 593–600. 10.1172/JCI109165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman IR, Tabata B, & Edelstone DI (1986). Blood Flow and Oxygen Delivery to the Organs of the Neonatal Lamb as a Function of Hematocrit. Pediatric Research, 20(12), 1274–1279. 10.1203/00006450-198612000-00016 [DOI] [PubMed] [Google Scholar]
- Jelkmann W (2011). Regulation of erythropoietin production: Erythropoietin production. The Journal of Physiology, 589(6), 1251–1258. 10.1113/jphysiol.2010.195057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelkmann W (2013). Physiology and Pharmacology of Erythropoietin. Transfusion Medicine and Hemotherapy, 40(5), 302–309. 10.1159/000356193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M, Oski FA, Nathan DG, & Bunn HF (1975). Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. Journal of Clinical Investigation, 55(3), 469–477. 10.1172/JCI107953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ, & Casey DP (2014). Muscle blood flow, hypoxia, and hypoperfusion. Journal of Applied Physiology, 116(7), 852–857. 10.1152/japplphysiol.00620.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A, Ao-ieong ESY, Williams AT, Jani VP, Muller CR, Yalcin O, & Cabrales P (2019). Increased Hemoglobin Oxygen Affinity With 5-Hydroxymethylfurfural Supports Cardiac Function During Severe Hypoxia. Frontiers in Physiology, 10, 1350. 10.3389/fphys.2019.01350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg PO, Hlastala MP, & Woodson RD (1979). Effect of increased blood-oxygen affinity on oxygen transport in hemorrhagic shock. Journal of Applied Physiology, 47(4), 889–895. 10.1152/jappl.1979.47.4.889 [DOI] [PubMed] [Google Scholar]
- Mangin O (2017). High oxygen affinity hemoglobins. La Revue de Médecine Interne, 38(2), 106–112. 10.1016/j.revmed.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Mazzeo RS (2008). Physiological Responses to Exercise at Altitude. Sports Medicine, 38(1), 1–8. 10.2165/00007256-200838010-00001 [DOI] [PubMed] [Google Scholar]
- Santbergen B, & van der Heul C (2014). At high altitude in the Netherlands: Secondary erythrocytosis due to HB-Malmo. Case Reports, 2014, bcr2014203701–bcr2014203701. 10.1136/bcr-2014-203701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JRA, Dominelli PB, Roy TK, Secomb TW, Hoyer JD, Oliveira JL, & Joyner MJ (2019). Modelling the relationships between haemoglobin oxygen affinity and the oxygen cascade in humans. The Journal of Physiology, 597(16), 4193–4202. 10.1113/JP277591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb KL, Dominelli PB, Baker SE, Klassen SA, Joyner MJ, Senefeld JW, & Wiggins CC (2022). Influence of High Hemoglobin-Oxygen Affinity on Humans During Hypoxia. Frontiers in Physiology, 12. 10.3389/fphys.2021.763933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow RM, Swenberg ML, Berger RL, Shrager RI, Luzzana M, Samaja M, & Rossi-Bernardi L (1977). Oxygen equilibrium curve of normal human blood and its evaluation by Adair’s equation. Journal of Biological Chemistry, 252(7), 2331–2337. 10.1016/S0021-9258(17)40559-X [DOI] [PubMed] [Google Scholar]
- Wranne B, Berlin G, Jorfeldt L, & Lund N (1983). Tissue oxygenation and muscular substrate turnover in two subjects with high hemoglobin oxygen affinity. Journal of Clinical Investigation, 72(4), 1376–1384. 10.1172/JCI111094 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.