Abstract
Rationale:
Hypertension is a common and serious adverse effect of calcineurin inhibitors, including cyclosporine and tacrolimus (FK506). Although increased sympathetic nerve discharges are associated with calcineurin inhibitor–induced hypertension, the sources of excess sympathetic outflow and underlying mechanisms remain elusive. Calcineurin (protein phosphatase-2B) is broadly expressed in the brain, including the paraventricular nuclear (PVN) of the hypothalamus, which is critically involved in regulating sympathetic vasomotor tone.
Objective:
We determined whether prolonged treatment with the calcineurin inhibitor causes elevated sympathetic output and persistent hypertension by potentiating synaptic N-methyl-D-aspartate (NMDA) receptor activity in the PVN.
Methods and Results:
Telemetry recordings showed that systemic administration of FK506 (3 mg/kg/day) for 14 days caused a gradual and profound increase in arterial blood pressure in rats, which lasted at least 7 days after discontinuing FK506 treatment. Correspondingly, systemic treatment with FK506 markedly reduced calcineurin activity in the PVN and circumventricular organs, but not rostral ventrolateral medulla, and increased the phosphorylation level and synaptic trafficking of NMDA receptors in the PVN. Immunocytochemistry labeling showed that calcineurin was expressed in presympathetic neurons in the PVN. Whole-cell patch clamp recordings in brain slices revealed that treatment with FK506 increased baseline firing activity of PVN presympathetic neurons; this increase was blocked by the NMDA or AMPA receptor antagonist. Also, treatment with FK506 markedly increased presynaptic and postsynaptic NMDA receptor activity of PVN presympathetic neurons. Furthermore, microinjection of the NMDA or AMPA receptor antagonist into the PVN of anesthetized rats preferentially attenuated renal sympathetic nerve discharges and blood pressure elevated by FK506 treatment. In addition, systemic administration of memantine, a clinically used NMDA receptor antagonist, effectively attenuated FK506 treatment–induced hypertension in conscious rats.
Conclusions:
Our findings reveal that normal calcineurin activity in the PVN constitutively restricts sympathetic vasomotor tone via suppressing NMDA receptor activity, which may be targeted for treating calcineurin inhibitor–induced hypertension.
Keywords: autonomic nervous system, immunosuppressant, neurogenic hypertension, synaptic plasticity, sympathetic nervous system
Graphical Abstract
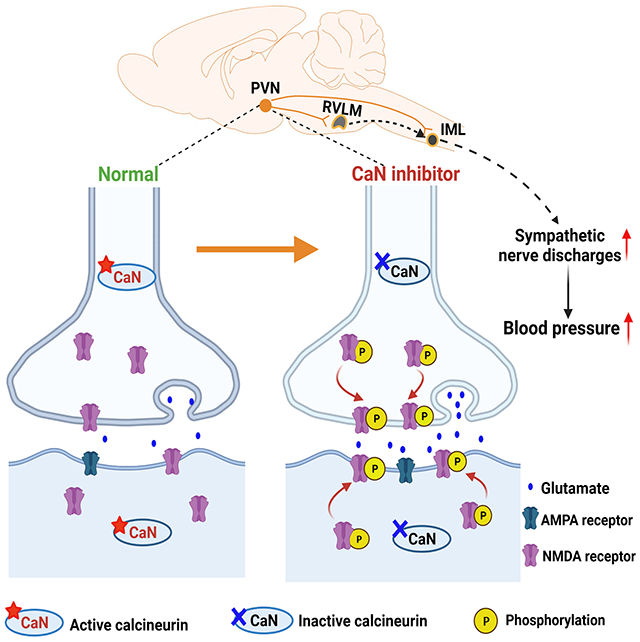
INTRODUCTION
Calcineurin inhibitors, including cyclosporine and tacrolimus (FK506), are used clinically as standard immunosuppressants in organ/tissue transplantation and in the treatment of autoimmune diseases, such as psoriasis and rheumatoid arthritis. Hypertension is a common and serious adverse effect that accompanies the prolonged use of calcineurin inhibitors. The incidence of calcineurin inhibitor–induced hypertension (CIH) is 30%–50% in patients with autoimmune diseases and 50%–100% in liver, renal, and heart transplant recipients.1–3 The mean arterial blood pressure (ABP) is 110–140 mmHg in most transplant recipients receiving calcineurin inhibitors,4, 5 and uncontrolled hypertension often extensively reduces graft survival and can lead to mortality.6, 7 In organ transplant recipients, CIH is associated with higher muscle sympathetic nerve activity.8 Also, treatment with cyclosporine increases renal sympathetic nerve activity and ABP in intact rats, which is blunted in pithed rats 9 and prevented by ganglionic blockade.10 However, previous work on neural mechanisms of CIH has largely focused on the acute effect of a single injection of calcineurin inhibitors on sympathetic nerve discharges.9–12 Although the sympathetic nervous system is closely involved in the development of CIH, the sources of elevated sympathetic outflow and underlying mechanisms remain elusive.
Calcineurin (also known as protein phosphatase-2B) is a Ca2+/calmodulin-dependent serine/threonine protein phosphatase, and calcineurin inhibitors suppress cytokine production in immune T cells to exert immunosuppressive effects. In addition to its presence in immune T cells, calcineurin is also broadly expressed in brain tissues, including the paraventricular nucleus (PVN) of the hypothalamus.13–15 Calcineurin plays a key role in neuronal functions, such as learning and memory, modulation of receptor function, and neuronal excitability.16, 17 The role of the brain calcineurin in CIH has been largely overlooked, because it was assumed that neither cyclosporine nor FK506 can easily cross the blood-brain barrier (BBB). However, both cyclosporine and FK506 are highly lipophilic drugs. Cyclosporine can cause hyperpermeability of the BBB,18, 19 and FK506 is readily detected in the brain upon systemic injection.20 At present, it is unknown whether and how the brain is involved in persistent hypertension caused by prolonged treatment with calcineurin inhibitors.
The paraventricular nuclear (PVN) of the hypothalamus represents the rostral extension of a serial connection of sympathetic-related neurons in the nervous system.21, 22 The PVN sends projections to vasomotor neurons in the rostral ventrolateral medulla (RVLM) in the brainstem and sympathetic preganglionic neurons in the intermediolateral cell column in the spinal cord,23, 24 which in turn regulate sympathetic nerve discharges. Although the PVN is not actively involved in ABP regulation under normal conditions, it is a major source of excitatory drive that augments sympathetic outflow in hypertensive conditions25–27 and in heart failure.28 The glutamate N-methyl-D-aspartate receptor (NMDAR) activity in the brain is critically regulated via phosphorylation by various protein kinases and phosphatases.17, 29, 30 In this study, we tested the hypothesis that prolonged treatment with the calcineurin inhibitor causes elevated sympathetic output and persistent hypertension by potentiating synaptic NMDAR activity in the PVN. Our study demonstrates for the first time the critical role of constitutive calcineurin activity in the brain in restraining sympathetic vasomotor tone. Our findings not only reveal the molecular substrates responsible for generating excess sympathetic outflow in CIH but also suggest a new molecular target for treating CIH.
METHODS
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Detailed methods can be found in the Supplemental Material.
Animal model
All experimental procedures were approved by Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center. Male Sprague-Dawley rats (9–11 weeks old) were purchased from Envigo. FK506 (#3631, Tocris Bioscience) was dissolved in dimethyl sulfoxide and injected intraperitoneally at a dose of 3 mg/kg once a day for 14 consecutive days. Memantine (#14184, Cayman Chemical) was administered via intraperitoneal injection or in the drinking water. ABP was measured in free-moving rats using a Millar telemetry system (Telemetry Research Ltd).13, 27 Heart rate (HR) values were derived from the blood pressure pulse signal.
Calcineurin activity measurement
Calcineurin activity in brain tissues was measured using a calcineurin cellular activity assay kit (#207007, Millipore).32 Briefly, fresh brain samples, including the hypothalamus, PVN, frontal cortex, hippocampus, organum vasculosum laminae terminalis (OVLT), subfornical organ (SFO), and RVLM, were obtained from FK506-treated and vehicle-treated rats. Calcineurin activity was calculated as the difference of free phosphate release between assays with and without EGTA.33
Synaptosome immunoprecipitation
Synaptosomes were isolated from the brain in rats as described previously.31, 34 Synaptosomal samples were incubated at 4°C overnight with Protein G beads prebound to rabbit anti-phosphoserine (#AB1603, Millipore) or rabbit anti-phosphothreonine antibodies (#AB1607, Millipore). Protein G beads prebound to rabbit IgG were used as controls. Samples were immunoblotted with a mouse anti-GluN1 antibody (1:500; #75-272, NeuroMab), mouse anti-GluN2A antibody (1:500; #75-288; NeuroMab), and mouse anti-GluN2B antibody (1:500; #75-097, NeuroMab).
Electrophysiological recording in brain slices
Spinally projecting and RVLM-projecting PVN neurons were retrogradely labeled as described previously.30, 38 The firing activity of labeled PVN neurons was recorded using a perforated whole-cell method, as we described previously.41, 42 The miniature excitatory postsynaptic currents (mEPSCs) were recorded at a holding potential of −60 mV.31 The postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)- and NMDAR-currents were elicited by puff application of AMPA (100 μM) or NMDA (100 μM) directly to the recorded PVN neuron.26, 29
PVN microinjection and recording of renal sympathetic nerve activity
Rats were anesthetized by intraperitoneal injection of a mixture of α-chloralose (60 mg/kg) and urethane (800 mg/kg). A branch of the left renal postganglionic sympathetic nerve was isolated under a surgical microscope. Renal nerve was cut distally to ensure that afferent nerve activity was not recorded.25, 35 A microinjection pipette was advanced into the PVN,25, 31 and the microinjection was performed using a Nanoject II injector.
Statistical analysis
All data were presented as means ± SEM. The firing rate and mEPSCs were analyzed using a peak detection program (MiniAnalysis, Synaptosoft). Two-tailed Student’s t test was used to determine differences between two groups. Repeated measures ANOVA followed by Dunnett’s post hoc test or two-way ANOVA followed by Bonferroni’s post hoc test was used to determine differences among three or more groups. All statistical analyses were performed using Prism software (GraphPad).
RESULTS
Prolonged treatment with a calcineurin inhibitor induces long-lasting hypertension
We first established a clinically relevant model of CIH to study the mechanism of persistent hypertension caused by calcineurin inhibitors. In patients with organ transplants, ABP increases progressively within the first few weeks after the start of calcineurin inhibitors.4, 5 However, previous studies mainly tested acute effects of a large dose of calcineurin inhibitors on sympathetic nerve activity.9, 10, 45, 46 FK506 is the preferred drug for modern immunosuppression regimens. To mimic CIH in patients, we instrumented rats with radiotelemetry and then subjected them to intraperitoneal injection of FK506 (3 mg/kg, once a day) for 14 days. This dose of FK506 can inhibit immune function and increase ABP in rodents.47–50 Radiotelemetry recordings showed that repeated treatment with FK506 gradually and profoundly increased ABP 7 days after beginning treatment (Figs. 1A and S1). Treatment with FK506 significantly elevated baseline mean ABP from 93.62 ± 1.92 mmHg to 120.05 ± 2.78 mmHg on the last day of treatment during the light cycle (p = 0.00503, F(4.45,22.26) = 20.01, n = 6 rats). Similarly, treatment with FK506 markedly increased heart rate (HR) in these rats (Fig 1B). Strikingly, the elevated ABP and HR in both light and dark cycles were persistent and remained stable at least 7 days after discontinuation of FK506 treatment (Figs. 1A,B and Fig. S1). Treatment with the vehicle had no significant effect on the ABP or HR during the entire period of experiments (n = 6 rats, Fig. 1A,B). These results demonstrate that prolonged treatment with a clinically relevant dose of the calcineurin inhibitor causes progressive and persistent hypertension in rats.
Figure 1. Repeated systemic treatment with FK506 leads to persistent hypertension and reduced calcineurin activity in the brain.
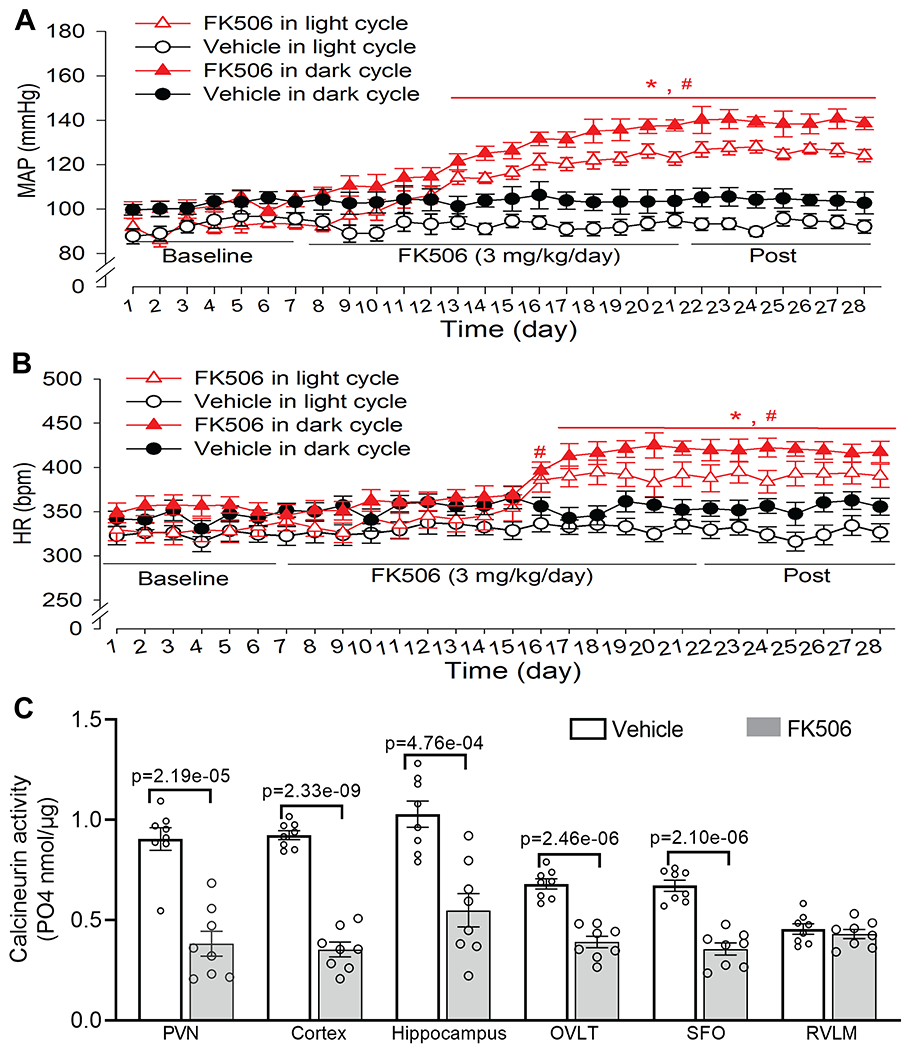
A and B, Time course of changes in mean arterial blood pressure (MAP, A) and heart rate (B) recorded using radiotelemetry in conscious rats treated with FK506 (3 mg/kg/day) or vehicle for 14 days (n = 6 rats per group). Telemetry data were averaged during the light (7:00 A.M. to 7:00 P.M.) and dark period (7:00 P.M. to 7:00 A.M. the next day) each day. Two-way ANOVA followed by Bonferroni’s post hoc test. C, Calcineurin activity in the PVN, frontal cortex, hippocampus, OVLT, SFO, and RVLM of rats 7 days after discontinuing treatment with FK506 or vehicle (n = 8 samples/rats per group). Two-tailed Student’s t test. Data are expressed as mean ± SEM.
Systemic treatment with FK506 diminishes calcineurin phosphatase activity in the forebrain
Calcineurin is extensively expressed in the brain,15 and FK506 can cross the blood-brain barrier upon systemic injection.20 We next determined whether repeated systemic treatment with FK506 has a prolonged effect on calcineurin activity in the brain. We treated rats with intraperitoneal injection of FK506 (3 mg/kg/day) or vehicle for 14 days. We obtained brain tissues in rats 7 days after discontinuing treatment. Compared with vehicle-treated rats, treatment with FK506 markedly reduced calcineurin phosphatase activity in the PVN (p = 2.19e-05, t(14) = 6.23, n = 8 samples per group), frontal cortex, hippocampus, and circumventricular organs (i.e., OVLT and SFO) (n = 8 samples per group, Fig. 1C). However, the calcineurin activity in the RVLM was relatively low compared with forebrain regions and did not differ significantly between FK506-treated and vehicle-treated groups (n = 8 samples per group, Fig. 1C). These findings suggest that repeated systemic treatment with FK506 causes a long-lasting reduction in calcineurin activity in the forebrain, which coincides with persistent hypertension.
Systemic treatment with FK506 increases NMDAR phosphorylation and synaptic expression in the PVN
Because the hypothalamic PVN is critically involved in the development of neurogenic hypertension,22, 25 the following experiments were focused on the role of NMDARs in the PVN in the pathogenesis of CIH. NMDARs are typically composed of two GluN1 and two GluN2 (GluN2A and/or GluN2B) subunits, and both GluN1 and GluN2 subunits contain serine/threonine residues that are subject to phosphorylation control by protein kinases and phosphatases.51, 52 We obtained PVN and RVLM tissues from rats 7 days after FK506 treatment was ended. We conducted immunoprecipitation assays to determine whether systemic treatment with FK506 affects phosphorylation levels of synaptic NMDARs. The anti-phosphoserine and anti-phosphothreonine antibodies precipitated GluN1, GluN2A, and GluN2B in synaptosomes isolated from PVN tissues of FK506-treated and vehicle-treated rats, whereas the irrelevant IgG did not precipitate any proteins of NMDAR subunits (Fig. 2A–C). Further analysis showed that the protein levels of phosphoserine (pSer)-GluN1 and phosphothreonine (pThr)-GluN1 in PVN synaptosomes were significantly higher in FK506-treated rats than in vehicle-treated rats (pSer-GluN1: p = 0.0013, t(10) = 4.41; pThr-GluN1: p = 0.0005, t(10) = 5.11; n = 6 rats per group, Fig. 2A). In contrast, the protein levels of pSer-GluN1 and pThr-GluN1 in the RVLM synaptosomes were very low and did not differ significantly between the two group (Fig. S2). Furthermore, there was no significant difference in protein levels of pSer/pThr-GluN2A or pSer/pThr-GluN2B in PVN synaptosomes between the two groups (Fig. 2B,C).
Figure 2. Repeated systemic treatment with FK506 increases NMDAR phosphorylation and synaptic trafficking in the PVN.
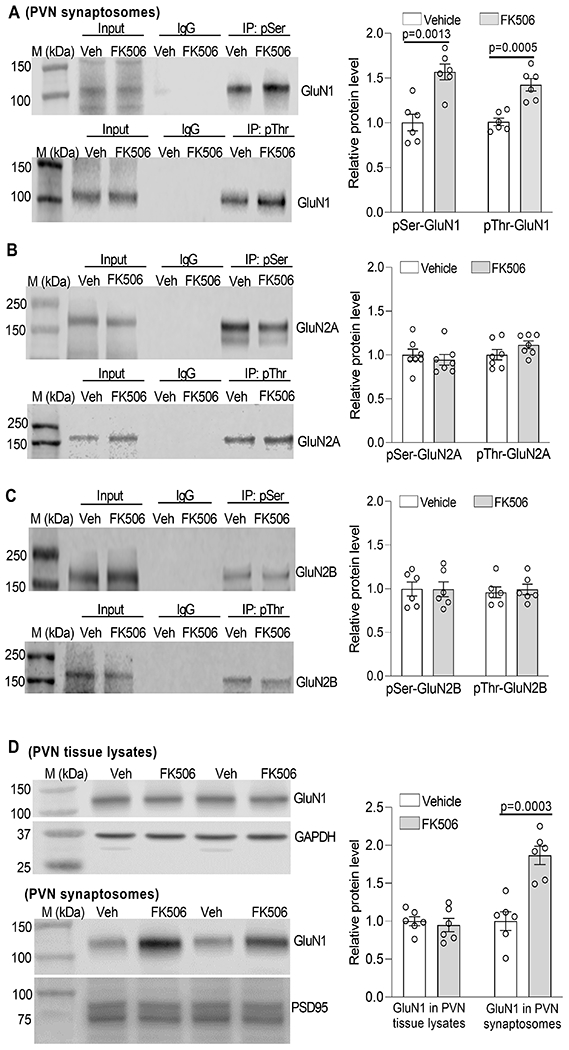
A-C, Representative blotting images and quantification show the protein levels of phosphoserine (pSer)/phosphothreonine (pThr)-GluN1 (A, n = 6 rats per group), pSer/pThr-GluN2A (B, n = 7 rats per group), and pSer/pThr-GluN2B (C, n = 6 rats per group) in PVN synaptosomes from rats 7 days after terminating treatment with FK506 or Veh. D, Representative blotting images and quantification show the protein levels of GluN1 in PVN tissue lysates and PVN synaptosomes from rats 7 days after stopping treatment with FK506 or Veh (n = 6 rats per group). Two-tailed Student’s t test. Data are expressed as mean ± SEM.
We then determined whether increased NMDAR phosphorylation by FK506 treatment affects the synaptic expression of NMDARs in the PVN. Immunoblotting using the PVN tissue lysates showed that the total protein level of GluN1, the obligatory subunit of NMDARs, was similar between FK506-treated and vehicle-treated rats (Fig. 2D). However, immunoblotting analysis of PVN synaptosomes revealed a significantly higher level of GluN1 in FK506-treated rats than in vehicle-treated rats (p = 0.0003, t(10) = 5.45, n = 6 rats per group, Fig. 2D). These results suggest that systemic treatment with the calcineurin inhibitor potentiates NMDAR phosphorylation and promotes NMDAR synaptic trafficking in the PVN.
Treatment with FK506 causes hyperactivity of PVN presympathetic neurons via increased glutamatergic input
To determine whether prolonged systemic treatment with FK506 affects excitability of PVN presympathetic neurons, we first examined whether calcineurin is expressed in spinally projecting PVN neurons. Spinally projecting PVN neurons were labeled with a retrograde tracer, Alexa Fluor 594-conjugated cholera toxin subunit-B (CTB), microinjected into the intermediolateral cell column of the thoracic spinal cord. We performed immunocytochemical labeling using a calcineurin antibody that recognizes calcineurin A and B subunits.53 Confocal images showed that CTB-labeled neurons were colocalized with calcineurin-immunoreactivity in the PVN (Fig. 3A).
Figure 3. Calcineurin inhibition increases the excitability of PVN presympathetic neurons by augmenting glutamatergic input.
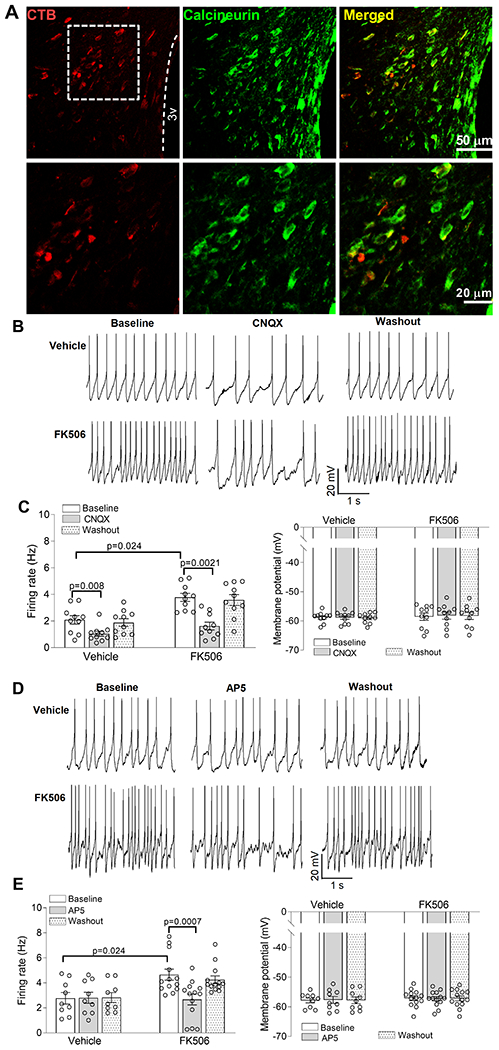
A, Representative confocal images show the presence of calcineurin immunoreactivity (green) in CTB (red)-labeled spinally projecting neurons in the rat PVN. 3V, third ventricle. B-E, Original recording traces and quantification show the effect of bath application of CNQX (n = 10 neurons from 5 rats per group; B and C) or AP5 (n = 9 neurons from 4 rats in the vehicle group; n = 13 neurons from 5 rats in the FK506 group; D and E) on the firing frequency and membrane potential of spinally projecting PVN neurons of rats 7 days after discontinuing treatment with FK506 or vehicle. Two-way ANOVA followed by Bonferroni’s post hoc test. Data are expressed as mean ± SEM.
Hyperactivity of PVN presympathetic neurons plays an important role in generating elevated sympathetic outflow in neurogenic hypertension.22, 25, 29, 35 We used perforated whole-cell recordings to measure spontaneous firing activity of spinally projecting PVN neurons in brain slices of rats 7 days after discontinuing the treatment with FK506 or vehicle. Compared with the conventional whole-cell approach, perforated recording can largely preserve the intracellular environment with minimal effects on Ca2+-dependent signaling.41, 42 The baseline firing rate of labeled PVN neurons was significantly higher in FK506-treated rats than in vehicle-treated rats (p = 0.024, F(1,20) = 3.96, n = 9 neurons in vehicle group; n = 13 neurons in FK506 group, Fig. 3B–E). However, the membrane potential of labeled PVN neurons were similar between the two groups (Fig. 3B–E).
Glutamate is the predominant excitatory neurotransmitter in the brain, and glutamate-mediated fast excitatory synaptic transmission is mainly mediated by AMPARs. We next determined the contribution of AMPARs to the hyperactivity of PVN presympathetic neurons in FK506-treated rats. Bath application of 20 μM CNQX,26 a specific AMPAR antagonist, for 6 min significantly decreased the firing activity of labeled neurons in both FK506-treated and vehicle-treated rats (vehicle group: p = 0.008, F(9,18) = 6.48; FK506 group: p = 0.0021, F(9,18) = 2.24; n = 10 neurons per group, Fig. 3B,C).
Although NMDARs are functionally inactive in the PVN under normal conditions, increased synaptic NMDAR activity augments glutamatergic input in the PVN in neurogenic hypertension.26, 29, 30 We thus determined whether NMDARs play a role in the hyperactivity of spinally projecting PVN neurons induced by FK506 treatment. Bath application of 50 μM AP5, a specific NMDAR antagonist, for 6 min had no effect on the firing activity of labeled PVN neurons in vehicle-treated rats (Fig. 3D,E). However, AP5 significantly reduced the firing activity of labeled neurons in FK506-treated rats (p = 0.0007, F(12,24) = 4.40, n = 13 neurons, Fig. 3D,E). Neither CNQX nor AP5 had any significant effect on the membrane potential of labeled PVN neurons in both groups (Fig. 3B–E). These results suggest that systemic treatment with a calcineurin inhibitor induces hyperexcitability of PVN presympathetic neurons by potentiating glutamatergic input.
Treatment with FK506 potentiates presynaptic and postsynaptic NMDAR activity of PVN presympathetic neurons
Because the hyperactivity of PVN presympathetic neurons induced by FK506 treatment is preferentially maintained by NMDARs, we subsequently determined the activity of presynaptic NMDARs in spinally projecting PVN neurons in brain slices of rats 7 days after repeated treatment with FK506 or vehicle. We measured miniature excitatory postsynaptic currents (mEPSCs), which reflects spontaneous quantal release of glutamate from presynaptic terminals.26, 54 Whole-cell voltage-clamp recordings revealed that the baseline frequency of mEPSCs in labeled spinally projecting PVN neurons was significantly greater in FK506-treated rats than in vehicle-treated rats (p = 0.0069, F(2,28) = 34.87, n = 8 neurons in each group, Fig. 4A–C). The amplitude of mEPSCs was similar between the two groups (Fig. 4A–C). Bath application of AP5 (50 μM)26 significantly reduced the frequency of mEPSCs in labeled PVN neurons in FK506-treated rats (p = 0.0003, F(7,14) = 8.207, n = 8 neurons, Fig. 4A–C), indicating that the increased presynaptic glutamate release is mediated by NMDARs. In contract, AP5 had no effect on the frequency of mEPSCs of labeled PVN neurons in vehicle-treated rats (Fig. 4A–C).
Figure 4. Calcineurin inhibition increases presynaptic and postsynaptic NMDAR activity of PVN presympathetic neurons.
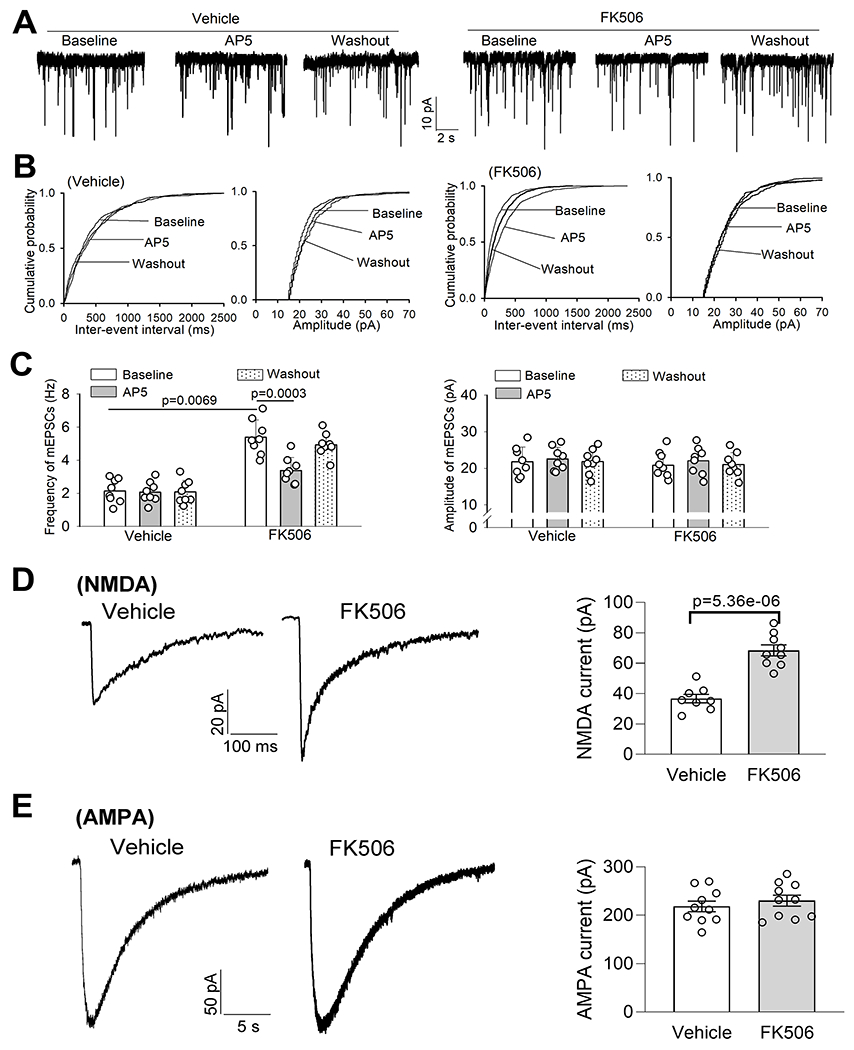
A-C, Original recording traces (A), cumulative probability plots (B), and quantification (C) show the effects of AP5 on the frequency and amplitude of mEPSCs of spinally projecting PVN neurons of rats 7 days after discontinuing treatment with FK506 or vehicle (n = 8 neurons from 3 rats per group). D and E, Representative recording traces and quantification show puff-elicited NMDA currents (D, n = 8 neurons from 3 rats in the vehicle group, n = 9 neuros from 3 rats in FK506 group) and puff-elicited AMPA currents (E, n = 10 neurons from 4 rats per group) of spinally projecting PVN neurons of rats 7 days after discontinuing treatment with FK506 or vehicle. C, two-way ANOVA followed by Bonferroni’s post hoc test; D and E, two-tailed Student’s t test. Data are expressed as mean ± SEM.
We also determined whether treatment with FK506 affects postsynaptic NMDAR activity in spinally projecting PVN neurons. Postsynaptic NMDAR currents were elicited by direct puff application of 100 μM NMDA to the recorded PVN neuron. The amplitude of puff-elicited inward NMDAR currents in labeled PVN neurons was significantly greater in FK506-treated rats than in vehicle-treated rats (p = 5.36e-06, t(15) = 6.87; n = 8 neurons in vehicle group; n = 9 neurons in FK506 group, Fig. 4D). Furthermore, we determined whether postsynaptic AMPAR activity in the PVN is altered by systemic treatment with FK506. The amplitude of AMPAR currents elicited by puff application of 100 μM AMPA to labeled PVN neurons did not differ significantly between the two groups (Fig. 4E).
In addition, we performed whole-cell recordings in labeled RVLM-projecting PVN neurons in brain slices of rats 7 days after repeated treatment with FK506 or vehicle. The frequency of mEPSCs in RVLM-spinally projecting neurons was much greater in FK506-treated rats than in vehicle-treated rats (n = 15 neurons per group, Fig. 5A–C). AP5 application significantly reduced the frequency of mEPSCs in labeled PVN neurons in FK506-treated rats but not in the vehicle group (Fig. 5A–C). Moreover, the amplitude of puff NMDAR currents in labeled PVN neurons were significantly larger in FK506-treated rats than in vehicle-treated rats (n = 11 neuron in vehicle-treated group, n = 10 neurons in FK506-treated group, Fig. 5D). However, the amplitude of puff AMPAR currents in labeled PVN neurons did not differ significantly between the two groups (Fig. 5E). Together, these findings suggest that systemic treatment with a calcineurin inhibitor augments the activity of presynaptic and postsynaptic NMDARs in PVN presympathetic neurons.
Figure 5. Calcineurin inhibition increases presynaptic and postsynaptic NMDAR activity of RVLM-projecting PVN presympathetic neurons.
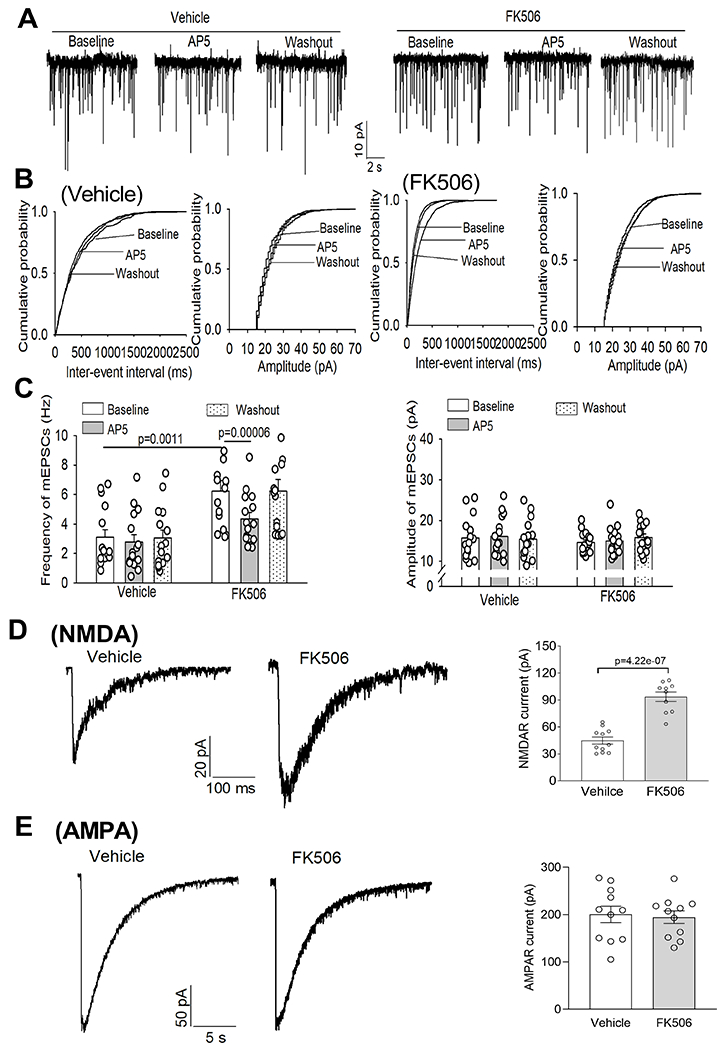
A-C, Original recording traces (A), cumulative probability plots (B), and quantification (C) show the effects of AP5 on the frequency and amplitude of mEPSCs of RVLM-projecting PVN neurons of rats 7 days after discontinuing treatment with FK506 or vehicle (n = 15 neurons from 5 rats per group). D and E, Representative recording traces and quantification show puff-NMDA elicited currents (D, n = 11 neurons from 4 rats in the vehicle group, n = 10 neuros from 4 rats in FK506 group) and puff-AMPA elicited currents (E, n = 11 neurons from 4 rats per group) of RVLM-projecting PVN neurons of rats 7 days after discontinuing treatment with FK506 or vehicle. C, two-way ANOVA followed by Bonferroni’s post hoc test; D and E, two-tailed Student’s t test. Data are expressed as mean ± SEM.
Sympathetic vasomotor tone is sustained by glutamatergic input and NMDAR activity in the PVN in CIH
To functionally relate calcineurin inhibitor–induced synaptic plasticity to regulation of sympathetic outflow in vivo, we determined whether blocking glutamatergic input in the PVN attenuates sympathetic vasomotor tone in rats 7 days after repeated treatment with FK506 or vehicle. Bilateral microinjection of CNQX (1.8 nmol/50 nL)25 into the PVN did not significantly change renal sympathetic nerve activity (RSNA), HR, or ABP in vehicle-treated rats (n = 7 rats; Fig. 6A,C,E). However, microinjection of CNQX significantly reduced RSNA, HR, and ABP in FK506-treated rats (n = 7 rats; Fig. 6B,D,E). The latency of the inhibitory responses was 2.11 ± 0.15 min after CNQX injection in FK506-treated rats, and the peak responses occurred within 10 min after microinjection. The maximal reductions were 25.68 ± 3.26% in RSNA, 33.11 ± 6.49 mmHg in ABP, and 42.96 ± 8.25 bpm in HR in FK506-treated rats after microinjection of CNQX (Fig. 6B,D).
Figure 6. Glutamatergic input in the PVN sustains elevated sympathetic vasomotor tone in CIH.
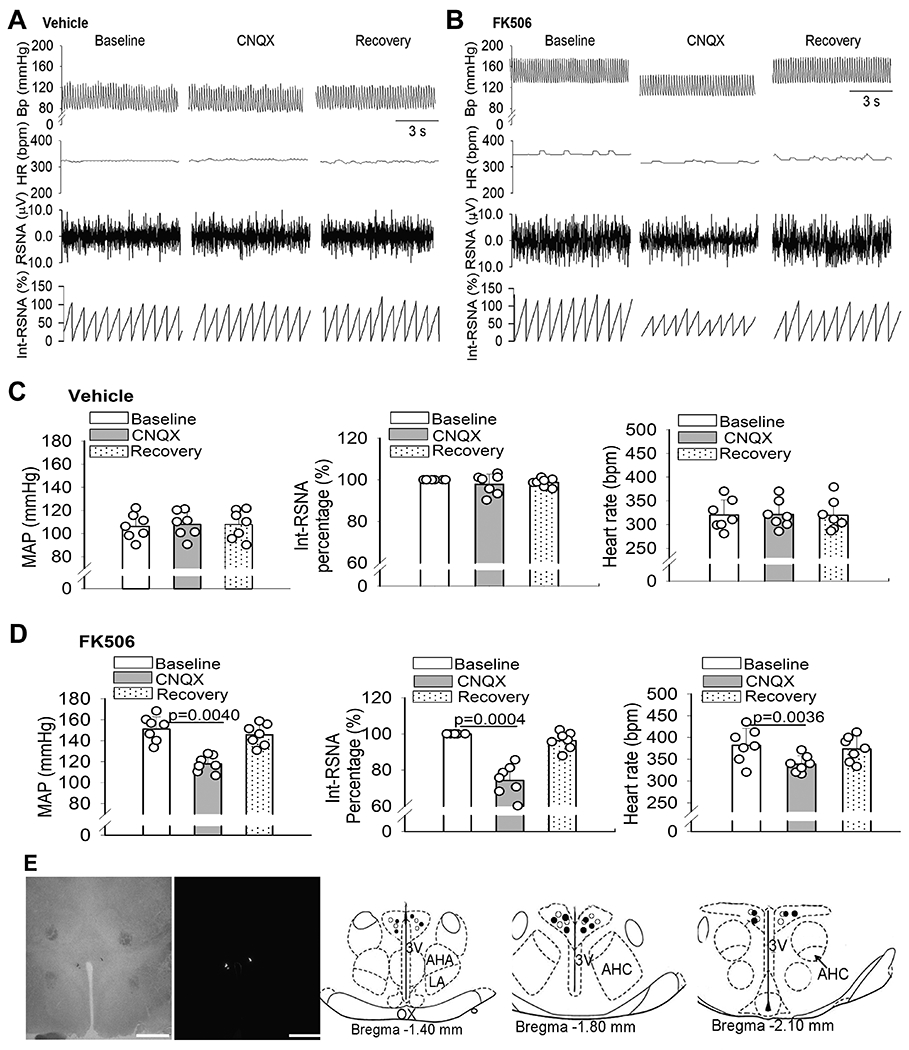
A-D, Original recording traces (A and B) and quantification (C and D) show the effects of microinjection of CNQX into the PVN on mean arterial blood pressure (MAP), heart rate (HR), and integrated renal sympathetic activity (Int-RSNA) in rats 7 days after discontinuing treatment with FK506 or vehicle (n = 7 rats per group). Repeated measures ANOVA followed by Dunnett’s post hoc test Data are expressed as mean ± SEM. E, Representative brightfield and fluorescence images and schematic drawings show the CNQX microinjection sites in the PVN in vehicle-treated (○) and FK506-treated rats (●). Scale bar, 1 mm. 3V, third ventricle; OX, optic chiasm; AHA, anterior hypothalamic area; LA, latero-anterior hypothalamus; AHC, central division of the anterior hypothalamus.
We next determined whether increased NMDAR activity in the PVN mediates elevated sympathetic output in CIH. Bilateral microinjection of AP5 (1.0 nmol, 50 nL)25 into the PVN had no significant effect on RSNA, HR, or ABP in 8 vehicle-treated rats (Figs. 7A,C,E). However, microinjection of the same amount of AP5 into the PVN significantly inhibited RSNA, HR, and ABP in 8 FK506-treated rats (Figs. 7B,D,E). The RSNA, HR, and ABP started to decrease 2.86 ± 0.51 min after AP5 microinjection in FK506-treated rats, and the peak responses of RSNA, ABP, and HR occurred within 10 min after microinjection. The maximal reductions were 19.63 ± 3.11% in RSNA, 22.03 ± 3.67 mmHg in ABP, and 42.32 ± 2.24 bpm in HR (Fig. 7D). These data suggest that CIH is associated with elevated sympathetic outflow, which is sustained by glutamatergic input mediated by NMDAR activity in the PVN. Thus, normal calcineurin activity constitutively suppresses synaptic NMDAR activity in the PVN and ensuing sympathetic outflow.
Figure 7. NMDAR activity in the PVN maintains high sympathetic outflow in CIH.
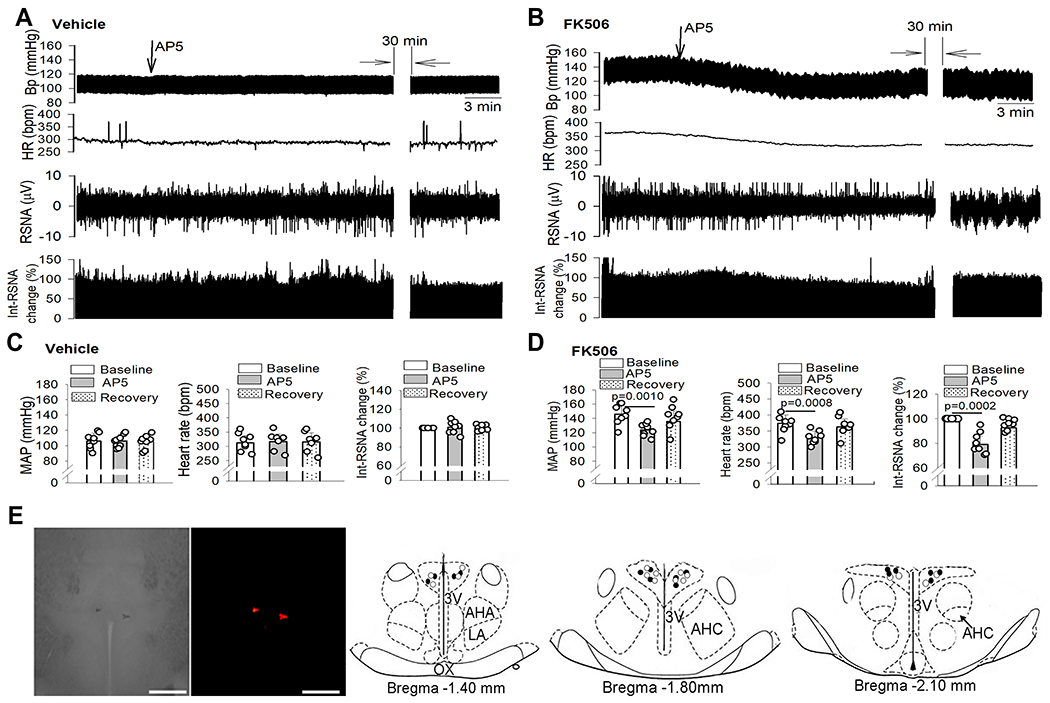
A-D, Original recording traces (A and B) and quantification (C and D) show the effects of microinjection of AP5 into the PVN on mean arterial blood pressure (MAP), heart rate (HR), and integrated renal sympathetic activity (Int-RSNA) in rats 7 days after discontinuing treatment with FK506 or vehicle (n = 8 rats per group). Repeated measures ANOVA followed by Dunnett’s post hoc test. Data are expressed as mean ± SEM. E, Representative brightfield and fluorescence images and schematic drawings show the AP5 microinjection sites in the PVN in vehicle-treated (○) and FK506-treated rats (●). Scale bar, 1 mm. 3V, third ventricle; OX, optic chiasm; AHA, anterior hypothalamic area; LA, latero-anterior hypothalamus; AHC, central division of the anterior hypothalamus.
Blocking NMDARs via systemic administration of memantine reduces CIH
To ascertain the potential clinical relevance of our findings, we determined whether targeting NMDARs via systemic treatment with an NMDAR antagonist is effective for CIH. Memantine is a clinically well-tolerated uncompetitive NMDAR antagonist for the treatment of Alzheimer disease with moderate affinity and rapid unblocking kinetics.55 We determined whether systemic administration of memantine is effective in lowering ABP in the rat model of CIH. A single intraperitoneal injection of memantine (10 mg/kg)56 had no significant effect on ABP or HR, measured with radiotelemetry, in conscious rats 7 days after repeated treatment with vehicle (n = 6 rats; Fig. 8A,B). In contrast, systemic treatment with memantine produced a significant reduction in ABP and HR in rats 7 days after repeated treatment with FK506 (n = 6 rats; Fig. 8A,B). The inhibitory effect of memantine occurred within 60 min, and the ABP returned to the baseline level 4 h after memantine injection.
Figure 8. Systemic treatment of memantine reduces CIH.
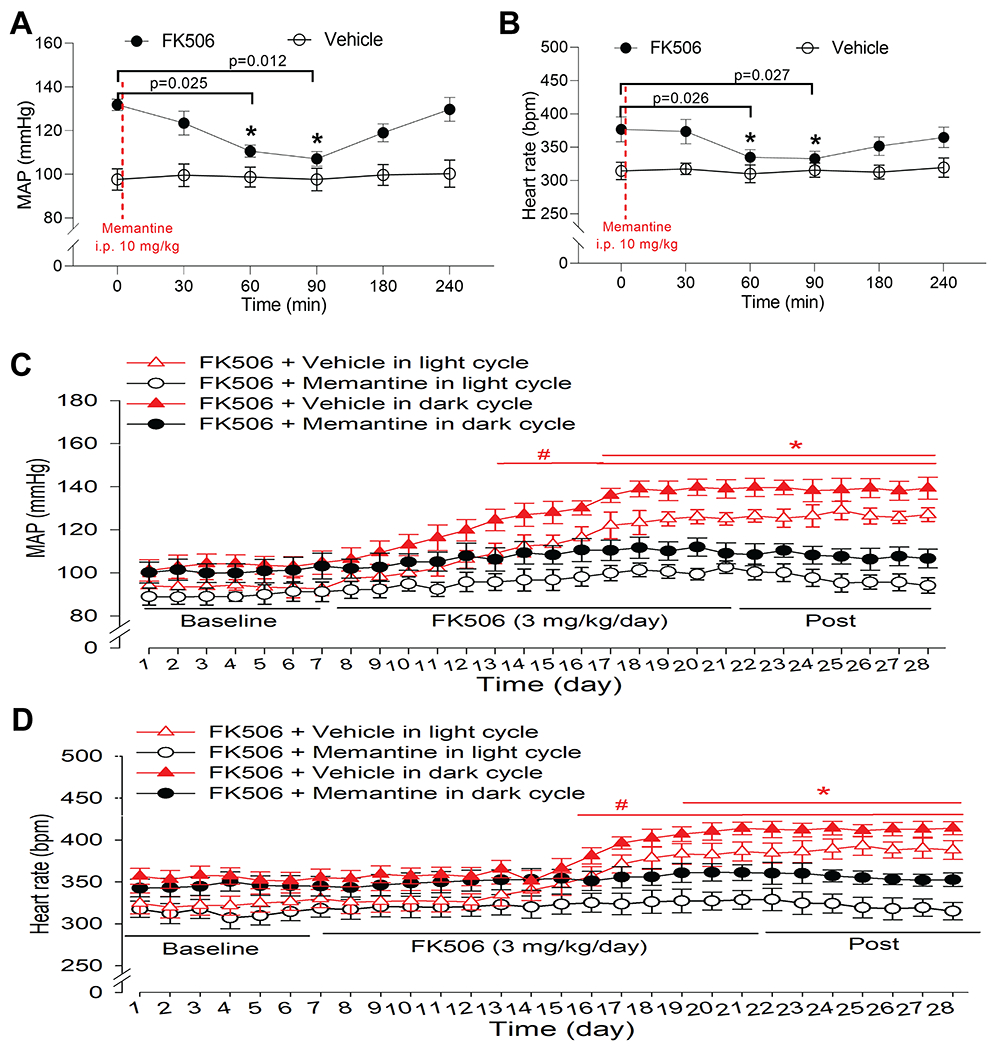
A and B, Time course of changes in mean arterial blood pressure (MAP, A) and heart rate (B), measured using telemetry in conscious rats, after a single intraperitoneal injection of memantine (10 mg/kg) 7 days after discontinuing treatment with FK506 (3 mg/kg/day for 14 days) or vehicle (n = 6 rats per group). Repeated measures ANOVA followed by Dunnett’s post hoc test. C and D, Time course of changes in MAP (C) and heart rate (D) recorded using radiotelemetry during light and dark cycles in conscious rats. Rats were cotreated with FK506 (3 mg/kg/day, intraperitoneal injection) and memantine (20 mg/kg/day in the drinking water) or vehicle for 14 days (n = 6 rats per group). Two-way ANOVA followed by Bonferroni’s post hoc test. Data are expressed as mean ± SEM.
Lastly, we determined whether concurrent treatment with memantine attenuates the development of hypertension induced by FK506. As expected, treatment with memantine (20 mg/kg/day56 in the drinking water) diminished the increase in ABP and HR caused by intraperitoneal injection of FK506 (3 mg/kg/day) for 14 days (n = 6 rats per group; Figs. 8C,D and S3). These results indicate that systemic treatment with the NMDAR antagonist effectively reduces hypertension induced by the calcineurin inhibitor.
DISCUSSION
Our study provides new evidence that sympathetic outflow in CIH is elevated and generated primarily by excitatory glutamatergic input to the PVN. Most previous studies examined the acute effect of calcineurin inhibitors on sympathetic nerve activity in animal models.9–12 Also, virtually nothing is known about the potential role of the brain in the development of CIH. In this study, we developed a clinically relevant animal model of CIH to determine how systemically administered calcineurin inhibitors cause persistent hypertension. We found that prolonged treatment with FK506 caused a progressive increase in ABP that was sustained long after FK506 treatment was ended. Calcineurin is the most abundant and the only Ca2+/calmodulin-dependent serine/threonine protein phosphatase in the nervous system. Consistent with extensive expression of calcineurin in the central nervous system,15 we showed that calcineurin activity in the forebrain regions was markedly reduced by FK506 treatment. Strikingly, coincide with persistent hypertension, the reduced calcineurin activity in the forebrain was still present 7 days after discontinuance of FK506 treatment. Consistent with previous reports,20, 57 our findings clearly indicate that calcineurin inhibitors can cross the BBB and cause extended impairment in normal calcineurin activity in the forebrain. We found that blocking glutamatergic input with the NMDAR or AMPAR antagonist in the PVN profoundly reduced ABP and sympathetic vasomotor tone potentiated by FK506 treatment. Thus, our study demonstrates for the first time that the central action of calcineurin inhibitors is the major pertinent mechanism underlying the pathogenesis of CIH.
A major finding of our study is that calcineurin critically regulates NMDAR phosphorylation in the PVN. Agents present in the systemic circulation can access the PVN via circumventricular organs, which do not have a well-formed BBB.58, 59 As a result, calcineurin activity in the PVN may be particularly susceptible to systemically administered calcineurin inhibitors. The PVN contains an abundance of glutamatergic nerve terminals and ionotropic glutamate receptors, including NMDARs and AMPARs.13, 26, 60 Protein phosphorylation by the coordinated activities of protein kinases and phosphatases is central to NMDAR regulation.17, 35, 61 Unknown is the specific phosphatase involved in the control of NMDAR activity in the PVN. Calcineurin serves as a molecular constraint on synaptic plasticity and memory62 and can negatively regulate NMDAR function in the hippocampus.17, 63 In the present study, we found that treatment with FK506 predominantly increased the phosphorylation level of the NMDAR subunit GluN1 in the PVN. These data are consistent with a previous report showing that microinjection of cyclosporine into the striatum increases GluN1 phosphorylation.64 We also showed that synaptic expression of NMDARs in the PVN is potentiated by FK505 treatment. Because phosphorylation plays a key role in the control of NMDAR trafficking,29, 52 calcineurin inhibition likely promotes synaptic trafficking of NMDARs via increased NMDAR phosphorylation in the PVN. Therefore, normal calcineurin activity constitutively suppresses synaptic expression of NMDARs by limiting their phosphorylation levels in the PVN.
Another important finding of our study is that calcineurin inhibition augments NMDAR activity at both presynaptic and postsynaptic sites in the PVN to cause hyperactivity of PVN presympathetic neurons. This is supported by our findings that the NMDAR-mediated frequency of glutamatergic mEPSCs and the amplitude of puff NMDA currents of presympathetic neurons in the PVN were significantly increased by treatment with FK506. We also showed that calcineurin inhibition with FK506 significantly increased the discharge activity of PVN presympathetic neurons, which was normalized by blocking NMDARs or AMPARs. Presynaptic NMDARs in the PVN are latent and not functionally active under normotensive conditions, but they become tonically activated to potentiate synaptic glutamate release in hypertensive conditions.25, 27, 29 The increased presynaptic glutamate release subsequently acts on postsynaptic NMDARs and AMPARs to increase the excitability of PVN presympathetic neurons in CIH. Thus, NMDARs and AMPARs have overlapping roles in augmenting glutamatergic input in the PVN in CIH.
Our study also documents a crucial role of the PVN in generating excess sympathetic outflow in CIH. We showed in this study that blocking NMDARs in the PVN normalized ABP and sympathetic nerve discharges elevated by FK506 treatment, indicating the functional significance of increased glutamatergic input in the PVN in maintaining high sympathetic outflow in CIH. Although glutamatergic input in the PVN normally is not involved in regulating basal ABP,22, 25, 29 it is a major source of excitatory drive that augments sympathetic outflow in spontaneously hypertensive rats, a genetic model of hypertension.25, 31 We found that blocking AMPARs with CNQX attenuated the firing activity of PVN presympathetic neurons in both control and FK506-treated rats. However, microinjection of CNQX into the PVN preferentially inhibited sympathetic vasomotor tone only in FK506-treated rats. Thus, it is the NMDAR-driven glutamatergic input that contributes predominantly to increased sympathetic outflow in CIH. Thus, our findings unravel the fundamental role of endogenous calcineurin in the PVN as a key “negative regulator” to tonically limit sympathetic outflow.
Because prolonged treatment with calcineurin inhibitors causes a general reduction in calcineurin activity in the forebrain, regions other than the PVN may be involved in augmenting sympathetic outflow in CIH. For example, circumventricular organs, including the SFO and OVLT, send projections to the PVN65, 66 and RVLM.67 Because treatment with FK506 markedly reduced calcineurin activity in circumventricular organs, this may provide additional excitatory glutamatergic input to the PVN and/or RVLM, contributing to elevated sympathetic outflow caused by calcineurin inhibitors in vivo. Furthermore, the PVN and RVLM are major loci of sympathetic-related neuronal network and that the RVLM is an important relay site in the descending presympathetic pathway from the PVN to the spinal cord.21, 23, 24 Although calcineurin activity in the RVLM was not reduced in this animal model of CIH, we found that treatment with FK506 similarly increased synaptic NMDAR activity of both spinally projecting and RVLM-projecting neurons in the PVN. Thus, the calcineurin inhibitor–induced NMDAR hyperactivity in the PVN likely augments sympathetic output via neuronal projections to both the RVLM and spinal cord. In addition to increased sympathetic nerve discharges, other mechanisms have been proposed to contribute to the development of CIH, such as vasoconstriction and renal sodium retention.68, 69 For example, cyclosporine-induced sodium retention seems to be caused by activation of the sympathetic nervous system, because renal denervation abrogates the sodium-retaining effect.10 Thus, calcineurin inhibitors may increase renal sympathetic nerve activity to cause renin secretion that activates the renin-angiotensin system, leading to sodium and water retention.70, 71 Nevertheless, CIH is still prevalent in patients with kidney transplants,4, 5 suggesting that the renal sympathetic nerve alone may not play a major role in CIH.
In conclusion, our study demonstrates for the first time that the PVN is a major source of excitatory drive for excess sympathetic outflow in CIH. Calcineurin inhibition primarily augments glutamatergic input via increasing phosphorylation and synaptic activity of NMDARs in the PVN, which results in sustained sympathetic vasomotor tone in CIH (Fig. S4). Therefore, our study provides a molecular framework for understanding the pathogenesis of CIH. Most organ transplant recipients have normal or low ABP, and even a moderate increase in ABP can greatly increase organ perfusion pressure and reduce allograft and patient survival.5, 72 Our findings also suggest that NMDARs could be targeted to reduce sympathetic output in patients with CIH. This notion is supported by our findings that systemic administration of memantine, an FDA-approved NMDAR antagonist, effectively attenuated hypertension caused by prolonged treatment with FK506. Further clinical studies are needed to validate the efficacy of memantine in treating CIH.
Supplementary Material
Novelty and Significance.
What Is Known?
Hypertension is a common and serious adverse effect that accompanies the prolonged use of calcineurin inhibitors, which are used as immunosuppressants in organ/tissue transplantation and in the treatment of autoimmune diseases.
Calcineurin inhibitors cause increased sympathetic nerve discharges in animal models and in patients.
What New Information Does This Article Contribute?
Repeated systemic treatment with tacrolimus (FK506) causes a gradual and long-lasting increase in arterial blood pressure and diminishes calcineurin activity in forebrain tissues.
Systemic treatment with FK506 augments phosphorylation, synaptic trafficking, and synaptic activity of N-methyl-D-aspartate (NMDA) receptors and potentiates NMDA receptor–dependent excitability of presympathetic neurons in the hypothalamus.
Blocking NMDA receptors in the hypothalamus reduces sympathetic nerve discharges and arterial blood pressure elevated by systemic treatment with FK506.
Summary of the novelty and significance
Calcineurin inhibitors, such as cyclosporine and tacrolimus, are immunosuppressants used clinically in organ/tissue transplantation and in the treatment of autoimmune disorders. However, prolonged use of these drugs often causes persistent hypertension, which reduces allograft and patient survival. Although increased sympathetic nerve activity is associated with calcineurin inhibitor–induced hypertension, little is known about where and how the sympathetic outflow is generated. Calcineurin is widely expressed in the brain, but its role in calcineurin inhibitor–induced hypertension has been overlooked. By using a new animal model, our study demonstrates for the first time the critical role of constitutive calcineurin activity in the brain, particularly the hypothalamus, in restraining sympathetic outflow and hypertension caused by calcineurin inhibitors. We showed that systemic treatment with tacrolimus diminishes calcineurin activity in various forebrain regions. Treatment with tacrolimus potentiates phosphorylation and synaptic trafficking and activity of glutamate NMDA receptors to increase the firing activity of sympathetic-related neurons in the hypothalamus. Importantly, memantine, a clinically available NMDA receptor antagonist, effectively reduces hypertension induced by tacrolimus. Therefore, our findings not only reveal the molecular substrates of increased sympathetic outflow by calcineurin inhibitors but also suggest NMDA receptors as a new therapeutic target for treating calcineurin inhibitor–induced hypertension.
Sources of Funding
This study was supported by grants (HL154512 and HL142133) from the National Institutes of Health and by the N.G. and Helen T. Hawkins Endowment.
Nonstandard Abbreviations and Acronyms:
- ABP
arterial blood pressure
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- AP5
2-amino-5-phosphonopentanoic acid
- aCSF
artificial cerebrospinal fluid
- BBB
blood-brain barrier
- CIH
calcineurin inhibitor–induced hypertension
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CTB
cholera toxin subunit-B
- HR
heart rate
- mEPSC
miniature excitatory postsynaptic current
- NMDAR
N-methyl-D-aspartate receptor
- OVLT
organum vasculosum laminae terminalis
- PVN
paraventricular nuclear
- RSNA
renal sympathetic nerve activity
- RVLM
rostral ventrolateral medulla
- SFO
subfornical organ
Footnotes
Disclosures/Conflict of interest
The authors declare no competing financial interest.
REFERENCES
- 1.Loughran TP Jr., Deeg HJ, Dahlberg S, Kennedy MS, Storb R and Thomas ED. Incidence of hypertension after marrow transplantation among 112 patients randomized to either cyclosporine or methotrexate as graft-versus-host disease prophylaxis. Br J Haematol. 1985;59:547–53. [DOI] [PubMed] [Google Scholar]
- 2.Snanoudj R, Kriaa F, Arzouk N, Beaudreuil S, Hiesse C, Durrbach A and Charpentier B. Single-center experience with cyclosporine therapy for kidney transplantation: analysis of a twenty-year period in 1200 patients. Transplant Proc. 2004;36:83s–88s. [DOI] [PubMed] [Google Scholar]
- 3.Taler SJ, Textor SC, Canzanello VJ and Schwartz L. Cyclosporin-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437–49. [DOI] [PubMed] [Google Scholar]
- 4.Textor SC, Taler SJ, Canzanello VJ, Schwartz L and Augustine JE. Posttransplantation hypertension related to calcineurin inhibitors. Liver Transpl. 2000;6:521–30. [DOI] [PubMed] [Google Scholar]
- 5.Hoorn EJ, Walsh SB, McCormick JA, Zietse R, Unwin RJ and Ellison DH. Pathogenesis of calcineurin inhibitor-induced hypertension. J Nephrol. 2012;25:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koomans HA and Ligtenberg G. Mechanisms and consequences of arterial hypertension after renal transplantation. Transplantation. 2001;72:S9–12. [DOI] [PubMed] [Google Scholar]
- 7.Kasiske BL, Anjum S, Shah R, Skogen J, Kandaswamy C, Danielson B, O’Shaughnessy EA, Dahl DC, Silkensen JR, Sahadevan M and Snyder JJ. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43:1071–81. [DOI] [PubMed] [Google Scholar]
- 8.Scherrer U, Vissing SF, Morgan BJ, Rollins JA, Tindall RS, Ring S, Hanson P, Mohanty PK and Victor RG. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323:693–9. [DOI] [PubMed] [Google Scholar]
- 9.Chiu PJ, Vemulapalli S, Sabin C, Rivelli M, Bernardino V and Sybertz EJ. Sympathoadrenal stimulation, not endothelin, plays a role in acute pressor response to cyclosporine in anesthetized rats. J Pharmacol Exp Ther. 1992;261:994–9. [PubMed] [Google Scholar]
- 10.Moss NG, Powell SL and Falk RJ. Intravenous cyclosporine activates afferent and efferent renal nerves and causes sodium retention in innervated kidneys in rats. Proc Natl Acad Sci U S A. 1985;82:8222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyson T, Ermel LD, Belshaw PJ, Alberg DG, Schreiber SL and Victor RG. Cyclosporine- and FK506-induced sympathetic activation correlates with calcineurin-mediated inhibition of T-cell signaling. Circ Res. 1993;73:596–602. [DOI] [PubMed] [Google Scholar]
- 12.Morgan BJ, Lyson T, Scherrer U and Victor RG. Cyclosporine causes sympathetically mediated elevations in arterial pressure in rats. Hypertension. 1991;18:458–66. [DOI] [PubMed] [Google Scholar]
- 13.Li DP, Byan HS and Pan HL. Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci. 2012;32:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takaishi T, Saito N, Kuno T and Tanaka C. Differential distribution of the mRNA encoding two isoforms of the catalytic subunit of calcineurin in the rat brain. Biochem Biophys Res Commun. 1991;174:393–8. [DOI] [PubMed] [Google Scholar]
- 15.Kuno T, Mukai H, Ito A, Chang CD, Kishima K, Saito N and Tanaka C. Distinct cellular expression of calcineurin A alpha and A beta in rat brain. J Neurochem. 1992;58:1643–51. [DOI] [PubMed] [Google Scholar]
- 16.Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF and Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–29. [DOI] [PubMed] [Google Scholar]
- 17.Tong G, Shepherd D and Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–2. [DOI] [PubMed] [Google Scholar]
- 18.Dohgu S, Sumi N, Nishioku T, Takata F, Watanabe T, Naito M, Shuto H, Yamauchi A and Kataoka Y. Cyclosporin A induces hyperpermeability of the blood-brain barrier by inhibiting autocrine adrenomedullin-mediated up-regulation of endothelial barrier function. Eur J Pharmacol. 2010;644:5–9. [DOI] [PubMed] [Google Scholar]
- 19.Takata F, Dohgu S, Yamauchi A, Sumi N, Nakagawa S, Naito M, Tsuruo T, Shuto H and Kataoka Y. Inhibition of transforming growth factor-beta production in brain pericytes contributes to cyclosporin A-induced dysfunction of the blood-brain barrier. Cell Mol Neurobiol. 2007;27:317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami Y, Takamatsu H, Noda A, Osoda K, Ichise R, Tatsumi M, Tabata K, Sawamoto T and Nishimura S. Pharmacokinetic animal PET study of FK506 as a potent neuroprotective agent. J Nucl Med. 2004;45:1946–9. [PubMed] [Google Scholar]
- 21.Strack AM and Loewy AD. Pseudorabies virus: a highly specific transneuronal cell body marker in the sympathetic nervous system. J Neurosci. 1990;10:2139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dampney RA, Michelini LC, Li DP and Pan HL. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am J Physiol Heart Circ Physiol. 2018;315:H1200–H1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranson RN, Motawei K, Pyner S and Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res. 1998;120:164–72. [DOI] [PubMed] [Google Scholar]
- 24.Pyner S and Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–56. [DOI] [PubMed] [Google Scholar]
- 25.Li DP and Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007;49:916–25. [DOI] [PubMed] [Google Scholar]
- 26.Li DP, Yang Q, Pan HM and Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol. 2008;586:1637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou JJ, Shao JY, Chen SR, Li DP and Pan HL. α2δ-1-dependent NMDA receptor activity in the hypothalamus is an effector of genetic-environment interactions that drive persistent hypertension. J Neurosci. 2021;41:6551–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li YF, Cornish KG and Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res. 2003;93:990–7. [DOI] [PubMed] [Google Scholar]
- 29.Li DP, Zhou JJ, Zhang J and Pan HL. CaMKII regulates synaptic NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Neurosci. 2017;37:10690–10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao X, Zhou JJ, Li DP and Pan HL. Src kinases regulate glutamatergic input to hypothalamic presympathetic neurons and sympathetic outflow in hypertension. Hypertension. 2017;69:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma H, Chen SR, Chen H, Zhou JJ, Li DP and Pan HL. α2δ-1 couples to NMDA receptors in the hypothalamus to sustain sympathetic vasomotor activity in hypertension. J Physiol. 2018;596:4269–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin Y, Gao D, Wang Y, Wang ZH, Wang X, Ye J, Wu D, Fang L, Pi G, Yang Y, Wang XC, Lu C, Ye K and Wang JZ. Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling. Proc Natl Acad Sci U S A. 2016;113:E3773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gooch JL, Toro JJ, Guler RL and Barnes JL. Calcineurin A-α but not A-β is required for normal kidney development and function. Am J Pathol. 2004;165:1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou JJ, Shao JY, Chen SR, Chen H and Pan HL. α2δ-1 protein promotes synaptic expression of Ca(2+) permeable-AMPA receptors by inhibiting GluA1/GluA2 heteromeric assembly in the hypothalamus in hypertension. J Neurochem. 2022;161:40–52. [DOI] [PubMed] [Google Scholar]
- 35.Ma H, Chen SR, Chen H and Pan HL. Endogenous AT1 receptor-protein kinase C activity in the hypothalamus augments glutamatergic input and sympathetic outflow in hypertension. J Physiol. 2019;597:4325–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J, Li L, Chen SR, Chen H, Xie JD, Sirrieh RE, MacLean DM, Zhang Y, Zhou MH, Jayaraman V and Pan HL. The α2δ-1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep. 2018;22:2307–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li DP, Atnip LM, Chen SR and Pan HL. Regulation of synaptic inputs to paraventricular-spinal output neurons by alpha2 adrenergic receptors. J Neurophysiol. 2005;93:393–402. [DOI] [PubMed] [Google Scholar]
- 38.Zhou JJ, Pachuau J, Li DP, Chen SR and Pan HL. Group III metabotropic glutamate receptors regulate hypothalamic presympathetic neurons through opposing presynaptic and postsynaptic actions in hypertension. Neuropharmacology. 2020;174:108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li DP, Chen SR and Pan HL. Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic GABA release. J Neurophysiol. 2002;88:2664–74. [DOI] [PubMed] [Google Scholar]
- 40.Li DP, Chen SR and Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003;23:5041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen SR, Zhu L, Chen H, Wen L, Laumet G and Pan HL. Increased spinal cord Na(+)-K(+)-2Cl(−) cotransporter-1 (NKCC1) activity contributes to impairment of synaptic inhibition in paclitaxel-induced neuropathic pain. J Biol Chem. 2014;289:31111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye ZY, Li DP, Byun HS, Li L and Pan HL. NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension. J Neurosci. 2012;32:8560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen SR, Chen H, Zhou JJ, Pradhan G, Sun Y, Pan HL and Li DP. Ghrelin receptors mediate ghrelin-induced excitation of agouti-related protein/neuropeptide Y but not pro-opiomelanocortin neurons. J Neurochem. 2017;142:512–520. [DOI] [PubMed] [Google Scholar]
- 44.Ye ZY, Li DP and Pan HL. regulation of hypothalamic presympathetic neurons and sympathetic outflow by group II metabotropic glutamate receptors in spontaneously hypertensive rats. Hypertension. 2013;62:255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryuzaki M, Stahl LK, Lyson T, Victor RG and Bishop VS. Sympathoexcitatory response to cyclosporin A and baroreflex resetting. Hypertension. 1997;29:576–82. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Li JL, Hosaka M, Janz R, Shelton JM, Albright GM, Richardson JA, Südhof TC and Victor RG. Cyclosporine A-induced hypertension involves synapsin in renal sensory nerve endings. Proc Natl Acad Sci U S A. 2000;97:9765–9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiasson VL, Talreja D, Young KJ, Chatterjee P, Banes-Berceli AK and Mitchell BM. FK506 binding protein 12 deficiency in endothelial and hematopoietic cells decreases regulatory T cells and causes hypertension. Hypertension. 2011;57:1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hošková L, Málek I, Kautzner J, Honsová E, van Dokkum RP, Husková Z, Vojtíšková A, Varcabová S, Cervenka L and Kopkan L. Tacrolimus-induced hypertension and nephrotoxicity in Fawn-Hooded rats are attenuated by dual inhibition of renin-angiotensin system. Hypertens Res. 2014;37:724–32. [DOI] [PubMed] [Google Scholar]
- 49.Murase N, Starzl TE, Demetris AJ, Valdivia L, Tanabe M, Cramer D and Makowka L. Hamster-to-rat heart and liver xenotransplantation with FK506 plus antiproliferative drugs. Transplantation. 1993;55:701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeda Y, Miyamori I, Furukawa K, Inaba S and Mabuchi H. Mechanisms of FK 506-induced hypertension in the rat. Hypertension. 1999;33:130–6. [DOI] [PubMed] [Google Scholar]
- 51.Chatterjea D, Hamid E, Leonard JP and Alford S. Phosphorylation-state-dependent regulation of NMDA receptor short-term plasticity modifies hippocampal dendritic Ca2+ transients. J Neurophysiol. 2010;104:2203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou MH, Chen SR, Wang L, Huang Y, Deng M, Zhang J, Zhang J, Chen H, Yan J and Pan HL. Protein kinase C-mediated phosphorylation and α2δ-1 interdependently regulate NMDA receptor trafficking and activity. J Neurosci. 2021;41:6415–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norris CM, Kadish I, Blalock EM, Chen K-C, Thibault V, Porter NM, Landfield PW and Kraner SD. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and alzheimer’s models. J Neurosci. 2005;25:4649–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sulzer D and Pothos EN. Regulation of quantal size by presynaptic mechanisms. Rev Neurosci. 2000;11:159–212. [DOI] [PubMed] [Google Scholar]
- 55.Farlow MR and Cummings JL. Effective pharmacologic management of Alzheimer’s disease. Am J Med. 2007;120:388–97. [DOI] [PubMed] [Google Scholar]
- 56.Chen SR, Samoriski G and Pan HL. Antinociceptive effects of chronic administration of uncompetitive NMDA receptor antagonists in a rat model of diabetic neuropathic pain. Neuropharmacology. 2009;57:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gottschalk S, Cummins CL, Leibfritz D, Christians U, Benet LZ and Serkova NJ. Age and sex differences in the effects of the immunosuppressants cyclosporine, sirolimus and everolimus on rat brain metabolism. Neurotoxicology. 2011;32:50–7. [DOI] [PubMed] [Google Scholar]
- 58.Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology. 2009;89:370–6. [DOI] [PubMed] [Google Scholar]
- 59.Pan HL. Brain angiotensin II and synaptic transmission. Neuroscientist. 2004;10:422–31. [DOI] [PubMed] [Google Scholar]
- 60.Herman JP, Eyigor O, Ziegler DR and Jennes L. Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat. J Comp Neurol. 2000;422:352–62. [PubMed] [Google Scholar]
- 61.Li DP, Zhou JJ and Pan HL. Endogenous casein kinase-1 modulates NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Physiol. 2015;593:4439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y, Fischer QS, Zhang Y, Baumgartel K, Mansuy IM and Daw NW. Reversible blockade of experience-dependent plasticity by calcineurin in mouse visual cortex. Nat Neurosci. 2005;8:791–6. [DOI] [PubMed] [Google Scholar]
- 63.Lieberman DN and Mody I. Regulation of NMDA channel function by endogenous Ca2+-dependent phosphatase. Nature. 1994;369:235–239. [DOI] [PubMed] [Google Scholar]
- 64.Choe ES, Shin EH and Wang JQ. Inhibition of protein phosphatase 2B upregulates serine phosphorylation of N-methyl-D-aspartate receptor NR1 subunits in striatal neurons in vivo. Neurosci Lett. 2005;384:38–43. [DOI] [PubMed] [Google Scholar]
- 65.Johnson AK and Gross PM. Sensory circumventricular organs and brain homeostatic pathways. Faseb J. 1993;7:678–86. [DOI] [PubMed] [Google Scholar]
- 66.Kawano H and Masuko S. Region-specific projections from the subfornical organ to the paraventricular hypothalamic nucleus in the rat. Neuroscience. 2010;169:1227–34. [DOI] [PubMed] [Google Scholar]
- 67.Stocker SD, Wenner MM, Farquhar WB and Browning KN. Activation of the organum vasculosum of the lamina terminalis produces a sympathetically mediated hypertension. Hypertension. 2022;79:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyer-Lehnert H and Schrier RW. Potential mechanism of cyclosporine A-induced vascular smooth muscle contraction. Hypertension. 1989;13:352–60. [DOI] [PubMed] [Google Scholar]
- 69.Curtis JJ, Luke RG, Jones P and Diethelm AG. Hypertension in cyclosporine-treated renal transplant recipients is sodium dependent. Am J Med. 1988;85:134–8. [DOI] [PubMed] [Google Scholar]
- 70.DiBona GF. Sympathetic nervous system influences on the kidney. Role in hypertension. Am J Hypertens. 1989;2:119s–124s. [DOI] [PubMed] [Google Scholar]
- 71.Pontes RB, Crajoinas RO, Nishi EE, Oliveira-Sales EB, Girardi AC, Campos RR and Bergamaschi CT. Renal nerve stimulation leads to the activation of the Na+/H+ exchanger isoform 3 via angiotensin II type I receptor. Am J Physiol Renal Physiol. 2015;308:F848–56. [DOI] [PubMed] [Google Scholar]
- 72.Mange KC, Cizman B, Joffe M and Feldman HI. Arterial hypertension and renal allograft survival. JAMA. 2000;283:633–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Detailed methods can be found in the Supplemental Material.


