Abstract
Objective:
The mechanisms through which cognitive-behavioral therapies (CBTs) for ARFID may work have yet to be elucidated. To inform future treatment revisions to increase parsimony and potency of CBT for ARFID (CBT-AR), we evaluated change in food neophobia during CBT-AR treatment of a sensory sensitivity ARFID presentation via a single case study.
Method:
An adolescent male completed 21, twice-weekly sessions of CBT-AR via live video delivery. From pre- to mid- to post-treatment and at 2-month follow-up, we calculated percent change in food neophobia and ARFID symptom severity measures. Via visual inspection, we explored trajectories of week-by-week food neophobia in relation to clinical improvements (e.g., when the patient incorporated foods into daily life).
Results:
By post-treatment, the patient achieved reductions across food neophobia (45%), and ARFID severity (53–57%) measures and no longer met criteria for ARFID, with sustained improvement at 2-month follow-up. Via visual inspection of week-by-week food neophobia trajectories, we identified that decreases occurred after mid-treatment and were associated with incorporation of a food directly tied to the patient’s main treatment motivation.
Discussion:
This study provides hypothesis-generating findings on candidate CBT-AR mechanisms, showing that changes in food neophobia were related to food exposures most connected to the patient’s treatment motivations.
Keywords: feeding and eating disorders, avoidant/restrictive food intake disorder, ARFID, telemedicine, video therapy, cognitive-behavioral therapy, food neophobia, exposure, case report
INTRODUCTION
Outpatient cognitive-behavioral therapy (CBT) approaches for avoidant/restrictive food intake disorder (ARFID) have been studied for children, adolescents, and adults, including a manualized 20- to 30-session CBT for ARFID (CBT-AR; Thomas and Eddy, 2019). CBTs for ARFID have been shown to be feasible and acceptable, with promising results for efficacy (Dumont, Jansen, Kroes, de Haan, & Mulkens, 2019; Thomas et al., 2021; Thomas, Becker, et al., 2020). Mechanisms through which CBTs for ARFID may work have yet to be elucidated.
Mechanisms of action are characteristics changed by interventions that then lead to symptom improvement (Kazdin, 2007). Understanding which treatment constituents lead to change in specific mechanisms (called target engagement; Kraemer, Wilson, Fairburn, and Agras, 2002) is crucial to inform treatment parsimony (Guastaferro & Collins, 2019). In CBT-AR, exposure is hypothesized to decrease food neophobia (i.e., fear of trying new foods), which in turn decreases food avoidance. In two single-arm clinical trials, there were large and significant pre- to post-treatment reductions in the hypothesized mechanism (food neophobia) and the target outcome (ARFID severity), and these changes were highly correlated (Thomas et al., 2021; Thomas, Becker, et al., 2020). However, because food neophobia was not measured at every session, the reasons for when and why food neophobia change in CBT-AR remain unknown. Identification of whether changes in food neophobia is a plausible mechanism could inform refinements to make CBT-AR briefer, more potent, and easier to disseminate.
We aimed to evaluate change in food neophobia during CBT-AR for the ARFID sensory sensitivity presentation via a single case study. In the following case, an adolescent male (“Sam”) was offered 21, twice weekly video-delivered sessions of CBT-AR. We explored via visual inspection whether change in weekly measured food neophobia aligned with clinical improvements such as incorporation of foods into daily life. We also reported changes in ARFID symptom severity by questionnaires from pre- to mid- to post-treatment and at 2-month follow-up.
METHOD
See Supplementary Methods for more information.
Ethics
The MassGeneralBrigham Human Research Committee did not require ethical review for the publication of Sam’s anonymized case data. Sam provided permission for and approved the report.
Participant
Sam was a 16-year-old male who presented to an outpatient eating disorder clinic with concerns that his eating behaviors were a burden to his friends and family. Not only was he limited by the few foods he felt comfortable eating, even the smell or appearance of non-preferred foods decreased his appetite. He skipped activities or events with others because his preferred foods might be unavailable. When he could not find anything to eat at an Italian restaurant where he took his girlfriend for a first date, he decided he had to seek help to expand his dietary variety.
Sam’s daily diet consisted primarily of cheese pizza, French fries, macaroni and cheese, grilled cheese, plain bagels, and chicken nuggets. He explained that he could, “with a gun to my head” eat celery, cucumbers, baby carrots, apples, oranges, grapes, raspberries, strawberries, watermelon, cantaloupe, peanuts, and cashews.
Sam met DSM-5 criteria for ARFID due to psychosocial impairment (e.g., difficulty eating with others, avoidance of social eating). His weight was 145lbs and height was 5’10” (BMI=20.8 kg/m2; 49th percentile), with no weight loss and no nutritional deficiencies. He had no history of other psychological disorders and had not sought prior treatment for his eating difficulties.
Treatment
Four stages comprise manualized CBT-AR—Stage 1 includes psychoeducation, self-monitoring, and early behavioral changes (tailored to the patient’s ARFID presentation); Stage 2 includes identifying foods for exposure; Stage 3 includes food exposures in and between sessions aligned with primary ARFID maintaining mechanisms (i.e., sensory sensitivity in Sam’s case); Stage 4 involves maintenance/relapse prevention (Thomas and Eddy, 2019). Sam’s treatment focused on expanding food variety via exposures and was conducted primarily individually with some parental support for purchasing and preparing foods for exposures. CBT-AR sessions were twice weekly conducted via live video through a Health Insurance Portability and Accountability Act-approved platform.
Measures
All measures were captured as part of routine clinical care. Weekly and at 2-month follow-up: the Food Neophobia Scale (FNS) which measures fear of trying new foods (Pliner & Hobden, 1992). At the beginning of treatment, mid-treatment, post-treatment, and 2-month follow-up: the Nine Item ARFID Screen (NIAS) (Zickgraf & Ellis, 2018) and the Pica, ARFID, Rumination Disorder Interview—Questionnaire (PARDI-AR-Q), which both measure ARFID severity (Thomas, Eddy, et al., 2020).
Data Analysis
We descriptively reported percent changes in scores across treatment (pre-, mid-, and post-treatment) and at a 2-month follow-up. We plotted week-by-week FNS scores and used visual inspection to identify when and why changes in FNS scores occurred.
RESULTS
Overall Self-Report Measure Changes
Table 1 displays self-report measures at pre-treatment, mid-treatment, post-treatment, and 2-month follow-up. At pre-treatment, Sam scored 67/70 on the FNS, 15/15 on the NIAS-picky eating, and screened positive for ARFID by the PARDI-ARQ, reporting difficulty eating with others, eating in social situations, and sensory-based avoidance. At mid-treatment, minimal decreases were seen in FNS (9%) and NIAS (13%); the PARDI-ARQ ARFID severity and sensory-based avoidance decreased, but Sam still screened positive for ARFID due to no change in social eating difficulty. At post-treatment, Sam no longer screened positive for ARFID on the PARDI-AR-Q, and had large decreases in the FNS, NIAS, and PARDI-ARQ (45–62%), which were all sustained at 2-month follow-up.
Table 1.
Changes in Self-Report Measures
| Measure | Pre-Treatment | Mid-Treatment (Week 6) | Post-Treatment1 (Week 10) |
2-month Follow-up1 | |
|---|---|---|---|---|---|
| Scale | Food Neophobia Scale (range=10–70)2 | 67 | 61 (9%) | 37 (45%) | 43 (34%) |
| Item 1 | I am constantly sampling new and different foods | 7 | 5 | 2 | 5 |
| Item 2 | I don’t trust new foods | 6 | 6 | 3 | 3 |
| Item 3 | If I don’t know what is in a food, I won’t try it | 7 | 6 | 2 | 2 |
| Item 4 | I like foods from different countries | 6 | 7 | 4 | 4 |
| Item 5 | I find ethnic food too weird to eat | 7 | 6 | 6 | 5 |
| Item 6 | At dinner parties, I will try a new food | 7 | 5 | 2 | 2 |
| Item 7 | I am afraid to eat things I have never had before | 6 | 6 | 5 | 5 |
| Item 8 | I am very particular about the foods I will eat | 7 | 6 | 3 | 5 |
| Item 9 | I will eat almost anything | 7 | 7 | 4 | 6 |
| Item 10 | I like to try new ethnic restaurants | 7 | 7 | 6 | 6 |
| Nine Item ARFID Screen 3 | |||||
| Subscale | Picky eating (range=0–15) | 15 | 13 (13%) | 7 (53%) | 10 (33%) |
| Item 1 | I don’t like to try new foods4 | 5 | 4 | 1 | 3 |
| Item 2 | I dislike most foods that other people eat easily | 5 | 4 | 3 | 3 |
| Item 3 | The list of foods that I will eat is shorter than the list of foods I won’t eat | 5 | 5 | 3 | 4 |
| Subscale | Appetite (range=0–15) | 3 | 0 | 0 | 0 |
| Subscale | Fear (range=0–15) | 0 | 0 | 0 | 0 |
| PARDI-AR-Q 5 | |||||
| Algorithm | ARFID diagnosis6 | Yes | Yes | No | No |
| Subscale | ARFID severity (range=0–6) | 3.5 | 2.5 (29%) | 1.5 (57%) | 1.5 (57%) |
| Item 22 | Difficult interactions with people | 2 | 0 | 0 | 0 |
| Item 23 | Difficult social situations | 5 | 5 | 3 | 3 |
| Subscale | Sensory-based avoidance (range=0–6) | 5.3 | 3.3 (38%) | 2.0 (62%) | 1.3 (75%) |
| Item 24 | Sensitivity to taste | 4 | 2 | 2 | 1 |
| Item 25 | Sensitivity to texture/consistency | 6 | 5 | 3 | 2 |
| Item 26 | Sensitivity to appearance | 6 | 3 | 1 | 1 |
| Subscale | Lack of interest in food and eating (range=0–6) | 0.7 | 0.3 | 0.3 | 0.0 |
| Subscale | Concern about aversive consequences (range=0–6) | 0.0 | 0.0 | 0.0 | 0.0 |
Note. PARDI-AR-Q=Pica, ARFID, and Rumination Disorder Interview-ARFID Questionnaire; ARFID=avoidant/restrictive food intake disorder. Where indicated, percent change represents percent change from pre-treatment.
Items 1, 4, 6, 9, and 10 are scored agree extremely (1) to disagree extremely (7). Items 2, 3, 5, 7, and 8 are scored disagree extremely (1) to agree extremely (7).
Items are scored strongly disagree (0) to strongly agree (5 with three subscales (each with three items, scored 0–15) that have validated cutoffs to detect likely ARFID —picky eating ≥10, appetite ≥9, fear ≥10 (Burton Murray et al., 2021). The patient did not score highly on the NIAS appetite or fear subscales, but had the highest possible score on the picky eating subscale; thus, individual items are only presented for the picky eating subscale.
Suggested item to replace the originally validated NIAS item 1 (“I am a picky eater”) via personal communication with the measure creator on 10/17/2017.
The patient did not score highly on the lack of interest and fear of aversive consequences subscales; thus, individual items are only presented for the sensory-based avoidance subscale.
The PARDI-ARQ screening algorithm maps onto DSM-5 criteria—if the respondent endorses any of these subcriteria and endorses that eating is a significant problem in their life, the PARDI-AR-Q algorithm produces a positive screen response, indicating likely ARFID and the need for a diagnostic interview.
Weekly Treatment Changes
Figure 1 displays week-by-week changes in the FNS to explore when and why changes in ARFID symptoms occurred. In Week 1, food intake monitoring, CBT-AR model of ARFID education, personalized functional model, and small flexibility challenges (e.g., re-introduction of dropped foods) were completed. In Week 2, Sam started a plan for food exposures by identifying foods he was willing to try, identifying a list of 85 foods. By the end of Week 2, he completed his first in-session exposure (using the CBT-AR 5 Steps for tasting new foods) with mango, lettuce, and angel hair pasta with butter. Figure 1 indicates ranges of time during which Sam was continuing to try particular foods. At the final Session (Week 10), the clinician and Sam collaboratively reviewed his progress and made a maintenance plan.
Figure 1.
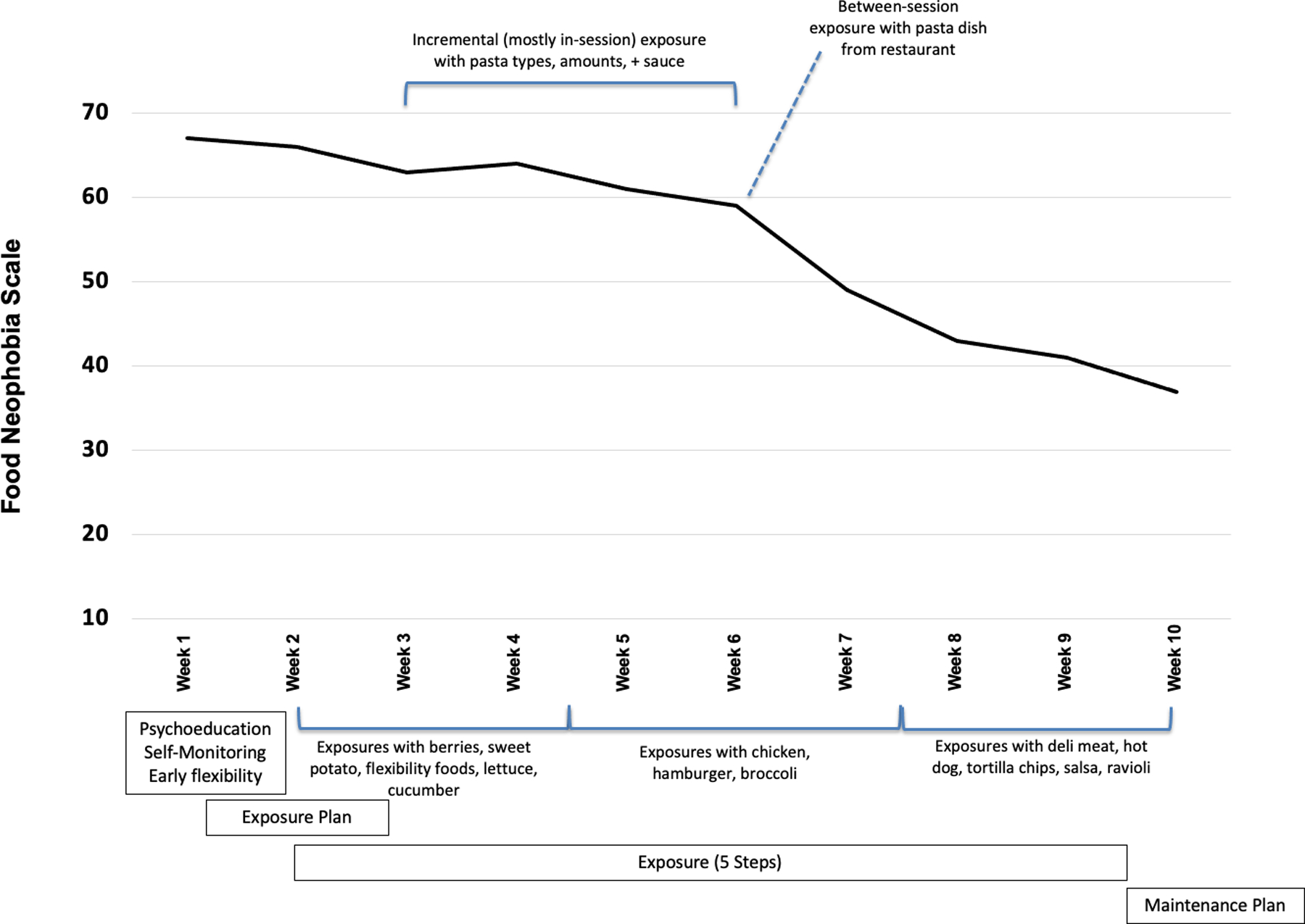
Weekly Change in the Food Neophobia Scale
Note. Pre-treatment period was 8 weeks, treatment period was 10 weeks, and post-treatment period was 8 weeks.
Visual inspection of the FNS score trajectory revealed that scores were relatively stable until Week 4. There was a small score decrease between Week 4 and Week 6 with a noticeably steeper decrease in the trajectory after Week 6. To explain why the trajectory changed after Week 6, we reviewed session notes.
Until Week 6, Sam tried a mix of foods within and between sessions that he had some familiarity with (e.g., fruits and vegetables) and within session tasted small amounts of different types of pastas and sauces—the foods that he had difficulty eating while out on a date and led him to pursue treatment. At Week 5, he ate a half serving of spaghetti with butter and at Week 6, he ate a full serving of penne with marinara sauce. After this, he was able to eat a full rigatoni pasta dish with marinara sauce. Prior to Week 6, all but two of Sam’s between-session exposures were with fruits/vegetables. After Week 6, both in- and between-session exposures focused on foods more distinct from his previous eating habits including variations on proteins (e.g., breaded chicken, chicken sandwich), as well as other pastas (e.g., ravioli).
DISCUSSION
We described CBT-AR for an adolescent male (Sam) with a sensory sensitivity presentation of ARFID due to psychosocial impairment. Sam achieved reductions across all ARFID measures pre- to post-treatment with sustained changes at 2-month follow-up. Notably between post-treatment and 2-month follow-up, scores increased on the FNS by 6 and on the NIAS—picky by 3, possible consequent of not being in treatment (e.g., FNS items 1 and 9 may reflect no longer doing planned exposures) or related to trait-based picky eating (e.g., NIAS item 1, FNS item 8). However, we identified an association between clinically meaningful change and willingness to try new foods (via decrease in FNS scores) due to Sam incorporating a new food (pasta) that was directly relevant to his main motivation for treatment. We view these findings as hypothesis-generating to examine CBT-AR mechanisms of action. We believe our findings are clinically relevant, suggesting that food exposures may be most potent when clearly connected to a patient’s treatment motivations.
Sam’s primary goal in treatment was to increase his ability to eat socially, after having difficulty eating at an Italian restaurant. The first three weeks of treatment focused on exposure to foods similar to the foods he could already eat. Between pre- and mid-treatment (Week 6) there was minimal change in the NIAS—picky subscale (13% decrease) and the FNS (9% decrease). On the PARDI-AR-Q, there was notable change in the sensory-based avoidance subscale (38% decrease), but he still screened positive for ARFID due to social impairment. The PARDI-AR-Q sensory-based avoidance subscale measures how much sensitivity to taste, texture, and appearance of food has affected eating. Decreases in this scale may reflect Sam’s exposure work (Thomas, Eddy, et al., 2020)—that despite having ARFID sensory sensitivity, he was trying foods anyway. At mid-treatment he reported eating a full dish of pasta with sauce from a restaurant. After this point, there was greater change in the FNS and NIAS-picky eating (34% and 40% further decreases by post-treatment, respectively) and he no longer screened positive for ARFID on the PARDI-AR-Q by post-treatment.
While goal setting and motivation for treatment is a part of CBT-AR delivery, our findings may suggest that goals directly related to treatment engagement be more explicitly woven into planning and perhaps harnessed to provide the earliest change in food neophobia and ARFID symptom improvements. In CBT-AR, important exposure for those who need to gain weight/grow or need to correct nutritional deficiencies naturally are to select foods that promote nutritional rehabilitation. It is also emphasized that foods that would decrease psychosocial impairment be tasted in treatment. However, our results could indicate that treatment may be most successful if goals to decrease psychosocial impairment are also rooted in the very reasons why patients are seeking treatment, as in the foods that patients think would make the most meaningful change in their life.
For patients with psychosocial impairment (like Sam), selection of foods the patient connects to the most meaningful quality of life improvement could be focused on early. Thus, CBT-AR and other CBTs for ARFID may increase potency (reducing time in treatment), if food exposures are prioritized by their greatest quality of life impact. For example, early exposures could be tailored to highest-priority foods (e.g., as one of the five foods brought to session even if in small quantities). This may be most effective with patients who have insight into psychosocial impairments. For future research to evaluate these hypotheses, willingness to try new foods (e.g., via FNS), may be a potential proximal mechanism that changes during the course of treatment to improve ARFID status/symptoms. We also think that evaluation of when an individual conquers their highest priority food could serve as another mechanism.
This report was limited by its single case nature and our findings may not generalize to other ARFID prototype presentations or individuals who require interventions to restore nutritional status. However, our findings provide preliminary support that progress with foods that the patient perceives to have the largest quality of life impact may be a candidate mechanism in CBTs for ARFID. It is possible that the video-delivery method also affected treatment outcomes, such as enhancing ability to do in-vivo food exposures with greater access to foods in the home. In addition, we only evaluated weekly change using the FNS. Other measures may be equally or more sensitive to change, as our findings suggest that some items related to sensory sensitivity on the PARDI-AR-Q may change prior to changes in food neophobia as well as diagnostic psychosocial impairment ARFID. We also did not evaluate other putative mechanisms that could be targeted in CBT-AR, such as disgust sensitivity. Future research is needed to both establish the efficacy of CBT-AR and examine mechanisms of action using trial design methods that evaluate target engagement.
Supplementary Material
Grant support:
This manuscript was supported by the National Institute of Mental Health, K23MH125143 (KRB) and the National Institute of Diabetes and Digestive and Kidney Diseases K23 DK131334 (HBM).
Footnotes
Conflicts of Interest: HBM, LB, and MD have no personal or financial conflicts to declare. JJT, KTE, and KRB receive royalties for book sales from Cambridge University Press for books on cognitive-behavioral therapy for avoidant/restrictive food intake disorder.
Public significance statement: Cognitive-behavioral therapies (CBTs) can be effective for treating avoidant/restrictive food intake disorder (ARFID). However, we do not yet have evidence to show how they work. This report of a single patient shows that willingness to try new foods (i.e., food neophobia), changed the most when the patient experienced a clinical improvement most relevant to his motivation for seeking treatment.
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Burton Murray H, Dreier MJ, Zickgraf HF, Becker KR, Breithaupt L, Eddy KT, & Thomas JJ (2021). Validation the Nine Item ARFID Screen (NIAS) subscales for distinguishing ARFID presentations and screening for ARFID International Journal of Eating Disorders, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont E, Jansen A, Kroes D, de Haan E, & Mulkens S (2019). A new cognitive behavior therapy for adolescents with avoidant/restrictive food intake disorder in a day treatment setting: A clinical case series. International Journal of Eating Disorders, 52(4), 447–458. doi: 10.1002/eat.23053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastaferro K, & Collins LM (2019). Achieving the Goals of Translational Science in Public Health Intervention Research: The Multiphase Optimization Strategy (MOST). American Journal of Public Health, 109(S2), S128–s129. doi: 10.2105/ajph.2018.304874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE (2007). Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology, 3, 1–27. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, & Agras WS (2002). Mediator of moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry, 59, 877–883. [DOI] [PubMed] [Google Scholar]
- Pliner P, & Hobden K (1992). Development of a scale to measure the trait of food neophobia in humans. Appetite, 19(2), 105–120. [DOI] [PubMed] [Google Scholar]
- Thomas JJ, Becker KR, Breithaupt L, Murray HB, Jo JH, Kuhnle MC, . . . Eddy KT (2021). Cognitive-behavioral therapy for adults with avoidant/restrictive food intake disorder. Journal of Behavioral and Cognitive Therapy, (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JJ, Becker KR, Kuhnle MC, Jo JH, Harshman SG, Wons OB, . . . Eddy KT (2020). Cognitive-behavioral therapy for avoidant/restrictive food intake disorder: Feasibility, acceptability, and proof-of-concept for children and adolescents. International Journal of Eating Disorders, 53(10), 1636–1646. doi: 10.1002/eat.23355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JJ, & Eddy K (2019). Cognitive-behavioral therapy for avoidant/restrictive food intake disorder: children, adolescents, and adults Cambridge: UK: Cambridge University Press. [Google Scholar]
- Thomas JJ, Eddy KT, Micali N, Kuhnle MC, Dreier MJ, Lawson EA, . . . Bryant-Waugh R (2020). Preliminary validation of a self-report questionnaire version of the pica, ARFD, and rumination disorder interview (PARDI-ARQ) Paper presented at the Eating Disorders Research Society Meeting, Sitges, Spain: (virtual). [Google Scholar]
- Zickgraf HF, & Ellis JM (2018). Initial validation of the Nine Item Avoidant/Restrictive Food Intake Disorder Screen (NIAS): A measure of three restrictive eating behaviors. Appetite(123), 32–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


