SUMMARY
Exploratory analysis of a phase 3 trial in esophageal cancer found that the patients who most contributed to an overall survival benefit from PD-1 blockade were not responders, but non-responders. The analysis has limitations but may have implications for investigating the optimal timing of immunotherapy relative to other treatments.
In this issue of Clinical Cancer Research, Okada and colleagues report long-term outcomes on anti-PD-1 monotherapy, as compared with chemotherapy, from the phase III ATTRACTION-03 trial of patients with previously treated advanced esophageal squamous cell carcinoma (aESCC)1. The updated results confirm that nivolumab improved overall survival (OS) over taxane (HR 0.79). These results have less practical relevance now, given recent evidence of an OS benefit from concurrent immune checkpoint inhibition (ICI) plus chemotherapy over chemotherapy alone in previously untreated aESCC2. However, exploratory results included in the updated report from ATTRACTION-03 might inform further research efforts to optimize the impact of immunotherapy.
A general understanding of the impact of ICI in aESCC has been that most of the OS benefit is driven by ICI responders who enrich the “tail” of the survival curves. The primary publication of ATTRACTION-03 reported that ICI responders had longer duration of response and longer time to response compared with chemotherapy responders. It would be reasonable to expect that ICI responders had significantly longer OS than chemotherapy responders and that, by extension, those whose best response on ICI was only progressive disease (PD) or stable disease (SD) would have derived minimal OS benefit from ICI.
Counterintuitively, the new results show that the patients in ATTRACTION-03 who most accounted for the improved OS do not appear to be responders to ICI, but rather non-responders (Figure 1). The investigators subcategorized patients based on their best overall response while on study therapy. Complete or partial response was infrequent in both arms, 16% each, and nivolumab was associated with a mild OS advantage (HR 0.84) in this group. By contrast, in nivolumab-treated patients who had PD or SD as best response, 44% and 15%, respectively, nivolumab was associated with profound improvement in OS (HR 0.59 and 0.41, respectively). The remaining group, those lacking measurable lesions at baseline, 19%, did not appear to contribute to nivolumab’s association with improved OS (HR 1.11).
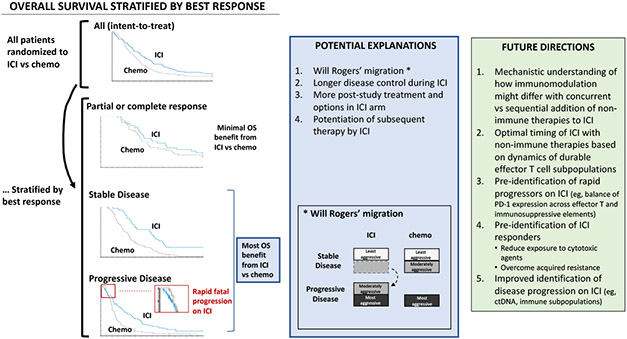
Figure. Apparent overall survival benefit from ICI in non-responders and its implications for future research.
On the left are results from the ATTRACTION-03 phase 3 trial in which patients with advanced esophageal squamous cell carcinoma were randomized to nivolumab vs taxane in the second-line setting. The primary endpoint of OS was met in the intent to treat population. Exploratory OS outcomes stratified by best response, usually determined within the first 1-4 months, are shown. Most of the OS benefit from ICI (vs chemotherapy) in the study was observed in patients whose best response was stable or progressive disease. Patients who lacked measurable lesions at baseline, 19% of the nivolumab arm, are not shown (survival curves not reported) and did not appear to contribute to nivolumab’s association with improved OS.
In the middle (blue box) are potential explanations for the apparent OS benefit from ICI in patients whose best response was stable or progressive disease.
Abbreviations: ICI, immune checkpoint inhibition; OS, overall survival.
There are several potential explanations for this apparent OS benefit favoring ICI over chemotherapy after PD and SD.
First, pseudo-progression does not appear to adequately explain it. Continuation of ICI beyond first radiographic PD was permitted, and only three patients (3.2% among 93 patients with PD) experienced suspected pseudo-progression, consistent with the low frequency observed in solid tumors.
Second, some of this apparent OS improvement in non-responders could be artifactual. The divergence of OS curves in the PD group could have arisen from a “Will Rogers’ phenomenon”—referring to an apparent epidemiologic paradox described by the eponymous humorist regarding the migration of populations: ie, that moving one element from one set to another set can raise the average values of both sets In this scenario, the PD group contains chemorefractory patients in the chemotherapy arm (n=51), who classically embody highly aggressive biology, and “immunorefractory” patients (n=93) in the ICI arm. The “immunorefractory” group is larger and may include both highly and moderately aggressive biology, the latter (more chemosensitive element) having “migrated” from the SD group. Comparing these two arms within the PD group would give the confounded impression that ICI outperformed chemo. Moreover, this “migration” of moderately aggressive biology out of the SD group would then concentrate the ICI arm of the SD group with the least aggressive biology and leave the chemotherapy arm of the SD group with both moderately and least aggressive biology. Comparing these two arms within the SD group would give the confounded impression that, again, ICI outperformed chemo. The distribution of reported baseline prognostic variables did not appear to be consistently over-represented in one arm vs another within the SD or PD group, which diminishes the extent to which this phenomenon played a role, but does not exclude it. The influence of this migration would increase to the degree that immunosensitivity and chemosensitivity do not overlap.
Third, after progression on study treatment, patients in the PD group of the ICI arm were more likely to receive post-study systemic therapy than their counterparts in the taxane arm (60% vs 35%). They also had more systemic options.
Fourth, OS benefit in the PD and SD groups would not be eliminated if we had a better predictive biomarker. A perfect biomarker of response—ie, identifies all ICI responders in ATTRACTION-03 and no one else—would exclude those who had at best PD or SD, despite their apparent OS benefit from ICI. A positive biomarker test result that predicts for OS benefit from ICI (vs chemotherapy) would need to include patients whose best response was PD, because the distribution of OS benefit from ICI was heavily weighted toward the PD group.
A final potential explanation is that the favorable OS observed in the ICI arm resulted from an impact of ICI that persisted beyond its cessation. ICI has a long half-life so that anti-cancer therapy delivered post-ICI could actually be concurrent. Alternatively, ICI might restore immune competence in a manner that enhances subsequent chemotherapy efficacy by invigorating dysfunctional CD8+ T cells3. This may be enhanced when ICI is given alone, rather than concurrently with cytotoxic agents, as the latter can also have disparate impacts on tumor immunity, capable of killing the very T cells invigorated by ICI4. In preclinical studies, ICI before—rather than concurrently with—paclitaxel/carboplatin led to greater suppression of tumor growth, accompanied by expansion of durable CX3CR1+ effector CD8+ T cells that later survived the toxic effects of subsequent chemotherapy through drug efflux4. Interestingly, the anti-PD-1/-CTLA-4 arm of CHECKMATE-648 had comparable 12-month OS rates as the anti-PD-1 plus chemotherapy arm, despite having shorter PFS and treatment duration2.
Moving forward, meta-analyses of phase 3 trials containing individual patient data, ideally molecularly annotated, could help parse the degree to which the observed post-study OS differences are due to confounding vs ICI’s potentiation of subsequent therapy. Such databases have been instrumental in colorectal cancer. Meanwhile, further mechanistic understanding of how ICI modulates systemic and local immune responses, particularly how immunomodulation might differ if non-immune therapies are added to ICI concurrently vs sequentially, could refine strategies of integrating these therapies. Understanding whether this immunomodulation differs based on the pre-existing immune contexture (eg, immune-inflamed, -excluded, -desert) could suggest profile-targeted therapies that can be added to ICI.
A particularly unmet need is the pre-identification of rapid progression on ICI. PD-L1 expression in the tumor microenvironment can identify some, but not all, of these patients2. Recent evidence suggests that the balance of PD-1 expression across effector T cells and Tregs can powerfully distinguish those with favorable and adverse response to ICI, which induces both recovery of dysfunctional PD-1+CD8+ T cells (“friend”) and enhanced PD-1+Treg–mediated immunosuppression (“foe”)5. The addition of concurrent chemotherapy to ICI appears to prevent rapid progression, but it remains unclear whether rapid progressors benefit from the addition of ICI to chemotherapy2. Ideally, their adverse immune-mediated response to ICI could be therapeutically exploited (eg, concomitant targeting of Tregs).
Another area of research is the development of immune-monitoring biomarkers to capture dynamic alterations of immune subpopulations (eg, CX3CR1+) that fluctuate in response to interval treatment, to guide immune therapies. As patients live longer, one can envision rationally sequenced therapies with increasingly cytotoxic-free periods, longitudinally designed to leverage the immediate and potentially prolonged impact of ICI based on real-time immune monitoring.
Financial Support
The authors are supported by the National Cancer Institute (1R01 CA248147-01 to Harry Yoon and 1R01 CA256927-01 to Haidong Dong).
Footnotes
Disclosure of conflict of interest
Dr. Harry H. Yoon reports relevant financial relationship(s) with industry (all honoraria paid to institution) OncXerna (advisory board), Merck (advisory board, steering committee), Zymeworks (advisory board), MacroGenics (advisory board, steering committee), BMS (advisory board), BeiGene (steering committee, advisory board, education symposium, research), and AstraZeneca (advisory board) and funding from Merck, BMS, MacroGenics, BeiGene, Boston Biomedical, Elevar Therapeutics, and CARsgen. Dr. Shi reports consulting/advisory role from Yiviva Inc, Boehringer Ingelheim Pharmaceuticals, Inc, Regeneron Pharmaceuticals, Inc., Hoosier Cancer Research Network, Honorarium/speaker role from Chugai Pharmaceutical Co., Ltd (to myself), research funds from Celgene/BMS, Roche/Genentech, Janssen, Novartis (to institution). Dr Dong reports no conflicts.
REFERENCES
- 1.Okada M, Kato K, Cho BC, et al. Three-year follow-up and response-survival relationship of nivolumab in previously treated patients with advanced esophageal squamous cell carcinoma (ATTRACTION-3). Clin Cancer Res. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doki Y, Ajani JA, Kato K, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med. 2022;386(5):449–462. [DOI] [PubMed] [Google Scholar]
- 3.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan Y, Cao S, Liu X, et al. CX3CR1 identifies PD-1 therapy-responsive CD8+ T cells that withstand chemotherapy during cancer chemoimmunotherapy. JCI Insight. 2018;3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumagai S, Togashi Y, Kamada T, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nature Immunology. 2020;21(11):1346–1358. [DOI] [PubMed] [Google Scholar]