Abstract
Background
Numerous studies have reported that eveningness is associated with increased alcohol consumption. However, biological markers of circadian timing, such as dim light melatonin onset (DLMO) and circadian photoreceptor responsivity (post‐illumination pupil response, PIPR), have rarely been assessed in the context of habitual alcohol consumption. This study aimed to examine sleep, circadian timing, and photoreceptor responsivity in adult alcohol drinkers.
Methods
Participants (21 to 45 years) included 28 light and 50 heavy drinkers. The 8‐day study consisted of a week of ad lib sleep monitored with wrist actigraphy, followed by a 9‐h laboratory session with a photoreceptor responsivity and circadian phase assessment.
Results
The heavy drinkers obtained on average 28 more minutes of sleep (p = 0.002) and reported more eveningness than the light drinkers (p = 0.029). There was a trend for a shorter DLMO‐midsleep interval (p = 0.059) in the heavy drinkers, reflecting a tendency for them to sleep at an earlier circadian phase. The PIPR in the heavy drinkers was significantly smaller than in the light drinkers (p = 0.032), suggesting reduced circadian photoreceptor responsivity in the heavy drinkers. A larger PIPR was significantly associated with a later DLMO in the light drinkers (r = 0.44, p = 0.019), but this relationship was absent in the heavy drinkers (r = −0.01, p = 0.94).
Conclusions
These results are consistent with earlier reports of more eveningness and a shorter DLMO‐midsleep interval being associated with heavier alcohol drinking. The novel finding of reduced circadian photoreceptor responsivity in heavy drinkers is consistent with prior rodent studies. Future studies should explore the impact of habitual alcohol consumption on other measures of circadian photoreceptor responsivity.
Keywords: alcohol, circadian, light, post‐illumination pupil response
This study of light and heavy alcohol drinkers found that heavy alcohol drinking was associated with more eveningness, and a trend towards more circadian misalignment, as reflected in a shorter interval between the dim light melatonin onset and midpoint of sleep. Heavy drinking was also associated with reduced circadian responsivity to light, as assessed with the post‐illumination pupil response. The relationship between circadian responsivity to light and circadian timing was also weaker in heavy drinkers than in light drinkers.

INTRODUCTION
Numerous studies have reported that later circadian timing in humans, as reflected in proxy markers of circadian timing such as evening chronotype or later sleep timing, is associated with increased alcohol consumption. For example, adolescents with an evening preference reported almost twice the lifetime drinking occasions as compared to those with intermediate or morning preference (Urban et al., 2011). Other studies have reported that a high evening preference and/or later sleep times in adolescents was significantly associated with more alcohol use (Gau et al., 2007; Negriff et al., 2011; Pieters et al., 2010), significantly higher AUDIT scores (i.e., self‐reported alcohol consumption, drinking behaviors, and alcohol‐related problems; Saxvig et al., 2012) and greater alcohol misuse (Glozier et al., 2014). One longitudinal study of adolescents (12 to 21 years) found that evening preference was associated with more binge drinking and at‐risk alcohol use at baseline but also predicted binge drinking 1 year later (Hasler et al., 2017). The reported effects in these studies remained significant even after analyses were adjusted for factors such as pubertal development, age, sex, race/ethnicity, socioeconomic status, educational level, and psychopathology. Similar associations have also been observed in adults (≥18 years). For example, adults with an evening preference and/or later sleep times were more likely to be alcohol drinkers (Whittier et al., 2014; Wittmann et al., 2006), consumed more alcohol (Adan, 1994; Kanerva et al., 2012; Tavernier & Willoughby, 2014; Van Reen et al., 2016), had higher AUDIT scores (Prat & Adan, 2011; Taylor et al., 2011; Taylor et al., 2020), and had higher alcohol dependence scores (Hasler et al., 2013). Thus, the relationship between proxy markers of circadian timing and alcohol consumption is consistently reported.
This literature on circadian timing and alcohol consumption is, however, limited in at least two ways. First, proxy circadian markers, such as questionnaires, have been largely used, instead of biological markers of circadian timing. This is likely because the assessment of such biological markers is a time‐intensive process. For example, the assessment of the dim light melatonin onset (DLMO) typically requires half‐hourly sampling of saliva in dim light (as light suppresses melatonin), in the 6 h prior to habitual bedtime (Benloucif et al., 2008). Nonetheless, the DLMO is considered the gold standard circadian phase marker in humans (Klerman et al., 2002; Lewy et al., 1999). In the only two studies to date that have assessed the DLMO in the context of habitual alcohol drinking, one found later DLMO timing in emerging adult alcohol drinkers, and this was significantly associated with more drinking on the following weekend (Hasler et al., 2019). In the other study, recently abstinent alcohol‐dependent individuals had later DLMOs than healthy controls (Conroy et al., 2012). These results are consistent with the literature, but more studies with larger sample sizes are needed to assess the DLMO in relation to habitual alcohol consumption.
Second, the role of the intrinsically photosensitive retinal ganglion cells (ipRGCs), the primary circadian photoreceptors, in the relationship between alcohol use and circadian timing remains unexplored. IpRGCs transmit the light signal to the circadian pacemaker and therefore play a key role in influencing circadian timing (Berson et al., 2002). IpRGCs express melanopsin, a primary photopigment that can respond directly to light (Provencio et al., 2000). The melanopsin response in ipRGCs can be quantified with chromatic pupillometry, which examines the pupil diameter during a post‐illumination period, termed the post‐illumination pupil response (PIPR, Gamlin et al., 2007). The PIPR reflects ipRGC responses, providing quantification of individual differences in melanopsin‐driven sensitivity to light (Kardon et al., 2009; Zele et al., 2019).
The aim of this study was to compare sleep, circadian timing, and circadian photoreceptor responsivity in two carefully defined and distinct groups of relatively healthy adults who regularly consumed alcohol but had significantly different habitual alcohol use patterns. With this approach, sleep, circadian timing, and circadian photoreceptor responsivity could then be directly compared between light and heavy drinkers. Based on the preexisting literature, it was hypothesized that relative to light drinkers, heavy drinkers would have later circadian timing as reflected in both proxy markers of circadian timing and in the gold standard DLMO. It was also hypothesized that such later circadian timing in the heavy drinkers would be positively associated with greater circadian photoreceptor responsivity (i.e., greater PIPR) to light in the afternoon/evening, as light exposure at this time of day is associated with phase delays.
MATERIALS AND METHODS
Participants
Participants were 78 alcohol drinking adults recruited through online advertisements who met the criteria for light or heavy drinker. The screening process consisted of an online survey, followed by a telephone interview and then an in‐person screening interview. Alcohol use had to be consistently reported on all three screening occasions for a candidate to be considered for the study with a consistent and predominant drinking pattern for a minimum of the past 1 year or longer. Based on prior work (Holdstock et al., 2000; King et al., 2002; King et al., 2011), inclusion criteria for heavy drinkers (n = 50) were consumption of ≥10 standard alcoholic drinks/week and at least one weekly binge drinking occasion per week as per the NIAAA definition of ≥5 drinks/occasion for male participants and ≥4 drinks/occasion for female participants (NIAAA, 2005). Further, over the past year, the majority of heavy drinkers (96%, 48/50) reported that the first 5 drinks (4 for women) were consumed within the first 2 h of an episode with fairly regular frequency. The inclusion criteria for light drinkers were consumption of 1 to 5 standard alcoholic drinks/week as the predominant drinking pattern and no/rare (≤3 per year) episodes of binge drinking in the past year, and any history of regular heavy drinking was exclusionary. For both drinking groups, participants could not report any past or current significant alcohol withdrawal symptoms (e.g., seizures), nor treatment for alcohol or substance abuse, or describe any plans to immediately change their drinking pattern.
Other inclusion criteria for both drinking groups were as follows: (1) age between 21 and 45 years; (2) body mass index between 19 and 35 kg/m2; (3) no significant chronic disease (e.g., heart, lung, gastrointestinal, vascular, endocrine, autoimmune disease, cancer); (4) no high likelihood of obstructive sleep apnea (Netzer et al., 1999) or restless leg syndrome (Hening & Allen, 2003); (5) no extremely short or long sleepers (defined as ≤5 h/night, ≥10 h/night); (6) no reported eye disease or colorblindness (assessed with Ishihara test; Clark, 1924); (7) no past or present psychotic or bipolar disorders, post‐traumatic stress disorder, or obsessive compulsive disorder; (8) no current suicidal ideation or intent; (9) no significant anxiety or depressive symptoms (Beck Depression Inventory II ≥17 (Beck et al., 1996), State‐Trait Anxiety Inventory‐Trait ≥80 (Spielberger et al., 1970)); (10) no use of prescription medications (contraceptives, acne medications, and inhalers for exercise‐induced asthma permitted), or supplemental melatonin; (11) no heavy cigarette smoking or vaping (≥10 cigarette equivalents/day); (12) no travel across time zones in the past month; and (13) no shift work currently or in past month. Female participants who were pregnant, breastfeeding, perimenopausal, or menopausal were excluded. At the in‐person screening interview, all participants had to have a breathalyzer reading of <0.000 g/dl and a negative urine toxicology screen (for cocaine, amphetamine, methamphetamine, marijuana [tetrahydrocannabinol], opiate, phencyclidine, barbiturates, and benzodiazepines) and were instructed to be drug‐free during the study. The overall goal was to generate two distinct and carefully characterized groups of relatively healthy adult alcohol drinkers, to better examine the sleep and circadian differences between them. See Table 1 for sample characteristics.
Table 1.
Sample characteristics
| Light drinkers (n = 28) | Heavy drinkers (n = 50) | p‐Value | |
|---|---|---|---|
| Age (mean, SD) | 27.9 (5.9) | 27.3 (5.1) | 0.66 |
| Sex assigned at birth (%) | |||
| Male | 32% | 56% | 0.04 |
| Female | 68% | 44% | |
| Race (%) | |||
| Asian | 18% | 16% | 0.10 |
| Black | 21% | 4% | |
| Other | 4% | 4% | |
| White | 57% | 76% | |
| Ethnicity (%) | |||
| Hispanic/Latinx | 7% | 12% | 0.50 |
| Beck Depression Inventory (mean, SD) | 1.26 (2.01) | 1.66 (2.36) | 0.46 |
| State‐Trait Anxiety Inventory‐State (mean, SD) | 40.93 (6.27) | 40.10 (7.01) | 0.61 |
| Laboratory session day of week (%) | |||
| Weekday | 42.9 | 40.0 | 0.92 |
| Weekend | 57.2 | 56.0 | |
| Photoperiod on day of laboratory session (mean, SD) | 12.39 (2.24) | 12.60 (1.96) | 0.68 |
| Season on day of laboratory session | |||
| Winter | 18% | 14% | 0.84 |
| Spring | 32% | 42% | |
| Summer | 25% | 24% | |
| Fall | 25% | 20% | |
| Circadian time of testing (mean, SD) a | 2.50 (1.16) | 3.10 (1.21) | 0.049 |
Note: The mood questionnaires were collected at the start of the laboratory session.
p Values <0.05 are bolded.
Circadian time of testing was the time interval from the first saliva sample to the later determined DLMO.
Design
The study consisted of an 8‐day protocol (Figure 1). On Day 1, participants attended the laboratory and received a wrist actigraphy monitor (30‐s epochs, Actiwatch Spectrum, Respironics) to wear on their non‐dominant wrist for the duration of the protocol. They were instructed to press the event marker on the monitor before and after ad‐lib sleep each night and completed daily sleep and event diaries which tracked sleep, caffeine, alcohol, and medication use during the protocol. During this visit, participants also participated in a practice PIPR assessment (see below) to become familiar with the procedure (data not analyzed).
Figure 1.
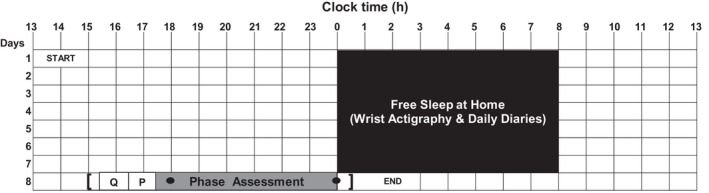
A representation of the 8‐day study protocol, for a participant with an average bedtime of midnight. On Day 1, participants attended the laboratory and received a wrist actigraphy monitor and were instructed on how to complete daily diaries while sleeping ad lib at home (participants were not required to follow a fixed 8‐h sleep schedule). On Day 8, participants arrived for the laboratory session 9 h before their habitual bedtime. They completed questionnaires (Q), completed a circadian photoreceptor responsivity assessment (P), and then began a circadian phase assessment (dots represent the timing of the first and last saliva sample). The time of arrival and departure from the laboratory on Day 8 is represented by square brackets
On Day 8, participants arrived at the laboratory 9 h prior to their habitual bedtime. They were instructed not to consume any alcohol or caffeine in the prior 24 h, and no non‐steroidal anti‐inflammatory drugs in the prior 72 h, to avoid confounding melatonin levels (see below). They were breathalyzed to confirm recent alcohol abstinence. Following this, their wrist actigraphy data were downloaded, and they completed questionnaires and underwent a circadian photoreceptor responsivity assessment (described below). Participants then completed a 6.5‐h circadian phase assessment session, which completed their laboratory session. They were not permitted to drive themselves home and so either traveled home via taxi/rideshare or with a friend or family member who drove them. Participants started the study protocol and their laboratory session on a weekday (Monday, Tuesday, Wednesday, or Thursday) or weekend (Saturday or Sunday). Friday nights were reserved for another study in the laboratory. Data collection occurred at both Rush University Medical Center and the University of Michigan, and the Institutional Review Boards at both institutions approved the study protocol. All participants gave written informed consent prior to participating. Participants were compensated $520 after they completed the 8‐day protocol.
Measures
Wrist actigraphy
Objective measures of sleep were derived from the 7 days of wrist actigraphy recordings made just prior to the laboratory session. Data were analyzed with the Actiware 6.0.9 program (medium sensitivity, Respironics, Bend, OR). The setting of nightly rest intervals for analysis was guided by the event markers, sleep diaries, light data, and activity levels (Patel et al., 2015). Objective actigraphic estimates of sleep timing (sleep onset time and final wake time), total sleep time (number of minutes scored as sleep in each rest interval), and sleep efficiency (proportion of time between sleep onset and final wake time scored as sleep in each rest interval, expressed as a percentage) were extracted for each study night and averaged over the 7 days.
Questionnaires
At the start of the laboratory session, participants completed multiple questionnaires. Sleep quality and insomnia symptoms were assessed with the Pittsburgh Sleep Quality Index (Buysse et al., 1989) and Insomnia Severity Index (Bastien et al., 2001). Circadian preference was assessed with the Morningness‐Eveningness Questionnaire (Horne & Ostberg, 1976) and chronotype and social jet lag were assessed with the Munich ChronoType Questionnaire (Roenneberg et al., 2003). Current depressive and anxiety symptoms were assessed with the Beck Depression Inventory (Beck et al., 1961) and State‐Trait Anxiety Inventory‐State Questionnaire (Spielberger et al., 1970). Attitudes to alcohol were assessed with a three‐item version of the Alcohol Purchase Task (Amlung et al., 2015) which asked if drinks were free, how many drinks would the participant consume right now, and what maximum dollar amount they would pay for a single drink and for the drinking session in total. Participants also completed the Anticipated Biphasic Effects of Alcohol Scale (A‐BAES) which assessed anticipated levels of stimulation and sedation after imagining drinking four alcoholic drinks (Earleywine & Martin, 1993) and the Anticipated Drug Effects Questionnaire (A‐DEQ) which asked how much they would feel, like, and want more alcohol after consuming four alcoholic drinks (Fridberg et al., 2017).
Circadian photoreceptor responsivity
The post‐illumination pupil response (PIPR) was then assessed with a laboratory‐made pupillometer, which consisted of blue (488 nm) and red (632 nm) LEDs. The light from the LEDs traveled through optic fibers before being combined in a spatial homogenizer and a diffuser, producing a highly uniform field. A field lens with a 2‐mm artificial pupil was used to create a Maxwellian view (Kelbsch et al., 2019). The LED light outputs were digitally controlled by software developed in Objective‐C on a Mac computer.
For the PIPR assessment, participants entered a dark room, sat in a height‐adjustable chair, and rested their chin on a chin rest, which was adjusted to position their right eye in front of the artificial pupil to view the LED light. Light pulses (500 msec) of red light (2000 Troland, which quantified retinal illuminance based on light luminance in cd/m2 and pupil size in mm) or blue light (2000 Td) were applied to the right eye, in a 30° circular field, with the central 10.5° blocked to minimize the effect of macular pigment's selective absorption. The diameter of the left pupil was recorded with an infrared camera from an Eyelink II eye tracker (SR Research) with a sampling rate of 250 Hz and spatial resolution of 0.1% of baseline pupil size. The test started with three trials of red LED light, followed by three trials of blue LED light. Each trial lasted 35.5 s (2 s before light pulse, 0.5 s light pulse, and 25 s after lights off). The obtained pupil response curve for each trial was normalized to the percentage of baseline pupil size (which was measured over 2 s before light pulse). Then, the normalized pupil response curve was averaged first for red LED and blue LED lights. Overall circadian photoreceptor responsivity for each participant was calculated as the difference between the red light (average of three trials) and blue light (average of three trials) pupil response curves at 6 s after each light pulse (i.e., net blue minus red, percent of baseline; Adhikari et al., 2016; Kelbsch et al., 2019).
Circadian phase assessment
After the pupil responsivity testing, at 6.5 h prior to habitual bedtime, participants entered a room in the laboratory specifically designed for circadian phase assessments. Participants remained awake and seated in dim lit (<5 lux, at level of eyes, in direction of gaze, measured every 2 h, Extech EA33 light meter) and were continuously monitored by staff. After 30 min in the dim light, subjects gave a saliva sample every 30 min using Salivettes (Sarstedt). Toothpaste or mouthwash was not allowed during the phase assessments. Small snacks and fluids were permitted, except in the 10 min before each sample, and subjects were required to brush their teeth and rinse with water while remaining seated 10 min before each sample if they had consumed food or drink. The samples were centrifuged immediately upon collection and frozen. The samples were later shipped in dry ice to SolidPhase, which radioimmunoassayed the samples for melatonin using the Buhlmann RIA assay, which is the most accurate assay for salivary melatonin (Kennaway, 2019). The assay sensitivity was 0.5 pg/ml. Intra‐assay and inter‐assay coefficients of variation for low levels of salivary melatonin are 20.1%, and 16.7%, respectively. A dim light melatonin onset (DLMO) was calculated for each participant, as the clock time (with linear interpolation) when the melatonin concentration exceeded the mean of three low, consecutive, daytime values plus twice the standard deviation of these points (Benloucif et al., 2008; Voultsios et al., 1997). This low threshold more closely tracks the initial rise of melatonin (Molina & Burgess, 2011). Like prior related studies (Conroy et al., 2012; Hasler et al., 2019) the alignment between sleep and circadian timing was also calculated, as the interval between DLMO and midsleep (halfway point between sleep onset and final wake time).
Statistical analysis
All analyses were conducted using Stata 15.1 (StataCorp). Independent samples t‐tests were used to compare continuous variables between the two drinking groups and chi‐square tests were used for categorical variables. Group differences and the relationships between variables were explored using linear regression, which adjusted for variables known to impact sleep and circadian variables: age, sex, photoperiod (daylength) on the day of the laboratory session, and day of week of laboratory assessment (weekday or weekend). Statistical significance was determined at p < 0.05.
RESULTS
Sample characteristics
The final sample consisted of 28 light drinkers and 50 heavy drinkers who participated from February 2017 to February 2020. Their demographics and baseline characteristics are shown in Table 1. There were plans to enroll more light drinkers, but the study ended abruptly with a laboratory shutdown due to the COVID‐19 pandemic. The average age of the sample was in the late 20s. There were significantly more male participants in the heavy drinking group. There were no significant group differences in race or ethnicity, and both groups showed minimal depressive and anxiety symptoms, as expected from the screening criteria. There was also no group difference in the photoperiod (daylength), season, or day of the week of the laboratory session. The clock time of the laboratory session was not different between groups—the first saliva sample (first dot in Figure 1) occurred at 18:05 ± 1.23 in light drinkers and at 18:10 ± 1.31 in the heavy drinkers (p = 0.82). The circadian time of the laboratory session was derived post hoc as the interval from the first saliva sample in the phase assessment (first dot in Figure 1) to the later calculated DLMO. There was a significant group difference in the circadian time of testing such that the heavy drinkers were assessed on average 36 min later relative to their DLMO than the light drinkers (Table 1).
Alcohol‐related variables
As expected, the heavy drinkers reported consuming more alcohol drinks per week, and having more alcohol binges per week, on an online 30‐day timeline followback questionnaire (Sobell & Sobell, 1995) collected at the in‐person screening interview (Table 2). Similarly, as reported on the daily diaries during the baseline week, the heavy drinkers continued to drink more than the light drinkers, even though drinking was limited with the instruction to abstain from alcohol in the 24 h prior to the laboratory session. The heavy drinkers had a significantly higher AUDIT score than the light drinkers, which reflected potentially harmful or hazardous alcohol consumption (Babor et al., 1989). Most of the heavy drinkers did not meet the criteria for an alcohol use disorder (AUD), with only three of them (6%) meeting the criteria for mild AUD 2 or 3 symptoms according to the DSM‐5 criteria. As expected on the Alcohol Purchase Task, heavy drinkers proposed drinking significantly more and paying significantly more for a drinking session than did light drinkers. Both groups reported similar levels of anticipated stimulation from 4 standard alcohol drinks, and the heavy drinkers anticipated significantly higher liking and wanting with lower levels of sedation and feeling alcohol effects, generally consistent with subjective responses measured in alcohol challenge research (King et al., 2011).
Table 2.
Alcohol‐related variables
| Light drinkers (n = 28) (mean, SD, range) | Heavy drinkers (n = 50) (mean, SD, range) | Adjusted p‐value | |
|---|---|---|---|
| Alcohol drinks/week from TLFB | 2.57 (1.09, 0.75 to 4.75) | 17.89 (6.84, 9.5 to 46.75) | <0.001 |
| Alcohol binges/week from TLFB | 0.02 (0.07, 0 to 0.25) | 2.0 (0.8, 0.75 to 5) | <0.001 |
| Alcohol drinks in baseline week | 3.50 (3.23, 0 to 12) | 14.06 (11.86, 0 to 56.5) | <0.001 |
| Alcohol binges in baseline week | 0.11 (0.31, 0 to 1) | 1.44 (1.33, 0 to 5) | <0.001 |
| AUDIT | 3.0 (1.2, 0 to 6) | 11.4 (4.2, 5 to 21) | <0.001 |
| Alcohol purchase task | |||
| Number of drinks | 2.48 (1.50, 0 to 6) | 5.89 (2.72, 2 to 14) | <0.001 |
| Maximum $ on single drink | 8.16 (3.83, 0 to 15) | 8.16 (3.29, 2 to 16) | 0.65 |
| Maximum $ on total drinks | 18.48 (9.93, 0 to 40) | 27.08 (9.93, 8 to 40) | <0.001 |
| Anticipated biphasic alcohol effects | |||
| Stimulation | 39.25 (16.98, 0 to 64) | 43.40 (10.07, 15 to 65) | 0.13 |
| Sedation | 30.93 (15.74, 3 to 70) | 11.88 (9.15, 0 to 36) | <0.001 |
| Anticipated drug effects | |||
| Feel effects of alcohol | 80.3 (23.0, 0 to 100) | 54.5 (21.8, 0 to 99.5) | <0.001 |
| Like effects of alcohol | 61.3 (21.8, 0 to 100) | 75.4 (18.1, 10.5 to 100) | 0.002 |
| Want more alcohol | 24.7 (24.1, 0 to 77) | 67.6 (19.9, 14 to 100) | <0.001 |
Note: TLFB = 30‐day timeline followback questionnaire administered during the in‐person screening interview. The AUDIT questionnaire was completed during the in‐person screening interview. p‐Values were adjusted for age, sex, photoperiod, and laboratory assessment day (weekend/weekday).
p Values <0.05 are bolded.
Sleep and circadian variables
On average, the heavy drinkers obtained 28 more minutes of sleep per night than the light drinkers (Table 3). This was mostly due to a later wake time in the heavy drinkers. The heavy drinkers reported more insomnia symptoms, but on average, both groups did not show clinically significant insomnia symptoms (average Insomnia Severity Index score ≤7). Likewise, both groups had good sleep quality and good sleep efficiency. A significantly greater tendency to eveningness was observed in the heavy drinkers. A later average chronotype was also observed in the heavy drinkers, but this was not statistically significant. There was a trend for the heavy drinkers to have more social jet lag, although both groups on average had a social jet lag of <2 h. The DLMO occurred on average 32 min later in the heavy drinkers, although this was not statistically significant. There was a trend for a shorter DLMO to midsleep interval in the heavy drinkers, reflecting that they slept at an earlier circadian phase. There were seven participants with missing DLMOs—all were heavy drinkers. Two participants may have had earlier DLMOs which could have been determined if the phase assessment had started earlier, two participants may have had later DLMOs which could have been determined if the phase assessment had ended later, and three participants showed a very erratic rise in melatonin such that a DLMO was not readily discernible. Lastly, the net difference between red and blue pupil response curves 6 s after the light pulse in the heavy drinkers was significantly smaller than in the light drinkers, suggesting reduced circadian photoreceptor responsivity in the heavy drinkers (Table 3, Figure 2).
Table 3.
Sleep and circadian variables
| Light drinkers (n = 28) | Heavy drinkers (n = 50) | Adjusted p‐value | |
|---|---|---|---|
| Sleep onset time (mean, SD) | 00:23 (1.3) | 00:15 (1.1) | 0.308 |
| Final wake time (mean, SD) | 07:40 (1.4) | 08:08 (1.3) | 0.218 |
| Total sleep time (h; mean, SD) | 6.71 (0.73) | 7.18 (0.77) | 0.002 |
| Sleep efficiency (%; mean, SD) | 92.22 (2.91) | 91.34 (3.19) | 0.483 |
| Pittsburgh Sleep Quality Index (mean, SD) | 3.00 (1.76) | 3.60 (1.94) | 0.205 |
| Insomnia Severity Index (mean, SD) | 2.43 (1.81) | 4.10 (3.38) | 0.009 |
| Morningness‐eveningness (mean, SD) | 52.71 (9.97) | 47.63 (7.80) | 0.029 |
| Munich chronotype (h; mean, SD) | 04:39 (1.41) | 05:15 (1.17) | 0.118 |
| Munich social jet lag (h; mean, SD) | 1.29 (1.28) | 1.75 (0.88) | 0.055 |
| Dim light melatonin onset (mean, SD) | 20:35 (1.5) | 21:07 (1.5) | 0.254 |
| Dim light melatonin onset to midsleep interval (h; mean, SD) | 7.44 (0.76) | 7.03 (1.0) | 0.059 |
| Post‐illumination pupil response (net difference, % baseline, 6 secs, mean, SD) a | 4.49 (2.55) | 2.87 (3.13) | 0.032 |
Note: p Values were adjusted for age, sex, photoperiod, and day of week of laboratory assessment (weekend/weekday).
p Values <0.05 are bolded.
The group comparison of PIPR was also adjusted for the circadian time of assessment (the time interval between the first saliva sample shortly after the PIPR and the DLMO, calculated post hoc).
Figure 2.
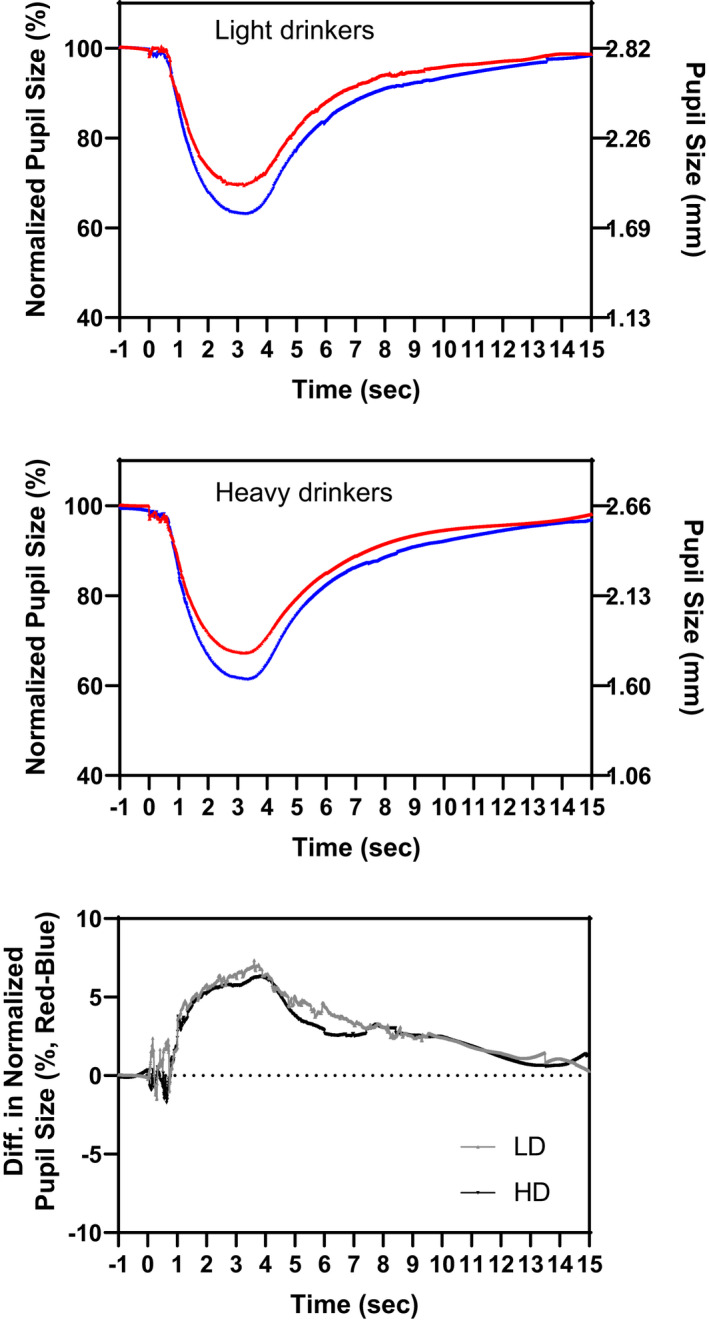
The averaged pupil response curves after red and blue LED lights in the light (top) and heavy alcohol drinkers (middle). The left y‐axis shows the pupil size normalized to baseline in percentage, and the right y‐axis shows the average corresponding pupil size in mm. The bottom figure shows the net red‐blue difference in each drinking group (LD (lighter line) = light drinkers, HD (heavier line) = heavy drinkers) and highlights the reduced difference in the heavy versus light drinkers at 6 s after the light pulses
Associations between circadian timing and circadian photoreceptor responsivity
In contrast to expectations, while heavy drinkers had a later average DLMO (but not statistically significantly so), they also had a significantly reduced PIPR, reflecting reduced circadian photoreceptor responsivity (Table 3, Figure 2). As the PIPR was assessed in the afternoon/evening hours, it was expected that more circadian photoreceptor responsivity, which should amplify phase‐delaying effects of light in the evening, would associate with a later DLMO. To further explore this, the relationship between DLMO and PIPR was separately explored in each drinking group (Figure 3). In the light drinkers, greater circadian photoreceptor responsivity (larger PIPR) was significantly associated with a later DLMO as expected (r = 0.44, p = 0.019). However, in the heavy drinkers, the correlation was neither significant nor meaningful (r = −0.01, p = 0.94). These associations between DLMO and PIPR in each group were still observed after adjusting the analyses for sex, age, photoperiod, day of week of laboratory, and even circadian time of testing (light drinkers, r = 0.44, p = 0.033; heavy drinkers r = −0.04, p = 0.80). Circadian time of testing was derived post hoc as the time interval from the first saliva sample to the DLMO. Thus, heavy alcohol consumption was associated with a smaller PIPR and the relationship between the DLMO and PIPR seen in the light drinkers was not present in the heavy drinkers.
Figure 3.
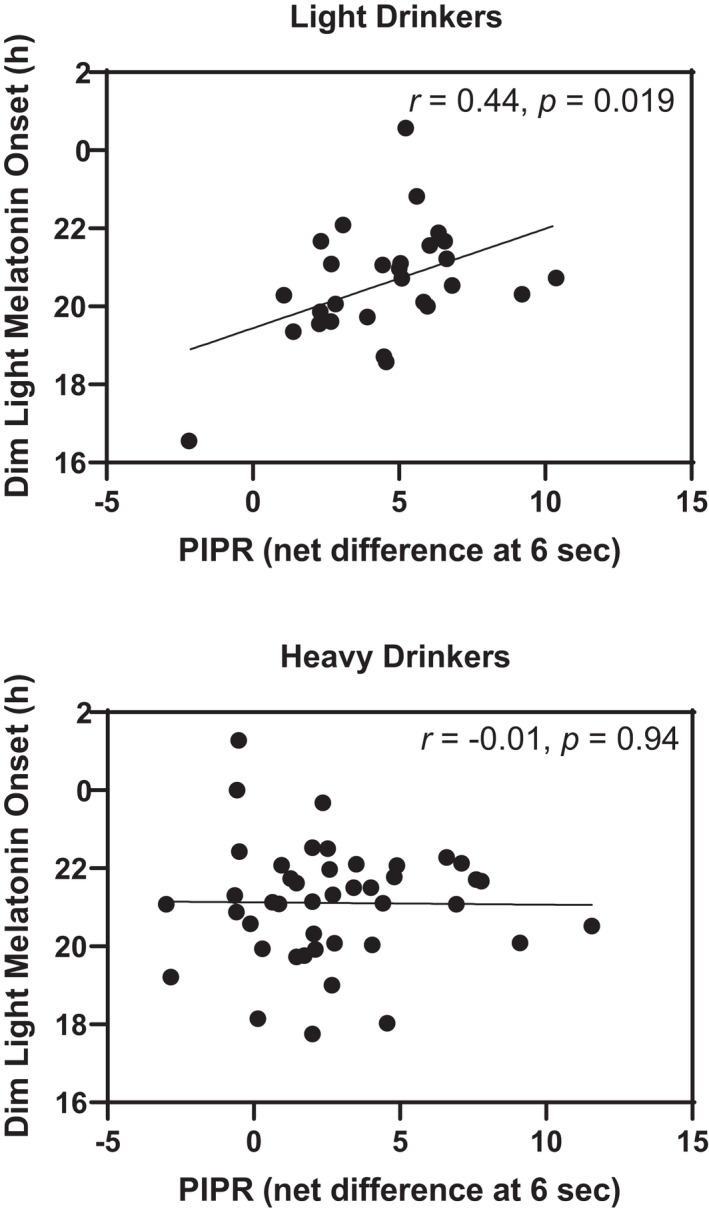
Scatterplots of the timing of the dim light melatonin onset (DLMO) versus the circadian photoreceptor responsivity (PIPR, which was calculated as the net difference at 6 s from pupil response curves between the red and blue LED lights) in the light and heavy alcohol drinkers. The unadjusted correlations are shown
DISCUSSION
In this study, two distinct and carefully characterized groups of relatively healthy adult alcohol drinkers were examined: light and heavy drinkers. Compared with the light drinkers, the heavy drinkers engaged in more alcohol drinking and binging behavior, both at screening and in the baseline week prior to the laboratory session. The heavy drinkers also reported more hazardous alcohol consumption on the AUDIT, were willing to pay more for a drinking session, and anticipated that after consuming four standard alcoholic drinks, they would feel less sedation, but like and want more alcohol, as compared with the light drinkers. The main sleep and circadian differences between the groups (after adjusting for age, sex, photoperiod, and day of week of laboratory assessment) were that the heavy drinkers woke about 30 min later and endorsed more eveningness. There was no group difference in the DLMO but a trend toward a shorter DLMO to midsleep interval in the heavy drinkers. The heavy drinkers also exhibited a smaller PIPR, suggesting reduced circadian photoreceptor responsivity to light.
This study is only the third study in the literature to examine the DLMO, the gold standard circadian phase marker in humans, in the context of habitual alcohol drinking. The finding of more eveningness in the heavy drinkers is consistent with the broader literature (Adan, 1994; Gau et al., 2007; Kanerva et al., 2012; Negriff et al., 2011; Pieters et al., 2010; Tavernier & Willoughby, 2014; Van Reen et al., 2016). However, this greater eveningness was not reflected in a significantly later DLMO. Instead, there was a trend observed for a shorter DLMO‐midsleep interval in association with heavy alcohol consumption which is consistent with the other DLMO and alcohol consumption studies (Conroy et al., 2012; Hasler et al., 2019). Indeed, the shorter DLMO‐sleep midpoint of about 25 min observed in the heavy versus light drinkers matches the difference in the DLMO‐sleep midpoint observed between recently abstinent alcohol‐dependent individuals and healthy controls (Conroy et al., 2012). Thus, heavy drinkers do not necessarily have later circadian timing per se but instead have later circadian timing relative to the timing of their sleep, potentially reflecting greater circadian misalignment (Hasler et al., 2019). Interestingly, the light and heavy drinkers did not meaningfully differ in their mood symptoms, nor in their sleep quality or sleep efficiency, and the heavy drinking was not associated with poor mood or sleep disturbance. In fact, the heavy drinkers actually obtained more sleep per night than the light drinkers. Thus, heavy drinking was not associated with later bedtimes and not simply due to the heavy drinkers having more evening hours available to them. One possibility is that circadian misalignment characterized by the shorter DLMO‐sleep midpoint interval (phase angle) was associated with altered reward functioning and impaired impulse control (Hasler et al., 2021), leading, in turn, to more alcohol consumption (Hasler & Clark, 2013). Indeed, eveningness has been repeatedly linked to greater global impulsivity (e.g., (Kang et al., 2015, Russo et al., 2012) and was recently found to associate with multiple subdimensions of impulsivity measured at the state level over and above the effects of actual sleep timing or duration (Hasler et al., 2022). Importantly, eveningness was associated with greater urgency (positive and negative), which has been particularly linked to alcohol use and related problems (Littlefield et al., 2014). This suggests that the clinical treatment of patients seeking treatment for heavy drinking may be enhanced by assessing circadian preference, as an eveningness tendency might indicate the utility of more attention to monitoring the role of impulsivity in the patients' alcohol use, as well as consideration of their sleep schedules. Preliminary evidence suggests that imposing circadian misalignment impairs the neural underpinnings of impulse control (Hasler et al., 2021), while correcting it may reduce impulsivity (Fargason et al., 2017). Lastly, there is evidence that later sleep/circadian timing may be associated with later timing of peak alcohol craving (Hisler et al., 2021), which could be informative for heavy drinking evening types.
In the light drinkers, the anticipated relationship between circadian photoreceptor responsivity (PIPR) and DLMO was observed—namely that higher circadian photoreceptor responsivity was associated with a later DLMO. However, this relationship was not observed in the heavy drinkers who displayed a significantly reduced circadian photoreceptor responsivity (reduced PIPR). The reduced circadian photoreceptor responsivity in the habitual heavy drinkers may indicate impaired photoentrainment, leading to a disrupted relationship between light input and circadian timing. While acute alcohol intake does not alter pupil diameter with a steady light exposure (Brown et al., 1977), acute alcohol exposure has been reported to modulate the sensitivity of retinal neurons to various neurotransmitters such as gamma‐aminobutyric acid (GABA) (Yeh & Kolb, 1997). The effects of an acute alcohol dose on PIPR have not yet been assessed. The possibility that a history of heavy alcohol use modulates circadian photoreceptor responsivity to light should be further explored with other methods, such as melatonin suppression to light (Phillips et al., 2019). While a single dose of alcohol was not shown to systematically alter circadian phase shifts to light in healthy humans (Burgess et al., 2016), robust evidence in rodents suggests that chronic alcohol consumption can reduce photic inputs into the clock, disrupt circadian entrainment, and alter circadian period (Brager et al., 2010; Ruby et al., 2009). Circadian phase shifts to light remain to be studied in habitual heavy drinkers, and future research should try to tease apart the effects of heavy alcohol use from the effects of circadian misalignment.
This study has several strengths and several limitations. In terms of strengths, this is the largest study to date to examine habitual alcohol drinking and the DLMO, and it corroborates the preexisting literature that suggests an association between eveningness, a shorter DLMO‐midsleep interval, and greater alcohol consumption. In addition, this is the first study to examine circadian photoreceptor responsivity in the context of habitual alcohol drinking, and it has revealed the possibility that heavy alcohol drinking may suppress melanopsin‐driven pupil responsivity. Important factors known a priori to impact sleep and circadian variables, such as age, sex, photoperiod (daylength), season, and day of week of laboratory assessment (weekday/weekend), were included in the analyses. Further, the study protocol timing was anchored to habitual bedtime, which led to participants being assessed around the same circadian time (average group difference of 36 min), thus likely reducing variance in the data. Finally, calculating the PIPR as a percentage of baseline reduces error due to individual differences in baseline pupil size, and using equal 2000 Td illuminance at the retina for red and blue light allowed for the adjustment of the response to blue light for non‐specific effects on the PIPR as measured by the response to red light (i.e., autonomic influence on the PLR (Kelbsch et al., 2019)). In terms of limitations, this study may be underpowered as the recruitment of light drinkers was cut short due to the COVID‐19 pandemic. Additionally, this is a cross‐sectional study, and the causation of the reported group differences and associations cannot be determined. In terms of the PIPR, the absence of eye disease was only determined from participant self‐report and ophthalmological examinations were not conducted to confirm this, although prior studies with similar sample sizes found no previously unreported retinal health conditions (Roecklein et al., 2021). Furthermore, PIPR was only assessed in the evening, and given the circadian rhythm in PIPR (Zele et al., 2011), group differences in the PIPR in the morning may or may not have been different to what was observed in the evening. Finally, the heavy alcohol drinkers were screened to be relatively healthy and therefore may not represent less healthy heavy alcohol drinkers who engage in other drug use such as cannabis or experience comorbid psychiatric disorders. Future research should compare these sleep and circadian variables between a healthy control group and patients meeting a diagnosis of AUD according to DSM‐5 criteria.
In summary, this study found that in generally healthy humans, heavy alcohol drinking was associated with more eveningness, a shorter DLMO‐midsleep interval, and reduced circadian photoreceptor responsivity, as compared to light drinkers. Heavy drinking in this sample was not associated with poor mood or sleep disturbance. Future research should interrogate whether heavy habitual alcohol consumption is either a consequence or cause of reduced melanopsin‐driven retinal responsivity.
CONFLICT OF INTEREST
HJB is a consultant for Natrol, LLC, Moving Mindz, Pty Ltd, and F. Hoffmann‐La Roche Ltd. All other authors report no financial or non‐financial interests to disclose.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R01 AA023839. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Burgess, H.J. , Rizvydeen, M. , Kikyo, F. , Kebbeh, N. , Tan, M. & Roecklein, K.A. et al. (2022) Sleep and circadian differences between light and heavy adult alcohol drinkers. Alcoholism: Clinical and Experimental Research, 46, 1181–1191. Available from: 10.1111/acer.14872
REFERENCES
- Adan, A. (1994) Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction, 89, 455–462. [DOI] [PubMed] [Google Scholar]
- Adhikari, P. , Feigl, B. & Zele, A.J. (2016) Rhodopsin and melanopsin contributions to the early redilation phase of the post‐illumination pupil response (PIPR). PLoS One, 11, e0161175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung, M. , Mccarty, K.N. , Morris, D.H. , Tsai, C.L. & Mccarthy, D.M. (2015) Increased behavioral economic demand and craving for alcohol following a laboratory alcohol challenge. Addiction, 110, 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor, T.F. , De La Fuente, J.R. , Saunders, J. & Grant, M. (1989) AUDIT the alcohol use disorders identification test: guidelines for use in primary health care. Geneva: WHO/MNH/DAT. [Google Scholar]
- Bastien, C.H. , Vallieres, A. & Morin, C.M. (2001) Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Medicine, 2, 297–307. [DOI] [PubMed] [Google Scholar]
- Beck, A.T. , Steer, R.A. & Brown, G.K. (1996) Manual for the Beck depression inventory‐II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck, A.T. , Ward, C.H. , Mendelson, M. , Mock, J. & Erbaugh, J. (1961) An inventory for measuring depression. Archives of General Psychiatry, 4, 53–63. [DOI] [PubMed] [Google Scholar]
- Benloucif, S. , Burgess, H.J. , Klerman, E.B. , Lewy, A.J. , Middleton, B. , Murphy, P.J. et al. (2008) Measuring melatonin in humans. Journal of Clinical Sleep Medicine, 4, 66–69. [PMC free article] [PubMed] [Google Scholar]
- Berson, D.M. , Dunn, F.A. & Takao, M. (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science, 295, 1070–1073. [DOI] [PubMed] [Google Scholar]
- Brager, A.J. , Ruby, C.L. , Prosser, R.A. & Glass, J.D. (2010) Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcoholism, Clinical and Experimental Research, 34, 1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, B. , Adams, A.J. , Haegerstrom‐Portnoy, G. , Jones, R.T. & Flom, M.C. (1977) Pupil size after use of marijuana and alcohol. American Journal of Ophthalmology, 83, 350–354. [DOI] [PubMed] [Google Scholar]
- Burgess, H.J. , Rizvydeen, M. , Fogg, L.F. & Keshavarzian, A. (2016) A single dose of alcohol does not meaningfully alter circadian phase advances and phase delays to light in humans. The American Journal of Physiology, 310(8), R759–R765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse, D.J. , Reynolds, C.F. , Monk, T.H. , Berman, S.R. & Kupfer, D.J. (1989) The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Clark, J.H. (1924) The Ishihara test for color blindness. American Journal of Physiological Optics, 5, 269–276. [Google Scholar]
- Conroy, D.A. , Hairston, I.S. , Arnedt, J.T. , Hoffmann, R.F. , Armitage, R. & Brower, K.J. (2012) Dim light melatonin onset in alcohol‐dependent men and women compared with healthy controls. Chronobiology International, 29, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earleywine, M. & Martin, C.S. (1993) Anticipated stimulant and sedative effects of alcohol vary with dosage and limb of the blood alcohol curve. Alcoholism, Clinical and Experimental Research, 17, 135–139. [DOI] [PubMed] [Google Scholar]
- Fargason, R.E. , Fobian, A.D. , Hablitz, L.M. , Paul, J.R. , White, B.A. , Cropsey, K.L. et al. (2017) Correcting delayed circadian phase with bright light therapy predicts improvement in ADHD symptoms: a pilot study. Journal of Psychiatric Research, 91, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridberg, D.J. , Rueger, S.Y. , Smith, P. & King, A.C. (2017) Association of anticipated and laboratory‐derived alcohol stimulation, sedation, and reward. Alcoholism, Clinical and Experimental Research, 41, 1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin, P.D. , Mcdougal, D.H. , Pokorny, J. , Smith, V.C. , Yau, K.W. & Dacey, D.M. (2007) Human and macaque pupil responses driven by melanopsin‐containing retinal ganglion cells. Vision Research, 47, 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau, S.S. , Shang, C.Y. , Merikangas, K.R. , Chiu, Y.N. , Soong, W.T. & Cheng, A.T. (2007) Association between morningness‐eveningness and behavioral/emotional problems among adolescents. Journal of Biological Rhythms, 22, 268–274. [DOI] [PubMed] [Google Scholar]
- Glozier, N. , O'dea, B. , Mcgorry, P.D. , Pantelis, C. , Amminger, G.P. , Hermens, D.F. et al. (2014) Delayed sleep onset in depressed young people. BMC Psychiatry, 14, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler, B.P. , Bruce, S. , Scharf, D. , Ngari, W. & Clark, D.B. (2019) Circadian misalignment and weekend alcohol use in late adolescent drinkers: preliminary evidence. Chronobiology International, 36, 796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler, B.P. & Clark, D.B. (2013) Circadian misalignment, reward‐related brain function, and adolescent alcohol involvement. Alcoholism, Clinical and Experimental Research, 37, 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler, B.P. , Franzen, P.L. , De Zambotti, M. , Prouty, D. , Brown, S.A. , Tapert, S.F. et al. (2017) Eveningness and later sleep timing are associated with greater risk for alcohol and marijuana use in adolescence: initial findings from the National Consortium on alcohol and neurodevelopment in adolescence study. Alcoholism, Clinical and Experimental Research, 41, 1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler, B.P. , Sitnick, S.L. , Shaw, D.S. & Forbes, E.E. (2013) An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Research, 214, 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler, B.P. , Soehner, A.M. , Wallace, M.L. , Logan, R.W. , Ngari, W. , Forbes, E.E. et al. (2021) Experimentally imposed circadian misalignment alters the neural response to monetary rewards and response inhibition in healthy adolescents. Psychological Medicine, 17, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler, B.P. , Wallace, M.L. , Graves, J.L. , Molina, B.S.G. & Pedersen, S.L. (2022) Circadian preference is associated with multiple domains of trait and state level impulsivity. Chronobiology International, 39, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hening, W.A. & Allen, R.P. (2003) Restless legs syndrome (RLS): the continuing development of diagnostic standards and severity measures. Sleep Medicine, 4, 95–97. [DOI] [PubMed] [Google Scholar]
- Hisler, G.C. , Rothenberger, S.D. , Clark, D.B. & Hasler, B.P. (2021) Is there a 24‐hour rhythm in alcohol craving and does it vary by sleep/circadian timing? Chronobiology International, 38, 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock, L. , King, A.C. & De Wit, H. (2000) Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcoholism, Clinical and Experimental Research, 24, 789–794. [PubMed] [Google Scholar]
- Horne, J.A. & Ostberg, O. (1976) A self‐assessment questionnaire to determine morningness‐eveningness in human circadian rhythms. International Journal of Chronobiology, 4, 97–110. [PubMed] [Google Scholar]
- Kanerva, N. , Kronholm, E. , Partonen, T. , Ovaskainen, M.L. , Kaartinen, N.E. , Konttinen, H. et al. (2012) Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiology International, 29, 920–927. [DOI] [PubMed] [Google Scholar]
- Kang, J.I. , Park, C.I. , Sohn, S.Y. , Kim, H.W. , Namkoong, K. & Kim, S.J. (2015) Circadian preference and trait impulsivity, sensation‐seeking and response inhibition in healthy young adults. Chronobiology International, 32, 235–241. [DOI] [PubMed] [Google Scholar]
- Kardon, R. , Anderson, S.C. , Damarjian, T.G. , Grace, E.M. , Stone, E. & Kawasaki, A. (2009) Chromatic pupil responses: preferential activation of the melanopsin‐mediated versus outer photoreceptor‐mediated pupil light reflex. Ophthalmology, 116, 1564–1573. [DOI] [PubMed] [Google Scholar]
- Kelbsch, C. , Strasser, T. , Chen, Y. , Feigl, B. , Gamlin, P.D. , Kardon, R. et al. (2019) Standards in pupillography. Frontiers in Neurology, 10, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway, D.J. (2019) A critical review of melatonin assays: past and present. Journal of Pineal Research, 67, e12572. [DOI] [PubMed] [Google Scholar]
- King, A.C. , De Wit, H. , Mcnamara, P.J. & Cao, D. (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of General Psychiatry, 68, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, A.C. , Houle, T. , De Wit, H. , Holdstock, L. & Schuster, A. (2002) Biphasic alcohol response differs in heavy versus light drinkers. Alcoholism, Clinical and Experimental Research, 26, 827–835. [PubMed] [Google Scholar]
- Klerman, E.B. , Gershengorn, H.B. , Duffy, J.F. & Kronauer, R.E. (2002) Comparisons of the variability of three markers of the human circadian pacemaker. Journal of Biological Rhythms, 17, 181–193. [DOI] [PubMed] [Google Scholar]
- Lewy, A.J. , Cutler, N.L. & Sack, R.L. (1999) The endogenous melatonin profile as a marker for circadian phase position. Journal of Biological Rhythms, 14, 227–236. [DOI] [PubMed] [Google Scholar]
- Littlefield, A.K. , Stevens, A.K. & Sher, K.J. (2014) Impulsivity and alcohol involvement: multiple, distinct constructs and processes. Current Addiction Reports, 1, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina, T.A. & Burgess, H.J. (2011) Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiology International, 28, 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol and Alcoholism . (2005) Helping patients who drink too much: a Clinician's guide. Bethesda, MD: National Institutes of Health. [Google Scholar]
- Negriff, S. , Dorn, L.D. , Pabst, S.R. & Susman, E.J. (2011) Morningness/eveningness, pubertal timing, and substance use in adolescent girls. Psychiatry Research, 185, 408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer, N.C. , Stoohs, R.A. , Netzer, C.M. , Clark, K. & Strohl, K.P. (1999) Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Annals of Internal Medicine, 131, 485–491. [DOI] [PubMed] [Google Scholar]
- Patel, S.R. , Weng, J. , Rueschman, M. , Dudley, K.A. , Loredo, J.S. , Mossavar‐Rahmani, Y. et al. (2015) Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep, 38, 1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, A.J.K. , Vidafar, P. , Burns, A.C. , Mcglashan, E.M. , Anderson, C. , Rajaratnam, S.M.W. et al. (2019) High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proceedings of the National Academy of Sciences of the United States of America, 116(24), 12019–12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters, S. , Van Der Vorst, H. , Burk, W.J. , Wiers, R.W. & Engels, R.C. (2010) Puberty‐dependent sleep regulation and alcohol use in early adolescents. Alcoholism, Clinical and Experimental Research, 34, 1512–1518. [DOI] [PubMed] [Google Scholar]
- Prat, G. & Adan, A. (2011) Influence of circadian typology on drug consumption, hazardous alcohol use, and hangover symptoms. Chronobiology International, 28, 248–257. [DOI] [PubMed] [Google Scholar]
- Provencio, I. , Rodriguez, I.R. , Jiang, G. , Hayes, W.P. , Moreira, E.F. & Rollag, M.D. (2000) A novel human opsin in the inner retina. The Journal of Neuroscience, 20, 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roecklein, K.A. , Franzen, P.L. , Wescott, D.L. , Hasler, B.P. , Miller, M.A. , Donofry, S.D. et al. (2021) Melanopsin‐driven pupil response in summer and winter in unipolar seasonal affective disorder. Journal of Affective Disorders, 291, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg, T. , Wirz‐Justice, A. & Merrow, M. (2003) Life between clocks: daily temporal patterns of human chronotypes. Journal of Biological Rhythms, 18, 80–90. [DOI] [PubMed] [Google Scholar]
- Ruby, C.L. , Brager, A.J. , Depaul, M.A. , Prosser, R.A. & Glass, J.D. (2009) Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. The American Journal of Physiology, 297, R729–R737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, P.M. , Leone, L. , Penolazzi, B. & Natale, V. (2012) Circadian preference and the big five: the role of impulsivity and sensation seeking. Chronobiology International, 29, 1121–1126. [DOI] [PubMed] [Google Scholar]
- Saxvig, I.W. , Pallesen, S. , Wilhelmsen‐Langeland, A. , Molde, H. & Bjorvatn, B. (2012) Prevalence and correlates of delayed sleep phase in high school students. Sleep Medicine, 13, 193–199. [DOI] [PubMed] [Google Scholar]
- Sobell, L.C. & Sobell, M.B. (1995) Alcohol timeline follow‐back users' manual. Toronto, ON: Addiction Research Foundation. [Google Scholar]
- Spielberger, C.D. , Gorsuch, R.L. & Lushene, R.E. (1970) The state‐trait anxiety inventory manual. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Tavernier, R. & Willoughby, T. (2014) Are all evening‐types doomed? Latent class analyses of perceived morningness‐eveningness, sleep and psychosocial functioning among emerging adults. Chronobiology International, 31, 232–242. [DOI] [PubMed] [Google Scholar]
- Taylor, B.J. , Bowman, M.A. , Brindle, A. , Hasler, B.P. , Roecklein, K.A. , Krafty, R.T. et al. (2020) Evening chronotype, alcohol use disorder severity, and emotion regulation in college students. Chronobiology International, 37, 1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, D.J. , Clay, K.C. , Bramoweth, A.D. , Sethi, K. & Roane, B.M. (2011) Circadian phase preference in college students: relationships with psychological functioning and academics. Chronobiology International, 28, 541–547. [DOI] [PubMed] [Google Scholar]
- Urban, R. , Magyarodi, T. & Rigo, A. (2011) Morningness‐eveningness, chronotypes and health‐impairing behaviors in adolescents. Chronobiology International, 28, 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reen, E. , Roane, B.M. , Barker, D.H. , Mcgeary, J.E. , Borsari, B. & Carskadon, M.A. (2016) Current alcohol use is associated with sleep patterns in first‐year college students. Sleep, 39, 1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voultsios, A. , Kennaway, D.J. & Dawson, D. (1997) Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. Journal of Biological Rhythms, 12, 457–466. [DOI] [PubMed] [Google Scholar]
- Whittier, A. , Sanchez, S. , Castaneda, B. , Sanchez, E. , Gelaye, B. , Yanez, D. et al. (2014) Eveningness chronotype, daytime sleepiness, caffeine consumption, and use of other stimulants among Peruvian university students. Journal of Caffeine Research, 4, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann, M. , Dinich, J. , Merrow, M. & Roenneberg, T. (2006) Social jetlag: misalignment of biological and social time. Chronobiology International, 23, 497–509. [DOI] [PubMed] [Google Scholar]
- Yeh, H.H. & Kolb, J.E. (1997) Ethanol modulation of GABA‐activated current responses in acutely dissociated retinal bipolar cells and ganglion cells. Alcoholism, Clinical and Experimental Research, 21, 647–655. [PubMed] [Google Scholar]
- Zele, A.J. , Adhikari, P. , Cao, D. & Feigl, B. (2019) Melanopsin and cone photoreceptor inputs to the afferent pupil light response. Frontiers in Neurology, 10, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zele, A.J. , Feigl, B. , Smith, S.S. & Markwell, E.L. (2011) The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS One, 6, e17860. [DOI] [PMC free article] [PubMed] [Google Scholar]


