Abstract
Purpose:
The PI3K pathway is dysregulated in the majority of triple-negative breast cancer(TNBCs), yet single agent inhibition of PI3K has been ineffective in TNBC. PI3K inhibition leads to an immediate compensatory up-regulation of the Wnt pathway. Dual targeting of both pathways is highly synergistic against TNBC models in vitro and in vivo. We initiated a Phase I clinical trial combining gedatolisib, a pan-class I isoform PI3K/mTOR inhibitor, and cofetuzumab pelidotin, an antibody-drug conjugate against the cell-surface PTK7 protein (Wnt pathway co-receptor) with an auristatin payload.
Experimental Design:
Participants(pts) had metastatic TNBC or ER low (ER and PgR<5%, HER2-negative) breast cancer, and had received at least one prior chemotherapy for advanced disease. The primary objective was safety. Secondary endpoints included objective response(ORR), clinical benefit at 18 weeks(CB18), progression-free survival(PFS), and correlative analyses.
Results:
18 pts were enrolled in 3 dose cohorts: gedatolisib 110 mg weekly + cofetuzumab pelidotin 1.4mg/kg every 3 weeks (n=4), 180mg + 1.4mg/kg (n=3), and 180mg + 2.8mg/kg (n=11). Nausea, anorexia, fatigue, and mucositis were common but rarely reached ≥Grade 3 severity. Myelosuppression was uncommon. ORR was 16.7% (3/18). An additional 3 pts had stable disease, of these 2 had stable disease for >18 weeks; CB18 was 27.8%. Median PFS was 2.0 months (95%CI for PFS:1.2-6.2). Pts with clinical benefit were enriched with genomic alterations in the PI3K and PTK7 pathways.
Conclusions:
The combination of gedatolisib + cofetuzumab pelidotin was well tolerated and demonstrated promising clinical activity. Further investigation of this drug combination in metastatic TNBC is warranted.
Trial Registration:
Keywords: PI3K, PTK7, Gedatolisib, Cofetuzumab Pelidotin, Triple-negative breast cancer
INTRODUCTION
Triple-negative breast cancer (TNBC) is a heterogeneous disease that comprises a minority (15-20%) of breast cancer cases, but has a disproptionally higher mortality (1). TNBC is characterized by the absence of estrogen-receptor (ER), progesterone-receptor (PR), and human epidermal growth factor receptor 2 (HER2) over-expression (2-4). Patients with TNBC have a higher probability of relapse in the first three years after surgery, higher rates of visceral metastases, and shortened overall survival upon onset of metastatic disease when compared to hormone receptor positive and HER2-positive breast cancers(5). Despite the recent FDA approval of immune checkpoint inhibition using pembrolizumab for early-stage (6) and late-stage disease (7), cytotoxic chemotherapy remains the mainstay treatment for metastatic TNBC. Previous attempts to introduce targeted therapies in TNBC by inhibiting the EGFR and c-KIT receptors were unsuccessful(8-11). PARP inhibitors are effective, but only in the subset of patients with deleterious BRCA1/2 mutations(12,13). Sacituzumab govitecan, an antibody drug conjugate (ADC) targeting TROP2 was recently approved by the (FDA) for metastatic TNBC, based on an overall response rate (ORR) of 33.3% in a single-arm Phase II study unselected for TROP2 expression(14). These results were recently confirmed in the Phase III ASCENT study demonstrating an ORR of 35% with sacituzumab compared to 5% with single-agent chemotherapy in patients treated with two prior lines of therapy(15). Clearly there remains a critical need to identify additional novel targeted therapies for TNBC.
The majority of TNBCs (~70%) harbor a genomic aberration in the PI3K pathway(16), most commonly an activating mutation or somatic copy number change in one of the canonical components(17). Somewhat surprisingly, single-agent inhibition of PI3K has been ineffective as a treatment for TNBC(18). We have previously reported that PI3K inhibition in TNBC results in an immediate compensatory upregulation of the Wnt pathway(19). Activation of the Wnt pathway is well-known for its role in cancer metastases and confers resistance to PI3K inhibition in TNBC, pancreatic, and colorectal cell lines(20-22). Simultaneous dual targeting of both pathways is synergistic against TNBC models in vitro and in vivo(19).
Building on our preclinical observations, we initiated a Phase I clinical trial of gedatolisib(23,24), a pan-class I isoform PI3K/mTOR inhibitor, and cofetuzumab pelidotin(25,26), an antibody-drug conjugate against the cell-surface PTK7 protein with an auristatin payload. PTK7 is a pseudo-receptor tyrosine kinase involved in non-canonical Wnt signaling that maintains developmental planar cell polarity in normal cells(27). PTK7 is over-expressed in a variety of cancers including TNBC(25), and its expression is induced by PI3K inhibition therefore providing rationale for exploring the combination of a PI3K inhibitor with a PTK7-targeting ADC in TNBC. The payload for cofetuzumab pelidotin, auristatin, is in itself synergistic with gedatolisib, providing another potential mechanism of synergy for the combination(28).
METHODS
Study population
Pts had metastatic TNBC or ER low (ER and PgR <5%, HER2 negative) breast cancer, and had received at least one prior chemotherapy for metastatic disease. Pts were excluded if previously treated with a PI3K or an mTOR inhibitor or if they had previous exposure to cofetuzumab pelidotin. Pts were required to have an ECOG PS ≤1 with adequate hematologic, hepatic, and renal function. Pts with treated and stable CNS involvement were allowed. Given the potential for hypergylcemia with gedatolisib, pts with uncontrolled diabetes were excluded.
Trial Design and Statistical analyses
This single-center, phase I dose-escalation trial utilized a traditional 3+3 schema with a small expansion cohort at the recommended phase II dose to better characterize safety (Supplementary Figure 1). The primary objective was safety as assessed by NCI CTC v4.0 criteria. Dose limiting toxicity (DLT) was defined as grade 4 neutropenia lasting ≥ 7 days; febrile neutropenia; grade 3 thrombocytopenia associated with bleeding or grade 4 thrombocytopenia lasting ≥ 4 days; any non-hematological grade 3 ≥ toxicity despite the use of adequate/maximal medical interventions and/or prophylaxis; ALT or AST > 3X upper limit of normal (ULN) and total bilirubin >2X ULN with serum alkaline phosphatase normal; any toxicity that resulted in a >14 day delay in treatment; or any death not clearly due to underlying disease or extraneous causes. Secondary endpoints included objective response rate (ORR), clinical benefit at 18 weeks (CB18, prospectively defined as all pts with a complete response, partial response, or stable disease for at least 18 weeks), and progression-free survival (PFS). Exploratory analyses probed for association between genomic/pathologic features and clinical efficacy to identify putative biomarkers for consideration in subsequent trials. The Kaplan-Meier method was used to analyze for PFS and OS (with median times and 95% confidence intervals calculated) using SAS Version 9.4 (Cary, NC). The study was approved by the Institutional Review Board (IRB) at Indiana University; all pts provided written informed consent prior to study entry. This study was conducted in accordance with United States Common Rule.
Treatment plan
The recommended phase 2 dose (RP2D) and toxicity profile had been established for each agent as monotherapy. As overlapping toxicity and pharmacokinetic interactions were not expected, we planned limited dose escalation, starting with 50% of the monotherapy RP2D for each agent in cohort one. The full RP2D of each agent as monotherapy was delivered in cohort 3 (Table 1). Gedatolisib was infused first followed by cofetuzumab pelidotin. Pts were treated prophylactically with a steroid mouthwash prior to administration of gedatolisib to minimize mucositis. Pts were evaluated clinically each week during the first 2 cycles and on day 1 of each subsequent cycle. Serum chemistry was obtained at the start of each cycle; complete blood counts were obtained prior to each gedatolisib infusion. Dose modifications for hematologic and non-hematologic toxicity were pre-specified. Response was evaluated according to RECIST 1.1 criteria every 2 cycles (6 weeks) through week 18, then every 3 cycles thereafter. Tumor biopsies for correlative analyses were obtained at screening and Cycle 1 Day 15 in pts with accessible lesions.
Table 1.
Treatment plan
| No. of pts |
Cohort | Gedatolisib | Cofetuzumab Pelidotin | ||||
|---|---|---|---|---|---|---|---|
| Dose | Frequency of administration |
Route of administration |
Dose | Frequency of administration |
Route of administration |
||
| 4 | 1 | 110 mg | D1,8,15 every 21 days | IV | 1.4 mg/kg | D1 every 21 days | IV |
| 3 | 2 | 180 mg | D1,8,15 every 21 days | IV | 1.4 mg/kg | D1 every 21 days | IV |
| 11 | 3 | 180 mg | D1,8,15 every 21 days | IV | 2.8 mg/kg | D1 every 21 days | IV |
PTK7 and p-AKT Immunohistochemistry
PTK7 immunohistochemistry (IHC) was performed by Flagship Biosciences (Westminster, CO) using a proprietary antibody for staining under a protocol developed by Pfizer(29). Staining for phospho-AKT (pAKT, Ser473), was performed using antibody #4060 procured from Cell Signaling Technology (Beverly, MA) as described previously(30). PTK7 and pAKT. IHC results were assessed by a certified pathologist and quantified by H-score.
Exome library preparation and sequencing
DNA from tumor and matched blood normal was extracted using the Qiagen AllPrep DNA/RNA FFPE kit and QIAamp DNA Blood Mini kit, respectively. DNA library preparation utilized the SureSelect XTHS Target Enrichment System for Illumina Paired-End Multiplexed Sequencing Version A1, July 2017 (Agilent Technologies). Libraries were then hybridized, captured, and amplified with the Agilent Human All Exon V7 probe set (48Mb, hg38). Libraries were sequenced on a NovaSeq 6000 sequencer using 150bp paired-end chemistry (Illumina, Inc.). A Phred quality score (Q score) was used to measure the quality of sequencing. More than 90% of the sequencing reads reached Q30 (99.9% base call accuracy).
Exome variant and copy number analysis
FASTQ files were aligned to the human reference genome (b37/hg19) using BWA (v. 0.7.17, https://bio-bwa.sourceforge.net/).(31) PCR duplicates were removed and coverage metrics were calculated using Picard-tools (v.2.21.2, https://picard.sourceforge.net/). The Genome Analysis Toolkit (GATK, v. 4.1.4.1, https://www.broadinstitute.org/gatk/) was used for SNP and INDEL discovery according to GATK best practices.(32) Copy number variants (CNV) were analyzed using CODEX2 (v.1.3.0).(33)
Data Availability
Raw sequencing data for this study were generated by The Center for Medical Genomics at Indiana University School of Medicine. Derived data supporting the findings of this study are available in the Supplementary Data.
RESULTS
Participant characteristics
We enrolled 18 pts; 10 in three dose escalation cohorts and 8 in expansion at cohort 3. Baseline characteristics are shown in Table 2. Pts were heavily pretreated with the majority having prior exposure to an anthracycline, taxane, platinum, and capecitabine.
Table 2.
Participant Characteristics
| Characteristic | Overall (N=18) |
|---|---|
| Age (year) | |
| Median (Range) | 53 (32-77) |
| Gender, n (%) | |
| Female | 18 (100%) |
| Race, n (%) | |
| White | 17 (94.4%) |
| Black or African American | 1 (5.6%) |
| Ethnicity | |
| Not Latino or Hispanic | 17 (94.4%) |
| Unknown | 1 (5.6%) |
| Prior Therapies | |
| Taxane | 18 (100%) |
| Platinum | 18 (100%) |
| Anthracycline | 17 (94.4%) |
| Capecitabine | 13 (72.2%) |
| Gemcitabine | 8 (44.4%) |
| Eribulin | 6 (33.3%) |
| Vinorelbine | 5 (27.8%) |
| Immunotherapy | 4 (22.2%) |
| PARP inhibitor | 3 (16.7%) |
| Other | 3 (16.7%) |
Safety
Dose escalation was completed with no dose limiting toxicities (DLTs); an additional pt was included in cohort 1 to accommodate an immediate clinical need for treatment. A small expansion cohort proceeded at the full RP2D for monotherapy for both agents. The most common adverse events of any grade were nausea (n=16, 89%), anorexia (n=13, 72%), constipation (n=12, 67%), fatigue (n=12), and mucositis (n=12). Grade 3 or greater toxicity (nausea, n=1; fatigue, n=2) and myelosuppression (Grade ≥3 neutropenia, n=2) were uncommon (Table 3).
Table 3.
Observed toxicities in >10% of pts or observed as grade ≥ 3 using CTCAE criteria.
| CTCAE Term | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total | Total Percent |
Grade 3-5 |
Grade 3-5 Percent |
|---|---|---|---|---|---|---|---|---|---|
| Nausea | 11 | 4 | 1 | 0 | 0 | 16 | 88.89 | 1 | 5.56 |
| Anorexia | 9 | 4 | 0 | 0 | 0 | 13 | 72.22 | 0 | 0.00 |
| Constipation | 10 | 2 | 0 | 0 | 0 | 12 | 66.67 | 0 | 0.00 |
| Fatigue | 2 | 8 | 2 | 0 | 0 | 12 | 66.67 | 2 | 11.11 |
| Mucositis oral | 10 | 2 | 0 | 0 | 0 | 12 | 66.67 | 0 | 0.00 |
| Back pain | 6 | 3 | 2 | 0 | 0 | 11 | 61.11 | 2 | 11.11 |
| Dyspnea | 6 | 1 | 3 | 1 | 0 | 11 | 61.11 | 4 | 22.22 |
| Vomiting | 6 | 2 | 3 | 0 | 0 | 11 | 61.11 | 3 | 16.67 |
| Alopecia | 4 | 6 | 0 | 0 | 0 | 10 | 55.56 | 0 | 0.00 |
| Pruritus | 9 | 0 | 0 | 0 | 0 | 9 | 50.00 | 0 | 0.00 |
| Diarrhea | 6 | 1 | 1 | 0 | 0 | 8 | 44.44 | 1 | 5.56 |
| Dyspepsia | 4 | 4 | 0 | 0 | 0 | 8 | 44.44 | 0 | 0.00 |
| Headache | 6 | 2 | 0 | 0 | 0 | 8 | 44.44 | 0 | 0.00 |
| Anemia | 1 | 4 | 2 | 0 | 0 | 7 | 38.89 | 2 | 11.11 |
| Cough | 7 | 0 | 0 | 0 | 0 | 7 | 38.89 | 0 | 0.00 |
| Insomnia | 6 | 1 | 0 | 0 | 0 | 7 | 38.89 | 0 | 0.00 |
| Neutrophil count decreased | 0 | 4 | 1 | 1 | 0 | 6 | 33.33 | 2 | 11.11 |
| Anxiety | 2 | 3 | 0 | 0 | 0 | 5 | 27.78 | 0 | 0.00 |
| Hyperglycemia | 5 | 0 | 0 | 0 | 0 | 5 | 27.78 | 0 | 0.00 |
| Pain | 1 | 2 | 2 | 0 | 0 | 5 | 27.78 | 2 | 11.11 |
| Blurred vision | 4 | 0 | 0 | 0 | 0 | 4 | 22.22 | 0 | 0.00 |
| Dysgeusia | 3 | 1 | 0 | 0 | 0 | 4 | 22.22 | 0 | 0.00 |
| Edema limbs | 4 | 0 | 0 | 0 | 0 | 4 | 22.22 | 0 | 0.00 |
| Hypokalemia | 1 | 2 | 1 | 0 | 0 | 4 | 22.22 | 1 | 5.56 |
| Pain in extremity | 3 | 1 | 0 | 0 | 0 | 4 | 22.22 | 0 | 0.00 |
| Peripheral sensory neuropathy | 2 | 2 | 0 | 0 | 0 | 4 | 22.22 | 0 | 0.00 |
| Aspartate aminotransferase increased | 2 | 1 | 0 | 0 | 0 | 3 | 16.67 | 0 | 0.00 |
| Chest wall pain | 1 | 2 | 0 | 0 | 0 | 3 | 16.67 | 0 | 0.00 |
| Chills | 3 | 0 | 0 | 0 | 0 | 3 | 16.67 | 0 | 0.00 |
| Lung infection | 1 | 0 | 2 | 0 | 0 | 3 | 16.67 | 2 | 11.11 |
| Mucosal infection | 1 | 1 | 1 | 0 | 0 | 3 | 16.67 | 1 | 5.56 |
| Rash acneiform | 3 | 0 | 0 | 0 | 0 | 3 | 16.67 | 0 | 0.00 |
| Rash maculo-papular | 2 | 0 | 1 | 0 | 0 | 3 | 16.67 | 1 | 5.56 |
| Urinary tract pain | 2 | 1 | 0 | 0 | 0 | 3 | 16.67 | 0 | 0.00 |
| Abdominal pain | 1 | 0 | 1 | 0 | 0 | 2 | 11.11 | 1 | 5.56 |
| Alanine aminotransferase increased | 2 | 0 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Allergic rhinitis | 2 | 0 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Bone pain | 1 | 1 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Dry mouth | 2 | 0 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Infections and infestations - Other, specify | 0 | 2 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Lymphedema | 2 | 0 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Nasal congestion | 1 | 1 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Oral pain | 2 | 0 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Paresthesia | 2 | 0 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Skin and subcutaneous tissue disorders - Other, specify | 2 | 0 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Thromboembolic event | 0 | 2 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Urinary tract infection | 0 | 2 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Urinary urgency | 1 | 1 | 0 | 0 | 0 | 2 | 11.11 | 0 | 0.00 |
| Colitis | 0 | 0 | 1 | 0 | 0 | 1 | 5.56 | 1 | 5.56 |
| Dehydration | 0 | 0 | 1 | 0 | 0 | 1 | 5.56 | 1 | 5.56 |
| Investigations - Other, specify | 0 | 0 | 1 | 0 | 0 | 1 | 5.56 | 1 | 5.56 |
| Respiratory failure | 0 | 0 | 0 | 1 | 0 | 1 | 5.56 | 1 | 5.56 |
| Skin infection | 0 | 0 | 1 | 0 | 0 | 1 | 5.56 | 1 | 5.56 |
| White blood cell decreased | 0 | 0 | 1 | 0 | 0 | 1 | 5.56 | 1 | 5.56 |
There were no grade 5 events observed
Efficacy
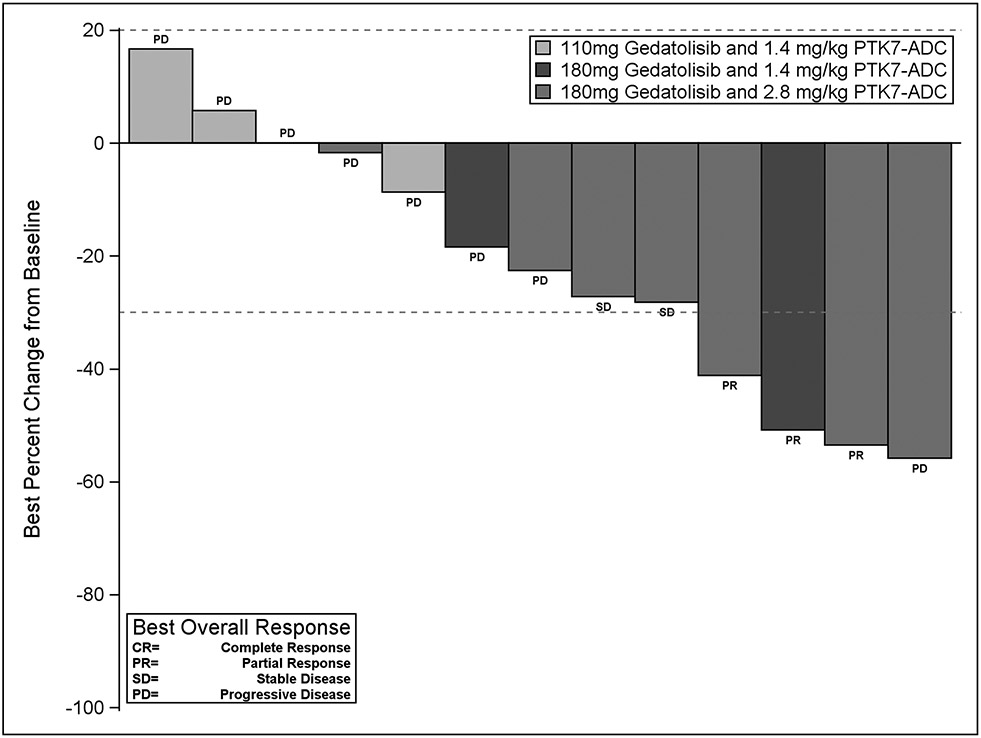
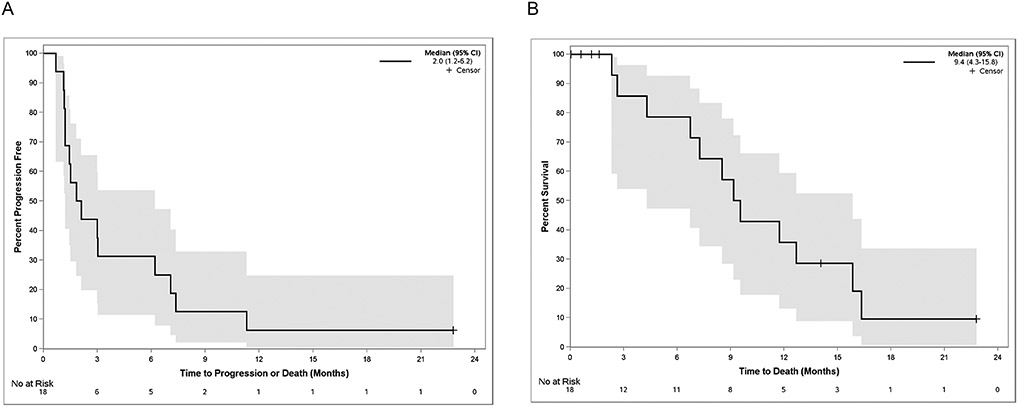
16 of 18 pts enrolled were evaluable for response; two pts discontinued treatment prior to the first disease assessment that was not due to toxicity or progression. Three pts achieved a confirmed partial response (ORR 16.7%, 3/18 including the two non-evaluable patients). An additional 3 pts had stable disease (SD), of these 2 had SD for > 18 weeks. The clinical benefit rate at 18 weeks (CB18) was 27.8% (5/18) (Figure 1). Of note, one pt with SD with Inflammatory TNBC (Pt #: 0613-25) had their only target lesion biopsied and therefore were not eligible for measurement per RECIST criteria, however has had a prolonged exceptional response of ~ 2 years. The pt has been off all anti-cancer treatment for > 15 months. Overall, after a median follow-up of 7.9 months, median progression-free survival (PFS) was 2.0 months (95% CI for PFS:1.2-6.2) (Figure 2A); median overall survival (OS) was 9.4 months (95% CI for OS:4.3-15.8, Figure 2B).
Figure 1.
Waterfall plot demonstrating best tumor response from baseline (y-axis). 18 pts enrolled on trial, 16 were evaluable for response, 13 pts with measurable disease reached at least the first pre-specified imaging timepoint for disease assessment. Pts are color-coded by dose cohort. PR=Partial Response; SD=Stable Disease; PD=Progressive Disease. Of note, the pt with best percent change for the target lesions, unfortunately, in the same visit had increasing/PD for the non-target lesions, resulting in overall best response as PD.
Figure 2.
(A) Kaplan-Meier plot of progression free survival (PFS) demonstrating a median PFS of 2.0 months (95% CI for PFS:1.2-6.2). (B) Kaplan-Meier plot of overall survival (OS) demonstrating a median OS of 9.4 months (95% CI for OS:4.3-15.8).
Correlative analyses
Using whole exome sequencing, we set out to observe if somatic DNA variants were associated with clinical response (Table 4). Of the 18 pts on trial, 15 had successful whole exome sequencing of the baseline biopsy sample. We did not detect abberations in the PI3K pathway or PTK7 in 6 out of 15 pts. In pts who had a partial response (n=3), we observed a PTEN I101N mutation in one pt and a high gain of AKT2 in another. In pts who had stable disease (n=3), one harbored a high gain of AKT2; another a PIK3CA E545A gain-of-function mutation and PTEN single copy deletion; and a third had high gains in AKT2, PIK3CG, and PTK7. In those pts with progressive disease (n=10), we observed an AKT2 S268L gain-of-function mutation in one pt, a PIK3CA H1047R gain-of-function mutation in one pt, a PTK7 variant (of unknown significance), and high gains of AKT2 in 3 pts and PIK3CB in 2 pts. Overall, 5 of 5 pts with either a PR or SD and matched exome sequencing harbored an aberration in the PI3K pathway or PTK7, compared to 4 of 9 pts with progressive disease.
Table 4.
Genomic alterations and immunohistochemistry results with best response. DNA aberrations and copy number variation of focus were in the PI3K pathway (PIK3CA, PIK3CB, PIK3CD, PIK3CG, AKT1, AKT2, AKT3, PTEN) and PTK7. N/A = samples did not have mutations in the PI3K or PTK7 pathway. NS = Not sequenced.
| Patient ID |
Dose Cohort |
Best Response | CB18 | PFS (Months) |
DNA variants |
Copy number variation | PTK7 H-score (Screen) |
PTK7 H-score (C1D15) |
NUC-pAKT (Screen) |
Cyto-pAKT (Screen) |
NUC-pAKT (C1D15) |
Cyto-pAKT (C1D15) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 1 | Progressive disease | 1.12 | N/A | N/A | 120 | 166 | 0 | 0 | 0 | 10 | |
| 3 | 1 | Progressive disease | 1.22 | N/A | N/A | 16 | 25 | 0 | 0 | 0 | 2 | |
| 4 | 1 | Progressive disease | 1.15 | N/A | High Gain: AKT2, PIK3CB | N/A | 232 | 0 | 0 | N/A | N/A | |
| 5 | 1 | Progressive disease | 2.1 | PTK7 E139Q | High Gain: AKT2, PIK3CB | 101 | 22 | 0 | 0 | 0 | 0 | |
| 7 | 2 | Progressive disease | 0.69 | AKT2 S268L | N/A | 197 | N/A | 20 | 1 | N/A | N/A | |
| 8 | 2 | Partial Response | Yes | 11.31 | PTEN I101N | N/A | 116 | N/A | 1 | 200 | 0 | 10 |
| 9 | 2 | Progressive disease | 1.22 | N/A | N/A | 224 | 116 | 0 | 0 | 10 | 0 | |
| 10 | 3 | Progressive disease | 1.84 | N/A | N/A | 68 | 82 | 0 | 0 | 0 | 6 | |
| 12 | 3 | Progressive disease | 1.51 | N/A | N/A | 111 | N/A | 2 | 60 | N/A | N/A | |
| 15 | 3 | Partial Response | Yes | 6.21 | N/A | High Gain: AKT2 | 144 | N/A | 0 | 20 | N/A | N/A |
| 16 | 3 | Progressive disease | 1.45 | PIK3CA H1047R | High Gain: AKT2 | 168 | N/A | 4 | 140 | N/A | N/A | |
| 18 | 3 | Not Evaluable | - | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| 19 | 3 | Partial Response | Yes | 7.36 | NS | NS | N/A | N/A | N/A | N/A | N/A | N/A |
| 20 | 3 | Stable disease | Yes | 7.07 | N/A | High Gain: AKT2 | 3 | 120 | 120 | 200 | N/A | N/A |
| 21 | 3 | Progressive disease | 3.02 | NS | NS | N/A | N/A | N/A | N/A | N/A | N/A | |
| 22 | 3 | Not Evaluable | - | NS | NS | N/A | N/A | N/A | N/A | N/A | N/A | |
| 25 | 3 | Stable disease | Yes | 22.78 | PIK3CA E545A | One copy deletion: PTEN | 0 | N/A | 0 | 0 | N/A | N/A |
| 29 | 3 | Stable disease | 2.99 | N/A | High Gain: AKT2, PIK3CG, PTK7 | 175 | 15 | 0 | 0 | 0 | 100 |
Of note, in our pt with exceptional response to therapy (Pt #: 0613-25) we observed that the pt had a concurrent PIK3CA E545A gain-of-function mutation and a PTEN deletion. These data demonstrate two mutations that are consistent with potential activation of the PI3K pathway and may help explain the exceptional response seen in this pt.
Tissues from research biopsies obtained prior to therapy and at C1D15 were stained for the expression of PTK7 as well as phospho-AKT, a pharmacodynamic marker of PI3K inhibition (Table 4). There was no correlation between IHC H-Scores for either marker and timepoint with clinical benefit. However, due to the small sample size this observation is preliminary.
DISCUSSION
In this study, we have shown that the novel combination of gedatolisib and cofetuzumab pelidotin can be delivered in combination at full doses with manageable toxicity and has clinical activity in pts with heavily pretreated metastatic TNBC. While the PI3K pathway is genomically and transcriptomically aberrant in the majority of TNBCs, single agent treatment with inhibitors of this pathway has resulted in only modest clinical activity, contrary to the theory of oncogene addiction(34,35). Transcriptome reprogramming has emerged as a common response mechanism in tumor cells when exposed to small molecule perturbations(36). Four independent groups have reported that up-regulation of the Wnt pathway is a common compensatory response to PI3K inhibition driving resistance(19-22). Inhibition of both pathways resulted in synergistic anti-tumor efficacy in preclinical models(19). We theorized that we could leverage this compensatory mechanism to inform clinical combination therapy by using a PI3K inhibitor (gedatolisib) to potentially drive up-regulation of PTK7, such that synergy would be achieved with co-treatment with a PTK7-ADC (cofetuzumab pelidotin). Further, previously reported data has also shown that the payload for cofetuzumab pelidotin, auristatin, is in itself synergistic with gedatolisib, providing another potential mechanism for synergistic action of this combination(28).
In this phase I trial, we report the first clinical experience of combining a PI3K pathway inhibitor with an agent that targets a component of the Wnt pathway. The trial successfully completed its dose escalation to RP2D of both agents with no DLTs. Safety data from the dose escalation and our expansion cohort found the combination to be generally well tolerated. Further, while our sample size was limited, we observed clinical activity of the combination with a CB18 of 27.8%. Given the paucity of targeted agents for the majority of patients with TNBC, this combination provides a promising avenue for increasing the clinical armamentarium. Our study was not powered to definitely detect biomarkers of response, however, patients with genomic aberrations in the PI3K pathway or PTK7 may preferentially benefit. Taken together, these data set the stage for larger Phase II studies of the combination in metastatic TNBC.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Targeted therapy options for metastatic triple-negative breast cancer (TNBC) are limited. Previous genomic analyses of large cohorts of TNBC have consistently demonstrated activation of the PI3K pathway. However, single-agent PI3K inhibition has had modest clinical efficacy. Preclinical data from our group and others have demonstrated that up-regulation of the Wnt pathway induces resistance to PI3K inhibition. Herein, we report a Phase I clinical trial of gedatolisib (PI3K/mTOR inhibitor) in combination with cofetuzumab pelidotin, an antibody-drug conjugate targeting the Wnt pathway receptor PTK7, in patients with metastatic TNBC. In this first clinical trial combining a PI3K and Wnt pathway agent we report a favorable safety profile and anti-tumor activity. Further clinical trials testing combinations targeting these two pathways are warranted.
Financial Support:
This work was supported by the NIH/NCI R21CA229951 (M. Radovich); Breast Cancer Research Foundation (K. Miller); 100 Voices of Hope; The Vera Bradley Foundation for Breast Cancer Research; The Catherine Peachey Fund, and the Indiana University Precision Health Initiative. Drug support was provided by Pfizer.
Footnotes
Meeting presentation: The results of this work were presented at the San Antonio Breast Cancer Symposium, December 2020
Disclosure of possible conflicts of interest: MR: Equity in LifeOmic, LLC and Tyme Technologies; Honoraria from Lilly; Contracted Research Support from Lilly, Boston Biomedical; Currently employed at Caris Life Sciences. JPS: Currently employed at Caris Life Sciences. CJB: Currently employed at Caris Life Sciences. BAH: Employed at LifeOmic, LLC. SMB: Employed at LifeOmic, LLC. TJB: Honorarium Medscape, Novartis. BPS: Lilly-Educational, Pfizer, Genentech, Exact Sciences, Foundation medicine-research. SB, SKA, AVS, KDM declare no conflicts of interest.
REFERENCES
- 1.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13(15 Pt 1):4429–34 doi 13/15/4429 [pii] 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 2.Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, et al. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res 2008;14(24):8010–8 doi 14/24/8010 [pii] 10.1158/1078-0432.CCR-08-1208. [DOI] [PubMed] [Google Scholar]
- 3.Pal SK, Childs BH, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat 2011;125(3):627–36 doi 10.1007/s10549-010-1293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol 2010;7(12):683–92 doi nrclinonc.2010.154 [pii] 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 5.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26(8):1275–81 doi JCO.2007.14.4147 [pii] 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 6.Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382(9):810–21 doi 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 7.Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020;396(10265):1817–28 doi 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 8.Modi S, D'Andrea G, Norton L, Yao TJ, Caravelli J, Rosen PP, et al. A phase I study of cetuximab/paclitaxel in patients with advanced-stage breast cancer. Clin Breast Cancer 2006;7(3):270–7. [DOI] [PubMed] [Google Scholar]
- 9.Modi S, Seidman AD, Dickler M, Moasser M, D'Andrea G, Moynahan ME, et al. A phase II trial of imatinib mesylate monotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat 2005;90(2):157–63 doi 10.1007/s10549-004-3974-0. [DOI] [PubMed] [Google Scholar]
- 10.O'Shaughnessy J, W D, V S, K McIntyre, K L, Holmes F, et al. Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer. San Antonio Breast Cancer Symposium 2007(Abstract 308). [Google Scholar]
- 11.Baselga J, Albanell J, Ruiz A, Lluch A, Gascon P, Guillem V, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol 2005;23(23):5323–33 doi JCO.2005.08.326 [pii] 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 12.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 2010;376(9737):235–44 doi S0140-6736(10)60892-6 [pii] 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 13.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361(2):123–34 doi NEJMoa0900212 [pii] 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 14.Kalinsky K, Diamond JR, Vahdat LT, Tolaney SM, Juric D, O'Shaughnessy J, et al. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol 2020;31(12):1709–18 doi 10.1016/j.annonc.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Bardia A, Tolaney S, Loirat Dea. ASCENT: A randomized phase III study of sacituzumab govitecan vs treatment of physician’s choice in patients with previously treated metastatic triple-negative breast cancer. ESMO Virtual Congress 2020. Abstract LBA17 Presented September 19, 2020 2020. [Google Scholar]
- 16.Radovich M, Clare SE, Atale R, Pardo I, Hancock BA, Solzak JP, et al. Characterizing the heterogeneity of triple-negative breast cancers using microdissected normal ductal epithelium and RNA-sequencing. Breast Cancer Res Treat 2014;143(1):57–68 doi 10.1007/s10549-013-2780-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comprehensive molecular portraits of human breast tumours. Nature 2012;490(7418):61–70 doi 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garrido-Castro AC, Saura C, Barroso-Sousa R, Guo H, Ciruelos E, Bermejo B, et al. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res 2020;22(1):120 doi 10.1186/s13058-020-01354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solzak JP, Atale RV, Hancock BA, Sinn AL, Pollok KE, Jones DR, et al. Dual PI3K and Wnt pathway inhibition is a synergistic combination against triple negative breast cancer. NPJ breast cancer 2017;3:17 doi 10.1038/s41523-017-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzeng HE, Yang L, Chen K, Wang Y, Liu YR, Pan SL, et al. The pan-PI3K inhibitor GDC-0941 activates canonical WNT signaling to confer resistance in TNBC cells: resistance reversal with WNT inhibitor. Oncotarget 2015;6(13):11061–73 doi 10.18632/oncotarget.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong Z, Sepramaniam S, Chew XH, Wood K, Lee MA, Madan B, et al. PORCN inhibition synergizes with PI3K/mTOR inhibition in Wnt-addicted cancers. Oncogene 2019;38(40):6662–77 doi 10.1038/s41388-019-0908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park YL, Kim HP, Cho YW, Min DW, Cheon SK, Lim YJ, et al. Activation of WNT/beta-catenin signaling results in resistance to a dual PI3K/mTOR inhibitor in colorectal cancer cells harboring PIK3CA mutations. Int J Cancer 2019;144(2):389–401 doi 10.1002/ijc.31662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Campo JM, Birrer M, Davis C, Fujiwara K, Gollerkeri A, Gore M, et al. A randomized phase II non-comparative study of PF-04691502 and gedatolisib (PF-05212384) in patients with recurrent endometrial cancer. Gynecologic oncology 2016. doi 10.1016/j.ygyno.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro GI, Bell-McGuinn KM, Molina JR, Bendell J, Spicer J, Kwak EL, et al. First-in-Human Study of PF-05212384 (PKI-587), a Small-Molecule, Intravenous, Dual Inhibitor of PI3K and mTOR in Patients with Advanced Cancer. Clin Cancer Res 2015;21(8):1888–95 doi 10.1158/1078-0432.CCR-14-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damelin M, Bankovich A, Bernstein J, Lucas J, Chen L, Williams S, et al. A PTK7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci Transl Med 2017;9(372) doi 10.1126/scitranslmed.aag2611. [DOI] [PubMed] [Google Scholar]
- 26.Tolcher AW, Calvo E, Doger B, Maitland ML, Gibson B, Xuan D, et al. A Phase I Study of PF-06647020, an Antibody-Drug Conjugate Targeting Protein Kinase 7 (PTK7), in Patients with Advanced Solid Tumors. Late Breaking Abstract, Oral Presentation, 2015 European Cancer Congress 2015. [Google Scholar]
- 27.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 2004;430(6995):93–8 doi 10.1038/nature02677nature02677 [pii]. [DOI] [PubMed] [Google Scholar]
- 28.Shor B, Kahler J, Dougher M, Xu J, Mack M, Rosfjord E, et al. Enhanced Antitumor Activity of an Anti-5T4 Antibody-Drug Conjugate in Combination with PI3K/mTOR inhibitors or Taxanes. Clin Cancer Res 2016;22(2):383–94 doi 10.1158/1078-0432.CCR-15-1166. [DOI] [PubMed] [Google Scholar]
- 29.Jackson-Fisher A, Mehra N, Gianani R, Whalen P, Vizcarra P, Deng S, et al. Abstract 4035: Protein tyrosine kinase 7 (PTK7) biomarker analysis in patients (pts) treated with PF-06647020, a PTK7 antibody-drug conjugate (ADC), in a phase I dose expansion study. Cancer Research 2019;79(13 Supplement):4035- doi 10.1158/1538-7445.Am2019-4035. [DOI] [Google Scholar]
- 30.Badve S, Collins NR, Bhat-Nakshatri P, Turbin D, Leung S, Thorat M, et al. Subcellular localization of activated AKT in estrogen receptor- and progesterone receptor-expressing breast cancers: potential clinical implications. Am J Pathol 2010;176(5):2139–49 doi 10.2353/ajpath.2010.090477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009;25(14):1754–60 doi 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics 2013;43:11 0 1– 0 33 doi 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Y, Wang R, Urrutia E, Anastopoulos IN, Nathanson KL, Zhang NR. CODEX2: full-spectrum copy number variation detection by high-throughput DNA sequencing. Genome Biol 2018;19(1):202 doi 10.1186/s13059-018-1578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinstein IB, Joe AK. Mechanisms of disease: Oncogene addiction--a rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol 2006;3(8):448–57 doi 10.1038/ncponc0558. [DOI] [PubMed] [Google Scholar]
- 35.Weinstein IB, Joe A. Oncogene addiction. Cancer Res 2008;68(9):3077–80; discussion 80 doi 10.1158/0008-5472.CAN-07-3293. [DOI] [PubMed] [Google Scholar]
- 36.Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X, et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017;171(6):1437–52 e17 doi 10.1016/j.cell.2017.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing data for this study were generated by The Center for Medical Genomics at Indiana University School of Medicine. Derived data supporting the findings of this study are available in the Supplementary Data.