Abstract
Mindfulness meditation has been shown to increase resting-state functional connectivity (rsFC) between the posterior cingulate cortex (PCC) and dorsolateral prefrontal cortex (DLPFC), which is thought to reflect improvements in shifting attention to the present moment. However, prior research in long-term meditation practitioners lacked quantitative measures of attention that would provide a more direct behavioral correlate and interpretational anchor for PCC–DLPFC connectivity and was inherently limited by small sample sizes. Moreover, whether mindfulness meditation primarily impacts brain function locally, or impacts the dynamics of large-scale brain networks, remained unclear. Here, we sought to replicate and extend prior findings of increased PCC–DLPFC rsFC in a sample of 40 long-term meditators (average practice = 3759 hr) who also completed a behavioral assay of attention. In addition, we tested a network-based framework of changes in interregional connectivity by examining network-level connectivity. We found that meditators had stronger PCC-rostrolateral prefrontal cortex (RLPFC) rsFC, lower connector hub strength across the default mode network, and better subjective attention, compared with 124 meditation-naive controls. Orienting attention positively correlated with PCC–RLPFC connectivity and negatively correlated with default mode network connector hub strength. These findings provide novel evidence that PCC–RLPFC rsFC may support attention orienting, consistent with a role for RLPFC in the attention shifting component of metacognitive awareness that is a core component of mindfulness meditation training. Our results further demonstrate that long-term mindfulness meditation may improve attention and strengthen the underlying brain networks.
INTRODUCTION
Mindfulness meditation is defined as the practice of focusing attention on present-moment experience (Kabat-Zinn, 1990), in contrast to mind-wandering or attending to thoughts about the past or future. Short- and long-term mindfulness meditation training have both been associated with increased resting-state functional connectivity (rsFC) between posterior cingulate cortex (PCC) and dorsolateral prefrontal cortex (DLPFC) and rostrolateral prefrontal cortex (RLPFC) (Creswell et al., 2016; Brewer et al., 2011). These brain regions are implicated in mind-wandering, attentional control, and attention shifting, respectively (Fox, Spreng, Ellamil, Andrews-Hanna, & Christoff, 2015; Hasenkamp, Wilson-Mendenhall, Duncan, & Barsalou, 2012; Smallwood, Brown, Baird, & Schooler, 2012; Spreng, Mar, & Kim, 2008; Burgess, Dumontheil, & Gilbert, 2007; MacDonald, Cohen, Stenger, & Carter, 2000). Increased PCC–DLPFC coupling is thought to reflect better attentional control over mind-wandering (Brewer & Garrison, 2014). However, prior research with long-term mindfulness meditators lacked measures of attention to test the hypothesis that increased PCC–DLPFC connectivity reflects improved attention, and was further limited by small sample sizes of less than 15 participants per group (Brewer et al., 2011). Therefore, the functional relevance of increased PCC–DLPFC connectivity among long-term mediation practitioners remained unclear.
The DLPFC plays a critical role in executive control as measured by behavioral measures, such as the Attention Network Task (ANT; Fan, McCandliss, Fossella, Flombaum, & Posner, 2005), and is a key node of the frontoparietal control network, which is involved in attentional control (Smallwood et al., 2012; MacDonald et al., 2000). Research also indicates a role for DLPFC in orienting attention to mental representations (Nobre et al., 2004). In a study with experienced meditators, DLPFC activation was associated with a shift in attention from mind-wandering to focused attention (Hasenkamp et al., 2012). Moreover, during a nonmeditative state, meditators with more experience had stronger rsFC within attention networks than meditators with less experience during a nonmeditative state (Hasenkamp et al., 2012). A meta-analysis of brain activations associated with open monitoring meditation, which was one of the primary forms of meditation practiced by participants in the current study, included foci in DLPFC and RLPFC (Fox et al., 2016). Finally, behavioral evidence also demonstrates attentional benefits of mindfulness meditation training, including for orienting attention and executive control (van den Hurk, Giommi, Gielen, Speckens, & Barendregt, 2010; Jha, Krompinger, & Baime, 2007; Tang et al., 2007).
The RLPFC is situated in Brodmann's area 10, which is a region that is activated across numerous cognitive tasks. However, taken together, the available evidence lends support to the hypothesis that RLPFC may switch attention between internal and external stimuli (e.g., between self-related processing and task- or goal-oriented processing [Burgess et al., 2007]). According to this hypothesis, RLPFC is part of a system that serves to determine the source of cognitive representations (e.g., internally or externally generated), exerting influence on attention allocation in open-ended situations when goals are self-generated or underspecified (e.g., the task-free setting of a resting-state scan), or when sustained attention is required (Burgess et al., 2007). This hypothesized function for RLPFC is consistent with a role in metacognitive awareness that is a core component of mindfulness meditation training. This form of meta-awareness is specifically cultivated in open monitoring style practices that emphasize broad awareness to present-moment experience while simultaneously monitoring for the presence of mind-wandering, and returning attention to the task of focusing on present-moment experience (Lutz, Jha, Dunne, & Saron, 2015).
In addition to mediation-related effects on frontal regions, a recent meta-analysis of functional neuroimaging studies of meditation practice found that focused attention meditation—an essential ingredient of mindfulness practice—reduced PCC activation (Fox et al., 2016). De-activation of PCC was associated with “undistracted awareness” and “concentration” based on a qualitative analysis of meditators' subjective reports during an fMRI neurofeedback task (Garrison et al., 2013). Moreover, long-term meditators were able to purposefully deactivate PCC through meditation during neurofeedback, whereas meditation-naive control participants were unable to do so (Garrison et al., 2013). Therefore, PCC likely plays a central role in meditation-related improvements in attention, and studies on long-term mindfulness practitioners may provide unique insights into the effects of mindfulness meditation on brain function and connectivity, shedding light on unique mechanisms of change as a function of specific stages of training (Kral et al., 2018; Brefczynski-Lewis, Lutz, Schaefer, Levinson, & Davidson, 2007).
The current study builds on this literature by examining relationships between long-term mindfulness meditation practice and PCC rsFC in a sample of 40 meditators compared to 124 meditation-naive controls who were later assigned to an intervention as part of a randomized controlled trial (RCT; as detailed in the work of Kral et al., 2019). Following prior work, we examined changes in PCC rsFC with three ROIs: left and right DLPFC, and left RLPFC. The DLPFC ROIs were anatomically defined based on the middle frontal gyrus (depicted in teal in Figure 1C), and overlapped with ROIs where long-term meditation practice was previously shown to relate to stronger rsFC with PCC (Brewer et al., 2011). Given the size and potential for functional heterogeneity within this DLPFC ROI, we conducted analysis of mean ROI connectivity, as well as voxelwise analysis within the DLPFC mask. The RLPFC ROI (depicted in green Figure 1C) was defined based on coordinates from a study showing a significant effect of mindfulness meditation training on PCC rsFC (Creswell et al., 2016) and was located rostral to the canonical DLPFC. We previously examined changes in PCC rsFC with this RLPFC ROI following mindfulness-based stress reduction (MBSR) in a subsample of participants from the current study (Kral et al., 2019), and the current study extends this analysis to test for effects of long-term mindfulness training. In addition, we tested for relationships between PCC rsFC and total lifetime home and retreat practice hours, separately, and hypothesized that more hours of lifetime meditation practice would be associated with a larger increase in PCC rsFC.
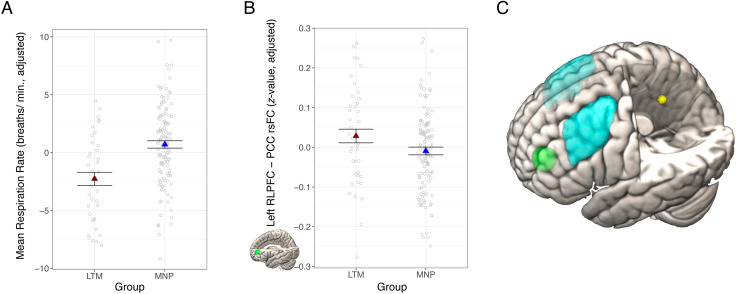
Figure 1. .
Long-term mindfulness meditation practice associated with slower respiration rate and stronger PCC-RLPFC rsFC. (A) Meditators had slower average respiration rate than nonmeditators. (B) Meditators had stronger PCC rsFC with RLPFC compared with meditation-naive participants. The RLPFC target region is depicted in green, and the PCC seed region is depicted in yellow. (C) The dorsolateral pFC ROIs are in light blue (based on the middle frontal gyrus from the Harvard–Oxford atlas [Craddock et al., 2012]), the RLPFC ROI is depicted in green, and the PCC seed is in yellow (with the latter two based on coordinates from a prior study (Creswell et al., 2016). Dependent variables and data points are adjusted for age and sex, and an additional covariate for scan acquisition version in (B). Error bars represent 1 standard error above and below the point estimates of the means. PCC = posterior cingulate cortex; rsFC = resting-state functional connectivity; LTM = long-term meditator; MNP = meditation naive participant; RLPFC = rostrolateral prefrontal cortex; min = minute.
We expected to replicate prior results of increased PCC resting-state connectivity with DLPFC and RLPFC in this larger sample of 40 long-term meditators relative to a meditation-naive, age-matched control group, which would comprise a substantial advancement over prior reports with limited sample sizes (e.g., less than 15 participants per group; Brewer et al., 2011). Because individual differences in physiology may affect fMRI measures (de la Cruz et al., 2019) and long-term meditation has been associated with lower respiratory rate (Wielgosz, Schuyler, Lutz, & Davidson, 2016), we also tested for group differences in heart rate and respiration rate, and conducted sensitivity analysis to test whether group differences in rsFC persisted after controlling for these physiological measures. We also sought to extend prior research findings in two critical ways. First, informed by our prior work, we examined relationships between PCC connectivity and measures of attention, which is critical for interpreting rsFC differences between long-term meditators and controls. To that end, we included two self-report measures and one behavioral measure: the attention scale of the Emotional Styles Questionnaire (ESQ; Kesebir, Gasiorowska, Goldman, Hirshberg, & Davidson, 2019) and experience sampling via text messaging (a.k.a., ecological momentary assessment), and the ANT. The ANT provided a well-validated, behavioral measure of three types of attention: alerting, orienting, and executive control (Fan et al., 2009). Prior research has found associations between long-term training in mindfulness meditation with faster orienting attention and better attentional efficiency (van den Hurk et al., 2010), as well as improvements in ANT performance following short-term meditation training (Trautwein, Kanske, Böckler, & Singer, 2020; Ainsworth, Eddershaw, Meron, Baldwin, & Garner, 2013).
Second, we examined group differences in graph theoretical metrics indexing network topography. We assessed whether rsFC differences associated with mindfulness meditation are regionally specific to the PCC, DLPFC, and RLPFC seeds, or whether they may instead reflect underlying differences in the overall dynamics of separate networks to which these regions belong. To that end, we focused on two graph theoretical measures obtained from resting connectivity estimates: within-module degree (WMD) and participation coefficient, which index within- and between-module(s) hub properties, respectively. Within-module degree indicates high local connectivity of a given node to other nodes within the same module, whereas participation coefficient denotes the diversity of intermodular connections. More specifically, a provincial hub is a node with high WMD. In contrast, a connector hub has a high participation coefficient and is posited to contribute to global intermodular integration (Rubinov & Sporns, 2010).
If higher PCC–RLPFC connectivity in meditators reflects a more general difference in network dynamics, such that these modules are more integrated, then we would expect to see higher participation coefficients for one or both of the corresponding networks (default mode network [DMN] and frontoparietal control network, respectively). Given the interpretation of higher PCC–RLPFC connectivity as reflecting increased attentional control by the frontoparietal control network on DMN, we hypothesized that meditators would have higher participation coefficients than meditation-naive participants in the frontoparietal control network, and lower WMD in the DMN. In addition, we assessed hub properties for the dorsal attention network, in which DLPFC participates.
In summary, we here sought to (a) replicate prior research showing mindfulness meditation-related increases in PCC functional connectivity with DLPFC and RLPFC, (b) extend the literature to determine whether such differences were associated with improvements in attentional measures, and (c) assess network connectivity metrics derived from graph theory.
METHODS
This study is registered as a clinical trial with ClinicalTrials.gov (NCT02157766). Although the details of the trial design of the current study are present in the clinical trial registration, the specific analysis described here, and the outcomes, were not registered. Some of the methods detailed below were previously described (Kral et al., 2019).
Participants
We recruited 183 healthy participants from a nonclinical population, composed of 140 meditation-naive participants and 43 meditators. Meditation-naive participants (average age 44.3 ± 12.8 years, 83 women) were recruited from Madison, WI, and the surrounding community using flyers, on-line advertisements, and advertisements in local media for a study researching “the impact of health wellness classes on the brain and body.” Sample size was determined based on a power analysis. The baseline data for meditation-naive participants (i.e., those in the RCT) served as a control group in this cross-sectional study. Seventeen meditation-naive participants had rsFC data excluded from analysis because of excessive motion (described below; n = 11) or anatomical brain abnormalities as determined by a neuroradiologist (n = 6), resulting in inclusion of 123 meditation-naive participants (average age ± SD = 42.4 ± 12.4 years, 74 women, 49 men) in analyses reported here.
Meditators were recruited from meditation centers and through related mailing lists throughout the United States, in addition to flyers and advertisements in newspapers similar to the recruitment strategy for meditation-naive participants. Meditation-related recruitment criteria included at least 5 years of daily practice (with an average practice of at least 200 min per week), experience with Vipassana, concentration, and compassion/loving-kindness meditations, and at least 5 weeks of retreat practice. Meditation retreats involve spending a continuous period of days or weeks (and in some cases, years) in meditation practice, often at a meditation or community center. Retreats often include group practice, in addition to solitary practice, and may include extended periods of silence. Lifetime hours of practice were calculated based on participants' reports of their average hours of formal meditation practice per week and their total years of practice (average = 3759 hr, range = 780–19,656 hr). Lifetime retreat practice hours were calculated by summing the practice hours that were reported for each retreat. Practice hours were log-transformed using the natural log, to correct for a highly right-skewed distribution. Three meditators had rsFC data excluded from analysis because of excessive motion (n = 2) or anatomical brain abnormalities (n = 1), resulting in 40 meditators in the final sample for analysis (average age ± SD = 44.1 ± 11.8 years, 15 women, 25 men).
Participants were excluded if any of the following applied, because of their potential impact on the current analyses or other aspects of the larger study in which they were enrolled: regular use of psychotropic or nervous system altering medication; psychiatric diagnosis in the past year or history of bipolar disorder, schizophrenia or schizoaffective disorder; color blindness; currently participating in another clinical trial (meditation-naive participants only); current asthma diagnosis; currently diagnosed with a sleep disorder or regularly taking prescribed sleeping medications; current night shift worker; significant training or practice in meditation or mind–body techniques such as yoga or Tai-Chi (meditation-naive participants only); expert in physical activity, music, or nutrition (meditation-naive participants only); any history of brain damage or seizures; and medical conditions that would affect the participant's ability to participate in study procedures. Written, informed consent was obtained from all participants according to the Declaration of Helsinki (World Medical Association, 2013), and the study was approved by the Health Sciences institutional review board at the University of Wisconsin–Madison.
Data Collection
Participants attended a 24-hr laboratory visit at the Waisman Laboratory for Brain Imaging and Behavior that included an MRI scan, behavioral testing, self-report data collection, and additional measures as part of a larger multisession, multiproject study. Experimenters were blind to the group assignment during data collection. All participants were given monetary compensation for their participation.
Emotional Style Questionnaire
The ESQ (Kesebir et al., 2019) consists of a 1–7 Likert scale with 1 = strongly disagree and 7 = strongly agree. One of the ESQ subscales provided a measure of attention that was most relevant to the hypotheses of the current study, and items included: “I do not get distracted easily, even when I am in a situation in which a lot is going on” and “I sometimes feel like I have very little control over where my attention goes” (reverse-coded). A subset of meditation-naive participants (n = 93, average age ± SD = 44.1 ± 11.8 years, 48 women, 45 men) completed the ESQ, which was introduced subsequent to the onset of data collection because of availability of the measure. All meditators completed the ESQ.
ANT
The ANT was performed outside the scanner. Stimuli were presented using E-prime 2.0 (E-Prime 2.0, 2012), and the task was administered as described in prior literature (Fan et al., 2009). We calculated RT for each condition after excluding error trials, and RTs from trials longer than 1200 msec (errors of omission) and shorter than 250 msec (impulsive responses) were also excluded. Two participants were excluded because of low accuracy (one participant had 18% accuracy on congruent trials, and one participant had 51% accuracy on incongruent trials).
MRI Acquisition
Images were acquired on a GE MR750 3.0 Tesla MRI scanner with a 32-channel head coil. Anatomical scans consisted of a high-resolution 3-D T1-weighted inversion recovery fast gradient echo image (inversion time = 450 msec, 256 × 256 in-plane resolution, 256-mm field of view, 192 × 1.0 mm axial slices). A 12-min. functional resting-state scan run was acquired using a gradient echo EPI sequence (360 volumes, repetition time/echo time/flip = 2000 msec/20 msec/75°, 224-mm field of view, 64×64 matrix, 3.5 × 3.5 mm in-plane resolution, 44 interleaved sagittal slices, 3-mm slice thickness with 0.5-mm gap). The in-plane resolution was decreased after the first 21 participants from 3.5 × 3.5 mm to 2.33 × 3.5 mm to better address sinus-related artifacts, resulting in a matrix of 96 × 64.
Experience Sampling
Experience sampling was conducted for 1 week following the laboratory visit. Participants provided their mobile phone numbers and availability for 8-hr periods for each of the 7 days. Participants had a choice of receiving text messages 6, 7, or 8 times a day, and received a text message every 90 min on average. The text message contained a question assessing mind-wandering: “Was your attention on the activity you were performing?” Participants were asked to respond with a number from 1 (attention is not on the task) to 9 (attention is completely on the task at hand). On average, participants responded to 82% of text messages they received. The response window was set to the time between two successive messages, such that participants were given until the next message arrived to respond to the current message. If participants sent two responses in-between messages, the second response was discarded. The ratings across all 7 days of the week were averaged to obtain a mean attention rating for each participant.
Data Analysis
Functional Image Processing and Analysis
Functional images were processed using a combination of Analysis of Functional NeuroImages (AFNI; Cox, 1996) Version 17.3 and FMRI Expert Analysis Tool Version 6.00, part of FMRIB's Software Library (Smith et al., 2004), using the following steps: removal of the first four volumes; motion correction with FMRIB's linear image registration tool (MCFLIRT; Jenkinson, Bannister, Brady, & Smith, 2002); skull removal with the brain extraction tool (BET; Smith, 2002); and registration of the individual's functional data to their anatomical image using the Boundary-Based Registration approach (Greve & Fischl, 2009). A 12-degree of freedom affine transformation using FMRIB's linear image registration tool (FLIRT; Jenkinson et al., 2002) was followed by FMRIB's nonlinear image registration tool (FNIRT) transformation to register each participant's functional data to Montreal Neurological Institute 152 space. Images were segmented into white matter, gray matter, and cerebrospinal fluid with FMRIB's automated segmentation tool for use as masks that were eroded using a 3 × 3 × 3 voxel kernel and then used to generate ROI-averaged time series, with white matter and cerebrospinal fluid time-series serving as nuisance regressors (along with their derivatives and the six motion regressors) with AFNI's 3dDeconvolve. Images were smoothed with a 5-mm FWHM Gaussian kernel.
We extracted the time-series from a spherical PCC seed with a 4-mm radius defined based on coordinates from Creswell et al. (2016; Figure 1C, in yellow). We regressed each time-series (separately) back onto each participant's data using AFNI's 3dDeconvolve, which also censored high-motion time-points (greater than 0.2-mm framewise displacement; Power et al., 2014). Participants were excluded from analysis if they had less than 6 min of data because of more than 50% of data points censored for motion. Two sets of target ROIs were defined for assessing PCC rsFC: a bilateral DLPFC ROI, based on medial frontal gyrus from the Harvard–Oxford atlas (Craddock, James, Holtzheimer, Hu, & Mayberg, 2012) thresholded at 50% probability for small-volume-corrected voxelwise analysis (Figure 1C, in light blue), which was split into left and right for ROI analysis; and a left RLPFC ROI defined as a 10-mm sphere around coordinates provided in the work of Creswell et al. (2016; Figure 1C, in green). There was no overlap between the RLPFC ROI defined from the literature and the anatomically defined DLFPC ROIs. Connectivity was assessed based on the Fisher-z transformed correlation between the seed and every other voxel in the brain for the voxelwise analysis, and separately for each of the target ROIs. Voxelwise analyses were thresholded at p < .05 controlling for family-wise error using threshold-free cluster enhancement with FMRIB's Software Library's Randomise (Winkler, Ridgway, Webster, Smith, & Nichols, 2014).
Graph Theoretical Network Analysis
We calculated hub connectivity metrics for the default mode (Figure 2B), frontoparietal control, and dorsal attention networks based on the Gordon connectivity atlas (Gordon et al., 2016). First, the mean resting-state time-series was extracted from each of the 333 nodes in the Gordon atlas, and then, we constructed a correlation matrix for each participant by computing pairwise Pearson correlations for each set of nodes. We used the correlation matrix to calculate both provincial and connector hub properties of each node using WMD and participation coefficient measures, respectively, (with nodes assigned to networks as defined in the Gordon atlas) following the procedures detailed by Hwang, Bertolero, Liu, and D'Esposito (2017). We tested for group differences in hub connectivity across all nodes in the respective network (default mode, frontoparietal control or dorsal attention), separately, by estimating linear mixed-effects models with the lmer and anova functions in R statistics (Kuznetsova, Brockhoff, & Christensen, 2017; R Core Team, 2013), which included by-subject random effects and covariates to control for age, sex, and scan acquisition version.
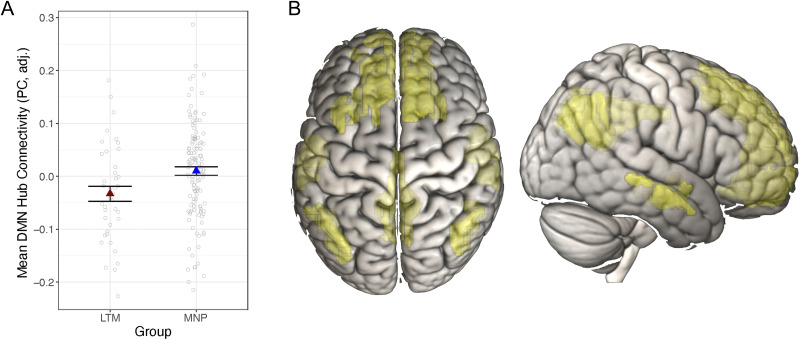
Figure 2. .
Long-term mindfulness meditation practice associated with lower DMN hub connectivity. (A) Meditators had lower DMN hub connectivity (assessed via participation coefficients [PC] across all DMN nodes) compared with meditation-naive participants. Dependent variables and data points are adjusted for age, sex, and scan acquisition version. Data points represent average (adjusted) PC values for each participant. Error bars represent 1 standard error above and below the point estimates of the means. (B) The DMN is in yellow based on the Gordon atlas of resting-state networks (Gordon et al., 2016). LTM = long-term meditator; MNP = meditation-naive participant; DMN = default mode network.
Physiological Measures
Respiration and heart rate data were collected during the concurrent resting-state fMRI scan, amplified using a BIOPAC MP-150 system, and digitized at 1000 Hz. Mean heart rate was assessed using CMetX (Hibbert, Weinberg, & Klonsky, 2012) and interbeat interval series were corrected for artifact and ectopic beats. Heart rate data were cleaned by interpolating over ectopic or artifactual interbeat interval using in-house MATLAB software (Allen, Chambers, & Towers, 2007). Respiration data were collected using a respiration belt placed over the thorax, data were inspected for artifact, and artifacts were rejected. Mean respiration rate was calculated with in-house MATLAB scripts using trough-to-trough measurements on the cleaned respiration series.
Statistical Analysis
Linear models were conducted using the lm function in the stats package in R (R Core Team, 2013), in which rsFC (or other dependent variable) was regressed on group (or other variables of interest). All analyses included covariates for age and sex, and analyses of rsFC included an additional covariate for the change in the resting-state scan acquisition (as described above). All results are reported after removing outliers based on Cook's d, with a cutoff threshold of 4/(n − p) for data points disconnected from the distribution (where n = sample size and p = number of parameters in the model) as determined by the modelCaseAnalysis function of the lmSupport package (Curtin, 2015) in R (R Core Team, 2013). The number of outliers removed from each analysis ranged from zero to five meditation-naive participants, and from zero to two meditators. Results remain consistent when outliers are included in the model, except in one case, as described below. We used a false discovery rate correction to control for multiple comparisons for each family of tests (e.g., across three ROIs), using the p.adjust function in R statistics, and corrected p values are indicated by p*.
RESULTS
Descriptive statistics for dependent variables by group are presented in Table 1.
Table 1. .
Descriptive Statistics
| Measure | Long-Term Meditators | Meditation-Naive Participants | ||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | Min | Max | M | SD | Min | Max | |
| R DLPFC-PCC rsFC (r) | 0.17 | 0.14 | −0.12 | 0.51 | 0.13 | 0.12 | −0.10 | 0.49 |
| L DLPFC-PCC rsFC (r) | 0.19 | 0.12 | −0.01 | 0.54 | 0.16 | 0.10 | −0.09 | 0.51 |
| L RLPFC-PCC rsFC (r) | 0.17 | 0.14 | −0.13 | 0.46 | 0.12 | 0.11 | −0.11 | 0.40 |
| DMN participation coeff. | 0.28 | 0.09 | 0.09 | 0.48 | 0.33 | 0.09 | 0.10 | 0.59 |
| FPN participation coeff. | 0.34 | 0.09 | 0.13 | 0.49 | 0.34 | 0.10 | 0.02 | 0.51 |
| DAN participation coeff. | 0.32 | 0.10 | 0.13 | 0.54 | 0.33 | 0.12 | 0.06 | 0.59 |
| DMN within-module deg. | 0.21 | 0.22 | −0.21 | 0.58 | 0.22 | 0.20 | −0.31 | 0.64 |
| FPN within-module deg. | −0.03 | 0.21 | −0.55 | 0.47 | 0.02 | 0.22 | −0.54 | 0.64 |
| DAN within-module deg. | −0.11 | 0.23 | −0.47 | 0.58 | −0.11 | 0.22 | −0.57 | 0.47 |
| Attention (ESQ) | 4.84 | 0.91 | 2.86 | 6.43 | 4.39 | 1.01 | 1.86 | 6.43 |
| Attention (ES) | 7.39 | 1.06 | 4.72 | 8.96 | 6.68 | 1.09 | 3.57 | 9.00 |
| ANT-R orienting RT (msec) | 93.2 | 42.0 | −0.27 | 192.8 | 101.0 | 40.1 | 9.21 | 206.7 |
| ANT-R executive RT (msec) | 135.5 | 41.3 | 38.5 | 270.0 | 137.4 | 41.6 | 8.8 | 307.4 |
| ANT-R alerting RT (msec) | 23.0 | 25.5 | −33.6 | 76.0 | 19.4 | 26.6 | −41.9 | 117.3 |
R = right; L = left; DLPFC = dorsolateral prefrontal cortex; PCC = posterior cingulate cortex; rsFC = resting-state functional connectivity; RLPFC = rostrolateral prefrontal cortex; DMN = default mode network; FPN = frontoparietal control network; DAN = dorsal attention network; coeff. = coefficient; deg. = degree; ESQ = Emotional Styles Questionnaire; ES = experience sampling; ANT-R = Attention Network Task–Revised; M = mean; SD = standard deviation; Min = minimum; Max = maximum.
Physiological Measures
Group differences in respiration rate and heart rate may contribute to or confound rsFC differences between meditators and meditation-naive participants (Chen et al., 2020; de la Cruz et al., 2019), and previous research has reported reduced respiration rate in long-term meditators (Wielgosz et al., 2016). Therefore, we examined group differences in respiration and heart rates in this sample. Meditators had significantly lower respiration rate than meditation-naive participants, t(157) = 4.60, p < .001, b = 3.04, CI [1.73, 4.34] (Figure 1A), consistent with prior research. There was no difference in heart rate between meditators and meditation-naive participants, t(148) = −0.46, p = .64, b = −0.70, CI [−3.68, 2.28].
Resting-State Brain Connectivity
ROI Analysis
We tested for differences in PCC rsFC between meditators and meditation-naive participants using a linear model in which we regressed average rsFC Fisher-z transformed r values (from DLPFC and RLPFC ROIs) on group, including covariates for age, sex, and scan resolution (which was increased partway through data collection to better address sinus-related artifacts. See Methods section for additional details). There were no differences in PCC rsFC between meditators and meditation-naive participants with the anatomically generated DLPFC ROIs (right DLPFC: t(155) = −1.57, p = .12, p* = .18, b = −0.04, CI [−0.08, 0.01]; left DLPFC: t(157) = −0.79, p = .43, p* = .43, b = −0.02, CI [−0.05, 0.02]). Thus, we failed to replicate prior findings of increased PCC-DLPFC functional connectivity associated with long-term meditation practice (Brewer et al., 2011).
Meditators had stronger rsFC than meditation-naive participants between PCC and left RLPFC, t(154) = −2.78, p = .007, p* = .021, b = −0.06, CI [−0.10, −0.02] (Figure 1B). These results remained significant when we added respiration rate and heart rate to the model, and when outliers were included in the model, except for a single long-term meditator outlier where the result was marginal when the outlier was included (p = .05). Subsequent ROI analyses focused on the RLPFC ROI in which we found significant group differences.
Network Analysis
We next examined network-level connectivity measures to test whether long-term mindfulness meditation training was associated with connectivity differences in the global brain networks in which PCC, DLPFC, and RLPFC are embedded. We regressed participation coefficients and WMDs (separately) for all nodes of the default mode, dorsal attention, and frontoparietal networks, onto group in a linear mixed effects model, including by-subject random intercepts. We included covariates for age, sex, and scan acquisition. As with the ROI analyses, we also conducted sensitivity analyses to test whether the results held when controlling for between-subjects variance in heart rate and respiration rate.
Meditators' DMN nodes had significantly lower participation coefficients compared with meditation-naive participants', F(167) = 7.02, p = .009, p* = .027 (Figure 2A). Descriptive figures of DMN graph properties are presented separately by group in Figure 3. Similar to the ROI analysis, we added heart rate and respiration rate to the model to test whether these physiological variables accounted for additional or overlapping variance in hub connectivity. Whereas there was no significant effect of heart rate (p = .51) or respiration rate (p = .63) on DMN participation coefficients, above and beyond effects of the other variables, the effect of group on participation coefficients in the DMN became marginal, F(151) = 3.62, p = .059, when including these covariates. Contrary to our hypothesis, there were no differences between groups for participation coefficients in the frontoparietal control network, F(143) = 0.40, p = .53, or in the dorsal attention network, F(154) = 0.14, p = .71. There were no group differences for WMD for any of the networks (dorsal attention network: F(154) < 0.010, p = .98; DMN: F(155) = 0.02, p = 0.89; fronto-parietal network: F(143) = 2.47, p = .12).
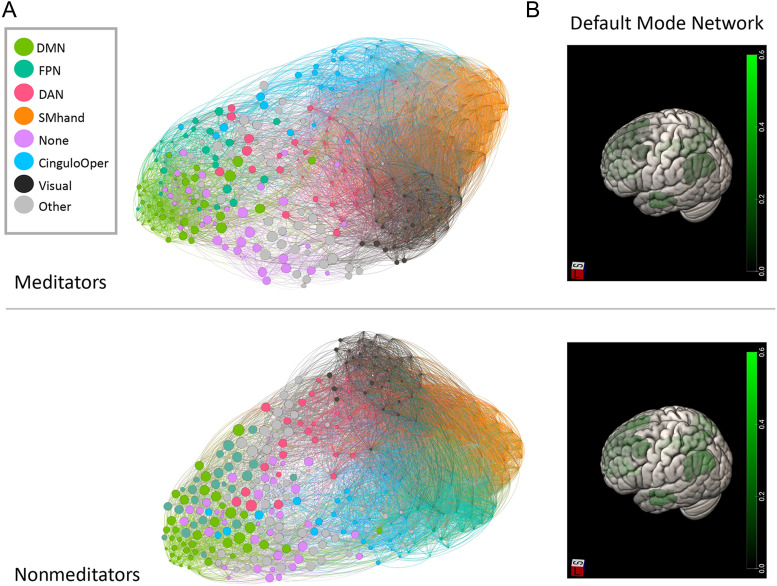
Figure 3. .
Descriptive figures of network participation coefficients by group. (A) Depiction of nodes scaled by the participation coefficient (PC) and colored by network with networks mapped to color in the legend. (B) Heat map of DMN participation coefficients by group. DMN = default mode network; FPN = fronto-parietal network; DAN = dorsal attention network; SMhand = supplementary motor, hand; CinguloOper = cingulo-opercular network; Other = other network or subcortical region.
Attention
We regressed each attention measure on group (separately), while controlling for age and sex.
Self-report Measures of Attention
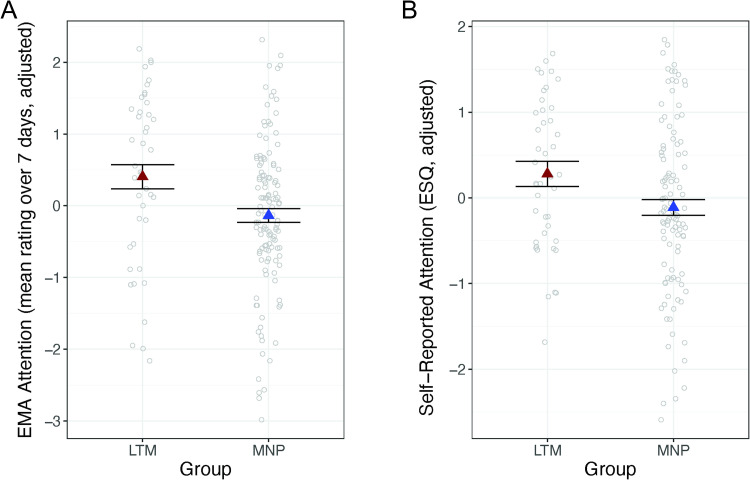
Meditators reported significantly greater attention to task than meditation-naive participants, on average, during 1 week of experience sampling, t(161) = −3.42, p < .001, b = −0.67, CI [−1.06, −0.28] (Figure 4A). Meditators also reported higher attention than meditation-naive participants on the ESQ, t(146) = −2.26, p = .026, b = −0.40, CI [−0.75, −0.05] (Figure 4B).
Figure 4. .
Long-term mindfulness meditation practice associated with improved self-reported attention. (A) Meditators had higher attention to task compared with meditation-naive participants based on experience sampling. (B) Meditators had higher self-reported attention than meditation-naive participants based on the Emotional Styles Questionnaire (ESQ) attention scale. Dependent variables and data points are adjusted for age and sex. Error bars represent 1 standard error above and below the point estimates of the means. LTM = long-term meditator; MNP = meditation-naive participant; ES = experience sampling; EMA = ecological momentary assessment.
Behavioral Measures of Attention: ANT
There were no differences between groups in RT on the ANT for orienting, t(169) = 1.45, p = .15, p* = .48, b = 10.41, CI [−3.81, 24.62] (Figure 5A), alerting, t(167) = −1.38, p = .17, p* = .48, b = −6.43, CI [−15.64, 2.78], or executive control, t(165) = 0.60, p = .55, p* = .55, b = 4.36, CI [−10.01, 18.72]. The results remained consistent (and nonsignificant) when using normalized RT, as in van den Hurk et al. (2010). Moreover, there were no group differences in accuracy for any trial type (orienting: t(178) = −0.60, p = .55; alerting: t(175) = 0.49, p = .62; executive control: t(175) = −1.62, p = .11).
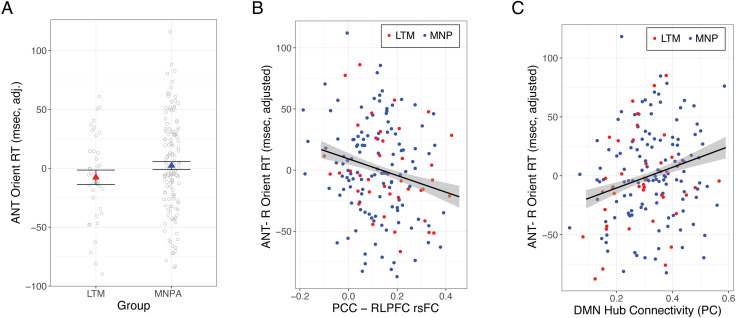
Figure 5. .
Long-term mindfulness meditation practice, orienting attention, and PCC–rlPFC rsFC. (A) There was no difference between meditators and meditation-naive participants in orienting attention RT on the ANT. (B) Stronger PCC–rlPFC rsFC was associated with faster orienting attention, across all participants. (C) Lower DMN hub connectivity (assessed via participation coefficients [PC]) was associated with faster orienting attention, across participants. Dependent variables and data points are adjusted for age and sex. Error bars represent 1 standard error above and below the point estimates of the means. LTM = long-term meditator; MNP = meditation-naive participant; PCC = posterior cingulate cortex; rlPFC = rostrolateral prefrontal cortex; rsFC = resting-state functional connectivity; ANT = Attention Network Task; DMN = default mode network.
Brain–Behavior Relationships
In order to directly test relationships between rsFC metrics and attention, we regressed each attention measure (separately) onto rsFC, controlling for age, sex, and scan acquisition. Stronger PCC–RLPFC connectivity was associated with faster attentional orienting, t(156) = −2.77, p = .007, p* = .03, b = −78,34, CI [−134.13, −22.55] (Figure 5B) across all participants, and this relationship remained significant when heart and respiration rate were added to the model (p = .005). Post hoc tests showed that the relationship between stronger PCC–RLPFC connectivity and faster orienting attention was significant within the meditation-naive group, t(117) = −2.03, p = .045, b = −72.79, CI [−143.82, −1.75], and marginal among meditators, t(35) = −1.78, p = .083, b = −91.85, CI [−196.38, 12.69], likely because of the smaller sample size and reduced statistical power in the latter group. There was no relationship between PCC–RLPFC connectivity and alerting, t(158) = 0.68, p = .68, p* = .50, b = 12.57, CI [−23.80, 48.75] or executive control, t(157) = 1.88, p = .062, p* = .09, b = 50.87, CI [−2.56, 104.29].
There was no relationship between either measure of self-reported attention and PCC rsFC in the voxelwise analysis, either whole-brain or small-volume corrected to DLPFC (ps > .05 corrected for FWE), nor in the ROI analysis with RLPFC (experience sampling: t(146) = −0.73, p = .47, p* = .99, b = −0.61, CI [−2.26, 1.04]; ESQ: t(143) = −1.05, p = .30, p* = .77, b = −0.80, CI [−2.32, 0.71]).
Lower average participation coefficients in the DMN were associated with faster orienting attention, t(164) = 2.75, p = .007, p* = .03, b = 90.19, CI [25.53, 154.86] (Figure 5C), and the result remained consistent when controlling for physiological variables (p = .011). There was no relationship between DMN participation coefficients and alerting, t(165) = −1.73, p = .086, p* = .14, b = −35.38, CI [−75.80, 5.05], or executive control, t(165) = −0.27, p = .78, p* = .78, b = −9.01, CI [−73.93, 55.91]. There was also no significant relationship between DMN participation coefficients and self-reported attention (experience sampling: t(152) = −0.05, p = .96, p* = .96, b = −0.04, CI [−1.88, 1.79]; ESQ: t(135) = −1.52, p = .13, p* = .26, b = −1.38, CI [−3.18, 0.41]).
Practice Time
There were no significant effects of practice time (either total hours of retreat or daily practice) on any brain or behavioral measures (ts < 2.30, p*s > .050).
Exploratory Voxelwise Connectivity Analysis
In order to test for subregions of the DLPFC within which meditators may have had stronger PCC rsFC than meditation-naive participants, we conducted small-volume corrected voxelwise analysis, in the bilateral, anatomically defined DLPFC mask, given the relatively large size and functional heterogeneity of this region. There were no statistically significant differences in PCC rsFC between meditators and meditation-naive participants within the DLPFC mask. There were no regions in which PCC rsFC differed for meditators compared with meditation-naive participants in whole-brain analysis, contrary to prior research (Brewer et al., 2011). Unthresholded statistical maps are available at Neurovault (Gorgolewski et al., 2015): https://neurovault.org/collections/8451/.
DISCUSSION
The current study partially replicated prior work (Brewer et al., 2011), showing stronger rsFC between PCC and RLPFC in participants with a long-term practice in mindfulness meditation compared with meditation-naive participants. These results are consistent with prior work showing increased PCC–RLPFC connectivity following a short-term mindfulness meditation intervention (Creswell et al., 2016), but which were not replicated in a separate analysis of the RCT with short-term meditation training among meditation-naive participants from the current study (Kral et al., 2019). Yet, we failed to replicate prior reports of stronger rsFC between PCC and DLPFC in association with long-term meditation practice, or to find moderation effects of practice time. We found that stronger PCC–RLPFC rsFC was associated with faster orienting attention, which lends further support for the interpretation that stronger PCC–RLPFC connectivity reflects better ability to shift attention to stimuli that arise in present-moment experience.
Activation of RLPFC and its connectivity with PCC could thus serve as a neural mechanism underlying subcomponents of meta-awareness that support attentional shifting and the ability to re-allocate attentional resources to examine internally generated cognitions as they arise, or conversely to return attentional focus to externally generated phenomena. Although there is a wealth of research and related paradigms examining phasic instances of meta-awareness in the context of error detection and noticing instances of mind-wandering (Schooler et al., 2011; Ullsperger, Harsay, Wessel, & Ridderinkhof, 2010; Hester, Foxe, Molholm, Shpaner, & Garavan, 2005), research and paradigms for assessing the neural basis for sustained meta-awareness are lacking and critical for our understanding of mechanisms of change with mindfulness-based meditation training.
Although meditators did not differ from controls in the ANT behavioral measure of orienting attention, they reported higher goal-directed attention on both questionnaire and experience sampling measures compared with nonmeditating controls. Prior research has reported improvements or associations in ANT orienting and executive function with mindfulness meditation training (Trautwein et al., 2020; Ainsworth et al., 2013; van den Hurk et al., 2010), although differences between these studies and the current research may account for the discrepancy in results. Two of these prior studies examined the effects of short-term mindfulness meditation training (Trautwein et al., 2020; Ainsworth et al., 2013), in contrast to the current study of the long-term meditation training. We would not expect the same attentional changes or benefits at short- versus long-term stages of meditation experience, given prior evidence for differential benefits and/or mechanisms at these different stages (Kral et al., 2018; Brefczynski-Lewis et al., 2007). Furthermore, the study that found associations between long-term meditation practice and ANT outcomes relied on small sample sizes of 20 participants per group, and reported on nonsignificant (trend-level) effects as group differences in the Abstract and Discussion sections (van den Hurk et al., 2010). The current study also differed from this prior work in that the sample populations were recruited from the United States of America and the Netherlands, respectively, although how this difference contributed to the findings is unknown. Further research is needed to rigorously and directly test whether the effects of meditation on ANT outcomes can be replicated.
We also examined resting connectivity at the network level, by testing group differences in provincial (i.e., within-module) and connector (i.e., between-module) hub properties. We found lower participation coefficients for the DMN in meditators compared with meditation-naive participants, reflecting a reduction in connector hubs in the DMN. This contrasts with the stronger PCC–RLPFC rsFC we found for meditators in seed-based analysis. This may reflect reduced information flow from the DMN to other networks, and highlights the potential specificity of increased rsFC between PCC and RLPFC in association with mindfulness meditation practice. Lower connector hub strength of the DMN was associated with faster orienting attention on the ANT, providing initial evidence that reduced information flow between DMN and other networks may contribute to aspects of improved attention. However, these results should be interpreted with caution, given that the effects became marginal when controlling for individual differences in heart rate and respiration rate that can confound resting-state fMRI analysis. There were no differences between groups for hub properties of the dorsal attention or frontoparietal control networks, nor for provincial hub properties (i.e., WMD) or overall strength of connectivity between DMN and the dorsal attention network or frontoparietal control network. Taken together, the combination of unique seed- and network-based results may indicate a regulatory role for RLPFC in restricting the participation of the DMN through its interaction with PCC. This interpretation is consistent with the gateway hypothesis of RLPFC, and with theory and evidence on meditation-related influences on attentional processes (Hasenkamp et al., 2012; Burgess et al., 2007).
Meditators had higher attention to task than meditation-naive participants, as assessed with both a self-report questionnaire and experience sampling via text messaging. Higher attention to task, and conversely, less mind-wandering, are consistent with the guidance for mindfulness meditation, namely, maintaining attention on present-moment experience. These results are also consistent with the interpretation of higher PCC–RLPFC resting connectivity among meditators as reflecting better attentional control of mind-wandering (Brewer & Garrison, 2014). Note, however, there was no association between rsFC and self-reported attention in the current study, although we previously found that increased self-reported attention was associated with increased PCC–DLPFC rsFC following MBSR (Kral et al., 2019). It is possible that changes in these measures of attention and functional connectivity track together because they share underlying mechanisms of change, whereas the same measures examined cross-sectionally are uncorrelated because of different functionality. It is also possible that the within-subject design in the prior RCT study may have been more sensitive for identifying correlations among changes without including variance in unrelated between-subjects factors. The current study is limited given the cross-sectional nature of the design, and the associated lack of a baseline for meditators with which we could compare the current measures to examine change over time associated with long-term mindfulness meditation practice.
The RLPFC region in which we found a significant group difference was defined based on coordinates from a study showing stronger rsFC with PCC following a short-term mindfulness meditation intervention (Creswell et al., 2016). There was no difference between meditators and meditation-naive participants in anatomically defined ROIs, which include a more canonical DLFPC region, and in which we previously found increased rsFC with PCC following MBSR compared with controls (Kral et al., 2019). There were also no differences between groups in any of the behavioral measures of attention, and self-reported attention may be biased by demand characteristics that are only partially addressed with experience sampling methods. Future research is needed combining multiple behavioral measures, including new measures to tap different aspects of meta-awareness together with naturalistic paradigms similar to prior research (Hasenkamp & Barsalou, 2012), to parse the potential differential influence of mindfulness meditation on functional connectivity of RLPFC and DLPFC.
The results of the current study provide additional evidence consistent with prior work indicating a relationship between mindfulness meditation practice and increased resting connectivity between nodes of the default mode and frontoparietal control networks (Kral et al., 2019; Creswell et al., 2016; Brewer et al., 2011). We also found evidence for a relationship between DMN–frontoparietal network functional connectivity at rest and attentional orienting, such that greater connectivity was associated with faster orienting. In addition, meditators reported higher goal-directed attention than meditation-naive participants in experience sampling and self-report measures. Changes in the dynamic interactions of PCC and RLPFC are a candidate neural mechanism underlying attentional improvements with mindfulness meditation training, although from this research it is clear that there is potentially important variation in the specific parameters of attention that improve with long-term mindfulness training.
Acknowledgments
We would like to thank Michael Anderle, Ron Fisher, Jane Sachs, Jeanne Harris, Mariah Brown, Elizabeth Nord, Kaley Ellis, Gina Bednarek, Kara Chung, Pema Lhamo, David Bachhuber, Amelia Cayo, Christopher Harty, Sonam Kindy, and Dan Dewitz for assistance with recruitment and/or data collection. We would also like to thank Katherine Bonus, Devin Coogan, Bob Gillespie, Diana Grove, Lori Gustafson, Matthew Hirshberg, Peggy Kalscheur, Chad McGehee, Vincent Minichiello, Laura Pinger, Lisa Thomas Prince, Kristi Rietz, Sara Shatz, Chris Smith, Heather Sorensen, Jude Sullivan, Julie Thurlow, Michael Waupoose, Sandy Wojtal-Weber, and Pam Young for teaching and/or coordinating the interventions, and John Koger, Ty Christian, David Thompson, and Nate Vack for technical assistance.
Reprint requests should be sent to Richard J. Davidson, Center for Healthy Minds, University of Wisconsin—Madison, 625 West Washington Avenue, Madison, WI, USA 53703 or via e-mail: rjdavids@wisc.edu.
Author Contributions
Tammi R. A. Kral: Conceptualization; Formal analysis; Investigation; Methodology; Software; Visualization; Writing—Original draft; Wring—Review & editing. Regina C. Lapate: Conceptualization; Methodology; Software; Visualization; Writing—Original draft; Writing—Review & editing. Ted Imhoff-Smith: Investigation; Methodology; Writing—Review & editing. Elena Patsenko: Investigation; Methodology. Daniel W. Grupe: Data curation; Investigation; Software; Writing—Review & editing. Robin Goldman: Data curation; Project administration; Writing—Review & editing. Melissa A. Rosenkranz: Funding acquisition; Methodology; Writing—Review & editing. Richard J. Davidson: Conceptualization; Funding acquisition; Methodology; Resources; Supervision; Writing—Review & editing.
Funding Information
This work was supported by the National Center for Complementary and Integrative Health (https://dx.doi.org/10.13039/100008460), grant number: P01AT004952 to R. J. D., the National Institute of Mental Health (https://dx.doi.org/10.13039/100000025), grant numbers: R01-MH43454, P50-MH084051 to R. J. D., Fetzer Institute (https://dx.doi.org/10.13039/100001614), grant number: 2407, and the John Templeton Foundation (https://dx.doi.org/10.13039/100000925), grant number: 21337 to R. J. D., Eunice Kennedy Shriver National Institute of Child Health and Human Development (https://dx.doi.org/10.13039/100009633), grant number: P30 HD003352-449015 to Albee Messing. T. R. A. K. was supported by the National Institute of Mental Health (https://dx.doi.org/10.13039/100000025), grant number: T32MH018931.
Diversity in Citation Practices
Retrospective analysis of the citations in every article published in this journal from 2010 to 2021 reveals a persistent pattern of gender imbalance: Although the proportions of authorship teams (categorized by estimated gender identification of first author/last author) publishing in the Journal of Cognitive Neuroscience (JoCN) during this period were M(an)/M = .407, W(oman)/M = .32, M/W = .115, and W/W = .159, the comparable proportions for the articles that these authorship teams cited were M/M = .549, W/M = .257, M/W = .109, and W/W = .085 (Postle and Fulvio, JoCN, 34:1, pp. 1–3). Consequently, JoCN encourages all authors to consider gender balance explicitly when selecting which articles to cite and gives them the opportunity to report their article's gender citation balance. The authors of this article report its proportions of citations by gender category to be as follows: M/M = .639; W/M = .194; M/W = .167; W/W = 0.
REFERENCES
- Ainsworth, B., Eddershaw, R., Meron, D., Baldwin, D. S., & Garner, M. (2013). The effect of focused attention and open monitoring meditation on attention network function in healthy volunteers. Psychiatry Research, 210, 1226–1231. 10.1016/j.psychres.2013.09.002, [DOI] [PubMed] [Google Scholar]
- Allen, J. J. B., Chambers, A. S., & Towers, D. N. (2007). The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biological Psychology, 74, 243–262. 10.1016/j.biopsycho.2006.08.005, [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis, J. A., Lutz, A., Schaefer, H. S., Levinson, D. B., & Davidson, R. J. (2007). Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of the National Academy of Sciences, U.S.A., 104, 11483–11488. 10.1073/pnas.0606552104, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer, J. A., & Garrison, K. A. (2014). The posterior cingulate cortex as a plausible mechanistic target of meditation: Findings from neuroimaging. Annals of the New York Academy of Sciences, 1307, 19–27. 10.1111/nyas.12246, [DOI] [PubMed] [Google Scholar]
- Brewer, J. A., Worhunsky, P. D., Gray, J. R., Tang, Y.-Y., Weber, J., & Kober, H. (2011). Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences, U.S.A., 108, 20254–20259. 10.1073/pnas.1112029108, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, P. W., Dumontheil, I., & Gilbert, S. J. (2007). The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Sciences, 11, 290–298. 10.1016/j.tics.2007.05.004, [DOI] [PubMed] [Google Scholar]
- Chen, J. E., Lewis, L. D., Chang, C., Tian, Q., Fultz, N. E., Ohringer, N. A., et al. (2020). Resting-state “physiological networks”. Neuroimage, 213, 116707. 10.1016/j.neuroimage.2020.116707, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance Neuroimages. Computers and Biomedical Research, 29, 162–173. 10.1006/cbmr.1996.0014, [DOI] [PubMed] [Google Scholar]
- Craddock, R. C., James, G. A., Holtzheimer, P. E., Hu, X. P., & Mayberg, H. S. (2012). A whole brain fMRI atlas generated via spatially constrained spectral clustering. Human Brain Mapping, 33, 1914–1928. 10.1002/hbm.21333, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell, J. D., Taren, A. A., Lindsay, E. K., Greco, C. M., Gianaros, P. J., Fairgrieve, A., et al. (2016). Alterations in resting-state functional connectivity link mindfulness meditation with reduced Interleukin-6: A randomized controlled trial. Biological Psychiatry, 80, 53–61. 10.1016/j.biopsych.2016.01.008, [DOI] [PubMed] [Google Scholar]
- de la Cruz, F., Schumann, A., Köhler, S., Reichenbach, J. R., Wagner, G., & Bär, K.-J. (2019). The relationship between heart rate and functional connectivity of brain regions involved in autonomic control. Neuroimage, 196, 318–328. 10.1016/j.neuroimage.2019.04.014, [DOI] [PubMed] [Google Scholar]
- Curtin, J. (2015). lmSupport: Support for linear models (2.9.2) [Computer software]. https://CRAN.R-project.org/package=lmSupport. [Google Scholar]
- E-Prime 2.0. (2012). Psychology Software Tools, Inc. [Google Scholar]
- Fan, J., Gu, X., Guise, K. G., Liu, X., Fossella, J., Wang, H., et al. (2009). Testing the behavioral interaction and integration of attentional networks. Brain and Cognition, 70, 209–220. 10.1016/j.bandc.2009.02.002, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, J., McCandliss, B. D., Fossella, J., Flombaum, J. I., & Posner, M. I. (2005). The activation of attentional networks. Neuroimage, 26, 471–479. 10.1016/j.neuroimage.2005.02.004, [DOI] [PubMed] [Google Scholar]
- Fox, K. C. R., Dixon, M. L., Nijeboer, S., Girn, M., Floman, J. L., Lifshitz, M., et al. (2016). Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations. Neuroscience & Biobehavioral Reviews, 65, 208–228. 10.1016/j.neubiorev.2016.03.021, [DOI] [PubMed] [Google Scholar]
- Fox, K. C. R., Spreng, R. N., Ellamil, M., Andrews-Hanna, J. R., & Christoff, K. (2015). The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage, 111, 611–621. 10.1016/j.neuroimage.2015.02.039, [DOI] [PubMed] [Google Scholar]
- Garrison, K., Santoyo, J., Davis, J., Thornhill, T., Kerr, C., & Brewer, J. (2013). Effortless awareness: Using real time neurofeedback to investigate correlates of posterior cingulate cortex activity in meditators' self-report. Frontiers in Human Neuroscience, 7, 440. 10.3389/fnhum.2013.00440, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, E. M., Laumann, T. O., Adeyemo, B., Huckins, J. F., Kelley, W. M., & Petersen, S. E. (2016). Generation and evaluation of a cortical area parcellation from resting-state correlations. Cerebral Cortex, 26, 288–303. 10.1093/cercor/bhu239, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski, K. J., Varoquaux, G., Rivera, G., Schwarz, Y., Ghosh, S. S., Maumet, C., et al. (2015). NeuroVault.Org: A web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers in Neuroinformatics, 9, 8. 10.3389/fninf.2015.00008, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve, D. N., & Fischl, B. (2009). Accurate and robust brain image alignment using boundary-based registration. Neuroimage, 48, 63–72. 10.1016/j.neuroimage.2009.06.060, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp, W., & Barsalou, L. (2012). Effects of meditation experience on functional connectivity of distributed brain networks. Frontiers in Human Neuroscience, 6, 38. 10.3389/fnhum.2012.00038, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp, W., Wilson-Mendenhall, C. D., Duncan, E., & Barsalou, L. W. (2012). Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. Neuroimage, 59, 750–760. 10.1016/j.neuroimage.2011.07.008, [DOI] [PubMed] [Google Scholar]
- Hester, R., Foxe, J. J., Molholm, S., Shpaner, M., & Garavan, H. (2005). Neural mechanisms involved in error processing: A comparison of errors made with and without awareness. Neuroimage, 27, 602–608. 10.1016/j.neuroimage.2005.04.035, [DOI] [PubMed] [Google Scholar]
- Hibbert, A. S., Weinberg, A., & Klonsky, E. D. (2012). Field validity of heart rate variability metrics produced by QRSTool and CMetX. Psychological Assessment, 24, 777–782. 10.1037/a0027284, [DOI] [PubMed] [Google Scholar]
- Hwang, K., Bertolero, M. A., Liu, W. B., & D'Esposito, M. (2017). The human thalamus is an integrative hub for functional brain networks. Journal of Neuroscience, 37, 5594–5607. 10.1523/JNEUROSCI.0067-17.2017, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17, 825–841. 10.1006/nimg.2002.1132, [DOI] [PubMed] [Google Scholar]
- Jha, A. P., Krompinger, J., & Baime, M. J. (2007). Mindfulness training modifies subsystems of attention. Cognitive, Affective, & Behavioral Neuroscience, 7, 109–119. 10.3758/CABN.7.2.109, [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn, J. (1990). Full catastrophe living (FEP Missing ed.). Delacorte Press. [Google Scholar]
- Kesebir, P., Gasiorowska, A., Goldman, R., Hirshberg, M. J., & Davidson, R. J. (2019). Emotional style questionnaire: A multidimensional measure of healthy emotionality. Psychological Assessment, 31, 1234–1246. 10.1037/pas0000745, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral, T. R. A., Imhoff-Smith, T., Dean, D. C., Grupe, D., Adluru, N., Patsenko, E., et al. (2019). Mindfulness-based stress reduction-related changes in posterior cingulate resting brain connectivity. Social Cognitive and Affective Neuroscience, 14, 777–787. 10.1093/scan/nsz050, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral, T. R. A., Schuyler, B. S., Mumford, J. A., Rosenkranz, M. A., Lutz, A., & Davidson, R. J. (2018). Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. Neuroimage, 181, 301–313. 10.1016/j.neuroimage.2018.07.013, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova, A., Brockhoff, P. B., & Christensen, R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82, 1–26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Lutz, A., Jha, A. P., Dunne, J. D., & Saron, C. D. (2015). Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. American Psychologist, 70, 632–658. 10.1037/a0039585, [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, A. W., Cohen, J. D., Stenger, V. A., & Carter, C. S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288, 1835–1838. 10.1126/science.288.5472.1835, [DOI] [PubMed] [Google Scholar]
- Nobre, A. C., Coull, J. T., Maquet, P., Frith, C. D., Vandenberghe, R., & Mesulam, M. M. (2004). Orienting attention to locations in perceptual versus mental representations. Journal of Cognitive Neuroscience, 16, 363–373. 10.1162/089892904322926700, [DOI] [PubMed] [Google Scholar]
- Power, J. D., Mitra, A., Laumann, T. O., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage, 84, 320–341. 10.1016/j.neuroimage.2013.08.048, [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing; https://www.R-project.org/. [Google Scholar]
- Rubinov, M., & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. Neuroimage, 52, 1059–1069. 10.1016/j.neuroimage.2009.10.003, [DOI] [PubMed] [Google Scholar]
- Schooler, J. W., Smallwood, J., Christoff, K., Handy, T. C., Reichle, E. D., & Sayette, M. A. (2011). Meta-awareness, perceptual decoupling and the wandering mind. Trends in Cognitive Sciences, 15, 319–326. 10.1016/j.tics.2011.05.006, [DOI] [PubMed] [Google Scholar]
- Smallwood, J., Brown, K., Baird, B., & Schooler, J. W. (2012). Cooperation between the default mode network and the frontal–parietal network in the production of an internal train of thought. Brain Research, 1428, 60–70. 10.1016/j.brainres.2011.03.072, [DOI] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155. 10.1002/hbm.10062, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E. J., Johansen-Berg, H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23(Suppl. 1), S208–S219. 10.1016/j.neuroimage.2004.07.051, [DOI] [PubMed] [Google Scholar]
- Spreng, R. N., Mar, R. A., & Kim, A. S. N. (2008). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience, 21, 489–510. 10.1162/jocn.2008.21029, [DOI] [PubMed] [Google Scholar]
- Tang, Y.-Y., Ma, Y., Wang, J., Fan, Y., Feng, S., Lu, Q., et al. (2007). Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences, U.S.A., 104, 17152–17156. 10.1073/pnas.0707678104, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein, F.-M., Kanske, P., Böckler, A., & Singer, T. (2020). Differential benefits of mental training types for attention, compassion, and theory of mind. Cognition, 194, 104039. 10.1016/j.cognition.2019.104039, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger, M., Harsay, H. A., Wessel, J. R., & Ridderinkhof, K. R. (2010). Conscious perception of errors and its relation to the anterior insula. Brain Structure and Function, 214, 629–643. 10.1007/s00429-010-0261-1, [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk, P. A. M., Giommi, F., Gielen, S. C., Speckens, A. E. M., & Barendregt, H. P. (2010). Greater efficiency in attentional processing related to mindfulness meditation. Quarterly Journal of Experimental Psychology, 63, 1168–1180. 10.1080/17470210903249365, [DOI] [PubMed] [Google Scholar]
- Wielgosz, J., Schuyler, B. S., Lutz, A., & Davidson, R. J. (2016). Long-term mindfulness training is associated with reliable differences in resting respiration rate. Scientific Reports, 6, 27533. 10.1038/srep27533, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M., & Nichols, T. E. (2014). Permutation inference for the general linear model. Neuroimage, 92, 381–397. 10.1016/j.neuroimage.2014.01.060, [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association. (2013). World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Journal of the American Medical Association, 310, 2191–2194. 10.1001/jama.2013.281053, [DOI] [PubMed] [Google Scholar]