Abstract
Temporal influence of electronic cigarettes (Ecigs) on blood vessels are poorly understood. This study evaluated a single-episode of cigarette versus Ecig exposure on middle cerebral artery (MCA) reactivity, and determined how long after the exposure MCA responses took to return to normal. We hypothesized cigarette and Ecig exposure would induce rapid (<4-hours) reduction in MCA endothelial function and would resolve within 24-hours. Sprague-Dawley rats (4-month-old) were exposed Air (n=5), traditional cigarettes (20-puffs, N=16) or Ecigs (20-puff group, N=16; or 60-puff group, N=12). Thereafter, cigarette and Ecig groups were randomly assigned for post-exposure vessel myography testing on Day-0 (D0, 1-4 hours post-exposure), Day-1 (D1, 24-28 hours post), Day-2 (D2, 48-52 hours post), and Day-3 (72-76 hours post). Greatest effect on endothelial-dependent-dilation (EDD) was observed within 24-hours of exposure (~50% decline between D0-D1) for both cigarette and Ecig groups, and impairment persisted with all groups for up to 3 days. Changes in endothelial-independent-dilation (EID) responses were less severe (~27%) and shorter-lived (recovering by D2) compared with EDD. Vasoconstriction in response to serotonin (5-HT) was similar to EID, with greatest impairment (~45% for all exposure groups) at D0-D1, and returning to normal by D2. These data show exposure to cigarettes and Ecigs trigger similar level/duration of cerebrovascular dysfunction following a single exposure. The finding that Ecig (without nicotine) and cigarette exposure (with nicotine) produce the same effects, suggests nicotine is not likely triggering MCA dysfunction and vaping (with/without nicotine) has potential to produce the same vascular harm and/or disease as smoking.
Keywords: vaping, brain, middle cerebral artery, extracellular vesicles, 3R4F cigarette
INTRODUCTION
Acute effects of using electronic cigarettes (Ecigs) are known to have transient effects on vascular function in humans (Vlachopoulos et al., 2016; Franzen et al., 2018; Caporale et al., 2019; Kerr et al., 2019; Kuntic et al., 2020; Wehrli et al., 2020; Chatterjee et al., 2021), animals (Kuntic et al., 2020; Rao et al., 2020; Jin et al., 2021) and on endothelial (and other) cells in culture (Putzhammer et al., 2016; Fetterman et al., 2018; Lee et al., 2019; Kerasioti et al., 2020). Consistent with this, long-term Ecig exposure has been shown to increase arterial stiffness and impair (both endothelial-dependent and -independent) blood vessel reactivity (Vlachopoulos et al., 2016; Franzen et al., 2018; Olfert et al., 2018; Kerr et al., 2019; El-Mahdy et al., 2021). We have also recently reported impaired cerebrovascular endothelial function in offspring indirectly exposed (in utero) to Ecigs from maternal exposure during pregnancy (Burrage et al., 2021). Impaired cerebrovascular function is particularly concerning as it is associated with lower cerebral blood flow (Tarumi et al., 2011; Jefferson et al., 2018), neurocognitive decline (Hanon et al., 2005; Mitchell et al., 2011; Pase et al., 2016; Thorin-Trescases et al., 2018), development of cerebral microbleeds (Ding et al., 2015), and greater overall risk for cerebrovascular and cardiovascular disease (Mitchell, 2015; Alzahrani et al., 2018) – all of which are promoting factors for onset of stroke and worsening of post-ischemic brain injury (Kaisar et al., 2017). Recent reports also find that vaping increases shedding of endothelial- and platelet-derived extracellular vesicles (EVs), which may be associated to underlying vascular changes and eventually lead to adverse health outcomes (Benedikter et al., 2018; Mobarrez et al., 2020)
The purpose of this study was to evaluate effects of a single exposure on reactivity of the middle cerebral artery (MCA), and time required for MCA responses to return to normal following a single direct exposure. The MCA was studied because it is a major cerebral artery that supplies ~50% of cerebral blood flow to the brain (Harper et al., 1984), and is the most common site for an ischemic stroke. We hypothesized that a single vape exposure would induce rapid impairment/blunting of MCA endothelial function (<4 hours) that would likely resolve within 24 hours. Indeed, we observed rapid blunting of MCA endothelial function (1-4 hours) following vaping, but recovery of MCA function was not restored for at least 72 hours after vaping. We also provide evidence that cigarette and Ecig exposure increase circulating extracellular vesicles (EVs) production demonstrating that a single exposure likely triggers maladaptive cellular/molecular responses. When compounded with chronic, daily, vaping it is not difficult to envisage that impaired vascular function may be prodromic for cerebrovascular disease.
MATERIALS AND METHODS
Ethical Approval
All procedures were conducted with approval from West Virginia University Animal Care and Use Committee (IACUC) approval (#16-05003053), and conformed to the previously described principles and regulations for animal experimentation (Grundy, 2015). All surgical procedures were terminal and performed under deep anesthesia (inhaled isoflurane) to minimize pain or discomfort.
Study design and exposure system
Male Sprague Dawley rats (4-month old) were purchased (Charles River, Wilmington, MA) and housed in pathogen-free vivarium facility at West Virginia University. They were provided standard rat chow and tap water and kept on 12:12 day:night cycle throughout the study.
Animals were randomly assigned to Air (n=5), Ecig 20-puffs (n=16), Ecig 60-puffs (n=12), or traditional cigarettes (i.e. 3R4F Reference cigarette, Univ. of Kentucky; n=16) groups, and subjected to single (one-time) exposure corresponding to the assigned group. After exposure to either E-cig or 3R4F Reference cigarette, animals were randomly assigned to post-exposure study days corresponding to Day 0 (D0, within 1-4 hours post exposure; n=3-5/exposure group), Day 1 (D1, 24-28 hours post exposure; n=3-5/exposure group), Day 2 (D2, 48-52 hours post exposure; n=3-5/exposure group), and Day 3 (72-76 hours post exposure; n=3-5/exposure group). Euthanasia occurred via exsanguination and organ removal under deep anesthesia to collect MCA vessel to be used for ex vivo studies with pressure arteriography (Living Systems, Scintica Inc.).
Exposures were performed using a whole-body chamber. Ecig exposure (n=4 per time group) and traditional cigarette smoke (n=4 per time group) exposure were generated from 20 puffs over a 1-hour exposure window. For Ecig exposure, an additional group was studied, whereby the 20-puff/hour regime was extended for 3 hours resulting in a total exposure to 60 puffs (n=3 per time point). Ecig aerosol was generated using Joyetech atomizer (0.5Ω) with a custom Ecig controller system that allowed precise and reliable activation of the Ecig device. Puff duration was set to 5-sec with an inhalation draw rate of ~1 LPM. Wattage (W) for heating coil was set at 17.5W, and 20 L/min bias flow of air was used to continuously flush the chamber throughout the exposure which provided an episodic exposure pattern.
E-liquid composition was a mixture by volume of 50% glycerin (Avantor, J.T. Baker #2143-01) and 50% propylene glycol (Fisher #P355-1) with no nicotine and no flavoring. Temperature and humidity were measured with probes inside the chamber (Table 1). Air group received handling at the same time and in the same way as exposed groups to replicate any associated stress. They remained in the same room as exposure groups but were only exposed to ambient air.
Table 1.
Chamber and exposure characteristics
| 3R4F |
E-cig |
|
|---|---|---|
| Chamber temperature (C) | 22.6 ± 0.3 | 21.8 ± 1.2 |
| Chamber relative humidity (%) | 46.3 ± 13.6 | 25.8 ± 7.5 |
| Aerosol/Smoke Concentration (mg/m3) | 7 ± 5 | 334 ± 110 |
| E-cig device | ||
| Watts (fixed setting with 0.5 Ω coil) | n/a | 17.5 |
| Volts | n/a | 3.0 ± 0.2 |
| Amps | n/a | 5.9 ± 0.4 |
n/a, not applicable
Aerosol Analysis
Aerosol concentration was measured using MicroDust Tracker (Model# CEl-712, Cesella, MA) and reported as the average concentration from the entire exposure time (Table 1).
Measurements of Vascular Reactivity in Isolated MCA
Rats were anesthetized by inhalation of isoflurane using an induction chamber (at 5%) and placed on nose cone used to maintain anesthesia (at 1.5%-3%) throughout the procedure. Animals were euthanized by cardiac puncture and exsanguination by flushing the vascular system with phosphate buffer saline (PBS) solution. Immediately thereafter, the brain was removed and a middle cerebral artery (MCA) was removed from Circle of Willis, cleaned and placed into an isolated microvessel chamber filled with cold physiological salt solution (PSS; 4°C). Both ends of the isolated MCA were cannulated within a heated chamber (37°C) that allowed the lumen and exterior of the vessel to be perfused and superfused, respectively, with heated PSS from separate reservoirs. PSS was equilibrated with a 21% O2, 5% CO2, and 74% N2 gas mixture and had the following composition (mM): 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 1.18 NaH2PO4, 24 NaHCO3, 0.026 EDTA, and 5.5 glucose.
Following cannulation, MCAs were extended to their in-situ length and equilibrated to ~70 mmHg (Lombard et al., 1999). Then the vessel was challenged using 10−4 M serotonin to check viability; thereafter, vessel reactivity was assessed in response to increasing concentrations of an endothelial-dependent dilator (acetylcholine, ACh; 10−9M – 10−4M), endothelial-independent dilator (sodium nitroprusside, SNP; 10−9M – 10−4M), and a potent cerebrovascular constrictor (5-hydroxytryptamine, 5-HT (serotonin); 10−9M – 10−4M). MCA responses to ACh were also measured following acute incubation (30 minutes) of vessels with nitro-L-arginine methylester (L-NAME, 10−4M; an inhibitor of NO synthase, Sigma Aldrich) to assess contributions of nitric oxide (NO) (Butcher et al., 2012). Likewise, we incubated vessels in TEMPOL (10−4M) in similar manner to assess the effect of oxidative stress in modulating MCA reactivity (Butcher et al., 2012).
A video dimension analyzer connected to the inverted microscope was used to measure lumen diameter (Table 2). Resistance vessels (such as the MCA) have endogenous smooth muscle tone therefore pre-constriction prior to assessing dilator responses is not required. Thus, dilation and constriction are assessed by subtracting the starting lumen diameter before each treatment and chambers were washed between each drug. Although addition of L-NAME and/or TEMPOL can alter lumen diameter, these responses (and previously noted) are assessed to the vessels starting diameter before treatment, and therefore any initial effect by the addition of L-NAME or TEMPOL does not change our finding or interpretation that these compounds have on ACh-mediated dilation.
Table 2.
MCA Starting Diameters
| Air | Ecig | 3R4F | ANOVA Exposure |
ANOVA Days |
ANOVA Interaction |
|
|---|---|---|---|---|---|---|
|
|
|
|
|
|||
| n= | 5 | 7 | 4 | |||
| Diameter (μm) | ||||||
| D0 | 108±23 | 122±15 | 111±11 | p=0.122 | p=0.514 | p=0.944 |
| D1 | 116±14 | 99±15 | ||||
| D2 | 120±16 | 115±17 | ||||
| D3 | 109±21 | 100±9 | ||||
| Min (μm) | 72 | 91 | 82 | |||
| Max (μm) | 126 | 147 | 127 | |||
Extracellular Vesicles (EVs)
Blood was obtained by cardiac puncture at sacrifice, and immediately spun at 3000 rpm, 4°C, for 10 minutes, in sterile 2-ml BD vacutainer tubes containing Lithium Heparin (BD #366664). After centrifugation, plasma was removed in 200 μl aliquots with 2 μl of DMSO to preserve vesicles, flash frozen in liquid N2 and stored at −80°C until processed. To obtain EVs, frozen samples were thawed on ice and spun at 1500 rpm, 4°C for 10 minutes to separate plasma and cell debris. Supernatant was removed placed in a new tube, with 1 μL taken and diluted in 1 mL of 1X sterile PBS. Diluted sample was immediately visualized with a Malvern Panalytical Nanosight NS300 for particle size and quantity in the WVU Flow Cytometry & Single Cell Core Facility (RRID:SCR 017738). Remaining plasma was spun at max speed (13K rpm) for 2 hours at 4°C. The supernatant was removed and 500 μL of 1X PBS was added. The sample was spun for another hour at max speed at 4°C. These samples were later used in an Exo-check antibody array (# EXORAY200A-4, Thermo Fisher Scientific) to confirm presence of EVs. Markers for exosomes represented in the kit included CD63, CD81, ALIX, FLOT1, ICAM1, EpCam, ANXA5 and TSG101. GM130, a cis-Golgi marker, was also present to visualize any cellular contaminations within the samples.
Zeta Potential (ZP) was used to measure surface electrostatic potential of EVs as an indicator of surface charge and colloidal stability influenced by surface chemistry. It was conducted by diluting purified EVs (1:1000 −1:10,000 with sterile 1X- PBS) to reach a number per frame of 50 to 500, ideal for Nano Tracking Analysis (NTA) and measured by Zetasizer Nano Z (Zetasizer Nano Z) at WVU core laboratory. Temperature was set at 25 °C and 5 cycles at high sensitivity settings were performed per measurement for a total of three to five measurements per sample.
Data and Statistical Analyses
All data are presented as mean±SD, except when noted. Reactivity of isolated MCA vessels to changes in drug dose concentration were first analyzed by repeated-measures two-way analysis of variance (ANOVA). Thereafter, Tukey post-hoc test was used to determine differences between doses of respective drugs used. Max response characteristics between groups were assessed using a multifactorial analysis of variance (ANOVA) with an interaction term (time-by-group), and a Tukey post-hoc test to determine differences between groups, as appropriate.
In all cases, p≤0.05 was taken to reflect statistical significance.
RESULTS
No significant differences (at any timepoint) were observed in measured outcomes between Ecig 20-puffs (N=16; n=4 each time point) versus Ecig 60-puffs (N=12; n=3 each time point) exposure groups (Figure 1). Therefore, data from these two Ecig groups were combined to represent a single Ecig group (n=7) for all further analyses.
Figure 1.
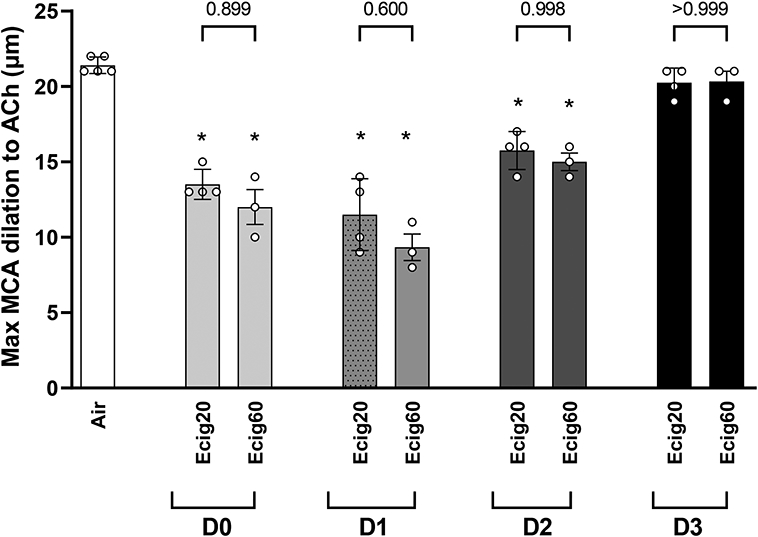
Graph showing maximal response to ACh (i.e. 10-4 M) in middle cerebral arteries (MCA) between Ecig exposure groups (Ecig 20-puffs n=4 and Ecig 60-puffs n=3) compared to responses in controls (Air, n=5). MCA data for Ecig groups reflects number of (D)ays after exposure, and show no difference in response between Ecig 20- vs 60-puffs at any time point. *signifies different compared to Air (p<0.05). Mean±SD.
Endothelial-dependent dilation (EDD)
EDD response of the MCA was significantly blunted in rats exposed to Ecig and 3R4F reference cigarette compared with Air controls (Figure 2A-C). For all exposure groups, the greatest decline (~50%) in EDD was observed with D0 or D1 (i.e. <24-hours post vaping)(Figure 2A&B). Although evidence of recovery began by D2, maximal MCA EDD function still remained blunted at D2 for all exposure groups compared with controls (Figure 2C). By D3, MCA EDD function had returned to the same levels found in non-exposed controls (Figure 2A-C).
Figure 2.
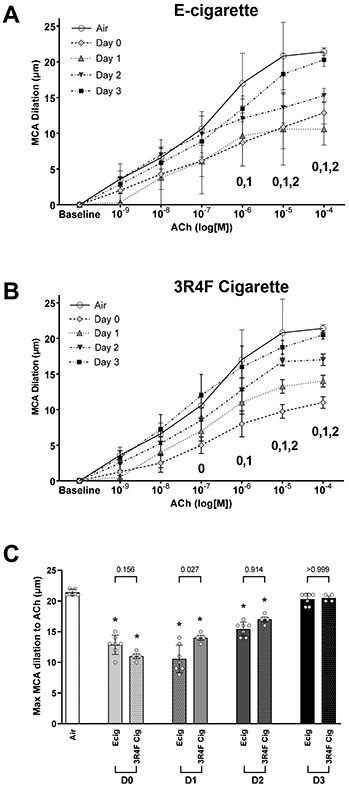
Middle cerebral arteries (MCA) reactivity assessed with ex vivo pressure myography. MCA dilation responses to increasing concentration of acetylcholine (ACh). Dose response curves reflects data corresponding to number of (D)ays after an exposure to either (A) Ecig (n=7) or (B) 3R4F Reference Cigarette (n=4) and compared to responses in controls (Air, n=5). Numeral notation in panel A & B indicates post hoc testing with significant difference (p<0.05) between Air vs Day 0 = “0”; Air vs Day 1 = “1”, and Air vs Day 2 = “2” for respective doses. (C) Summary graph showing maximal response to ACh (i.e., [10−4] data point from dose response curves in A & B) showing similar blunting and overall time course for vessel recovery between Ecig and 3R4F Cigarette. *signifies different compared to Air (p<0.05). Post-hoc testing also revealed no differences between respective Ecig and 3R4F Cigarette groups at all time points, except D1 where a small, but statistically significant, difference between exposure groups was seen. Mean±SD.
To evaluate the effect of nitic oxide (NO) on EDD we incubated the MCA with L-NAME (an inhibitor of NO synthase, Sigma Aldrich) and then stimulated with ACh. Here we observed, in Air control animals, L-NAME inhibition of NO production accounts for half (~50%) of EDD stimulated response (Figure 3A). Within 24 hours of Ecig exposure (i.e. D0 and D1), the blunting effect with or without L-NAME in Ecig or 3R4F cigarettes exposed animals was not different, suggesting that majority (81-91%) of the impairment in MCA vessels at D0 and D1 was with due to reduced NO bioavailability (Figure 3A). The blunting effect of L-NAME remain consistent at all time points, even as partial and full recovery in MCA reactivity was observed at D2 and D3, respectively, in both Ecig and 3R4F Cigarette animals (Figure 3A).
Figure 3.
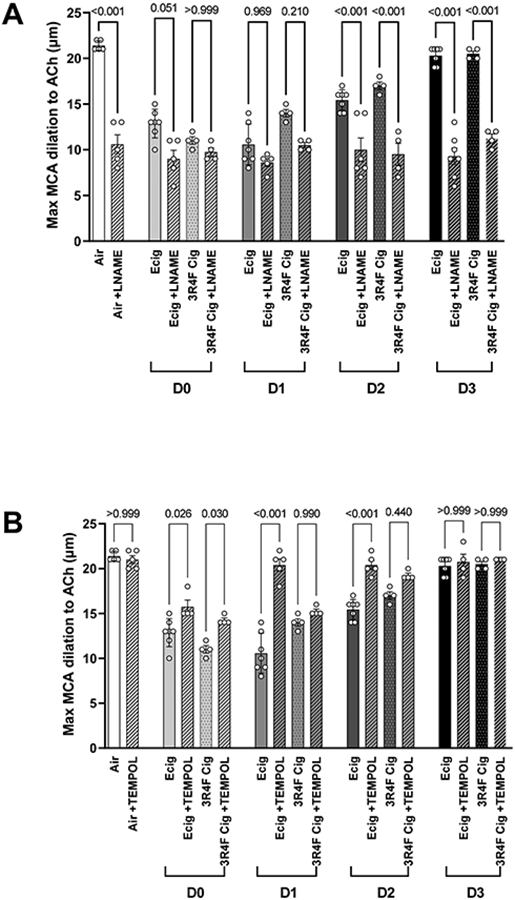
Data showing the temporal response of maximal [10−4] ACh-stimulated middle cerebral artery (MCA) incubated with (A) L-NAME, a nitric oxide (NO) inhibitor, or (B) TEMPOL, a superoxide dismutase mimetic (i.e. scavenger for superoxide anion). In (A), data show the inhibition of NO production in Air (control) animals results in ~50% impairment in MCA reactivity. Similar degree of impairment (i.e. ~50%) is observed in Ecig and 3R4F Cigarette exposed animals on D0 and D1, with or without L-NAME, suggesting the impairment is mostly attributable to reduced NO bioavailability. Partial recovery of the MCA reactivity (for both Ecig and 3R4F) begins by D2, and is fully recovered by D3, but again, is lost in the presence of L-NAME, further supporting the importance of altered NO underpinning the vascular impairment observed in response to Ecig and 3R4F on D0, D1 and D2. In (B), data show TEMPOL has small effect by improving MCA reactivity on D0, and then fully restores MCA dilation response on D1-3. In contrast, TEMPOL does not rescue the impaired reactivity in 3R4F Cigarette exposed animals, suggesting that superoxide-induced ROS activity plays a role with vascular dysfunction associated with Ecigs but not cigarettes.
We also evaluated the effect of TEMPOL on MCA reactivity to gain insight into the role of superoxide-mediated oxidative stress toward EDD response. For Ecig exposure, we observed MCA dysfunction was fully rescued by TEMPOL incubation on D1, D2 and D3; with only small (but statistically significant) improvement on D0 (Figure 3B). In contrast, 34RF Cigarette group, was not rescued at any time point with TEMPOL. However, as seen with Ecig group, there was also small (but statistically significant) improvement seen on D0 for 3R4F cigarette group (Figure 2B).
Endothelial-independent dilation (EID)
EID response of the MCA was similarly impaired between Ecig and 3R4F Cigarettes groups (Figure 4A-C). Impairment for both Ecig and 3R4F Cigarette was only seen on D0 and D1 (p<0.01 for both Ecig and 3R4F groups), while EID responses on D2 and D3 were not different that non-exposed control (Figure 4C).
Figure 4.
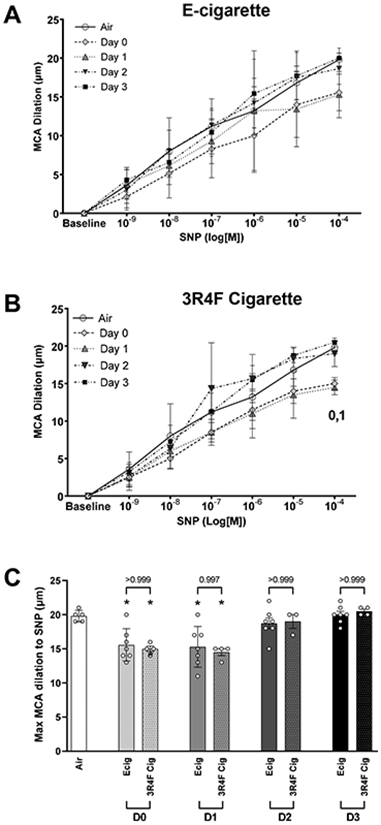
Middle cerebral arteries (MCA) reactivity assessed with ex vivo pressure myography. MCA dilation responses to increasing concentration of sodium nitroprusside (SNP). Dose response curve reflects data corresponding to number of (D)ays after an exposure to either (A) Ecig (n=7) or (B) 3R4F Reference Cigarette (n=4) and compared to responses in controls (Air, n=5). Numeral notation in panel A & B indicates post hoc testing with significant difference (p<0.05) between Air vs Day 0 = “0”, Air vs Day 1 = “1”. (C) Summary graph showing maximal response to SNP (i.e., [10−4] data point from dose response curves in A & B) showing no difference in overall time course to vessel recovery between Ecig and 3R4F Cigarette. * signifies different compared to Air (p<0.05). Post-hoc testing also revealed no differences between respective Ecig and 3R4F Cigarette groups at any time point. Mean±SD.
Vasoconstriction responses
Vasoconstrictor response to increasing concentration of serotonin (5HT) are shown in Figure 5. As seen with EDD and EID, greatest impairment (~45%) for exposure to Ecig and cigarettes occurred within 24-hours after exposure (between D0 and D1). Blunting of the vasoconstriction response (~19% average for vape/smoke groups, p<0.05) persisted at D2, except for Ecig 20-puff group (p=n.s.). By D3, MCA vasoconstriction responses to 5HT had returned to control levels in air exposed animals (Figure 5).
Figure 5.
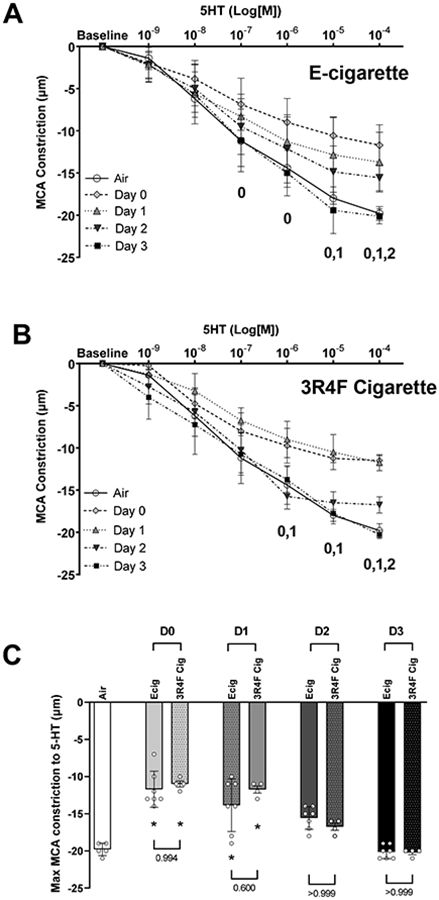
Middle cerebral arteries (MCA) reactivity assessed with ex vivo pressure myography. Data shows MCA constriction response to increasing concentration of serotonin (5-HT). Dose response curve reflects data corresponding to number of (D)ays after an exposure to either (A) Ecig (n=7) or (B) 3R4F Reference Cigarette (n=4) and compared to responses in controls (Air, n=5). Numeral notation in panel A & B indicates post hoc testing with significant difference (p<0.05) between Air vs Day 0 = “0”, Air vs Day 1 = “1”, vs Air and Day 2 = “2”. (C) Summary graph showing maximal response to SNP (i.e., [10−4] data point from dose response curves in A & B) showing no difference in overall time course to vessel recovery between Ecig and 3R4F Cigarette. * signifies different compared to Air (p<0.05). Post-hoc testing also revealed no differences between respective Ecig and 3R4F Cigarette groups at any time point. Mean±SD.
Extracellular Vesicles (EVs)
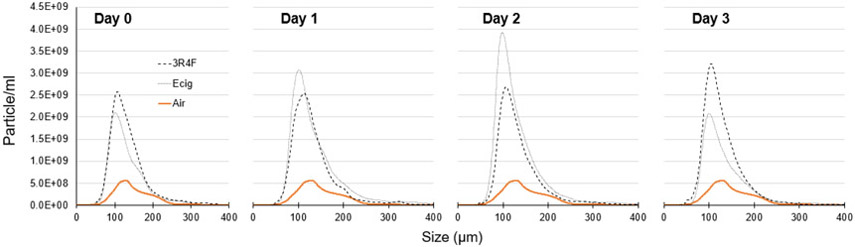
Circulating plasma EVs were found to be elevated in Ecig and 3R4F Cigarette exposed groups on D1, D2 and D3 when compared with Air exposed controls (Figure 6 and Table 3). Peak values of circulating EVs showed elevated EVs for both Ecig and 3R4F Cigarettes (compared to Air controls) on D1-D3, and only 3R4F cigarettes on D0 (Table 3). When assessing area-under-curve (AUC) for Ecig and 3R4F Cigarette compared to Air, AUC was also greater for Ecig on D1 and D2, and for 3R4F Cigarette on D0 and D3, but not statistically significant for 3R4F on D1 and D2 compared to Air (Table 3). Although an ANOVA main effect for exposure condition was seen for EV sizes (correlating to peak circulating EVs), post-hoc testing showed no significant difference between Ecig or 3R4F conditions compared Air on any day post exposure (Figure 6 and Table 3).
Figure 6.
Data showing temporal expression of circulating extracellular vesicles (EVs) number and size-distribution in plasma collected from Air (n=5), Ecig (n=7) and 3R4F Cigarette (n=4) exposed animals. ANOVA main effect for exposure condition (Ecig or 3R4F vs Air) is significant at all time points (i.e. D0-D3) when assessing peak EV values, EV size (at peak value) and Area-Under-Curve (AUC). Post-hoc testing and summary statistics are shown in Table 3.
Table 3.
Extracellular Vesicles Summary data
| Air | Ecig | 3R4F | ANOVA Exposure |
ANOVA Days |
ANOVA Interaction |
|
|---|---|---|---|---|---|---|
|
|
|
|
|
|||
| n= | 5 | 7 | 4 | |||
| Peak Distribution Value (x108 | ||||||
| D0 | 7.41±8.90 | 22.3±14.9 | 27.1±5.8* | p<0.001 | p=0.629 | p=0.446 |
| D1 | 32.8±15.5* | 28.1±7.3* | ||||
| D2 | 39.0±15.4* | 26.9±5.5* | ||||
| D3 | 26.1±13.4* | 33.6±9.9* | ||||
| Size @Peak (um) | ||||||
| D0 | 124±16 | 110±46 | 115±12 | p=0.005 | p=0.839 | p=0.992 |
| D1 | 101±9 | 108±11 | ||||
| D2 | 100±2 | 108±2 | ||||
| D3 | 106±13 | 105±6 | ||||
| Area Under Curve (x107 | ||||||
| D0 | 6.32±7.50 | 15.3±12.1 | 20.8±3.4* | p<0.001 | p=0.661 | p=0.387 |
| D1 | 23.5±9.3* | 20.2±4.3□ | ||||
| D2 | 27.0±9.7* | 19.5±1.6□ | ||||
| D3 | 15.5±1.1 | 22.5±7.4* | ||||
Tukey post-hoc test
p<0.05 and
p=0.075-0.05 compared to Air
DISCUSSION
To our knowledge this is the first report to describe temporal effects of Ecig exposure on cerebrovascular function. Our data demonstrates that a single 20-puff session of Ecig or cigarette use rapidly impairs MCA function (within 1-4 hours), and that impairment in EDD takes up to 72 hours after exposure to recover (for both cigarette smoke or Ecig aerosol exposure). This temporal response was the same even when increasing dose of Ecig exposure three-fold (going from 20- to 60-puffs, Fig. 1). The overall respective MCA responses (EDD, EID and vasoconstriction) were nearly identical between cigarette vs Ecig, suggesting that exposure to Ecigs likely confers similar risk/harm potential to cerebrovasculature that is known from cigarette use.
A major difference in the recovery of blunted responses we observed was the recovery (reactivity similar to air exposed control levels) for EID and vasoconstriction responses occurred earlier (by D2, i.e. 48 hours after exposure), whereas recovery for EDD responses did not occur until D3 (72 hours after exposure). The reason for the difference between EDD vs EID (or vasoconstriction) is not clear, but suggests that mechanisms with direct impact to smooth muscle function induced by EID are shorter-lived than that to endothelial cells, or that it takes a greater insult to blunt smooth muscle mechanisms rather than endothelium. In chronic conditions, such as chronic stress (Brooks et al., 2018), diabetes/metabolic syndrome (Phillips et al., 2005), and chronic inhalation to Ecigs or cigarettes (Olfert et al., 2018) impaired EDD is often accompanied by enhanced vascular smooth muscle vasoconstriction, which has been linked to reduced bioavailability of NO, the production of vasoconstrictor peptides (angiotensin II, endothelin-1), the formation of oxygen-derived free radicals (e.g. superoxide anions) and/or the release of vasoconstrictor metabolites of arachidonic acid. However, we show that the impaired EDD of the MCA was accompanied by a blunted, and not enhanced, vasoconstriction response. An explanation that might account for this difference is that chronic conditions associated with vascular dysfunction would also be expected to have vascular remodeling (e.g. changes in elastin and collagen deposition) that are not present (or have had sufficient time to develop) in the acute phase. But we also cannot rule out that receptors or enzymes in smooth muscle regulation are also directly impaired with acute exposure to Ecig and cigarettes. Thus, acute effects toward smooth muscle could be very different from those after chronic exposures.
Our data (LNAME+ACh, Figure 3A) establish that impairment in NO bioavailability is a major contributor to MCA dysfunction we observed. This finding is consistent with existing reports that both smoking (Golbidi et al., 2020) and vaping (Carnevale et al., 2016) reduces NO bioavailability. Numerous studies have also shown that Ecig aerosol produce cytotoxicity and oxidative stress to endothelial cells (as well as epithelial cells, fibroblasts, and many other cells)(Lerner et al., 2015; Lerner et al., 2016; Putzhammer et al., 2016; Sancilio et al., 2016). Indeed, when examining MCA response to ACh in the presence of TEMPOL (a stable synthetic compound that mimics superoxide dismutase) we found that TEMPOL improved MCA function at all time points, and fully rescued impairment on D1-D3 (Figure 3B). This supports the notion that oxidative stress is a principal contributor to the MCA dysfunction induced by Ecig exposure. The fact that TEMPOL only partially rescued MCA dysfunction within the first few hours after exposure (i.e. D0, Figure 3B) indicates that a different mechanism is likely at play in this early response. In contrast to Ecig, TEMPOL only partially improved MCA dysfunction induced by cigarettes at the D1-D2 time points, suggesting that superoxide may not the main mechanism at play with cigarette exposure. However, it should be noted, TEMPOL not only reduces superoxide, but it may increase H2O2 in blood vessels (Chen et al., 2003). In turn, H2O2 may produce vasodilation (Gao et al., 2003), vasoconstriction (Rodríguez-Martínez et al., 1998; Gao & Lee, 2001) or a biphasic effect (Gao et al., 2003; Csekő et al., 2004) depending on the vascular bed and experimental conditions. As such, we cannot rule out that the acute TEMPOL incubation of the MCA with the aim to lower the presence of superoxide, may have improved the MCA endothelial-dependent dilation via the modulating actions of H2O2.
One important difference between our Ecig and cigarette exposed groups was the presence of nicotine in 3R4F cigarettes. Nicotine acts on nicotinic acetylcholine receptors (NAChRs) which are broadly present in the body (i.e. central and peripheral nervous system, endothelial cells, immune cells, and many other organs/tissue), and have well documented cardiovascular effects (i.e. increasing heart rate, blood pressure, sympathetic predominance). Rather, the role and contribution of nicotine in the etiology for impaired EDD is more questionable. Several studies (Palpant et al., 2015; Carnevale et al., 2016; Vlachopoulos et al., 2016; Chaumont et al., 2018; Franzen et al., 2018), including our own work (Olfert et al., 2018), have reported that Ecigs (with or without nicotine) produces similar EDD blunting effects as that occurring with smoking. These data are consistent with extensive literature showing chronic nicotine administration via transdermal application does not increase risk of cardiovascular disease (Working Group Report, 1994; Joseph et al., 1996; Tzivoni et al., 1998; Meine et al., 2005). We have also previously reported TEMPOL is able to rescue MCA function in adolescent and adult rat offspring that have MCA dysfunction secondary to maternal Ecig exposure during pregnancy (with or without nicotine)(Burrage et al., 2021). Thus, it is not immediately clear why we see TEMPOL rescue blunted EDD on D1-D3 for Ecigs, but not for cigarettes in the present study. We can only postulate that additional constituents in cigarette smoke could be triggering MCA dysfunction that are not responsive to actions of TEMPOL.
From a toxicological point of view, it is necessary to consider both the physical and chemical constituents present in the smoke/aerosol cloud delivered to the lungs. In our previous work (Burrage et al., 2021) we have reported that nearly all aerosol produced by Ecigs is in the fine range (less than 2.5 μm, PM2.5), and that ~10-20% are in the ultrafine range (PM less than 0.1 um, PM0.1). Both PM2.5 and PM0.1 are recognized in the etiology of vascular dysfunction (Brook et al., 2002; Kampa & Castanas, 2008; D'Errico & Stapleton, 2018), however PM0.1 is of particular interest as they can elicit cardiovascular responses independent of any effects in the lung (see review (Riediker et al., 2019)). Thus, Ecigs are designed to deliver aerosol that is optimal for deposition into the lung periphery. Yet, it is interesting to note, despite significant differences in aerosol concentration between 3R4F cigarette and Ecigs groups (ranging from 7 to 334 mg/m3)(Table 1), we do not find major differences in magnitude or temporal outcomes observed (Figures 1-6). These data suggest the differences in aerosol concentration (in this range and size distribution) does not worsen MCA function, indicating that the threshold that triggers vascular dysfunction is likely to be very low.
Numerous studies suggest that carbonyl compounds may play a significant role with vaping-induced vascular dysfunction (Bekki et al., 2014; Kosmider et al., 2014; Farsalinos & Gillman, 2017; Ogunwale et al., 2017; Jin et al., 2021). Indeed, many studies have reported on chemical composition associated with Ecig aerosol generation, but it is often difficult or impossible to compare these studies to clinical outcomes due to differences in Ecig device, coil material, composition of e-liquid, and/or puff topology. However a recent report (Jin et al., 2021) finds that aerosolized formaldehyde produces a similar impairment in aortic EDD as seen with Ecig exposure, providing evidence that reactive aldehydes at the same concentration found within Ecig aerosol can trigger vascular dysfunction. Conklin and colleagues (Conklin et al., 2018) have also shown that changing the ratio of VG:PG can influence relative concentration of formaldehyde (and other reactive aldehydes) in each Ecig puff, raising the possibility that the ratio of VG:PG e-liquid could have an influence effecting the degree of vascular impairment. While the purpose of this study was not meant to uncover the mechanistic links responsible for MCA dysfunction, we believe the temporal responses and outcomes we are reporting are valuable to ensure the appropriate attention is made to the timing after inhalation exposures that investigator use in future (more mechanistic) studies. Moreover, our data and emerging literature provides strong rationale to more fully characterize the chemical constituents found in Ecig aerosols and their effect on clinical and physiological outcomes in future studies.
This is wide recognition EVs can provide important endocrine signaling in health and disease (Stahl & Raposo, 2019). Previous work from our lab (Burrage et al., 2021) and others (Antoniewicz et al., 2016; Benedikter et al., 2018; Mobarrez et al., 2020) have found Ecig exposure increase circulating EVs. Accordingly, we evaluated the temporal release of plasma EVs in our exposure groups. Our data show both Ecig and 3R4F cigarettes increase circulating EVs by 3-4.5 fold within the time course we studied, and remained elevated on D3 (even after our MCA reactivity returned to control levels)(Figure 6). The implications of this remains unclear and will require further investigation, but raise the possibility epigenetic signaling events have much longer half-life than the clinical/physiological impairments observed. Ecig and 3R4F responses were not different within the first 24 hrs (i.e. D0-D1), but were different on D2 and D3. Again, the significance of this (if any) is not presently clear. It is notable, a recent human study reports (a one-time 30-puff Ecig exposure) increases EVs within 2-6 hours after vaping, but only with Ecig+nicotine, and not with vaping without nicotine. Our study provides a longer temporal window of evaluation, showing that circulating EVs peaked after 2- or 3-days, for Ecig and Cigrettes respectively (Figure 6, Table 2). Although we do not have Ecig+nicotine to report, our 3R4F cigarettes contained nicotine, and showed increases of EVs were similarly elevated at D0-D1 compare to our Ecig groups (which did not have nicotine. Regardless of the temporal difference observed, these data provide compelling evidence that exposure to Ecigs (even without nicotine) produced significant biological stress to trigger increases in circulating EVs. Once released, EVs can be taken up by many different cell types (including endothelial cells) and thus are believed to represent early indicator of cellular changes that lead to chronic disease (Benedikter et al., 2018). Although we have not identified the cellular/tissue source(s) of EVs in this study, our data add to a growing number of studies that suggest Ecig exposure induces significant physiologic perturbations (similar to that seen with smoking) that indicate Ecigs should not be considered safe.
Human Relevance and Study limitations
Given that a relatively low exposure (one-time 20-puffs) produced significant blunting (~50%) of MCA function, and that the overall effect was similar with cigarette exposure (20-puffs), it does not seem logical to conclude that vaping is risk-free, or that vaping will have meaningful harm reduction benefits toward vascular pathologies. Although the exposure in this acute study is not sufficient (nor designed) to induce any vascular pathologies per se, it clearly demonstrates even low-levels of Ecig or cigarette exposure alters the normal physiological responses (homeostasis) of cerebrovascular bed for several days. Thus, it is not difficult to envisage that repeated daily smoking, or vaping, could lead to damage and/or remodeling that ultimately result in vascular disease, such as stroke, peripheral artery disease atherosclerosis, coronary heart disease, etc. (Freund et al., 1993). Indeed, studies show that both vaping and smoking produces endothelial dysfunction in humans as evidenced by impaired flow-mediated dilation (FMD)(Carnevale et al., 2016; Biondi-Zoccai et al., 2019; Caporale et al., 2019; Chatterjee et al., 2021) and increases in pulse wave velocity (PWV, an index of arterial stiffness)(Vlachopoulos et al., 2016; Franzen et al., 2018; Kerr et al., 2019). In vivo animals models also show increased FMD (Rao et al., 2020), increased PWV (Olfert et al., 2018; Szostak et al., 2020), as well as impaired vascular reactivity with chronic Ecig and cigarette exposures (Olfert et al., 2018; Burrage et al., 2021; Jin et al., 2021). Thus, although it is too early to know whether E-cigs will produce the same long-term outcomes on humans as smoking, based on evidence in this study and existing literature it seems unlikely to expect that Ecigs will be different/better for humans than smoking.
In this study, we did not include nicotine (or any flavors) in our e-liquid. While this is potential limitation in our study, we would also point out our findings provide insight that is relevant to all E-cig users. Indeed, our data underscores the point that the base solution (i.e. delivery vehicle itself) is a major contributor to vascular dysfunction. This does not mean that nicotine, or even other additives (such as flavors)(Fetterman et al., 2018), are not important and/or may be potentially harmful in their own right. To the contrary, nicotine alone can have significant influence on addiction and cardiovascular responses (as previously mentioned). Likewise, thermal degradation of flavors added to e-liquid can also produce harmful chemicals. User preferences toward nicotine concentration, choice of flavors, device voltage- or temperature-setting, vaping topology and frequency of use, are likely add further (not less) harm.
Conclusions
E-cigs have only been widely available in USA/Europe markets since 2007, thus long-term consequences of Ecig use in humans are still being debated. While proponents for Ecigs argue that they provide significant health benefits compared with smoking (Benowitz & Burbank, 2016; Benowitz & Fraiman, 2017), there is growing evidence showing similarity in responses between Ecig and cigarette exposure that cannot be ignored. Our data show that acute MCA dysfunction is associated with exposure to Ecig and cigarettes and persists for up to 3 days after a single exposure. The observation that Ecig (without nicotine) and cigarette (with nicotine) exposure results in the same overall magnitude and temporal response for recovery of MCA reactivity, suggests that factor(s) other than nicotine are likely responsible for the sequalae leading to MCA dysfunction. When taken in the broader context of health, our data showing MCA dysfunction in correlation with increases in circulating EV concentration adds to the growing body of evidence that counters the narrative that Ecigs are ‘safe’, or even ‘safer’ than cigarettes. These data highlight the need for novel tobacco products to be evaluated with a more holistic view of health, and to look beyond just the potential for lung injury when evaluating the harm potential from inhalation exposures.
New Findings.
What is the central question of this study?
Acute exposure to electronic cigarettes (Ecigs) triggers abnormal vascular responses in systemic arteries, however effects on cerebral vessels are poorly understood and time for recovery is not known. We hypothesized that exposure to cigarettes or Ecigs would trigger rapid (<4-hours) impairment of middle cerebral artery (MCA) but that this would resolve by 24-hours.
What is the main finding and its importance?
Cigarettes and Ecigs caused similar degree and duration of MCA impairment. We find it takes up to 72-hours after exposure for MCA function to return to normal. Our finding suggest that Ecig use likely produces similar adverse vascular health outcomes as seen with cigarette smoke.
Acknowledgements
We gratefully acknowledge assist by Dr. Kathy Brundage and the WVU Flow Cytometry & Single Cell Core Facility for technical assistance in conducting assessment of extracellular vesicles.
Grants
Funding support was provided, in part, by NIH U54-GM104942-05S1 (IMO), NIH 1R21-ES033026-01 (IMO, PDC, JB), American Heart Association CSA 20 35320107 (IMO, DD, JB, PDC), and resources provided the WVU Flow Cytometry & Single Cell Core Facility (NIH GM109098, NIH GM103434).
Footnotes
Disclosures
The authors have no conflict of interest, financial or otherwise, to declare.
Data Availability Statement
The data that supports the findings of this study are presented in Figures 1-6 and Tables 1-3, and are available in the supporting information for this article – see statistical summary table.
References
- Alzahrani T, Pena I, Temesgen N & Glantz SA (2018). Association Between Electronic Cigarette Use and Myocardial Infarction. American Journal of Preventive Medicine 55, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniewicz L, Bosson JA, Kuhl J, Abdel-Halim SM, Kiessling A, Mobarrez F & Lundback M (2016). Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis 255, 179–185. [DOI] [PubMed] [Google Scholar]
- Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H & Kunugita N (2014). Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health 11, 11192–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedikter BJ, Wouters EFM, Savelkoul PHM, Rohde GGU & Stassen FRM (2018). Extracellular vesicles released in response to respiratory exposures: implications for chronic disease. J Toxicol Environ Health B Crit Rev 21, 142–160. [DOI] [PubMed] [Google Scholar]
- Benowitz NL & Burbank AD (2016). Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends in Cardiovascular Medicine 26, 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL & Fraiman JB (2017). Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol 14, 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi-Zoccai G, Sciarretta S, Bullen C, Nocella C, Violi F, Loffredo L, Pignatelli P, Perri L, Peruzzi M, Marullo AGM, Falco ED, Chimenti I, Cammisotto V, Valenti V, Coluzzi F, Cavarretta E, Carrizzo A, Prati F, Carnevale R & Frati G (2019). Acute Effects of Heat-Not-Burn, Electronic Vaping, and Traditional Tobacco Combustion Cigarettes: The Sapienza University of Rome-Vascular Assessment of Proatherosclerotic Effects of Smoking (SUR-VAPES) 2 Randomized Trial. Journal of the American Heart Association 8, e010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S & Silverman F (2002). Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 105, 1534–1536. [DOI] [PubMed] [Google Scholar]
- Brooks S, Branyan KW, DeVallance E, Skinner R, Lemaster K, Sheets JW, Pitzer CR, Asano S, Bryner RW, Olfert IM, Frisbee JC & Chantler PD (2018). Psychological stress-induced cerebrovascular dysfunction: the role of metabolic syndrome and exercise. Exp Physiol 103, 761–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrage EN, Aboaziza E, Hare L, Reppert S, Moore J, Goldsmith WT, Kelley EE, Mills A, Dakhlallah D, Chantler PD & Olfert IM (2021). Long-term cerebrovascular dysfunction in the offspring from maternal electronic cigarette use during pregnancy. Am J Physiol Heart Circ Physiol 321, H339–H352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher JT, Goodwill AG & Frisbee JC (2012). The ex vivo Isolated Skeletal Microvessel Preparation for Investigation of Vascular Reactivity. JoVE, e3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale A, Langham MC, Guo W, Johncola A, Chatterjee S & Wehrli FW (2019). Acute Effects of Electronic Cigarette Aerosol Inhalation on Vascular Function Detected at Quantitative MRI. Radiology 293, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G & Frati G (2016). Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest 150, 606–612. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Caporale A, Tao JQ, Guo W, Johncola A, Strasser AA, Leone FT, Langham MC & Wehrli FW (2021). Acute e-cig inhalation impacts vascular health: a study in smoking naive subjects. Am J Physiol Heart Circ Physiol 320, H144–H158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont M, de Becker B, Zaher W, Culié A, Deprez G, Mélot C, Reyé F, Van Antwerpen P, Delporte C, Debbas N, Boudjeltia KZ & van de Borne P (2018). Differential Effects of E-Cigarette on Microvascular Endothelial Function, Arterial Stiffness and Oxidative Stress: A Randomized Crossover Trial. Scientific Reports 8, 10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-F, Allen W Cowley J & Zou A-P (2003). Increased H2O2 counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 285, R827–R833. [DOI] [PubMed] [Google Scholar]
- Conklin DJ, Ogunwale MA, Chen Y, Theis WS, Nantz MH, Fu X-A, Chen L-C, Riggs DW, Lorkiewicz P, Bhatnagar A & Srivastava S (2018). Electronic cigarette-generated aldehydes: The contribution of e-liquid components to their formation and the use of urinary aldehyde metabolites as biomarkers of exposure. Aerosol science and technology : the journal of the American Association for Aerosol Research 52, 1219–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csekő C, Bagi Z & Koller A (2004). Biphasic effect of hydrogen peroxide on skeletal muscle arteriolar tone via activation of endothelial and smooth muscle signaling pathways. Journal of Applied Physiology 97, 1130–1137. [DOI] [PubMed] [Google Scholar]
- D'Errico JN & Stapleton PA (2018). Developmental onset of cardiovascular disease-Could the proof be in the placenta? Microcirculation, e12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Mitchell GF, Bots ML, Sigurdsson S, Harris TB, Garcia M, Eiriksdottir G, Buchem MAv, Gudnason V & Launer LJ (2015). Carotid Arterial Stiffness and Risk of Incident Cerebral Microbleeds in Older People. Arteriosclerosis, Thrombosis, and Vascular Biology 35, 1889–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mahdy MA, Mahgoup EM, Ewees MG, Eid MS, Abdelghany TM & Zweier JL (2021). Long-term electronic cigarette exposure induces cardiovascular dysfunction similar to tobacco cigarettes: role of nicotine and exposure duration. Am J Physiol Heart Circ Physiol 320, H2112–H2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE & Gillman G (2017). Carbonyl Emissions in E-cigarette Aerosol: A Systematic Review and Methodological Considerations. Front Physiol 8, 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterman JL, Weisbrod RM, Feng B, Bastin R, Tuttle ST, Holbrook M, Baker G, Robertson RM, Conklin DJ, Bhatnagar A & Hamburg NM (2018). Flavorings in Tobacco Products Induce Endothelial Cell Dysfunction. Arterioscler Thromb Vasc Biol 38, 1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen KF, Willig J, Cayo Talavera S, Meusel M, Sayk F, Reppel M, Dalhoff K, Mortensen K & Droemann D (2018). E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: A randomized, double-blinded pilot study. Vascular Medicine 23, 419–425. [DOI] [PubMed] [Google Scholar]
- Freund KM, Belanger AJ, D'Agostino RB & Kannel WB (1993). The health risks of smoking. The Framingham Study: 34 years of follow-up. Ann Epidemiol 3, 417–424. [DOI] [PubMed] [Google Scholar]
- Gao Y-J, Hirota S, Zhang D-W, Janssen LJ & Lee RMKW. (2003). Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. British Journal of Pharmacology 138, 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ & Lee RMKW (2001). Hydrogen peroxide induces a greater contraction in mesenteric arteries of spontaneously hypertensive rats through thromboxane A2 production. British Journal of Pharmacology 134, 1639–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbidi S, Edvinsson L & Laher I (2020). Smoking and Endothelial Dysfunction. Curr Vasc Pharmacol. Volume 18, Number 1, 2020, pp. 1–11(11) [DOI] [PubMed] [Google Scholar]
- Working Group Report (1994). Nicotine replacement therapy for patients with coronary artery disease. Arch Intern Med 154, 989–995. [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. Exp Physiol 100, 755–758. [DOI] [PubMed] [Google Scholar]
- Hanon O, Haulon S, Lenoir H, Seux M-L, Rigaud A-S, Safar M, Girerd X & Forette F (2005). Relationship Between Arterial Stiffness and Cognitive Function in Elderly Subjects With Complaints of Memory Loss. Stroke 36, 2193–2197. [DOI] [PubMed] [Google Scholar]
- Harper SL, Bohlen HG & Rubin MJ (1984). Arterial and microvascular contributions to cerebral cortical autoregulation in rats. American Journal of Physiology-Heart and Circulatory Physiology 246, H17–H24. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Cambronero FE, Liu D, Moore EE, Neal JE, Terry JG, Nair S, Pechman KR, Rane S, Davis LT, Gifford KA, Hohman TJ, Bell SP, Wang TJ, Beckman JA & Carr JJ (2018). Higher Aortic Stiffness Is Related to Lower Cerebral Blood Flow and Preserved Cerebrovascular Reactivity in Older Adults. Circulation 138, 1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Lynch J, Richardson A, Lorkiewicz P, Srivastava S, Theis W, Shirk G, Hand A, Bhatnagar A, Srivastava S & Conklin DJ (2021). Electronic cigarette solvents, pulmonary irritation, and endothelial dysfunction: role of acetaldehyde and formaldehyde. Am J Physiol Heart Circ Physiol 320, H1510–H1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Norman SM, Ferry LH, Prochazka AV, Westman EC, Steele BG, Sherman SE, Cleveland M, Antonuccio DO, Hartman N & McGovern PG (1996). The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med 335, 1792–1798. [DOI] [PubMed] [Google Scholar]
- Kaisar MA, Villalba H, Prasad S, Liles T, Sifat AE, Sajja RK, Abbruscato TJ & Cucullo L (2017). Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is Metformin a viable countermeasure? Redox biology 13, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa M & Castanas E (2008). Human health effects of air pollution. Environ Pollut 151, 362–367. [DOI] [PubMed] [Google Scholar]
- Kerasioti E, Veskoukis AS, Skaperda Z, Zacharias A, Poulas K, Lazopoulos G & Kouretas D (2020). The flavoring and not the nicotine content is a decisive factor for the effects of refill liquids of electronic cigarette on the redox status of endothelial cells. Toxicol Rep 7, 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DMI, Brooksbank KJM, Taylor RG, Pinel K, Rios FJ, Touyz RM & Delles C (2019). Acute effects of electronic and tobacco cigarettes on vascular and respiratory function in healthy volunteers: a cross-over study. J Hypertens 37, 154–166. [DOI] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J & Goniewicz ML (2014). Carbonyl Compounds in Electronic Cigarette Vapors: Effects of Nicotine Solvent and Battery Output Voltage. Nicotine & Tobacco Research 16, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntic M, Oelze M, Steven S, Kroller-Schon S, Stamm P, Kalinovic S, Frenis K, Vujacic-Mirski K, Bayo Jimenez MT, Kvandova M, Filippou K, Al Zuabi A, Bruckl V, Hahad O, Daub S, Varveri F, Gori T, Huesmann R, Hoffmann T, Schmidt FP, Keaney JF, Daiber A & Munzel T (2020). Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2). Eur Heart J 41, 2472–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Ong SG, Zhou Y, Tian L, Bae HR, Baker N, Whitlatch A, Mohammadi L, Guo H, Nadeau KC, Springer ML, Schick SF, Bhatnagar A & Wu JC (2019). Modeling Cardiovascular Risks of E-Cigarettes With Human-Induced Pluripotent Stem Cell-Derived Endothelial Cells. J Am Coll Cardiol 73, 2722–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Rutagarama P, Ahmad T, Sundar IK, Elder A & Rahman I (2016). Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochemical and Biophysical Research Communications 477, 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R & Rahman I (2015). Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE 10, e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard JH, Liu Y, Fredricks KT, Bizub DM, Roman RJ & Rusch NJ (1999). Electrical and mechanical responses of rat middle cerebral arteries to reduced P O 2 and prostacyclin. American Journal of Physiology-Heart and Circulatory Physiology 276, H509–H516. [DOI] [PubMed] [Google Scholar]
- Meine TJ, Patel MR, Washam JB, Pappas PA & Jollis JG (2005). Safety and effectiveness of transdermal nicotine patch in smokers admitted with acute coronary syndromes. Am J Cardiol 95, 976–978. [DOI] [PubMed] [Google Scholar]
- Mitchell GF (2015). Arterial stiffness: insights from Framingham and Iceland. Current Opinion in Nephrology and Hypertension 24, 1–7. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V & Launer LJ (2011). Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility – Reykjavik Study. Brain 134, 3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobarrez F, Antoniewicz L, Hedman L, Bosson JA & Lundbäck M (2020). Electronic cigarettes containing nicotine increase endothelial and platelet derived extracellular vesicles in healthy volunteers. Atherosclerosis 301, 93–100. [DOI] [PubMed] [Google Scholar]
- Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ & Fu XA (2017). Aldehyde Detection in Electronic Cigarette Aerosols. ACS Omega 2, 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfert IM, DeVallance E, Hoskinson H, Branyan KW, Clayton S, Pitzer CR, Sullivan DP, Breit MJ, Wu Z, Klinkhachorn P, Mandler WK, Erdreich BH, Ducatman BS, Bryner RW, Dasgupta P & Chantler PD (2018). Chronic exposure to electronic cigarettes results in impaired cardiovascular function in mice. J Appl Physiol (1985) 124, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palpant NJ, Hofsteen P, Pabon L, Reinecke H & Murry CE (2015). Cardiac development in zebrafish and human embryonic stem cells is inhibited by exposure to tobacco cigarettes and e-cigarettes. PLoS ONE 10, e0126259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S & Mitchell GF (2016). Aortic Stiffness and the Risk of Incident Mild Cognitive Impairment and Dementia. Stroke 47, 2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SA, Sylvester FA & Frisbee JC (2005). Oxidant stress and constrictor reactivity impair cerebral artery dilation in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 288, R522–530. [DOI] [PubMed] [Google Scholar]
- Putzhammer R, Doppler C, Jakschitz T, Heinz K, Forste J, Danzl K, Messner B & Bernhard D (2016). Vapours of US and EU Market Leader Electronic Cigarette Brands and Liquids Are Cytotoxic for Human Vascular Endothelial Cells. PLoS ONE 11, e0157337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Liu J & Springer ML (2020). JUUL and Combusted Cigarettes Comparably Impair Endothelial Function. Tobacco Regulatory Science 6, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediker M, Zink D, Kreyling W, Oberdorster G, Elder A, Graham U, Lynch I, Duschl A, Ichihara G, Ichihara S, Kobayashi T, Hisanaga N, Umezawa M, Cheng TJ, Handy R, Gulumian M, Tinkle S & Cassee F (2019). Particle toxicology and health - where are we? Part Fibre Toxicol 16, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Martínez MA, García-Cohen EC, Baena AB, González R, Salaíces M & Marín J (1998). Contractile responses elicited by hydrogen peroxide in aorta from normotensive and hypertensive rats. Endothelial modulation and mechanism involved. British Journal of Pharmacology 125, 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancilio S, Gallorini M, Cataldi A & di Giacomo V (2016). Cytotoxicity and apoptosis induction by e-cigarette fluids in human gingival fibroblasts. Clin Oral Investig 20, 477–483. [DOI] [PubMed] [Google Scholar]
- Stahl PD & Raposo G (2019). Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology (Bethesda) 34, 169–177. [DOI] [PubMed] [Google Scholar]
- Szostak J, Wong ET, Titz B, Lee T, Wong SK, Low T, Lee KM, Zhang J, Kumar A, Schlage WK, Guedj E, Phillips B, Leroy P, Buettner A, Xiang Y, Martin F, Sewer A, Kuczaj A, Ivanov NV, Luettich K, Vanscheeuwijck P, Peitsch MC & Hoeng J (2020). A 6-month systems toxicology inhalation study in ApoE(−/−) mice demonstrates reduced cardiovascular effects of E-vapor aerosols compared with cigarette smoke. Am J Physiol Heart Circ Physiol 318, H604–h631. [DOI] [PubMed] [Google Scholar]
- Tarumi T, Shah F, Tanaka H & Haley AP (2011). Association Between Central Elastic Artery Stiffness and Cerebral Perfusion in Deep Subcortical Gray and White Matter. Am J Hypertension 24, 1108–1113. [DOI] [PubMed] [Google Scholar]
- Thorin-Trescases N, Montgolfier Od, Pinçon A, Raignault A, Caland L, Labbé P & Thorin E (2018). Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. American Journal of Physiology-Heart and Circulatory Physiology 314, H1214–H1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T & Brunel P (1998). Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther 12, 239–244. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Ioakeimidis N, Abdelrasoul M, Terentes-Printzios D, Georgakopoulos C, Pietri P, Stefanadis C & Tousoulis D (2016). Electronic Cigarette Smoking Increases Aortic Stiffness and Blood Pressure in Young Smokers. J Am Coll Cardiol 67, 2802–2803. [DOI] [PubMed] [Google Scholar]
- Wehrli FW, Caporale A, Langham MC & Chatterjee S (2020). New Insights From MRI and Cell Biology Into the Acute Vascular-Metabolic Implications of Electronic Cigarette Vaping. Front Physiol 11, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are presented in Figures 1-6 and Tables 1-3, and are available in the supporting information for this article – see statistical summary table.