Abstract
The AlkS protein activates transcription from the PalkB promoter, allowing the expression of a number of genes required for the assimilation of alkanes in Pseudomonas oleovorans. We have identified the promoter from which the alkS gene is transcribed, PalkS, and analyzed its expression under different conditions and genetic backgrounds. Transcription from PalkS was very low during the exponential phase of growth and increased considerably when cells reached the stationary phase. The PalkS −10 region was similar to the consensus described for promoters recognized by Escherichia coli RNA polymerase bound to the alternative sigma factor ςS, which directs the expression of many stationary-phase genes. Reporter strains containing PalkS-lacZ transcriptional fusions showed that PalkS promoter is very weakly expressed in a Pseudomonas putida strain bearing an inactivated allele of the gene coding for ςS, rpoS. When PalkS was transferred to E. coli, transcription started at the same site and expression was higher in stationary phase only if ςS-RNA polymerase was present. The low levels of AlkS protein generated in the absence of ςS were enough to support a partial induction of the PalkB promoter. The −10 and −35 regions of PalkS promoter also show some similarity to the consensus recognized by ςD-RNA polymerase, the primary form of RNA polymerase. We propose that in exponential phase PalkS is probably recognized both by ςD-RNA polymerase (inefficiently) and by ςS-RNA polymerase (present at low levels), leading to low-level expression of the alkS gene. ςS-RNA polymerase would be responsible for the high level of activity of PalkS observed in stationary phase.
Like many other microorganisms, soil bacteria live in environments of frequently changing conditions and have evolved mechanisms to withstand unfavorable situations, such as famine periods or other stressful circumstances. When nutrients become limited, cells stop growing and enter the so-called stationary phase, a process that involves important changes in the global pattern of gene expression and protein turnover (reviewed in reference 21). One of the key elements whose synthesis and activity increases at the onset of stationary phase is the alternative sigma factor ςS, encoded by the rpoS gene. This factor binds to the RNA polymerase (RNAP) core, substituting for the vegetative (primary) factor ςD, changing in this way the promoter specificity of RNAP holoenzyme and directing it towards a subset of stationary-phase promoters (reviewed in reference 26). Interest in ςS has grown considerably in the last few years, although efforts have been almost exclusively directed towards the case of Escherichia coli. Although genes homologous to the E. coli rpoS have been described in soil bacteria such as Pseudomonas putida (34), Pseudomonas fluorescens (36), and Pseudomonas aeruginosa (39), very little information is available about ςS-dependent promoters in pseudomonads. This is an important group of bacteria because of its wide distribution in many different environments, its great nutritional and metabolic versatility (which gives it an important role in the degradation of chemicals and in the carbon cycle), and because it includes several pathogens for plants and animals.
We have found that ςS is involved in the regulation of the pathway for the assimilation of n-alkanes encoded in the OCT plasmid of Pseudomonas oleovorans. The genetics and enzymology of the metabolism of medium-chain-length n-alkanes (C6 to C12) in P. oleovorans have been well characterized; the enzymes involved oxidize the alkanes to the corresponding terminal acyl-coenzyme A derivatives, which then enter the β-oxidation cycle (reviewed in reference 42). Expression of the genes coding for these enzymes is controlled by the AlkS protein, a transcriptional regulator which, in the presence of alkanes, activates the expression of the PalkB promoter (9, 19, 44). It has been shown that the PalkB promoter is correctly expressed, maintaining its regulation by AlkS, when transferred to P. putida and to E. coli (10, 45). In addition, PalkB promoter activity, and therefore that of the alkane degradation pathway, is modulated by catabolic repression depending on the carbon source being used, both in P. oleovorans (14, 38) and when transferred to P. putida (45). We show in this report that the gene coding for the AlkS protein is expressed at levels in the stationary phase much higher than those in the exponential phase of growth, and that it is transcribed from a ςS-dependent promoter. This suggests that the expression of the genes required for the metabolism of alkanes is connected to the metabolic status of the cell through at least two checkpoints: the growth phase (through ςS) and the carbon source being used (through catabolic repression).
MATERIALS AND METHODS
Bacterial strains and plasmids.
The strains and plasmids used throughout this work are listed in Table 1. The PalkS-lacZ transcriptional fusion used included positions −344 to +53 relative to the PalkS transcriptional start site defined in this work. The PalkB-lacZ transcriptional fusion contained positions −525 to +66 relative to the PalkB transcription start site (45). These fusions were delivered to the chromosome of P. putida or E. coli cells with plasmids pTPS16 and pPBK2, respectively, two mini-Tn5-based suicide donor vectors which contain the above fusions (45). Plasmid pRSP1 was obtained by cloning at the EcoRI site of the broad-host-range vector pKT231 an EcoRI DNA segment from plasmid pMIR13450 containing the P. putida rpoS gene.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description or relevant phenotype | Reference or source |
|---|---|---|
| E. coli strains | ||
| CC118(λpir) | CC118 lysogenized with λpir phage | 16 |
| TG1 | Host for DNA manipulations | 35 |
| MC4100 | Wild type for the rpoS gene | 37 |
| RH90 | rpoS derivative of MC4100 | 23 |
| ER2 | MC4100 with a PalkS-lacZ fusion in the chromosome | This work |
| ERS2 | RH90 with a PalkS-lacZ fusion in the chromosome | This work |
| P. putida strains | ||
| KT2442 | hdsR; Rifr derivative of KT2440 | 13 |
| PBS4 | KT2442 with a PalkB-lacZ fusion and alkS in the chromosome | 45 |
| PS16 | KT2442 with a PalkS-lacZ fusion in the chromosome | 45 |
| 5.2 | rpoS derivative of KT2442 (Smr gene inserted into rpoS) | M. I. Ramos-González |
| C1R1 | rpoS derivative of KT2440 (luxA,B genes inserted into rpoS) | 34 |
| PSS1 | Strain 5.2 with a PalkS-lacZ fusion in the chromosome | This work |
| PSS5 | C1R1 with a PalkS-lacZ fusion in the chromosome | This work |
| PSPS1 | Strain 5.2 with PalkB-lacZ fusion and alkS in the chromosome | This work |
| CRSP1 | C1R1 with PalkB-lacZ fusion and alkS in the chromosome | This work |
| Plasmids | ||
| pRK2013 | Kmr Mob+ Tra+; donor of transfer functions | 12 |
| pKT231 | Smr Kmr; broad-host-range RSF1010-derived vector | 2 |
| pTPS16 | Apr Telr; PalkS-lacZ fusion cloned into a suicide mini-Tn5-based delivery vector | 45 |
| pPBK2 | Apr Kmr; PalkB-lacZ fusion cloned into a suicide mini-Tn5-based delivery vector | 45 |
| pTLS1 | Apr Telr; alkS gene cloned at the NotI site of pJMT6 | 45 |
| pTS1 | Apr; contains the alkS gene and 559 nt upstream from it | 45 |
| pUJPS16 | Apr; contains the PalkS-lacZ transcriptional fusion | 45 |
| pRSP1 | Kmr; contains the wild-type P. putida rpoS gene | This work |
General procedures for DNA manipulations were as previously described (35). Plasmid DNA was introduced into P. putida by conjugation, using plasmid pRK2013 as the donor of transfer functions in triparental matings, as described previously (7).
Media and culture conditions.
Cells were grown at 30°C in rich Luria-Bertani (LB) medium or in minimal salts M9 medium (35), the latter supplemented with citrate (30 mM) as the carbon source and with trace elements (3). Where indicated, the nonmetabolizable inducer dicyclopropylketone (DCPK), which mimics the inducing effect of alkanes, was added up to 0.05% (vol/vol) to induce the expression of PalkB promoter. Antibiotics were used at the following concentrations (in micrograms per milliliter): ampicillin, 100; kanamycin, 50; tetracycline, 12; streptomycin, 50. Potassium tellurite was used at 80 μg/ml.
Assay for β-galactosidase.
An overnight culture of cells harboring either a PalkS-lacZ fusion or a PalkB-lacZ fusion and the alkS gene was diluted to a final turbidity of about 0.04 in fresh LB medium or in minimal salts M9 medium supplemented with citrate as the carbon source. Cultures were grown at 30°C and, at different times, aliquots were taken and β-galactosidase activity was measured as described by Miller (30). In the case of cells bearing the PalkB-lacZ fusion, expression of PalkB promoter was induced by the addition of the nonmetabolizable inducer DCPK up to 0.05% (vol/vol) when cultures reached a turbidity of 0.08 (at 600 nm).
S1 nuclease analyses of mRNAs.
Total RNA was isolated from bacterial cultures as previously described (32). S1 nuclease reactions were performed as previously described (1), using 25 μg of total RNA and an excess of a 5′ end-labeled single-stranded DNA (ssDNA) hybridizing to the 5′ region of the mRNA. This ssDNA probe was generated by linear PCR as described before (45), using as substrate either plasmid pUJPS16 (which contains the PalkS-lacZ fusion) or pTS1 (which contains the complete alkS gene and 627 nucleotides [nt] upstream from the alkS translation start site). Prior to being used as template for the amplification reactions, plasmids were cut with NotI (pUJPS16) or HindIII (pTS1), whose targets are located more than 400 nt upstream from the PalkS start site.
PCR amplification.
Primers used to amplify an internal region of the P. putida and P. oleovorans rpoS gene were 5′-AGACCATCGA(G/A)CGGGC(G/C)ATCAT and 5′-CG(A/G)TCGTCGGT(G/C)A(G/C)GGTATC, whose 5′ ends anneal to positions 473 and 743, respectively, of the P. putida rpoS gene. Amplification was performed using standard protocols (annealing temperature, 65°C; elongation temperature, 70°C; 30 cycles) by which we obtained a DNA fragment 270 bp in length corresponding to a region of the P. putida rpoS gene coding for ςS residues 159 to 247 (34).
RESULTS
Localization and characterization of the promoter for the alkS gene.
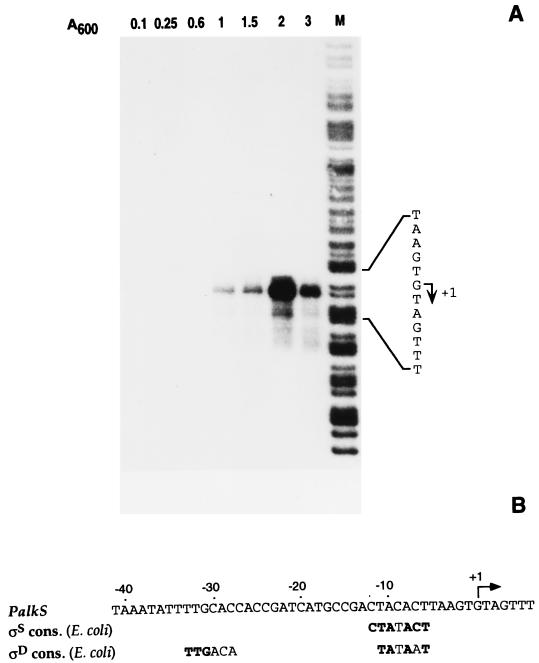
The DNA sequences required for a normal expression of the alkS gene have been narrowed down to about 400 nt upstream from the alkS initiation codon (9, 44), which indicates that the promoter for this gene should lie within this 400-nt DNA region. Accordingly, a fusion to lacZ that included the sequences immediately upstream from the alkS ribosome binding site and up to position −408 relative to the translation initiation codon was constructed (45). This fusion was introduced into the chromosome of P. putida KT2442, in which the alk system is correctly expressed, maintaining its regulatory features (45). Analysis of the expression of β-galactosidase in the resulting strain, named PS16, suggested that alkS is expressed at higher levels when cells enter into stationary phase than when they grow exponentially (45). To investigate this observation, which could be important for the regulation of the alk pathway, we sought to localize and characterize the promoter for the alkS gene. Total RNA was purified from cells of P. putida PS16 at different moments of the culture growth, and the alkS transcription start site was investigated by S1 nuclease mapping. As shown in Fig. 1, a clear unique signal was observed, suggesting a putative transcription start site located 68 nt upstream from the alkS initiation codon. Transcription was barely detectable during the exponential phase of growth, increasing considerably when cells entered into stationary phase (Fig. 1). mRNA levels decreased in late stationary phase. The same results were obtained when the assay was performed with a P. putida derivative containing the complete alkS gene (strain PBS4; not shown).
FIG. 1.
Identification of the promoter for the alkS gene. (A) P. putida PS16 was grown in LB and when the culture reached a turbidity (A600) of 0.1, 0.25, 0.6, 1, 1.5, 2, or 3, aliquots were collected and processed to obtain total RNA. The transcripts arising from the promoter region for the alkS gene at each cell density were analyzed by S1 mapping, using equal amounts of total RNA and an excess of ssDNA probe in all cases. This allows the simultaneous detection of the transcription start site and the amount of transcript generated. The gel shows the signal obtained at each cell density; samples were run in parallel with a DNA size ladder (lane M) obtained by chemical sequencing of the same ssDNA used as probe, as described previously (29). The transcription start site (+1) is indicated. (B) Comparison of the sequences upstream from the transcription start site (the PalkS promoter) with the consensus sequences of E. coli promoters for ςD-RNAP and for ςS-RNAP. Boldface letters indicate the positions of the consensus that are present at the PalkS promoter.
Inspection of the region upstream from the detected transcription start site showed sequences with certain homology to the consensus for the vegetative ςD-RNAP; although only 50% of the positions matched the consensus (Fig. 1), these were the most conserved in vegetative E. coli promoters (25). In addition, sequences in the −10 region showed high similarity to the consensus proposed for the promoters recognized by ςS-RNAP in E. coli (11, 26, 40). Considering that the transcriptional activity observed matched that of stationary-phase ςS-dependent promoters (expression is low in exponential phase and high in stationary phase), it seemed likely that the start site detected corresponded to the promoter for the alkS gene, which we named PalkS and which is probably recognized by ςS-RNAP. The presence of sequences resembling the consensus for the vegetative RNAP suggested that ςD-RNAP may also recognize this promoter. We next investigated these possibilities in more detail.
Efficient transcription from the PalkS promoter requires ςS-RNAP.
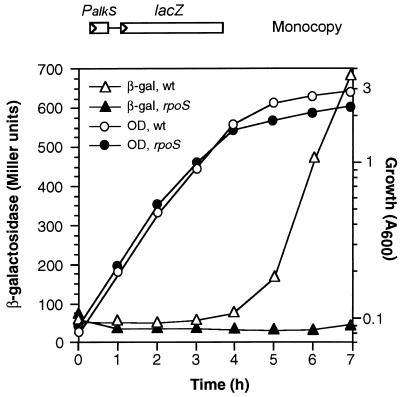
The gene coding for the alternative sigma factor ςS (rpoS) in P. putida has recently been identified and P. putida strains bearing an inactivated rpoS allele have been obtained (34). To determine in vivo whether the expression of PalkS promoter indeed relies on ςS-RNAP, the PalkS-lacZ transcriptional fusion was introduced into the chromosome of P. putida 5.2, in which the rpoS gene has been interrupted by a cassette specifying resistance to streptomycin and is, therefore, a ςS-deficient strain (Table 1). The suicide donor plasmid pTPS16 was used for this purpose, since it contains the transcriptional fusion cloned into a mini-Tn5 transposon. The strain thus obtained, which contained the PalkS-lacZ fusion in a rpoS-deficient background, was named PSS1. The isogenic strain PS16, containing the PalkS-lacZ fusion and a wild-type rpoS allele, was used as a control (strains 5.2 and PS16 both derive from P. putida KT2442; Table 1). Figure 2 shows that expression of β-galactosidase in the wild-type strain (PS16) was very low in the exponential phase and increased considerably (up to 14-fold) in the stationary phase, in agreement with the amounts of transcripts generated from PalkS at different growth stages observed in Fig. 1. In the case of the rpoS-deficient strain (PSS1), the levels of β-galactosidase were very low both in the exponential and in the stationary phases of growth (between 40 and 50 Miller units), although an overnight incubation of the cultures allowed the accumulation of small amounts of β-galactosidase (around 150 to 180 Miller units). The same behavior was observed in several independent transconjugants, in which the PalkS-lacZ fusion should be inserted into different locations of the chromosome, indicating that the low expression of PalkS in the rpoS-deficient strain PSS1 is not the result of the particular chromosomal region into which the PalkS-lacZ fusion is inserted. To further verify this result, we introduced the PalkS-lacZ fusion into a different rpoS-deficient P. putida strain, named C1R1, which derives from P. putida KT2440 (the parental strain of KT2442). Analyses of β-galactosidase expression in the resulting strain, named PSS5, showed results similar to those described above for strain PSS1 (data not shown). Finally, the introduction of a plasmid containing the wild-type P. putida rpoS gene (plasmid pRSP1) into the rpoS-deficient strain PSS1 restored the correct expression of the PalkS promoter in stationary phase, yielding an expression pattern essentially identical to that obtained in the wild-type PS16 strain (not shown). Altogether, the results obtained strongly suggest that efficient expression of the alkS gene requires the presence of ςS-RNAP.
FIG. 2.
Expression of the PalkS promoter in P. putida cells devoid of ςS. P. putida strains PS16 (wild-type for rpoS) and PSS1 (bearing an inactivated rpoS allele), both containing the PalkS-lacZ fusion inserted into the chromosome, were grown in LB medium, and levels of β-galactosidase were measured at different cell densities. The plot shows the amount of β-galactosidase (in Miller units; represented by triangles) and the cell density (OD600; represented by circles) observed at each sampling time. Open symbols correspond to strain PS16, and filled symbols correspond to strain PSS1. A minimum of three independent assays were performed for each strain; representative assays are shown. wt, wild type.
The rpoS gene is present in P. oleovorans.
P. oleovorans and P. putida belong to the group of Pseudomonas sensu stricto, and the sequences of their 16S rDNA show a 97.6% similarity (33). The alk system is expressed similarly in both species, showing the same regulatory features (10, 45), which suggests that PalkS should be recognized by ςS-RNAP not only in P. putida but in P. oleovorans as well. Nevertheless, the presence of the rpoS gene in P. oleovorans had not been reported. To investigate this issue, two oligonucleotides whose 5′ ends annealed to positions 473 and 743, respectively, of the P. putida rpoS gene were designed. This rpoS region codes for a segment of ςS that is highly conserved among Pseudomonas species (34). The use of total DNA from P. putida as template in PCR allowed us to obtain a single amplification product of the expected size (270 bp). When the input DNA corresponded to P. oleovorans, a single DNA fragment of the same size was obtained. Sequencing of a 230-bp region of the amplification product from P. oleovorans showed that it was 93.2% identical to the homologous region of the P. putida rpoS gene, the translated product being 100% identical to the corresponding segment of the P. putida ςS protein (not shown). Therefore, rpoS is present in P. oleovorans.
The PalkS promoter has similar behavior in E. coli and P. putida.
ςS-dependent promoters have been studied mainly in E. coli (8, 11, 17, 20, 40, 43). To analyze the behavior of the PalkS promoter in E. coli, the PalkS-lacZ transcriptional fusion was introduced into the chromosome of the E. coli isogenic strains MC4100 (wild type for rpoS) and RH90 (rpoS deficient), using the delivery vector pTPS16. As in P. putida, PalkS expression in wild-type E. coli cells for rpoS was significantly higher in stationary phase than in exponential phase, and the higher activity in stationary phase was not observed in rpoS-deficient cells (Fig. 3A). Nevertheless, PalkS expression seemed to be more efficient in E. coli than in P. putida in all cases (compare Fig. 3A and 2). An S1-mapping analysis indicated that transcription from PalkS initiated in E. coli at the same site as in P. putida (Fig. 3B), although a fraction of the transcripts initiated 2 bp upstream. Transcripts were hardly detectable during the exponential phase and increased significantly in the stationary phase (Fig. 3). Again, essentially no transcription from PalkS was detected in the rpoS-deficient E. coli strain. Therefore, as in P. putida, efficient expression of the alkS promoter in E. coli depends on ςS-RNAP.
FIG. 3.
Expression of the PalkS promoter in E. coli: influence of ςS. E. coli strains ER2 (wild type for rpoS) and ERS2 (bearing an inactivated rpoS allele), both containing the PalkS-lacZ fusion inserted into the chromosome, were grown in LB medium. At different cell densities, aliquots were taken and cells were processed either for measurement of β-galactosidase levels or for purification of total RNA. (A) Plot showing the amount of β-galactosidase (in Miller units; represented by triangles) and the cell density (OD600; represented by circles) observed at each sampling time. Open and filled symbols correspond to strain ER2 and to strain ERS2, respectively. (B) PalkS transcription start site and amount of transcript produced in each strain at the indicated cell densities, analyzed by S1 mapping in the presence of an excess of labeled ssDNA probe. Equal amounts of total RNA were used in each sample. Lane M is a DNA size ladder obtained as for Fig. 1. wt, wild type.
The low expression levels of alkS in the absence of ςS allow for a substantial induction of the PalkB promoter.
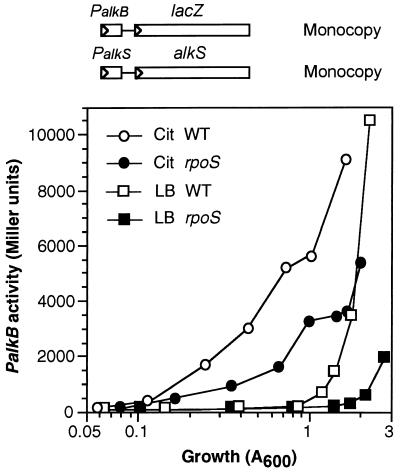
It is believed that some E. coli ςS-dependent promoters can also be recognized by the vegetative ςD-RNAP, at least under certain conditions (8, 20, 22, 40). In the case of PalkS, although the levels of mRNA arising from it in P. putida rpoS-deficient cells were essentially undetectable (not shown), some weak expression occurred since low but detectable amounts of β-galactosidase were synthesized in rpoS-deficient cells harboring the PalkS-lacZ fusion (Fig. 2). To investigate whether the PalkS promoter could be recognized by RNAP in the absence of ςS, we analyzed whether the AlkS protein levels produced when the alkS gene is introduced into a P. putida rpoS-deficient background are enough to activate the PalkB promoter. To this end, the alkS gene (including its own promoter) and a PalkB-lacZ transcriptional fusion were introduced by means of mini-Tn5 transposon-delivery vectors into the chromosome of the P. putida rpoS-deficient strain 5.2, yielding strain PSPS1, and the levels of β-galactosidase produced in the absence or presence of the nonmetabolizable inducer DCPK were analyzed. For comparison, the isogenic strain PBS4 was used, which also contains the PalkB-lacZ fusion and the alkS gene inserted into the chromosome and bears a wild-type rpoS allele. Analyses with cells growing both in rich LB medium and in minimal salts medium supplemented with citrate as carbon source were performed, since it is known that the induction of PalkB in exponential phase by DCPK and AlkS is strongly down-regulated by catabolic repression when cells are grown in rich LB medium but not when grown on minimal salts medium containing citrate as the carbon source (45). Repression in LB medium is relieved when cells reach stationary phase. It should be mentioned that expression of the alkS gene is not affected by catabolic repression (45). As shown in Fig. 4, when cells were grown in minimal salts medium with the inducer DCPK, the levels of AlkS present in the strain lacking ςS were enough to activate transcription from the PalkB promoter, although the induction was about two- or threefold lower than when a wild-type rpoS allele was present. When cells were grown in LB medium, activation of PalkB was restricted to the stationary phase, when catabolic repression is relieved (45), and again the induction was considerably more efficient when ςS was present (around 20-fold, depending on the culture density considered; Fig. 4). Similar results were observed when several transconjugants were analyzed and when the PalkB-lacZ fusion and the alkS gene were inserted into the P. putida rpoS-deficient strain C1R1 (not shown), suggesting that the expression of alkS that occurs in the absence of ςS takes place from its own promoter, and not by readthrough from chromosomal sequences located upstream from it. Therefore, the results presented here indicate that in the absence of ςS-RNAP the alkS gene is still expressed, although at very low levels. This low-level expression nevertheless generates enough AlkS protein to allow a partial induction of the PalkB promoter in the presence of an inducer. As discussed below, transcription from PalkS in the absence of ςS should depend on a RNAP holoenzyme bound to another sigma factor, most likely to the vegetative ςD.
FIG. 4.
Activation of the PalkB promoter by AlkS in P. putida cells devoid of ςS. P. putida PBS4 (wild type for rpoS) and PSPS1 (bearing an inactivated rpoS allele), both harboring a PalkB-lacZ fusion and the alkS gene integrated into the chromosome, were grown in duplicate in LB medium (represented by squares) or in minimal salts medium supplemented with citrate (Cit; represented by circles). At a cell density of about 0.08, the nonmetabolizable inducer DCPK was added to one of the flasks, leaving the other one as a noninduced control. Aliquots were taken at different times from both flasks, and levels of β-galactosidase were measured. The plot shows the expression of PalkB observed in induced cultures as a function of cell density (A600). The levels of β-galactosidase observed in the absence of inducer were in the range of 30 to 100 Miller units, slowly increasing with cell density, and are not shown. Open symbols correspond to strain PBS4, and filled symbols to strain PSPS1. A minimum of three independent assays were performed for each medium; representative assays are shown. WT, wild type.
DISCUSSION
Expression of the pathways for the degradation of aliphatic and aromatic hydrocarbons is normally regulated, most often at the transcriptional level. Since these compounds are not preferred growth substrates, regulation is frequently also subject to overimposed levels of control which connect the activity of individual promoters of these pathways to the physiological status of the cell (reviewed in reference 4). The pathway for the degradation of alkanes of P. oleovorans is a good example of this interconnection between promoter activity and cell metabolism. It is known that its expression is modulated by catabolic repression by certain organic acids (succinate or lactate) and by amino acids (14, 38, 45). In this work, we show that regulation of this pathway may have an additional link to the metabolic status of the cell since transcription from the promoter leading to the expression of the alkS gene, PalkS, is very low during the exponential phase and much higher in stationary phase. Many promoters that are activated at the onset of stationary phase rely on a form of RNAP that contains the alternative sigma factor ςS, the amounts of which are subject to a complex regulation at the levels of transcription, translation, and protein stability (24), being higher in stationary phase (18). We present several lines of evidence indicating that PalkS is a ςS-dependent promoter. First, the −10 region of PalkS is highly similar to the consensus proposed for E. coli promoters recognized by ςS-RNA polymerase (11, 26, 43). In addition, P. putida reporter strains devoid of ςS and bearing a PalkS-lacZ transcriptional fusion supported a very low expression of the PalkS promoter, indicating that ςS-RNAP is required for its efficient expression. Normal expression of PalkS was restored when a wild-type rpoS allele was supplied in trans. Finally, PalkS showed similar behavior when transferred to E. coli, its expression being much lower in ςS-deficient cells.
The PalkS promoter shows also some sequence similarities with promoters recognized by the vegetative ςD-RNAP. Indeed, the −10 region of E. coli ςS- and ςD-dependent promoters has been shown to be rather similar, and some promoters are recognized by both ςS-RNAP and ςD-RNAP, at least in vitro (40, 41). Factors such as the decrease of DNA supercoiling that occurs at the onset of stationary phase or changes in medium osmolarity are believed to determine whether such promoters are preferentially recognized by ςS-RNAP of by ςD-RNAP (8, 22). In cells having a disrupted rpoS gene, which are therefore devoid of ςS-RNAP, PalkS was very weakly expressed, although this low-level expression led to AlkS protein levels high enough to allow partial induction of the promoter it activates, PalkB. The low levels of AlkS present in rpoS-deficient cells probably arise from the recognition of the PalkS promoter by another form of RNAP holoenzyme. Considering that the −10 and −35 regions of PalkS show certain similarity to the consensus recognized by the vegetative RNAP, the most likely candidate would be ςD-RNAP. Therefore, it is likely that the low levels of AlkS produced in exponential phase arise from recognition of PalkS by both ςD-RNAP (inefficiently) and by ςS-RNAP which, at least in E. coli, is believed to be present at low but detectable levels in exponential phase (18). The total amount of transcripts arising from PalkS in exponential phase is nevertheless low. In stationary phase, transcription from the PalkS promoter is much more efficient. Since PalkS is a ςS-dependent promoter, it is likely that, as has been shown for E. coli (18), the levels of ςS-RNAP in P. putida are probably higher in stationary phase than in exponential phase.
Most of what is known about ςS-RNAP comes from studies performed in E. coli. As mentioned above, the behavior of PalkS promoter in E. coli was similar to that observed in P. putida: its activity was lower in exponential phase than in stationary phase, transcription started at the same site, and efficient expression required also the alternative sigma factor ςS. Another Pseudomonas promoter, the Pm promoter from the P. putida TOL plasmid, has been shown to depend on ςS both in E. coli and in P. putida (27, 31). Therefore, as more promoters are studied it will not be surprising to find that Pseudomonas ςS-dependent promoters behave analogously to the corresponding E. coli promoters.
Our results suggest that P. putida needs very little expression of the alkS gene to induce the pathway for the degradation of alkanes. Although stationary-phase cells have a much higher level of expression of the gene coding for the regulator, this does not lead to gratuitous induction of the alk pathway in the absence of inducers, since basal levels of expression of the PalkB promoter in stationary phase increase very little, if at all (reference 45 and this work). We suspect that even when present at high levels, AlkS is unable to activate transcription in the absence of an appropriate inducer.
Several hypotheses as to what could be the advantage of having AlkS under the control of ςS-RNAP can be proposed. It should be taken into account that ςS is more than just a stationary-phase sigma factor but rather a global regulator involved in adaptation to situations of stress (15). It could be argued that cells may detect high concentrations of alkanes as a stress signal, so that recruitment of the AlkS regulator under the control of ςS would help to trigger a fast response to the stress signal. Nevertheless, it has been shown that alkanes by themselves do not have a negative effect on cell growth; rather, it is the alkane hydroxylase (a membrane protein) and the products of alkane oxidation that are detrimental for the cell (5, 6). Another possibility is that under conditions of starvation a higher concentration of the activator protein could help to speed up a response when alkanes that can be used as a growth substrate enter the cell. In this sense, it has been shown that AlkS is present in the cell in limiting amounts during the exponential phase (45). Whether higher AlkS levels lead to a more efficient induction of PalkB promoter in stationary phase has not been investigated. It should be mentioned that starvation has been shown to lead to increased expression of several catabolic enzymes even if their respective substrates are absent, or present at very low concentrations (reviewed in reference 28). This response to is believed to confer on the cell an enhanced scavenging capacity for scarce substrates. Similarly, higher AlkS levels in stationary phase could confer a superior scavenging ability for trace amounts of alkanes entering the cell. Finally, AlkS could be less stable in stationary phase than in exponential phase, a problem that could be circumvented if the alkS gene is expressed more efficiently in stationary phase. This possibility has not been explored. Whatever the reason for needing higher amounts of AlkS in stationary phase, it is reasonable to predict that the alkS gene is under the influence of a ςS-dependent promoter as a way to assure a fast response to the presence of alkanes under situations of famine.
ACKNOWLEDGMENTS
We are grateful to Maribel Ramos for providing the P. putida rpoS strains and to Victor de Lorenzo and José Pérez-Martín for stimulating discussions.
This work was supported by grant BIO97-0645-C02-01 from the Comisión Interministerial de Ciencia y Tecnología and grant 07M/0720/1997 from the Comunidad Autónoma de Madrid.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 2.Bagdasarian M, Lurz R, Rückert B, Franklin F C H, Bagdasarian M M, Frey J, Timmis K N. Specific-purpose plasmid cloning vectors. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 3.Bauchop T, Eldsen S R. The growth of microorganisms in relation to their energy supply. J Gen Microbiol. 1960;23:457–569. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- 4.Cases I, de Lorenzo V. Expression systems and physiological control of promoter activity in bacteria. Curr Opin Microbiol. 1998;1:303–310. doi: 10.1016/s1369-5274(98)80034-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Janssen D B, Witholt B. Growth on octane alters the membrane lipid fatty acids of Pseudomonas oleovorans due to the induction of alkB and synthesis of octanol. J Bacteriol. 1995;177:6894–6901. doi: 10.1128/jb.177.23.6894-6901.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Q, Janssen D B, Witholt B. Physiological changes and alk gene instability in Pseudomonas oleovorans during induction and expression of alk genes. J Bacteriol. 1996;178:5508–5512. doi: 10.1128/jb.178.18.5508-5512.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lorenzo V, Timmis K N. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 8.Ding Q, Kusano S, Villarejo M, Ishihama A. Promoter selectivity control of Escherichia coli RNA polymerase by ionic strength: differential recognition of osmoregulated promoters by EςD and EςS holoenzymes. Mol Microbiol. 1995;16:649–656. doi: 10.1111/j.1365-2958.1995.tb02427.x. [DOI] [PubMed] [Google Scholar]
- 9.Eggink G, Engel H, Meijer W G, Otten J, Kingma J, Witholt B. Alkane utilization in Pseudomonas oleovorans. Structure and function of the regulatory locus alkR. J Biol Chem. 1988;263:13400–13405. [PubMed] [Google Scholar]
- 10.Eggink G, Lageveen R G, Latenburg B, Witholt B. Controlled and functional expression of the Pseudomonas oleovorans alkane utilizing system in Pseudomonas putida and Escherichia coli. J Biol Chem. 1987;262:17712–17718. [PubMed] [Google Scholar]
- 11.Espinosa-Urgel M, Chamizo C, Tormo A. A consensus structure for ςS-dependent promoters. Mol Microbiol. 1996;21:657–659. doi: 10.1111/j.1365-2958.1996.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 12.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin F C H, Bagdasarian M, Bagdasarian M M, Timmis K N. Molecular and functional analysis of the TOL plasmid pWW0 from Pseudomonas putida and cloning of the genes for the entire regulated aromatic ring meta-cleavage pathway. Proc Natl Acad Sci USA. 1981;78:7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grund A, Shapiro J, Fennewald M, Bacha P, Leahy J, Markbreiter K, Nieder M, Toepfer M. Regulation of alkane oxidation in Pseudomonas putida. J Bacteriol. 1975;123:546–556. doi: 10.1128/jb.123.2.546-556.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hengge-Aronis R. Back to log phase: ςS as a global regulator in osmotic control of gene expression in Escherichia coli. Mol Microbiol. 1996;21:887–893. doi: 10.1046/j.1365-2958.1996.511405.x. [DOI] [PubMed] [Google Scholar]
- 16.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiratsu K, Shinagawa H, Makino K. Mode of promoter recognition by the Escherichia coli RNA polymerase holoenzyme containing the ςS subunit: identification of the recognition sequence of the fic promoter. Mol Microbiol. 1995;18:841–850. doi: 10.1111/j.1365-2958.1995.18050841.x. [DOI] [PubMed] [Google Scholar]
- 18.Jishage M, Iwata A, Ueda S, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of the four species of sigma subunit under various growth conditions. J Bacteriol. 1996;178:5447–5451. doi: 10.1128/jb.178.18.5447-5451.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kok M, Oldenhuis R, van der Linden M P G, Raatges P, Kingma J, van Lelyveld P H, Witholt B. The Pseudomonas oleovorans alkane hydroxylase gene. Sequence and expression. J Biol Chem. 1989;264:5435–5441. [PubMed] [Google Scholar]
- 20.Kolb A, Kotlarz D, Kusano S, Ishihama A. Selectivity of the Escherichia coli RNA polymerase Eς38 for overlapping promoters and ability to support CRP activation. Nucleic Acids Res. 1995;23:819–826. doi: 10.1093/nar/23.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolter R, Siegele D A, Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 22.Kusano S, Ding Q, Fujita N, Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase Eς70 and Eς38 holoenzymes. J Biol Chem. 1996;271:1998–2004. doi: 10.1074/jbc.271.4.1998. [DOI] [PubMed] [Google Scholar]
- 23.Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991;5:49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 24.Lange R, Hengge-Aronis R. The cellular concentration of the ςS subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation and protein stability. Genes Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- 25.Lisser S, Margalit H. Compilation of E. coli mRNA promoter sequences. Nucleic Acids Res. 1993;21:1507–1516. doi: 10.1093/nar/21.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loewen P C, Hengge-Aronis R. The role of the sigma factor ςS (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 27.Marqués S, Gallegos M T, Ramos J L. Role of ςS in transcription from the positively controlled promoter of the TOL plasmid of Pseudomonas putida. Mol Microbiol. 1995;18:851–857. doi: 10.1111/j.1365-2958.1995.18050851.x. [DOI] [PubMed] [Google Scholar]
- 28.Matin A. Role of alternative sigma factors in starvation protein synthesis-novel mechanisms of catabolite repression. Res Microbiol. 1996;147:494–505. doi: 10.1016/s0923-2508(96)90151-5. [DOI] [PubMed] [Google Scholar]
- 29.Maxam A M, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 30.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 31.Miura K, Inouye S, Nakazawa A. The rpoS gene regulates OP2, an operon for the lower pathway of xylene catabolism on the TOL plasmid, and stress response in Pseudomonas putida mt-2. Mol Gen Genet. 1998;259:72–78. doi: 10.1007/s004380050790. [DOI] [PubMed] [Google Scholar]
- 32.Monsalve M, Mencía M, Rojo F, Salas M. Transcription regulation in bacteriophage φ29: expression of the viral promoters throughout the infection cycle. Virology. 1995;207:23–31. doi: 10.1006/viro.1995.1048. [DOI] [PubMed] [Google Scholar]
- 33.Moore E R B, Mau M, Arnscheidt A, Böttger E C, Hutson R A, Collins M D, van de Peer Y, de Wacther R, Timmis K N. The determination and comparison of the 16S rRNA gene sequences of species of the genus Pseudomonas (sensu stricto) and estimation of the natural intrageneric relationships. Syst Appl Microbiol. 1996;19:478–492. [Google Scholar]
- 34.Ramos-González M I, Molin S. Cloning, sequencing and phenotypic characterization of the rpoS gene from Pseudomonas putida KT2440. J Bacteriol. 1998;180:3421–3431. doi: 10.1128/jb.180.13.3421-3431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 36.Samiguet A, Kraus J, Henkels M D, Muehlchen A M, Loper J E. The sigma factor sigma S affects antibiotic production and biological control activity of Pseudomonas fluorescens Pf-5. Proc Natl Acad Sci USA. 1995;92:12255–12259. doi: 10.1073/pnas.92.26.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 38.Staijen I E. The alkane hydroxylase system of Pseudomonas oleovorans: expression and activity in recombinant Escherichia coli and the native host. Ph.D. thesis. Groningen, The Netherlands: University of Groningen; 1996. [Google Scholar]
- 39.Tanaka K, Takahashi H. Cloning, analysis and expression of an rpoS homologue gene from Pseudomonas aeruginosa PAO1. Gene. 1994;150:81–85. doi: 10.1016/0378-1119(94)90862-1. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka K, Kusano S, Fujita N, Ishihama A, Takahashi H. Promoter determinants for Escherichia coli RNA polymerase holoenzyme containing ς38 (the rpoS gene product) Nucleic Acids Res. 1995;23:827–834. doi: 10.1093/nar/23.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. Heterogeneity of the principal sigma factor in Escherichia coli: the rpoS gene product, ς38, is a second principal sigma factor of RNA polymerase in stationary phase Escherichia coli. Proc Natl Acad Sci USA. 1993;90:3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Beilen J B, Wubbolts M G, Witholt B. Genetics of alkane oxidation by Pseudomonas oleovorans. Biodegradation. 1994;5:161–174. doi: 10.1007/BF00696457. [DOI] [PubMed] [Google Scholar]
- 43.Wise A, Brems R, Ramakrishnan V, Villarejo M. Sequences in the −35 region of the Escherichia coli rpoS-dependent genes promote transcription by EςS. J Bacteriol. 1996;178:2785–2793. doi: 10.1128/jb.178.10.2785-2793.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wubbolts M. Xylene and alkane monooxygenases from Pseudomonas putida. Ph.D. thesis. Groningen, The Netherlands: University of Groningen; 1994. [Google Scholar]
- 45.Yuste L, Canosa I, Rojo F. Carbon-source-dependent expression of the PalkB promoter from the Pseudomonas oleovorans alkane degradation pathway. J Bacteriol. 1998;180:5218–5226. doi: 10.1128/jb.180.19.5218-5226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]