Abstract
Objective:
Emerging evidence from animal experiments indicate that factors secreted by the placenta are critical for normal fetal organ development. Our objective was to characterize the umbilical vein and artery proteome in preterm infants and identify proteins that decrease in the neonatal circulation following delivery.
Methods:
Cord blood at delivery and neonatal blood at 48–72 hours of life was collected in 25 preterm infants. Plasma protein abundance was determined using the SomaLogic platform.
Results:
When comparing protein levels of umbilical venous to arterial cord blood, 434 proteins were significantly higher indicating placental secretion into the fetal circulation. Moreover, when comparing neonatal blood to umbilical vein levels, 142 proteins were significantly lower. These proteins included Endoplasmic reticulum resident protein 29, CD59, Fibroblast growth factor 2 and Dynactin subunit 2, which are involved in brain development and prevention of brain damage as well as Fibroblast growth factor 1 which prevents lung fibrosis.
Conclusion:
The late second trimester human placenta secretes proteins into the fetal circulation which decrease following delivery. Many of these proteins are predicted to be important in the development of fetal organs. Further studies are needed to directly link placental proteins to organ development and poor outcomes in preterm infants.
INTRODUCTION
Each year 12–18 million preterm infants (before 37 weeks of gestation) are born worldwide 1–3, representing approximately 10–11% of all live births 1–4. Despite improvements in clinical management within the field of perinatal medicine, such as antenatal steroid administration and surfactant replacement, prematurity remains a leading cause of infant morbidity and mortality 2 with a survival rate of 45% at 23 weeks and 82% at 25 weeks of gestation 5. Moreover, those that survive are at increased risk for developing major morbidities including early or late sepsis, necrotizing enterocolitis, chronic lung disease, severe intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), or severe retinopathy of prematurity (ROP) 6. Importantly, with each additional co-morbidity, specifically brain injury, ROP, or lung injury, there is an independent association with poor outcome later in life defined as death or disability 7–9. Thus, given the large burden of disease in preterm infants, there is an urgent need for the development of novel intervention strategies and treatments.
One of the most fundamental differences between fetal and postnatal life is the instantaneous discontinuation of the umbilical circulation at delivery. In addition to providing nutrients and oxygen to the growing fetus, the human placenta secretes >100 proteins into the maternal circulation, which are involved in the maternal metabolic and cardiovascular adaptations to pregnancy 10,11. Moreover, emerging evidence from animal experiments indicate that factors secreted by the placenta into the fetal circulation are critical for normal organ development of the fetus. For example, the development of the fetal forebrain in mice is critically dependent on placental serotonin synthesis 12, and altered placental serotonin secretion causes abnormal neurodevelopment in adult offspring 12,13. Recently, we identified 341 proteins which were significantly higher in the umbilical vein compared to the umbilical artery in normal term infants, representing proteins secreted by the human placenta into the fetal circulation at the end of gestation 14. However, it is currently unknown if these or other proteins are secreted by the placenta into the fetal circulation earlier in gestation and if the preterm neonate can produce these proteins once separated from the placental circulation.
In the present study, we characterized the extremely and very preterm infant proteome as well as identified proteins with significantly decreased circulating concentrations in neonatal as compared to umbilical vein blood.
METHODS
Study subjects
Pregnant women aged 18–45 years who were admitted to labor and delivery at 23 to 31 weeks of gestation due to suspected preterm labor, premature rupture of membranes, pre-eclampsia, and/or other maternal complications were recruited from July 2018 to March 2020 following written informed consent. The Institutional Review Board at University Hospital Colorado and Children’s Hospital Colorado approved the study (IRB #18–0637). Exclusion criteria included delivery after 32 and 0/7 weeks of gestation, infants with a congenital cardiac disease (did not include patent ductus arteriosus, patent foramen ovale, or atrial heart defect), malformations or syndromes with unknown genetic defect, genetic abnormality including but not limited to Trisomy 13, 18, and 21, prenatal ultrasound with findings of absent or reverse end-diastolic flow in the umbilical artery, congenital infections including HIV, Hepatitis B or C, or CMV, metabolic disorder, maternal recreational drug use except for alcohol use, infants requiring a blood transfusion prior to the neonatal blood sample, and/or any neonate who was deemed by the medical staff as too ill. These exclusions were chosen given they may have impacted placental/neonatal development, placenta blood flow, or altered the neonatal plasma proteome.
Collection of blood samples & data collection
Within 30 minutes following delivery, umbilical vein and arterial blood was collected from the placenta after clamping of the cord and processed immediately by centrifugation at 2200 x g for 15 minutes at 4°C. Plasma was then collected and subsequently aliquoted and stored at −80°C for later batch analysis. If the neonate met criteria, at 48–72 hours of life, 0.6 mL of neonatal blood was obtained, and plasma was collected as above. All samples were de-identified for storage and after collection of relevant data from the electronic medical record. Data on the current pregnancy, mother, and infant at birth and at the time of discharge or death of the patient was recorded in a REDCAP electronic database hosted at Colorado Clinical & Translational Sciences Institute (CCTSI) 15,16.
SOMOLogic proteomics
Quantification of proteins was performed on paired blood samples (umbilical vein versus umbilical artery and umbilical vein versus neonatal blood) using the SomaLogic platform (Boulder, CO) as previously described 17,18. Briefly, each plasma sample was incubated with modified aptamers, which are chemically modified nucleotides that bind to each of the proteins being measured, to generate modified aptamer-protein complexes which are highly specific and stable. Following a series of washes, which disrupt all non-specific interactions, the SOMAmers were placed in a denaturing buffer, releasing them from their target-protein complexes. The released SOMAmers were then hybridized to their complementary sequences on a microarray chip and quantified by fluorescence using an Agilent hybridization array (Agilent Technologies). The resulting signal intensity is directly correlated to the protein levels of the original sample 17,18.
Intra-assay variability was estimated and found to be <3% for 50% of the proteins and <8% for 95% of proteins. Inter-assay CVs are not available for our study. However rigorous estimates of inter-assay variability for this particular SOMAscan Assay (1.3K) were recently reported to be as low as <7% for 50% of the proteins and <15% for 95% of proteins, when using Hyb.Cal normalized data 19.
RNA Isolation and qRT-PCR analysis
To assess gene expression of placental proteins, placentas were collected within 60 minutes of delivery. Three samples of villous tissue (~0.5cm) were dissected from the placenta and rinsed in ice cold physiologic saline and placed in RNAlater (Qiagen, Venlo, Netherlands) at 4°C for 24–48 hours. The RNAlater was removed and samples were stored at −80°C until batch analysis. RNA was extracted from the villous tissue using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands). We selected 6 candidate proteins with the largest concentration differences between the umbilical venous and neonatal circulation with predicted roles in neurogenesis and lung development (Table 5) which included CD59, Dynactin subunit 2, Endoplasmic reticulum resident protein 29 (ERp29), Ephrin-B1, Fibroblast growth factor 1 (FGF1), and Fibroblast growth factor 2 (FGF2). qRT-PCR was carried out using primers from ThermoFisher Scientific, Waltham, MA and RNA analysis was performed as previously described 20; beta actin and 18S (ThermoFisher Scientific, Waltham, MA) were used as housekeeping genes.
Table 5.
Proteins significantly decreased in neonatal-to-umbilical venous blood with predicted roles in development.
| PROTEIN NAME | UNIPRO | Adjusted P Value | % DIFFERENCE (CI) | SIGNIFICANCE |
|---|---|---|---|---|
| HCG |
P01215 P01233 |
<0.0001 | −96 (−97, −92) |
• Synthesized by the placenta to produce progesterone to maintain the corpus luteum [41] • Involved in the development of the placenta by stimulating angiogenesis, trophoblast cell differentiation, amongst other functions [41] |
| CD59 | P13987 | <0.0001 | −38 (−44, −30) |
• Knock out mice models suggest this protein protects against ischemic brain damage [50] • Spinal cords of mice deficient in this protein had enhanced inflammation, demyelination, and axonal injury [51] • Deficits of this protein are associated with neuritic losses similar to what is seen in Alzheimer's disease [52] |
| DCTN2 | Q13561 | <0.0001 | −37 (−43, −29) |
• Present in the nerve growth cone of mice suggesting a role in synapse formation during brain development [53] |
| Ephrin-B1 | P98172 | 0.0001 | −25 (−32, −17) |
• Knock down of this protein in rats suggest that it functions in the formation of synapses [54] |
| ERp29 | P30040 | <0.0001 | −49 (−56, −42) |
• In a rat model of spinal cord injury, high expression was associated with an increase in motor neuronal survival and axonal regeneration [55,56] |
| FGF1 | P05230 | 0.0001 | −56 (−67, −41) |
• Induces early lung development in the embryonic phase of lung development at the stage of budding [42, 43] • In an animal model, attenuates TGF-pi-induced pulmonary fibrosis when overexpressed [44] |
| FGF2 | P09038 | 0.0062 | −36 (−50, −19) |
• Knock out models in mice show cerebral cortex defects at birth [57] |
HCG, human chorionic gonadotropin; DCTN2, Dynactin subunit 2; ERp29, Endoplasmic reticulum resident protein 29; FGF1, Fibroblast growth factor 1; FGF2, Fibroblast growth factor 2; CI, Confidence Interval
Statistics
Differences in relative abundance of proteins in paired umbilical venous blood and neonatal plasma and umbilical venous and artery blood were compared using paired t-tests. Additionally, we used two-sample t-tests to compare differences in umbilical venous plasma in newborns for the following clinical characteristics: prematurity, placental pathology for chorioamnionitis, delivery method, premature rupture of membranes, pre-eclampsia, intubation at delivery, and sex. The Benjamini-Hochberg procedure was performed to control for false discovery rate given multiple comparisons. Data were transformed using log base 2 and differences are reported as % change with confidence intervals. Significance was set at 0.05. R version 4.0.2 software (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/) was used for analysis.
Bioinformatics & Text Mining Methods
To screen for the most significant pathways of the proteins with the highest or lowest abundance in neonatal blood when compared to the umbilical vein, the Reactome, a pathway database that is utilized to link human proteins by their known molecular functions or to determine novel functional relationships 21,22, was utilized. The UNIPRO identifiers for the proteins of interest were introduced into the database and the pathway analysis report was obtained. In addition, we also tested for enrichment of proteins associated with relevant keywords in MEDLINE abstracts such as the development of the brain, lung, intestine, cardiovascular system, retina, neurogenesis, and angiogenesis using text mining methods 23.
RESULTS
Clinical Characteristics
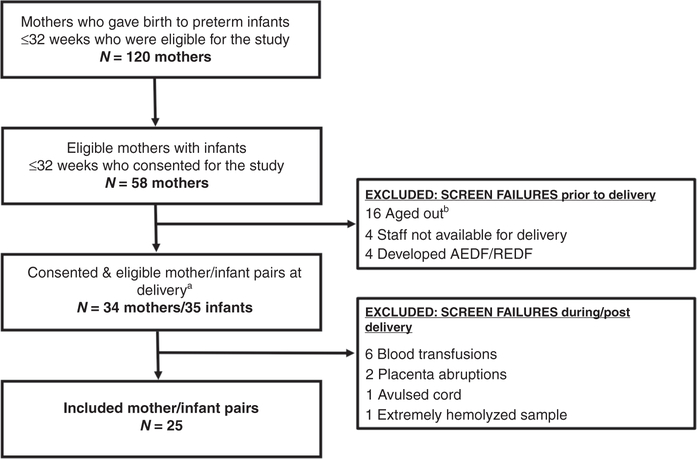
As shown in Figure 1, of the 58 consented patients, 24 patients were screen failures prior to delivery leaving 34 eligible mothers and 35 eligible infants at delivery. This included 4 twin deliveries, with only one set having both twins initially eligible. Ten additional study subjects were excluded during or after delivery due to marked hemolysis in the collected blood sample, inability to collect cord blood secondary to placental abruption or an avulsed cord, or severe anemia in the infant resulting in a blood transfusion, which included one infant where both twins were initially eligible. As a result, 25 mother/infant pairs completed the study.
Figure 1. Flow diagram of patient population.
a Four sets of twins were included in the study; 3 sets only had 1 twin eligible at the start of the study. One set initially had both twins eligible, however 1 infant required a blood transfusion secondary to extreme anemia and became a screen failure. b Aged out indicated those mothers who stayed pregnant past 32 weeks 0 days gestation and therefore were no longer eligible for the study. AEDF/REDF, Absent end-diastolic flow/ reverse end-diastolic flow.
Baseline and demographic characteristics of the 25 mothers and infants at birth are presented in Table 1. Median maternal age was 33 years with a range of 23 to 39 years. The majority of mothers were non-Hispanic (68%) and white (72%). Preterm premature rupture of membranes complicated 48% of pregnancies, whereas 32% of women in the cohort had pre-eclampsia. Basic infant characteristics at birth included a male predominance (60% males) and a median gestational age at delivery of 30 weeks (range of 24 to 32 weeks). Within the first 6 hours of life, 44% of infants required intubation and 52% of infants received antibiotics.
Table 1.
Characteristics of mothers and infants at birth.
| MATERNAL CHARACTERISTICS | |
| Maternal Age, years, median (range) | 33 (23 – 39) |
| Hispanic Ethnicity, n (%) | 8 (32%) |
| Race: | |
| White, n (%) | 18 (72%) |
| African American, n (%) | 2 (8%) |
| Asian, n (%) | 1 (4%) |
| Unknown, n (%) | 4 (16%) |
| Cesarean Delivery, n (%) | 17 (68%) |
| Single Gestation, n (%) | 21 (84%) |
| Pre-eclampsia, n (%) | 8 (32%) |
| PPROM, n (%) | 12 (48%) |
| Hypertension, n (%) | 5 (20%) |
| Diabetes, n (%) | 3 (12%) |
| Alcohol Use, n (%) | 2 (8%) |
| Betamethasone: | |
| 0–1 dose, n (%) | 0 (0%) |
| 2 doses, n (%) | 12 (48%) |
| 3 doses, n (%) | 3 (12%) |
| 4 doses, n (%) | 10 (40%) |
| Chorioamnionitis: | |
| Clinically + Placental Pathology, n (%) | 2 (8%) |
| Placental Pathology only, n (%) | 13 (52%) |
| NEONATAL CHARACTERISTICS AT BIRTH | |
| Gestational age, weeks [median (range)] | 30 (24 – 32) |
| Male Gender, n (%) | 15 (60%) |
| Growth parameters at delivery | |
| Birth Weight, grams [median (IQR)] | 1355 (1040 – 1700) |
| Length, cm [median (IQR)] | 39 (34 – 41) |
| Head Circumference, cm [median (IQR)] | 28 (25 – 30) |
| Respiratory Support at delivery | |
| HFOV, n (%) | 2 (8%) |
| Conventional Ventilation, n (%) | 9 (36%) |
| Non-invasive support, n (%) | 14 (56%) |
| Additional Support | |
| Surfactant, n (%) | 10 (40%) |
| Blood pressor support, n (%) | 3 (12%) |
| Antibiotics, n (%) | 13 (52%) |
N= 25 mothers/infants; IQR Interquartile range; PPROM Preterm premature rupture of membranes; HFOV High frequency oscillatory ventilation.
Infant outcomes at discharge are summarized in Table 2. Moderate or severe chronic lung disease was diagnosed in 21.7% of infants, grade III/IV IVH in 13%, stage III-V ROP in 16.7%, and medical and surgical necrotizing enterocolitis in 4% of neonates, respectively.
Table 2.
Clinical characteristics of neonates at discharge.
| NEONATAL CHARACTERISTICS AT DISCHARGE | |
|---|---|
| Living, n/N (%) | 24/25 (96%) |
| DOL at discharge, median (IQR) a | 67 (47–85) |
| Postmenstmal age, median (IQR) a | 39 (38–41) |
| Chronic Lung Disease a | |
| None, n/N (%) | 1/23 (4.3%) |
| Mild, n/N (%) | 17/23 (73.9%) |
| Moderate, n/N (%) | 1/23 (4.3%) |
| Severe, n/N (%) | 4/23 (17.4%) |
| IVH Grade III/VI, n/N (%) b | 3/23 (13.0%) |
| ROP Grade III-V, n/N (%) c | 3/18 (16.7%) |
| ROP Any Intervention, n/N (%) | 0/18 (0%) |
| Medical NEC, n/N (%) | 1/25 (4%) |
| Surgical NEC, n/N (%) | 1/25 (4%) |
| Medical intervention for PDA, n/N (%) | 3/25 (12%) |
| PDA Coil, n/N (%) | 1/25 (4%) |
| PDA ligation, n/N (%) | 0/25 (0%) |
Two patients were transferred to a level 2 NICU and therefore their discharge information was incomplete.
One patient declined the evaluation and one patient transferred prior to the second head ultrasound
Six patients did not qualify for ROP exams given they were >30 weeks and birthweight >1500g and one patient unknown since transferred prior to exam.
DOL, Day of Life; IQR, Interquartile Range; IVH, Intraventricular hemorrhage; ROP, Retinopathy of prematurity; NEC, Necrotizing enterocolitis; PDA, Patent ductus arteriosus
Proteome of the very and extremely preterm infant
Of the 1,317 proteins on the SomaLogic proteomic platform, 434 proteins were significantly higher in the umbilical vein as compared to the umbilical artery representing secretion or release by the placenta into the fetal circulation with 139 proteins having a ≥100% and 18 proteins with a ≥500% higher abundance in the umbilical vein (Table 3a, Supplemental Table 1). There were also 302 proteins significantly lower in the umbilical vein as compared to the artery suggesting uptake by the placenta with 84 proteins having a ≥50% and 15 proteins having a ≥65% lower abundance in the umbilical vein (Table 3b, Supplemental Table 2). In infant blood collected 48–72 hours after delivery, the abundance of 142 proteins were significantly lower when compared to the umbilical vein with 25 proteins having a ≥50% decrease in neonatal blood when compared to umbilical venous blood (Table 4a, 5, and Supplemental Table 3). In contrast, 126 proteins were significantly higher in infant blood 48–72 hours post-delivery as compared to the umbilical vein with 24 proteins having a ≥100% increase in neonatal blood (Table 4b and Supplemental Table 4). Of the 142 proteins significantly decreased in neonatal blood 48–72 hours of life, 63 proteins were also secreted or released by the placenta (Supplemental Table 5). When comparing the results of the current study with our previous report on secreted proteins in the term human placenta 14, we found that 265 of the 434 proteins secreted by the preterm placenta were also released into the fetal circulation by the term placenta (Supplemental Table 6).
Table 3.
Proteins with the largest umbilical vein-to-artery differences in relative abundance.
| A. Proteins with Higher Relative Abundance in the Umbilical Vein | | ||||
| Target Full Name | UniProt | % Difference (CI) | P-value | Adjusted P-value |
| Netrin receptor UNC5D | Q6UXZ4 | 2004 (407, 8637) | 0.0006 | 0.0111 |
| Ephrin-B2 | P52799 | 1837 (335, 8531) | 0.0011 | 0.0111 |
| SLIT and NTRK-like protein 5 | O94991 | 1534 (286, 6813) | 0.0013 | 0.0111 |
| Osteopontin | P10451 | 1375 (262, 5909) | 0.0014 | 0.0111 |
| Leucine-rich repeat transmembrane protein FLRT3 | Q9NZU0 | 844 (182, 3058) | 0.0018 | 0.0111 |
| Collectin-12 | Q5KU26 | 787 (202, 2504) | 0.001 | 0.0111 |
| Properdin | P27918 | 692 (164, 2280) | 0.0016 | 0.0111 |
| Cystatin-D | P28325 | 662 (195, 1872) | 0.0006 | 0.0111 |
| Nucleoside diphosphate kinase B | P22392 | 663 (217, 1735) | 0.0003 | 0.0111 |
| Thyroid Stimulating Hormone | P01215.P01222 | 629 (174, 1837) | 0.0009 | 0.0111 |
| Growth factor receptor-bound protein 2 | P62993 | 562 (169, 1529) | 0.0007 | 0.0111 |
| Brother of CDO | Q9BWV1 | 560 (153, 1625) | 0.0012 | 0.0111 |
| Granulocyte colony-stimulating factor | P09919 | 551 (147, 1616) | 0.0014 | 0.0111 |
| Semaphorin-6A | Q9H2E6 | 531 (149, 1500) | 0.0011 | 0.0111 |
| Importin subunit beta-1 | Q14974 | 528 (142, 1528) | 0.0014 | 0.0111 |
| Adapter molecule crk | P46108 | 520 (144, 1475) | 0.0012 | 0.0111 |
| Inorganic pyrophosphatase | Q15181 | 521 (139, 1508) | 0.0014 | 0.0111 |
| Xaa-Pro aminopeptidase 1 | Q9NQW7 | 513 (168, 1304) | 0.0005 | 0.0111 |
| B. Proteins with Lower Relative Abundance in the Umbilical Vein | ||||
| Target Full Name | UniProt | % Difference (CI) | P-value | Adjusted P-value |
| GTP-binding nuclear protein Ran | P62826 | −93 (−98, −76) | 0.0006 | 0.0111 |
| 40S ribosomal protein S3 | P23396 | −83 (−94, −50) | 0.0041 | 0.0128 |
| 6-phosphogluconate dehydrogenase, decarboxylating | P52209 | −76 (−89, −48) | 0.0019 | 0.0111 |
| Peroxiredoxin-6 | P30041 | −71 (−85, −44) | 0.0016 | 0.0111 |
| Platelet factor 4 | P02776 | −71 (−90, −17) | 0.0255 | 0.0465 |
| Histone H1.2 | P16403 | −70 (−89, −18) | 0.0229 | 0.0431 |
| DNA topoisomerase 1 | P11387 | −69 (−86, −35) | 0.0051 | 0.0146 |
| Protein S100-A6 | P06703 | −68 (−82, −41) | 0.0016 | 0.0111 |
| Aflatoxin B1 aldehyde reductase member 2 | O43488 | −67 (−82, −41) | 0.0015 | 0.0111 |
| Ubiquitin-conjugating enzyme E2 N | P61088 | −67 (−82, −39) | 0.0021 | 0.0111 |
| Peroxiredoxin-1 | Q06830 | −66 (−81, −39) | 0.002 | 0.0111 |
| Ubiquitin+1, truncated mutation for UbB | P62979 | −66 (−81, −39) | 0.0017 | 0.0111 |
| Histone acetyltransferase type B catalytic subunit | O14929 | −66 (−83, −32) | 0.0058 | 0.0159 |
| Non-histone chromosomal protein HMG-14 | P05114 | −65 (−84, −27) | 0.0093 | 0.0216 |
| Casein kinase II 2-alpha:2-beta heterotetramer | P68400.P67870 | −65 (−80, −37) | 0.0021 | 0.0111 |
Proteins significantly increased in relative abundance by ≥500% (A) or significantly decreased by ≥65% (B) following multiple comparisons with adjusted p-value of ≤0.05 when comparing venous cord blood to arterial cord blood in neonates ≤ 32 weeks of gestation (N=12) using the SomaLogic platform. CI, Confidence Interval
Table 4.
Proteins with the largest neonatal-to-umbilical vein blood differences in relative abundance.
| A. Proteins with Lower Relative Abundance in the Neonatal Blood as Compared to the Umbilical Vein | ||||
| Target Full Name | UniProt | % Difference | P-value | Adjusted P-value |
| Human Chorionic Gonadotropin | P01215.P01233 | −96 (−97, −92) | <0.0001 | <0.0001 |
| Annexin A1 | P04083 | −82 (−90, −66) | <0.0001 | 0.0002 |
| Nucleoside diphosphate kinase B | P22392 | −81 (−92, −54) | 0.0007 | 0.0071 |
| Thyroid Stimulating Hormone | P01215.P01222 | −78 (−90, −53) | 0.0004 | 0.0044 |
| Granulocyte colony-stimulating factor | P09919 | −73 (−89, −34) | 0.0061 | 0.0353 |
| Annexin A2 | P07355 | −71 (−83, −49) | 0.0001 | 0.0017 |
| Chorionic somatomammotropin hormone | P0DML2.P0DML3 | −69 (−79, −54) | <0.0001 | 0.0001 |
| Plasminogen activator inhibitor 1 | P05121 | −67 (−84, −31) | 0.0046 | 0.0278 |
| Mitogen-activated protein kinase 8 | P45983 | −63 (−72, −51) | <0.0001 | <0.0001 |
| Inosine-5’-monophosphate dehydrogenase 1 | P20839 | −61 (−72, −47) | <0.0001 | 0.0001 |
| Activated Protein C | P04070 | −59 (−71, −42) | <0.0001 | 0.0004 |
| Fibroblast growth factor 1 | P05230 | −56 (−67, −41) | <0.0001 | 0.0001 |
| Dynein light chain roadblock-type 1 | Q9NP97 | −55 (−70, −33) | 0.0004 | 0.0046 |
| Fibroblast growth factor 23 | Q9GZV9 | −55 (−73, −24) | 0.0044 | 0.027 |
| Tyrosine-protein kinase Lyn, isoform B | P07948 | −55 (−73, −26) | 0.0029 | 0.0197 |
| Vesicular integral-membrane protein VIP36 | Q12907 | −55 (−62, −46) | <0.0001 | <0.0001 |
| Insulin-like growth factor-binding protein 1 | P08833 | −55 (−72, −25) | 0.0031 | 0.0207 |
| R-spondin-3 | Q9BXY4 | −54 (−66, −38) | <0.0001 | 0.0004 |
| Thrombin | P00734 | −54 (−71, −27) | 0.002 | 0.0148 |
| Adenylosuccinate lyase | P30566 | −53 (−72, −23) | 0.0048 | 0.0294 |
| Glutathione S-transferase P | P09211 | −52 (−70, −23) | 0.0041 | 0.0259 |
| Protein FAM3B | P58499 | −52 (−61, −41) | <0.0001 | <0.0001 |
| Tyrosine-protein kinase Fer | P16591 | −52 (−72, −18) | 0.0099 | 0.0489 |
| Phosphoglycerate kinase 1 | P00558 | −51 (−70, −18) | 0.0082 | 0.0425 |
| Interleukin-23 | P29460.Q9NPF7 | −50 (−69, −19) | 0.0069 | 0.038 |
| B. Proteins with Higher Relative Abundance in Neonatal Blood as Compared to the Umbilical Vein | ||||
| Target Full Name | UniProt | % Difference | P-value | Adjusted P-value |
| Trefoil factor 1 | P04155 | 482 (286, 782) | <0.0001 | <0.0001 |
| Serum amyloid P-component | P02743 | 403 (261, 596) | <0.0001 | <0.0001 |
| C-reactive protein | P02741 | 363 (143, 782) | 0.0001 | 0.001 |
| Protein-glutamine gamma-glutamyltransferase E | Q08188 | 338 (123, 751) | 0.0001 | 0.0018 |
| Complement component C9 | P02748 | 281 (128, 536) | <0.0001 | 0.0004 |
| Troponin I, fast skeletal muscle | P48788 | 218 (88, 439) | 0.0001 | 0.0018 |
| Ectonucleoside triphosphate diphosphohydrolase 5 | O75356 | 203 (151, 263) | <0.0001 | <0.0001 |
| Midkine | P21741 | 203 (77, 421) | 0.0003 | 0.0036 |
| Integrin alpha-IIb: beta-3 complex | P08514.P05106 | 168 (99, 258) | <0.0001 | <0.0001 |
| Interleukin-17 receptor B | Q9NRM6 | 166 (114, 227) | <0.0001 | <0.0001 |
| Eotaxin | P51671 | 160 (92, 253) | <0.0001 | <0.0001 |
| C-X-C motif chemokine 13 | O43927 | 153 (91, 236) | <0.0001 | <0.0001 |
| Interleukin-1 receptor-like 1 | Q01638 | 148 (60, 281) | 0.0002 | 0.0031 |
| Alpha-1-antichymotrypsin complex | P01011 | 131 (74, 210) | <0.0001 | 0.0001 |
| Troponin T, cardiac muscle | P45379 | 131 (80, 199) | <0.0001 | <0.0001 |
| Bone sialoprotein 2 | P21815 | 130 (48, 256) | 0.0007 | 0.0065 |
| Phospholipase A2, membrane associated | P14555 | 128 (53, 243) | 0.0003 | 0.0039 |
| C-C motif chemokine 28 | Q9NRJ3 | 123 (79, 179) | <0.0001 | <0.0001 |
| Peptide YY | P10082 | 123 (72, 189) | <0.0001 | 0.0001 |
| Collagenase 3 | P45452 | 122 (44, 239) | 0.0008 | 0.0074 |
| Lymphocyte activation gene 3 protein | P18627 | 120 (65, 197) | <0.0001 | 0.0002 |
| Kallikrein-7 | P49862 | 119 (73, 177) | <0.0001 | <0.0001 |
| Parathyroid hormone | P01270 | 116 (61, 189) | <0.0001 | 0.0003 |
| Alpha-1 -antichymotrypsin | P01011 | 108 (65, 166) | <0.0001 | 0.0001 |
Proteins significantly decreased in relative abundance by ≥50% (A) or increased by ≥100% (B) following multiple comparisons with adjusted p-value of ≤0.05 when comparing neonatal blood to venous cord blood in neonates ≤ 32 weeks of gestation (N=25) using the SomaLogic platform. CI, Confidence Interval
Sub-analysis of specific prenatal and post-natal characteristics can be found in Supplemental Table 7. The comparison of the proteomic profiles of venous cord blood from extremely and very preterm infants were similar apart from 3 proteins which were significantly higher in the very preterm infants’ venous cord blood when compared to the extremely preterm infants when adjusted for multiple comparisons and 3 proteins which were significantly lower. Additionally, in infants born to mothers with placental pathology positive for chorioamnionitis, 7 proteins were significantly higher in comparison to venous cord blood from infants without chorioamnionitis. Comparing venous cord blood from infants born with the following characteristics: delivery method, premature rupture of membranes, pre-eclampsia, intubation at delivery, and sex, the proteomic profiles between each of the above groups were similar following adjustment for multiples comparisons (Supplemental Table 7).
Bioinformatics
Using the Reactome to determine the biological networks for the 142 proteins that were lower in neonatal blood as compared to the umbilical vein, the immune system, intracellular signaling by second messengers, axon guidance, and nervous system development were some of the most significant pathways (Supplementary Table 8a and Reference 24). Similarly, for the 126 proteins that were increased in the neonate, the immune system, complement cascade, extracellular matrix organization, platelet degranulation, and clotting cascade were identified (Supplementary Table 8b and Reference 25). Using text mining methods on the 142 proteins with significantly lower levels in neonatal than in umbilical vein blood, we found that many of these proteins are associated with the development and/or function of the brain, lung, intestine, cardiovascular system, or retina. A selected number of these proteins, including CD59, Dynactin subunit 2, Ephrin-B1, ERp29, FGF1, and FGF2, and the associated developmental related literature are presented in Table 5.
Messenger RNA Analysis
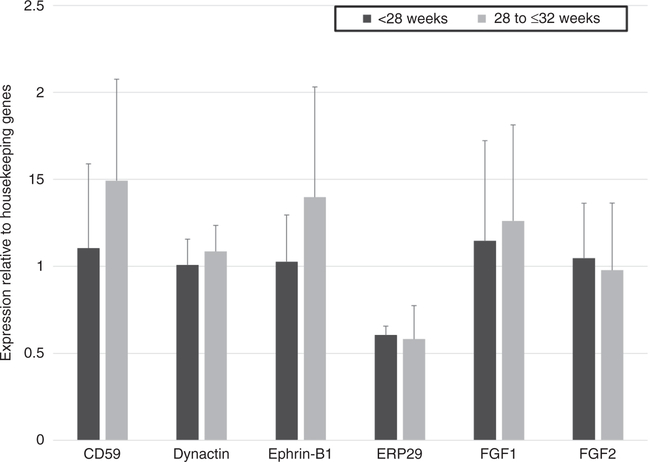
CD59, Dynactin subunit 2, Ephrin-B1, ERp29, FGF1, and FGF2 were found to be expressed on the mRNA level in preterm placentas, indicating they are produced in the placenta. We found similar mRNA expression levels in very and extremely preterm placentas (Figure 2).
Figure 2. Placental gene expression.
Relative placental expression of genes encoding candidate proteins were expressed at similar levels in extremely and very preterm infants (23 to <28 weeks, N=6; 28 to <32 weeks, N=8) using qPCR. Student t test was used to determine significance between groups. Error bars represents +1SD. ERp29, Endoplasmic reticulum resident protein 29; FGF1, Fibroblast growth factor 1; FGF2, Fibroblast growth factor 2.
DISCUSSION
We report for the first time the umbilical and post-delivery proteome of extremely and very premature infants using the SOMAscan aptamer-based platform. Our data supports the concept that the late second trimester human placenta secretes proteins into the fetal circulation and using bioinformatics we predict that many of these proteins are involved in the development of fetal organs, including the brain and lung. We speculate that when born prematurely, infants are deprived of these developmentally active proteins, which may contribute to their poor outcomes.
We previously reported that 341 proteins are secreted by the human placenta into the fetal circulation at term 14. Interestingly, of these 341 proteins, 265 were also found to secreted by the preterm placenta into the fetal circulation in the current study, suggesting that the human placental secretome into the fetal circulation does not change markedly in the second half of pregnancy. Suski and colleagues identified 245 proteins at delivery in umbilical cord blood from infants ≤30 weeks of gestation as well as at term using liquid chromatography-mass spectrometry with 58–65 of those proteins being significantly higher in concentration in infants ≤30 weeks when compared to term 26. When comparing late preterm infants (32 to 36 weeks of gestation) with and without respiratory distress syndrome, Hu and colleagues found that umbilical cord blood from infants with respiratory disease had 139 upregulated and 112 downregulated peptides, many of which were involved in processes of respiratory failure, atelectasis, and maturation of endothelial cells 27. Here we sought to further analyze the preterm umbilical cord plasma proteome as well as determine to what extent the circulating concentrations of these proteins changed in the circulation of preterm infants following delivery. The analysis consisted of approximately 1,300 known proteins in the SOMAmer (Slow Off-rate Modified Aptamer) panel. With ~ 20,000 protein coding genes and at least 17,000 identified nonmodified human proteins 28, it can be estimated that the SOMALogic platform used in the current study, represents a 7–8% coverage of the total proteome. Although this platform included only a fraction of all proteins, representing a limitation of our study, the proteins on this platform have been preselected with a focus on secreted proteins 18. And since our study focused on identifying proteins secreted by the human preterm placenta into the fetal circulation, this provided the rationale for using this proteomic approach in our study. Additionally, the human plasma proteome is highly complex 29,30 with multiple posttranslational modifications and a profound dynamic range in the concentrations of different plasma proteins 31, which constitute a major analytical challenge using traditional LC/MS-based proteomics approaches to identify proteins. For example, in order to measure proteins that are present in plasma in lower concentrations it is necessary to first remove high-abundance proteins, such as albumin, which may result in the concomitant removal of low-abundance proteins, such as cytokines, peptide hormones and lipoproteins. The SOMALogic proteomic platform, however, allows for measurement of proteins in small volumes of serum or plasma with very high sensitivity and dynamic range thereby circumventing some of the major problems with traditional proteomics in plasma 18. As a consequence, the SOMALogic platform is now widely used in high impact human proteomics studies 32–37, including studies in children 38–40.
Here we report that 434 proteins were found in significantly higher relative abundance in the umbilical vein when compared to the artery, consistent with placental release of these proteins into the fetal circulation. Also, 302 proteins were significantly lower in the umbilical vein as compared to the artery suggesting uptake by the placenta. This could represent fetal proteins that are removed by the placenta for metabolism or excretion to the mother or constitute fetal signals to the placenta. Moreover, the abundance of 142 proteins were significantly lower in infant blood collected 48–72 hours after delivery as compared to the umbilical vein. On further evaluation of these proteins, 25 proteins had a ≥50% lower relative abundance in neonatal blood including beta human chorionic gonadotropin, a hormone which is synthesized by the placenta to stimulate progesterone synthesis by the corpeus luteum in order to maintain early pregnancy 41. Our data demonstrate that hCG is also secreted into the fetal circulation in the second trimester and decreased by 96% following delivery, representing a good ‘internal control’ for a protein likely to be synthesized almost exclusively by the placenta.
Critical stages of lung development occur between 20 to 30 weeks of gestation, placing an infant delivered in late second trimester at increased risk of chronic lung disease. Indeed, up to 40% of extremely low birth weight infants develop lung complications 4. In our study, 21.7% of patients were affected with moderate to severe chronic lung disease diagnosed at 36 weeks. We speculate that proteins secreted by the placenta may be important for fetal lung development and that the discontinuation of a placental source of these proteins in the infant delivered prematurely may contribute to the development of chronic lung disease. FGF1 may be of importance in this context. First, the relative abundance of this protein was markedly lower in neonatal as compared to umbilical vein blood (56% decrease) and the late second trimester human placenta expressed FGF1 mRNA. These data support the placenta as an important source of FGF1 in the umbilical circulation. Second, FGF1 has previously been shown to induce early lung development at the stage of budding in the embryonic phase of lung development 42,43. Moreover, FGF1 has been reported to attenuate TGF-β1-induced pulmonary fibrosis in an animal model 44, which is notable given that one of the hallmarks of chronic lung disease in premature infants is increased interstitial fibrosis 45. Albeit speculative, we propose that FGF1 secreted from the placenta plays a critical role in fetal lung development and could be developed into a future intervention to attenuate fibrosis in the lungs in premature infants and improve lung related outcomes.
Infants born extremely or very prematurely are at risk for brain damage, including intraventricular hemorrhage (IVH) and periventricular leukomalacia (PVL), which are associated with long-term sequelae including cerebral palsy and learning difficulties 6–8,46. Horbar and colleagues found that in infants <1500g, 6.1% developed IVH grades III and IV and 2.7 % were diagnosed with PVL 6. In our small cohort, we had a slightly higher percentage (13%) of premature infants with severe IVH. We found several proteins secreted by the placenta into the fetal circulation with known associations to nervous system development, including ERp29, Dynactin subunit 2, FGF2, and CD59 (Table 5). These proteins demonstrated a 36–49% decrease in neonatal blood as compared to umbilical levels, supporting their placental origin. Thus, we speculate that decreased circulating levels of ERp29, Dynactin subunit 2, FGF2, and/or CD59 following premature delivery and loss of the placental circulation, contribute to an increased risk of brain damage in these infants. It is important to note, however, although we have highlighted here proteins that decreased significantly in neonatal blood as compared to the umbilical vein with known roles in brain or lung development in the literature, it is plausible that a smaller but statistically significant decrease in one protein or the combined effect of a decrease in many proteins may be more significant in neonatal development.
Another interesting finding was the 126 proteins that were significantly increased following delivery which may be important in postnatal growth or in dysregulated processes. For example, Midkine (203% increase) is known to be associated with postnatal lung growth 47 and Eotaxin (160% increase) has been associated with autism in full term infants 48 as well as is increased in extremely low birth weight infants who die or go on to develop bronchopulmonary disease 49.
Limitations of this study include that some of the most ill infants were excluded given they required a blood transfusion, which introduced adult proteins before the neonatal blood sample could be taken. Furthermore, given the novelty of our study, the optimal timepoint for the neonatal blood draw is unknown. Although these samples were taken when other samples were needed clinically, it is possible that in order to detect significant decreases in proteins with a longer half-life blood sampling at a later timepoint would be required. Another limitation is that the proteomic platform only evaluates proteins. Many other substances, for example, lipids, carbohydrates, micronutrients, miRNA in exosomes, amongst others may be clinically important substances that are secreted from the placenta and were not analyzed during this study. Lastly for our sub-analysis data, our numbers were small, and data should be considered preliminary.
In conclusion, our data show that a substantial number of placental proteins predicted to be critical for brain and lung development are higher in the umbilical vein compared to the umbilical artery and decrease rapidly in the neonatal circulation following delivery of premature infants. These findings are consistent with the possibility that the human placenta secretes proteins that influence normal development of fetal organs. Importantly, these observations imply that premature infants may be deprived of these critical developmental factors, which likely contribute to poor outcomes. Well-designed animal studies are needed to mechanistically link placental proteins to the development of the fetal brain and lung and to test the hypothesis that administration of selected proteins of placental origin could improve outcomes in prematurity.
Supplementary Material
Impact Statement:
Prematurity remains a leading cause of morbidity and mortality requiring the development of novel treatments. Emerging evidence from animal studies suggest that factors secreted from the placenta may be critical in the development of the fetus.
We report that the preterm human placenta secretes an array of proteins into the fetal circulation. Some of these proteins are predicted to be involved in the development of the brain and the lung.
When born prematurely, infants are deprived of these placental proteins, which may contribute to their poor outcomes.
Statement of financial support:
The work was supported by NIH HD104914, the Alessia Rose Foundation and Teigen Everts Parmet Foundation, a microgrant granted by the Clinical & Translational Research Center, and the Marshall Klaus Perinatal Research Award from the American Academy of Pediatrics. This work was supported in part by the REDCAP electronic database hosted at Colorado Clinical & Translational Sciences Institute (CCTSI) with the Development and Informatics Service Center (DISC) grant support (NIH/NCRR Colorado CTSI Grant Number UL1 RR025780).
Footnotes
Disclosure Statement: The authors have no conflicts of interest relevant to this article to disclose.
Category of Study: Basic Science
Consent Statement: IRB #18-0637 approved by the Ethics Committees at Colorado Children’s Hospital and University Hospital Colorado. All patients consented to participation with a written informed consent.
References
- 1.Blencowe H et al. National, Regional, and Worldwide Estimates of Preterm Birth Rates in the Year 2010 with Time Trends since 1990 for Selected Countries: A Systematic Analysis and Implications. Lancet 379, 2162–2172 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Partridge EA, Davey MG, Hornick MA & Flake AW An Extrauterine Environment for Neonatal Development: Extending Fetal Physiology Beyond the Womb. Semin Fetal Neonatal Med 22, 404–409 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Platt MJ Outcomes in Preterm Infants. Public Health 128, 399–403 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Pereira KD et al. Tracheostomy in the Extremely Premature Neonate: A Multi-Institutional Study. Otolaryngol Head Neck Surg, 194599820905528 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Rysavy MA et al. Assessment of an Updated Neonatal Research Network Extremely Preterm Birth Outcome Model in the Vermont Oxford Network. JAMA pediatrics, e196294 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horbar JD et al. Mortality and Neonatal Morbidity among Infants 501 to 1500 Grams from 2000 to 2009. Pediatrics 129, 1019–1026 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Schmidt B et al. Prediction of Late Death or Disability at Age 5 Years Using a Count of 3 Neonatal Morbidities in Very Low Birth Weight Infants. J Pediatr 167, 982–986.e982 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Schmidt B et al. Impact of Bronchopulmonary Dysplasia, Brain Injury, and Severe Retinopathy on the Outcome of Extremely Low-Birth-Weight Infants at 18 Months: Results from the Trial of Indomethacin Prophylaxis in Preterms. JAMA 289, 1124–1129 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Holsti A, Serenius F & Farooqi A Impact of Major Neonatal Morbidities on Adolescents Born at 23–25 Weeks of Gestation. Acta Paediatr 107, 1893–1901 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Burton GJ & Jauniaux E What Is the Placenta? Am J Obstet Gynecol 213, S6.e1, S6–8 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Burton GJ & Fowden AL The Placenta: A Multifaceted, Transient Organ. Philos Trans R Soc Lond B Biol Sci 370, 20140066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnin A et al. A Transient Placental Source of Serotonin for the Fetal Forebrain. Nature 472, 347–350 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ansorge MS, Zhou M, Lira A, Hen R & Gingrich JA Early-Life Blockade of the 5-Ht Transporter Alters Emotional Behavior in Adult Mice. Science 306, 879–881 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Michelsen TM, Henriksen T, Reinhold D, Powell TL & Jansson T The Human Placental Proteome Secreted into the Maternal and Fetal Circulations in Normal Pregnancy Based on 4-Vessel Sampling. FASEB J, fj201801193R (2018). [DOI] [PubMed] [Google Scholar]
- 15.Harris PA et al. Research Electronic Data Capture (Redcap)--a Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J Biomed Inform 42, 377–381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA et al. The Redcap Consortium: Building an International Community of Software Platform Partners. J Biomed Inform 95, 103208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganz P et al. Development and Validation of a Protein-Based Risk Score for Cardiovascular Outcomes among Patients with Stable Coronary Heart Disease. JAMA 315, 2532–2541 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Gold L et al. Aptamer-Based Multiplexed Proteomic Technology for Biomarker Discovery. PLoS One 5, e15004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Candia J et al. Assessment of Variability in the Somascan Assay. Sci Rep 7, 14248 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaccioli F et al. Expression and Functional Characterisation of System L Amino Acid Transporters in the Human Term Placenta. Reprod Biol Endocrinol 13, 57 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jassal B et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res 48, D498–d503 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabregat A et al. Reactome Pathway Analysis: A High-Performance in-Memory Approach. BMC Bioinformatics 18, 142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Liang Y & Wishart D Polysearch2: A Significantly Improved Text-Mining System for Discovering Associations between Human Diseases, Genes, Drugs, Metabolites, Toxins and More. Nucleic Acids Res 43, W535–542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.“Pathway Analysis Report”, Reactome, Version #76, <https://reactome.org/PathwayBrowser/#/ANALYSIS=MjAyMTA1MjEyMDI4NDZfNjIyNzM%3D> (21/05/2021).
- 25.“Pathway Analysis Report”, Reactome, Version #76, <https://reactome.org/PathwayBrowser/#/ANALYSIS=MjAyMTA1MjAxNTA1MDBfNjE1ODY%3D> (21/05/2021).
- 26.Suski M et al. Plasma Proteome Changes in Cord Blood Samples from Preterm Infants. J Perinatol 38, 1182–1189 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Hu Y et al. Peptidomics Analysis of Umbilical Cord Blood Reveals Potential Preclinical Biomarkers for Neonatal Respiratory Distress Syndrome. Life Sci 236, 116737 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Kim MS et al. A Draft Map of the Human Proteome. Nature 509, 575–581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson NL & Anderson NG The Human Plasma Proteome: History, Character, and Diagnostic Prospects. Mol Cell Proteomics 1, 845–867 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Anderson NL et al. The Human Plasma Proteome: A Nonredundant List Developed by Combination of Four Separate Sources. Mol Cell Proteomics 3, 311–326 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Nedelkov D, Kiernan UA, Niederkofler EE, Tubbs KA & Nelson RW Investigating Diversity in Human Plasma Proteins. Proc Natl Acad Sci U S A 102, 10852–10857 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apps R et al. Multimodal Immune Phenotyping of Maternal Peripheral Blood in Normal Human Pregnancy. JCI Insight 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrannini G et al. Coronary Artery Disease and Type 2 Diabetes: A Proteomic Study. Diabetes Care 43, 843–851 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Williams SA et al. Plasma Protein Patterns as Comprehensive Indicators of Health. Nat Med 25, 1851–1857 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penn-Nicholson A et al. Discovery and Validation of a Prognostic Proteomic Signature for Tuberculosis Progression: A Prospective Cohort Study. PLoS Med 16, e1002781 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chirinos JA et al. Reduced Apolipoprotein M and Adverse Outcomes across the Spectrum of Human Heart Failure. Circulation 141, 1463–1476 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun BB et al. Genomic Atlas of the Human Plasma Proteome. Nature 558, 73–79 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hewitson L et al. Blood Biomarker Discovery for Autism Spectrum Disorder: A Proteomic Analysis. PLoS One 16, e0246581 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Dong L et al. Aptamer Based Proteomic Pilot Study Reveals a Urine Signature Indicative of Pediatric Urinary Tract Infections. PLoS One 15, e0235328 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynch AM et al. The Relationship of Novel Plasma Proteins in the Early Neonatal Period with Retinopathy of Prematurity. Invest Ophthalmol Vis Sci 57, 5076–5082 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borisova MA, Moiseenko DY & Smirnova OV Human Chorionic Gonadotropin: Unknown About Known. Fiziol Cheloveka 43, 97–110 (2017). [PubMed] [Google Scholar]
- 42.Lebeche D, Malpel S & Cardoso WV Fibroblast Growth Factor Interactions in the Developing Lung. Mech Dev 86, 125–136 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Cardoso WV, Itoh A, Nogawa H, Mason I & Brody JS Fgf-1 and Fgf-7 Induce Distinct Patterns of Growth and Differentiation in Embryonic Lung Epithelium. Dev Dyn 208, 398–405 (1997). [DOI] [PubMed] [Google Scholar]
- 44.Shimbori C et al. Fibroblast Growth Factor-1 Attenuates Tgf-Beta1-Induced Lung Fibrosis. J Pathol 240, 197–210 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Voynow JA “New” Bronchopulmonary Dysplasia and Chronic Lung Disease. Paediatr Respir Rev 24, 17–18 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Novak CM, Ozen M & Burd I Perinatal Brain Injury: Mechanisms, Prevention, and Outcomes. Clin Perinatol 45, 357–375 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Matsuura O et al. Midkine Expression Is Associated with Postnatal Development of the Lungs. Cell Struct Funct 27, 109–115 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Heuer LS et al. An Exploratory Examination of Neonatal Cytokines and Chemokines as Predictors of Autism Risk: The Early Markers for Autism Study. Biol Psychiatry 86, 255–264 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kandasamy J, Roane C, Szalai A & Ambalavanan N Serum Eotaxin-1 Is Increased in Extremely-Low-Birth-Weight Infants with Bronchopulmonary Dysplasia or Death. Pediatr Res 78, 498–504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harhausen D et al. Membrane Attack Complex Inhibitor Cd59a Protects against Focal Cerebral Ischemia in Mice. J Neuroinflammation 7, 15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mead RJ et al. Deficiency of the Complement Regulator Cd59a Enhances Disease Severity, Demyelination and Axonal Injury in Murine Acute Experimental Allergic Encephalomyelitis. Lab Invest 84, 21–28 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Hochsmann B & Schrezenmeier H Congenital Cd59 Deficiency. Hematol Oncol Clin North Am 29, 495–507 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Abe TK, Tanaka H, Iwanaga T, Odani S & Kuwano R The Presence of the 50-Kda Subunit of Dynactin Complex in the Nerve Growth Cone. Biochem Biophys Res Commun 233, 295–299 (1997). [DOI] [PubMed] [Google Scholar]
- 54.McClelland AC, Sheffler-Collins SI, Kayser MS & Dalva MB Ephrin-B1 and Ephrin-B2 Mediate Ephb-Dependent Presynaptic Development Via Syntenin-1. Proc Natl Acad Sci U S A 106, 20487–20492 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLaughlin T, Falkowski M, Wang JJ & Zhang SX Molecular Chaperone Erp29: A Potential Target for Cellular Protection in Retinal and Neurodegenerative Diseases. Adv Exp Med Biol 1074, 421–427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu R et al. Endoplasmic Reticulum Protein 29 Protects Cortical Neurons from Apoptosis and Promoting Corticospinal Tract Regeneration to Improve Neural Behavior Via Caspase and Erk Signal in Rats with Spinal Cord Transection. Mol Neurobiol 50, 1035–1048 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Dono R, Texido G, Dussel R, Ehmke H & Zeller R Impaired Cerebral Cortex Development and Blood Pressure Regulation in Fgf-2-Deficient Mice. EMBO J 17, 4213–4225 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.