Abstract
In this work, we evaluated the removal efficiency of diclofenac by Chlorella vulgaris OW-01, Nannochloropsis oculata CCAP 849/7, Scenedesmus acutus UTEX 72, and Scenedesmus obliquus CCAP 276/2. Each microalga was grown in media with different concentrations (50 and 100% of the original formulation) of carbon, nitrogen, and phosphorus, to evaluate their effect on the removal of diclofenac. We also evaluated the photodegradation of diclofenac under the same conditions. The diclofenac removed from the media ranged from 59 to 92%, obtaining the highest removal with S. obliquus. The diclofenac adsorbed on the cell walls ranged from 12.2 to 26.5%, obtaining the highest adsorption with S. obliquus. The diclofenac degraded by light ranged from 15 to 28%. The nutrient deficit showed no influence on the removal of diclofenac in any of the microalgae under study. These results indicate that S. obliquus is the best alternative for the bioremediation of diclofenac.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03268-2.
Keywords: Phycoremediation, Pharmaceuticals, Adsorption, Photodegradation
Introduction
The presence of emerging contaminants (ECs) in wastewater has attracted great interest due to the potential undesirable effects of these pollutants in the environment and living organisms (Arnold et al. 2013; Kumar et al. 2022). The ECs include pharmaceuticals, personal care products, pesticides, among others. There is no legislation covering the discharge of these compounds into the environment. While commonly present in water, only recently ECs have been identified as significant water pollutants (Bonnefille et al. 2018; Samal et al. 2022). In general, these products are obtained by organic syntheses and their passage through biological systems does not guarantee their complete biotransformation (Nicolaou et al. 2007; Croom 2012).
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most self-medicated products worldwide. They constitute approximately 5 to 10% of all the medications prescribed each year. Diclofenac is one of the NSAIDs mostly used for its anti-inflammatory, analgesic, and antipyretic effects. Once consumed, more than 90% of diclofenac is excreted in 72 h, with 35% excreted in the bile and 65% in the urine (Mullan et al. 2017; Wongrakpanich et al. 2018; Ribeiro et al. 2022).
The conventional wastewater treatment plants (WWTPs) are ineffective for the removal of diclofenac, due to the physical chemical properties of this drug (Sophia and Lima 2018; Alessandretti et al, 2021). Therefore, diclofenac enters the environment where it can be incorporated into the trophic chain, generating toxic effects in aquatic organisms, even at low environmental concentrations (Lee et al. 2011; Gröner et al. 2017; Cuellar-Bermudez et al. 2017; Xu et al. 2019; Szopinska et al. 2022).
Phytoremediation involves the application of microalgae for bioremediation (biosorption, bioconcentration, assimilation, sequestration, biotransformation) of pollutants from water in a sustainable manner, without generating secondary hazardous compounds. This process has gained great interest over the past few years (Koul et al. 2022: Samal et al. 2022). Phycoremediation is an interesting stage for treating wastewater since it provides tertiary biotreatment while producing potentially valuable biomass that may be used for a variety of applications (Priyadharshini et al. 2021). The microalgae used in this treatment not only assimilate inorganic nitrogen and phosphorus to grow (Ruiz-Marin et al. 2010; Martínez et al. 2000) but can also remove heavy metal ions (Zhou et al. 2012), endocrine disrupting chemicals, pharmaceuticals, and other compounds used in personal care products (Liu et al. 2010; Zhou et al. 2013; Goswami et al. 2022).
Similar to bacteria, fungi and algae are capable to remove organic contaminants via adsorption, absorption, and degradation processes (Li et al. 2009; Norvill et al. 2016; Xiong et al.2018; Samal et al. 2022). However, diclofenac removal by microalgal-based technologies has not been widely studied and limited literature is available (Cuellar-Bermudez et al. 2017). Various studies have evaluated the toxicity of diclofenac in concentrations ranging from 2 to 200 μg mL−1, using different species of microalgae such as Desmodesmus subspicatus, Chlorella sorokiniana, and Chlorella pyrenoidosa, among others, with authors reporting growth inhibition at concentrations higher than 40 μg mL−1. Lower diclofenac concentrations result stimulant for the growth of some microalgae (Cleuvers 2004; de Wilt et al. 2016; Zhu et al. 2014; Ben Ouada et al. 2019; Zhang et al. 2019). Previous studies have shown that biodegradation is the most effective way by which microalgae eliminate pharmaceuticals, including diclofenac (Norvill et al. 2016; Cuellar-Bermudez et al. 2017). However, photolysis is also an important removal mechanism (Xiong et al. 2018; Kunkel and Radke 2012; Alharbi et al. 2022).
Microalgae require nutrients, mainly carbon (C), nitrogen (N), and phosphorous (P), which determine their growth rate and chemical composition. In particular, carbon has a critical role in microalgal growth and in essential biochemical processes; can be supplied to the culture as carbon dioxide (CO2), and have significant effects on the microalgal growth, metabolite content, and composition in microalgal cells (Wang et al 2018). On the other hand, nitrogen is an essential component of proteins, chlorophylls, nucleic acid, enzymes, and other compounds that are indispensable in maintaining the microalgal growth. Nitrogen abundance promotes cell growth and cell division due to the high efficiency of the photosynthesis (Jerez et al 2016). While the phosphorus is an indispensable nutrient for the formation of nucleic acids, phospholipids, and high energy molecules in microalgal cells. Compared to nitrogen, phosphorus starvation has little detrimental effect on the microalgal growth, however, extremely low concentrations of phosphorus are unable to support microalgal growth and thereby result in reduced concentrations of biomass. In contrast, when phosphorus is in excess it is deposited as polyphosphate in microalgal cells (Yu et al. 2019; Anne-Marie et al. 2020).
It has been postulated that the biodegradability of ECs correlates well with C, N and P ratio of the wastewater in the absence of inhibitory or recalcitrant compounds (Posadas et al. 2014; Maryjoseph and Ketheesan, 2020). However, supplementation of these essential nutrient would impose additional cost to bioremediation process. Therefore, a strategy that includes nutrient deficiency would be beneficial.
Until now, no studies have been reported on the relationship between nutrient deficiency (C, N, P) and diclofenac removal by microalgae. In the present work, a microalgae cultivation study under controlled conditions was developed using four strains (Chlorella vulgaris OW-01, Nannochloropsis oculata CCAP 849/7, Scenedesmus acutus UTEX 72, and Scenedesmus obliquus CCAP 276/2) to evaluate their potential for diclofenac removal from the growth media. Considering adsorption and photodegradation processes, this study was carried out independently for each microalga, modifying the initial concentrations (from 100 to 50% of the original composition) of the macronutrients carbon, nitrogen, and phosphorous to evaluate their impact in the removal of diclofenac.
Materials and methods
Background of the microalgae species
The genus Chlorella are green with a high concentration of chlorophyll a and b. Their status in trophic chains as primary producers makes them ideal organisms to evaluate their ability to accumulate metal ions and other contaminants. They can be found in marine environments, fresh water, and flooded soils (Cai et al. 2013).
The genus Nannochloropsis can be found in marine environments, but they are also found in fresh and brackish water. Species of this genus have a high content of polyunsaturated fatty acids, produce large amounts of triglycerides, and grow fast. Moreover, they constitute the main phototrophic microorganisms used to produce biofuels. In recent years, several strains of Nannochloropsis have been investigated to remove contaminants (Li et al. 2014; Zuorro et al. 2017).
The genus Scenedesmus can grow in urban wastewater; registering growth rates similar to those reported when grown in synthetic media. Species from this genus tolerate a wide temperature range (they are viable at − 3 ℃ and their mobility ceases at − 18 ℃) and they can grow at pH values between 5.5 and 8, with 6.8 as the optimum. All of these features make this genus versatile for the purification of residual water (Martínez et al. 2000).
Microalgae cultivation
The Autonomous University of Aguascalientes provided the freshwater microalgae Chlorella vulgaris OW-01, Nannochloropsis oculata CCAP 849/7, Scenedesmus acutus UTEX 72, and Scenedesmus obliquus CCAP 276/2. A modified Bold basal medium (BBM) without calcium (Ca) was used in this study (Montes and Pulido 2012). Included in the original composition of the medium, calcium interferes in the analysis by HPLC when quantifying the removal of diclofenac. Moreover, previous experiments in our laboratory have shown that the absence of calcium does not interfere with the growth of the four microalgae (Figure S1). 200 mL of BBM were inoculated independently with each microalga in sterile conditions at a volume ratio of 10% (Vinoculum/Vmedium), the system was stirred at 10 g and kept at 25 ºC for two weeks in a culture room under a photoperiod of 16 h of light and 8 h of darkness using LED lamps of 14 W (FLCLED-03, Tecnolite, Monterrey, México).
To generate the inoculum necessary for the following experiments, the microalgae culture was adjusted to an optical density of 0.25 using a GloMax® MultiMicroplate microplate reader (Promega Corporation, Madison, WI) at a wavelength of 750 nm.
Experimental design
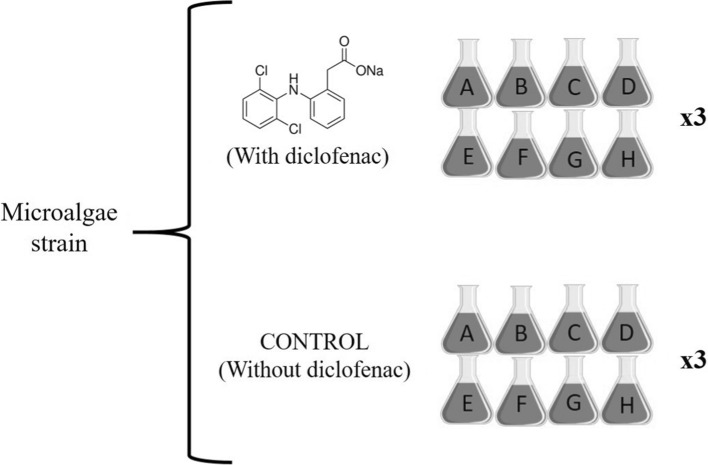
In Erlenmeyer flasks of 100 mL, 70 mL of BBM were added and inoculated with 10% of the microalgae in suspension (Vinoculum/Vmedium). The experiments were carried out in batches of 8 flasks, varying the initial concentration of the macronutrients: carbon, nitrogen, and phosphorus (set to 50 or 100% of their original composition in the BBM) to evaluate their effect on the removal of diclofenac. The combination of nutrients is shown in (Table 1). This procedure was performed for each microalga independently. Figure 1 shows the complete design for each of the 4 strains described. For each experiment, 3 biological replicates were made.
Table 1.
Experimental design showing the different concentrations in percentage for each nutrient
| Experiment | Nutrients (%) | ||
|---|---|---|---|
| Carbon | Nitrogen | Phosphorus | |
| A | 50 | 50 | 50 |
| B | 100 | 50 | 50 |
| C | 50 | 100 | 50 |
| D | 100 | 100 | 50 |
| E | 50 | 50 | 100 |
| F | 100 | 50 | 100 |
| G | 50 | 100 | 100 |
| H | 100 | 100 | 100 |
Concentration: 100% N = 250 mg L−1; 100% P = 105 mg L−1; 100% C = 0.33 mg L−1
Fig. 1.
Complete experimental design for each microalga (C. vulgaris, N. oculata, S. acutus, and S. obliquus). Each letter in the flasks indicates a different combination of nutrients (carbon, nitrogen, phosphorous) and concentrations (50, 100%)
The initial diclofenac concentration used when testing S. acutus and S. obliquus was 8.7 µg mL−1, while for C. vulgaris and N. oculata was 10 µg mL−1. These concentrations were previously established after performing phycotoxicity tests, where different concentrations of diclofenac were evaluated independently for each microalga, kept in BBM without modified the nutrients concentrations and without added a carbon source; selecting the concentration that induced the greatest growth, are found in the supplementary material (Figure S2).
A diclofenac solution was prepared with diclofenac sodium salt (Sigma-Aldrich, St. Louis, MO) dissolved in 3 mL of ethanol and diluted with distilled water until obtaining a concentration of 250 µg mL−1. The diclofenac solution was added to the flasks for initial concentrations of 8.7 or 10 µg mL−1 according to each microalga as described previously. A positive control for each microalgae tested was included and incubated at the same conditions, but without diclofenac. An aeration system was designed using plastic hoses connected to an air pump operating at 2.2 L min−1 to add CO2 and to stir the cultures. The growth was allowed until the biomass reached the stationary phase. A sample of 2.5 mL was withdrawn from each flask every 48 h to evaluate the growth kinetics, and the concentrations of chlorophyll a and of diclofenac.
Growth kinetics and chlorophyll A determination
The optical density was used as an indirect indicator of the microalga growth. Samples of 200 μL (by triplicate) were employed to quantify their optical density by spectrophotometry at 750 nm with a GloMax-Multi Microplate Reader (Promega Corp., Madison, WI).
The concentration of chlorophyll a was measured as an indicator of the physiological state of the microalgae. Samples of 1 mL were centrifuged to separate the biomass, which was washed with 1 mL of sterile distilled water and contacted with 1.5 mL of absolute methanol. The sample was heated 10 min at 65 °C and incubated 24 h at 4 °C under dark conditions to favor the release of chlorophyll. Afterward, the samples were centrifuged to obtain the supernatant (Henriques et al. 2007), whose absorbance was recorded using a spectrophotometer Jenway 6705 (Bibby Ltd., Stone, UK) at wavelengths of 652, 665, and 750 nm. The concentration of chlorophyll a of each sample was calculated as described by Porra et al. (1989).
Diclofenac removal by Chlorella vulgaris, Nannochloropsis oculata, Scenedesmus acutus, and Scenedesmus obliquus
Every 48 h, under sterile conditions, a 500 μL sample was withdrawn from the culture of Chlorella vulgaris, Nannochloropsis oculata, Scenedesmus acutus, and Scenedesmus obliquus and centrifuged 5 min at 15,600g. The supernatant was transferred to another tube and concentrated with a Vacufuge Plus (Eppendorf, Hamburg, Germany) during 3 h at 30 °C. The residue obtained by evaporation was resuspended in 500 µL of ethanol and sonicated at an amplitude of 40%. Once the mixture was centrifuged 5 min at 15,600g and the supernatant completely evaporated, the sample was resuspended again in 500 µL of ethanol.
The samples obtained were analyzed by high-performance liquid chromatography (HPLC) using a 1260 Infinity System (Agilent, Santa Clara, CA) having a diode array detector (DAD) and a column packed with spherical silica (Agilent, Eclipse XDB C-18). The column was fed with a 0.1% trifluoroacetic acid (TFA) aqueous solution (mobile phase A). A gradient of acetonitrile was generated over a period of 10 min using 0.1% TFA in acetonitrile (mobile phase B) from 0 to 100%. Finally, the equipment was fed with mobile phase A to restore the initial conditions in the column. Detection of diclofenac was performed at 275 nm.
A calibration standard was elaborated with concentrations of 0.5, 1, 5, 10, and 15 µg mL−1 using a diclofenac standard (Sigma-Aldrich, St. Louis, MO). The injection volume for standards and samples was 100 µL and the flow rate was 0.6 mL min−1.
Diclofenac removal by adsorption
To evaluate the adsorption of diclofenac on the cell walls of the microalgae, 40 mL of each culture were collected at the end of the study and centrifuged 20 min at 2300g, the supernatant discarded, and the biomass dried at 70 °C for 24 h, with the dry weight registered to homogenize each culture of microalga.
A first wash was performed on the dried biomass using 1 M NaCl, adding 500 µL and stirring 30 s with a vortex mixer. The sample was centrifuged 5 min at 15,600g and the supernatant transferred to a new tube. A second wash was performed to the microalgae pellet by adding 500 µL of a 0.1% Tween 20 solution. To the supernatant recovered from each wash, 500 µL of ethanol were added, an incubation was performed at 4 °C for 24 h. Finally, the samples were centrifuged 5 min at 15,600g and the supernatants analyzed by HPLC to quantify the concentration of diclofenac.
Diclofenac degradation by photolysis
To evaluate the abiotic degradation of diclofenac, i.e., without algae cells, the same culture conditions were maintained as for the biotic experiments. For this, 77 mL of BBM in flasks were supplied with 8.7 or 10 µg mL−1 of diclofenac, without the addition of microalgae. For the diclofenac concentration of 8.7 µg mL−1, samples were withdrawn on days 21 and 25, while for the concentration of 10 µg mL−1, samples were withdrawn on days 27 and 29. These days corresponded to the end of the growth kinetics of each microalga. As a control, the same experiment was performed in parallel but without light. The samples were subsequently analyzed by HPLC to quantify the concentration of diclofenac.
Statistical analysis
All experiments were performed in triplicate with three biological replicas. The data obtained were subjected to an analysis of variance (ANOVA) with three factors using the Fisher’s test at a significance level of 0.05 (p < 0.05) using the Minitab® v.16 software.
Results and discussion
Biomass growth
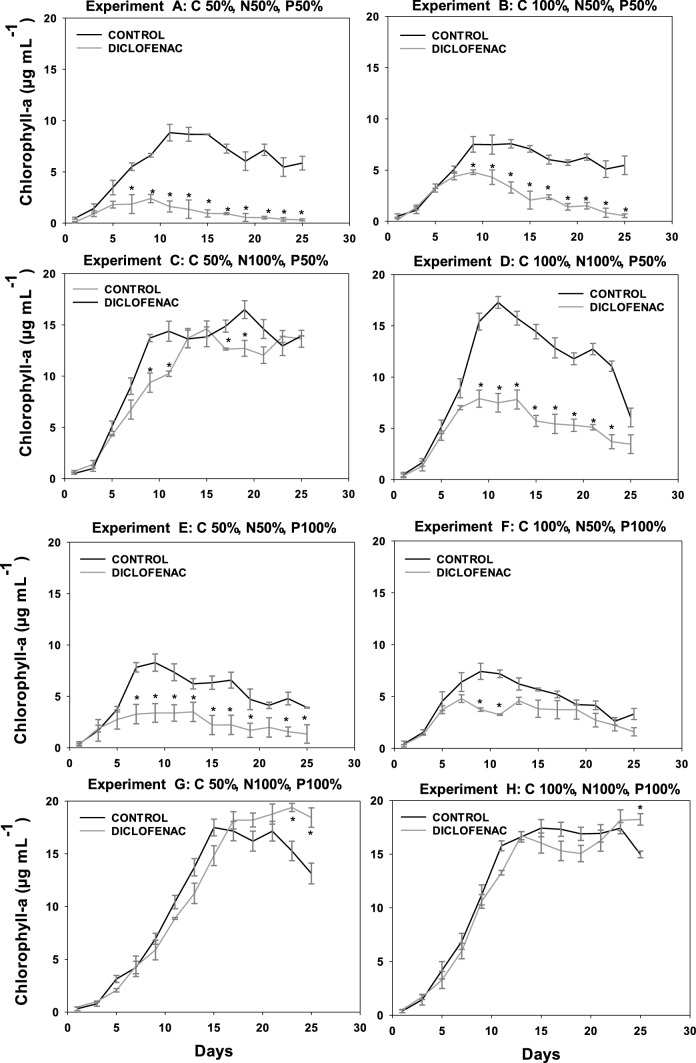
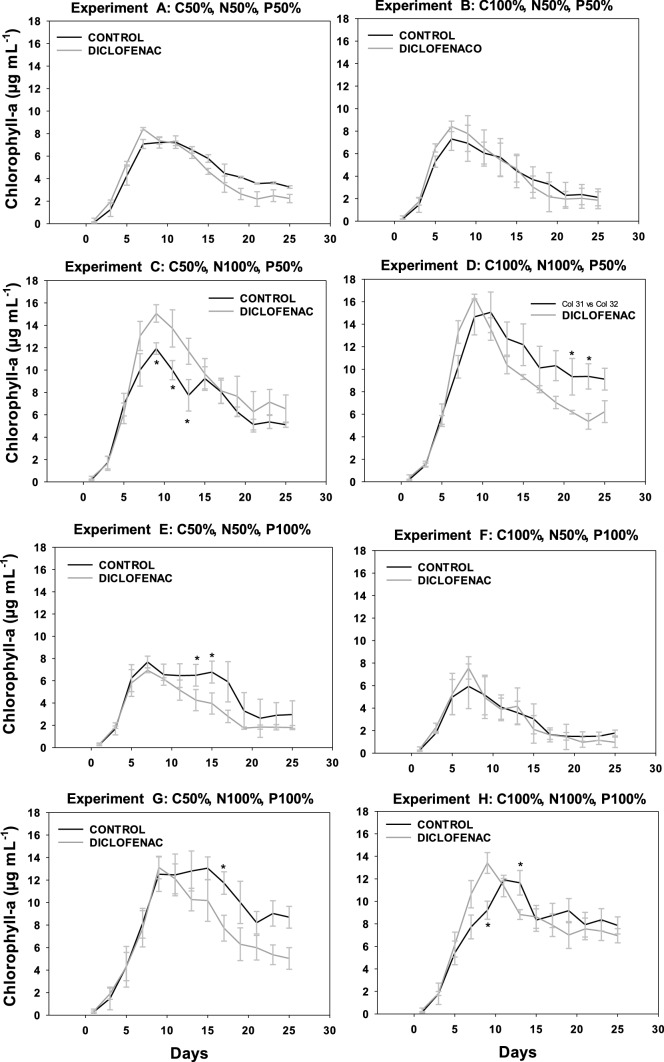
The effect of diclofenac in the growth of the four microalgae analyzed was determined by optical density (OD750), with the growth kinetics available in the supplementary material (Figures S3 to S6). The concentration of chlorophyll was measured as an indicator of the physiological state of the microalgae, given that it is an important pigment for the photosynthesis and plays a significant role in energy capture and transfer (Figs. 2, 5). In general, the four microalgae studied presented a similar behavior, starting with adaptation phases lasting 3 days, as well as diauxic growth in some cultures, attributed to their mixotrophic metabolism.
Fig. 2.
Content of chlorophyll a in the culture of C. vulgaris OW-01 for the eight experiments with different initial concentrations of C, N, and P in absence or presence of diclofenac (8.7 µg mL.−1). *Indicates statistically significant differences relative to the control (ANOVA, p < 0.05, Fisher test)
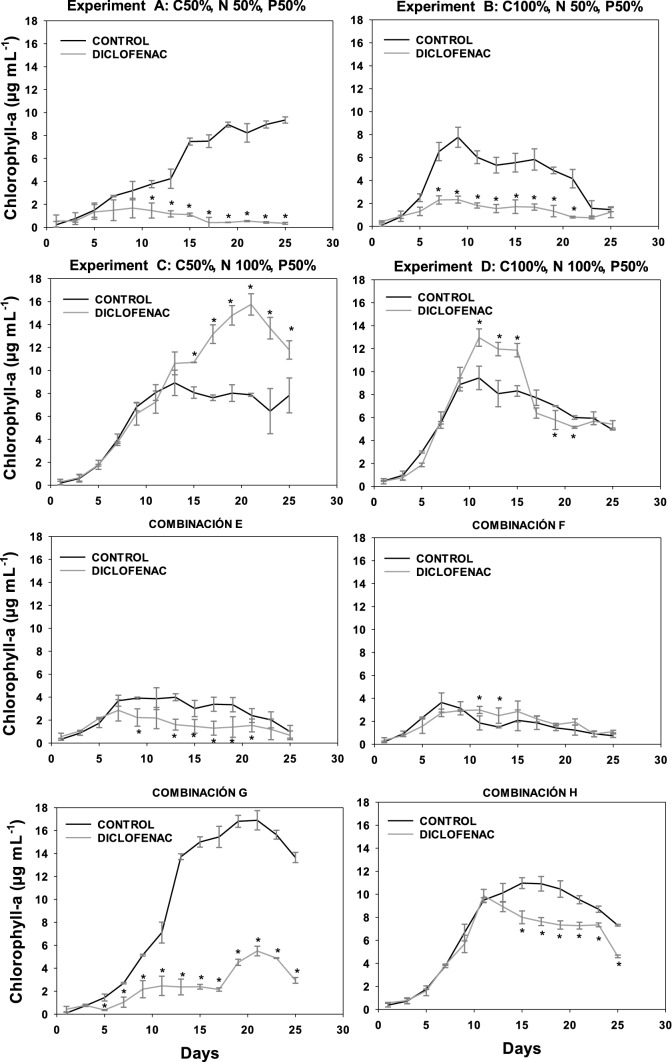
Fig. 5.
Content of chlorophyll a in the culture of S. obliquus CCAP 276/2 for the eight experiments with different concentrations of C, N, and P in absence or presence of diclofenac (8.7 µg mL.−1). *Indicates statistically significant differences relative to the control (ANOVA, p < 0.05, Fisher test)
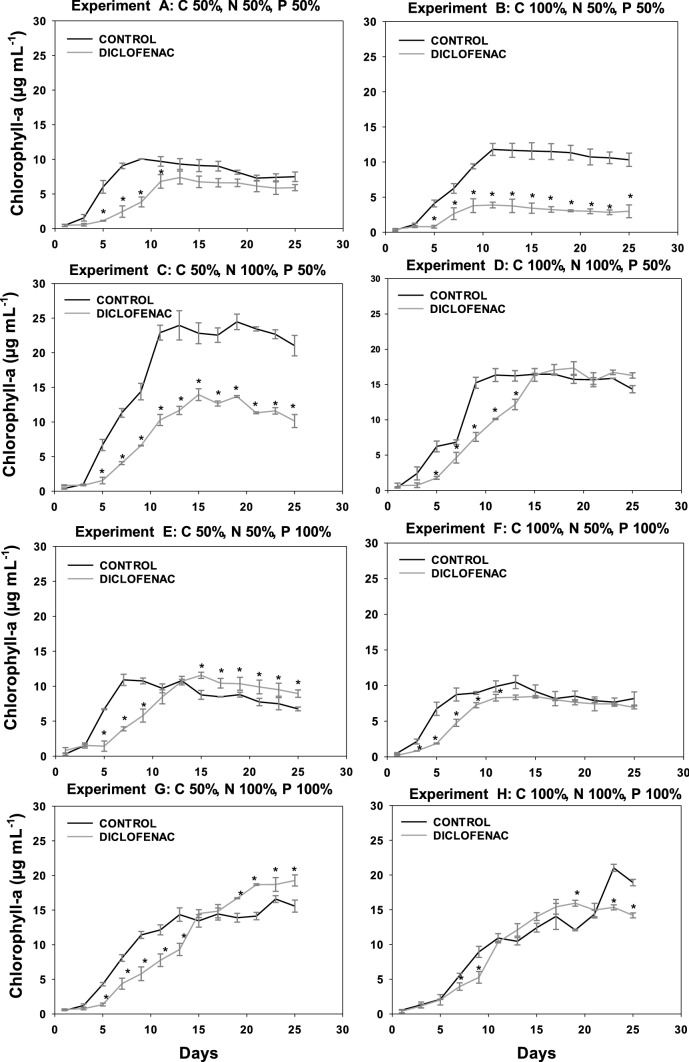
In the graphs corresponding to the chlorophyll content of the four microalgae, experiments A (C50 N50 P50), B (C100 N50 P50), E (C50 N50 P100), and F (C100 N50 P100), the nitrogen deficit significantly decreased the growth of both the control culture (without diclofenac) and the cultures containing diclofenac, when compared to experiments C (C50 N100 P50), D (C100 N100 P50), G (C50 N100 P100), and H (C100 N100 P100) that contained 100% of nitrogen. The nitrogen-deficient cultures showed an inhibition in growth with respect to those having 100% of this nutrient; this was observed for all microalgae studied. Nitrogen is the nutrient that determines microalgae growth (Perez et al. 2011) since it is necessary for the fixation of CO2 in autotrophic cultures or for carbon assimilation in heterotrophic cultures. In addition, at the biochemical level, the limitation of nitrogen directly influences the formation of amino acids, which restricts the transcription of mRNA and therefore reduces the synthesis of proteins. On the other hand, the efficiency of the photosystem II (PSII) decreases as a consequence of the thermal dissipation of the excitation energy absorbed in the pigmentary bed, resulting in a reduction of the photosynthesis rate, leading to a reduction in the respiration rate and affecting the growth of microalgae (Barsanti 2006).
Regarding the carbon deficit, in the case of N. oculata (Fig. 3) and S. acutus (Fig. 4) no significant difference in the content of chlorophyll a was observed in both the control culture and the cultures with diclofenac. However, in C. vulgaris, when comparing experiments C (C50 N100 P50) and D (C100 N100 P50) in cultures with diclofenac, a lower content of chlorophyll a was observed in the latter, which can be attributed to the addition of diclofenac since the respective control cultures showed no significant difference between experiments C and D. As for S. obliquus (Fig. 5), it presented a significant difference in the content of chlorophyll a in the diclofenac culture for experiment G (C50 N100 P100) when compared to experiment H (C100 N100 P100), similar to N. oculata. This difference could be attributed to the relationship between the concentrations of nutrients and the addition of diclofenac. Nevertheless, comparing the control cultures of both experiments no significant difference was found. A trend is observed in cultures with diclofenac modifying the content of chlorophyll a, although not statistically confirmed. Some genera of microalgae can combine autotrophic and heterotrophic metabolisms when carrying out photosynthesis in addition to ingesting organic materials such as glucose (Dragone 2022). Therefore, these microalgae are not strictly dependent on light or on organic substrates to grow (Liang et al. 2009; Yeh and Chang 2012; Leong et al. 2022). Mixotrophic metabolism limits the impact of biomass loss during respiration and reduces the amount of organic substrate needed to grow.
Fig. 3.
Content of chlorophyll a in the culture of N. oculata CCAP 849/7 for the eight experiments with different concentrations of C, N, and P in absence or presence of diclofenac (8.7 µg mL.−1). *Indicates statistically significant differences relative to the control (ANOVA, p < 0.05, Fisher test)
Fig. 4.
Content of chlorophyll a in the culture of S. acutus UTEX 72 for the eight experiments with different initial concentrations of C, N, and P in absence or presence of diclofenac (8.7 µg mL.−1). *Indicates statistically significant differences relative to the control (ANOVA, p < 0.05, Fisher test)
C. vulgaris, N. oculata, and S. acutus did not show a significant difference in the content of chlorophyll a in the experiments with deficiency of phosphorous. For S. obliquus, experiments A (C50 N50 P50) and B (C100 N50 P50), limited in phosphorous, showed a higher content of chlorophyll a in the control culture when compared to experiments E (C50 N50 P100) and F (C100 N50 P100), containing 100% of it. This effect can be caused by the relationship between phosphorus and other nutrients; although it has also been reported that phosphorus at low concentrations can limit the growth of some algal species. Conversely, other microalgae absorb phosphorus in excess and can survive for some time in phosphate-deficient waters (Round 1973). Many genera of algae have the ability to produce and store polyphosphates in small vacuoles that can be catabolized by enzymatic activity to release phosphate molecules for their use in the cellular metabolism (Brönmark and Hansson 2005).
The addition of diclofenac did not affect the content of chlorophyll a in S. acutus. However, in C. vulgaris, N. oculata, and S. obliquus a trend was observed in diclofenac cultures, where the content of chlorophyll a was modified compared to their respective controls (cultures without diclofenac). This effect could be associated with an excess of the carbon source related to the addition of diclofenac; considering that it was absorbed by microalgae. According to Sánchez-Torres et al. (2008), microalgae can uptake organic compounds present in the medium and can internalize them as nutrients for their growth. Several authors have reported the effect of diclofenac and photosynthetic activity on the growth of different microalgae such as Desmodesmus subspicatus, Chlamydomonas reinhardtii, Chlorella pyrenoidosa, Scenedesmus obliquus at concentrations ranging from 7.5 to 147 mg L−1. In these studies, the authors report an inhibition in growth and photosynthetic activity in percentages between 30 and 50% (Cleuvers 2004; Escher et al. 2005; Zhu et al. 2014; Majewska et al. 2018; Weissmannova et al. 2018; Zhang et al. 2019). Some authors have reported that the toxicity of diclofenac in microalgae could be related to its damaging effects on the membrane due to its weak solubility in water and related lipophilicity (Escher et al. 2005; Corcoll et al. 2014). Therefore, if the synthesis of pigment is linked to the chloroplast membranes, the membrane damage could be a reason for the low pigment content (Lemoine and Schoefs 2010).
Due to the particular characteristics of each microorganism, they showed different degrees of tolerance to distinct compounds, in this case to diclofenac. In summary, we were able to observe that nitrogen is the limiting factor on the growth of the microalgae studied, since its deficiency decreased the content of chlorophyll a, in both cultures, with diclofenac and the control. Moreover, the addition of diclofenac affected the growth of most of the cultures in a negative way.
Diclofenac removal from culture media
The elimination of diclofenac from the media by C. vulgaris, N. oculata, S. acutus, and S. obliquus in the 8 experiments was analyzed at the end of the kinetics (Table 2). The strains with the best removal capacity were S. obliquus and C. vulgaris, followed by S. acutus, while N. oculata had the lowest removal. Removal percentages greater than 90% were obtained by S. obliquus (91.1%) and C. vulgaris (90%) in experiment F (100C 50 N 100P). For S. acutus, the highest percentage (88.7%) was reached in experiment C (50C 100 N 50P) after 21 days, while N. oculata achieved 77.9% removal in experiment H (100C 100 N 100P) after 27 days. No significant difference was found between the removal percentages obtained in the 8 experiments for each of the microalga (ANOVA, p < 0.05, Fisher test). Nevertheless, there is a significant difference in experiment H of N. oculata with respect to the percentage obtained using S. obliquus.
Table 2.
Percentage of diclofenac removal C. vulgaris OW-01, N. oculata CCAP 849/7, S. acutus UTEX 72, and S. obliquus CCAP 276/2 grown in media with different initial concentrations of carbon, nitrogen, and phosphorus
| Experiment | Removal (%) | |||
|---|---|---|---|---|
| C. vulgaris | N. oculata | S. acutus | S. obliquus | |
|
A 50C 50 N 50P |
80.3a ± 3.7 | 59.8bc ± 2.4 | 80.08a ± 7.4 | 81.4a ± 4.8 |
|
B 100C 50 N 50P |
84.7a ± 14.1 | 60.6ac ± 6.3 | 77.9a ± 11.4 | 79.9a ± 0.07 |
|
C 50C 100 N 50P |
87.6a ± 8.7 | 72.4ac ± 10.7 | 88.7a ± 8.3 | 87.8a ± 10.5 |
|
D 100C 100 N 50P |
84.9a ± 11.6 | 74.9ac ± 10.2 | 87.7a ± 8.1 | 90.6a ± 8.0 |
|
E 50C 50 N 100P |
87.8a ± 10.9 | 70.3ac ± 3.9 | 85.7a ± 9.8 | 88.2a ± 4.9 |
|
F 100C 50 N 100P |
90.0a ± 12.8 | 77.5ac ± 15.5 | 83.3a ± 11.3 | 91.1a ± 8.2 |
|
G 50C 100 N 100P |
84.4a ± 14.1 | 68.8ac ± 15.4 | 81.9a ± 8.9 | 88.7a ± 10.5 |
|
H 100C 100 N 100P |
83.5ab ± 2.0 | 77.9bc ± 2.6 | 80.6ab ± 4.8 | 86.2a ± 4.3 |
The values with different letters (a, ab, bc, ac) indicate statistically significant differences in the percentage of removal between microalgae for each experiment (ANOVA, p < 0.05, Fisher test)
When comparing experiments H (100C 100 N 100P) and A (50C 50 N 50P), a higher removal percentage was obtained with experiment H, reaching 83.5, 77.9, 80.6, and 86.2% removal with C. vulgaris, N. oculata, S. acutus, and S. obliquus, respectively, while for experiment A the corresponding percentages were 80.3, 59.8, 80, and 81.4%. At the beginning of the study, our hypothesis was that the experiment A would achieve higher elimination percentages by having a deficit of the three nutrients, forcing the microalgae to use diclofenac as carbon source. Nonetheless, after analyzing our results, the nutrient difference did not influence the removal. A suitable comparison cannot be performed since there is not enough information on the removal of diclofenac with different species of microalgae and data about other microorganisms are scarce. The results obtained in our work are comparable to those reported by Santos et al. (2017), who evaluated the removal capacity of Chlorella sorokiniana, Chlorella vulgaris, and Scenedesmus obliquus with an initial concentration of 25 µg mL−1 of diclofenac in the Mann and Myers medium over 10 days. With S. obliquus and C. vulgaris they reported 98 and 69% removal, respectively, while in our work it reached 91% for the former and from 80 to 90% for the latter. The differences between the two works are due to the exposure time, the initial concentration of diclofenac, and the variation of nutrients in the culture medium. Another study reported by Wilt et al. (2016) used the microalgae C. sorokiniana, in batch cultures for 23 days, evaluating the removal of a drug mixture that included diclofenac. Additionally, they evaluated the degradation of drugs in abiotic cultures. These authors reported diclofenac removal percentages from 40 to 60% with the microalga studied. Nonetheless the results obtained in abiotic cultures were similar to those experiments with the microalga; concluding that the removal of the drug was mediated by photodegradation. Another study, reported by Matamoros et al. (2015), used a microalgal consortium to evaluate the elimination of 26 compounds (including diclofenac) in two seasons (warm and cold seasons) at exposure times of 4 and 8 days. The diclofenac removal ranged from 21 to 92%; concluding that photodegradation is the most important removal processes.
In our study we observed that the nutrient deficit does not influence the removal of diclofenac for any of the four microalgae studied, obtaining the highest percentage of removal by the microalgae S. obliquus (91%). Therefore, even in nutrient deficient media, a high percentage of removal of this drug can be achieved.
Desorption of diclofenac from the cell wall of C. vulgaris, N. oculata, S. acutus, and S. obliquus
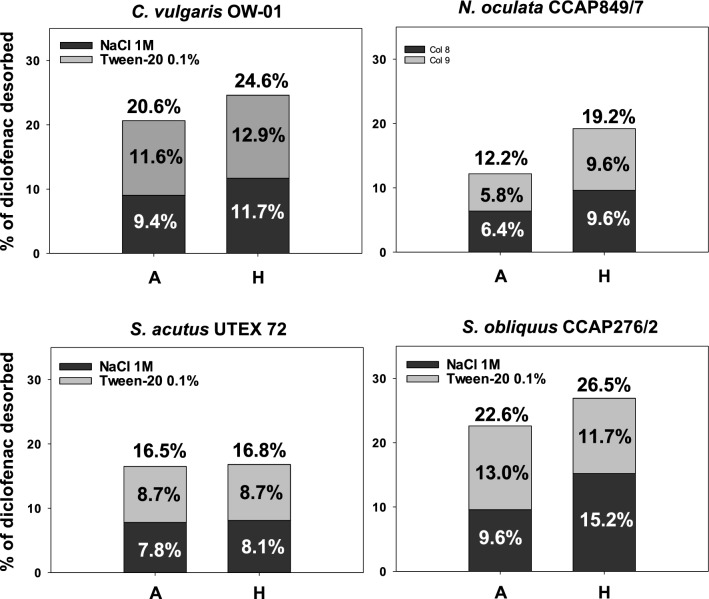
The amount of diclofenac adsorbed on the cell wall of all microalgae at the end of the growth kinetics was analyzed after washing with 1 M NaCl and 0.1% Tween 20 (Fig. 6). Only experiments A and H for each microalga were analyzed since no significant difference were observed in previous analyses (ANOVA, p < 0.05, Fisher test). The experiment H had higher percentage of desorbed diclofenac, indicating that adsorption removal is related to the amount of biomass obtained for each microalga, since they correspond to the experiments where the microalgae had higher biomass concentration. However, in the statistical analysis, no significant difference was found in the percentages of desorbed diclofenac between the experiments for each microalga, nor among the microalgae analyzed.
Fig. 6.
Diclofenac desorption from the cell wall of each microalga studied under the conditions of experiments A (50C 50 N 50P) and H (100C 100 N 100P). A C. vulgaris OW-01, B N. oculata CCAP 849/7, C S. acutus UTEX 72, and D S. obliquus CCAP 276/2 (ANOVA, p < 0.05, Fisher test)
S. obliquus obtained the highest desorption, at 26.5% for experiment H, of which 15.2% was desorbed using 1 M NaCl and 11.7% using 0.1% Tween 20. N. oculata had the lowest desorption of diclofenac, being 12.2% for experiment A, of which 6.4% was desorbed using 1 M NaCl and 5.8% using 0.1% Tween 20. The adsorption percentages did not show significant differences either between experiments A and H for each microalga or among the different microalgae. In a study by de Wilt et al. (2016) with C. sorokiniana, they reported the adsorption of diclofenac from 5.5 to 7.5%, which are smaller than the values we obtained with C. vulgaris. Even though both microalgae belong to the same genus and a similar behavior would have been expected, there is a significant difference with our work since percentages from 20.6 to 24.6% were reached. In 2019, Ben-Ouada et al., evaluated the removal of diclofenac by two green algae, Picocystis sp. and Graesiella sp., obtaining diclofenac removal percentages from 25 to 73%, but reporting less than 1% of diclofenac adsorbed. These results contrast with those obtained in our work, as each group used different species of microalgae with diverse components in the cell wall of each microalga establishing different interactions with diclofenac. Earlier reports show that the adsorption process varies significantly according to the hydrophobicity, structure, and functional groups present in the contaminating compounds and on the cell surface of the microalgae (Xiong et al. 2018).
In the experiments carried out in this work, the pH started at 6.8 and slightly increased, reaching 7.2 at the end of the study. As diclofenac has a pKa of 4.7 (Kamal et al. 2007), this compound has a high tendency to ionize, rendering most molecules negatively charged, therefore repulsive towards negatively charged compounds (De Oliveira et al. 2016). For N. gaditana, it has been established that amino acids exist within its cell wall (Scholz et al. 2014). If this is the case for the microalgae studied in this work, diclofenac could be interacting electrostaticaly with basic amino acids contained in their cell wall. Moreover, for N. oculata it has been established that its cell wall contains algaenans, highly aliphatic polymers (Zhang and Volkman 2017). Once again if this condition can be extended to the microalgae studied, protonated diclofenac molecules could interact hydrophobically with these polymers, despite having less than 1% of protonated diclofenac molecules due to their low water solubility (Fini et al. 2012).
For the four microalgae here studied, there are both hydrophilic and hydrophobic interactions involved in the adsorption of diclofenac; since the percentages of diclofenac desorbed by 1 M NaCl and 0.1% Tween 20 were similar. According to the results obtained, it is considered that adsorption did not represent the main mechanism of diclofenac removal for C. vulgaris, N. oculata, S. acutus, and S. obliquus and suggest that these microalgae contain compounds in the cell wall that allow them to sustain both hydrophobic and electrostatic interactions with diclofenac. The low percentage of diclofenac adsorbed on the cell wall indicates that other mechanisms could be participating in the degradation of the drug such as intracellular absorption and degradation of the drug to use it as a carbon source, as well as extracellular degradation. As reported before, microalgae can excrete polymeric substances including saccharides, proteins, enzymes, methyl and acetyl groups, and lipids, which generate a matrix that keeps extracellular enzymes close to the microalgae, favoring the degradation of organic compounds (Xiong et al. 2018; Maryjoseph and Ketheesan 2020; Samal et al. 2022).
Unfortunately, there are not enough reports that analyze the behavior of microalgae when adsorbing compounds to their cell wall or internalizing them to the cell. As in the previous study, the nutrient deficit does not influence the adsorption of diclofenac to the cell wall and that the interaction of this drug with the four microalgae analyzed is both electrostatic and hydrophobic, having a higher percentage of removal by adsorption with S. obliquus (26%).
Photolysis test of diclofenac
We evaluated the abiotic degradation of diclofenac in the media studied. To obtain the percentage of diclofenac degraded with the initial concentration of 8.7 µg mL−1, samples were withdrawn on days 21 and 25, corresponding to the final days of the kinetics for the microalgae studied, and resulting in a degradation range of 15 to 28%. For the initial concentration of 10 µg mL−1, samples were withdrawn on days 27 and 29, resulting in a degradation ranging from 19 to 25%. Several studies have shown that several drugs can be photodegraded since they generally have aromatic rings, heteroatoms, and other functional groups; allowing the absorption of solar radiation or facilitating reactions with photosensitive compounds present in the medium that induce their photodegradation (Kunkel and Radke 2012; Rivera-Utrilla et al 2013). Ben-Ouada et al. (2019) evaluated the elimination of diclofenac using initial concentrations of 25, 50 and 100 µg mL−1 by Picocystis sp. and Graesiella sp., while blank controls without algae cells served to quantify the abiotic removal of diclofenac. While these abiotic cultures had incubation conditions similar to those of our work, the abiotic removal did not exceed 8% after 5 days regardless of the initial diclofenac concentration. In a study by Zhang et al. (2013), the removal of diclofenac in mesocosms of Scirpus validus was evaluated, growing in hydroponic conditions and mesocosms without plants. In the latter, an 80% reduction in diclofenac concentration was observed after 7 days. The authors indicated that the elimination of diclofenac in aquatic systems can be mainly attributed to photodegradation. The results obtained in our work vary from those reported and the difference may arise from the fact that in photodegradation studies the compounds in solution are exposed to a continuous light source with controlled light intensity, in addition to the effect of the initial concentration of the drug. When analyzing by HPLC what was obtained in the control experiment (without light), a significant decrease in the initial concentrations of diclofenac was not observed.
Conclusions
The addition of diclofenac did not modify the growth of S. acutus, whereas for C. vulgaris, N. oculata and S. obliquus, a change in their growth was observed in cultures exposed to diclofenac compared with their corresponding control cultures (without diclofenac). Nitrogen proved to be a limiting factor in the growth of all the microalgae in cultures with and without diclofenac, but it did not affect the elimination of diclofenac. Similarly, the variation of the initial nutrient concentrations (nitrogen, phosphorus, and carbon) did not affect the elimination of diclofenac from the media. The percentage of diclofenac removal varied from 59 to 91% among the four microalgae analyzed; obtaining the highest removal in experiment F (100C 50 N 100P) with S. obliquus. The percentages of diclofenac desorption from the cell wall were between 12 and 26% among all microalgae with the highest desorption percentage for S. obliquus in the experiment with 100% C, 100% N, and 100% P; therefore, it is suggested that other processes are involved in the elimination of diclofenac. Finally, the percentage of diclofenac degraded by photolysis ranged from 15 to 28% within 25 to 29 days. Based on the results obtained, S. obliquus was the best microalga alternative for diclofenac removal.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Sincere thanks are due to Dr. Roberto Rico Martínez from the Autonomous University of Aguascalientes for kindly providing the microalgae used in this project and to Dra. Luz María Teresita Paz Maldonado from the Bioreactors Engineering Laboratory at the Autonomous University of San Luis Potosí for providing equipment to perform the necessary analyses in this project. Also thanks to the National Council of Science and Technology (CONACyT) for the grant awarded with registration number 252858.
Author contributions
SS: conceptualization, methodology, formal analysis, investigation, writing-original draft, visualization. GO: resources, formal analysis, writing-review and editing. GC: and VM: resources, writing-review and editing, supervision. SG: conceptualization, validation, resources, writing-review and editing, supervision, project administration, funding acquisition.
Funding
The authors did not receive support from any organization for the submitted work.
Data availability
All data are incorporated into the article and its online supplementary material.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethics approval
Not applicable.
Consent to participle
Not applicable.
Consent to publication
Not applicable.
References
- Alessandretti I, Toniciolli-Rigueto CV, Torres-Nazari M, Rosseto M, Dettmer A. Removal of diclofenac from wastewater: a comprehensive review of detection, characteristics and tertiary treatment techniques. J Environ Chem Eng. 2021;9:106743. doi: 10.1016/j.jece.2021.106743. [DOI] [Google Scholar]
- Alharbi SK, Ansari AJ, Nghiem LD, Price WE. New transformation products from ozonation and photolysis of diclofenac in the aqueous phase. Process Saf Environ. 2022;157:106–114. doi: 10.1016/j.psep.2021.10.050. [DOI] [Google Scholar]
- Anne-Marie K, Yee W, Loh SH, Thye AA, Cha S. Effects of excess and limited phosphate on biomass, lipid and fatty acid contents and the expression of four fatty acid desaturase genes in the tropical Selenastraceaen Messastrum gracile SE-MC4. Appl Biochem Biotechol. 2020 doi: 10.1007/s12010-019-03182-z. [DOI] [PubMed] [Google Scholar]
- Arnold KE, Boxall ABA, Brown AR, Cuthbert RJ, Gaw S, Hutchinson TH, Jobling S, Madden JC, Metcalfe CD, Naidoo V, Shore RF, Smits JE, Taggart MA, Thompson HM. Assessing the exposure risk and impacts of pharmaceuticals in the environment on individuals and ecosystems. Biol Lett. 2013;9:20130492. doi: 10.1098/rsbl.2013.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsanti L. Algae. Anatomy, biochemistry and biotechnology. Boca Raton: CRC Press; 2006. [Google Scholar]
- Ben-Ouada S, Ben-Ali R, Cimetiere N, Leboulanger C, Ben-Ouada H, Sayadi S. Biodegradation of diclofenac by two green microalgae: Picocystis sp. y Graesiella sp. Ecotoxicol Environ Saf. 2019;186:109769. doi: 10.1016/j.ecoenv.2019.109769. [DOI] [PubMed] [Google Scholar]
- Bonnefille B, Courant F, Gomez E. Diclofenac in the marine environment: a review of its occurrence and effects. Mar Pollut Bull. 2019;131(Part A):496–506. doi: 10.1016/j.marpolbul.2018.04.053. [DOI] [PubMed] [Google Scholar]
- Brönmark C, Hansson LA. The biology of lakes and ponds. Oxford: University Press; 2005. [Google Scholar]
- Cai T, Park SY, Li Y. Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sust Energ Rev. 2013;19:360–369. doi: 10.1016/j.rser.2012.11.030. [DOI] [Google Scholar]
- Cleuvers M. Mixture toxicity of the anti-Inflammatory drugs diclofenac, ibuprofen, naproxen and acetylsalicylic acid. Ecotoxicol Environ Saf. 2004;59:309–315. doi: 10.1016/S0147-6513(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Corcoll N, Acuña V, Barceló D, Casellas M, Guasch H, Huerta B, Petrovic M, Ponsatí L, Rodríguez-Mozaz S, Sabater S. Pollution-induced community tolerance to non-steroidal anti—inflammatory drugs (NSAIDs) in fluvial biofilm communities affected by WWTP effluents. Chemosphere. 2014;112:185–193. doi: 10.1016/j.chemosphere.2014.03.128. [DOI] [PubMed] [Google Scholar]
- Croom E. Chapter Three - Metabolism of Xenobiotics of Human Environments. Chapter Three-Metabolism Xenobiotics Human Environ. 2012;112:31–88. doi: 10.1016/B978-0-12-415813-9.00003-9. [DOI] [PubMed] [Google Scholar]
- Cuellar-Bermudez SP, Alemán-Nava GS, Chandra R, García-Pérez JS, Contreras-Angulo JR, Markou G, Muylaert K, Rittmann BE, Parra-Saldivar R. Nutrients utilization and contaminants removal. A review of two approaches of algae and cyanobacteria in wastewater. Algal Res. 2017;24:438–449. doi: 10.1016/j.algal.2016.08.018. [DOI] [Google Scholar]
- De Oliveira T, Guégan R, Thiebault T, Milbeau CL, Le Milveau C, Muller F, Teixeira V, Giovanela M, Boussafir M. Adsorption of diclofenac onto orgnoclays: effects of surfactant and environmental (pH and temperature) conditions. J Hazar Mater. 2016;323:558–566. doi: 10.1016/j.jhazmat.2016.05.001. [DOI] [PubMed] [Google Scholar]
- de Wilt A, Butkovskyi A, Tuantet K, Leal LH, Fernandes TV, Langenhoff A, Zeeman G. Micropollutant removal in an algal treatment system fed with source separated wastewater streams. J Hazar Mater. 2016;304:84–92. doi: 10.1016/j.jhazmat.2015.10.033. [DOI] [PubMed] [Google Scholar]
- Dragone G. Challenges and opportunities to increase economic feasibility and sustainability of mixotrophic cultivation of green microalgae of the genus Chlorella. Renew Sustain Energy Rev. 2022;160:112284. doi: 10.1016/j.rser.2022.112284. [DOI] [Google Scholar]
- Escher B, Bramaz N, Eggen R, Richter M. In vitro assessment of modes of toxic action of pharmaceuticals in aquatic life. Environ Sci Technol. 2005;39(9):3090–3100. doi: 10.1021/ess048590e. [DOI] [PubMed] [Google Scholar]
- Fini A, Bassini G, Monastero A, Cavallari C. Diclofenac salts, VIII. Effect of the counterions on the permeation through porcine membrane from aqueous saturated solutions. Pharmaceutics. 2012;4:413–429. doi: 10.3390/pharmaceutics4030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami RK, Agrawal K, Verma P. Microalgal-based remediation of wastewater: a step towards environment protection and management. Environ Qual Manag. 2022 doi: 10.1002/tqem.21850. [DOI] [Google Scholar]
- Gröner F, Höhne C, Kleiner W, Kloas W. Chronic diclofenac exposure affects gill integrity and pituitary gene expression and displays estrogenic activity in nile tilapia (Oreochromis niloticus) Chemosphere. 2017;166:473–481. doi: 10.1016/j.chemosphere.2016.09.116. [DOI] [PubMed] [Google Scholar]
- Henriques M, Silva A, Rocha J. Extraction and quantification of pigments from a marine microalga: a simple and reproducible method. Commun Curr Res Educ Topics Trends Appl Microbiol Formatex. 2007;2:586–593. [Google Scholar]
- Jerez CG, Malapascua JR, Sergejevová M, Figueroa FL, Masojídek J. Effect of nutrient starvation under high irradiance on lipid and starch accumulation in Chlorella fusca (Chlorophyta) Marine Biotechnol. 2016;18:24–36. doi: 10.1007/s10126-015-9664-6. [DOI] [PubMed] [Google Scholar]
- Kamal MA, Iimura N, Nabekura T, Kitagawa S. Enhanced skin permeation of diclofenac by ion-pair formation and further enhancement by microemulsion. Chem Pharm Bull. 2007;55:368–371. doi: 10.1248/cpb.55.368. [DOI] [PubMed] [Google Scholar]
- Koul B, Sharma K, Shah M. Phycoremediation: a sustainable alternative in wastewater treatment (WWT) regime. Environ Technol Innovation. 2022;25:102040. doi: 10.1016/j.eti.2021.102040. [DOI] [Google Scholar]
- Kumar R, Qureshi M, Kumar-Vishwakarma D, Al-Ansari N, Kuriqi A, Elbeltagi A, Saraswat A. A review on emerging water contaminants and the application of sustainable removal technologies. Case Stud Chem Env Eng. 2022;6:100219. doi: 10.1016/j.cscee.2022.100219. [DOI] [Google Scholar]
- Kunkel U, Radke M. Fate of pharmaceuticals in rivers: deriving a benchmark dataset at favorable attenuation conditions. Water Res. 2012;46:5551–5565. doi: 10.1016/j.watres.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Lee J, Ji K, Lim Kho Y, Kim P, Choi K. Chronic exposure to diclofenac on two freswater cladocerans and Japanese medaka. Ecotoxicol Environ Saf. 2011;74:1216–1225. doi: 10.1016/j.ecoenv.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Lemoine Y, Schoefs B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynth Res. 2010;106:155–177. doi: 10.1007/s11120-010-9583-3. [DOI] [PubMed] [Google Scholar]
- Leong WH, Mohamad-Saman NA, Kiatkittipong W, Assabumrungrat S, Najdanovic-Visak V, Wang J, Shiong-Khoo J, Kee-Lam M, Mohamad M, Wei-Lim J. Photoperiod-induced mixotrophic metabolism in chlorella vulgaris for high biomass and lipid to biodiesel productions using municipal wastewater medium. Fuel. 2022;313:123052. doi: 10.1016/j.fuel.2021.123052. [DOI] [Google Scholar]
- Li R, Chen GZ, Tam NFY, Luan TG, Shin PKS, Cheung S, Liu T. Toxicity of bisphenol A and its bioaccumulation and removal by a marine microalga Stephanodiscus hantzschii. Ecotox Environ Saf. 2009;72:321–328. doi: 10.1016/j.ecoenv.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Li F, Gao D, Hu Hanhua H. High-efficiency nuclear transformation of the oleaginous marine Nannochloropsis species using PCR product. Biosci Biotechnol Biochem. 2014;78:812–817. doi: 10.1080/09168451.2014.905184. [DOI] [PubMed] [Google Scholar]
- Liang Y, Sarkany N, Cui Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett. 2009;31:1043–1049. doi: 10.1007/s10529-009-9975-7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Guan YT, Gao QT, Tam NFY, Zhu WP. Cellular responses, biodegradation and bioaccumulation of endocrine disrupting chemicals in marine diatom Navicula incerta. Chemosphere. 2010;80:592–599. doi: 10.1016/j.chemosphere.2010.03.042. [DOI] [PubMed] [Google Scholar]
- Majewska M, Harshkova D, Gucsiora M, Aksmann A. Phytotoxic activity of diclofenac: evaluation using a model green algal Chlamydomonas reinhardtii with atrazine as a reference substance. Chemosphere. 2018;209:989–997. doi: 10.1016/j.chemosphere.2018.06.156. [DOI] [PubMed] [Google Scholar]
- Martínez ME, Sánchez S, Jiménez JM, Yousfi FE, Muñoz L. Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Biotechnol Rep. 2000;73:263–272. doi: 10.1016/j.btre.2016.04.003. [DOI] [Google Scholar]
- Maryjoseph S, Ketheesan B. Microalgae based wastewater treatment for the removal of emerging contaminants: a review of challenges and opportunities. Case Stud Chem Env Eng. 2020;2:100046. doi: 10.1016/j.cscee.2020.100046. [DOI] [Google Scholar]
- Matamoros V, Gutierrez R, Ferrer I, García J, Bayona J. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: a pilot-scale study. J Hazard Mater. 2015;288:34–42. doi: 10.1016/j.jhazmat.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Montes JP, Pulido M. Obtención de protocolos para el aislamiento, cultivo y extracción de ADN de Chlorella vulgaris Beyerinck. El Astrolabio. 2012;11:47–54. [Google Scholar]
- Mullan J, Weston KMBA, Burns P, Mullan J, Rudd R. Con-sumer knowledge about over-the-counter NSAIDs: they don’t know what they don’t know. Aust New Zealand J Public Health. 2017;41:210. doi: 10.1111/1753-6405.12589. [DOI] [PubMed] [Google Scholar]
- Nicolaou A, Meric S, Fatta D. Occurrence pattern of pharmaceuticals in water and wastewater environments. Anal Bioanal Chem. 2007;387(4):1225–1234. doi: 10.1007/s00216-006-1035-8. [DOI] [PubMed] [Google Scholar]
- Norvill ZN, Shilton A, Guieysse B. Emerging contaminant degradation and removal in algal wastewater treatment ponds: Identifying the research gaps. J Hazard Mater. 2016;313:291–309. doi: 10.1016/j.jhazmat.2016.03.085. [DOI] [PubMed] [Google Scholar]
- Pérez O, Escalante FM, de Bashan LE, Bashan Y. Heterotrophic cultures of microalgae: metabolism and potential products. Water Res. 2011;45:11–36. doi: 10.1016/j.watres.2010.08.037. [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA Bioenergetics. 1989;975:384–394. doi: 10.1016/S0005-2728(89)80347-0. [DOI] [Google Scholar]
- Posadas E, Bochon S, Coca M, García-González MC, García-Encina PA, Muñoz R. Microalgae-based agro-industrial wastewater treatment: a preliminary screening of biodegradability. J Appl Phycol. 2014;26:2335–2345. doi: 10.1016/j.cscee.2020.100046. [DOI] [Google Scholar]
- Priyadharshini SD, Babu PS, Manikandan S, Subbaiya R, Govarthanan M, Karmegam N. Phycoremediation of wastewater for pollutant removal: a green approach to environmental protection and long-term remediation. Environ Poll. 2021;290:117989. doi: 10.1016/j.envpol.2021.117989. [DOI] [PubMed] [Google Scholar]
- Ribeiro H, Rodrigues I, Napoleao L, Lira L, Marques D, Veríssimo M, Andrade JP, Dourado M. Non-steroidal anti-inflammatory drugs (NSAIDs), pain and aging: Adjusting prescription to patient features. Biom Pharm. 2022;150:112958. doi: 10.1016/j.biopha.2022.112958. [DOI] [PubMed] [Google Scholar]
- Rivera-Utrilla J, Sanchez-Polo M, Ferro-Garcia MA, Prados-Joya G, Ocampo-Perez R. Pharmaceuticals as emerging contaminants and their removal from water. A Review Chemosphere. 2013;93:1268–1287. doi: 10.1016/j.chemosphere.2013.07.059. [DOI] [PubMed] [Google Scholar]
- Round FE. Biologia das Algas. Rio de Janeiro: Editora Guanabara Dois S.A; 1973. [Google Scholar]
- Ruiz-Marin A, Mendoza-Espinosa LG, Stepherson T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour Technol. 2010;101:58–64. doi: 10.1016/j.biortech.2009.02.076. [DOI] [PubMed] [Google Scholar]
- Samal K, Mahapatra S, Hibzur Ali MD. Pharmaceutical wastewater as emerging contaminants (EC): treatment technologies, impact on environment and human health. Energy Nexus. 2022;6:100076. doi: 10.1016/j.nexus.2022.100076. [DOI] [Google Scholar]
- Sánchez-Torres H, Juscamaita J, Vargas J. Crecimiento mixotrófico de la microalga Nannochloropsis oculata en ensilado biológico de pescado. The Biologist. 2008;6:94–100. [Google Scholar]
- Santos CE, de Coimbra NR, Paniagua-Bermejo S, García-Perez AI, Otero-Cabero M (2017) Comparative assessment of pharmaceutical removal from wastewater by the microalgae Chlorella sorokiniana, Chlorella vulgaris y Scenedesmus obliquus. In: Farooq R, Ahmad Z (eds) Biological wastewater treatment and resource recovery. London, IntechOpen. Available from: https://www.intechopen.com/chapters/53770. 10.5772/66772
- Scholz MJ, Weiss TL, Jinkerson RE, Jing J, Roth R, Goodenough U, Posewitz MC, Gerken HG. Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot Cell. 2014;13:1450–1464. doi: 10.1128/EC.00183-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sophia AC, Lima EC. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol Environ Saf. 2018;150:1–17. doi: 10.1016/j.ecoenv.2017.12.026. [DOI] [PubMed] [Google Scholar]
- Szopinska M, Potapowicz J, Jankowska K, Luczkiewicz A, Svahn O, Björklund E, Nannou C, Lambropoulou D, Polkowska Z. Pharmaceuticals and other contaminants of emerging concern in Admiralty Bay as a result of untreated wastewater discharge: Status and possible environmental consequences. Sci Total Environ. 2022;835:155400. doi: 10.1016/j.scitotenv.2022.155400. [DOI] [PubMed] [Google Scholar]
- Wang S, Zheng L, Han X, Yang B, Li J, Sun C. Lipid accumulation and CO2 utilization of two marine oil-rich microalgal strains in response to CO2 aeration. Acta Oceanol Sin. 2018;37:119–126. doi: 10.1007/s13131-018-1171-y. [DOI] [Google Scholar]
- Weissmannova HD, Pavlosky J, Fiserova L, Kosarova H. Toxicity of diclofenac: cadmium binary mixtures to algae Desmodesmus subspicatus using nomalization method. Bulletin of Environ Contam Toxicol. 2018;101:205–213. doi: 10.1007/s000128-018-2384-7. [DOI] [PubMed] [Google Scholar]
- Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami JA. Comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143–150. doi: 10.14336/AD.2017.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JQ, Kurade MB, Jeon BH. Can microalgae remove pharmaceutical contaminants from water? Trends Biotechnol. 2018;36:30–44. doi: 10.1016/j.tibtech.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Xu M, Huang H, Li N, Li F, Wang D, Luo Q. Occurrence and ecological risk of pharmaceuticals and personal care products (PPCPs) and pesticides in typical surface watersheds, China. Ecotoxicol Environ Saf. 2019;175:289–298. doi: 10.1016/j.ecoenv.2019.01.131. [DOI] [PubMed] [Google Scholar]
- Yeh K, Chang J. Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalgae Chlorella vulgaris ESP-31. Bioresour Technol. 2012;105:120–127. doi: 10.1016/j.biortech.2011.11.103. [DOI] [PubMed] [Google Scholar]
- Yu SJ, Hu H, Zheng H, Wang YQ, Pan SB, Zeng RJ. Effect of different phosphorus concentrations on biodiesel production from Isochrysis zhangjiangensis under nitrogen sufficiency or deprivation condition. Appl Microbiol Biotechnol. 2019;103:5051–5059. doi: 10.1007/s00253-019-09814-y. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Volkman JK. Algaenan structure in the microalga Nannochloropsis oculata characterized from stepwise pyrolysis. Org Geochem. 2017;104:1–7. doi: 10.1016/j.orggeochem.2016.11.005. [DOI] [Google Scholar]
- Zhang DQ, Gersberg RM, Hua T, Zhu J, Goyal MK, Ng WJ, Tan SK. Fate of pharmaceutical compounds in hydroponic mesocosms planted with Scirpus validus. Environm Pollut. 2013;181:98–106. doi: 10.1016/j.envpol.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo J, Yao T, Zhang Y, Zhou X, Chu H. The influence of four pharmaceuticals on Chlorella pyrenoidosa culture. Sci Rep. 2019;9:1624. doi: 10.1038/s41598-018-36609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou GJ, Peng FQ, Zhang LJ, Ying GG. Biosorption of zinc and copper from aqueous solutions by two freshwater green microalgae Chlorella pyrenoidosa and Scenedesmus obliquus. Environ Sci Pollut Res. 2012;19:2918–2929. doi: 10.1007/s11356-012-0800-9. [DOI] [PubMed] [Google Scholar]
- Zhou GJ, Peng FQ, Zhang LJ, Ying GG. Cellular responses and bioremoval of nonylphenol and octylphenol in the freshwater green microalga Scenedesmus obliquus. Ecotoxicol Environ Saf. 2013;87:10–16. doi: 10.1016/j.ecoenv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Zhu S, Liu B, Chen B, Zhang J, Liu W. Effects of three pharmaceuticals and personal care products on growth and photosystem II in Scenedesmus obliquus. Zhongshan Daxue Xuebao/acta Scientiarum Natralium Universitatis Sunyatseni. 2014;53(1):121–126+134. [Google Scholar]
- Zuorro A, Maffei G, Lavecchia R. Kinetic modeling of azo dye adsorption on non-living cells of Nannochloropsis oceanica. J Environ Chem Engineer. 2017;5:4121–4127. doi: 10.1016/j.jece.2017.07.078. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are incorporated into the article and its online supplementary material.
Not applicable.