Abstract
Purpose:
Patients discontinuing immuno-oncology regimens may experience periods of disease control without need for ongoing anticancer therapy, but toxicity may persist. We describe treatment-free survival (TFS), with and without toxicity.
Patients and Methods:
Data were analyzed from the randomized phase III CheckMate 214 trial of nivolumab plus ipilimumab (n = 550) versus sunitinib (n = 546) for treatment-naïve, advanced renal cell carcinoma (aRCC). TFS was estimated by the 42-month restricted mean times defined by the area between Kaplan–Meier curves for two time-to-event endpoints defined from randomization: time to protocol therapy cessation and time to subsequent systemic therapy initiation or death. TFS was subdivided as TFS with and without toxicity by counting days with ≥1 grade ≥3 treatment-related adverse event (TRAE).
Results:
At 42 months since randomization, 52% of nivolumab plus ipilimumab and 39% of sunitinib intermediate/poor-risk patients were alive; 18% and 5% surviving treatment-free, respectively. Among favorable-risk patients, 70% and 73% of nivolumab plus ipilimumab and sunitinib patients were alive; 20% and 9% treatment-free. Over the 42-month period, mean TFS was over twice as long after nivolumab plus ipilimumab than sunitinib for intermediate/poor-risk (6.9 vs. 3.1 months) and three times as long for favorable-risk patients (11.0 vs. 3.7 months). Mean TFS with grade ≥3 TRAEs was a small proportion of time for both treatments (0.6 vs. 0.3 months after nivolumab plus ipilimumab vs. sunitinib for intermediate/poor-risk, and 0.9 vs. 0.3 months for favorable-risk patients).
Conclusions:
Patients initiating first-line nivolumab plus ipilimumab for aRCC spent more survival time treatment-free without toxicity versus those on sunitinib, regardless of risk group.
Translational Relevance.
We documented longer treatment-free survival (TFS) with nivolumab plus ipilimumab versus sunitinib in CheckMate 214. After accounting for the possibility that toxicity of immune checkpoint inhibitors (ICI) persisted or arose after discontinuation, nivolumab provided longer TFS without toxicity. Given the durable response and survival with ICI–ICI combination relative to VEGF receptor tyrosine kinase inhibitor–targeted therapy, this integrated analysis of CheckMate 214 demonstrated the value of describing the quality of survival time as a part of comparing their value and provided additional insight for individual decision-making about initiation of first-line therapy for advanced renal cell carcinoma. Clinical trials investigating ICI agents should assess TFS to describe quality of survival time for clinical decision-making when initiating therapy.
Introduction
Immune checkpoint inhibitors (ICI) produce unique patterns of antitumor response and toxicity (1–3) as compared with targeted therapy–based regimens. While patients discontinuing ICIs may experience periods of durable disease control without the need for subsequent systemic therapy, treatment-related adverse events (TRAE) may also persist beyond or emerge after ICI discontinuation. Too often, investigators focus data presentations on positive treatment outcomes (e.g., duration of response) that occur after treatment initiation in a subset of patients. We recently proposed a novel outcome—treatment-free survival (TFS)—to characterize the antitumor activity and the toxicity experienced during the period after ending ICI therapy until starting subsequent systemic therapy, or death, for the entire trial cohort (4). The outcome was developed in the setting of comparing ICIs as monotherapy or in combination in two randomized double-blind trials. In our previous analyses in advanced melanoma based on the CheckMate 067 and CheckMate 069 trials, TFS in the nivolumab plus ipilimumab group was greater than with either nivolumab or ipilimumab alone (5, 6).
TFS has been analyzed as part of an integrated summary of how overall survival (OS) time is spent for all patients from the start of trial-assigned first-line therapy (4). The motivation to include all patients was to avoid overemphasizing a selected subgroup of patients who fared extremely well, by examining a time horizon starting at the initial clinical decision point about first-line therapy. The analysis incorporated occurrence of side effects and/or persistence after cessation of initial therapy to balance therapeutic efficacy and toxicity.
In advanced renal cell carcinoma (aRCC), the approvals of dual immuno-oncology (IO) combinations and IO-VEGF–targeted therapy combinations offer multiple choices for first-line therapy, creating a need to understand how patients spend survival time after initiation of these regimens (7). Results from the randomized phase III CheckMate 214 trial in previously untreated, predominantly clear cell aRCC showed OS to be significantly longer with nivolumab plus ipilimumab than with sunitinib among patients with International Metastatic Renal Cell Carcinoma Database Consortium (IMDC; ref. 8) intermediate or poor prognostic risk (9). The OS improvement persisted with longer follow-up (10, 11). After a minimum follow-up of 42 months (10), 52% and 39% of intermediate/poor-risk patients were alive in the nivolumab plus ipilimumab and sunitinib groups, respectively. Patients with favorable-risk aRCC had 70% and 73% 42-month OS probability with nivolumab plus ipilimumab and sunitinib, respectively. TRAEs leading to treatment discontinuation were more frequent with nivolumab plus ipilimumab versus sunitinib, occurring in 22% and 13% of patients, respectively (10); however, nivolumab plus ipilimumab was associated with better health-related quality of life (HRQoL) throughout treatment (12, 13).
We estimated TFS in the CheckMate 214 trial, providing the first analysis of this novel outcome in a trial comparing a dual IO regimen with a targeted therapy. This investigation of CheckMate 214 methodologically extends our previous work (4) to also characterize toxicity during protocol therapy. With analysis of all IMDC risk cohorts, we gain insight about TFS as a part of assessing efficacy and toxicity tradeoffs in different prognostic scenarios of OS benefit and move closer to the creation of an outcome that can further inform clinical decision-making.
Patients and Methods
The study population comprised patients enrolled in the randomized phase III CheckMate 214 trial of nivolumab plus ipilimumab versus sunitinib for treatment-naïve, predominantly clear cell aRCC (ClinicalTrials.gov, NCT02231749). A total of 1,096 patients were randomly assigned in a 1:1 ratio to receive either unblinded nivolumab (3 mg/kg of body weight) plus ipilimumab (1 mg/kg) intravenously every 3 weeks for four doses, followed by nivolumab (3 mg/kg) every 2 weeks, or sunitinib (50 mg) orally once daily for 4 weeks of each 6-week cycle. Protocol treatment was continued until occurrence of progressive disease or unacceptable toxicity; a protocol amendment in 2017 after primary data disclosure allowed discontinuation of nivolumab plus ipilimumab after 2 years of therapy without progression or toxicity. Subsequent therapies after therapy discontinuation were noted, apart from cases of withdrawn consent. Most patients enrolled (77%; 847/1,096) had IMDC intermediate- or poor-risk disease. The minimum follow-up from treatment initiation for this analysis was 42 months (10).
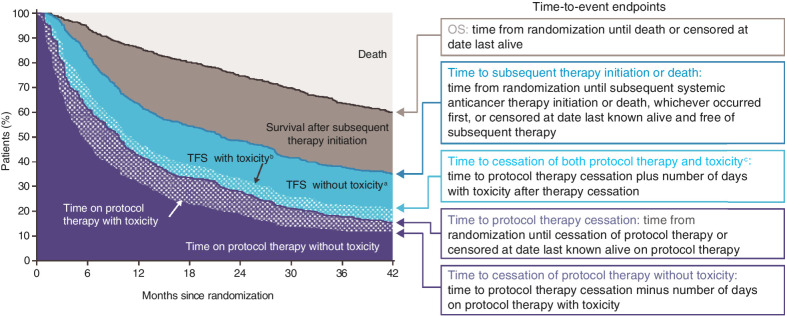
The analysis included all randomized patients (550 received nivolumab plus ipilimumab and 546 received sunitinib). We estimated the distributions of time-to-event endpoints, each defined from randomization, by the Kaplan–Meier method. The median time-to-event as a summary captures only one point of the endpoint distribution, and thus is not a suitable measure for estimating the TFS outcome of interest. Instead, for this analysis we estimated restricted mean times for endpoints, which equates to areas under the Kaplan–Meier curves restricted to follow-up through 42 months. As described previously (4), TFS was defined as the time between two time-to-event endpoints: time to protocol therapy cessation and time to subsequent therapy initiation or death. These endpoints partition survival time (the area under the OS curve) into three states (Fig. 1): time on protocol therapy, TFS, and survival after subsequent therapy initiation. Each survival state was characterized as an area between Kaplan–Meier curves, and estimated as differences between 42-month restricted mean times.
Figure 1.
Characterizing how patients spent OS time: schematic illustration defining endpoints that partition area under the OS curve into TFS and other survival states. Areas under and between Kaplan–Meier curves are restricted mean times. The probability of surviving treatment-free (i.e., in TFS) at 42 months from randomization was estimated by the difference in Kaplan–Meier estimates of 42-month event-free times of the two defining endpoints (14). aTime after cessation of protocol therapy without toxicity, before initiation of subsequent systemic anticancer therapy, or death. bTime after cessation of protocol therapy with toxicity while treatment-free. cIncludes toxicity persisting since protocol therapy and toxicity newly presenting after protocol therapy cessation.
TFS and time on protocol therapy were further partitioned into survival states with and without toxicity. We investigated two definitions of TRAE: grade ≥2 or grade ≥3, each assessed per National Cancer Institute Common Terminology Criteria for Adverse Events version 4. We examined TRAEs reported between randomization and start of subsequent therapy and counted the number of unique days with one or more TRAEs reported during the relevant period. Figure 1 and the Supplementary Material provide more detail on survival state definitions.
Between-group comparisons were based on the between-group absolute differences in 42-month restricted mean times with bootstrapped 95% confidence intervals (CI), also expressed as relative differences (nivolumab plus ipilimumab/sunitinib) in mean times.
The CheckMate 214 trial was approved by the institutional review board or ethics committee at each site and was conducted according to Good Clinical Practice guidelines, defined by the International Conference on Harmonisation. All patients provided written informed consent that was based on the Declaration of Helsinki principles.
Data availability statement
Bristol Myers Squibb's policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Results
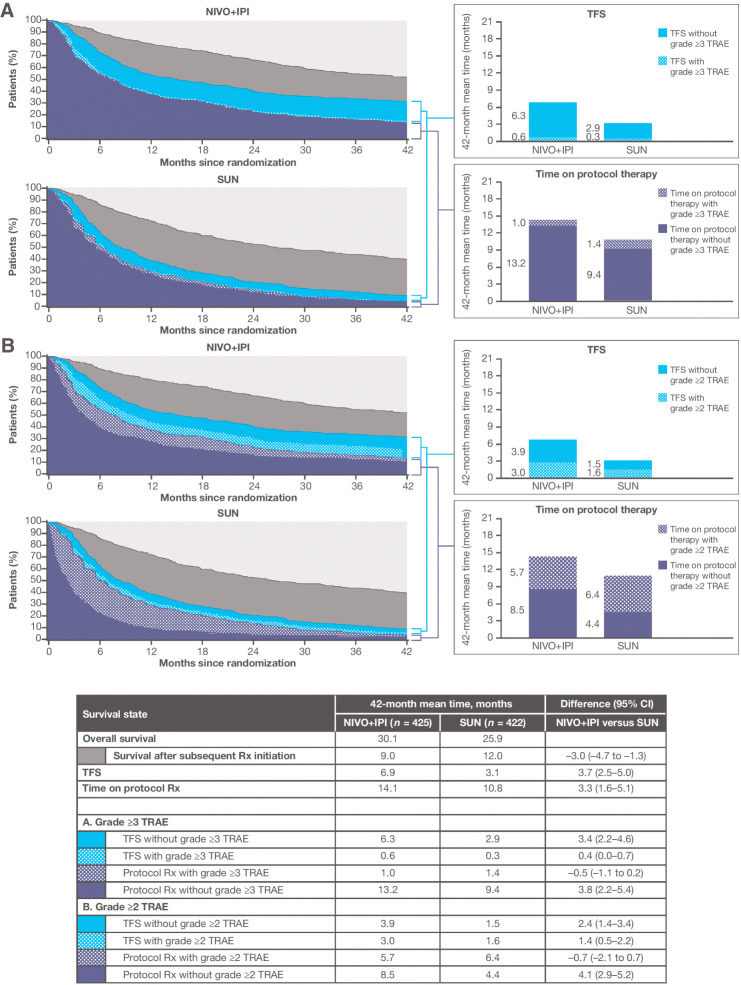
Among the 847 patients with IMDC intermediate or poor prognostic risk aRCC, the Kaplan–Meier estimates of OS at 42 months since randomization were 52% of nivolumab plus ipilimumab and 39% of sunitinib patients—31% and 9% were free of subsequent therapy, and 14% and 4.1% remained on their assigned protocol therapy, respectively (Supplementary Fig. S1). The probabilities of being treatment-free at 42 months were 18% and 4.9%, respectively (Supplementary Table S1). Supplementary Fig. S2 depicts the observed individual treatment-free intervals using graphical patient histories, with the x-axis origin reoriented as the end of protocol therapy, with patients sorted by treatment-free duration. With follow-up time restricted to 42 months since randomization, the mean OS and TFS were longer with nivolumab plus ipilimumab than with sunitinib (Fig. 2). The 42-month mean TFS was 6.9 months with nivolumab plus ipilimumab versus 3.1 months with sunitinib [difference, 3.7 months (95% CI, 2.5–5.0)]. This difference resulted from a 7.1-month longer mean time from randomization to subsequent therapy initiation or death (21.0 vs. 13.9 months; Supplementary Fig. S1), and despite longer mean time on protocol therapy.
Figure 2.
Estimates of TFS, with and without toxicity, and other survival states over the 42-month period since randomization, according to treatment group, among 847 IMDC intermediate- and poor-risk patients. Toxicity is defined alternatively by grade ≥3 TRAEs (A) and grade ≥2 TRAEs (B). NIVO+IPI, nivolumab plus ipilimumab; Rx, therapy; SUN, sunitinib.
The 42-month mean TFS without toxicity remained more than twice as long with nivolumab plus ipilimumab than with sunitinib on the basis of either definition of toxicity [TFS without grade ≥3 TRAEs: difference, 3.4 months (95% CI, 2.2–4.6); TFS without grade ≥2 TRAEs: difference, 2.4 months (95% CI, 1.4–3.4); Fig. 2]. Grade ≥2 TRAEs that persisted or were newly reported after protocol therapy cessation resulted in 42-month mean TFS with toxicity of 3.0 versus 1.6 months after nivolumab plus ipilimumab versus sunitinib therapy, respectively. The 42-month mean TFS with grade ≥3 TRAEs was <1 month for both treatment groups.
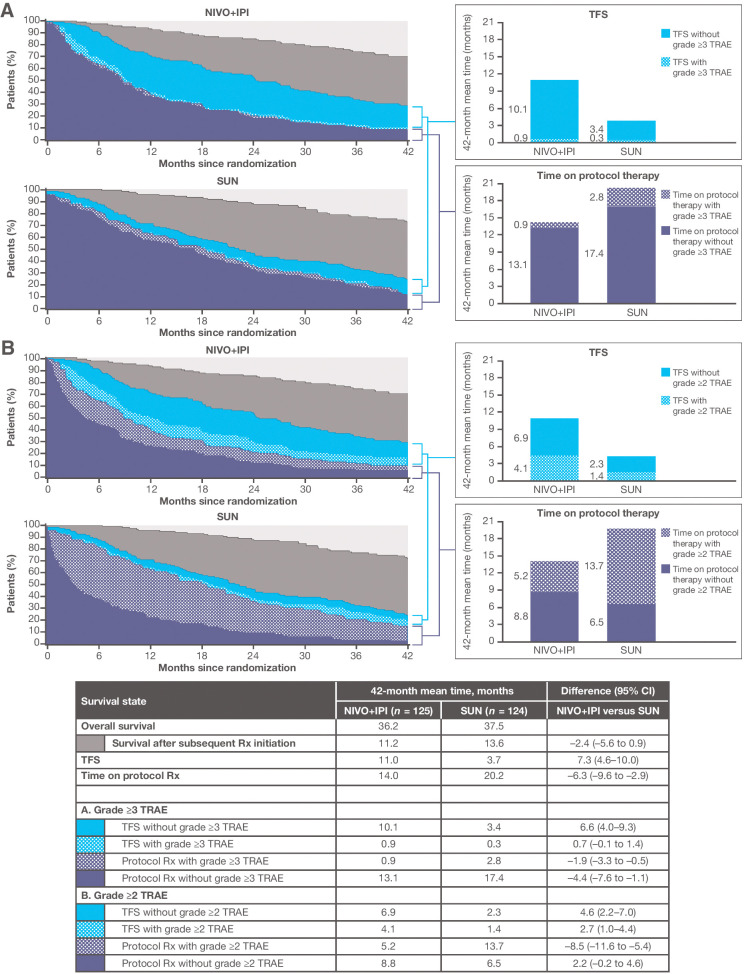
Among the 249 patients with IMDC favorable prognostic risk, 70.1% of nivolumab plus ipilimumab and 73% of sunitinib patients were alive, and 29% and 24% were free of subsequent therapy initiation, with 9.6% and 15% remaining on protocol therapy; thus, 19.5% and 8.8% were treatment-free, respectively, at 42 months since randomization (Supplementary Fig. S1). For favorable-risk patients, the pattern of how survival time was spent differed between treatment groups (Fig. 3). The 42-month mean TFS was approximately three times longer with nivolumab plus ipilimumab than with sunitinib [11.0 vs. 3.7 months; difference, 7.3 months (95% CI, 4.6–10.0)]. The difference in TFS resulted from shorter mean protocol therapy duration for the nivolumab plus ipilimumab group (14.0 vs. 20.2 months, respectively) and similar mean time from randomization to subsequent therapy initiation or death (Supplementary Fig. S1) over the 42-month period.
Figure 3.
Estimates of TFS, with and without toxicity, and other survival states over the 42-month period since randomization, according to treatment group, among IMDC favorable-risk patients. Toxicity is defined alternatively by grade ≥3 TRAEs (A) and grade ≥2 TRAEs (B). NIVO+IPI, nivolumab plus ipilimumab; Rx, therapy; SUN, sunitinib.
Although the mean TFS with grade ≥2 TRAEs was longer with nivolumab plus ipilimumab versus sunitinib (4.1 vs. 1.4 months, respectively; Fig. 3), the mean TFS without toxicity remained three times longer with nivolumab plus ipilimumab [6.9 vs. 2.3 months; difference, 4.6 months (95% CI, 2.2–7.0)]. TFS with grade ≥3 TRAEs was 0.9 month for the nivolumab plus ipilimumab group and 0.3 month for the sunitinib group on average. During nivolumab plus ipilimumab treatment, favorable-risk patients spent an average of 5.2 and 8.8 months with and without grade ≥2 TRAEs, respectively, versus mean times of 13.7 and 6.5 months during sunitinib treatment with and without grade ≥2 TRAEs. The mean times on protocol therapy with grade ≥3 TRAEs were 0.9 and 2.8 months with nivolumab plus ipilimumab versus sunitinib, respectively.
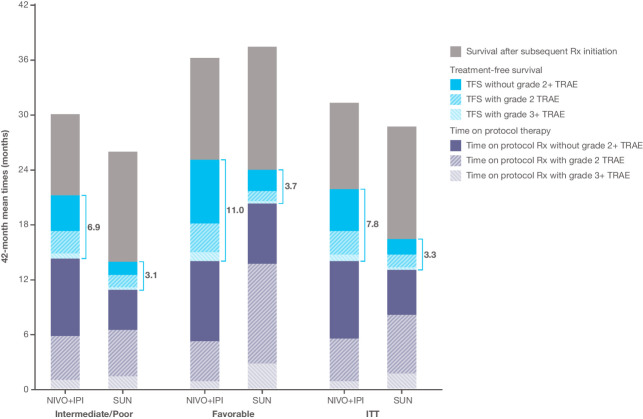
Results for the overall intent-to-treat population were similar to those of the intermediate/poor-risk population (Fig. 4). The mean TFS was 4.6 months longer (95% CI, 3.4–5.7) over the 42-month period with nivolumab plus ipilimumab versus sunitinib (7.8 vs. 3.3 months; Supplementary Fig. S3). Mean TFS without toxicity remained more than twice as long with nivolumab plus ipilimumab than with sunitinib.
Figure 4.
Summary of estimated mean TFS and survival states over the 42-month period since randomization according to treatment group, in the overall intent-to-treat population and by IMDC subgroup. The total 42-month mean TFS, with and without toxicity, is indicated by the blue bracket. The height of the bars represents the 42-month mean overall survival. NIVO+IPI, nivolumab plus ipilimumab; Rx, therapy; SUN, sunitinib.
Discussion
The novel TFS outcome complements OS, progression-free survival, and other outcomes that might impact clinical decision-making with an integrated summary of how OS time is spent. The TFS analysis describes the experience of an entire clinical trial population from the point of treatment initiation. This approach can aid decision-making prior to any knowledge about an individual patient's tolerance and tumor responsiveness to therapy. It is in direct contrast to analyses focusing on selected patient subgroups that emerge after therapy has been initiated, such as responders. Such analyses may inform later decision-making, for example about continuation or discontinuation of therapy if a patient experiences complete response (15).
We observed clinically meaningful TFS among patients with aRCC receiving first-line therapy with nivolumab plus ipilimumab in CheckMate 214, regardless of IMDC prognostic risk. In patients with IMDC intermediate or poor prognostic risk, nivolumab plus ipilimumab provided significantly longer survival than sunitinib (10). The overall 42-month mean TFS was more than doubled after nivolumab plus ipilimumab versus sunitinib, despite longer mean protocol treatment duration, because of delayed time to initiating subsequent therapy for nivolumab plus ipilimumab versus sunitinib. This increase in TFS was accomplished without increasing toxicity. The mean protocol treatment time with grade ≥2 TRAEs as a proportion of total therapy duration was less with nivolumab plus ipilimumab than with sunitinib (40% vs. 59%).
Nivolumab plus ipilimumab is approved by the FDA for treatment of intermediate/poor-risk patients; the trial was not designed to demonstrate improvements in outcomes in the favorable-risk patients (9, 16). While OS was similar in patients with IMDC favorable prognostic risk who were treated with nivolumab plus ipilimumab versus sunitinib, the patterns of how patients spent OS were notably different. At 42 months, 20% and 9% of nivolumab plus ipilimumab and sunitinib-treated patients were treatment-free, respectively. Over the 42 months, sunitinib patients spent more time on protocol therapy, and on average, approximately two thirds of that time with grade ≥2 TRAEs (14% of that time with grade ≥3 TRAEs). In contrast, nivolumab plus ipilimumab patients spent more time treatment-free, and approximately two thirds of that TFS time without grade ≥2 TRAEs. In this patient subgroup, by definition with minimal symptoms of their cancer, the tradeoffs of time on therapy and AEs of the therapy as well as their HRQoL implications are key features of decision-making when initiating first-line therapy (17, 18). The HRQoL of patients in CheckMate 214 was better throughout treatment with nivolumab plus ipilimumab versus sunitinib, including domains of disease-related symptoms, treatment side effects, and functional well-being (12, 13). After discontinuation of protocol therapy in CheckMate 214, collection of HRQoL data was limited. Future trials should continue HRQoL assessments that are sensitive to symptoms and side effects to fully assess HRQoL after therapy discontinuation.
Whereas in advanced melanoma we investigated TFS in three ICI regimens of nivolumab and ipilimumab in combination or as monotherapies, our analysis of TFS in patients with aRCC from the CheckMate 214 trial compared an ICI combination with a targeted therapy. Most patients treated with a VEGF-receptor tyrosine kinase inhibitor (TKI) continue therapy until progression, and durable response off-treatment is rare (7, 19, 20). This analysis of CheckMate 214 provides the first insight into interpreting TFS involving a non-ICI control group, which is valuable in the context of TFS comparisons in future trials involving IO and non-IO regimens. Combinations of an ICI with VEGF-targeted therapy have been approved regardless of IMDC risk (21–25), and have been similarly prescribed until progressive disease or unacceptable toxicity. The comprehensive characterization of how OS time was spent with ICI/VEGF inhibitor regimens may provide insight into whether these combinations can achieve similar TFS results, or whether persistent VEGF suppression is required to achieve durable disease control.
The estimated mean TFS of approximately 3 months in the sunitinib group may be longer than anticipated for patients' transitions from first- to second-line therapy, indicating the possible presence of rare patients with disease control after stopping VEGF-targeted therapy, or regional therapy such as surgery or radiation required before initiating subsequent systemic therapy. In addition, TFS represents the interval between discontinuation of first-line therapy and death for patients who may have been receiving supportive care but never received second-line systemic anticancer therapy, whether because it was not prescribed or not available. Therefore, a complete collection of data on the initiation of any subsequent systemic anticancer therapy is critical in future trials to most accurately assess TFS.
A TFS analysis can also be employed to capture how changing the dose and schedule of established regimens may have differential impact. The nivolumab plus ipilimumab induction dosing regimen used in CheckMate 214 differed from that for patients with advanced melanoma initiating nivolumab plus ipilimumab in the CheckMate 067 and CheckMate 069 trials (4). Both regimens were designed to be given until progressive disease or unacceptable toxicity, and in both protocols, patients who required discontinuation of nivolumab plus ipilimumab induction treatment could not continue to nivolumab maintenance therapy. The trial protocols did not initially anticipate stopping therapy for clinical benefit, so the time on protocol therapy was linked to treatment toxicity or efficacy and in turn to the start of the TFS period. TRAE-related discontinuation of the aRCC nivolumab plus ipilimumab regimen was about half as frequent as for the melanoma regimen previously analyzed (5, 6, 10). In advanced melanoma, nearly 40% of patients discontinued nivolumab plus ipilimumab for TRAEs (5, 6), and mean TFS represented approximately one third of the 36-month period since initiation of therapy (4). The aRCC regimen was better tolerated, likely because of a lower ipilimumab dose, with 22% of patients discontinuing for TRAEs (10), and mean TFS was approximately 20% of the 42-month period since treatment initiation overall.
It is possible that future gains in TFS can be achieved by limiting treatment duration of ICI-based combination therapy. Historically, high-dose IL2 was administered during a finite period and could achieve durable response in a subset of patients with aRCC (26, 27). The CheckMate 214 results must be interpreted in the context of the design to continue treatment until disease progression, and the protocol-defined specifications for dose modification and treatment discontinuation for each treatment group. Approximately half and two thirds of patients in the nivolumab plus ipilimumab and sunitinib groups, respectively, discontinued treatment for disease progression, as did one third and one fifth for TRAEs or non-TRAEs, respectively (10). Dose reductions were not permitted for nivolumab plus ipilimumab, while sunitinib dose reductions were allowed (9). Discontinuation of nivolumab plus ipilimumab after 2 years was infrequent, as it was allowed only after protocol amendment (10), and treatment holidays were not allowed in either treatment group. It is conceivable that many, if not all, patients who were continuing to respond to nivolumab plus ipilimumab could have had their treatment stopped at 2 years or even earlier, perhaps adding considerable months to the TFS state. Combination therapy regimens aim to achieve a durable response; doing so with a shorter duration of therapy could be considered an objective unique to ICI therapy, as it would not only increase TFS but also potentially decrease the financial cost of treatment (28).
Differences in ICI and VEGF receptor TKI TRAE types, incidence, and duration are well documented (7, 9–11, 17). The analysis incorporated the number of days with TRAEs during protocol therapy, as in the original Q-TWiST analysis (29, 30), and any TRAEs that persisted after cessation or were newly reported during TFS before initiation of second-line therapy. To estimate TFS accurately with and without toxicity, TRAEs (or more generally, events used to define toxicity days) should be followed until resolution and collected between protocol therapy cessation and initiation of subsequent systemic therapy, or death.
The fuller characterization of toxicity in the integrated analysis was also motivated by the inclusion of patients with varied prognoses in CheckMate 214. At 42 months since initiation of nivolumab plus ipilimumab, 52% and 70% of patients in the intermediate/poor-risk and favorable-risk cohorts, per IMDC prognostic risk criteria, were alive, respectively (8, 10). It remains to be explored whether the IMDC criteria, which were developed in context of VEGF-targeted therapy, should be refined since the introduction of ICIs (31). Nevertheless, IMDC risk groups provide a contrast of estimating TFS and protocol therapy time with and without toxicity in settings with different clinical decision-making (17).
In addition to longer OS demonstrated in intermediate/poor-risk patients and the overall population of patients with aRCC initiating first-line nivolumab plus ipilimumab (10), we documented longer TFS with nivolumab plus ipilimumab versus sunitinib in all IMDC risk groups. Even accounting for the possibility that toxicity of ICIs persisted or arose after ICI discontinuation, nivolumab plus ipilimumab provided longer TFS without toxicity. Given the durable response and survival with ICI–ICI combination relative to VEGF receptor TKI targeted therapy, this integrated analysis of CheckMate 214 demonstrated the value of describing the quality of survival time as a part of comparing their value for both intermediate/poor-risk and favorable-risk populations, and provided additional insight for individual decision-making about initiation of first-line therapy for aRCC.
Authors' Disclosures
M.M. Regan reports grants from Bristol Myers Squibb (BMS) and personal fees from BMS during the conduct of the study. M.M. Regan also reports grants from Novartis, Pfizer, Ipsen, TerSera, Merck, Ferring, Pierre Fabre, Roche, AstraZeneca, Bayer, and BMS; other support from Ipsen/Debiopharm; and personal fees from Tolmar outside the submitted work. C.M. Mantia reports non-financial support and other support from BMS during the conduct of the study. T. Powles reports personal fees from AstraZeneca, BMS, Exelixis, Incyte, Ipsen, Merck, MSD, Novartis, Pfizer, Seattle Genetics, Merck Serono, Astellas, Johnson & Johnson, Eisai, and Roche; grants from AstraZeneca, Roche, BMS, Exelixis, Ipsen, Merck, MSD, Novartis, Pfizer, Seattle Genetics, Merck Serono, Astellas, Johnson & Johnson, and Eisai; and other support from Roche, Pfizer, MSD, AstraZeneca, and Ipsen outside the submitted work. R.J. Motzer reports grants from BMS during the conduct of the study; R.J. Motzer also reports grants and personal fees from Pfizer, Novartis, Eisai, Exelixis, Merck, and Genentech/Roche, as well as grants from Lilly, Incyte, EMD Serono Research and Development Institute, and Aveo outside the submitted work. N.M. Tannir reports grants from BMS during the conduct of the study, as well as personal fees from BMS, Pfizer, Nektar, Exelixis, Eisai Medical Research, Eli Lilly, Oncorena, Calithera Biosciences, Novartis, Ipsen, and Merck Sharp & Dohme outside the submitted work. C.-H. Lee reports grants and personal fees from BMS, Exelixis, Eisai, Merck, Pfizer; personal fees from Amgen and EMD Serono; and grants from Calithera and Eli Lilly outside the submitted work. Y. Tomita reports personal fees from BMS; grants from Pfizer; and grants and personal fees from Ono Pharmaceutical during the conduct of the study. Y. Tomita also reports grants from Chugai, Eisai, and Taiho, as well as grants and personal fees from Astellas and Takeda outside the submitted work. M.H. Voss reports grants and personal fees from Pfizer and Roche Genentech, as well as personal fees from Corvus, Exelixis, Eisai, Calithera, Aveo, Chengdu, and Novartis outside the submitted work. E.R. Plimack reports personal fees from Aveo, Merck, BMS, Calithera, Exelixis, MEI, Novartis, and Pfizer, as well as grants from Aveo, Merck, BMS, Exelixis, Pfizer, and Peloton during the conduct of the study; E.R. Plimack also reports personal fees from AstraZeneca, Eli Lilly, Infinity Pharma, Synergene, Pfizer, Clovis, Flatiron, Genentech, Horizon Pharma, Incyte, Inovio, Janssen, Merck, Roche, and Seattle Genetics outside the submitted work. T.K. Choueiri reports grants, personal fees, non-financial support, and other support from Merck, BMS, Pfizer, EMD Serono, and Roche during the conduct of the study, as well as grants, personal fees, non-financial support, and other support from Roche, Pfizer, BMS, Merck, and EMD Serono outside the submitted work. In addition, T.K. Choueiri has a patent for biomarkers of activity/toxicity from IO pending, is speaker and on planning committees for ASCO/ESMO planning committees, and is involved in NCCN guidelines and NCI GU steering Committee. B.I. Rini reports grants, personal fees, and non-financial support from BMS during the conduct of the study; B.I. Rini also reports grants, personal fees, and non-financial support from Merck and Pfizer, as well as personal fees from Exelixis outside the submitted work. In addition, B.I. Rini reports institutional research funding from Pfizer, F. Hoffman-La Roche, Incyte, AstraZeneca, Taris, Seattle Genetics, Arrowhead Pharmaceuticals, Immunomedics, BMS, Mirati Therapeutics, Merck, Surface Oncology, Dragonfly Therapeutics, Aravive, and Exelixis; consulting from BMS, Pfizer, GNE/Roche, Aveo, Synthorx, Compugen, Merck, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, GSK, Shionogi, Eisai, Nikang Therapeutics; and stock from PTC Therapeutics. H.J. Hammers reports personal fees from BMS outside the submitted work. B. Escudier reports grants and personal fees from BMS during the conduct of the study; B. Escudier also reports grants and personal fees from Ipsen, as well as personal fees from Pfizer and Oncorena outside the submitted work. L. Albiges reports other support from Pfizer, Novartis, BMS, Ipsen, Astellas, MSD, Merck & Co., Janssen, AstraZeneca, and Eisai outside the submitted work. S. Huo is an employee of BMS. V. Del Tejo is an employee of BMS. B. Stwalley reports other support from BMS during the conduct of the study. M.B. Atkins reports grants and personal fees from BMS and Merck, as well as personal fees from Novartis, Genentech/Roche, Pfizer, Eisai, Exelixis, AstraZeneca, Agenus, Adagene, Aveo, Fathom, Pyxis Oncology, Lead BioPharma, Werewolf, Elpis, Asher Bio, PACT, Iovance, Idera, Apexigen, Arrowhead, Neoleukin, Immunocore, Surface, ScholarRock, Calithera, SeaGen, Sanofi, and Takeda outside the submitted work; M.B. Atkins also reports stock options from Werewolf and Pyxis Oncology. D.F. McDermott reports personal fees from BMS, Merck, Genentech, Pfizer, and Exelixis during the conduct of the study, as well as personal fees from Janssen, Clinigen, Alkermes, and Iovance outside the submitted work. No disclosures were reported by the other authors.
Supplementary Material
Acknowledgments
The CheckMate 214 trial is supported by Bristol Myers Squibb and Ono Pharmaceutical Company Ltd. This research was funded by a grant from Bristol Myers Squibb (to M.M. Regan, D.F. McDermott) and funded in part by NIH/NCI Cancer Center Support Grant P30 CA006516 (to M.M. Regan, O.A. Jegede, C.M. Mantia, T.K. Choueiri, D.F. McDermott) and NIH/NCI SPORE Grant P50 CA101942 (to D.F. McDermott, O.A. Jegede, T.K. Choueiri, M.B. Atkins). Authors received no financial support or compensation for publication of this manuscript. Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant (Core Grant, number P30 CA008748). The University of Texas MD Anderson Cancer Center is supported by the NIH (grant P30 CA016672). The Georgetown-Lombardi Comprehensive Cancer Center is supported by the NIH/NCI Cancer Center Support Grant P30 CA051008. We thank the patients and their families for making this study possible, the clinical study teams who participated in the study, and Bristol Myers Squibb and Ono Pharmaceutical Company Ltd. Professional medical writing and editorial assistance were provided by Nicolette Belletier, PhD, of Parexel, funded by Bristol Myers Squibb.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Contributions
M.M. Regan: Conceptualization, formal analysis, validation, methodology, writing–original draft, writing–review and editing. O.A. Jegede: Formal analysis, methodology, writing–review and editing. C.M. Mantia: Conceptualization, formal analysis, writing–original draft, writing–review and editing. T. Powles: Writing–review and editing. L. Werner: Formal analysis, writing–review and editing, methodology. R.J. Motzer: Writing–review and editing. N.M. Tannir: Writing–review and editing. C.-H. Lee: Writing–review and editing. Y. Tomita: Writing–review and editing. M.H. Voss: Writing–review and editing. E.R. Plimack: Writing–review and editing. T.K. Choueiri: Writing–review and editing. B.I. Rini: Writing–review and editing. H.J. Hammers: Writing–review and editing. B. Escudier: Writing–review and editing. L. Albiges: Writing–review and editing. S. Huo: Conceptualization, data curation, funding acquisition, writing–review and editing. V. Del Tejo: Conceptualization, funding acquisition, writing–review and editing. B. Stwalley: Conceptualization, funding acquisition, writing–review and editing. M.B. Atkins: Conceptualization, formal analysis, writing–original draft, writing–review and editing. D.F. McDermott: Conceptualization, formal analysis, writing–original draft, writing–review and editing.
References
- 1. Puzanov I, Diab A, Abdallah K, Bingham CO III, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017;376:354–66. [DOI] [PubMed] [Google Scholar]
- 3. Ravi P, Bakouny Z, Schmidt A, Choueiri TK. Novel therapeutic approaches and the evolution of drug development in advanced kidney cancer. Cancer J 2020;26:464–70. [DOI] [PubMed] [Google Scholar]
- 4. Regan MM, Werner L, Rao S, Gupte-Singh K, Hodi FS, Kirkwood JM, et al. Treatment-free survival: a novel outcome measure of the effects of immune checkpoint inhibition-a pooled analysis of patients with advanced melanoma. J Clin Oncol 2019;37:3350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;17:1558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mantia CM, McDermott DF. Vascular endothelial growth factor and programmed death-1 pathway inhibitors in renal cell carcinoma. Cancer 2019;125:4148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heng DYC, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–9. [DOI] [PubMed] [Google Scholar]
- 9. Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Motzer RJ, Escudier B, McDermott DF, Aren Frontera O, Melichar B, Powles T, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer 2020;8:e000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Motzer RJ, Rini BI, McDermott DF, Aren Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019;20:1370–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cella D, Escudier B, Saggi SS, Blum S, Ejzykowicz F, Ivanescu C. Time to deterioration in quality of life in previously untreated patients with advanced renal cell carcinoma (aRCC) in CheckMate 214. Ann Oncol 2020;31:S562. Abstract 714P. [Google Scholar]
- 13. Cella D, Grünwald V, Escudier B, Hammers HJ, George S, Nathan P, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol 2019;20:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang B, Tian L, McCaw ZR, Luo X, Talukder E, Rothenberg M, et al. Analysis of response data for assessing treatment effects in comparative clinical studies. Ann Intern Med 2020;173:368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthelemy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020;5:e001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Opdivo (nivolumab) [package insert]. Bristol Myers Squibb, Princeton, NJ: 2021. [Google Scholar]
- 17. Rini BI, Battle D, Figlin RA, George DJ, Hammers H, Hutson T, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J Immunother Cancer 2019;7:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong MK, Mohamed AF, Hauber AB, Yang JC, Liu Z, Rogerio J, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ 2012;15:1139–48. [DOI] [PubMed] [Google Scholar]
- 19. Kalra S, Rini BI, Jonasch E. Alternate sunitinib schedules in patients with metastatic renal cell carcinoma. Ann Oncol 2015;26:1300–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Powles T, Kayani I, Sharpe K, Lim L, Peters J, Stewart GD, et al. A prospective evaluation of VEGF-targeted treatment cessation in metastatic clear cell renal cancer. Ann Oncol 2013;24:2098–103. [DOI] [PubMed] [Google Scholar]
- 21. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116–27. [DOI] [PubMed] [Google Scholar]
- 22. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bavencio (avelumab) [package insert]. EMD Serono, New York, NY; 2020. [Google Scholar]
- 24. Keytruda (pembrolizumab) [package insert]. Merck, Kenilworth, NJ; 2020. [Google Scholar]
- 25. Choueiri TK, Powles T, Burotto M, Bourlon MT, Zurawski B, Juárez VMO, et al. Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. Ann Oncol 2020;31:S1159. Abstract 696O_PR. [Google Scholar]
- 26. McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 2005;23:133–41. [DOI] [PubMed] [Google Scholar]
- 27. Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289–96. [DOI] [PubMed] [Google Scholar]
- 28. Ambavane A, Yang S, Atkins MB, Rao S, Shah A, Regan MM, et al. Clinical and economic outcomes of treatment sequences for intermediate- to poor-risk advanced renal cell carcinoma. Immunotherapy 2020;12:37–51. [DOI] [PubMed] [Google Scholar]
- 29. Gelber RD, Goldhirsch A, Cole BF. Evaluation of effectiveness: Q-TWiST. the international breast cancer study group. Cancer Treat Rev 1993;19(suppl A):73–84. [DOI] [PubMed] [Google Scholar]
- 30. Goldhirsch A, Gelber RD, Simes RJ, Glasziou P, Coates AS. Costs and benefits of adjuvant therapy in breast cancer: a quality-adjusted survival analysis. J Clin Oncol 1989;7:36–44. [DOI] [PubMed] [Google Scholar]
- 31. Motzer RJ, Banchereau R, Hamidi H, Powles T, McDermott D, Atkins MB, et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell 2020;38:803–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Bristol Myers Squibb's policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.