Abstract
Patients with SARS-CoV-2 infection, exhibit various clinical manifestations and severity including respiratory and enteric involvements. One of the main reasons for death among covid-19 patients is excessive immune responses directed toward cytokine storm with a low chance of recovery. Since the balanced gut microbiota could prepare health benefits by protecting against pathogens and regulating immune homeostasis, dysbiosis or disruption of gut microbiota could promote severe complications including autoimmune disorders; we surveyed the association between the imbalanced gut bacteria and the development of cytokine storm among COVID-19 patients, also the impact of probiotics and bacteriophages on the gut bacteria community to alleviate cytokine storm in COVID-19 patients. In present review, we will scrutinize the mechanism of immunological signaling pathways which may trigger a cytokine storm in SARS-CoV2 infections. Moreover, we are explaining in detail the possible immunological signaling pathway-directing by the gut bacterial community. Consequently, the specific manipulation of gut bacteria by using probiotics and bacteriophages for alleviation of the cytokine storm will be investigated. The tripartite mutualistic cooperation of gut bacteria, probiotics, and phages as a candidate prophylactic or therapeutic approach in SARS-CoV-2 cytokine storm episodes will be discussed at last.
Keywords: SARS-CoV2, Signaling pathways, Cytokine storm, Gut bacteria, Bacteriophage, Probiotics
Abbreviations
- ACE2
angiotensin-converting enzyme-2
- SARS-CS
SARS-CoV-2 cytokine storm
- PRRs
pattern recognition receptors
- TLRs
Toll-like receptors
- RLRs
RIGI-like receptors
- MDA-5
melanoma differentiation-associated protein 5
- NETs
neutrophil traps
- ROS
reactive oxygen species
- ARDS
acute response syndrome
- TGF- β:
transforming growth factor-β
- MQs
Macrophages
- DCs
dendritic cells
- NK
natural killer
- APCs
antigen presenting cells
- GM-CSF
granulocyte-macrophage colony stimulating factor
- TNF- α:
tumor necrosis factor
- NO
nitric oxide
- SCFAs
Short-chain fatty acids
- AHR
aryl hydrocarbon receptor
- PSA
Polysaccharide A
- HDACs
histone deacetylases
- FOXP3
forkhead box P3
1. Introduction
The emergence of SARS-CoV-2 as a global pandemic brings up the Coronaviridae alongside the Orthomyxoviridae as two virus families with the ability to threaten the lives of human beings around the world. Although the estimated patient fatality rate due to COVID-19 has been lower than the Spanish influenza pandemic, SARS-CoV-2 has by far the highest socio-economic impact than other infectious agents that humans have ever encountered to date. There have been almost over than 555 million confirmed cases of SARS-CoV-2 infection, including more than 6.3 million deaths till July 11, 2022, as reported by WHO.
SARS-CoV-2 infects target cells via the ACE2 receptor, a transmembrane protein found in a variety of human tissues such as the small intestine, testis, heart, and lung [1]. In a SARS-CoV-2 infected person, the virus exacerbates mainly respiratory involvement in the form of viral pneumonia. The status of Immune response against viral components depends on various factors including age, sex, and underlying diseases [2,3]. The severity of COVID-19 disease is likely due to not only viral replication but also the status of the host immune responses. Meanwhile, one of the main causes of death in COVID-19 patients is due to aggressive inflammatory responses by hyper-production of an array of pro-inflammatory cytokines that are intensely associated with lung damage and multiorgan dysfunction with a low chance of recovery. The clinical condition is referred as to SARS-CoV-2 cytokine storm (SARS-CS) [4,5]. In the lack of sufficient therapeutical interventions, the SARS-CS comes to be a life-threatening situation with a series of serious clinical manifestations. The exact mechanisms of triggering cytokine storm scenarios among vulnerable high-risk groups are not yet well understood. Nevertheless, dysregulation of immune homeostasis plays an important role in the development of COVID-19 severity among high-risk groups [6,7]. The gut microbiota provides essential health benefits by regulating immune homeostasis. There is growing evidence, which has illustrated dysbiosis or disruption of gut microbiota could promote severe complications including asthma, cardiovascular disease, and autoimmune disorders [8,9]. Moreover, several layers of investigations have been conducted to assess the impact of gut microbiota dysbiosis (genetics, underlying disease, diet, antibiotics consumption, etc.), which results in cytokine storm in COVID-19 patients [10,11]. Furthermore, recent studies have demonstrated that COVID-19 patients have represented gut microbiota dysbiosis, which also could result in cytokine storm [12,13]. Unrevealing the cross-talk between gut bacteria and immune response could provide efficient strategies to intervene or prevent cytokine storm in SARS-CoV-2 patients. Although some aspects of this topic have been partly reviewed previously, a comprehensive view of SARS-CS to facilitate its treatment is still lacking with unmet clinical needs [14,15]. Herein, we provide an updated scenario of immunological signaling pathways of SARS-CoV-2 infection and SARS-CS. Initially, we are explaining the currently identified immunological signaling pathway features directed by the gut bacterial community, and also cross-talk between gut bacteria and SARS-CoV-2. Consequently, the feasibility of specific manipulation of imbalanced gut bacteria by using the probiotics and bacteriophages through their mechanism of action for alleviation of drastic inflammatory responses resulting in cytokine storm will be investigated. Overall, we aimed to suggest the tripartite mutualistic cooperation of gut bacteria, phages, and probiotics as a candidate prophylactic or therapeutic approach in SARS-CS episodes.
2. Immunopathology of SARS-CoV-2
2.1. Innate immune response
The first line of innate immune defense against viral infections includes a set of PRRs, including TLRs and RLRs that recognize the viral RNA genome and its replication intermediaries. Upon recognition, a variety of mediators and cell types can be induced by TLR pathways. This activates of inflammatory signaling pathways, results in a cytokine storm and widespreads damage to the host inducing the stimulation of antiviral interferon response. TLR9/MyD88 leads to the production of type I interferons (IFN-α and IFN-β) and pro-inflammatory cytokines (IL-6, IL-8, IL-10, IL-17, and TNF-α), through SARS-CoV-2 infection [16,17]. TLR3, TLR7/8, and RIG-I/MDA-5 tpassasse through ACE2, detect PAMPSs, and trigger downstream signaling by binding the adaptor proteins MyD88 and MAVS, which cause the transcription factors IRF3/7 and NF-κB to become activated, resulting in the production of IFN-I and pro-inflammatory cytokines. Also, the NF-κB pathway can be activated by TLR2, which detects Spike (S) protein and causes the production of inflammatory cytokines and chemokines [[18], [19], [20]]. Active viral replication leads to activation of neutrophils and monocytes/macrophages (MQ), which results in the overproduction of pro-inflammatory cytokines. Neutrophils are an early representative of SARS-CoV-2 infection, and the development of extracellular NETs is one of the processes by which neutrophils clear the infection. NETs may contribute to cytokine release and progression to respiratory failure. Activated neutrophils also release leukotrienes and ROS, thereby inducing cytotoxicity such as a cytokine storm [21,22]. In addition, activated neutrophils appropriately express properdin, factors B and C3, thereby promoting complement activation, a hallmark of severe COVID-19 disease [21]. Complement is a key player in the innate immune system's antiviral response, but its excessive activation can lead to pro-inflammatory responses and tissue damage. A recent study on SARS-CoV-2 showed that activation of the complement component C3 exacerbates disease in SARS-CoV-2 associated with cytokine storm and ARDS [23].
COVID-19 manifests with MQ infiltration into lung tissue, with epithelial cell and lung cell apoptosis, NF-κB pathway induction, and cytokine production. This inflammatory response is required to initiate immune responses against SARS-CoV-2 and excessive inflammation in the form of a cytokine storm contributes to COVID-19-related mortality such as TGF-β that promotes fibroblast proliferation and contributes to pulmonary fibrosis in COVID-19 patients [24,25]. In addition, cytokines such as IL-12 secreted by MQs and DCs can promote NK cell proliferation, cytotoxicityand immunostimulatory cytokine secretion such as; IFN-γ. NK cell stimulation may play a role in limiting SARS-CoV-2 infection [26]. SARS-CoV-2 also enhances the production of pro-inflammatory cytokines by DCs in response to signals activated by bacterial LPS via TLR-4, which further contributes to the induction of a destructive inflammatory response. The upregulation of IL-6 is an established effect of TLR-4 signaling, which occurs through the NF-κB signaling pathways [27,28]. In addition, innate APCs, such as DCs and MQs, provide an important bridge connecting innate and adaptive immune responses and present viral antigens to virus-specific T cells at the site of infection. This triggers the host's adaptive immunity, which is mediated by virus-specific B (humoral immunity) and T (cellular immunity) cells [29,30]. Although DC infection appears to play an important role in driving the cytokine storm and in regulating the T-cell response to SARS-CoV-2, the exact mechanisms used by the virus to change DC function demand more investigation [31,32].
2.2. Adaptive immune response
Once the innate immune system has been activated, the adaptive immune system runs in. The adaptive immune system comprises three main cell types: CD4 + T helper cells (TH), CD8 + Cytotoxic T cells (CTL), and B cells. Normally, during infection, activated Th1 effector T cells produce pro-inflammatory cytokines (IFN-γ, IL-2, and GM-CSF) and antiviral cytokines, such as granzyme B, TNF-α, TNF-β, and TNF-κ to stimulate responses of T cells. In addition, GM-CSF activates the CD14 + inflammatory monocytes to produce large amounts of IL-6, TNF-α, and other cytokines. This is followed by an infiltration of macrophages and neutrophils for pulmonary tissues, resulting in a cytokine storm [33]. It has been reported that significant increases in typical Th2 cytokines, including IL-4, IL-5, IL-10, and IL-13. Both IL-4 and IL-13 are primarily involved in inflammatory fibroblast remodeling, while Th1 cells exert anti-fibroblast activity by secreting IFN-γ and IL-2 [34].
Circulating CD8 + T cells specific for SARS-CoV-2 were observed less consistently than CD4+ T cells. CD8+ T cells are important in clearing many viral infections because of their ability to target the infected cells. During acute COVID-19, SARS-CoV2 specific CD8 + T cells express high levels of molecules involved in potent cytotoxic function, such as IFN-γ, granzyme B, and perforin, [35]. A decrease in peripheral blood T cells associated with disease severity and inflammation is now well documented in COVID-19. The number of T cells was inversely correlated with IL-6, IL-10, and TNF-α levels. High concentrations of TNF-α, IL-6, and IL-10 in cytokine storm induce negative regulatory effects on T cell survival and proliferation [[36], [37], [38]].
CD4+ T cells drives B lymphocytes to produce virus-specific antibodies such as IgG and IgM. In addition to producing virus-specific antibodies, activated B cells also secrete IL-1, IL-6, IL-8, TNF-α, GMCSF, and other cytokines, which can intensify the cytokine storm. As a result, viral replication will not be inhibited and viral infection will spread throughout the body, leading to further imbalance in the immune response and the release of many pro-inflammatory cytokines by non-specific immune cells [33,39]. In addition, a Th17 response has been detected and confirmed in patients with COVID-19. There is increasing evidence that Th17 cells that produce the inflammatory cytokine IL-17 recruit more monocytes/MQs and neutrophils to the site of infection and stimulate other cytokine cascades, such as IL-1β and IL-6, among others [40]. Elevated levels of IL-17 have been reported in COVID-19 patients as part of a cytokine storm, and are associated with viral load and disease severity [37]. In addition, the number of Treg cells, which regulate the induction and proliferation of effector T cells, was significantly lower in COVID-19 patients and this manifests as a lack of functional immunosuppression. Treg populations suppress innate and adaptive activation of immune cells by multiple mechanisms and secretion of immunosuppressive cytokines (IL-10, TGF-β, and IL-35) [41]. The loss of peripheral Tregs in COVID-19 patients may maintain a delicate balance between regulatory arms and immune system effects, leading to significant growth and activation of neutrophils, MQ, DCs, mast cells, and Th17 cells [42]. Locally and during SARS-CoV-2 infection, uncontrolled innate inflammatory responses and disturbances in Treg/Th17 balance can promote tissue damage [43]. Soluble substances, especially NO and TGF-β decrease the proliferation and activation of Th1 and Th17 cell lines and decrease IFN-γ and IL-17 production. They also reduce direct damage to lung parenchyma by inhibiting the activation of cytotoxic CD8+ cells. TGF-β would be able to increase the release of IL-4 and IL-6, as well as IL-13 by alveolar MQ, and accelerate fibrosis. On the other hand, inhibition of TGF-β is thought to decrease the inflow of neutrophils, MQs, and lymphocytes to the site of injury. TGF-β promotes redox imbalance by increasing ROS levels and decreasing antioxidant enzymes on the one hand. ROS also promotes the fibrinogenic effects of TGF-β by inducing it [44,45]. Besides aforementioned immune modulators, some investigations have mentioned the key role of the balanced gut microbiota in providing immune homeostasis [14,46,47]. The Immunopathology pathway of SARS-CoV-2 with host cells has been illustrated in Fig. 1 .
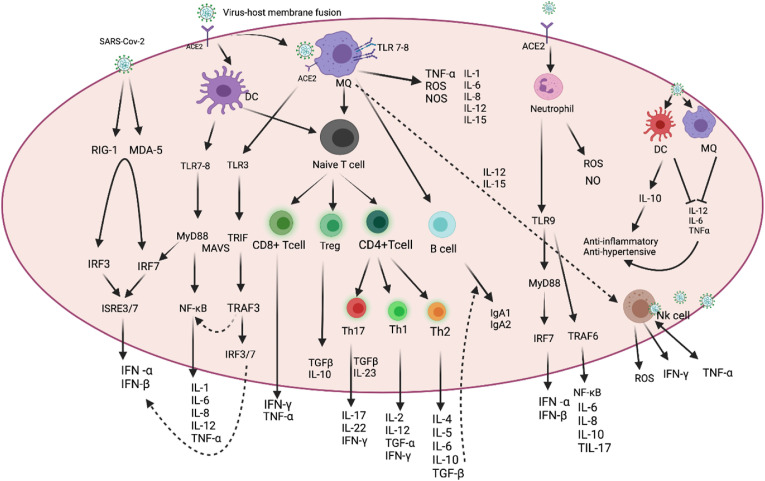
Fig. 1.
Immune response to SARS-CoV-2. SARS-CoV-2 is first recognized by the ACE2 receptor that present on respiratory epithelial cells and allows virus entry. Recognition of SARS-CoV-2 by PRRs stimulates the production of IFN-I and proinflammatory cytokines (e.g., IL-6 and TNF-α), respectively. NK cells, MQs, and APCs are in lung tissue to produce inflammatory factors. TNF-α, IL-1β, IL-6, and IL-8 are considered the main components of cytokine-storm. CD4 + T and CD8 + T cells play an important role in this defense. The cells produce pro-inflammatory cytokines to help the defending cells. In addition, CD4 + T cells stimulate B cells to produce virus-specific antibodies even as CD8 + T cells can directly target the virus-infected cells. TNF-α would be produced by NK cells, MQs, and activated CD4 and CD8 T cells. T cells also secrete IFN-γ. Also, Treg and type II macrophages could secret IL-10 and TGF-β, which then reduce the inflammatory response. (Figure has been created in biorender).
3. Gut bacteria in human health and SARS-CoV-2 cytokine storm
Commensal bacteria regulate innate and adaptive immune responses and affect the activation threshold for pathogenic stimulations, in massive part by producing small molecules that mediate host-microbial relations, such as SCFAs, AHR, PSA, and Polyamines. SCFAs are inhibitors of HDACs and act as signaling molecules that increase FOXP3 expression in an HDAC-dependent manner to raise tolerogenic anti-inflammatory cells by inactivating NF-κB, decreasing TNF-α production [48]. Gut bacteria induce a noticeable response of the gut immune system to the production of IgA by B cells, which has an important role in the regulation of gut bacterial populations in the small intestine [49]. SCFAs decrease the expression of T cell-activating molecules on antigen-presenting cells, such as DCs, and increase the number and function of colonic Treg cells and production of anti-inflammatory cytokines TGF-β and IL-10, which causes colon homeostasis. Also, SCFAs activate inflammasome assembly and increase the production of the downstream inflammatory cytokine IL-18 [48]. Additionally, they are also vital for maintaining mucosal immunity via improving IEC barrier function and immunological tolerance [50]. Gut bacteria can be affected by some factors, such as the use of antibiotics, underlying disease, aging, stress, bad dietary habits, and lifestyle [51]. Broad-spectrum antibiotic usage disrupts SCFAs and leads to hyperactivation of intestinal macrophages and an increase of pro-inflammatory Th cells. Microbiota disruption by antibiotics can cause the enhancement of pathogen-specific Th1 cell responses, tissue injury, and also reduction of Tregs and Th2 upregulation associated with immune responses and inflammation during infection [52].
Some bacteria, notably Lactobacilli spp., can produce AHR ligands and metabolize dietary tryptophan, which has potent inhibitory effects on the TNF-β response and can also boost Type 3 innate lymphoid cells [48]. PSA has pleiotropic modulatory effects on innate and adaptive immune cells, and because it interacts with TLR2 on DCs, it can keep the balance between Th1 and Th 2 cells. PSA can decrease inflammation in preclinical models of abscess formation and colitis by promoting IL-10 production via activated CD4+ T cells and increasing the activity of Treg cells [52]. Increased levels of circulating and colonic polyamines were associated with lower levels of colonic TNF-α and IL-6 and reinforcing epithelial barrier function [53,54].
Indeed, TLRs protect the host from hyper-inflammation by limiting the access of bacterial products via inhibiting NF-κB activation in the intestinal epithelium. According to a study, L. Plantarum is a potential important participant that interacts closely with human phagocytes and relies on this connection to activate IFN-I responses [55]. As a result, NOD1 is another receptor in innate immunuty that aids in the development of adaptive lymphoid tissues and the preservation of intestinal homeostasis. The stimulation of NOD2 by commensal bacteria enhances the survival and regeneration of gut epithelial stem cells. NOD2 helps to avoid small intestine inflammation by limiting the proliferation of the commensal Bacteroides Vulgatus [56,57]. TLR5 could identify bacteria flagellin, activate NF-κB, and control the expression of several inflammation-associated cytokines [52]. Gut dysbiosis has been reported to associate with a reduction in the production of gut bacteria-derived SCFAs such as butyrate resulting in enhanced gut permeability. This eases the translocation of microbiota-derived lipopolysaccharides (LPS), particularly from gram-negative bacteria and inflammatory substances to general circulation resulting in immune activation and inflammatory responses [58]. TLR4, which has been known for its ability to bind with bacterial LPS, is induced by Fusobacterium and results in stimulation of inflammatory genes expression via NF-κB signaling, which also could cause cytokine storm [59,60]. Peptidoglycan and teichoic acid of Streptococci attach to TLR-2 leading to the production of IL-1b, IL-6, IL-8, TNF-α, and inflammatory response [61]. As dysbiosis of the gut bacteria populations can result in many diseases, such as inflammatory bowel disease, obesity, and cancer [51] the analysis of fecal samples in 15 patients of SARS-CoV-2 has represented major changes in the fecal microbiome in comparison with controls [62]. Dysbiosis of gut bacteria disrupts the integrity of the gut barrier and induces the translocation of SARS-CoV-2 from the lung into the gut through the circulatory and lymphatic systems. It seems that the level of Enterobacteriaceae has raised during inflammatory conditions and exaggerated release of ROS and nitrogen intermediates by epithelial cells and transmigrating neutrophils in the gut lumen, which enhance inflammatory response [61].
Moreover, SARS-CoV-2 infection results in dysbiosis of gut flora and causes dominance of the pathogenic commensal bacterial. Higher levels of Klebsiella, Streptococcus, and Ruminococcus gnavus in SARS-CoV-2 patients have been associated with some proinflammatory cytokines (IFN-γ, TNF-α), which causes cytokine storm and Th1 cell response activation [63]. Magalhães et al., reported in their study that people belonging to risk groups for COVID-19-related death showed hyperimmune activation in the intestine, increased Th17 cells and IL-17 production. These patients also showed an increase in the circulating levels of bacterial endotoxins such as LPS, as well as pro-inflammatory cytokines including IL-1β, IL-6, and TNF [64]. Also, studies have demonstrated that COVID-19 patients present with decreased levels of Lactobacillus and Bifidobacterium. The high levels of Lactobacillus spp. correlate with increased anti-inflammatory IL-10 cytokine [14]. The interaction between gut bacteria and host cells is showed in Fig. 2 .
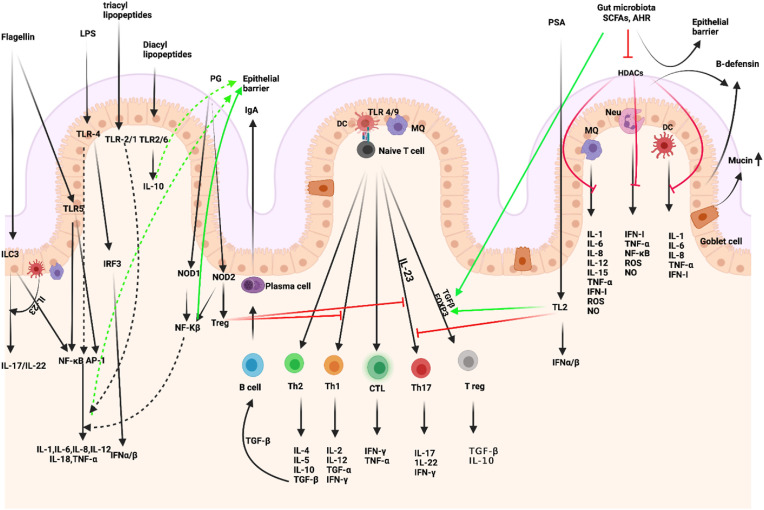
Fig. 2.
Intestinal microbiota-immunity interplay in immune homeostasis. Microbiome-derived TLR and NOD ligands and metabolites (e.g., SCFA, AHR ligands) act directly and indirectly. The effects of SCFAs include enhanced epithelial barrier function and immune tolerance, which promote gut homeostasis through specific mechanisms: 1. enhanced production of mucus by intestinal goblet cells, 2. inhibition of NF-κB; production of IL-18, 3. increased secretion of secretory IgA by B cells, 4. reduced expression of T cell-activating molecules on antigen-presenting cells, such as DCs, 5. and increased number and function of colonic Treg cells, including their expression of FOXP3 and their production of anti-inflammatory cytokines (TGF-β and IL-10). PSA is taken up by lamina propria DCs through a TLR2-dependent mechanism and presented to naïve CD4+ T cells. In the simultaneous presence of activated TGF-β, these cells can differentiate to Treg. IL-10 produced by these cells promotes immune homeostasis. Contrarily, IL-23 licensed through the same cascade promotes the expansion of pro-inflammatory Th17 cells. IECs-released factors such as retinoic acid (RA) and TGF-β, promote the development in the lamina propria of tolerogenic DCs that stimulate the differentiation of T cells into Treg. B cells differentiate into plasma cells (PCs) secreting IgA that translocate through the epithelium and are released into the mucus layer where control bacteria adhesion to host tissues. MQs, stimulated by signals such as flagellin, release IL-23, which in turn promotes the production of IL-22 by ILC3. (Figure has been created in biorender).
4. Probiotics and SARS-CoV2 cytokine storm
Balancing the immune responses and enhancing host immunity is paramount in COVID-19 pandemic scenario. Probiotics consumption upsurges immunological protection in the human host by maintaining the balance between the types of T cell responses (Th1/Th2, Th17, and Treg) and has a vital role in limiting various pathogens [65,67,68]. In vivo effects of probiotics evaluation has illustrated the increased peripheral immunoglobin production and decreased pro-inflammatory cytokine production. As discussed before, SARS-CoV-2 infection leads to a cytokine storm that causes deterioration in patient's lung condition and gastrointestinal tract infections. Therefore, these probiotic strains may facilitate the alleviation of cytokine storm by balancing cellular and humoral immune responses as seen in animal experiments [65,69,70]. There are not any available data verifying the effectiveness of probiotics on SARS-CoV-2 infection, but previously proven antiviral action of probiotics against different respiratory viruses could suggest probiotics as a harmless and accessible complementary medicine against COVID-19 disease. It has been illustrated that upon infection with influenza virus, many cytokines such as IL-12, IFN-γ, IL-4, IL-10, IL-1α, IL-1β, IL-6, TNF-α, IFN-α, and IFN-β are produced in the respiratory tract. Research on alleviating influenza infection and symptoms attempt to correct the imbalance caused by the out-of-control cytokine storm after infection [66,71]. Kawashima's group studied the antiviral effect of the YU strain of Lactobacillus plantarum (L. plantarum) in a mouse model of influenza virus (IFV). These probiotic strains activate the Th1 immune response, leading to increased levels of IFV-specific secretory IgA and neutralizing antibodies, resulting in the eradication of IFV from the lungs and other infection sites. These results confirm that the application of probiotic strains has a protective effect on IFV infection [72]. Bottari et al. reported that immune benefits of probiotics in SARS-CoV-2 infection could be obtained by developing mucosal immunity via the stimulation of IgA secretion, improvement of the biological functions of phagocytosis, and MQs, and adjustment of regulatory cells [73]. Additionally, there are scientific evidences that confirm the role of probiotics in enhancing immune functions [74].
Songa's group conducted a study to analyze the antiviral efficacy of L. rhamnosus in IFV infection. They found higher survival rate after administration lives probiotic bacteria. Treatment with live bacteria increased the production of secretory IgA and decreased the expression levels of the pro-inflammatory cytokines TNF-α and IL-6 in the lungs of infected mice. IL-6 is the major component of the cytokines released by activated MQs, and an IL-6 storm may increase the mass release of various inflammatory cytokines in patients with severe COVID-19 [75]. Pham et al. showed that L. rhamnosus EH8 strain yielded butyric acid that inhibits the secretion of IL-6 in MQs. In addition, this bacterium leads to the activation of free fatty acid receptor 2 (Ffar2) on the gut axis to suppress IL-6 signaling, which could be a target to treat the cytokine storm in COVID-19 [76]. Interestingly, a retrospective, single-center study of 311 consecutive heavy patients with confirmed COVID-19 showed that probiotics cannot lower IL-6 levels, but control the ability to moderate immunity and reduce the incidence of secondary infection in COVID-19 patients [77]. Also, Hou et al. showed that the presence of excess IL-6 promotes Th17 cell formation; IL-6 and IL-17 synergistically promote viral replication and may be important targets for anti-coronaviral therapy [78]. However, Colaneri et al. reported that anti-IL-6 therapy alone did not reduce ICU admissions and mortality in COVID-19 patients [79]. The findings represent a strategy that includes a more comprehensive approach to immune modulation rather than the suppression of host cytokines that could be more effective against the cytokine storm in virus-infected individuals. IL-17 and IL-6, stimulate viral persistence by immune cross-talk through autophagy [79]. Schett et al. reported that blocking IL-17 could decrease viral replication in COVID-19 patients [80]. The pathogenesis of the immune coronavirus responses is similar to Th17–Th1 driven autoimmune diseases and these interactions seem to perform a significant role in virus replication. Immune modulating effects of Bifidobacteria species, including an anti-IL-17 effect, could be important in both treatment and vaccine development [79]. Also, some clinical studies in humans have illustrated the favorable role of probiotic interventions in fighting against viral infections. Treatment with L. reuteri ATCC 55730 improves the mucosal expression level of IL-10 and decreases the inflammatory cytokine expression, including TNF- α, IL-1 β, and IL-8 [81,82]. Bibiloni's group stated the efficacy of VSL#3 in patients who suffer from clinical remission of ulcerative colitis. VSL#3 is a probiotic that is a mixture of eight strains, which modulate the secretion of the anti-inflammatory cytokine, IL-10, and inhibit the secretion of IL-6, IL-8, TNF-α, and IFN-γ. Thus, probiotic consumption strengthens gut barrier integrity and improves inflammatory responses by various signaling pathways [83]. In a clinical survey, the administration of probiotics resulted in IL-6 and C-reactive protein (CRP) decrease, while there were increased levels of IL-10 in multiple sclerosis patients' sera [84]. Another study has shown that some Bifidobacterium species can promote TGF-β signaling and increase the number of peripheral Tregs. L. plantarum NCU116 induced the expression of Th17 and Treg immune responses. This strain enhances the immunity of the intestinal mucosa and modulates the Th17/Treg balance which is ascribed to the TLR pathway in DC. After the hyper inflammation, there is a rapid increase in anti-inflammatory mediators such as Treg cells and IL-10 in the lungs that reduce lung damage. Together, they suggest an immunomodulatory potential in reducing cytokine storms. Thus, the use of anti-inflammatory probiotics can keep the gut microecology in balance and prevent secondary COVID-19 infection [[85], [86], [87]].
The probiotic strains Clostridium butyricum MIYAIRI 588, L. plantarum CAI6, L. rhamnosus GG, and VSL#3 can successfully control redox homeostasis in the host cell, resulting in an overall antioxidant capacity enhancement. This significant aspect of probiotic bacteria offers a unique opportunity to manage COVID-19 as redox homeostasis performs a vital role in slowing down the disease progression [65]. Regarding the effects of probiotics on respiratory disease, two randomized controlled trials found that critically ill, ventilated patients who received probiotics (L. rhamnosus GG, live Bacillus subtilis, and Enterococcus faecalis) had significantly less ventilator-associated pneumonia compared to placebo; therefore, it can be assumed that COVID-19 pneumonia can be alleviated in the same way [88,89]. It has been shown that the use of certain Lactobacillus strains can improve the gut microbial population by increasing mucus secretion and also prevent the breakdown of tight junction proteins by reducing the number of LPS [84]. Some studies have reported that SARS-CoV-2 can directly interact with LPS via S protein. While, neither the S protein alone nor the LPS alone induce any activation of pro-inflammatory NF-κB, the combination of the S protein, even at low LPS levels, dose-dependently increases NF-κB activation followed by the cytokine response in monocytes in vitro. Furthermore, ACE2 has been reported to exert a protective effect against LPS-induced acute lung injury in mice; therefore, viral ACE2 suppression may lead to a stronger inflammatory response in the lungs [90,91]. Probiotics likely interfere with the treatment of viral infections such as COVID-19. Therefore, it is important to better understand the immunomodulatory mechanisms of probiotic function to improve the targeting of probiotic interventions by selecting the optimal strain (s). Talking about probiotics without mentioning bacteriophages is impaired discussion, due to the crucial phage performance both in probiotic industry and also human microbiota. Cross talk between probiotics and host immune is represented in Fig. 3 .
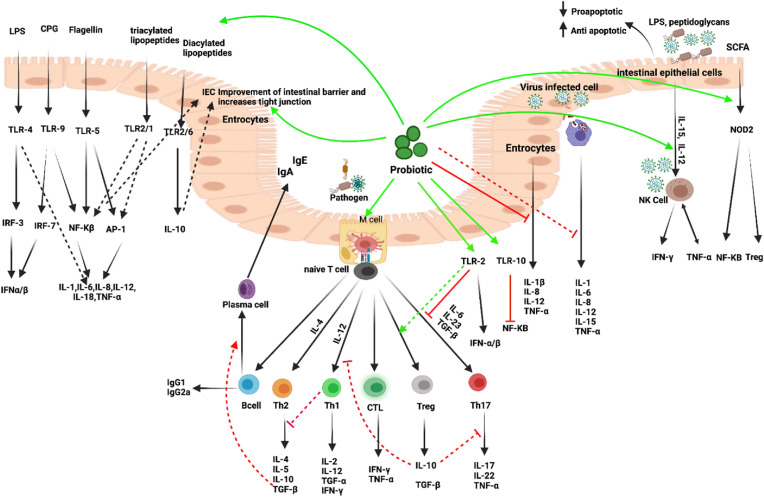
Fig. 3.
Immune mechanisms of probiotics. A nonspecific antiviral effect through the stimulation of innate immune cells: increase in the cytotoxic activity of NK cells and MQ phagocytosis. These bacteria can enhance epithelial barrier function via intercellular junctions or PRRs. Probiotic bacteria can shape the mucosal immune system toward a noninflammatory, tolerogenic pattern through the induction of T cells with regulatory properties. Probiotics can also downregulate Th1 responses and inhibit the production of proinflammatory cytokines, IL-12, TNF-α, and IFN-γ by DC. The predominant cytokine profile depends on the nature of the stimulus and the types of probiotic bacteria. Probiotics modulate the humoral response (increase IgA and decrease IgE), balance cell-mediated immune response (increase Treg cells and decrease Th2 response). It has been shown that Tregs play a role in maintaining the epithelial barrier via the production of TGF-b and IL-10., Probiotics can modulate the Th1 and Th2 responses, resulting in the restoration of the immune homeostasis. (Figure has been created in biorender).
5. Bacteriophages and SARS-CoV2 cytokine storm
Dominated by bacteria, the gut microbiome is made up of different taxonomic groups including viruses, fungi, and archaea which are also important members of the community with potential effects on human health [92,93]. Bacteriophages not only acted as particularly insensitive bacteria, but they can also have facilitative and inhibitory functions in the surrounding gut microbiota, Yet, phage interactions with bacterial hosts and the immune system in the human gut remains poorly described [94,95]. The ubiquity of phages in the gut and their ability to modulate bacterial communities in other ecosystems suggest that they could be active players in human health and interact with the host immune system. Phages can enhance phagocytosis of bacteria by MQs, due to administered phages together with the host bacteria, which were able to incite bacterial phagocytosis. This was referred to opsonization of bacterial cells by phages, where the phage covers the bacteria and makes it more recognizable to the immune system [96,97]. A survey showed that T4 phage in E. coli cells by binding to LPS could prevent LPS induced production of proinflammatory cytokines in mice [98]. The phage-mediated changes in gut bacterial communities could have downstream effects on immune signaling. Bacteriophages are very specific to their hosts, which minimize the risk of secondary infections, with no side effects during or after phage administration, while resistant bacteria, allergies, and secondary infections are common side effects of antibiotic treatment [99]. One of the critical problems of a viral infection is the super infection caused by pathogenic bacteria. To solve this problem, antibiotics come first in therapeutic methods. Antibiotics usually cause intestinal dysbiosis and lead to inflammation and cytokine storms. Hence, phage therapy can act as a targeted therapy only to kill specific pathogenic bacteria, help maintain balance in the gut microbiome, and may prevent cytokine storms. A study by Prazak et al. found that pneumonia can be treated with nebulized bacteriophages. Prophylactic administration of bacteriophages reduced the bacterial load in the lungs and improved the resistance of antibiotic-resistant animals infected with Staphylococcus aureus (S. aureus) to ventilation-related pneumonia [100]. An investigation has reported that administration of a combination of Gentamicin, Meropenem, and Vancomycin caused an increase in the Enterobacteriaceae and other pathogenic frequencies, besides, it leads to a decrease in Bifidobacterium and butyrate-producing species [101]. One of the main uses of antibiotics is in COVID-19 hospitalized patients. Antibiotic-naive patients with COVID-19 were enriched in opportunistic pathogens known to cause bacteremia, including Clostridium, Actinomyces viscosus, and Bacteroides nordii and accompanied by a further shift of the gut microbiome away from a healthy microbiome [102]. The finding of Yeoh et al., in 100 patients with COVID-19 showed that gut microbiota composition during hospitalization is associated with disease severity and plasma concentrations of several cytokines and inflammatory markers, which has suggested that antibiotics improve patient outcomes [103]. Therefore, phage therapy could be a possible advanced option that could play an important role in eliminating the pathogen as well as preventing gut dysbiosis, which can lead to a cytokine storm. Several studies have confirmed that phages have not only antibacterial but also antiviral properties. Anti-immunoregulatory and anti-inflammatory activity has also been demonstrated by phage particles, and these features may be useful in restoring immune homeostasis [104,105]. Recently, it has been shown that Pf phages endocytosed by leukocytes activate TLR3-dependent pattern recognition receptors and inhibit TNF-α driving IFN I production [106,107]. There is also data suggesting that T4 phage via inhibiting activation of NF-κB and ROS production can reduce extreme inflammatory reactions in pathology and clinical course of SARS-CoV-2 [108]. Phages also work to activate NK cells, which could be a key feature in their therapeutic actions. A study of staphylococcal phages on the expression of genes that are involved in antimicrobial immunity suggested that there is an upregulated translation of IL-2, which improves the activity of NK cells and hence causes a progressive cellular immune response [109]. Another investigation has shown that a Pseudomonas phage upregulates IL-10 expression in human mononuclear cells, while a Staphylococcus phage does not [110,111]. It must be ensured that bacteriophages are selected correctly which target both optimal bacteria and should be most effective against the growth of the bacterial population. Bacteriophages should not interfere with the patient's innate or adaptive immunity. It is also very important to exclude that the patient does not use antibodies to bacteriophages and does not produce antibodies to bacteriophages to get rid of the bacteriophage earlier than against SARS-CoV-2 [109,112]. Laboratory studies, clinical trials, and randomized steps of one to three human trials can be conducted to prove their therapeutic utility [113]. Phage therapy may also show promise as a treatment for SARS-CoV-2. Due to the data have been mentioned in Table 1 &2 , similar modulatory function on the immune response of probiotics and bacteriophages casts light on co-administration of them to quench cytokine storm in patients with extreme immune response, or ameliorate immune system in patients with immune deficiency, which both lead into the death in some chronic or acute cases. To develop this stance, it could be suggested that co-administration of phages, such as T4 and Pf4 with probiotics such as L.rhamnosus, L.paracasei, and L.fermentum L930BB, may help to modulate the immune response. Phage direct and indirect responses have been illustrated in Fig. 4 .
Table 1.
The proposed function of Gut bacteria and probiotics in COVID-19 patients.
| GUT | Probiotic | Function | References |
|---|---|---|---|
| Lactobacillus | L. plantarum | gastrointestinal barrier function, IgA ↑ | [114] |
| L. plantarum NCIMB8826 | IgG2a, IFN-γ ↑ | [115] | |
| Th2 ↓ | |||
| L. plantarum WCFS1 | TLR1/2, TLR2/6, TLR10, anti apoptotic, tight junction, intestinal barrier ↑ | [116] | |
| NF-κβ ↓ | |||
| L. plantarum MB452 | tight junction ↑ | [117] | |
| L. reuteri | Modulation of DC function, IL-10, Tregs ↑ | [118] | |
| L. reuteri ATCC 23272 | Modulation of TLR9, IL-5, and IL-13 ↓ | [119] | |
| L. reuteri DSM 17938 | Treg ↑ | [120] | |
| Th1 ↓ | |||
| L. rhamnosus | Phagocytosis, tight junction, IgA ↑ | [121,122] | |
| IL-6, TNF-α, TLR2 ↓ | |||
| L. rhamnosus GG | Phagocytosis, Th1 (IL-12 and IFN-γ), Tregs, TGF-β, IgA ↑ | [[123], [124], [125]] | |
| Neutrophil, eosinophils, IL-6, TNF-α, TLR2, Th2 (IL-4, IL-5, and IL-13) ↓ | |||
| L. casei | Modulation of DC function, TLR2, Tregs, IgA, IFN-γ, TNF-α, IL-10 ↑ | [118,126] | |
| NF-κβ ↓ | |||
| L. casei IBSO41 | Stimulation of DCs, TGF-β ↑ | [127] | |
| L. paracasei | MQ2 ↑ | [128] | |
| NF-κβ ↓ | |||
| L. fermentum L930BB | TLR1/2, TLR2/6, TLR10, anti apoptotic, tight junction, intestinal barrier ↑ | [129,130] | |
| NF-κβ ↓ | |||
| L. fermentum | IL-10, IFN- γ, TGF-β ↑ | [131,132] | |
| TLR4,IL-1β, IL-6,IL-8,MCP-1, TNF-α ↓ | |||
| L. paragasseri K7 | TLR1/2, TLR2/6, TLR10, anti apoptotic, tight junction, intestinal barrier ↑ | [129] | |
| NF-κβ ↓ | |||
| L. amylovorus | TLR4 ↑ | [131] | |
| IL-1β, IL-8 ↓ | |||
| L. bulgaricus | IL-10, IL-12, IFN-γ, IgA ↑ TNF-α ↓ |
[69,133] | |
| L. acidophilus | Phagocytosis, improved the Treg/Th17 imbalance IL-1, IL-6, IFN-γ, IgA ↑ | [131,134,135] | |
| IL-2, IL-4 ↓ | |||
| L. acidophilus AD031 | Stimulation of DCs, restored the balance of inflammatory cytokines and Th17/Treg cells, Treg, TGF-β, IgA ↑ | [127] | |
| Bifidobacterium | B. breve | IgA ↑ | [121,136] |
| TNF-α ↓ | |||
| B. longum | tight junction ↑ | [137] | |
| IL-6, TNF-a, TLR2 ↓ | |||
| B. longum ATCC | Neutrophil, IL-6, TNF-a, TLR2 ↓ | [123] | |
| B. lactis Bb-12 | Tregs, TGF-β ↑ | [138] | |
| B. infantis | mucin, IgA ↑ | [139] | |
| B. animalis IM386 | TLR1/2, TLR2/6, TLR10, anti-apoptotic, tight junction, intestinal barrier ↑ | [129] | |
| NF-κβ ↓ | |||
| Streptococcus | S. thermopiles | IgA ↑ | [140] |
| TNF-α ↓ | |||
| S. thermopiles ST28 | TLR-9, IFN-γ, modulation of Th1/Th17 balance ↑ | [141] | |
| Th17, IL-17 ↓ | |||
| Enterococcus | E. faeciumNM113/NM213 | TLR2, IL-6, TNF-α ↑ | [142] |
| NOD2 ↓ | |||
| E. faecalis CECT7121/PEF121 | IL-6,IL-10,IL-12, TNF-α ↑ | [143] | |
| Lactococcus | L.G50 | IL-12, IFN-γ, Th1 ↑ | [144] |
| Th2, IgG1, IgE ↓ | |||
| L. lactis KC24 | IL-10, Anti-inflammatory effects ↑ | [145] | |
| NO ↓ |
Table 2.
The proposed acts of some bacteriophages with anti-bacterial and anti-viral function.
| bacteriophage | Anti-bacterial function | Anti-virus function | Reference |
|---|---|---|---|
| Lytic Lactococcus phage | Anti-inflammatory response ↑ | [98] | |
| Pf4 (Pseudomonas aeruginosa phages) | TLR3, IL-1α/β, IL-6, IL-8, IFNI ↑ | TLR3, IL-12, IFNI ↑ | [98,110] |
| TNF-α ↓ | |||
| T4 phage | TLR2, IL-10 ↑ | hBD2, defensin-2, TLR2 ↑ | [108,109] |
| TLR4, T cell, IL-2, IL-6, NF-κβ, TNF-α, apoptosis ↓ | Virus attachment, ROS ↓ | ||
| Escherichia coli phages | IL-6, IL-8, IL-10, IFN-γ, TNF-α ↑ | TLR9, Th, CTL, IL-12, IFN-γ ↑ | [146,147] |
| Staphylococcus aureus phages | TNF-α, IL-1β, IFN-γ, IL-10 ↑ | [110] | |
| Staphylococcus aureus phages VB-SauM-JS25 | NF-κβ, pro-inflammatory cytokines ↓ | [148] | |
| A5/80 | TLR10, TLR2, IL-2, NK cell, anti-inflammatory effect ↑ |
IL-2, NK cell ↑ | [109,149] |
| TLR4 ↓ | |||
| M13 | TLRs ↑ |
[150,151] | |
| Apoptosis ↓ | |||
| MS2 phages | IFN-γ ↑ | [152] | |
| Bacteroides phages | TLR9, IFN-γ ↑ | [153] | |
| Acinetobacter.baumannii phage Βφ-R2096 | IL-6, TNF-α ↓ | [153] | |
| φkm18p phage | IL-6, TNF-α ↓ | [154] |
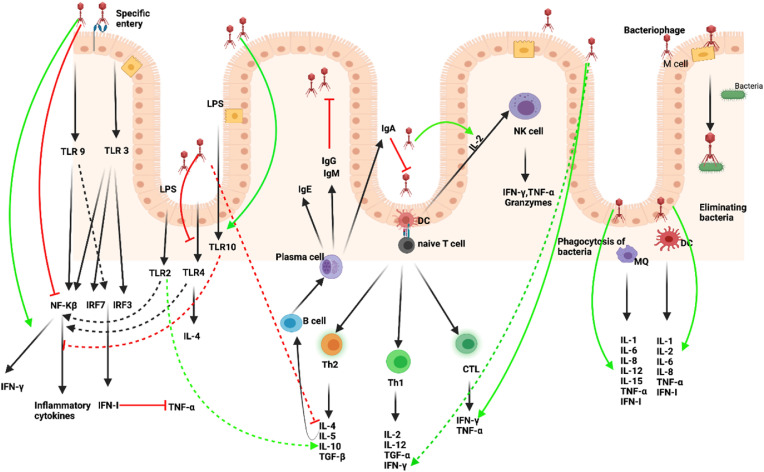
Fig. 4.
Interaction between phages and immune system. Phage infection led to the release of PAMPs which translocate the gut epithelium and trigger TLR pathways and induce a transcriptional response in monocytes, MQs, DCs, and particularly improved the transcription of IL-1, IL-6, and TNF-α. Besides, by inhibiting activation of NF-κB and ROS production, phages can reduce extreme inflammatory reactions. Phages induce antiviral immunity via activating NK cells, TLR3, and TLR-9, and inhibiting TNF-α driving IFN I production. Phages can trigger a strong immune response via activation of Th1 (IFN-γ and TNF-α), Th2 (IL-4, IL,-6 and IL-10) cells, and also B cells. The activation of Th1 generated a CTL response. (Figure has been created in biorender).
6. Concluding remarks: a glimpse into the future
The human gut microbiota is an exclusive community of microorganisms including bacteria, Mycobiome, Virome, Archaeome, and Eukaryotic Parasites that has received much attention because of their impacts on human health and diseases. SARS-CoV-2 infection can lead to widespread tissue damage resulting in multi-organ failure and death. This is due to an aggressive inflammatory response caused by releasing many proinflammatory cytokines, known as a cytokine storm. The gut microbiota regulates multiple host functions, including immune homeostasis. By contrast, imbalances in their community composition are associated with several human diseases or conditions. In the present study, we tried to discuss how the imbalanced gut bacteria could contribute to the development of cytokine storms in COVID-19 patients. We then suggested targeted interventions using probiotics and bacteriophages to mitigate this in COVID-19 patients by restoring balanced gut bacterial communities. In vitro and in vivo evidence supports the potential role of gut microbiota in regulating the immune system, suggesting a definitive role for probiotics and bacteriophages in viral infections including COVID-19. Since one of the main reasons for death among covid-19 patients is excessive immune responses directed toward cytokine storm we introduced the potential probiotics and bacteriophages that could alleviate the immune responses via induction of Treg, Th1, IFN-α, and IgA responses and suppression of proinflammatory responses including IL-6 and Th2. This can be a safe and promising low-cost method to balance the gut microbiota by supplementation of specific probiotics and phages to reduce the severity of COVID-19 morbidity and mortality. Probiotics and phages can prevent the formation of cytokine storms by simultaneously boosting innate immunity and avoiding the exaggeration of adaptive immunity challenged to respond quickly to viral propagation. Suppression of inflammatory cytokine responses by administration of probiotics and phages can prevent both the severity and development of ARDS. Since there are no effective therapy methods in SARS-CoV2 CS scenarios, it seems the targeted manipulation of the gut microbiome especially in high-risk groups in the early days of infection could potentially decrease the risk of development of cytokine storm in these patients.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Author's contribution
TZ, NF and MS wrote the first draft of the manuscript. MS and FJ coordinated the writing of the manuscript. FMGH and MKHM contributed to manuscript writing. All contributed to the editing and refinement of the final version of the manuscript. The author(s) read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
CRediT authorship contribution statement
Tahereh Zeinali: Visualization, Conceptualization, Writing – review & editing. Niloofar Faraji: Visualization, Data curation, Writing – review & editing. Farahnaz Joukar: Supervision, Project administration. Mohammadali Khan Mirzaei: Supervision. Hossnieh Kafshdar Jalali: Writing – review & editing. Mohammad Shenagari: Supervision, Conceptualization, Project administration, Writing – review & editing. Fariborz Mansour-Ghanaei: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors gratefully acknowledge the support contributors to this study.
Data availability
Data will be made available on request.
References
- 1.Beyerstedt S., Casaro E.B., Rangel É.B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021:1–15. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultze J.L., Aschenbrenner A.C. COVID-19 and the human innate immune system. Cell. 2021;184(7):1671–1692. doi: 10.1016/j.cell.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim S., Bae J.H., Kwon H.-S., Nauck M.A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L., Xie X., Tu Z., Fu J., Xu D., Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Targeted Ther. 2021;6:1–20. doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song P., Li W., Xie J., Hou Y., You C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H.-J., Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microb. 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B., Yao M., Lv L., Ling Z., Li L. The human microbiota in health and disease. Engineering. 2017;3:71–82. [Google Scholar]
- 10.Vodnar D.-C., Mitrea L., Teleky B.-E., Szabo K., Călinoiu L.-F., Nemeş S.-A., et al. Coronavirus disease (Covid-19) caused by (sars-cov-2) infections: a real challenge for human gut microbiota. Front. Cell. Infect. Microbiol. 2020;10:786. doi: 10.3389/fcimb.2020.575559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y., Shi X., Fu W., Xiang F., He X., Yang B., et al. Gut microbiota dysbiosis correlates with abnormal immune response in moderate COVID-19 patients with fever. J. Inflamm. Res. 2021;14:2619. doi: 10.2147/JIR.S311518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira C., Viana S.D., Reis F. Is gut microbiota dysbiosis a predictor of increased susceptibility to poor outcome of COVID-19 patients? An update. Microorganisms. 2021;9:53. doi: 10.3390/microorganisms9010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aktas B. Gut microbiota dysbiosis and COVID-19: possible links. Ref. Module Food Sci. 2021 doi: 10.1016/B978-0-12-819265-8.00072-3. [DOI] [Google Scholar]
- 14.Chhibber-Goel J., Gopinathan S., Sharma A. Interplay between severities of COVID-19 and the gut microbiome: implications of bacterial co-infections? Gut Pathog. 2021;13:1–6. doi: 10.1186/s13099-021-00407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saad N., Moussa S. Immune response to COVID-19 infection: a double-edged sword. Immunol. Med. 2021:1–10. doi: 10.1080/25785826.2020.1870305. [DOI] [PubMed] [Google Scholar]
- 16.Mishra K.P., Singh A.K., Singh S.B. Hyperinflammation and immune response generation in COVID-19. Neuroimmunomodulation. 2020:1–7. doi: 10.1159/000513198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bezemer G.F., Garssen J. TLR9 and COVID-19: a multidisciplinary theory of a multifaceted therapeutic target. Front. Pharmacol. 2021;11:1958. doi: 10.3389/fphar.2020.601685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng M., Karki R., Williams E.P., Yang D., Fitzpatrick E., Vogel P., et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021:1–10. doi: 10.1038/s41590-021-00937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S.-W., Wang C.-Y., Jou Y.-J., Huang S.-H., Hsiao L.-H., Wan L., et al. SARS coronavirus papain-like protease inhibits the TLR7 signaling pathway through removing Lys63-linked polyubiquitination of TRAF3 and TRAF6. Int. J. Mol. Sci. 2016;17:678. doi: 10.3390/ijms17050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taefehshokr N., Taefehshokr S., Hemmat N., Heit B. Covid-19: perspectives on innate immune evasion. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.580641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussman J.P. Cellular and molecular pathways of COVID-19 and potential points of therapeutic intervention. Front. Pharmacol. 2020;11:1169. doi: 10.3389/fphar.2020.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., et al. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20:343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gustine J.N., Jones D. Immunopathology of hyperinflammation in COVID-19. Am. J. Pathol. 2021;191:4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yim J., Lim H.H., Kwon Y. COVID-19 and pulmonary fibrosis: therapeutics in clinical trials, repurposing, and potential development. Arch Pharm. Res. (Seoul) 2021:1–15. doi: 10.1007/s12272-021-01331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Eeden C., Khan L., Osman M.S., Cohen Tervaert J.W. Natural killer cell dysfunction and its role in COVID-19. Int. J. Mol. Sci. 2020;21:6351. doi: 10.3390/ijms21176351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magro G. SARS-CoV-2 and COVID-19: is interleukin-6 (IL-6) the ‘culprit lesion’of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine: X. 2020;2 doi: 10.1016/j.cytox.2020.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanmohammadi S., Rezaei N. Role of Toll‐like receptors in the pathogenesis of COVID‐19. J. Med. Virol. 2021;93:2735–2739. doi: 10.1002/jmv.26826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosseini A., Hashemi V., Shomali N., Asghari F., Gharibi T., Akbari M., et al. Biomedicine & Pharmacotherapy; 2020. Innate and Adaptive Immune Responses against Coronavirus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jordan S.C. Innate and adaptive immune responses to SARS‐CoV‐2 in humans: relevance to acquired immunity and vaccine responses. Clin. Exp. Immunol. 2021;204:310–320. doi: 10.1111/cei.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou R., To K.K.-W., Wong Y.-C., Liu L., Zhou B., Li X., et al. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53:864–877. doi: 10.1016/j.immuni.2020.07.026. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Gomez A., Vitalle J., del Carmen Gasca-Capote M., Gutierrez-Valencia A., Trujillo-Rodriguez M., Serna-Gallego A., et al. bioRxiv; 2021. Dendritic Cell Deficiencies Persist Seven Months after SARS-CoV-2 Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen R., Lan Z., Ye J., Pang L., Liu Y., Wu W., et al. Cytokine storm: the primary determinant for the pathophysiological evolution of COVID-19 deterioration. Front. Immunol. 2021;12:1409. doi: 10.3389/fimmu.2021.589095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Paula C.B.V., de Azevedo M.L.V., Nagashima S., Martins A.P.C., Malaquias M.A.S., dos Santos Miggiolaro A.F.R., et al. IL-4/IL-13 remodeling pathway of COVID-19 lung injury. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-75659-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sette A., Crotty S. Cell; 2021. Adaptive Immunity to SARS-CoV-2 and COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rha M.-S., Shin E.-C. Activation or exhaustion of CD8+ T cells in patients with COVID-19. Cell. Mol. Immunol. 2021:1–9. doi: 10.1038/s41423-021-00750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jing Y., Luo L., Chen Y., Westerberg L.S., Zhou P., Xu Z., et al. SARS-CoV-2 infection causes immunodeficiency in recovered patients by downregulating CD19 expression in B cells via enhancing B-cell metabolism. Signal Transduct. Targeted Ther. 2021;6:1–13. doi: 10.1038/s41392-021-00749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct. Targeted Ther. 2020;5:1–8. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y., Zheng J., Islam M.S., Yang Y., Hu Y., Chen X. The role of CD4+ FoxP3+ regulatory T cells in the immunopathogenesis of COVID-19: implications for treatment. Int. J. Biol. Sci. 2021;17:1507. doi: 10.7150/ijbs.59534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H., Wang Z., Cao W., Wu Q., Yuan Y., Zhang X. Regulatory T cells in COVID-19. Aging Dis. 2021;12(7):1545–1553. doi: 10.14336/AD.2021.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muyayalo K.P., Huang D.H., Zhao S.J., Xie T., Mor G., Liao A.H. COVID‐19 and Treg/Th17 imbalance: potential relationship to pregnancy outcomes. Am. J. Reprod. Immunol. 2020;84 doi: 10.1111/aji.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahimzadeh M., Naderi N. Toward an understanding of regulatory T cells in COVID‐19: a systematic review. J. Med. Virol. 2021;93:4167–4181. doi: 10.1002/jmv.26891. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira-Gomes M., Kruglov A., Durek P., Heinrich F., Tizian C., Heinz G.A., et al. SARS-CoV-2 in severe COVID-19 induces a TGF-β-dominated chronic immune response that does not target itself. Nat. Commun. 2021;12:1–14. doi: 10.1038/s41467-021-22210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farhan C., Sohail M.U., Abdelhafez I., Salman S., Attique Z., Kamareddine L., et al. 2021. SARS-CoV-2 and Immune-Microbiome Interactions: Lessons from Respiratory Viral Infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y., Cheng X., Jiang G., Tang H., Ming S., Tang L., et al. Altered oral and gut microbiota and its association with SARS-CoV-2 viral load in COVID-19 patients during hospitalization. npj Biofilms and Microbiomes. 2021;7:1–9. doi: 10.1038/s41522-021-00232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pabst O., Slack E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 2020;13:12–21. doi: 10.1038/s41385-019-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takiishi T., Fenero C.I.M., Câmara N.O.S. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers. 2017;5 doi: 10.1080/21688370.2017.1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y.-J., Li S., Gan R.-Y., Zhou T., Xu D.-P., Li H.-B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015;16:7493–7519. doi: 10.3390/ijms16047493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamura A., Kurihara S., Takahashi D., Ohashi W., Nakamura Y., Kimura S., et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat. Commun. 2021;12:1–14. doi: 10.1038/s41467-021-22212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kibe R., Kurihara S., Sakai Y., Suzuki H., Ooga T., Sawaki E., et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci. Rep. 2014;4:1–11. doi: 10.1038/srep04548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gutierrez-Merino J., Isla B., Combes T., Martinez-Estrada F., Maluquer de Motes C. Beneficial bacteria activate type-I interferon production via the intracellular cytosolic sensors STING and MAVS. Gut Microb. 2020;11:771–788. doi: 10.1080/19490976.2019.1707015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agace W.W., McCoy K.D. Regionalized development and maintenance of the intestinal adaptive immune landscape. Immunity. 2017;46:532–548. doi: 10.1016/j.immuni.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Ramanan D., San Tang M., Bowcutt R., Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41:311–324. doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vignesh R., Swathirajan C.R., Tun Z.H., Rameshkumar M.R., Solomon S.S., Balakrishnan P. Could perturbation of gut microbiota possibly exacerbate the severity of COVID-19 via cytokine storm? Front. Immunol. 2021;11:3752. doi: 10.3389/fimmu.2020.607734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou S., Fang L., Lee M.-H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol. Rep. 2018;6:1–12. doi: 10.1093/gastro/gox031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guijarro-Muñoz I., Compte M., Álvarez-Cienfuegos A., Álvarez-Vallina L., Sanz L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway and proinflammatory response in human pericytes. J. Biol. Chem. 2014;289:2457–2468. doi: 10.1074/jbc.M113.521161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chattopadhyay I., Shankar E.M. SARS-CoV-2-indigenous microbiota nexus: does gut microbiota contribute to inflammation and disease severity in COVID-19? Front. Cell. Infect. Microbiol. 2021;11:96. doi: 10.3389/fcimb.2021.590874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajput S., Paliwal D., Naithani M., Kothari A., Meena K., Rana S. COVID-19 and gut microbiota: a potential connection. Indian J. Clin. Biochem. 2021:1–12. doi: 10.1007/s12291-020-00948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Lelie D., Taghavi S. COVID-19 and the gut microbiome: more than a gut feeling. mSystems. 2020;5 doi: 10.1128/mSystems.00453-20. e00453-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magalhães N.S., Savino W., Silva P.M.R., Martins M.A., Carvalho V.F. Gut microbiota dysbiosis is a crucial player for the poor outcomes for COVID-19 in elderly, diabetic and hypertensive patients. Front. Med. 2021:1318. doi: 10.3389/fmed.2021.644751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh K., Rao A. Probiotics: a potential immunomodulator in COVID-19 infection management. Nutr. Res. 2021;87:1–12. doi: 10.1016/j.nutres.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahooti M., Miri S.M., Abdolalipour E., Ghaemi A. The immunomodulatory effects of probiotics on respiratory viral infections: a hint for COVID-19 treatment? Microb. Pathog. 2020 doi: 10.1016/j.micpath.2020.104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mortaz E., Adcock I.M., Folkerts G., Barnes P.J., Paul Vos A., Garssen J. Probiotics in the management of lung diseases. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/751068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan F., Polk D. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011;27:496. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azad M., Kalam A., Sarker M., Wan D. Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/8063647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahooti M., Miri S.M., Abdolalipour E., Ghaemi A. The immunomodulatory effects of probiotics on respiratory viral infections: a hint for COVID-19 treatment? Microb. Pathog. 2020;148 doi: 10.1016/j.micpath.2020.104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurian S.J., Unnikrishnan M.K., Miraj S.S., Bagchi D., Banerjee M., Reddy B.S., et al. Archives of medical research; 2021. Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawashima T., Hayashi K., Kosaka A., Kawashima M., Igarashi T., Tsutsui H., et al. Lactobacillus plantarum strain YU from fermented foods activates Th1 and protective immune responses. Int. Immunopharm. 2011;11:2017–2024. doi: 10.1016/j.intimp.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 73.Bottari B., Castellone V., Neviani E. Probiotics and covid-19. Int. J. Food Sci. Nutr. 2021;72:293–299. doi: 10.1080/09637486.2020.1807475. [DOI] [PubMed] [Google Scholar]
- 74.Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metabol. Syndr.: Clin. Res. Rev. 2020;14:367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Youn H.-N., Lee D.-H., Lee Y.-N., Park J.-K., Yuk S.-S., Yang S.-Y., et al. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antivir. Res. 2012;93:138–143. doi: 10.1016/j.antiviral.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Pham M.T., Yang A.J., Kao M.-S., Gankhuyag U., Zayabaatar E., Jin S.-L.C., et al. Gut probiotic Lactobacillus rhamnosus attenuates PDE4B-mediated interleukin-6 induced by SARS-CoV-2 membrane glycoprotein. J. Nutr. Biochem. 2021;98 doi: 10.1016/j.jnutbio.2021.108821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Q., Cheng F., Xu Q., Su Y., Cai X., Zeng F., et al. The role of probiotics in coronavirus disease-19 infection in Wuhan: a retrospective study of 311 severe patients. Int. Immunopharm. 2021;95 doi: 10.1016/j.intimp.2021.107531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hou W., Jin Y.-H., Kang H.S., Kim B.S. Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function. J. Virol. 2014;88:8479–8489. doi: 10.1128/JVI.00724-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bozkurt H.S., Quigley E.M. The probiotic Bifidobacterium in the management of Coronavirus: a theoretical basis. Int. J. Immunopathol. Pharmacol. 2020;34 doi: 10.1177/2058738420961304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schett G., Sticherling M., Neurath M.F. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat. Rev. Immunol. 2020;20:271–272. doi: 10.1038/s41577-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y., Fatheree N.Y., Mangalat N., Rhoads J.M. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G1087–G1096. doi: 10.1152/ajpgi.00124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oliva S., Di Nardo G., Ferrari F., Mallardo S., Rossi P., Patrizi G., et al. Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment. Pharmacol. Ther. 2012;35:327–334. doi: 10.1111/j.1365-2036.2011.04939.x. [DOI] [PubMed] [Google Scholar]
- 83.Bibiloni R., Fedorak R.N., Tannock G.W., Madsen K.L., Gionchetti P., Campieri M., et al. VSL# 3 probiotic-mixture induces remission in patients with active ulcerative colitis. Official journal of the American College of Gastroenterology| ACG. 2005;100:1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 84.Morshedi M., Hashemi R., Moazzen S., Sahebkar A., Hosseinifard E.-S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: a systematic review. J. Neuroinflammation. 2019;16:1–11. doi: 10.1186/s12974-019-1611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie J., Nie S., Yu Q., Yin J., Xiong T., Gong D., et al. Lactobacillus plantarum NCU116 attenuates cyclophosphamide-induced immunosuppression and regulates Th17/Treg cell immune responses in mice. J. Agric. Food Chem. 2016;64:1291–1297. doi: 10.1021/acs.jafc.5b06177. [DOI] [PubMed] [Google Scholar]
- 86.Zheng B., van Bergenhenegouwen J., Overbeek S., van de Kant H.J., Garssen J., Folkerts G., et al. Bifidobacterium breve attenuates murine dextran sodium sulfate-induced colitis and increases regulatory T cell responses. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elekhnawy E., Negm W.A. The potential application of probiotics for the prevention and treatment of COVID-19. Egypt. J. Med. Human Genetics. 2022;23:1–9. doi: 10.1186/s43042-022-00252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morrow L.E., Kollef M.H., Casale T.B. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am. J. Respir. Crit. Care Med. 2010;182:1058–1064. doi: 10.1164/rccm.200912-1853OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeng J., Wang C.-T., Zhang F.-S., Qi F., Wang S.-F., Ma S., et al. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med. 2016;42:1018–1028. doi: 10.1007/s00134-016-4303-x. [DOI] [PubMed] [Google Scholar]
- 90.Kruglikov I.L., Scherer P.E. Preexisting and inducible endotoxemia as crucial contributors to the severity of COVID-19 outcomes. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petruk G., Puthia M., Petrlova J., Samsudin F., Strömdahl A.-C., Cerps S., et al. SARS-CoV-2 Spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J. Mol. Cell Biol. 2020;12:916–932. doi: 10.1093/jmcb/mjaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matijašić M., Meštrović T., Čipčić Paljetak H., Perić M., Barešić A., Verbanac D. Gut microbiota beyond bacteria—mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int. J. Mol. Sci. 2020;21:2668. doi: 10.3390/ijms21082668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang J.-Z., Du W.-T., Xu Y.-L., Cheng S.-Z., Liu Z.-J. Gut microbiome-based medical methodologies for early-stage disease prevention. Microb. Pathog. 2017;105:122–130. doi: 10.1016/j.micpath.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 94.Thursby E., Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vemuri R., Shankar E.M., Chieppa M., Eri R., Kavanagh K. Beyond just bacteria: functional biomes in the gut ecosystem including virome, mycobiome, archaeome and helminths. Microorganisms. 2020;8:483. doi: 10.3390/microorganisms8040483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kurzępa A., Dąbrowska K., Skaradziński G., Górski A. Bacteriophage interactions with phagocytes and their potential significance in experimental therapy. Clin. Exp. Med. 2009;9:93–100. doi: 10.1007/s10238-008-0027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Podlacha M., Grabowski Ł., Kosznik-Kawśnicka K., Zdrojewska K., Stasiłojć M., Węgrzyn G., et al. Interactions of bacteriophages with animal and human organisms—safety issues in the light of phage therapy. Int. J. Mol. Sci. 2021;22:8937. doi: 10.3390/ijms22168937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sinha A., Maurice C.F. Bacteriophages: uncharacterized and dynamic regulators of the immune system. Mediat. Inflamm. 2019;2019 doi: 10.1155/2019/3730519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang S., Gao X., Meng J., Zhang A., Zhou Y., Long M., et al. Metagenomic analysis of bacteria, fungi, bacteriophages, and helminths in the gut of giant pandas. Front. Microbiol. 2018;9:1717. doi: 10.3389/fmicb.2018.01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prazak J., Valente L., Iten M., Grandgirard D., Leib S.L., Jakob S.M., et al. Nebulized bacteriophages for prophylaxis of experimental ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus. Crit. Care Med. 2020;48:1042–1046. doi: 10.1097/CCM.0000000000004352. [DOI] [PubMed] [Google Scholar]
- 101.Ngoc B.V.T., Bich H.H., Galazzo G., Viet D.V.T., Oomen M., Minh T.N.N., et al. 2021. Cross-sectional Analysis of the Microbiota of the Human Gut and Their Direct Environment (Exposome) in a Household Cohort in Northern Vietnam. bioRxiv. [Google Scholar]
- 102.Zuo T., Zhang F., Lui G.C., Yeoh Y.K., Li A.Y., Zhan H., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955. doi: 10.1053/j.gastro.2020.05.048. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yeoh Y.K., Zuo T., Lui G.C.-Y., Zhang F., Liu Q., Li A.Y., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Górski A., Bollyky P.L., Przybylski M., Borysowski J., Międzybrodzki R., Jończyk-Matysiak E., et al. Perspectives of phage therapy in non-bacterial infections. Front. Microbiol. 2019;9:3306. doi: 10.3389/fmicb.2018.03306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Górski A., Dąbrowska K., Międzybrodzki R., Weber-Dąbrowska B., Łusiak-Szelachowska M., Jończyk-Matysiak E., et al. Phages and immunomodulation. Future Microbiol. 2017;12:905–914. doi: 10.2217/fmb-2017-0049. [DOI] [PubMed] [Google Scholar]
- 106.Sweere J.M., Van Belleghem J.D., Ishak H., Bach M.S., Popescu M., Sunkari V., et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science. 2019;363 doi: 10.1126/science.aat9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Górski A., Borysowski J., Miȩdzybrodzki R. Bacteriophage interactions with epithelial cells: therapeutic implications. Front. Microbiol. 2020:11. doi: 10.3389/fmicb.2020.631161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Górski A., Międzybrodzki R., Żaczek M., Borysowski J. Future Medicine; 2020. Phages in the Fight against COVID-19? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mishra V.N., Kumari N., Pathak A., Chaturvedi R.K., Gupta A.K., Chaurasia R.N. Possible role for bacteriophages in the treatment of SARS-CoV-2 infection. Int. J. Microbiol. 2020:2020. doi: 10.1155/2020/8844963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Belleghem J.D., Clement F., Merabishvili M., Lavigne R., Vaneechoutte M. Pro-and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-08336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sausset R., Petit M., Gaboriau-Routhiau V., De Paepe M. New insights into intestinal phages. Mucosal Immunol. 2020;13:205–215. doi: 10.1038/s41385-019-0250-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Febvre H.P., Rao S., Gindin M., Goodwin N.D., Finer E., Vivanco J.S., et al. PHAGE study: effects of supplemental bacteriophage intake on inflammation and gut microbiota in healthy adults. Nutrients. 2019;11:666. doi: 10.3390/nu11030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shahin K., Zhang L., Mehraban M.H., Collard J.-M., Hedayatkhah A., Mansoorianfar M., et al. Clinical and experimental bacteriophage studies: recommendations for possible approaches for standing against SARS-CoV-2. Microb. Pathog. 2022 doi: 10.1016/j.micpath.2022.105442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garcia-Gonzalez N., Battista N., Prete R., Corsetti A. Health-promoting role of lactiplantibacillus plantarum isolated from fermented foods. Microorganisms. 2021;9:349. doi: 10.3390/microorganisms9020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Toh Z.Q., Anzela A., Tang M.L., Licciardi P.V. Probiotic therapy as a novel approach for allergic disease. Front. Pharmacol. 2012;3:171. doi: 10.3389/fphar.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van den Nieuwboer M., van Hemert S., Claassen E., de Vos W.M. Lactobacillus plantarum WCFS 1 and its host interaction: a dozen years after the genome. Microb. Biotechnol. 2016;9:452–465. doi: 10.1111/1751-7915.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martens K., Pugin B., De Boeck I., Spacova I., Steelant B., Seys S., et al. Probiotics for the airways: potential to improve epithelial and immune homeostasis. Allergy. 2018;73:1954–1963. doi: 10.1111/all.13495. [DOI] [PubMed] [Google Scholar]
- 118.Feleszko W., Jaworska J., Rha R.D., Steinhausen S., Avagyan A., Jaudszus A., et al. Probiotic‐induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory‐dependent mechanisms in a murine model of asthma. Clin. Exp. Allergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 119.Mayer S., Raulf M.-K., Lepenies B. C-type lectins: their network and roles in pathogen recognition and immunity. Histochem. Cell Biol. 2017;147:223–237. doi: 10.1007/s00418-016-1523-7. [DOI] [PubMed] [Google Scholar]
- 120.Hoang T.K., He B., Wang T., Tran D.Q., Rhoads J.M., Liu Y. Protective effect of Lactobacillus reuteri DSM 17938 against experimental necrotizing enterocolitis is mediated by Toll-like receptor 2. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G231–G240. doi: 10.1152/ajpgi.00084.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Piqué N., Berlanga M., Miñana-Galbis D. Health benefits of heat-killed (Tyndallized) probiotics: an overview. Int. J. Mol. Sci. 2019;20:2534. doi: 10.3390/ijms20102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Plaza-Diaz J., Ruiz-Ojeda F.J., Gil-Campos M., Gil A. Mechanisms of action of probiotics. Adv. Nutr. 2019;10:S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Strober W., Murray P.J., Kitani A., Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 124.Wu C.-T., Chen P.-J., Lee Y.-T., Ko J.-L., Lue K.-H. Effects of immunomodulatory supplementation with Lactobacillus rhamnosus on airway inflammation in a mouse asthma model. J. Microbiol. Immunol. Infect. 2016;49:625–635. doi: 10.1016/j.jmii.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 125.Ludwig I.S., Broere F., Manurung S., Lambers T.T., van der Zee R., van Eden W. Lactobacillus rhamnosus GG-derived soluble mediators modulate adaptive immune cells. Front. Immunol. 2018;9:1546. doi: 10.3389/fimmu.2018.01546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Galdeano C.M., Perdigon G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine Immunol. 2006;13:219–226. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim J.Y., Park M.S., Ji G.E. Probiotic modulation of dendritic cells co-cultured with intestinal epithelial cells. World J. Gastroenterol.: WJG. 2012;18:1308. doi: 10.3748/wjg.v18.i12.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun K.-Y., Xu D.-H., Xie C., Plummer S., Tang J., Yang X.F., et al. Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine. 2017;92:1–11. doi: 10.1016/j.cyto.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 129.Paveljšek D., Ivičak‐Kocjan K., Treven P., Benčina M., Jerala R., Rogelj I. Distinctive probiotic features share common TLR2‐dependent signalling in intestinal epithelial cells. Cell Microbiol. 2021;23 doi: 10.1111/cmi.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Paveljšek D., Juvan P., Košir R., Rozman D., Hacin B., Ivičak-Kocjan K., et al. Lactobacillus fermentum L930BB and Bifidobacterium animalis subsp. animalis IM386 initiate signalling pathways involved in intestinal epithelial barrier protection. Benef. Microbes. 2018;9:515–525. doi: 10.3920/BM2017.0107. [DOI] [PubMed] [Google Scholar]
- 131.Llewellyn A., Foey A. Probiotic modulation of innate cell pathogen sensing and signaling events. Nutrients. 2017;9:1156. doi: 10.3390/nu9101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ding Y.-H., Qian L.-Y., Pang J., Lin J.-Y., Xu Q., Wang L.-H., et al. The regulation of immune cells by Lactobacilli: a potential therapeutic target for anti-atherosclerosis therapy. Oncotarget. 2017;8 doi: 10.18632/oncotarget.18346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sheikhi A., Giti H., Heibor M.R., Jafarzadeh A., Shakerian M., Baharifar N., et al. Lactobacilus delbrueckii subsp. bulgaricus modulates the secretion of Th1/Th2 and Treg cell-related cytokines by PBMCs from patients with atopic dermatitis. Drug research. 2017;67:724–729. doi: 10.1055/s-0043-117612. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Y., Li A-l, Sun Y-q, Du P., Liu L-b, Li S., et al. Lactobacillus acidophilus regulates STAT3 and STAT5 signaling in bovine β-lg-sensitized mice model. Dairy Sci. Technol. 2016;96:501–512. [Google Scholar]
- 135.Djaldetti M., Bessler H. Probiotic strains modulate cytokine production and the immune interplay between human peripheral blood mononucear cells and colon cancer cells. FEMS Microbiol. Lett. 2017;364:fnx014. doi: 10.1093/femsle/fnx014. [DOI] [PubMed] [Google Scholar]
- 136.Plaza-Diaz J., Gomez-Llorente C., Fontana L., Gil A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J. Gastroenterol.: WJG. 2014;20 doi: 10.3748/wjg.v20.i42.15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meng D., Zhu W., Ganguli K., Shi H.N., Walker W.A. Anti-inflammatory effects of Bifidobacterium longum subsp infantis secretions on fetal human enterocytes are mediated by TLR-4 receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;311:G744–G753. doi: 10.1152/ajpgi.00090.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rigaux P., Daniel C., Hisbergues M., Muraille E., Hols P., Pot B., et al. Immunomodulatory properties of Lactobacillus plantarum and its use as a recombinant vaccine against mite allergy. Allergy. 2009;64:406–414. doi: 10.1111/j.1398-9995.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 139.Ewaschuk J.B., Diaz H., Meddings L., Diederichs B., Dmytrash A., Backer J., et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G1025–G1034. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 140.Menard S., Candalh C., Bambou J., Terpend K., Cerf-Bensussan N., Heyman M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut. 2004;53:821–828. doi: 10.1136/gut.2003.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ogita T., Tanii Y., Morita H., Suzuki T., Tanabe S. Suppression of Th17 response by Streptococcus thermophilus ST28 through induction of IFN-γ. Int. J. Mol. Med. 2011;28:817–822. doi: 10.3892/ijmm.2011.755. [DOI] [PubMed] [Google Scholar]
- 142.Mansour N.M., Heine H., Abdou S.M., Shenana M.E., Zakaria M.K., El‐Diwany A. Isolation of Enterococcus faecium NM113, Enterococcus faecium NM213 and Lactobacillus casei NM512 as novel probiotics with immunomodulatory properties. Microbiol. Immunol. 2014;58:559–569. doi: 10.1111/1348-0421.12187. [DOI] [PubMed] [Google Scholar]
- 143.Sparo M., Delpech G., Batisttelli S., Já Basualdo. Immunomodulatory properties of cell wall extract from Enterococcus faecalis CECT7121. Braz. J. Infect. Dis. 2014;18:551–555. doi: 10.1016/j.bjid.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kimoto H., Mizumachi K., Okamoto T., Ji Kurisaki. New Lactococcus strain with immunomodulatory activity: enhancement of Th1‐type immune response. Microbiol. Immunol. 2004;48:75–82. doi: 10.1111/j.1348-0421.2004.tb03490.x. [DOI] [PubMed] [Google Scholar]
- 145.Lee N.-K., Han K.J., Son S.-H., Eom S.J., Lee S.-K., Paik H.-D. Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT--Food Sci. Technol. 2015;64:1036–1041. [Google Scholar]
- 146.Engelsöy U., Rangel I., Demirel I. Impact of proinflammatory cytokines on the virulence of uropathogenic Escherichia coli. Front. Microbiol. 2019;10:1051. doi: 10.3389/fmicb.2019.01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Singh A.K., Gaur V., Kumar A. Bacteriophages: IntechOpen; 2021. Role of Phage Therapy in COVID-19 Infection: Future Prospects. [Google Scholar]
- 148.Zhang L., Hou X., Sun L., He T., Wei R., Pang M., et al. Staphylococcus aureus bacteriophage suppresses LPS-induced inflammation in MAC-T bovine mammary epithelial cells. Front. Microbiol. 2018;9:1614. doi: 10.3389/fmicb.2018.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Borysowski J., Przybylski M., Międzybrodzki R., Owczarek B., Górski A. The effects of bacteriophages on the expression of genes involved in antimicrobial immunity. Adv. Hygiene Exp. Med/Postepy Higieny I Medycyny Doswiadczalnej. 2019;73 [Google Scholar]
- 150.Sanmukh S., Dos Santos S., Felisbino S. Natural bacteriophages T4 and M13 down-regulates Hsp90 gene expression in human prostate cancer cells (PC-3) representing a potential nanoparticle against cancer. Virol. Res. J. 2017;1(1):21–23. Virol Res J 2017 Volume 1 Issue. 2017;1. [Google Scholar]
- 151.Krut O., Bekeredjian-Ding I. Contribution of the immune response to phage therapy. J. Immunol. 2018;200:3037–3044. doi: 10.4049/jimmunol.1701745. [DOI] [PubMed] [Google Scholar]
- 152.Wang G., Liu Y., Feng H., Chen Y., Yang S., Wei Q., et al. Immunogenicity evaluation of MS2 phage-mediated chimeric nanoparticle displaying an immunodominant B cell epitope of foot-and-mouth disease virus. PeerJ. 2018;6 doi: 10.7717/peerj.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jeon J., Park J.-H., Yong D. Efficacy of bacteriophage treatment against carbapenem-resistant Acinetobacter baumannii in Galleria mellonella larvae and a mouse model of acute pneumonia. BMC Microbiol. 2019;19:1–14. doi: 10.1186/s12866-019-1443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang J.-L., Kuo C.-F., Yeh C.-M., Chen J.-R., Cheng M.-F., Hung C.-H. Efficacy of φkm18p phage therapy in a murine model of extensively drug-resistant Acinetobacter baumannii infection. Infect. Drug Resist. 2018;11:2301. doi: 10.2147/IDR.S179701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Data will be made available on request.