Abstract
Although the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant has spread, data on the clinical characteristics of infected patients are limited. In this study, the demographic, clinical characteristics, and laboratory data of 310 SARS-CoV-2 Omicron variant patients treated at Haihe Hospital of Tianjin were collected and analyzed. Information on these patients was compared to 96 patients with the Delta variant of concern (VOC) and 326 patients with the Beta VOC during the previous coronavirus disease 2019 (COVID-19) outbreak in Harbin. Of the 310 patients infected with the Omicron variant, the median age was 35 years. Most patients were clinically classified as mild (57.74%), and the most common symptoms were cough (48.71%), fever (39.35%), and sore throat (38.26%). The results for different vaccination groups in the Omicron group showed that the median of “SARS-CoV-2 specific IgG” after 2 or 3 doses of vaccination was higher than the unvaccinated group (all Ps < 0.05). Older age was associated with a higher proportion of moderate cases and lower asymptomatic and mild cases based on clinical classifications. Compared to the Delta and Beta groups, the median age of the Omicron group was younger. The total number of asymptomatic patients and mild patients in the Omicron virus group was higher than the Delta and Beta groups (60.97% vs. 54.17% vs. 47.55%). This study presented the clinical characteristics of the first group of patients infected with the Omicron variant in Tianjin, China, and compared their clinical features with patients infected by the Delta and Beta variants, which would increase our understanding of the characteristics of SARS-CoV-2 Omicron variant.
Keywords: SARS-CoV-2, Omicron, Clinical features, Vaccine, Age, Disease severity
Highlights
-
•
Clinical features of the Chinese first group of patients infected Omicron variants were studied.
-
•
The effects of vaccine and age on Omicron group were analyzed.
-
•
Compared with the Delta and Beta groups, the symptoms in Omicron group were less severe.
1. Introduction
Coronavirus disease 2019 (COVID-19) was caused by a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak in December 2019. As of July 14, 2022 over 555 million confirmed cases and over 6.3 million deaths have been reported worldwide (WHO, 2022a). SARS-CoV-2 has evolved and emerged into multiple variants. Variants of concern (VOCs), as previously defined by the World Health Organization (WHO), include Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2) variants. A new variant named Omicron (B.1.1.529) was confirmed as the fifth VOC by the WHO on November 26, 2021, and it attracted global attention because of the large number of mutations (WHO, 2022b).
Omicron has become the main variant in many countries within a short period because of its super-viral transmission capacity (GISAID, 2022). The infectivity and antibody resistance of SARS-CoV-2 is primarily determined by the binding of angiotensin-converting enzyme 2 (ACE2) and spike protein receptor-binding domain (RBD) complexes. The Omicron variant has 15 mutations in the RBD (Walls et al., 2020; Yan et al., 2020; Chen et al., 2022) that lead to higher viral transmission capacity and immune evasion (Greaney et al., 2021; Harvey et al., 2021). Therefore, the Omicron variant may be more contagious than other variants. However, information on the clinical characteristics of patients infected by the Omicron variant is limited and additional research is needed.
The clinical characteristics of patients with the Omicron variant have been described in research from the United Kingdom, South Korea, and Norway, and the prominent clinical manifestations include runny nose, headache, fatigue, cough, sore throat, fever, and expectoration (Brandal et al., 2021; Iacobucci, 2021; Kim et al., 2022). However, corresponding research has not been performed in China. After the first case of Omicron infection was found on January 8 in Tianjin, China, we continuously collected data from 310 patients infected by the Omicron variant. Our research examined the clinical characteristics and the effects of age and vaccination dose on Omicron-infected patients. We also compared their clinical features with those of patients infected by the Delta and Beta variants to explore whether this new variant had stronger pathogenicity.
2. Materials and methods
2.1. Study design and participants
We performed a retrospective study to analyze the clinical characteristics of patients infected with the SARS-CoV-2 Omicron variant and the differences between the Omicron variant and the Delta and Beta variants.
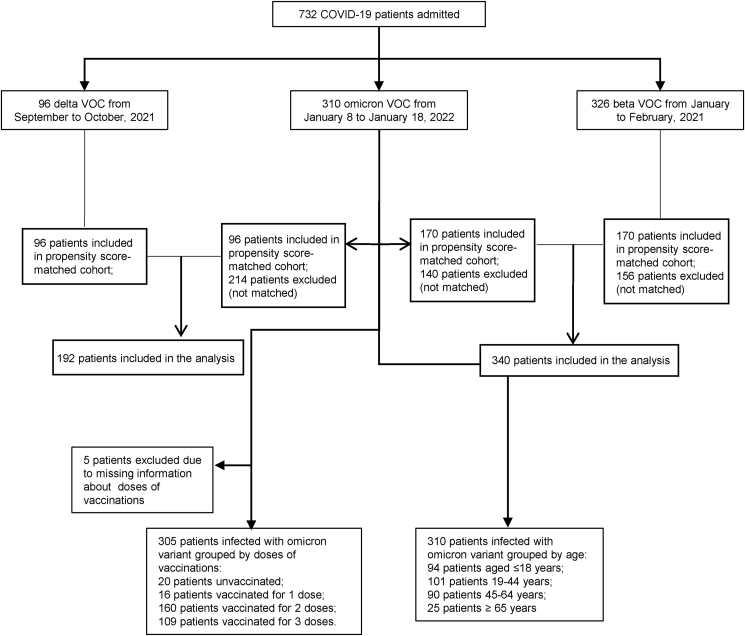
A total of 732 COVID-19 patients were preliminarily involved in this study. The 310 Omicron variant cases (from the Haihe Hospital of Tianjin, admission date from January 8 to January 18, 2022) were included in the Omicron group; the 96 Delta variant cases (from the First Affiliated Hospital of Harbin Medical University, admission date from September 20 to October 27, 2021) were included in the Delta VOC group; and the 326 Beta variant cases (from the First Affiliated Hospital of Harbin Medical University, admission date from January to February 2021) were included in the Beta group.
All procedures in the studies involving human participants were performed in accordance with the ethical standards of the institutional research committee and complied with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
2.2. Data collection
The demographic information, clinical characteristics, and laboratory findings of each patient were obtained and collected from the Haihe Hospital of Tianjin and the First Affiliated Hospital of Harbin Medical University. The data were recorded from the first day of admission (Fig. 1). The patients were grouped by age and vaccination doses.
Fig. 1.
Flow chart.
All diagnoses and clinical classifications of COVID-19 were based on the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 8) issued by the Chinese National Health Committee. Clinically, the disease is divided into four types of cases: mild, moderate, severe, and critical. Mild cases were defined as cases with mild clinical symptoms and no sign of pneumonia on imaging. Moderate cases were defined as cases with a fever and respiratory symptoms with radiological findings of pneumonia. Severe COVID-19 cases were defined as cases with one of the following criteria: (a) respiratory distress with respiratory frequency ≥ 30/min; (b) pulse oximeter oxygen saturation ≤ 93% at rest; and (c) oxygenation index (artery partial pressure of oxygen/inspired oxygen fraction, PaO2/FiO2) ≤ 300 mm Hg. There were no critical COVID-19 patients in this study.
2.3. Statistical analysis
SAS 9.4 software was used for the statistical analyses. Quantitative data with a normal distribution are statistically described as the means ± standard deviation (), and two independent-samples t-tests were performed for comparisons between two groups (the statistic is the t value). Analysis of variance was used to compare three groups (the statistic is F value), and quantitative data with a skewed distribution are statistically described by the medians and interquartile range [M(P25, P75)]. The Wilcoxon rank-sum test was used for comparisons between two groups (statistic is Z value), the Kruskal-Wallis test was used for comparisons between three groups (statistic is H value), and the Bonferroni method was used for pairwise comparisons between multiple groups. The frequency (percentage) was used for the statistical description of qualitative data, and the chi-squared test or Fisher's exact probability method was used to compare the composition between two groups (statistic is χ2 value). The test level of the hypothesis test was α = 0.05, and P < 0.05 indicated that the difference was statistically significant. Ordinal logistic regression models were used to analyze the factors that differed between patients of different ages within the Omicron group, and multinomial logistic regression models were used to analyze the factors that differed between patients infected with different variants (using the Omicron group as a control and splitting the model into two dichotomous logistic regression models to facilitate the presentation of results). The Spearman rank correlation coefficient was calculated for correlation analysis of quantitative data, and the Cramer V coefficient was calculated for correlation analysis of qualitative data. Correlation analysis of two ordered categorical variables calculated gamma coefficients.
3. Results
3.1. Clinical characteristics of patients infected with the Omicron VOC
The demographic and clinical characteristics of the 310 Omicron variant confirmed patients are summarized in Table 1. The median age was 35 years, and 94 patients (30.32%) were children aged <18 years. A total of 174 patients (56.13%) were women. A total of 269 patients (88.20%) received two or three doses of the vaccine (the COVID-19 vaccination status of five patients was not recorded) (Table 1).
Table 1.
Demographics, baseline characteristics, and clinical features of 310 patients infected with the SARS-CoV-2 B.1.1.529 (Omicron) variant.
| Characteristics | No. (%) of patients |
|---|---|
| Age groups(y) | |
| ≤ 18 | 94 (30.32) |
| 19–44 | 101 (32.58) |
| 45–64 | 90 (29.03) |
| ≥ 65 | 25 (8.06) |
| Gender | |
| Male | 136 (43.87) |
| Female | 174 (56.13) |
| Vaccination | |
| Without record | 5 (1.61) |
| Unvaccinated | 20 (6.45) |
| 1 Dose | 16 (5.16) |
| 2 Doses | 160 (51.61) |
| 3 Doses | 109 (35.16) |
| Clinical classifications | |
| Asymptomatic | 10 (3.23) |
| Mild | 179 (57.74) |
| Moderate | 118 (38.06) |
| Severe | 3 (0.97) |
| Laboratory findings | |
| IgG | 20.17 (44.05) |
| IgM | 26.58 (49.37) |
| Symptoms | |
| Fever | 122 (39.35) |
| Cough | 151 (48.71) |
| Sore Throat | 118 (38.26) |
| Feeble | 29 (9.35) |
| Headache | 21 (6.78) |
| Muscular Soreness | 16 (5.16) |
| Stuffy Nose | 28 (9.03) |
| Running Nose | 31 (10.00) |
| Taste Loss | 2 (0.65) |
| Anosmia | 5 (1.61) |
| Diarrhea | 10 (3.23) |
Note: Data are shown as number (%) or mean (standard deviation).
Most patients were clinically classified as mild (57.74%). Other clinical classifications were asymptomatic (3.23%), moderate (38.06%), and severe (0.97%). The most common symptoms were cough (48.71%), fever (39.35%), sore throat (38.26%), runny nose (10.00%), feeble (9.35%), and stuffy nose (9.03%) (Table 1).
3.2. Results of the comparisons between clinical features and symptoms for different vaccination groups in the Omicron group
According to the status of vaccinations, 305 out of 310 patients with vaccination records in the Omicron group were divided into four groups: the unvaccinated group, the 1-dose group, the 2-dose group, and the 3-dose group. Statistically significant differences were observed in the “SARS-CoV-2 specific IgG/IgM, age and C-reactive protein (CRP)" status between the four groups (P < 0.05). A further comparison revealed that the median “SARS-CoV-2-specific IgG” value after 2 or 3 doses of vaccination was higher than the unvaccinated group (1.61/0.56 vs. 0.08, all P < 0.05) and the median “SARS-CoV-2-specific IgM” value of the 2-dose group was higher than the unvaccinated group (1.49 vs. 0.09, P < 0.05). The median “CRP” value in the 3-dose group was higher than the 2-dose group (4.83 vs. 1.94, P < 0.05) (Table 2).
Table 2.
Comparison results of clinical features and symptoms for different vaccination groups in the Omicron group.
| Unvaccinated (n = 20) | 1 dose (n = 16) | 2 doses (n = 160) | 3 doses (n = 109) | F/H/χ2 | P value | |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| Age | 29 (2.5–66)f | 39 (15.5–50) | 16 (10–46) | 45 (35–56) | 44.2179 | < 0.0001 |
| Gender | 2.2694 | 0.5184 | ||||
| Female | 13 (65.00) | 7 (43.75) | 93 (58.13) | 58 (53.21) | ||
| Male | 7 (35.00) | 9 (56.25) | 67 (41.88) | 51 (46.79) | ||
| Clinical classifications | 0.0583 | |||||
| Asymptomatic | 1 (5.00) | 0 (0.00) | 6 (3.75) | 3 (2.75) | ||
| Mild | 8 (40.00) | 9 (56.25) | 102 (63.75) | 57 (52.29) | ||
| Moderate | 10 (50.00) | 6 (37.50) | 52 (32.50) | 48 (44.04) | ||
| Severe | 1 (5.00) | 1 (6.25) | 0 (0.00) | 1 (0.92) | ||
| WBC, × 109/L | 5.37 (4.21–7.3) | 6.05 (4.71–7.25) | 5.35 (4.26–6.71) | 5.41 (4.55–7.01) | 2.6866 | 0.4425 |
| LYMPH, × 109/L | 1.61 (0.73–2.48) | 1.85 (0.9–2.05) | 1.61 (1.06–2.18) | 1.32 (0.93–1.76) | 6.7698 | 0.0796 |
| N%, % | 50.65 ± 16.10 | 57.41 ± 16.51 | 53.84 ± 14.88 | 58.21 ± 11.61 | 2.92 | 0.0343 |
| CRP, mg/L | 1.83 (0.51–8.44)f | 4.64 (1.32–12.86) | 1.94 (0.72–4.72) | 4.83 (1.78–8.77) | 15.3650 | 0.0015 |
| IL–6, pg/mL | 9.85 (4–16.55) | 8.5 (4.7–10.1) | 6.15 (4.4–11.6) | 7.1 (4.7–8.8) | 0.6339 | 0.8886 |
| PCT, ng/mL | 0.07 (0.05–0.12) | 0.09 (0.07–0.09) | 0.05 (0.04–0.07) | 0.05 (0.04–0.08) | 4.8213 | 0.1854 |
| CD4, cell/μL | / | 662.85 (535.6–756.7) | 528.1 (267.44–770.9) | 599.15 (396.25–842.9) | 1.2230 | 0.5425 |
| CD8, cell/μL | / | 689.68 (347.07–888.41) | 295.55 (186.65–481.16) | 357.35 (213.42–480.06) | 2.7537 | 0.2524 |
| IgG | 0.08 (0.06–0.09)b,c | 0.43 (0.34–1.94) | 1.61 (0.26–22.92) | 0.56 (0.23–23.48) | 12.2657 | 0.0065 |
| IgM | 0.09 (0.06–0.11)b | 3.08 (0.62–25.79) | 1.49 (0.29–24.88) | 11.33 (0.14–40.16) | 8.3235 | 0.0398 |
| Symptoms | ||||||
| Fever | 7.2410 | 0.0646 | ||||
| Yes | 4 (20.00) | 7 (43.75) | 72 (45.00) | 36 (33.03) | ||
| No | 16 (80.00) | 9 (56.25) | 88 (55.00) | 73 (66.97) | ||
| Cough | 0.8427 | 0.8392 | ||||
| Yes | 10 (50.00) | 6 (37.50) | 79 (49.38) | 53 (48.62) | ||
| No | 10 (50.00) | 10 (62.50) | 81 (50.63) | 56 (51.38) | ||
| Sore throat | 17.6809 | 0.0005 | ||||
| Yes | 3 (15.00)c,f | 4 (25.00) | 51 (31.88) | 57 (52.29) | ||
| No | 17 (85.00) | 12 (75.00) | 109 (68.13) | 52 (47.71) | ||
| Feeble | 0.6880 | |||||
| Yes | 2 (10.00) | 0 (0.00) | 15 (9.38) | 12 (11.01) | ||
| No | 18 (90.00) | 16 (100.00) | 145 (90.63) | 97 (88.99) | ||
| Headache | 0.6214 | |||||
| Yes | 1 (5.00) | 0 (0.00) | 14 (8.81) | 6 (5.50) | ||
| No | 19 (95.00) | 16 (100.00) | 145 (91.19) | 103 (94.50) | ||
| Muscular soreness | 0.6508 | |||||
| Yes | 1 (5.00) | 0 (0.00) | 7 (4.38) | 8 (7.34) | ||
| No | 19 (95.00) | 16 (100.00) | 153 (95.63) | 101 (92.66) | ||
| Stuffy nose | 0.2583 | |||||
| Yes | 3 (15.00) | 0 (0.00) | 12 (7.50) | 13 (11.93) | ||
| No | 17 (85.00) | 16 (100.00) | 148 (92.50) | 96 (88.07) | ||
| Running nose | 1.0000 | |||||
| Yes | 2 (10.00) | 1 (6.67) | 17 (10.69) | 11 (10.09) | ||
| No | 18 (90.00) | 14 (93.33) | 142 (89.31) | 98 (89.91) | ||
| Taste loss | 0.3494 | |||||
| Yes | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (1.83) | ||
| No | 20 (100.00) | 16 (100.00) | 160 (100.00) | 107 (98.17) | ||
| Anosmia | 0.3044 | |||||
| Yes | 0 (0.00) | 0 (0.00) | 1 (0.63) | 4 (3.67) | ||
| No | 20 (100.00) | 16 (100.00) | 159 (99.38) | 105 (96.33) | ||
| Diarrhea | 0.2370 | |||||
| Yes | 0 (0.00) | 0 (0.00) | 3 (1.90) | 7 (6.48) | ||
| No | 20 (100.00) | 15 (100.00) | 155 (98.10) | 101 (93.52) | ||
| Others | 0.1889 | |||||
| Yes | 1 (5.88) | 0 (0.00) | 16 (17.78) | 4 (7.02) | ||
| No | 16 (94.12) | 7 (100.00) | 74 (82.22) | 53 (92.98) | ||
Note: "a" indicates that the difference between the unvaccinated group and receipt of 1 dose group are statistically significant; "b" indicates that the difference between the unvaccinated group and receipt of 2 doses group are statistically significant; "c" indicates that the difference between the unvaccinated group and receipt of 3 doses group are statistically significant; "d" indicates that the difference between the receipt of 1 dose and 2 doses group are statistically significant; "e" indicates that the difference between the receipt of 1 dose and 3 doses group are statistically significant; "f" indicates that the difference between the receipt of 2 doses and 3 doses group are statistically significant.
WBC: white blood cell; LYMPH: lymphocyte count; N%: neutrophil percentage; CRP: C-reactive protein; PCT: procalcitonin.
Data are shown as number (%), mean ± standard deviation, or mean (range). P < 0.05 considered the difference to be statistically significant in the four groups.
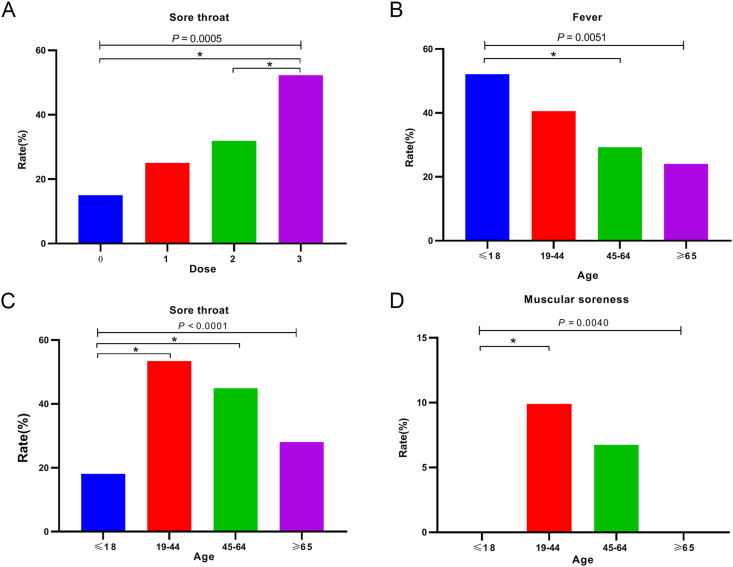
A statistically significant difference was observed in the occurrence of “sore throat” in the four groups (P < 0.005). Further pairwise comparisons showed that there was a statistically significant difference in the occurrence of sore throat between the 2-dose vaccination group and the 3-dose vaccination group (51 vs. 57, P < 0.005) (Fig. 2A, Table 2).
Fig. 2.
A Comparison results of sore throat symptoms in different inoculation dose groups of Omicron group. B Comparison of fever symptoms in different age groups in Omicron group. C Comparison of sore throat symptoms in different age groups in Omicron group. D Comparison of muscular soreness symptoms in different age groups in Omicron group. ∗ stands for P < 0.05.
3.3. Results of the comparison between the clinical features and symptoms for different age groups in the Omicron group
We divided the Omicron group into four age groups based on a previous study: children, < 18 years (n = 94); youth, 19–44 years (n = 101); middle-aged, 45–64 years (n = 90); and elderly, > 65 years old (n = 65) (Luo et al., 2020). The comparison results between the four groups stratified by age are shown in Table 3. Statistically significant differences were observed in the clinical characteristics between the four groups: SARS-CoV-2-specific IgM value (P = 0.0070), white blood cell (WBC) count (P = 0.0152), CRP (P < 0.0001), interleukin-6 (IL-6) content (P = 0.0097) and cluster of differentiation 8 (CD8) (P = 0.0071). A further comparison revealed that the ≤ 18 years group and 19–44 years group had specific differences in clinical classification (mild: 81.91% vs. 60.4%; moderate: 10.64% vs. 36.63%), lymphocyte count (LYMPH) (1.76 vs. 1.38), neutrophil percentage (N%) (51.32 ± 15.94 vs. 58.14 ± 13.60), CRP (1.40 vs. 3.48), and IL-6 (4.15 vs. 6.55). The ≤ 18 years age group and the 19–44 years age group had specific differences in their clinical classification (mild: 81.91% vs. 37.78%; moderate: 10.64% vs. 58.89%), LYMPH (1.76 vs. 1.34), CRP (1.40 vs. 3.42), and IL-6 (4.15 vs. 6.9). The ≤ 18 years group and ≥ 65 years group presented specific differences in clinical classification: mild (81.91% vs. 28%); moderate: (10.64% vs. 72%), WBC (5.64 vs. 4.61), LYMPH (1.76 vs. 1.14), CRP (1.40 vs. 3.79), IL-6 (4.15 vs. 9), CD8 (662.15 ± 65.92 vs. 204.41 ± 85.78), and IgM (1.24 vs. 0.09). The 19–44 years group and 45–64 years group had specific differences in clinical classification as mild (60.4% vs. 37.78%) and moderate (36.63% vs. 58.89%). The 19–44 age group and ≥ 65 age group had specific differences in IgM (6.49 vs. 0.09) (Table 3).
Table 3.
Comparison results of clinical features and symptoms for different age groups in the omicron group.
| ≤ 18 years (n = 94) | 19–44 years (n = 101) | 45–64 years (n = 90) | ≥ 65 years (n = 25) | F/H/χ2 | P value | |
|---|---|---|---|---|---|---|
| Baseline | 4.4651 | 0.2154 | ||||
| Gender | ||||||
| Female | 50 (53.19) | 55 (54.46) | 58 (64.44) | 11 (44.00) | ||
| Male | 44 (46.81) | 46 (45.54) | 32 (35.56) | 14 (56.00) | ||
| Vaccination, dose | 2 (2–2)a,b,e,f | 3 (2–3) | 3 (2–3) | 2 (1–2) | 66.7077 | <0.0001 |
| Clinical classifications | <0.0001 | |||||
| Asymptomatic | 7 (7.45)a,b,c,d | 1 (0.99) | 2 (2.22) | 0 (0.00) | ||
| Mild | 77 (81.91) | 61 (60.40) | 34 (37.78) | 7 (28.00) | ||
| Moderate | 10 (10.64) | 37 (36.63) | 53 (58.89) | 18 (72.00) | ||
| Severe | 0 (0.00) | 2 (1.98) | 1 (1.11) | 0 (0.00) | ||
| WBC, × 109/L | 5.64 (4.45–7.49)c | 5.71 (4.49–7.08) | 5.15 (4.24–6.6) | 4.61 (3.62–5.82) | 10.4331 | 0.0152 |
| LYMPH, × 109/L | 1.76 (1.29–2.49)a,b,c | 1.38 (0.94–1.86) | 1.34 (0.86–1.96) | 1.14 (0.7–1.62) | 24.9929 | <0.0001 |
| N%, % | 51.32 ± 15.94a | 58.14 ± 13.60 | 55.53 ± 11.95 | 58.66 ± 12.13 | 4.44 | 0.0045 |
| CRP, mg/L | 1.40 (0.41–3.53)a,b,c | 3.48 (0.90–8.77) | 3.42 (1.73–7.98) | 3.79 (2.00–12.10) | 22.8248 | <0.0001 |
| IL–6, pg/mL | 4.15 (3.1–5.05)a,b,c | 6.55 (4.8–9.6) | 6.9 (4.5–11) | 9 (5.9–12.3) | 11.4077 | 0.0097 |
| PCT, ng/mL | 0.69 (0.11–28) | 0.05 (0.04–0.07) | 0.04 (0.04–0.08) | 0.07 (0.04–0.09) | 10.1330 | 0.0175 |
| CD4, cell/μL | / | 563.95 (356.35–742.23) | 747.9 (426.68–870.2) | 408.51 (360.67–509.91) | 5.0635 | 0.0795 |
| CD8, cell/μL | 662.15 ± 65.92c | 354.92 ± 200.01 | 426.48 ± 236.06 | 204.41 ± 85.78 | 4.37 | 0.0071 |
| IgG | 6.76 (0.27–28.58) | 0.47 (0.23–1.34) | 0.7545 (0.14–24.86) | 2.13 (1.22–12.00) | 6.4003 | 0.0937 |
| IgM | 1.24 (0.22–18.92)c,e | 6.49 (0.56–51.91) | 1.93 (0.12–40.02) | 0.09 (0.05–0.31) | 12.1038 | 0.0070 |
| Symptoms | ||||||
| Fever | 12.7788 | 0.0051 | ||||
| Yes | 49 (52.13)b | 41 (40.59) | 26 (29.21) | 6 (24.00) | ||
| No | 45 (47.87) | 60 (59.41) | 63 (70.79) | 19 (76.00) | ||
| Cough | 4.4808 | 0.2140 | ||||
| Yes | 38 (40.43) | 52 (51.49) | 46 (51.69) | 15 (60.00) | ||
| No | 56 (59.57) | 49 (48.51) | 43 (48.31) | 10 (40.00) | ||
| Sore throat | <0.0001 | |||||
| Yes | 17 (18.09)a,b | 54 (53.47) | 40 (44.94) | 7 (28.00) | ||
| No | 77 (81.91) | 47 (46.53) | 49 (55.06) | 18 (72.00) | ||
| Feeble | 0.2678 | |||||
| Yes | 5 (5.32) | 10 (9.90) | 10 (11.24) | 4 (16.00) | ||
| No | 89 (94.68) | 91 (90.10) | 79 (88.76) | 21 (84.00) | ||
| Headache | 0.1092 | |||||
| Yes | 3 (3.19) | 12 (11.88) | 5 (5.68) | 1 (4.00) | ||
| No | 91 (96.81) | 89 (88.12) | 83 (94.32) | 24 (96.00) | ||
| Muscular soreness | 0.0040 | |||||
| Yes | 0 (0.00)a | 10 (9.90) | 6 (6.74) | 0 (0.00) | ||
| No | 94 (100.00) | 91 (90.10) | 83 (93.26) | 25 (100.00) | ||
| Stuffy nose | 0.4196 | |||||
| Yes | 10 (10.64) | 10 (9.90) | 8 (8.99) | 0 (0.00) | ||
| No | 84 (89.36) | 91 (90.10) | 81 (91.01) | 25 (100.00) | ||
| Running nose | 0.1085 | |||||
| Yes | 14 (15.05) | 11 (10.89) | 4 (4.49) | 2 (8.33) | ||
| No | 79 (84.95) | 90 (89.11) | 85 (95.51) | 22 (91.67) | ||
| Taste loss | 0.8005 | |||||
| Yes | 0 (0.00) | 1 (0.99) | 1 (1.12) | 0 (0.00) | ||
| No | 94 (100.00) | 100 (99.01) | 88 (98.88) | 25 (100.00) | ||
| Anosmia | 0.2035 | |||||
| Yes | 0 (0.00) | 3 (2.97) | 1 (1.12) | 1 (4.00) | ||
| No | 94 (100.00) | 98 (97.03) | 88 (98.88) | 24 (96.00) | ||
| Diarrhea | 0.2411 | |||||
| Yes | 1 (1.08) | 6 (6.06) | 3 (3.41) | 0 (0.00) | ||
| No | 92 (98.92) | 93 (93.94) | 85 (96.59) | 25 (100.00) | ||
| Others | 0.4144 | |||||
| Yes | 8 (14.55) | 3 (6.00) | 8 (16.00) | 2 (11.11) | ||
| No | 47 (85.45) | 47 (94.00) | 42 (84.00) | 16 (88.89) | ||
Note: "a" indicates that the difference between the two groups of ≤ 18 years and 19–44 years are statistically significant; "b" indicates that the difference between the two groups of ≤ 18 years and 45–64 years are statistically significant; "c" indicates that the difference between the two groups of ≤ 18 years and ≥ 65 years is statistically significant; "d" means the difference between the two groups of 19–44 years and 45–64 years is statistically significant; "e" means the difference between the two groups of 19–44 years and ≥65 years is statistically significant; “f" means that the difference between the 45–64 years and ≥ 65 years old is statistically significant.
WBC: white blood cell; LYMPH: lymphocyte count; N%: neutrophil percentage; CRP: C-reactive protein; PCT: procalcitonin.
Data are shown as number (%), mean ± standard deviation, or mean (range). P < 0.05 considered the difference to be statistically significant in the four groups.
Statistically significant differences were observed in the symptoms of fever (P = 0.0051), sore throat (P < 0.0001), and muscular soreness (P = 0.0040) between the four groups. Children were more prone to fever than the middle-aged group (52.13% vs. 29.21%) and rarely presented muscle soreness compared with to youth group (0 vs. 9.9%). The youth and middle-aged groups were more likely to have a sore throat than the children group (53.47%/44.94% vs. 18.09%) (Fig. 2B–D).
We performed univariate and multivariate logistic regression analyses in different age groups to explore different indicators. The results of univariate regression showed that the differences in eight indicators in patients of different ages were statistically significant: severity classification, number of vaccinations, WBC, N%, LYMPH, fever, sore throat, and stuffy nose. After performing multivariate logistic regression on the above factors, the differences in the following indicators remained statistically significant: severity classification (OR = 4.5677, 95% CI = 1.1405–18.2937, P = 0.0319), LYMPH (OR = 0.4887, 95% CI = 0.3659–0.6529, P < 0.0001), and fever (OR = 0.5251, 95% CI = 0.3279–0.8409, P = 0.0073) (Table 4).
Table 4.
Logistic regression analysis of different age groups in the omicron group.
| Univariable OR (95% CI) | P value | Multivariable OR (95% CI) | P value | |
|---|---|---|---|---|
| Clinical classifications (compared with asymptomatic) | ||||
| Mild | 2.7178 (0.7271–10.1582) | 0.1372 | 4.5677 (1.1405–18.2937) | 0.0319 |
| Moderate | 15.4785 (4.0069–59.7929) | <0.0001 | 22.2082 (5.3828–91.6265) | <0.0001 |
| Severe | 7.7149 (0.6727–88.4781) | 0.1007 | 8.6452 (0.441–169.469) | 0.1554 |
| Vaccination, dose (Compared with 0 dose) | ||||
| 1 | 1.1306 (0.3421–3.7367) | 0.8406 | 1.1496 (0.3017–4.3803) | 0.8381 |
| 2 | 0.3586 (0.1527–0.8422) | 0.0186 | 0.3226 (0.122–0.853) | 0.0226 |
| 3 | 1.8266 (0.7651–4.3606) | 0.1748 | 1.3926 (0.5262–3.6856) | 0.5048 |
| Fever | 0.4637 (0.3038–0.7077) | 0.0004 | 0.5251 (0.3279–0.8409) | 0.0073 |
| Sore throat | 1.777 (1.1674–2.7049) | 0.0073 | – | – |
| Running nose | 0.4604 (0.2302–0.9209) | 0.0283 | – | – |
| WBC, × 109/L | 0.8377 (0.7578–0.9259) | 0.0005 | – | – |
| N%, % | 1.0184 (1.0034–1.0335) | 0.0158 | – | – |
| LYMPH, × 109/L | 0.4801 (0.3649–0.6316) | <0.0001 | 0.4887 (0.3659–0.6529) | <0.0001 |
We performed a Spearman rank correlation analysis on severity classification, age, and the status of vaccinations among 310 patients within the Omicron group. The correlation coefficient between age and severity classification of COVID-19 patients was 0.44019 (P < 0.05). Within the Omicron group, age positively correlated with the severity classification of COVID-19 patients (Table 5).
Table 5.
Correlation analysis between age, clinical classifications, and vaccination.
| Age | Clinical classifications | Vaccination | |
|---|---|---|---|
| Age | 1 | – | – |
| Clinical classifications | 0.44019a | 1 | – |
| Vaccination | 0.29946a | 0.03140 | 1 |
Stands for P < 0.05.
3.4. Comparison between the Omicron, Delta, and Beta groups
The comparison results between the three groups are shown in Table 4. Significant differences were observed in age, clinical classification, WBC, N%, CRP, IL-6, PCT, and SARS-CoV-2-specific IgG/IgM between the three groups (P < 0.05). The mean age of patients was ordered as follows: Omicron group (35 years) < Delta group (42.5 years) < Beta group (52.5 years). The proportion of moderate-type patients was lower in the Omicron group than in the Delta and Beta groups (38.06% vs. 44.79% vs. 52.45%). The total number of asymptomatic patients and mild patients in the Omicron virus group was higher than in the Delta and Beta groups (60.97% vs. 54.17% vs. 47.55%). The Omicron patients had higher levels of CRP (2.86 vs. 2.44 vs. 0.50, P < 0.0001), PCT (0.05 vs. 0.03 vs. 0.03, P < 0.0001) and SARS-CoV-2-specific IgG (0.87 vs. 0.49 vs. 0.39, P < 0.0001) than the Delta and Beta patients. The IL-6 levels of patients in the Omicron group were higher than those in the Delta group (6.50 vs. 2.84, P < 0.0001) (Table 6).
Table 6.
Comparison between the omicron group and the Delta and Beta groups.
| Omicron (n = 310) | Delta (n = 96) | Beta (n = 326) | F/H/χ2 | P value | |
|---|---|---|---|---|---|
| Age(y) | 35 (11–53)a,b,c | 42.5 (28–56.5) | 52.5 (42–64) | 104.1613 | <0.0001 |
| Gender | |||||
| Female | 174 (56.13) | 53 (55.21) | 158 (48.47) | ||
| Male | 136 (43.87) | 43 (44.79) | 168 (51.53) | ||
| Vaccination, dose | 2 (2–3) | 2 (2–2) | 0 (0–0) | 556.4369 | <0.0001 |
| Clinical classifications | <0.0001 | ||||
| Asymptomatic | 10 (3.23)b,c | 4 (4.17) | 102 (31.29) | ||
| Mild | 179 (57.74) | 48 (50.00) | 53 (16.26) | ||
| Moderate | 118 (38.06) | 43 (44.79) | 171 (52.45) | ||
| Severe | 3 (0.97) | 1 (1.04) | 0 (0.00) | ||
| WBC, × 109/L | 5.405 (4.36–7.01)a,b,c | 6.18 (5–7.35) | 5.04 (4.05–6.25) | 24.6653 | <0.0001 |
| LYMPH, × 109/L | 1.48 (1–2.01) | 1.2 (0.5–2.5) | 1.555 (1.19–1.95) | 4.8005 | 0.0907 |
| N%, % | 55.35 (45.7–64.65) | 59.7 (50.8–67.2) | 56.95 (50.8–64.3) | 6.2088 | 0.0449 |
| CRP mg/L | 2.86 (0.91–7.37)b,c | 2.44 (0.50–7.68) | 0.5 (0.499–5.67) | 23.2142 | <0.0001 |
| IL–6, pg/mL | 6.5 (4.7–10.1) | 2.84 (1–5.62) | 5.22 (2.43–16.87) | 47.6800 | <0.0001 |
| PCT, ng/mL | 0.05 (0.04–0.08)a,b | 0.03 (0.02–0.04) | 0.03 (0.02–0.05) | 86.2541 | <0.0001 |
| CD4, cell/μL | 548.42 (366.23–788.86) | 618.5 (425–776) | 581.5 (462.5–841.5) | 1.5923 | 0.4511 |
| CD8, cell/μL | 334.28 (186.65–519.16) | 372 (286–526) | 347 (241–490) | 2.5548 | 0.2788 |
| IgG | 0.87 (0.24–20.99)a,b | 0.49 (0.43–0.58) | 0.39 (0.06–6.25) | 20.6799 | <0.0001 |
| IgM | 2.0625 (0.195–29.94)b,c | 3.15 (0.47–17.11) | 1.1 (0.08–7.41) | 22.0542 | <0.0001 |
| Symptoms | |||||
| Fever | 75.2992 | <0.0001 | |||
| Yes | 122 (39.35) a,b,c | 59 (61.46) | 58 (17.79) | ||
| No | 188 (60.65) | 37 (38.54) | 268 (82.21) | ||
| Cough | 73.0771 | <0.0001 | |||
| Yes | 151 (48.71) a,b,c | 82 (85.42) | 117 (35.89) | ||
| No | 159 (51.29) | 14 (14.58) | 209 (64.11) | ||
| Sore throat | 87.0197 | <0.0001 | |||
| Yes | 118 (38.06) b,c | 30 (31.25) | 24 (7.36) | ||
| No | 192 (61.94) | 66 (68.75) | 302 (92.64) | ||
| Feeble | 1.0775 | 0.5835 | |||
| Yes | 29 (9.35) | 7 (7.29) | 35 (10.74) | ||
| No | 281 (90.65) | 89 (92.71) | 291 (89.26) | ||
| Headache | 7.8568 | 0.0197 | |||
| Yes | 21 (6.77)a | 15 (15.63) | 25 (7.67) | ||
| No | 289 (93.23) | 81 (84.38) | 301 (92.33) | ||
| Muscular soreness | 0.4752 | ||||
| Yes | 16 (5.16) | 7 (7.29) | 14 (4.29) | ||
| No | 294 (94.84) | 89 (92.71) | 312 (95.71) | ||
| Stuffy nose | 36.9124 | <0.0001 | |||
| Yes | 28 (9.03) a,b,c | 20 (20.83) | 8 (2.45) | ||
| No | 282 (90.97) | 76 (79.17) | 318 (97.55) | ||
| Running nose | 18.8361 | <0.0001 | |||
| Yes | 31 (10.00) b,c | 17 (17.71) | 14 (4.29) | ||
| No | 279 (90.00) | 79 (82.29) | 312 (95.71) | ||
| Taste loss | 82.6751 | <0.0001 | |||
| Yes | 2 (0.65) a,c | 21 (21.88) | 9 (2.76) | ||
| No | 308 (99.35) | 75 (78.13) | 317 (97.24) | ||
| Anosmia | 93.0728 | <0.0001 | |||
| Yes | 5 (1.61) a,c | 23 (23.96) | 6 (1.84) | ||
| No | 305 (98.39) | 73 (76.04) | 320 (98.16) | ||
| Diarrhea | 22.1437 | <0.0001 | |||
| Yes | 10 (3.23) a,c | 14 (14.58) | 12 (3.68) | ||
| No | 300 (96.77) | 82 (85.42) | 314 (96.32) | ||
| Others | 254.8405 | <0.0001 | |||
| Yes | 21 (6.77) a,b,c | 81 (84.38) | 64 (19.63) | ||
| No | 289 (93.23) | 15 (15.63) | 262 (80.37) | ||
Note: "a" indicates that the difference between omicron group and delta group are statistically significant; "b" indicates that the difference between omicron group and beta group are statistically significant; "c" indicates that the difference between delta group and beta group are statistically significant.
WBC: white blood cell; LYMPH: lymphocyte count; N%: neutrophil percentage; CRP: C-reactive protein; IL-6: interleukin-6.
Data are shown as number (%), mean ± standard deviation, or mean (range). P < 0.05 considered the difference to be statistically significant in the three groups.
The most common symptoms in the three groups were fever, cough, and sore throat. However, there were statistically significant differences in the incidence of clinical symptoms between the three groups. The incidence of sore throat in Omicron patients was higher than in the Delta and Beta groups (38.06% vs. 31.25% vs. 7.35%, P < 0.0001). However, the Omicron group had a lower incidence of headache (6.77% vs. 15.63% vs. 7.67%, P = 0.0197), diarrhea (3.23% vs. 14.58% vs. 3.68%, P < 0.0001), taste loss (0.65% vs. 21.88% vs. 2.76%, P < 0.0001), and anosmia (1.61% vs. 23.96% vs. 1.84%, P < 0.0001) than the other two groups (Table 6).
We performed univariate and multivariate logistic regression analyses. Between the Omicron group and the Delta group, univariate analysis showed that 14 factors were statistically significant (P < 0.05), including CRP, IL-6, PCT, IgG, and other indicators. However, because the values were within the normal range, the clinical significance was small. Fever, cough, stuffy nose, taste loss, anosmia, diarrhea, and other symptoms were significantly different between the two groups (P < 0.05). Multivariate regression analysis was performed on these indicators, and the differences in these symptoms between groups were no longer statistically significant (Table 7). Between the Omicron and Beta groups, the results of univariate analysis showed statistically significant differences in age, severity classification, WBC, PCT, and percentage of neutrophils (P < 0.05). After multivariate analysis of these indicators, the results showed significant differences in age, type, and sore throat between the two groups (Table 8).
Table 7.
Logistic regression analysis of patients in the Delta group and the Omicron group.
| Univariable OR (95% CI) | P value | Multivariable OR (95% CI) | P value | |
|---|---|---|---|---|
| Age | 1.0166 (1.0052–1.028) | 0.0041 | – | – |
| Vaccination, dose (compared with 0 dose) | ||||
| 1 | 0.4614 (0.0897–1.9525) | 0.3863 | – | – |
| 2 | 0.9207 (0.3978–2.2363) | 0.9817 | – | – |
| 3 | 0.0127 (0–0.0642) | <0.0001 | – | – |
| IL–6, pg/mL | 0.8827 (0.828–0.9411) | 0.0001 | – | – |
| PCT, ng/mL | – | <0.0001 | – | – |
| IgG | 0.7216 (0.5706–0.9126) | 0.0065 | – | – |
| Fever | 2.4572 (1.5359–3.9314) | 0.0002 | – | – |
| Cough | 6.1675 (3.3546–11.339) | <0.0001 | – | – |
| Headache | 2.5485 (1.2568–5.1677) | 0.0095 | – | – |
| Stuffy nose | 2.6504 (1.4155–4.9627) | 0.0023 | – | – |
| Running nose | 1.9367 (1.0191–3.6806) | 0.0436 | – | – |
| Taste loss | 43.1109 (9.8918–187.8876) | <0.0001 | – | – |
| Anosmia | 19.219 (7.0684–52.2565) | <0.0001 | – | – |
| Diarrhea | 5.1217 (2.1947–11.9525) | 0.0002 | – | – |
| Others | 74.3057 (36.6452–150.67) | <0.0001 | 17.7151 (7.4181–42.3056) | <0.0001 |
Note: The PCT univariate or value is not accurate because the variable is continuous and the degree of variation is small, resulting in the inability to accurately estimate the change multiple of the PCT by 1 unit due to the PCT increment.
We use stepwise regression to screen variables. Therefore, a meaningful index in the univariate logistic regression may not have corresponding results in the multivariate logistic regression, and "–" is used to indicate that the index has no corresponding parameters in the multivariate logistic regression analysis.
Table 8.
Logistic regression analysis of patients in the Beta group and the Omicron group.
| Univariable OR (95% CI) | P value | Multivariable OR (95% CI) | P value | |
|---|---|---|---|---|
| Age | 1.0509 (1.0406–1.0614) | < 0.0001 | 1.0300 (1.0034–1.0574) | 0.0271 |
| Clinical classifications (compared with asymptomatic) | ||||
| Mild | 0.0294 (0.0128–0.0614) | < 0.0001 | 0.0472 (0.0099–0.2255) | 0.0001 |
| Moderate | 0.1426 (0.0637–0.2881) | < 0.0001 | 0.1642 (0.0337–0.7997) | 0.0253 |
| Severe | 0.0286 (0–0.1962) | < 0.0001 | – | – |
| WBC, × 109/L | 0.8524 (0.7823–0.9289) | 0.0003 | – | – |
| N%, % | 1.0143 (1.0013–1.0275) | 0.0313 | – | – |
| PCT, ng/mL | 0.0026 (0–0.4848) | 0.0257 | – | – |
| IgM | 0.9851 (0.9781–0.9922) | < 0.0001 | – | – |
| Fever | 0.3336 (0.2318–0.48) | < 0.0001 | – | – |
| Cough | 0.5895 (0.4291–0.8098) | 0.0011 | – | – |
| Sore throat | 0.1293 (0.0805–0.2079) | < 0.0001 | 0.0751 (0.0302–0.1866) | <0.0001 |
| Stuffy nose | 0.2534 (0.1136–0.565) | 0.0008 | – | – |
| Running nose | 0.404 (0.2106–0.7749) | 0.0064 | – | – |
| Others | 3.454 (2.0517–5.8147) | < 0.0001 | – | – |
Note: we use stepwise regression to screen variables. Therefore, a meaningful index in the univariate logistic regression may not have corresponding results in the multivariate logistic regression, and "–" is used to indicate that the index has no corresponding parameters in the multivariate logistic regression analysis.
To minimize the effect of potential confounding factors, we also performed propensity score matching to analyze patients of similar sex and age (Thomas et al., 2020; Lee et al., 2021). Patient age and sex were no longer significantly different after matching. A total of 192 patients (96 one-to-one matched patients in each cohort) were compared after the Omicron and Delta groups were matched. After matching, there were significant differences in the number of vaccinations, CRP, IL-6, PCT, fever, cough, nasal congestion, loss of taste, loss of smell, diarrhea, and other symptoms between the two groups (P < 0.05). The number of people who received 3 doses of vaccine in the Omicron group was significantly higher than in the Delta group (41.67% vs. 0%). However, the Delta variant was prevalent in the period when the development of the third dose vaccine was not yet mature. The incidence of fever, cough, stuffy nose, taste loss, anosmia, and diarrhea remained lower in the Omicron group than in the Delta group. The difference in the incidence of sore throat was no longer statistically significant (Table 9).
Table 9.
Comparison of one-to-one matched patients in the Delta group and Omicron group.
| Delta | Omicron | F/H/χ2 | P value | |
|---|---|---|---|---|
| Baseline | ||||
| Age(y) | 42.30 ± 18.87 | 42.01 ± 17.92 | 0.11 | 0.9127 |
| Gender | 0.3346 | 0.5629 | ||
| Female | 53 (55.21) | 49 (51.04) | ||
| Male | 43 (44.79) | 47 (48.96) | ||
| Vaccination, dose | <0.0001 | |||
| 0 | 11 (11.46) | 7 (7.29) | ||
| 1 | 4 (4.17) | 6 (6.25) | ||
| 2 | 81 (84.38) | 43 (44.79) | ||
| 3 | 0 (0.00) | 40 (41.67) | ||
| Clinical classifications | 0.5648 | |||
| Asymptomatic | 4 (4.17) | 1 (1.04) | ||
| Mild | 48 (50.00) | 53 (55.21) | ||
| Moderate | 43 (44.79) | 41 (42.71) | ||
| Severe | 1 (1.04) | 1 (1.04) | ||
| WBC, × 109/L | 6.18 (5–7.35) | 5.6 (4.31–7.07) | 1.5805 | 0.1140 |
| LYMPH, × 109/L | 1.2 (0.5–2.5) | 1.36 (1.03–2.06) | −1.1083 | 0.2677 |
| N%, % | 59.7 (50.8–67.2) | 57 (47.8–64) | 1.6016 | 0.1092 |
| CRP, mg/L | 2.44 (0.499–7.68) | 3.012 (1.18–7.95) | 0.8959 | 0.3703 |
| IL–6, pg/mL | 2.84 (1–5.62) | 5.95 (4.25–10.15) | 4.8635 | <0.0001 |
| PCT, ng/mL | 0.03 (0.02–0.04) | 0.0485 (0.04–0.073) | 6.0970 | <0.0001 |
| CD4, cell/μL | 618.5 (425–776) | 639.77 (381.88–861.89) | 0.5250 | 0.5996 |
| CD8, cell/μL | 37 2 (286–526) | 381.87 (196.44–574.97) | −0.1457 | 0.8842 |
| IgG | 0.49 (0.43–0.58) | 0.509 (0.22–2.19) | 0.2067 | 0.8362 |
| IgM | 3.15 (0.47–17.11) | 2.2305 (0.435–25.92) | −0.1803 | 0.8569 |
| Symptoms | ||||
| Fever | 7.5282 | 0.0061 | ||
| Yes | 59 (61.46) | 40 (41.67) | ||
| No | 37 (38.54) | 56 (58.33) | ||
| Cough | 28.9406 | <0.0001 | ||
| Yes | 82 (85.42) | 47 (48.96) | ||
| No | 14 (14.58) | 49 (51.04) | ||
| Sore throat | 3.7352 | 0.0533 | ||
| Yes | 30 (31.25) | 43 (44.79) | ||
| No | 66 (68.75) | 53 (55.21) | ||
| Feeble | 0.0723 | 0.7880 | ||
| Yes | 7 (7.29) | 8 (8.33) | ||
| No | 89 (92.71) | 88 (91.67) | ||
| Headache | 1.1497 | 0.2836 | ||
| Yes | 15 (15.63) | 10 (10.42) | ||
| No | 81 (84.38) | 86 (89.58) | ||
| Muscular soreness | 0.0723 | 0.7880 | ||
| Yes | 7 (7.29) | 8 (8.33) | ||
| No | 89 (92.71) | 88 (91.67) | ||
| Stuffy nose | 7.2835 | 0.0070 | ||
| Yes | 20 (20.83) | 7 (7.29) | ||
| No | 76 (79.17) | 89 (92.71) | ||
| Running nose | 2.8471 | 0.0915 | ||
| Yes | 17 (17.71) | 9 (9.38) | ||
| No | 79 (82.29) | 87 (90.63) | ||
| Taste loss | 20.5348 | <0.0001 | ||
| Yes | 21 (21.88) | 1 (1.04) | ||
| No | 75 (78.13) | 95 (98.96) | ||
| Anosmia | 23.0476 | <0.0001 | ||
| Yes | 23 (23.96) | 1 (1.04) | ||
| No | 73 (76.04) | 95 (98.96) | ||
| Diarrhea | 7.8091 | 0.0052 | ||
| Yes | 14 (14.58) | 3 (3.13) | ||
| No | 82 (85.42) | 93 (96.88) | ||
| Others | 118.2266 | <0.0001 | ||
| Yes | 81 (84.38) | 6 (6.25) | ||
| No | 15 (15.63) | 90 (93.75) | ||
Note: WBC: white blood cell; LYMPH: lymphocyte count; N%: neutrophil percentage; CRP: C-reactive protein; PCT: procalcitonin. Data are shown as number (%), mean ± standard deviation, or mean (range). P < 0.05 considered the difference to be statistically significant in the two groups.
A total of 340 patients (170 one-to-one matched patients in each cohort) were compared after the Omicron and Beta groups were matched. After matching, the severity classification, LYMPH, CRP, PCT, sore throat, muscle soreness, stuffy nose, and other symptoms of the two groups were significantly different (P < 0.05). The proportion of asymptomatic and mild patients in the Omicron group was higher than in the Beta group (45.30% vs. 37.65%), and the proportion of moderate and severe patients was lower than in the Beta group (54.70% vs. 62.35%). Compared to the Beta group, the Omicron group remained more prone to sore throat (45.88% vs. 8.82%, P < 0.0001), and the difference in the incidence of symptoms, such as diarrhea and headache, was no longer statistically significant (Table 10).
Table 10.
Comparison of one-to-one matched patients in the Beta group and Omicron group.
| Beta | Omicron | F/H/χ2 | P Value | |
|---|---|---|---|---|
| Baseline | ||||
| Age (y) | 50 (39–58) | 48 (36–58) | 0.8732 | 0.3826 |
| Gender | 0.0118 | 0.9136 | ||
| Female | 89 (52.35) | 88 (51.76) | ||
| Male | 81 (47.65) | 82 (48.24) | ||
| Vaccination, dose | < 0.0001 | |||
| 0 | 170 (100.00) | 7 (4.19) | ||
| 1 | 0 (0.00) | 9 (5.39) | ||
| 2 | 0 (0.00) | 61 (36.53) | ||
| 3 | 0 (0.00) | 90 (53.89) | ||
| Clinical classifications | < 0.0001 | |||
| Asymptomatic | 23 (13.53) | 2 (1.18) | ||
| Mild | 41 (24.12) | 75 (44.12) | ||
| Moderate | 106 (62.35) | 90 (52.94) | ||
| Severe | 0 (0.00) | 3 (1.76) | ||
| WBC, × 109/L | 5.17 (4.08–6.25) | 5.23 (4.37–6.77) | 1.5074 | 0.1317 |
| LYMPH, × 109/L | 1.5 (1.18–1.95) | 1.36 (0.95–1.92) | −2.0411 | 0.0412 |
| N%, % | 57.93 ± 10.06 | 56.56 ± 12.42 | 1.11 | 0.2666 |
| CRP, mg/L | 0.5 (0.50–5) | 3.79 (1.68–8.77) | 5.6320 | < 0.0001 |
| IL–6, pg/mL | 5.22 (2.29–12.88) | 6.9 (4.7–11.2) | −1.8719 | 0.0612 |
| PCT, ng/mL | 0.03 (0.02–0.05) | 0.049 (0.04–0.08) | 7.3176 | < 0.0001 |
| CD4, cell/μL | 593.5 (463–856) | 618.83 (393.75–815.6) | 0.5495 | 0.5827 |
| CD8, cell/μL | 345 (260.5–533) | 321.09 (170.4–499.19) | 0.8349 | 0.4038 |
| IgG | 1.73 (0.06–7.98) | 0.64 (0.213–8.98) | 0.7575 | 0.4487 |
| IgM | 2.48 (0.1–11.93) | 2.036 (0.14–34.12) | 1.7923 | 0.0731 |
| Symptoms | ||||
| Fever | 7.3138 | 0.0068 | ||
| Yes | 34 (20.00) | 56 (32.94) | ||
| No | 136 (80.00) | 114 (67.06) | ||
| Cough | 3.4343 | 0.0639 | ||
| Yes | 68 (40.00) | 85 (50.00) | ||
| No | 102 (60.00) | 85 (50.00) | ||
| Sore throat | 58.7462 | < 0.0001 | ||
| Yes | 15 (8.82) | 78 (45.88) | ||
| No | 155 (91.18) | 92 (54.12) | ||
| Feeble | 0.2396 | 0.6245 | ||
| Yes | 23 (13.53) | 20 (11.76) | ||
| No | 147 (86.47) | 150 (88.24) | ||
| Headache | 0.0377 | 0.8461 | ||
| Yes | 15 (8.82) | 14 (8.24) | ||
| No | 155 (91.18) | 156 (91.76) | ||
| Muscular soreness | 4.1111 | 0.0426 | ||
| Yes | 6 (3.53) | 15 (8.82) | ||
| No | 164 (96.47) | 155 (91.18) | ||
| Stuffy nose | 8.5774 | 0.0034 | ||
| Yes | 4 (2.35) | 17 (10.00) | ||
| No | 166 (97.65) | 153 (90.00) | ||
| Running nose | 2.7316 | 0.0984 | ||
| Yes | 6 (3.53) | 13 (7.65) | ||
| No | 164 (96.47) | 157 (92.35) | ||
| Taste loss | 0.4482 | |||
| Yes | 5 (2.94) | 2 (1.18) | ||
| No | 165 (97.06) | 168 (98.82) | ||
| Anosmia | 0.1141 | 0.7355 | ||
| Yes | 4 (2.35) | 5 (2.94) | ||
| No | 166 (97.65) | 165 (97.06) | ||
| Diarrhea | 0.5017 | 0.4787 | ||
| Yes | 11 (6.47) | 8 (4.71) | ||
| No | 159 (93.53) | 162 (95.29) | ||
| Others | 15.9759 | < 0.0001 | ||
| Yes | 36 (21.56) | 11 (6.47) | ||
| No | 131 (78.44) | 159 (93.53) | ||
Note: longitudinal comparison of clinical classification: the difference between the asymptomatic and mild cases between the two groups was statistically significant; the difference between the common type and the severe type was not statistically significant. Longitudinal comparison results of clinical classification before matching: the rates of asymptomatic, mild and normal types were significantly different between the two groups, and there was no significant difference in the rates of severe between the two groups. WBC: white blood cell; LYMPH: lymphocyte count; N%: neutrophil percentage; CRP: C-reactive protein; PCT: procalcitonin. Data are shown as number (%), mean ± standard deviation, or mean (range). P < 0.05 considered the difference to be statistically significant in the two groups.
4. Discussion
This analysis included 310 Omicron-infected patients from Tianjin and represented the clinical characteristics of the first group of patients infected by Omicron variant in China and the effects of vaccine doses and age. We also assessed whether the Omicron variant was more severe than the Delta and Beta variants. These data have important implications for the public to deepen their understanding of Omicron.
Evaluating the preventive ability of a COVID-19 vaccine against the rapid spread of the Omicron variant is critical for public health guidance. Our study found that the number of vaccinations was significantly related to the level of antibodies in the Omicron group. A significant increase was observed in the SARS-CoV-2-specific IgG levels, which suggested that increasing the number of vaccinations increased antibody levels in patients infected with Omicron, which is similar to Levi's study. However, Riccardo Levi's study found that one dose of vaccine was sufficient to maintain a relatively high level of immunity in the body, but these results may have been influenced by different populations and vaccination types (Levi et al., 2021). The mutated SARS-CoV-2 Omicron strain may weaken the effectiveness of current vaccine. Therefore, the protective ability of the vaccine for Omicron patients was lower than for Delta patients, as indicated in a study by Accorsi (Accorsi et al., 2022). Second, our study found that the number of vaccinations in this study had little effect on the clinical symptoms (such as fever and cough) of Omicron infection. The reason for this result may be the small sample size of the Omicron group, and the number of unvaccinated patients in the Omicron group that we included was very small, which resulted in unstable results. Finally, we found that the number of vaccinations had a certain relationship with the effect of the vaccine and the level of antibodies. However, insufficient data are available on the preventive effect of the current COVID-19 vaccine on the Omicron variant, and the specific effect needs further research.
This study also showed that the clinical characteristics and laboratory parameters of Omicron patients were closely related to the different age groups. These indicators could provide physicians worldwide with new information on Omicron variants and may help diagnose the disease. Consistent with related reports (Liu et al., 2020; Löhr et al., 2021), the lymphocyte levels in patients were inversely correlated with age, with older patients having significantly lower lymphocyte levels than younger patients. Lymphocyte levels are generally elevated during viral infection and abnormally reduced in the Omicron variant, as observed in the other variants (He et al., 2005; Zhang et al., 2020). Low levels of lymphocytes may be an indicator of the severity of the Omicron variant. Other laboratory indicators, such as WBC, CRP, PCT, IL6, and CD8, also showed obvious age-related trends to a certain extent, although we could not determine the exact relationship between these changes and the clinical features in patients. The results of our multivariate logistic regression analysis showed that there were still differences in the severity classification of COVID-19 patients of different ages when other factors were held constant. The results of Spearman rank correlation analysis also showed a positive correlation between age and severity classification. We also found that the Omicron variant was consistent with Delta and Beta variants in terms of the correlation between age and disease severity; moreover, older people were more prone to more severe clinical manifestations (Li et al., 2020), which may be due to the weakened immune function or underlying diseases in the elderly.
The question of whether the Omicron variant will cause more severe cases than previous SARS-CoV-2 variants has attracted much attention. According to our data, the clinical characteristics of the Omicron group were milder than the Delta and Beta groups. Compared to the Delta and Beta groups, the Omicron group was more prone to sore throat, but the incidence of headache, diarrhea, taste loss and anosmia was all lower. A prospective study by Menni et al. found that two symptoms were more common in Omicron patients compared to Delta, sore throat and hoarseness, and sore throat had an incidence as high as 70.5%, which is similar to our results (Menni et al., 2022). This reminds us that the symptoms of sore throat should be given sufficient attention, but the specific mechanism must be further explored. Therefore, the findings of our research indicated that the Omicron variant may cause fewer severe cases than the Delta and Beta variants, which is consistent with the results of recent studies in South Africa and Korea (Abdullah et al., 2022; Kim et al., 2022). These results may be related to the improvement of COVID-19 vaccine coverage and the lower mean age of Omicron patients. However, inflammatory indicators, such as CRP and IL-6, were higher in the Omicron patients than in the other two groups, but their values were within the normal range. The Omicron variant must be further observed and studied.
We performed propensity score matching to identify patients of similar age and gender for comparison. We found a difference in age but no sex difference in patients before matching, and after the age difference disappeared, the difference in disease severity between the Omicron and Delta groups was no longer statistically significant. Therefore, the lower disease severity of patients in the Omicron group in our sample was likely related to the overall younger age of the Omicron-infected patients. Some related studies also confirmed that the severity classification of COVID-19 was more severe with increasing age (Luo et al., 2020). However, after matching, the disease severity of the Omicron group remained lower than the beta group. We speculated that there may be other reasons in addition to age that make Omicron less severe, such as the lower virulence of the Omicron variant (Ulloa et al., 2022). Because our sample size was not sufficiently large and observation indicators were limited, more follow-up studies are needed to analyze these possible reasons. Our correlation test on the overall sample also verified that the severity classification of COVID-19 patients was related to age and variants (Table 11).
Table 11.
Correlation analysis of clinical classification with age, vaccination doses and virus strains.
| rs/V/G | P | |
|---|---|---|
| Age | 0.2990 | <0.0001 |
| Vaccination, dose | −0.0529 | 0.2009 |
| Virus strain | 0.3452 | <0.0001 |
Note: rs represents the rank correlation coefficient; V represents the Cramer V coefficient, an indicator of the degree of association between two categorical variables; G represents the Gamma coefficient, an indicator of the degree of association between two ordinal categorical variables.
Our research has certain limitations. First, the average age of the Omicron group was younger than that of the other two groups and few elderly people were included in the Omicron group. The prognosis was worse in older patients. Therefore, to more accurately determine the disease severity of the Omicron variant, larger samples of older adults must be investigated. Second, we only collected samples from 310 patients infected with the Omicron variant. The sample size was not sufficiently large, and there were very few unvaccinated patients, which may have affected our results. Studies with larger sample sizes may reveal clinical features of Omicron that were not observed in our research.
5. Conclusions
The median age of Omicron-infected patients in Tianjin was younger than the age of patients with previous variants, and the common symptoms were fever, cough, and sore throat. Within the Omicron group, SARS-CoV-2-specific IgG levels were higher in patients vaccinated with 2-dose and 3-dose vaccines than in the unvaccinated group, and older people were more prone to more severe clinical classifications. Compared to the Delta and Beta groups, the Omicron variant was less severe.
Data availability
The original data includes the laboratory test results and symptoms of each patient. The data are available from the corresponding author, Changsong Wang (changsongwangicu@163.com), upon reasonable request.
Ethics statement
The study was approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University.
Author contributions
Wei Yang: investigation, data curation, writing - original draft. Songliu Wang: investigation, data curation. Lei Wang: investigation, data curation. Yuxin Zhou: data curation, writing - original draft. Yu xin: writing - original draft. Hongxu Li: writing - original draft. Wenjing Mu: methodology. Qi Wu: investigation. Lei Xu: investigation. Mingyan Zhao: conceptualization, writing - review&editing. Changsong Wang: conceptualization, writing - review&editing, supervision. Kaijiang Yu: conceptualization, writing - review&editing, supervision.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by the Novel Coronavirus Pneumonia Emergency Treatment and Diagnosis Technology Research Project of the Heilongjiang Provincial Science and Technology Department and the Natural Science Foundation for Distinguished Young Scholars of Heilongjiang Province (GA20C001 and JQ2021H003).
Contributor Information
Mingyan Zhao, Email: mingyan1970@126.com.
Changsong Wang, Email: changsongwangicu@163.com.
Kaijiang Yu, Email: drkaijiang@163.com.
References
- Abdullah F., Myers J., Basu D., Tintinger G., Ueckermann V., Mathebula M., Ramlall R., Spoor S., de Villiers T., Van der Walt Z., Cloete J., Soma-Pillay P., Rheeder P., Paruk F., Engelbrecht A., Lalloo V., Myburg M., Kistan J., van Hougenhouck-Tulleken W., Boswell M.T., Gray G., Welch R., Blumberg L., Jassat W. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in Tshwane, South Africa. Int. J. Infect. Dis. 2022;116:38–42. doi: 10.1016/j.ijid.2021.12.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accorsi E.K., Britton A., Fleming-Dutra K.E., Smith Z.R., Shang N., Derado G., Miller J., Schrag S.J., Verani J.R. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the Sars-CoV-2 omicron and delta variants. JAMA. 2022;327:639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandal L.T., MacDonald E., Veneti L., Ravlo T., Lange H., Naseer U., Feruglio S., Bragstad K., Hungnes O., Ødeskaug L.E., Hagen F., Hanch-Hansen K.E., Lind A., Watle S.V., Taxt A.M., Johansen M., Vold L., Aavitsland P., Nygård K., Madslien E.H. Outbreak caused by the sars-cov-2 omicron variant in Norway, november to december 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.50.2101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang R., Gilby N.B., Wei G.W. Omicron variant (b.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J. Chem. Inf. Model. 2022;62:412–422. doi: 10.1021/acs.jcim.1c01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GISAID Initiative . 2022. Tracking of variants.https://www.gisaid.org/hcov19-variants/ Accessed July 16, 2022. [Google Scholar]
- Greaney A.J., Starr T.N., Gilchuk P., Zost S.J., Binshtein E., Loes A.N., Hilton S.K., Huddleston J., Eguia R., Crawford K., Dingens A.S., Nargi R.S., Sutton R.E., Suryadevara N., Rothlauf P.W., Liu Z., Whelan S., Carnahan R.H., Crowe J.E., Jr., Bloom J.D. Complete mapping of mutations to the Sars-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29:44–57. doi: 10.1016/j.chom.2020.11.007. e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., COVID-19 Genomics UK (COG-UK) Consortium. Peacock S.J., Robertson D.L. Sars-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Zhao C., Dong Q., Zhuang H., Song S., Peng G., Dwyer D.E. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int. J. Infect. Dis. 2005;9:323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci G. Covid-19: runny nose, headache, and fatigue are commonest symptoms of omicron, early data show. BMJ. 2021;375:n3103. doi: 10.1136/bmj.n3103. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Lee B., Choi Y.Y., Um J., Lee K.S., Sung H.K., Kim Y., Park J.S., Lee M., Jang H.C., Bang J.H., Chung K.H., Jeon J. Clinical characteristics of 40 patients infected with the sars-cov-2 omicron variant in korea. J. Kor. Med. Sci. 2022;37:e31. doi: 10.3346/jkms.2022.37.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.W., Ha E.K., Yeniova A.Ö., Moon S.Y., Kim S.Y., Koh H.Y., Yang J.M., Jeong S.J., Moon S.J., Cho J.Y., Yoo I.K., Yon D.K. Severe clinical outcomes of covid-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut. 2021;70:76–84. doi: 10.1136/gutjnl-2020-322248. [DOI] [PubMed] [Google Scholar]
- Levi R., Azzolini E., Pozzi C., Ubaldi L., Lagioia M., Mantovani A., Rescigno M. One dose of sars-cov-2 vaccine exponentially increases antibodies in individuals who have recovered from symptomatic covid-19. J. Clin. Invest. 2021;131 doi: 10.1172/JCI149154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Wu J., Wu F., Guo D., Chen L., Fang Z., Li C. The clinical and chest ct features associated with severe and critical covid-19 pneumonia. Invest. Radiol. 2020;55:327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Mao B., Liang S., Yang J.W., Lu H.W., Chai Y.H., Wang L., Zhang L., Li Q.H., Zhao L., He Y., Gu X.L., Ji X.B., Li L., Jie Z.J., Li Q., Li X.Y., Lu H.Z., Zhang W.H., Song Y.L., Qu J.M., Xu J.F., Shanghai Clinical Treatment Experts Group for COVID-19 Association between age and clinical characteristics and outcomes of covid-19. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.01112-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhr P., Schiele S., Arndt T.T., Grützner S., Claus R., Römmele C., Müller G., Schmid C., Dennehy K.M., Rank A. Impact of age and gender on lymphocyte subset counts in patients with covid-19. Cytometry. 2021 doi: 10.1002/cyto.a.24470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Liu S., Wang Y., Phillips-Howard P.A., Ju S., Yang Y., Wang D. Age differences in clinical features and outcomes in patients with COVID-19, Jiangsu, China: a retrospective, multicentre cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C., Valdes A.M., Polidori L., Antonelli M., Penamakuri S., Nogal A., Louca P., May A., Figueiredo J.C., Hu C., Molteni E., Canas L., Österdahl M.F., Modat M., Sudre C.H., Fox B., Hammers A., Wolf J., Capdevila J., Chan A.T., David S.P., Steves C.J., Ourselin S., Spector T.D. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID study. Lancet. 2022;399:1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L., Li F., Pencina M. Using propensity score methods to create target populations in observational clinical research. JAMA. 2020;323:466–467. doi: 10.1001/jama.2019.21558. [DOI] [PubMed] [Google Scholar]
- Ulloa A.C., Buchan S.A., Daneman N., Brown K.A. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286–1288. doi: 10.1001/jama.2022.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the sars-cov-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization WHO Coronavirus disease (COVID-19) pandemic. 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- World Health Organization Tracking SARS-CoV-2 variants. 2022. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of sars-cov-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., Akdis C.A., Gao Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data includes the laboratory test results and symptoms of each patient. The data are available from the corresponding author, Changsong Wang (changsongwangicu@163.com), upon reasonable request.