Abstract
BACKGROUND:
Vitamin D deficiency has been linked to various medical conditions such as bone loss, decreased mineralization, endocrine disorders, and central nervous system disorders, including epilepsy. Vitamin D deficiency is prevalent among patients with epilepsy (PWE). However, the specific association between vitamin D levels and age in PWE is unclear.
OBJECTIVES:
Identify the relation between vitamin D level and age in PWE and evaluate factors that may play a role in seizure control.
DESIGN:
Retrospective analytical medical record review
SETTING:
Outpatient epilepsy research clinic in Saudi Arabia
PATIENTS AND METHODS:
Between November 2016 and April 2020, we selected eligible PWE aged older than 14 years whose vita-min D levels were recorded at least once after reviewing 1550 patient electronic files. We analyzed data on serum vitamin D level by age and other factors, vitamin D supplement use, seizure classification, and conducted a multivariate logistic regression to assess associations with seizure control.
MAIN OUTCOME MEASURES:
Relationships between vitamin D levels and age and factors that might affect seizure control.
SAMPLE SIZE:
524 patients
RESULTS:
The prevalence of low serum vitamin D levels was high (86.8%). The median vitamin D level in all patients was low (38 nmol/L), and was lower in young PWE than in adult PWE (P<.01). Only 146 patients received vitamin D supplements. High vitamin D levels were associated with a 40% seizure reduction.
CONCLUSION:
Vitamin D deficiency is underestimated in PWE in Saudi Arabia, and is more prevalent among young adults and patients on polytherapy than in other PWE. Patients with high vitamin D levels had good seizure control compared with those with low levels. The effect of vitamin D supplements on seizure control should be further investigated in randomized control trials.
LIMITATIONS:
Retrospective study and no categorization by presence of supplementation.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Until the last decade, the increasing worldwide prevalence of vitamin D deficiency has been overlooked.1 It has been linked to various medical conditions such as weakened bone health, decreased mineralization, endocrine disorders, immune-mediated diseases, and central nervous system disorders, including cognitive impairment and epilepsy.2 Vitamin D has been reported to have neuroprotective, developmental, and immunomodulatory effects.3
Vitamin D deficiency is highly prevalent and has been reported in more than half of the adult population of a recent study.4 Furthermore, it is considered to be more prevalent among the elderly population, with clear evidence demonstrating an association between low vitamin D levels and abnormal bone mineralization and fractures.5
Low vitamin D levels are often found in patients with epilepsy (PWE),6 and hence, they are at a greater risk of osteoporosis and fractures.7 Calcium and vitamin D replacement as well as bone density are important factors to consider in minimizing the risk of fractures.8 Some studies have reported an association between low vita-min D levels and antiepileptic drugs (AEDs), especially cytochrome P-450 enzyme inducers; however, results have been inconsistent and predominantly observed among adult PWE. Moreover, there are data that support improvement in seizure frequency due to vitamin D supplementation in pediatric PWE.9 However, a recent study assessing the anticonvulsant effect of vita-min D showed no definite association between vitamin D supplementation and seizure control.10 Because the relationship of low vitamin D levels with age, duration of illness, and epilepsy has not been well studied, this study aimed to identify the relation of vitamin D level and age of PWE and further evaluate factors that may play a role in seizure control.
PATIENTS AND METHODS
In this retrospective medical record review we included PWE aged 14 years and older whose serum vita-min D levels were tested and recorded at least once during regular follow-up at an outpatient clinic of King Faisal Specialist Hospital and Research Center between November 2016 and April 2020. The most recent results were obtained in cases where patients had more than one reading. Demographic data collected included age, gender, age at seizure onset, seizure classification, duration of illness, risk factors, use of AEDs, vitamin D level, vitamin D supplementation if administered with dosage, and brain imaging and electroencephalography (EEG) findings. Vitamin D (25-hydroxy vitamin D) 25-OH D level was categorized into low (<75 nmol/L) and normal (≥75 nmol/L). The type of vitamin D was also entered. All findings regarding vitamin D levels were obtained from the same laboratory. Most of the PWE were on AED before starting vitamin D replacement. Data were entered electronically by trained personnel from the epilepsy department and were reviewed by an epilepsy consultant. Follow-up was performed for 3 to 42 months. The study was approved by the Institutional review board of King Faisal Specialist Hospital, and the need for consent was waived owing to the retrospective nature of the study. Confidentiality and anonymity of participants’ data were affirmed, and enrollment in the study did not affect the provision of the best management for the patients.
Statistical analysis, graphical representation, and regression analysis were performed using IBM SPSS version 21.0 for Windows (Armonk, New York, United States: IBM Corp). Vitamin D levels were categorized as normal or abnormal. We compared vitamin D levels with 2017 epilepsy classification,11 age, gender, duration of epilepsy, and antiepileptic medication use. For all statistical analyses, P values <.05 were considered significant.
RESULTS
Of 1550 PWE who attended the outpatient clinic, we identified 524 patients who were eligible for the study (Table 1). The median age was 32 years (IQR, 23-42;range 9-86) (Figure 1). Most were females (n=300, 57.3%); The median age of epilepsy onset was 15 years (IQR, 7-27). More than two-thirds of the patients (363) had one or more risk factors for epilepsy. In total, 216 (41%) patients used a single AED and 286 (55%) used two or more AEDs, and only 22 patients (4%) did not use any AEDs. Structural epilepsy contributed to 67% of identified epilepsy etiologies according to the current international League against Epilepsy classifications. Furthermore, EEG abnormalities were observed in more than 70% (364) of patients, and more than half of the EEG findings showing focal interictal epileptiform discharges. Sixty-two percent (325 cases) of brain magnetic resonance imaging (MRI) findings were abnormal, and vascular lesions and mesial temporal sclerosis were noted in around one-third of the cases (Table 1).
Table 1.
Demographic and clinical characteristics of the sample (n=524).
| Age categories (years) | |
| ≤20 | 91 (18.8) |
| 21 to ≤40 | 282 (58.1) |
| 41 to ≤60 | 111 (22.9) |
| >60 | 40 (8.2) |
| Gender | |
| Male | 300 (57.3) |
| Female | 224 (42.7) |
| Duration of illness (years) | 14 (11) |
| Classification | |
| Structural | 351 (67) |
| Genetic | 79 (15.1) |
| Infectious | 10 (1.9) |
| Metabolic | 1 (.2) |
| Immune | 9 (1.7) |
| Unknown | 74 (14.1) |
| Regimen | |
| Monotherapy | 216 (41.5) |
| Polytherapy | 285 (54.5) |
| None | 21 (4) |
| Electroencephalography | |
| Focal | 306 (59.8) |
| Generalized | 62 (12.1) |
| Normal | 144 (28.1) |
Data are n (%) for age (P<.01)
Figure 1.
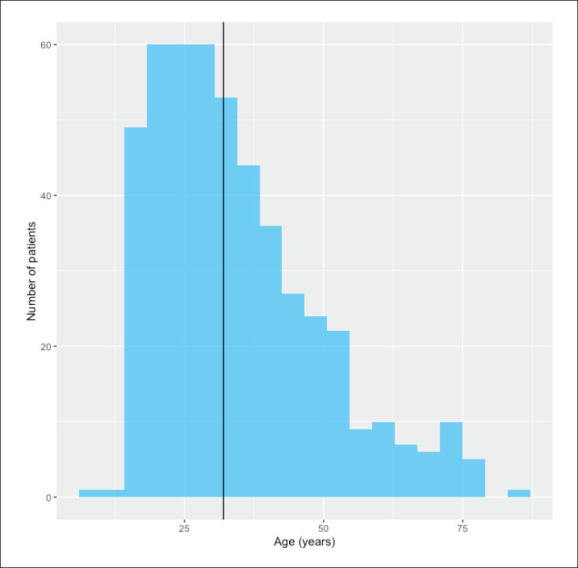
Age distribution of the population (vertical line is median value) (n=485 due to some missing data).
Low serum vitamin D level was found in 455 participants, with a prevalence of 86.8%. The median serum vitamin D level was 38 nmol/L (range; 6–180 nmol/L), which is lower than normal levels of 75 nmol/L (Figure 2).
Figure 2.
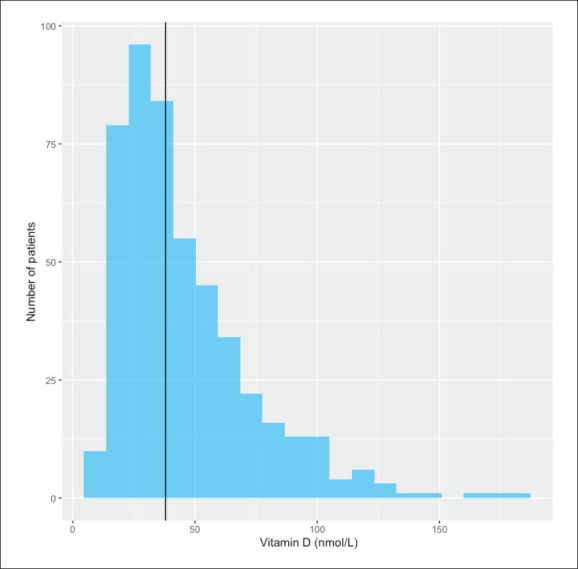
Serum vitamin D distribution (vertical line is median value).
Only 146 (28%) patients received vitamin D supplementation. Serum vitamin D levels varied according significantly to age, with a significant inverse correlation being observed between age at onset and vitamin
D levels (r=0.26, P<.001, 95% confidence interval [CI]; 0.19, 0.34). A similar correlation was also identified between age at epilepsy onset and vitamin D levels (correlation coefficient=0.24, P<.001, 95% CI; 0.15, 0.32). Furthermore, in categorizing ages at presentation into groups of 20 years duration, serum vitamin D levels were significantly higher in patients older than 60 years, than in those younger than 20 years (P<.01, 95% CI; 17.7, 37.7 nmol/L). A similar association, but to a lesser extent, was noted when comparing patients aged between 41 and 60 years with those younger than 20 years (9.3 nmol/L higher, P=.01, 95% CI; 2.1, 16.6 nmol/L). Other patient characteristics did not show any association with serum vitamin D levels. Figures 3-8 show vitamin D levels by various factors for most patients (n=485).
Figure 3.
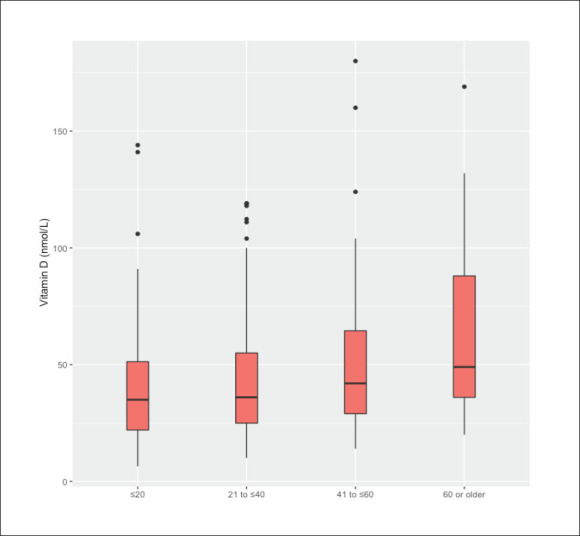
Age categories (years) by serum vitamin D levels.
Figure 8.
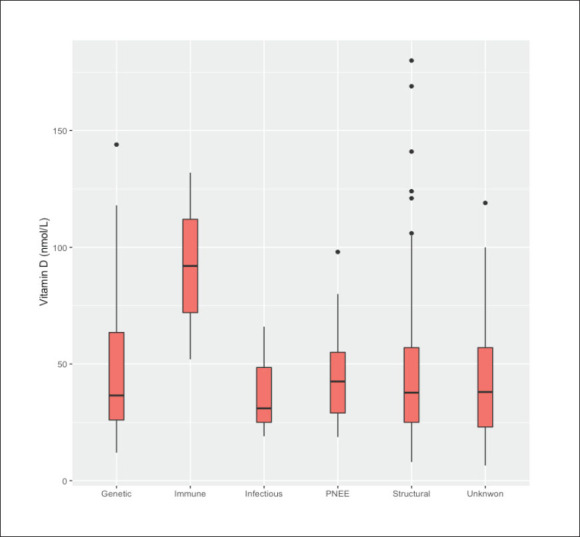
Epilepsy etiology by serum vitamin D levels.
Figure 4.
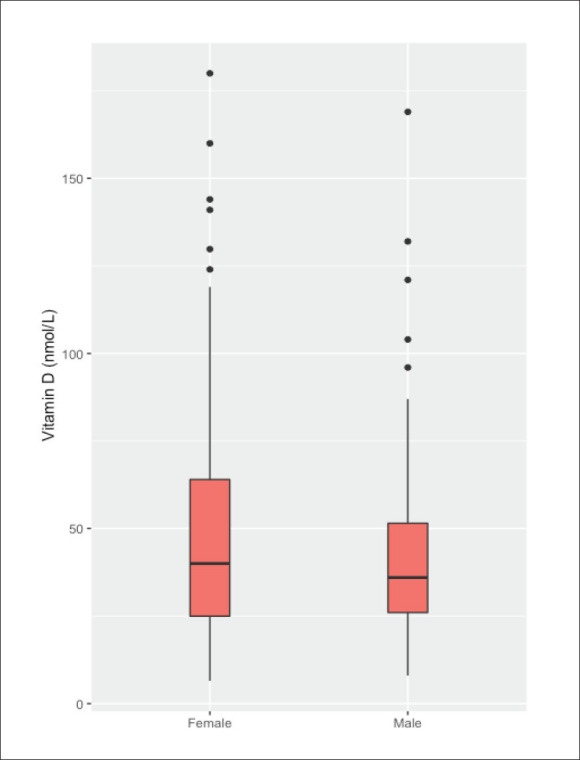
Serum vitamin D by sex.
Figure 5.
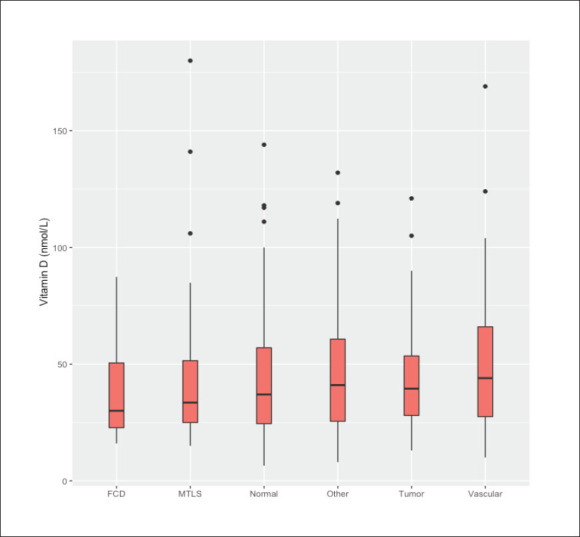
MRI brain findings by serum vitamin D levels.
Figure 6.
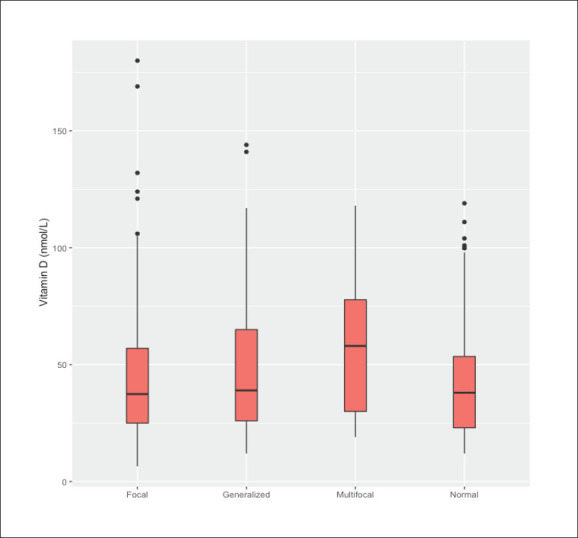
Electroencephalography findings by serum vitamin D levels.
Figure 7.
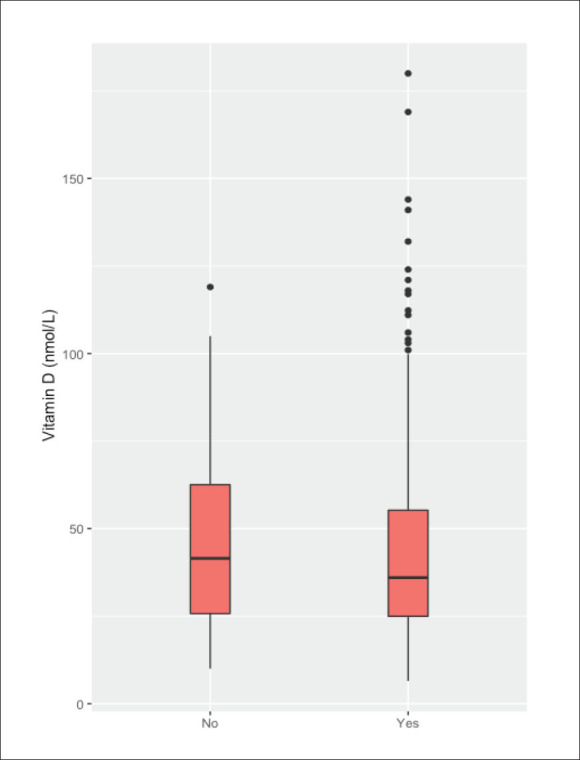
Epilepsy risk factors by serum vitamin D levels.
Over the minimum follow-up of 3 months, 237 patients (45%) were seizure free. On an average, seizure-free patients had slightly but insignificantly higher vitamin D levels (47.8 vs. 43.4 nmol/L, P=.06). Similarly, vitamin D supplementation had a crude effect of 40% increase in seizure freedom (unadjusted odds ratio [OR]=1.4, P=.05, 95% CI; 1.1, 2.1). This association was not observed when adjusted for age, AED regimen, sex, age at epilepsy onset, and brain MRI findings (adjusted OR=1.5, P=.08, 95% CI; 0.9, 2.3) in a multivariate logistic regression. With respect to age, the effect of vitamin D supplementation on seizure control was observed only in patients aged 20 years and younger (adjusted OR=2.9, P=.06, 95% CI; 1.0, 9.7), but those in other age groups did not show any association between vitamin D supplementation and seizure freedom for at least 18 months. The aforementioned findings suggest an association between higher serum vitamin D levels or vitamin D supplementation and seizure control in our studied population.
DISCUSSION
We found that low serum vitamin D levels was prevalent in 86.8% of the PWE, with a median level of 38 nmol/L. This finding was consistent with the results of the study by Teagarden et al who found a high prevalence of vitamin D deficiency among PWE, with 54% of patients using enzyme-inducing AEDs and 37% using non-enzyme-inducing AEDs.12 The effects of AEDs have been investigated in multiple trials, and most studies reported negative effects of AEDs, especially enzyme-inducing AEDs.13,14 In 2001, Al Rajeh et al estimated that there are more than six million PWE in Saudi Arabia.15 Since then, there has been no study that assessed the prevalence and effect of low vitamin D levels in PWE in Saudi Arabia. A recent study from Egypt in newly diagnosed pediatric patients with idiopathic generalized epilepsy showed that 40% of patients had vitamin D deficiency and 38% had vitamin D insufficiency.16
In our cohort, we found that young adult PWE younger than 20 years old had lower vitamin D levels than adult PWE with an average high level of 27.7 nmol/L. This result was concordant with that of a pediatric epilepsy population with a prevalence of vita-min D deficiency reaching up 62.6%.7 Vitamin D levels in the pediatric population were low, with one-third of the patients having low serum vitamin D levels.17 Adolescent PWE had a high prevalence of vitamin D deficiency than younger aged PWE.18
Patients taking polytherapy were at greater risk of having low vitamin D (P<.001) compared to those taking a single antiepileptic drug (P=.09). A study conducted in Malaysian pediatric PWE found that females, those receiving polytherapy, and patients of Indian ethnicity were at a greater risk of vitamin D deficiency than other patients. Other studies found that high body mass index,19 being female,17 and using AEDs, especially enzyme-inducing AEDs,13 were associated with vitamin D deficiency in pediatric PWE. Another study was conducted in a pediatric population in South Korea found that polytherapy, longer duration of using AED, tube feeding and overweight were independent risk factors for low serum vitamin D.20
We observed that a high serum vitamin D level either by using supplements or via sunlight exposure was associated with reduced seizure frequency by 40%. The anticonvulsant effect of vitamin D was also observed in animal models administered vitamin D supplements, and vitamin D supplementation has been reported to be associated with improved seizure control in mice.21,22 Zantta et al found that vitamin D modulated L-calcium channels in young rats, possibly indicating the use of vitamin D as an adjunctive therapy in PWE.23 Nevertheless, the effect of vitamin D supplementations in controlling seizures in human models remains unknown, although it has been reported to show a reduction in seizure frequency by at least 40% in a small group of participants.3 An increase in seizure frequency (more than four seizure events a month) was associated with low vitamin D levels in patients with generalized epilepsy.14 However, a large cohort study of PWE followed up for 2 years showed no benefits of vitamin D supplementation on seizure control in drug-resistant PEW.10 Although the information on the relationship between vitamin D levels and their effects on PWE is inconsistent, a study conducted in 2014 found that vitamin D prevents neuronal damage in the hippocampus, thereby contributing to the reduction in seizure frequency.24 In our study, we found no association between vitamin D levels and epilepsy classification, gender, radiological findings, and duration of illness. A recently published study reported low vitamin D levels in pediatric and adolescent patients with newly diagnosed generalized epilepsy, with no difference between the two age groups.25
Vitamin D deficiency has been associated with bone mineral density changes in the adult and elderly populations.26 Compared with the general population, epilepsy patients have a 2-6 times greater risk of fractures, and the risk seems to increase with a cumulative duration of exposure to AEDs.27 A recently published study from Egypt found that children with epilepsy had a risk of having low bone mineral density, especially when using enzyme-inducing AEDs and polytherapy (P<.001).28 Vitamin D supplementation was associated with improved bone mineral density, especially in PWE.29,30
This study had limitations. First, we did not assess the effect and duration of AEDs (neither enzyme-inducing and non-enzyme-inducing AEDs) on vitamin D level. Second, there was no categorization for patients taking supplementation, especially elderly patients. Lastly, we did not include a pediatric population in the study. Khalifah et al studied the effectiveness of vitamin D supplementation in preventing vitamin D deficiency in high-risk patients.31 We believe these findings might influence our practice in the near future.
In conclusion, vitamin D deficiency in PWE is underestimated in our region, with only one-third of participants having at least one vitamin D level measured during the follow-up period. The prevalence of low serum vitamin D in our study was 86.8%. Although vitamin D deficiency is frequent among adult PWE, especially young adults, and patients on polytherapy. Patients with high vitamin D levels had better seizure control than those with low levels. The effect of vitamin D supplements on seizure control shoud be further investigated in randomized control trials.
Funding Statement
Funding: None.
REFERENCES
- 1.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96(7):1911–30. 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 2.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al. The role of vitamin D in cancer prevention. Am J Public Health 2006;96(2):252–61. 10.2105/ajph.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holló A, Clemens Z, Kamondi A, Lakatos P, Szűcs A.. Correction of vitamin D deficiency improves seizure control in epilepsy: A pilot study. Epilepsy Behav 2012;24(1):131–3. 10.1016/j.yebeh.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Miratashi Yazdi SA, Abbasi M, Miratashi Yazdi SM.. Epilepsy and vitamin D: A comprehensive review of current knowledge. Rev Neurosci 2017;28(2):185–201. 10.1515/revneuro-2016-0044. [DOI] [PubMed] [Google Scholar]
- 5.Al-Alyani H, Al-Turki HA, Al-Essa ON, Alani FM, Sadat-Ali M.. Vitamin D deficiency in Saudi Arabians: A reality or simply hype: A meta-analysis (2008–2015). J Fam Community Med 2018;25(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamberg-Allardt CJE, Outila TA, Kärk-käinen MUM, Rita HJ, Valsta LM.. Vitamin D deficiency and bone health in healthy adults in Finland: Could this be a concern in other parts of Europe? J Bone Miner Res 2001;16(11):2066–73. 10.1359/jbmr.2001.16.11.2066. [DOI] [PubMed] [Google Scholar]
- 7.Nicolaidou P, Georgouli H, Kotsalis H, Matsinos Y, Papadopoulou A, Fretzayas A, et al. Effects of anticonvulsant therapy on vita-min D status in children: Prospective monitoring study. J Child Neurol 2006;21(3):205–9. 10.2310/7010.2006.00050. [DOI] [PubMed] [Google Scholar]
- 8.Shellhaas RA, MD, MS, Joshi SM, MD, MS.. Vitamin D and bone health among children with epilepsy. Pediatr Neurol 2010;42(6):385–93. 10.1016/j.pediatrneurol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Grzonka P, Rybitschka A, De Marchis GM, Marsch S, Sutter R.. Bone fractures from generalized convulsive seizures and status epilepticus-A systematic review. Epilepsia 2019;60(5):996–1004. 10.1111/epi.14738. [DOI] [PubMed] [Google Scholar]
- 10.Tombini M, Palermo A, Assenza G, Pellegrino G, Benvenga A, Campana C, et al. Calcium metabolism serum markers in adult patients with epilepsy and the effect of vitamin D supplementation on seizure control. Seizure 2018;58:75–81. 10.1016/j.seizure.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Scheffer, I.E., Berkovic, S., Capovilla, G., Connolly, M.B., French, J., Guilhoto, L., Hirsch, E., Jain, S., Mathern, G.W., Moshé, S.L., Nordli, D.R., Perucca, E., Tomson, T., Wiebe, S., Zhang, Y.-H. and Zuberi, S.M. (2017), ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia, 58: 512–521. 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teagarden DL, Meador KJ, Loring DW.. Meador, David W. Loring. Low vitamin D levels are common in patients with epilepsy. Epilepsy Res 2014;108(8):1352–6. 10.1016/j.eplepsyres.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verrotti A, Greco R, Latini G, Morgese G, Chiarelli F.. Increased bone turnover in prepubertal, pubertal, and postpubertal patients receiving carbamazepine. Epilepsia 2002;43(12):1488–92. 10.1046/j.1528-1157.2002.13002.x. [DOI] [PubMed] [Google Scholar]
- 14.Holló A, Clemens Z, Lakatos P.. Epilepsy and vitamin D. Int J Neurosci 2014;124(6):387–93. 10.3109/00207454.2013.847836. [DOI] [PubMed] [Google Scholar]
- 15.Al Rajeh S, Awada A, Bademosi O, Ogunniyi A.. The prevalence of epilepsy and other seizure disorders in an Arab population: A community-based study. Seizure 2001;10(6):410–4. 10.1053/seiz.2001.0602. [DOI] [PubMed] [Google Scholar]
- 16.Elmazny A, Amer H, Rashed L, Khalil S, Magdy R.. Vitamin D status of untreated children and adolescent Egyptian patients with genetic generalized epilepsy: A case–control study. Epilepsy Behav 2020;103:106840. 10.1016/j.yebeh.2019.106840. [DOI] [PubMed] [Google Scholar]
- 17.Shellhaas RA, Barks AK, Joshi SM.. Prevalence and risk factors for vitamin D insufficiency among children with epilepsy. Pediatr Neurol 2010;42(6):422–6. 10.1016/j.pediatrneurol.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SH, Yu J.. Risk factors of vitamin D deficiency in children with epilepsy taking anticonvulsants at initial and during follow-up. Ann Pediatr Endocrinol Metab 2015;20(4):198–205. 10.6065/apem.2015.20.4.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniels ZS, Nick TG, Liu C, Cassedy A, Glauser TA.. Obesity is a common comorbidity for pediatric patients with untreated, newly diagnosed epilepsy. Neurology 2009;73(9):658–64. 10.1212/wnl.0b013e3181ab2b11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, Y.-J., Park, K. M., Kim, Y. M., Yeon, G. M., & Nam, S. O. (2015). Longitudinal Change of Vitamin D Status in Children With Epilepsy on Antiepileptic Drugs: Prevalence and Risk Factors. Pediatric Neurology, 52(2), 153–159. doi: 10.1016/j.pediatrneurol.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 21.Pendo K, DeGiorgio MD, Michael C.. Vitamin D for the treatment of epilepsy: basic mechanisms, animal models and clinical trials. Front Neurol 2016;7:218. 10.3389/fneur.2016.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borowicz KK, Morawska M, Furmanek-Karwowska K, Luszczki JJ, Czuczwar SJ.. Cholecalciferol enhances the anticonvulsant effect of conventional antiepileptic drugs in the mouse model of maximal electroshock. European Journal of Pharmacology 2007;573(1–3):111–5. 10.1016/j.ejphar.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 23.Zanatta L, Goulart PB, Gonçalves R, Pierozan P, Winkelmann-Duarte EC, Woehl VM, et al. 1,25-Dihydroxyvitamin D3 mechanism of action: Modulation of L-type calcium channels leading to calcium uptake and intermediate filament phosphorylation in cerebral cortex of young rats. Biochim Biophys Acta 2012;1823(10):1708–19. 10.1016/j.bbamcr.2012.06.023 [DOI] [PubMed] [Google Scholar]
- 24.Dursun E, Gezen-Ak D, Yilmazer S.. The influence of vitamin D treatment on the inducible nitric oxide synthase (INOS) expression in primary hippocampal neurons. Noro Psikiyatr Ars 2014;51(2):163–8. 10.4274/npa.y7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonmez FM, Donmez A, Namuslu M, Canbal M, Orun E.. Vitamin D deficiency in children with newly diagnosed idiopathic epilepsy. J Child Neurol 2015;30(11):1428–32. 10.1177/0883073814566627. [DOI] [PubMed] [Google Scholar]
- 26.Cranney A, Horsley T, O’Donnell S, Weiler H, Puil L, Ooi D, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 2007;158:1–235. [PMC free article] [PubMed] [Google Scholar]
- 27.Nakken KO, Taubøll E.. Bone loss associated with use of antiepileptic drugs. Expert Opin Drug Saf 2010;9(4):561–71. 10.1517/14740331003636475 [DOI] [PubMed] [Google Scholar]
- 28.Osman NMM, Abdel Aziz RA, Soliman GT, Gamal Mohamed AG.. Bone mineral density evaluation of epileptic children on anti-epileptic medications. Egypt J Radiol Nucl Med 2017;48(4):1083–90. 10.1016/j.ejrnm.2017.07.006. [DOI] [Google Scholar]
- 29.Al-Saleh Y, Sulimani R, Sabico S, Raef H, Fouda M, Alshahrani F, et al. 2015 Guidelines for osteoporosis in Saudi Arabia: Recommendations from the Saudi Osteoporosis Society. Ann Saudi Med 2015;35(1):1–12. 10.5144/0256-4947.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikati MA, Dib L, Yamout B, Sawaya R, Rahi AC, Fuleihan Gel -H.. Two randomized vitamin D trials in ambulatory patients on anticonvulsants: Impact on bone. Neurology 2006;67(11):2005–14. 10.1212/01.wnl.0000247107.54562.0e. [DOI] [PubMed] [Google Scholar]
- 31.Khalifah RA, Hudairi A, Homyani DA, Hamad MH, Bashiri FA.. Vitamin D supplementation to prevent vitamin D deficiency for children with epilepsy: Randomized pragmatic trial protocol. Medicine 2018;97(40):e12734. 10.1097/md.0000000000012734. [DOI] [PMC free article] [PubMed] [Google Scholar]


