Abstract
BACKGROUND:
Stroke mortality and related functional disability have been declining over the last two decades, but stroke continues to represent the second leading cause of cardiovascular death worldwide and the number one cause for acquired long-term disability.
OBJECTIVES:
Assess short- and long-term health outcomes after acute ischemic stroke and analyze factors associated with poor survival and functional outcomes.
DESIGN:
Retrospective and survival analysis
SETTING:
Inpatient unit at a tertiary care referral hospital.
PATIENTS AND METHODS:
All patients admitted with acute ischemic stroke from 1 January 2017 to 31 August 2018 were included in the study. Functional status was assessed using the modified Rankin Scale (mRS). Other demographic and clinical variables were obtained from medical records. Data were analyzed by multivariable logistic regression, Cox proportional hazards, and the Kaplan-Meier method. Long-term follow-up data, including mortality and mRS was collected by follow-up phone call.
MAIN OUTCOME MEASURES:
Functional dependency and factors associated with mortality.
SAMPLE SIZE AND CHARACTERISTICS:
110 with mean age of 67.0 (14.7) years; 59 patients (53.6%) were males.
RESULTS:
Hypertension (75.5%), diabetes mellitus (54.6%), and dyslipidemia (29.1%) were common. Sixty-five patients (59.1%) had mRS >2 upon discharge including 18 patients (16.4%) who died during the hospital stay. The cumulative mortality rate was 25.4% (28/110) at 12 months and 30.0% (33/110) at 24 months. Twenty-nine stroke survivors (29/70, 41.4%) remained physically dependent (mRS >2) at the end of follow-up. Old age, atrial fibrillation, history of prior stroke, chronic kidney disease, and peripheral arterial disease were associated with increased mortality and functional dependence.
CONCLUSIONS:
Patients in Oman with acute ischemic stroke tend to have a high comorbidity burden, and their functional dependency and mortality are higher compared to patients from developed countries. Therefore, evidence-based measures such as establishing stroke units are essential to improve the health outcomes of patients with acute ischemic stroke.
LIMITATIONS:
Retrospective at single center.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Stroke mortality and related functional disability have been declining over the last two decades due to advances in the management of acute ischemic stroke.1,2 Such advances include but are not limited to thrombolytic therapy, mechanical thrombectomy, acute stroke units, and post-stroke rehabilitation programs, which have been shown to reduce both mortality and functional disability.2,3 However, stroke continues to represent the second leading cause of cardiovascular death worldwide and the number one cause for acquired long-term disability, resulting in a global annual economic burden.4 Around 25-50% of stroke survivors continue to have a severe functional disability.5 Furthermore, comorbidity is prevalent in patients with ischemic stroke. Comorbidity is associated with increased mortality and morbidity.6,7 Old age, pre-stroke functional dependency, atrial fibrillation, and high comorbidity are associated with worse post-stroke survival rate and functional independence.7
In the Middle Eastern countries, including Oman, studies addressing health outcomes after ischemic stroke are sparse;8-10 hence studying ischemic stroke health outcomes and analyzing factors associated with poor outcomes are essential elements of the health-care quality improvement process. We aimed to assess short- and long-term health outcomes after acute ischemic stroke. Also, we analyzed factors associated with poor survival and functional outcomes.
PATIENTS AND METHODS
The study was a retrospective study with a survival analysis that included all patients admitted to Sultan Qaboos University Hospital (SQUH) with acute ischemic stroke (within seven days of the onset of the symptoms) from 1 January 2017 to 31 August 2018. Functional status was assessed using the modified Rankin Scale (mRS), and functional dependency was defined as mRS >2.11 As relevant determinants of prognosis in ischemic stroke, the following variables were collected from the medical records: age, sex, treatment, atrial fibrillation (AF), ischemic heart disease, smoking status, hypertension, hyperlipidaemia, chronic kidney disease (CKD), diabetes mellitus, previous stroke, length of hospitalization, and mRS on discharge. In addition, long-term follow-up data, including mortality and mRS was done through a phone follow-up call in November 2020. The study was approved by the Medical Research Ethics Committee (MREC) of the College of Medicine and Health Sciences at Sultan Qaboos University (MERC. No.1949)
Categorical variables are reported as number and percentage. Continuous variables are expressed as mean and standard deviation (SD) for normally distributed data or median and interquartile range (IQR) for non-normally distributed data. Continuous variables between the two groups were compared using the t test for normally distributed variables or Wilcoxon rank-sum for non-normally distributed variables. The Fisher exact test was used to assess the association between categorical variables (due to the small sample size). Relevant clinical characteristics with P values <.25 were included in a multivariable logistic regression to identify independent predictors associated with severity of mRS (on discharge and follow up). Multivariable Cox proportional hazards regression was performed to identify variables related to mortality. In addition, long-term mortality outcome was analyzed as time-to-event using the Kaplan–Meier method and by using log-rank tests for comparisons. Hazard and odds ratios were calculated with 95% confidence intervals (CIs). Two-sided P values <.05 were considered to be statistically significant. Statistical calculations were performed using the Stata v. 16.1 software package (StataCorp LLC, USA).
RESULTS
One hundred ten patients were admitted with ischemic stroke during the study period (Table 1). The mean age was 67.0 (14.7) years, and 59 patients (53.6%) were males. Hypertension (75.5%), diabetes mellitus (54.6%), dyslipidemia (29.1%) were common. Only eight patients (7.3%) were treated with thrombolytic therapy, and 82 patients (74.6%) were discharged on aspirin and clopidogrel. The median length of hospitalization was 7 days (IQR: 5-11). Sixty-five patients (59.1%) had mRS >2 upon discharge including 18 patients (16.4%) who died during the index admission. Twenty-two patients died after discharge (20%). Multivariate logistic regression showed that old age (P=.002), AF (P<.001), and previous stroke (P=.013) were associated with an increased risk of functional dependency on discharge (mRS>2). The mean follow-up duration was 25.4 (1.3) months. There were 66 patients (60.0%) who had mRS>2 on the follow-up date, including 22 patients (20%) who died during the follow-up period.
Table 1.
Baseline characteristics, treatment and health outcomes of patients admitted with acute ischemic stroke.
| Total cohort (n=110) | mRS (≤2) (n=45) | mRS (>2) (n=65) | P | |
|---|---|---|---|---|
| Age (years) | 67.0 (14.7) | 60.1 (13.9) | 71.8 (13.4) | <.001 |
| Male | 59 (53.6) | 23 (51.1) | 36 (55.4) | .700 |
| Hypertension | 83 (75.5) | 31 (68.9) | 52 (80.0) | .183 |
| Smoking | 18 (16.4) | 11 (16.9) | 7 (15.6) | .183 |
| Diabetes mellitus | 60 (54.6) | 25 (55.6) | 35 (53.9) | .859 |
| Dyslipidemia | 32 (29.1) | 13 (28.9) | 19 (29.2) | .969 |
| Atrial fibrillation | 14 (12.7) | 0 | 14 (21.5) | <.001 |
| Ischemic heart disease | 24 (21.8) | 7 (15.7) | 17 (26.2) | .217 |
| Chronic kidney disease | 19 (17.3) | 6 (13.3) | 13 (20.0) | .450 |
| Peripheral Arterial disease | 4 (3.6) | 1 (2.2) | 3 (4.6) | .510 |
| Prior stroke | 27 (24.5) | 4 (8.9) | 23 (35.4) | <.001 |
| Thrombolytic therapy | 8 (7.3) | 3 (6.7) | 5 (7.7) | .839 |
| Aspirin or clopidogrel | 25 (22.7) | 12 (26.7) | 13 (20.0) | .701 |
| Aspirin and clopidogrel | 82 (74.6) | 32 (71.1) | 50 (77.0) | .701 |
| Anticoagulant alone | 19 (17.3) | 5 (11.1) | 14 (21.5) | .380 |
| Antiplatelet + anticoagulant | 4 (3.6) | 2 (3.1) | 2 (3.1) | |
| Median length of hospitalization (days) | 7 (5–11) | 7 (5–9) | 7 (5–12) | .8644 |
| Median follow up (months) | 29.3 (23.9) | 34.0 (10.5) | 25.4 (25.8) | <.001 |
| mRS on discharge | 3.3 (2.1) | 1.1 (0.8) | 4.9 (0.9) | <.001 |
| mRS on follow up | 3.4 (2.9) | 1 (0–3) | 4.6 (4.9) | <.001 |
| Mortality | 40 (36.4) | 4 (8.9) | 36 (55.38) | <.001 |
Data are n (%) or mean (standard deviation) for age, or median (interquartile range) for length of hospitalization and follow-up time. mRS: modified Rankin Scale; IQR: interquartile range.
The cumulative mortality rate was 25% at 12 months and 30% at 24 months (Figure 1). After excluding overall death (n=40), 41.4% of stroke survivors (29/70) remain physically dependent (mRS >2). Old age (P<.001), atrial fibrillation (AF) (P<.001), history of prior stroke (P<.001) were more common in patients who had an ischemic stroke resulting in functional dependency (mRS >2). Patients with functional dependency on discharge (mRS >2) had increased mortality (P<.001) and poor functional outcome during the follow-up period (P<.001). Also, old age (P=.02), AF (P<.001), and previous stroke (P=.02), were associated with poor functional outcome (mRS>2) on follow up.
Figure 1.
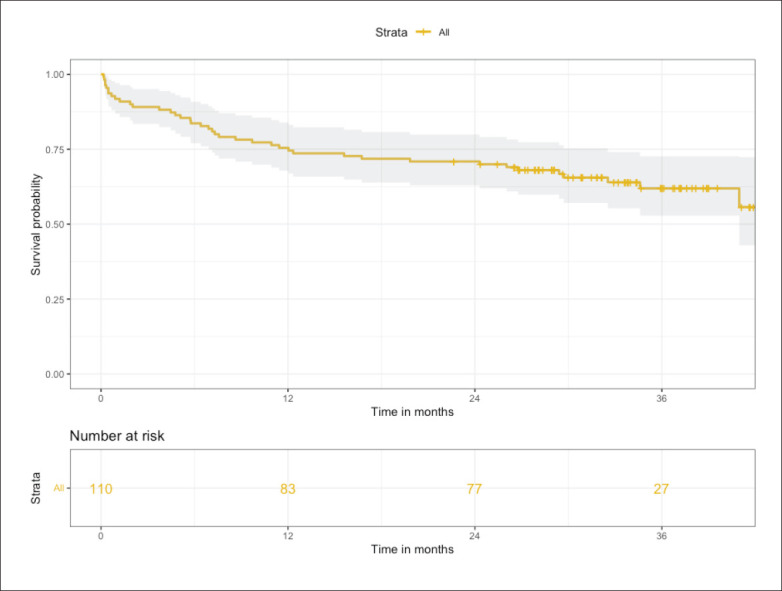
Kaplan-Meier survival analysis for patients with acute ischemic stroke (n=110).
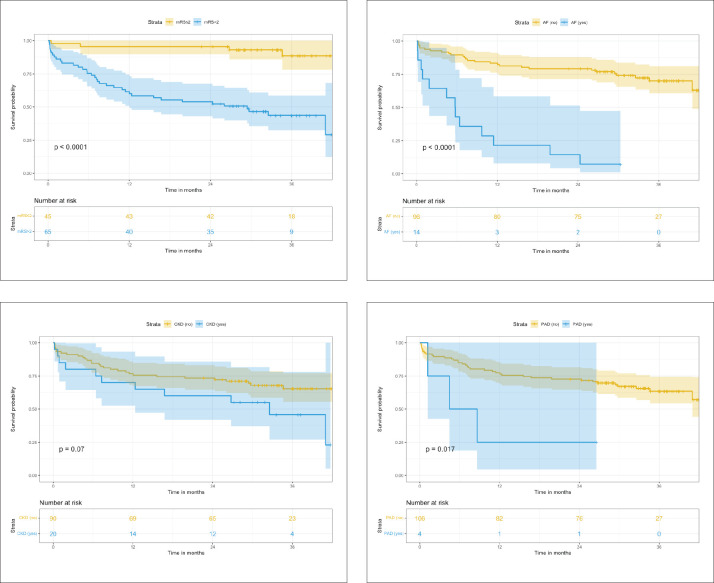
Forty patients died during the index admission or the follow-up period, and the reported underlying cause of deaths were ischemic stroke (n=28), sepsis (n=4), ischemic heart disease (n=3), malignancy (n=2), diabetes mellitus (n=1) and unknown causes (n=2). In addition, the log rank test in the Cox regression showed that mRS>2 (P=.002), AF (P<.0001), chronic kidney disease (CKD) (P=.07), peripheral arterial disease (P=.017) were associated with increased mortality (Figure 2).
Figure 2.
Kaplan-Meier survival analysis for patients with acute ischemic stroke by modified Rankin scale (mRS), atrial fibrillation (AF), chronic kidney disease (CKD) and peripheral arterial disease (PAD).
DISCUSSION
This study provides important data on the health outcomes of patients who suffered from acute ischemic stroke in this region of the world. The mean age of the patients with acute ischemic stroke was 67 years, 16.4% of the patients died during the index admission, 20% died during follow up, and 41.4% of stroke survivors remained physically dependent (mRS >2). Old age, AF, CKD, physical dependency on discharge (mRS >2), peripheral artery disease, and previous stroke were associated with poor health outcomes in patients with acute ischemic stroke.
Most of the patients were males (53.6%), which is similar to the findings of previous studies. This could be explained by the protective effect of estrogen in females and the higher prevalence of cardiovascular risk factors on males including smoking, and hypertension.12,13 Also, the patients in our cohort had a high burden of comorbidities, with the most common being hypertension (75.5%), followed by diabe tes mellitus (54.6%) and dyslipidemia (29.1%). These findings are similar to findings of previous studies in the Middle East, including studies in Saudi Arabia.14-18
Hypertension is the most prevalent and modifiable risk factor for stroke and is associated with worsened outcomes.19 In general, a high comorbidity burden is associated with the complexity of clinical management, poor health outcomes and increased health care cost. In patients with stroke, an increased morbidity index is associated with increased mortality; however, there are very few studies to assess the impact of comorbidity in other stroke outcomes.1,20,21
Only 7.3% of patients received thrombolytic therapy, similar to the reported figures in some developing countries.22 A recent study from Saudi Arabia showed that only 8.6% of patients with acute ischemic stroke received thrombolytic therapy.23 In contrast, some studies from Europe showed that more than 20% of patients received thrombolytic therapy for acute ischemic stroke.24 Thrombolytic therapy is associated with improved health outcomes in patients with acute ischemic stroke.25,26 The low percentage of patients who received thrombolytic therapy could be explained by delayed presentation to the hospital due to several factors, including the poor awareness of stroke symptoms among patients at increased risk.8,22
Also, endovascular thrombectomy has become a standard treatment for patients with large vessel occlusion related acute ischemic stroke. However, this is not readily available in our center.27 The majority of the patients were prescribed dual antiplatelets, which reflects adherence to the current recommendation of acute ischemic stroke management.28
Eighteen patients (16.4%) died during hospitalization; the reported in-hospital mortality rate after acute stroke ranged between 1.8-41% in developing countries and 0-22.0% in developed countries.22,29-32 A study from Japan reported a 30-day mortality rate of 9.8% after acute ischemic stroke.33 Also, a study from Saudi Arabia reported an inpatient mortality rate of 9.7% after acute ischemic stroke.34 This variation in the inpatient mortality rate for stroke is primarily due to delayed presentation, poor access to essential medications and other health care facilities in countries with poor health systems.29,35
The median follow-up duration was 29.3 (23.9) months. Another 22 patients died during the follow-up period. The cumulative mortality rate was 25% at 12 months and 30% at 24 months which is lower than the mortality rate in some of the developing countries but slightly higher than the reported rate in some developed countries.36-38
AF was associated with increased stroke severity and higher mortality, which is similar to findings of previous studies.39-41 The Framingham study analyzed stroke severity in AF; the 30-day mortality was greater in AF strokes than non-AF strokes (25% vs 14%). In addition, the AF group had poor survival and more risk of recurrence of stroke in the one-year follow up; hence the use of anticoagulation is crucial.42,43
Also, severe disability on discharge (mRS >2), old age, chronic kidney disease, peripheral arterial disease were associated with increased mortality in this study. Similarly, previous studies identified old age, smoking, female sex, dyslipidemia dementia, chronic kidney disease, AF, diabetes mellitus, peripheral artery disease, chronic heart disease and malignancy as factors associated with increased stroke mortality.32,37,44 Most patients died due to the sequel of ischemic stroke (e.g. aspiration pneumonia, infected pressure ulcer) or due to ischemic heart disease. This finding is similar to the findings of previous studies.45 Appropriate management of cardiovascular risk factors (e.g. hypertension and diabetes) and prevention of aspiration pneumonia are important measures to improve the survival of patients with ischemic stroke.22
Around 41% of stroke survivors remained physically dependent (mRS>2). The reported incidence of physical dependence among stroke survivors varies between 15-30% in the USA to 45% in China.46 We found that old age, previous stroke, AF, and high mRS>2 at discharge were associated with an increased functional disability during the follow-up period.
Our study has several limitations; first, it was a retrospective, single center and hospital-based. Some important data were not collected, including Glasgow coma scale, socioeconomic status and The National Institutes of Health Stroke Scale score. However, the study, being the first of its kind in the region, provides important data that can be used to improve care for patients with stroke.
This study suggests that stroke mortality rate and physical dependence post-stroke in this part of the world are higher than reported numbers from developed countries. There are very few studies addressing the stroke outcomes in the Middle East region. Increasing public awareness of risk factors of stroke, symptoms of stroke and establishment of emergency transportation services are key factors to increase the rate of thrombolysis and hence improving stroke health outcomes.8,22,35 In addi tion, establishing a stroke unit, access to endovascular intervention, and well-structured multi-disciplinary rehabilitation service are essential elements to optimize the care for patients with acute ischemic stroke.22 Our study findings should provide insights to health care managers and stakeholders to plan the necessary changes like allocating health care resources and implementing the necessary improvement in the current health care settings.
In conclusion, our study is one of the few studies reporting short- and long-term outcomes in patients with acute ischemic stroke from our region. The patients with acute ischemic stroke tend to have a high comorbidity burden, and their functional dependency and mortality were higher compared to patients from developed countries. Therefore, important measures including increasing public awareness of stroke, improving emergency transportation services, establishing stroke units and optimizing multi-disciplinary rehabilitation services are important elements to improve the health outcomes of patients with acute ischemic stroke. Our study findings should provide insights to health care managers and stakeholders to plan the nec-essary changes like allocating health care resources and implementing the necessary improvement in the current health care settings.
Funding Statement
Funding: None.
REFERENCES
- 1.Corraini P, Szepligeti SK, Henderson VW, Ording AG, Horvath-Puho E and Sorensen HT. Comorbidity and the increased mortality after hospitalization for stroke: a population-based cohort study. J Thromb Haemost. 2018;16:242–252. [DOI] [PubMed] [Google Scholar]
- 2.Marshall IJ, Wang Y, Crichton S, McKevitt C, Rudd AG and Wolfe CD. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol. 2015;14:1206–18. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy DJ, Diaz A, Sheinberg DL, Snelling B, Luther EM, Chen SH, Yavagal DR, Peterson EC and Starke RM. Long-Term Outcomes of Mechanical Thrombectomy for Stroke: A Meta-Analysis. Scientific World-Journal. 2019;2019:7403104. [DOI] [PMC free article] [PubMed]
- 4.Katan M and Luft A. Global Burden of Stroke. Semin Neurol. 2018;38:208–211. [DOI] [PubMed] [Google Scholar]
- 5.Wafa HA, Wolfe CDA, Bhalla A and Wang Y. Long-term trends in death and dependence after ischaemic strokes: A retrospective cohort study using the South London Stroke Register (SLSR). PLoS Med. 2020;17:e1003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt M, Jacobsen JB, Johnsen SP, Botker HE and Sorensen HT. Eighteen-year trends in stroke mortality and the prognostic influence of comorbidity. Neurology. 2014;82:340–50. [DOI] [PubMed] [Google Scholar]
- 7.Sennfalt S, Pihlsgard M, Norrving B, Ullberg T and Petersson J. Ischemic stroke patients with prestroke dependency: Characteristics and long-term prognosis. Acta Neurol Scand. 2021;143:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Shafaee MA, Ganguly SS and Al Asmi AR. Perception of stroke and knowledge of potential risk factors among Omani patients at increased risk for stroke. BMC Neurol. 2006;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YG, Miyazawa K, Wolff A, Zubaid M, Alsheikh-Ali AA, Sulaiman K and Lip GYH. One-year risks of stroke and mortality in patients with atrial fibrillation from different clinical settings: The Gulf SAFE registry and Darlington AF registry. Int J Cardiol. 2019;274:158–162. [DOI] [PubMed] [Google Scholar]
- 10.Kamran S, Bener AB, Deleu D, Khoja W, Jumma M, Al Shubali A, Inshashi J, Sharouqi I and Al Khabouri J. The level of awareness of stroke risk factors and symptoms in the Gulf Cooperation Council countries: Gulf Cooperation Council stroke awareness study. Neuroepidemiology. 2007;29:235–42. [DOI] [PubMed] [Google Scholar]
- 11.Banks JL and Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–6. [DOI] [PubMed] [Google Scholar]
- 12.Appelros P, Stegmayr B and Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–90. [DOI] [PubMed] [Google Scholar]
- 13.Roy-O’Reilly M and McCullough LD. Age and Sex Are Critical Factors in Ischemic Stroke Pathology. Endocrinology. 2018;159:3120–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Hajj M, Salameh P, Rachidi S and Hosseini H. The epidemiology of stroke in the Middle East. Eur Stroke J. 2016;1:180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qawasmeh MA, Aldabbour B, Momani A, Obiedat D, Alhayek K, Kofahi R, Yassin A and El-Salem K. Epidemiology, Risk Factors, and Predictors of Disability in a Cohort of Jordanian Patients with the First Ischemic Stroke. Stroke Res Treat. 2020;2020:1920583. [DOI] [PMC free article] [PubMed]
- 16.Tran J, Mirzaei M, Anderson L and Leeder SR. The epidemiology of stroke in the Middle East and North Africa. J Neurol Sci. 2010;295:38–40. [DOI] [PubMed] [Google Scholar]
- 17.Rukn SA, Mazya MV, Hentati F, Sassi SB, Nabli F, Said Z, Faouzi B, Hashim H, Abd-Allah F, Mansouri B, Kesraoui S, Gebeily S, Abdulrahman H, Akhtar N, Ahmed N, Wahl-gren N, Aref H, Almekhlafi M and Moreira T. Stroke in the Middle-East and North Africa: A 2-year prospective observational study of stroke characteristics in the region-Results from the Safe Implementation of Treatments in Stroke (SITS)-Middle-East and North African (MENA). Int J Stroke. 2019;14:715–722. [DOI] [PubMed] [Google Scholar]
- 18.Basri R, Issrani R, Hua Gan S, Prabhu N and Khursheed Alam M. Burden of stroke in the Kingdom of Saudi Arabia: A soaring epidemic. Saudi Pharm J. 2021;29:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cipolla MJ, Liebeskind DS and Chan SL. The importance of comorbidities in ischemic stroke: Impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab. 2018;38:2129–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elamy AH, Shuaib A, Carriere KC and Jeerakathil T. Common Comorbidities of Stroke in the Canadian Population. Can J Neurol Sci. 2020;47:314–319. [DOI] [PubMed] [Google Scholar]
- 21.Appelros P, Materne M, Jarl G and Arvidsson-Lindvall M. Comorbidity in Stroke-Survivors: Prevalence and Associations with Functional Outcomes and Health. J Stroke Cerebrovasc Dis. 2021;30:106000. [DOI] [PubMed] [Google Scholar]
- 22.Brainin M, Teuschl Y and Kalra L. Acute treatment and long-term management of stroke in developing countries. Lancet Neurol. 2007;6:553–61. [DOI] [PubMed] [Google Scholar]
- 23.Khatri IA, AlSkaini M, AlDayel A, Qamra A, Masuadi E, AlShammari M, AlKhalaf A, AlRasheed D, AlKhathaami A, AlOtaibi N, Tarawneh M and AlHizan K. Patterns and outcomes of stroke thrombolysis in a large tertiary care hospital in Riyadh, Saudi Arabia. Neurosciences (Riyadh). 2021;26:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandettini di Poggio M, Finocchi C, Brizzo F, Altomonte F, Bovis F, Mavilio N, Serrati C, Malfatto L, Mancardi G and Balestrino M. Management of acute ischemic stroke, thrombolysis rate, and predictors of clinical outcome. Neurol Sci. 2019;40:319–326. [DOI] [PubMed] [Google Scholar]
- 25.Campbell BC, Mitchell PJ, Yan B, Parsons MW, Christensen S, Churilov L, Dowling RJ, Dewey H, Brooks M, Miteff F, Levi C, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Kleinig T, Scroop R, Chryssidis S, Barber A, Hope A, Moriarty M, McGuinness B, Wong AA, Coulthard A, Wijeratne T, Lee A, Jannes J, Leyden J, Phan TG, Chong W, Holt ME, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM and investigators E-I. A multicenter, randomized, controlled study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits with Intra-Arterial therapy (EXTEND-IA). Int J Stroke. 2014;9:126–32. [DOI] [PubMed] [Google Scholar]
- 26.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, Tilley B, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Whiteley W, del Zoppo GJ, Baigent C, Sandercock P, Hacke W and Stroke Thrombolysis Trialists’ Collaborative G. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomized trials. Lancet. 2014;384:1929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P and Fiehler J. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur stroke J. 2019;4:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatia K, Jain V, Aggarwal D, Vaduga-nathan M, Arora S, Hussain Z, Uberoi G, Tafur A, Zhang C, Ricciardi M and Qamar A. Dual Antiplatelet Therapy Versus Aspirin in Patients With Stroke or Transient Ischemic Attack: Meta-Analysis of Randomized Controlled Trials. Stroke. 2021;52:e217-e223. [DOI] [PubMed] [Google Scholar]
- 29.Rost NS, Bottle A, Lee JM, Randall M, Middleton S, Shaw L, Thijs V, Rinkel GJ, Hemmen TM and Global Comparators Stroke Gc. Stroke Severity Is a Crucial Predictor of Outcome: An International Prospective Validation Study. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed]
- 30.Abdo R, Abboud H, Salameh P, El Hajj T and Hosseini H. Mortality and Predictors of Death Poststroke: Data from a Multicenter Prospective Cohort of Lebanese Stroke Patients. J Stroke Cerebrovasc Dis. 2019;28:859–868. [DOI] [PubMed] [Google Scholar]
- 31.Kimura K, Kazui S, Minematsu K, Yamaguchi T and Japan Multicenter Stroke Investigator's C. Analysis of 16,922 patients with acute ischemic stroke and transient ischemic attack in Japan. A hospital-based prospective registration study. Cerebrovasc Dis. 2004;18:47–56. [DOI] [PubMed] [Google Scholar]
- 32.Heuschmann PU, Kolominsky-Rabas PL, Misselwitz B, Hermanek P, Leffmann C, Janzen RW, Rother J, Buecker-Nott HJ, Berger K and German Stroke Registers Study G. Predictors of in-hospital mortality and attributable risks of death after ischemic stroke: the German Stroke Registers Study Group. Arch Intern Med. 2004;164:1761–8. [DOI] [PubMed] [Google Scholar]
- 33.Shigematsu K, Nakano H, Watanabe Y, Sekimoto T, Shimizu K, Nishizawa A, Makino M, Okumura A, Bando K and Kitagawa Y. Characteristics, risk factors and mortality of stroke patients in Kyoto, Japan. BMJ Open. 2013;3. [DOI] [PMC free article] [PubMed]
- 34.Alhazzani AA, Mahfouz AA, Abolyazid AY, Awadalla NJ, Katramiz K, Faraheen A, Khalil SN and Aftab R. In Hospital Stroke Mortality: Rates and Determinants in Southwestern Saudi Arabia. Int J Environ Res Public Health. 2018;15. [DOI] [PMC free article] [PubMed]
- 35.Femi OL and Mansur N. Factors associated with death and predictors of one-month mortality from stroke in Kano, Northwestern Nigeria. J Neurosci Rural Pract. 2013;4:S56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartmann A, Rundek T, Mast H, Paik MC, Boden-Albala B, Mohr JP and Sacco RL. Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study. Neurology. 2001;57:2000–5. [DOI] [PubMed] [Google Scholar]
- 37.Koton S, Tanne D, Green MS and Born-stein NM. Mortality and predictors of death 1 month and 3 years after first-ever ischemic stroke: data from the first national acute stroke Israeli survey (NASIS 2004). Neuroepidemiology. 2010;34:90–6. [DOI] [PubMed] [Google Scholar]
- 38.Ekeh B, Ogunniyi A, Isamade E and Ekrikpo U. Stroke mortality and its predictors in a Nigerian teaching hospital. Afr Health Sci. 2015;15:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaarisalo MM, Immonen-Raiha P, Marttila RJ, Salomaa V, Kaarsalo E, Salmi K, Sarti C, Sivenius J, Torppa J and Tuomilehto J. Atrial fibrillation and stroke. Mortality and causes of death after the first acute ischemic stroke. Stroke. 1997;28:311–5. [DOI] [PubMed] [Google Scholar]
- 40.Yang XM, Rao ZZ, Gu HQ, Zhao XQ, Wang CJ, Liu LP, Liu C, Wang YL, Li ZX, Xiao RP and Wang YJ. Atrial Fibrillation Known Before or Detected After Stroke Share Similar Risk of Ischemic Stroke Recurrence and Death. Stroke. 2019;50:1124–1129. [DOI] [PubMed] [Google Scholar]
- 41.Bjerkreim AT, Khanevski AN, Thomassen L, Selvik HA, Waje-Andreassen U, Naess H and Logallo N. Five-year readmission and mortality differ by ischemic stroke subtype. J Neurol Sci. 2019;403:31–37. [DOI] [PubMed] [Google Scholar]
- 42.Dulli DA, Stanko H and Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology. 2003;22:118–23. [DOI] [PubMed] [Google Scholar]
- 43.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ and D’Agostino RB. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–4. [DOI] [PubMed] [Google Scholar]
- 44.Wankowicz P, Golab-Janowska M and Nowacki P. Risk factors for death by acute ischaemic stroke in patients from West-Pomerania, Poland. Neurol Neurochir Pol. 2020;54:150–155. [DOI] [PubMed] [Google Scholar]
- 45.Kimura K, Minematsu K, Kazui S, Yamaguchi T and Japan Multicenter Stroke Investigators C. Mortality and cause of death after hospital discharge in 10,981 patients with ischemic stroke and transient ischemic attack. Cerebrovasc Dis. 2005;19:171–8. [DOI] [PubMed] [Google Scholar]
- 46.Yao YY, Wei ZJ, Zhang YC, Li X, Gong L, Zhou JW, Wang Y, Zhang YY and Wang RP. Functional Disability After Ischemic Stroke: A Community-Based Cross-Sectional Study in Shanghai, China. Front Neurol. 2021;12:649088. [DOI] [PMC free article] [PubMed] [Google Scholar]