Abstract
A hallmark of ARDS is progressive shrinking of the ‘baby lung,’ now referred to as the ventilator-induced lung injury (VILI) ‘vortex.’ Reducing the risk of the VILI vortex is the goal of current ventilation strategies; unfortunately, this goal has not been achieved nor has mortality been reduced. However, the temporal aspects of a mechanical breath have not been considered. A brief expiration prevents alveolar collapse, and an extended inspiration can recruit the atelectatic lung over hours. Time-controlled adaptive ventilation (TCAV) is a novel ventilator approach to achieve these goals, since it considers many of the temporal aspects of dynamic lung mechanics.
Keywords: Acute respiratory distress syndrome (ARDS), Ventilator-induced lung injury (VILI), Protective mechanical ventilation
Introduction
The acute respiratory distress syndrome (ARDS) remains a significant clinical problem with primary management being supportive use of mechanical ventilation (MV) [1]. However, inappropriate use of MV may result in ventilator-induced lung injury (VILI), which significantly increases ARDS mortality [2]. The use of MV for ARDS patients must balance its life-preserving attributes against its potential for harm. Strategies for optimizing this balance may vary substantially across patients, and even within the same patient over a given clinical course.
The shrinking baby lung
The ARDS lung has been conceptualized as being composed of two distinct, gravitationally separated compartments: (1) dependent regions consisting atelectatic and/or edematous airspaces and (2) normally inflated tissue in less dependent regions comprising the so-called baby lung [3]. This conceptualization led to the hypothesis that ventilating patients with ARDS using a reduced tidal volume (VT) would protect the baby lung from volutrauma caused by overdistension, while simultaneously allowing the atelectatic compartment to rest and [ideally] recover [4]. It was also assumed that an appropriate level of positive end-expiratory pressure (PEEP), based on oxygenation, would avoid atelectrauma [4, 5]. This ARDSNet method was studied in a NIH clinical trial in 2000 [4] and showed a significant reduction in ARDS mortality using volume control [assist-control] mode with a VT of 6 mL kg−1 compared to 12 mL kg−1 of ideal body weight. The use of 6 mL kg−1 soon became the standard of care for patients with ARDS.
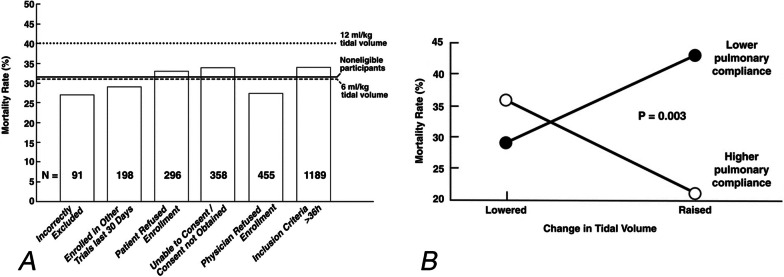
Recent statistical analyses suggest, however, that this low VT (LVT) strategy has not lived up to its initial promise in reducing mortality in ARDS [6–9]. Deans et al. [10] analyzed data from 2587 patients that were screened but excluded from the ARDSNet Acute Respiratory Management Approach (ARMA) trial for technical reasons but were followed and treated with VT of ~ 10 mL kg−1, which was the standard of care for ventilation at the time. The group with VT ~ 10 mL kg had the same mortality as the LVT (6 mL kg1) group (Fig. 1A). Also, a VT greater than 6 mL kg−1 was not always associated with increased mortality, nor was LVT always associated with reduced mortality. Rather, in patients with lower respiratory system compliance (CRS), raising VT increased mortality compared to LVT (42% for LVT vs. 29% for high VT), while raising VT in patients with higher CRS reduced mortality (21% for high VT vs. 37% for LVT; p = 0.003; Fig. 1B) [10].
Fig. 1.
A Comparison of mortality rates in patients included and excluded from the ARDSNetwork low VT trial (ARMA). The overall mortality rates of non-eligible patients who received standard of care mechanical ventilation (solid line; n = 2587), the 12 mL kg−1 tidal volume (VT) group (dotted line; n = 429), and the 6 mL kg−1 VT group (dashed line; n = 432) are shown. Mortality was consistent across the non-eligible patients for the six exclusion reasons (vertical bars) and similar to that in the 6 mL kg−1 VT group. Data provided to the Office of Human Research Protections from ARDSNet investigators from the ARMA trial for use at the June 9–11, 2003, consultants meeting. Available under the Freedom of Information Act [10]. B Pulmonary compliance plays a critical role in mortality with changes in VT size. There was a significant interaction between pulmonary compliance and mortality rate in the ARMA trial (p = 0.003). Raising VT increased mortality compared with lowering VT (filled circles; 42% vs. 29%) in patients with lower pulmonary compliance. In contrast, raising VT decreased mortality compared with lowering VT (unfilled circles; 21% vs. 37%) in patients with higher pulmonary compliance [10]. (Permission to republish requested)
These findings demonstrate that a patient’s individual lung pathophysiology is critically important for clinical outcome. Thus, there cannot be a single weight-based value of VT that is best for all patients either at the initiation of MV or as their clinical course evolves. Appreciation of this fact is evidenced by the recent interest in driving pressure (ΔP), calculated as the difference between plateau and end-expiratory pressures. ΔP can evolve over a patient’s clinical course and is approximated by the ratio VT/CRS, which explains why letting VT be influenced by CRS has proven to be better at stratifying ARDS-related mortality risk compared to weight-based VT [7, 11–15].
These observations beg the question as to whether ΔP should replace VT as the key factor guiding protective ventilation strategies [7, 11–16]. Dramatic reductions in ΔP can be achieved with high-frequency oscillatory ventilation (HFOV), because it uses VT that are less than the anatomic dead space volume. However, when tested in randomized controlled trials [17–19], HFOV failed to reduce ARDS-related mortality below that in the ARMA study [4]. Such a disappointing result may be related to the heterogeneous way that ventilation is distributed throughout the lung when cycled at high frequencies [20, 21] and the resultant heterogeneous distributions of parenchymal strain [20, 21]. Moreover, studies have shown that normal lung tissue, which is presumed to comprise the baby lung, is resistant to tissue damage induced by overdistension [22–30]. Also, although some studies suggest VILI occurs when a threshold of mechanical power applied to the lung is exceeded, studies in animal models suggest that VILI in the normal lung is only initiated when atelectrauma is allowed to occur regardless of VT [26, 31, 32].
The baby lung concept, which established the rationale for LVT, was originally based on CT imaging [3]. However, more recent studies pairing CT with 3He or 129Xe magnetic resonance imaging (MRI) have shown that pathologic airspaces develop heterogeneously throughout the ARDS lung [33–36], contrary to the notion of a normal baby lung compartment. Furthermore, it has recently been shown that the principle mechanism of VILI at the tissue level is regional alveolar instability [5], defined as cyclic alveolar collapse causing scattered micro-atelectasis to develop throughout the lung [33–38]. In addition, Broche et al. using high-resolution synchrotron phase-contrast computerized tomography (CT) showed that acute lung injury significantly increased small airway (1.7–0.21 mm) closure and that this closure was time dependent. They suggest that the airway pressure release ventilation (APRV) mode, with a very short expiratory duration, may help to keep these small airways open [39]. Since micro-atelectasis cannot be seen in conventional chest radiographs or CT images [32, 40], its importance as a VILI mechanism may not have been fully appreciated until recently.
An assumption of the current protective ventilation approach is that the injured lung can be easily compartmentalized into simple opened and closed components. However, several studies have demonstrated that the injured lung has a far more heterogeneous distribution of parenchymal mechanical properties. Techniques that may be used to further assess such intraparenchymal heterogeneity include electrical impedance tomography [39, 41], computed tomographic image registration [42, 43] and oscillometric measurements of respiratory impedance [44]. How any of these approaches may be used to further refine the ventilation modality will of course require further investigation.
Unshrinking the baby lung
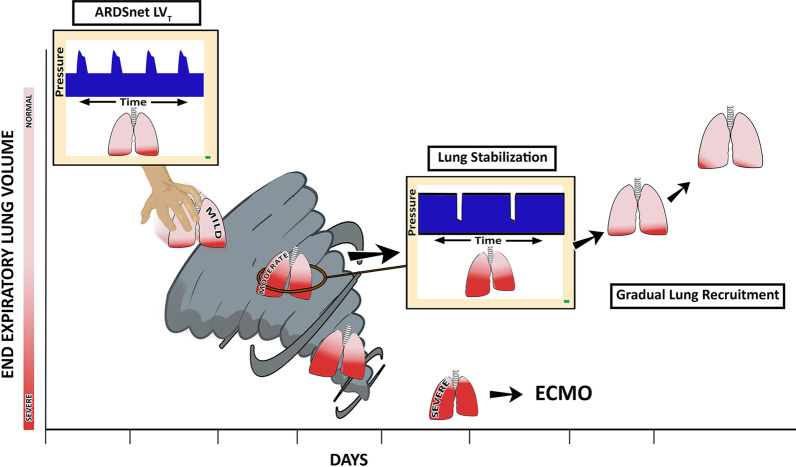
Marini and Gattinoni recently described the progression of VILI as a shrinking of the baby lung whereby tissue moves from the open to the atelectatic compartment, a process labeled the ‘VILI vortex’ (Fig. 2) [45]. They hypothesize that as the baby lung continues to lose normal tissue as a result of alveolar instability and collapse, increasing stress and strain from a fixed VT will be placed on the remaining open tissue, amplify existing lung injury. The VILI vortex concept was recently supported in a clinical study showing COVID-19-induced ARDS (CARDS) resulted in progressive lung collapse over a 3-week period [46].
Fig. 2.
The evolution of ventilator-induced lung injury (VILI) can be described as an ever-shrinking baby lung known as a VILI vortex [45]. The ‘patient’ with mild ARDS with mostly open lung tissue (pink) and a lesser amount of collapsed tissue (red) is placed on ARDSNet LVT ventilation. The LVT strategy is designed to shield the ‘baby lung’ from overdistension. However, this strategy using low VT and low airway pressures allows acutely injured tissue to continually collapse pushing it into the VILI vortex. As normal tissue progressively shrinks (pink → red), lung pathogenesis moves from mild-to-moderate ARDS. If unchecked, lung injury will progress into severe ARDS, at which point rescue methods such as extracorporeal membrane oxygenation (ECMO) may be necessary. ARDS causes the lung to become time and pressure dependent. This means that it will take more time for alveoli to open and less time for them to collapse at any given airway pressure. Thus, inspiratory and expiratory time can be used to accelerate alveolar opening and to minimize alveolar collapse. Using the ARDSNet approach, the short time at inspiration is not adequate to open collapsed alveoli, while the extended time at expiration will not prevent alveolar collapse (upper left ARDSNet LVT, Pressure/Time curve on the ventilator monitor). The open lung approach (OLA) using higher PEEP with and without recruitment maneuvers to rapidly (seconds or minutes) open the collapsed ARDS lung has not been successful at reducing ARDS-related mortality. Our group and others have shown the ability of inspiratory and expiratory duration to open and stabilize alveoli. Multiple studies using time-controlled ventilation strategies have confirmed that an extended inspiratory time will progressively recruit alveoli and a very brief expiratory time will prevent re-collapse [63, 66, 68, 71–83, 85, 86, 90]. An ventilator method to rapidly stabilize the lung (Center, Lung Stabilization, Pressure/Time curve on the ventilator monitor) using a very brief expiratory duration (Fig. 4B, Release Phase) has been shown to stabilize alveoli (Fig. 6, APRV 75%) and prevent progressive lung collapse pulling the lung from the Vortex. Once removed from the vortex, the collapsed tissue can be reopened slowly (gradual lung recruitment) over hours or day depending on the level of lung pathophysiology (Fig. 4B, CPAP Phase) [63, 66, 68, 71–83, 85, 86, 90]
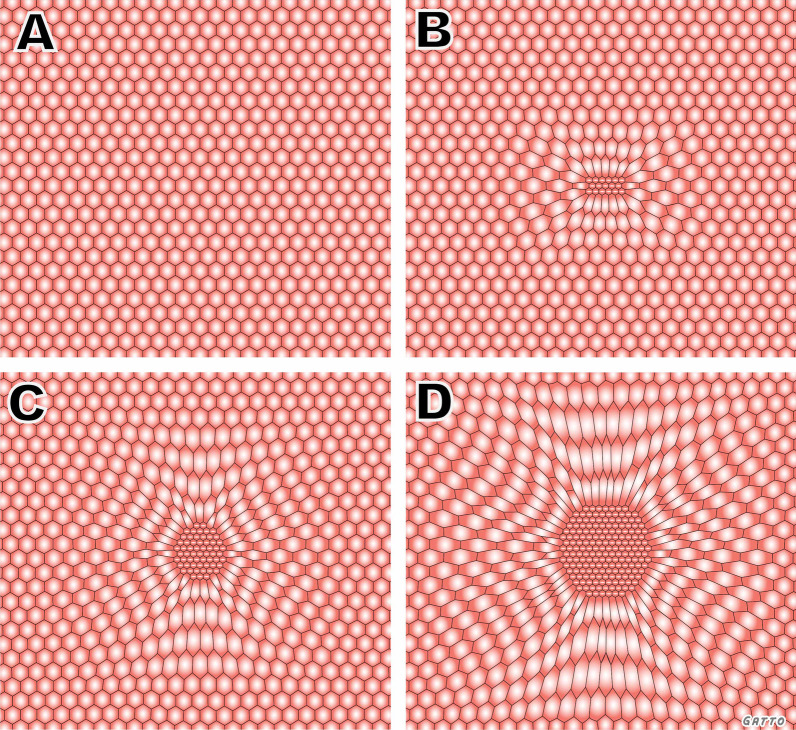
Unfortunately, fixing the VILI vortex is unlikely to be achieved solely with the use of a LVT strategy because the parenchymal tissues at the interfaces between normal and atelectatic regions experience particularly high distortional forces. These forces predispose the interfacial tissues to being the site of VILI pathogenesis through a permeability-originated obstruction response (POOR) that is self-reinforcing (Fig. 3) [37]. This means that VILI will continue to progress at the interfacial regions even if the baby lung as a whole is not over-inflated. It also leads to the conjecture that the only way of preventing VILI progression is through a substantial reduction in the total burden of POOR regions, something that requires the immediate mitigation of alveolar instability followed by steps to progressively and safely reopen densely atelectatic lung tissue (Fig. 2) [38].
Fig. 3.
Ventilator-induced lung injury (VILI) in the microenvironment arises through a permeability-originated obstruction response (POOR) that is self-reinforcing (POOR-becomes-POORer). A In normal homogeneously ventilated lung, alveoli (hexagons) are uniformly open and stress is evenly distributed. B Isolated POOR areas of edema-filled or collapsed alveoli (center) that occur in early lung injury concentrate stress in adjacent patent alveoli, causing overdistension and instability. C The size of the POOR area expands due to collapse and flooding of surrounding alveoli, leading to the POOR region becoming POORer. D As the size of the POORer region expands, the stress applied to the surrounding alveoli is amplified and, unless this pathogenesis is interrupted, tissue damage secondary to VILI will continue to spread rapidly. (Permission to republish requested)
The above supposition is not new, and indeed, it motivated the open lung approach (OLA) to protective MV. The OLA attempts to open the majority of collapsed lung by applying high levels of PEEP, either with or without periodic recruitment maneuvers (RM) [47–50]. Several large clinical trials using the OLA, however, failed to show a reduction in ARDS-related mortality [47–50] despite preliminary data suggesting that opening the ARDS lung reduces VILI. While this might seem puzzling given that the OLA has an apparently well-founded physiologic rationale, there are two critical factors that potentially account for its lack of success. First, while recruitment/derecruitment (R/D) is a process normally associated with excursions in lung volume over the lower end of its functional range, these processes can manifest at increasingly elevated volumes as the lung becomes progressively more injured and may eventually even extend over the entire lung volume range [51, 52]. Thus, in some ARDS cases there may be no safe level of PEEP at which R/D is eliminated during MV.
The second factor not addressed by the OLA is that recruitment is not solely determined by the amount of airway pressure applied but also depends on inspiratory and expiratory time. Indeed, lung reopening may take a long time, sometimes hours or days, to occur [53]. The OLA uses RMs that rely on high inspiratory pressure applied transiently (seconds to minutes) to achieve rapid reopening, but lung recruitment can be challenging to achieve quickly even with very high pressures due to accumulation of surfactant deactivation and edema in the airspaces [38, 54]. Also, the nondurable recruitment produced by the OLA has the potential for increasing the number of unstable airspaces if adequate PEEP is not applied.
Similarly, reducing airway pressure may not cause immediate derecruitment because collapse also takes time to occur. The timescale for alveolar collapse is typically much faster than the timescale for alveolar reopening, and the delay before collapse begins may be < 0.5 s [55]. If PEEP is set too low and/or expiratory time too long, the resulting progressive lung collapse is reflected in transient increases in lung elastance (i.e., reductions in CRS) observed immediately following a RM [56]. Furthermore, the rates at which both alveolar opening and collapse occur are functions of the nature and severity of lung injury [57], as well as the level of airway pressure applied [58]. In general, the more severe the lung injury, the more rapid and extensive derecruitment will be at a given pressure [59]. As conventionally practiced, the administration of a RM does not consider how rapidly the lung derecruits following each maneuver. If atelectatic regions are recruited but not stabilized, then cyclic R/D will continue to cause atelectrauma [60].
The above considerations suggest that a non-injurious ventilation strategy for the ARDS lung must first be able to halt ongoing derecruitment and then progressively pull the lung out of the VILI vortex by gradually opening atelectatic lung (Fig. 2). To do this, such a strategy must have the following two key attributes:
It must rapidly stabilize alveoli that are actively undergoing atelectrauma, such that susceptible airspaces do not have enough time to close during expiration and thus are prevented from having to reopen again during the subsequent inspiration. This prevents the accumulation of breath-to-breath atelectrauma and so pulls the lung from the VILI vortex.
It must progressively recruit atelectatic lung tissue in a sustained manner over a period of hours or even days. This minimizes the amount of excessively distorted parenchyma at the interfaces between patent and atelectatic regions of the lung that are frequently the sites of VILI initiation [61]. Once gradual airspace opening begins, it can spread to adjacent collapsed regions via the forces of parenchymal tethering and interdependence [62].
Neither attribute is likely to be realized in the injured lung during conventional MV because expiration is usually long enough to allow rapidly closing lung units sufficient time to derecruit unless very high PEEP is applied, and inspiration is too brief to recruit collapsed alveoli that open slowly over time (Figs. 4A, 5A). The above two attributes can be realized, however, if the pattern of ventilation is allowed to depart from traditional mechanical ventilation in the following two ways (Figs. 4B, 5B):
Expiration must be sufficiently brief that derecruitment does not have enough time to occur prior to the beginning of the next inspiration (Fig. 4B, Release Phase).
Inspiratory duration and pressure must be sufficient to progressively recruit atelectatic lung over an extended period of time, but not so high as to be injurious to the parenchyma or have adverse hemodynamic consequences (Fig. 4B, CPAP Phase). Pressure must be sustained in a manner that is capable of recruiting lung units gradually, and this pressure must be applied for hours or days until the lung is fully open and stable (Fig. 5B–D).
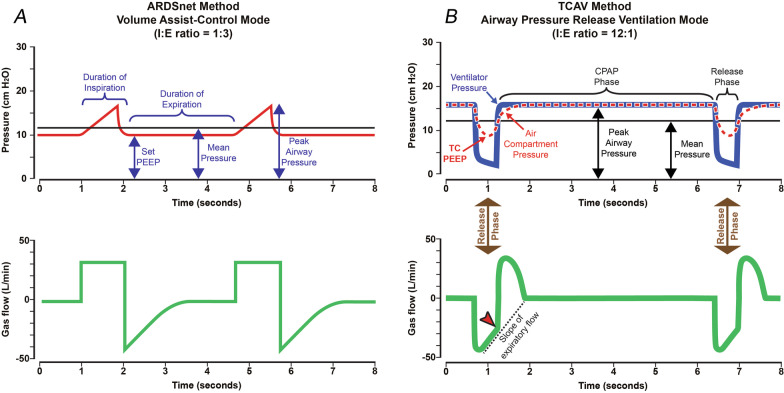
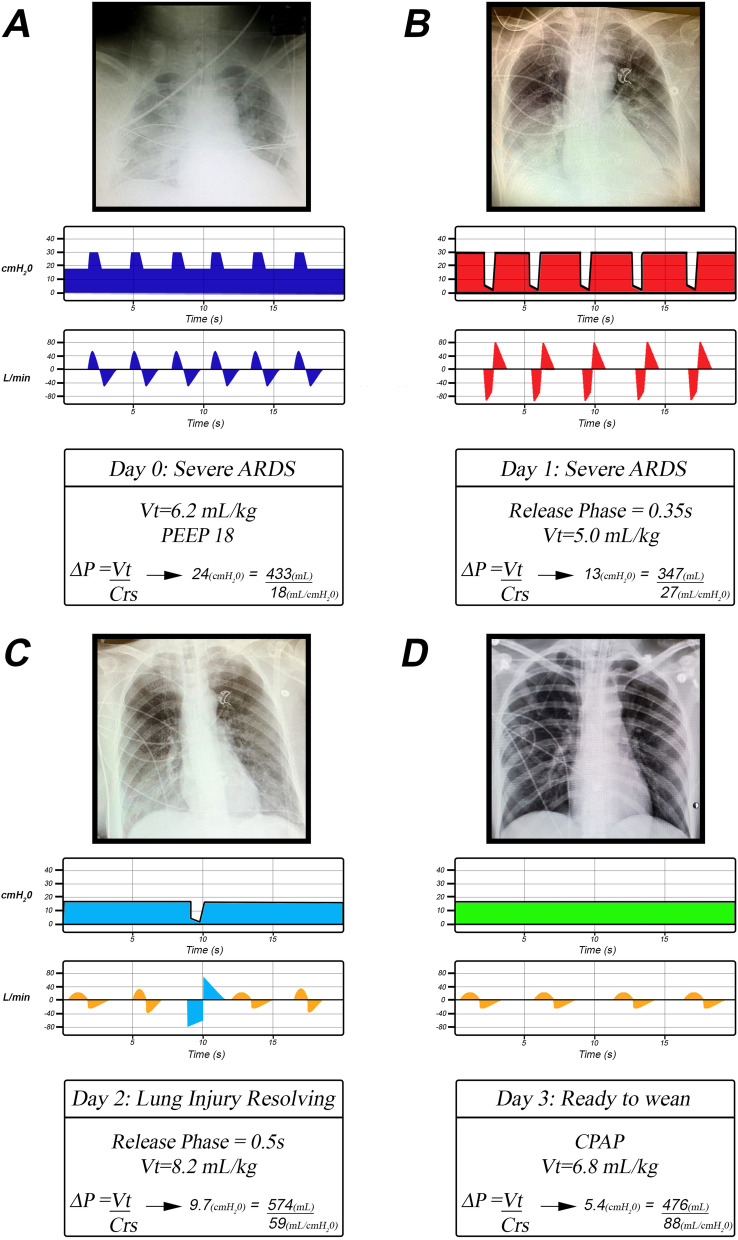
Fig. 4.
A Pressure/Time and Gas Flow/Time curves for Volume Assist-Control mode set and adjusted using the ARDSNet method. Key features include an inspiratory/expiratory ratio of 1:3. Plateau pressure is not extended, so peak inspiratory pressure is brief. Positive end-expiratory pressure (set-PEEP) and FiO2 are adjusted using oxygenation as the trigger for change [4]. B Pressure/Time and Gas Flow/Time curves for the airway pressure release ventilation (APRV) mode that is set and adjusted using the time-controlled adaptive ventilation (TCAV) method. Key features include an inspiratory/expiratory ratio as high as ~ 12:1, generating a prolonged inspiratory and short expiratory time. The continuous positive airway pressure (CPAP) phase is often ~ 90% of each breath. A tidal volume (VT) is not set, rather it is influenced by changes in (i) respiratory system compliance (CRS), (ii) the CPAP Phase pressure, and (iii) the duration of the Release Phase. The Release Phase is set as a percentage (75%) of the peak expiratory gas flow, which creates a very brief expiratory duration (Flow/Time curve, red arrowhead). Although this percentage is the same for most patients, the duration of the Release Phase can vary substantially in response to changes in CRS. The slope of the expiratory flow curve in the Gas Flow/Time curve provides a breath-to-breath measure of CRS. The lower the CRS, the steeper the slope of the expiratory flow curve, and the shorter the Release Phase. The slope of the expiratory flow curve becomes less steep as the patient’s CRS improves, which causes the Release Phase to lengthen (Fig. 7). The short Release Phase does not allow the lung time to depressurize fully, maintaining a time-controlled positive end-expiratory pressure (TC-PEEP, red dotted line). TC-PEEP is ~ 50% of the CPAP Phase pressure [91]. (Permission to republish requested)
Fig. 5.
A representative illustration of the sequence of events typically seen during the progressive ‘Unshrinking the Baby Lung’ using the TCAV method to set and adjust the APRV mode. Using this method, the lung is first stabilized using a brief expiratory duration (B) and then gradually recruits collapsed lung tissue over hours or days using an extended inspiratory duration (B). A Patient with severe ARDS with extensive lung collapse and edema (X-ray) ventilated using the ARDSNet low VT method showing typical Pressure/Time and Flow/Time curves (blue) as seen on a ventilator monitor. Even with a low VT (6.2 mL kg−1), the driving pressure (ΔP) is elevated (24 cmH2O) because the CRS is very low (18 mL/cmH2O). B The first day after the patient has been switched to the TCAV method, and the lung has begun to reopen showing typical Pressure/Time and Flow/Time curves as seen on a ventilator monitor (red). Although the lung has partially opened, it is still unstable and would rapidly re-collapse at lower airway pressures or if expiratory time was greater than the 0.35 s Release Phase. The CRS has improved (27 mL cmH2O−1) because of the recruited lung tissue (X-ray). Nevertheless, the CRS is low and lung recoil remains high, so the Release Phase is brief. The brief Release Phase generates a small VT (5.0 mL kg−1), which maintains the ΔP (13 cmH2O) within the safe range. At this time, there are no spontaneous breathing efforts. C On Day 2, the lung is nearing full recruitment, as evidenced by a markedly improved chest X-ray. The patient is breathing spontaneously (Flow/Time curve gold waves) and is contributing to the total minute ventilation (MVe). Although the TLow is still set to 75%, the release time is now 0.5 s and VT have increased (8.2 mL kg−1) with a more fully recruited lung. The ΔP has decreased to 9.7 cmH2O, even with a VT of 8.2 mL kg−1 because of an increase in CRS (59 mL cmH2O−1). Only an occasional Release Phase is needed to facilitate CO2 removal (Flow/Time curve light blue wave), since most of the MVe is generated by the patient’s spontaneous breathing (Flow/Time curve gold waves). Spontaneous VT average 6.0 mL kg. D On Day 3, the patient is ready to be weaned with restored lung volume. The Release Phase has been eliminated and the patient is generating all of their MVe with spontaneous breathing (Flow/Time curve gold waves). As a result, VT has increased further (6.8 mL kg−1), and ΔP (5.4 cmH2O) and CRS (88 mL cmH2O−1) are within their normal ranges. Note that a VT greater than 6 mL kg−1 is not harmful (i.e., normal ΔP) when delivered into a fully inflated lung with high CRS [10]. Also, note VT remains proportional to CRS. VT = tidal volume, PEEP = positive end-expiratory pressure, CRS = respiratory system compliance, MVe = minute ventilation, and ΔP = Driving Pressure (VT/CRS).
A mode of MV that potentially meets the above two requirements is APRV, although it must be administered in a very particular manner (Fig. 4B). The most critical APRV parameter is the duration of the expiratory Release Phase (TLow), which must be brief enough to prevent collapse of even the most rapidly closing alveoli, thereby attending to the first requirement. It is important to realize, however, that extending TLow from its optimal value by even a fraction of a second can increase expiratory derecruitment dramatically, with potentially disastrous results for the lung (Fig. 6A, B—APRV 10%) [63].
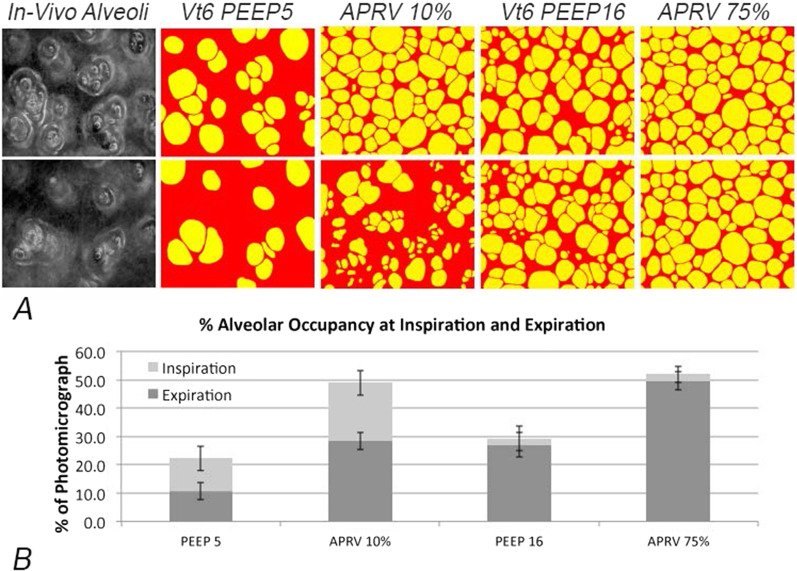
Fig. 6.
A Photomicrograph of in vivo subpleural alveoli (in vivo alveoli) at inspiration (top panel) and expiration (bottom panel) following lung injury in rats. Alveoli in the four ventilation treatment groups are depicted in yellow, while collapsed alveoli are in red. The images show the impact of ventilation strategies on alveolar recruitment and collapse using a conventional ventilation strategy with a low tidal volume (LVT) of 6 mL kg−1 (Vt6) combined with either PEEP 5 cmH2O (Vt6 PEEP5) or 16 cmH2O (Vt6 PEEP 16). Also shown for comparison are the results of using APRV with an extended PHigh (CPAP Phase, Fig. 4B) combined with two expiratory durations set at either 75% of peak expiratory flow (APRV 75%) or 10% of peak expiratory flow (APRV 10%). APRV 75% has a very short expiratory duration, while APRV 10% has a much longer expiratory duration (Fig. 4B, Release Phase). B The impact of each ventilation strategy on alveolar recruitment at inspiration (light gray bar) and derecruitment at expiration (dark gray bar) expressed as the percent (%) of the microscopic field. Conventional ventilation using LVT did not effectively recruit alveoli (PEEP5 and PEEP16). Increasing to PEEP16 reduced the number of alveoli that collapsed during expiration (difference between the light and dark gray bars), but did not recruit as many alveoli as APRV with an extended CPAP Phase. However, using APRV with an extended expiratory duration (APRV 10%) caused many of the newly recruited alveoli to re-collapsed. Alveolar collapse at expiration was prevented with the use of a brief expiratory duration (APRV 75%) [58]. (Permission to republish requested)
The second requirement can be met by an appropriate level of inspiratory airway pressure (PHigh) applied for an extended duration (THigh) (Fig. 4B, CPAP Phase). This effectively applies CPAP to the lung, initiating a gradual and sustained alveolar reopening over a prolonged period of time (Fig. 5B–D). Since the time required to recruit some regions of the lung can be extremely long, the benefits of opening the lung in this manner may not be evident for hours or even days (Fig. 5B–D) [32–35]. The level of pressure applied during expiration (PLow) is less critical to the derecruitment process because the brief TLow prevents the lungs from completely emptying by the end of expiration, resulting in a degree of time-controlled PEEP (Fig. 4B, TC-PEEP). Setting PLow to 0 cmH2O maximizes expiratory flow and facilitates CO2 elimination with each breath, thus helping to maintain normocarbia. At the exhalation termination point (TLow), the lung is rapidly re-inflated to the CPAP Phase (Fig. 4B, Gas Flow/Time curve, red arrowhead).
The values of PHigh, THigh, PLow, and TLow thus collectively define how APRV is administered. Using appropriate values for these parameters will mitigate some of the adverse effects of ventilating a baby lung. The question remains, however, as to how these values should be chosen, particularly TLow. Figure 7 shows: (A) normal expiratory flow/time curve, (B) the changes caused by ARDS, and (C) how this information can be used to set TLow necessary to stabilize alveoli and maintain a normal functional residual capacity (FRC). Passive exhalation in the normal lung is slow (~ 2 s) due a high CRS and low lung recoil (Fig. 7A—thin springs). A functioning pulmonary surfactant system maintains a normal FRC at atmospheric pressure. ARDS-induced surfactant deactivation reduces lung CRS and increases recoil (thick springs) causing a rapid exhalation (~ 1 s) of gas during expiration (Fig. 7B—ARDS). The FRC can decrease by up to 45% in lungs with severe ARDS [64]. Thus, allowing the injured lung to collapse to atmospheric pressure will result in a much lower FRC than in the normal lung. Experience in both the animal laboratory and the intensive care unit (ICU) has demonstrated that the expiratory gas flow curve using the APRV mode can be used in a patient-targeted and adaptable approach to predict the TLow duration necessary to stabilize alveoli.
Fig. 7.
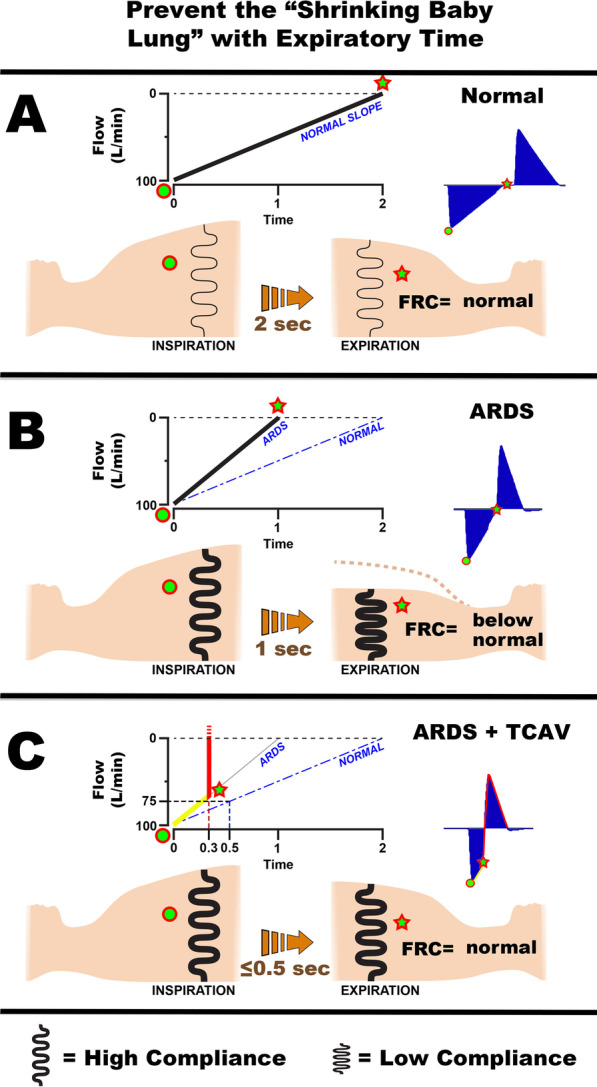
Using expiratory time and the airway pressure release ventilation (APRV) mode to maintain a normal functional residual capacity (FRC) and prevent progressive collapse of the ARDS lung (VILI vortex). A The Flow/Time curve (Fig. 4B) at the beginning of expiration (green dot) and at end expiration (Release Phase, red star). The normal lung is allowed to fully empty (Flow 0 L/min) to atmospheric pressure (0 cmH2O). The respiratory system compliance (CRS) in the normal lung is high, and therefore, the lung recoil is low (thin black spring). The slope of the expiratory flow curve (SlopeEF) is shallow (NORMAL SLOPE) taking ~ 2 s for the lung to fully empty. A functioning pulmonary surfactant system prevents lung collapse at atmospheric pressure, and FRC remains normal. The Flow/Time curve that would be seen on the ventilator monitor in blue (Fig. 4B, Gas Flow/Time). B ARDS diminishes surfactant function and dramatically decreases CRS, leading to increased lung recoil (thick black spring). This results in a steep SlopeEF (ARDS, solid black line) compared with a normal slope (NORMAL, dashed blue line). As a result, the lung empties rapidly (~ 1 s), and there is a marked reduction in FRC seem as large reduction in chest volume (green star). The Flow/Time curve that would be seen on the ventilator monitor in blue (Fig. 4B, Gas Flow/Time). C The TCAV method uses a fraction (75%) of the peak expiratory flow (100 L/min) to set the expiratory duration. Changes in the SlopeEF with decreasing CRS will modify the expiratory duration using this method (ARDS = 0.3 s; Normal = 0.5 s at the same 75% fraction). Note that in the ARDS lung the SlopeEF (yellow line) is steep and 75% is reached very rapidly (0.3 s) at which point the lung is rapidly re-inflated to the CPAP Phase (red line). In the normal lung, the SlopeEF is shallower and takes 0.5 s to reach 75% of the peak expiratory flow. The brief expiratory duration does not give the lung time to depressurize (Fig. 4B, TC-PEEP) or alveoli time to collapse (Fig. 6A, B, APRV 75%) maintaining a near normal FRC and preventing progressive lung collapse (VILI vortex). The Flow/Time curve that would be seen on the ventilator monitor in blue (Fig. 4B, Gas Flow/Time)
Using this approach, TLow is set such that expiration terminates when the magnitude of expiratory flow has fallen to a fixed percentage (75%) of its peak value at the start of expiration (Fig. 5C) [65, 66]. This results in TLow values that vary across patients and disease states. For example, patients with severe ARDS typically have less compliant lungs that empty quickly, thus reaching their expiratory termination points (Fig. 7C, 0.3 s) earlier than patients with more compliant lungs (Fig. 7C, 0.5 s). This effect can be appreciated by examining the slope of the expiratory gas flow/time curve (SlopeEF) from lungs with differing CRS (Fig. 7C—ARDS and NORMAL) [67]. As lung health improves and CRS increases, TLow may be increased accordingly (Fig. 5C—ARDS = 0.3 s; NORMAL = 0.5 s). Thus, setting TLow according to the SlopeEF affords a personalized approach to ventilating the injured lung that adapts to a patient’s changing physiology. Finally, similar to normalizing VT to CRS (i.e., driving pressure) to reduce mortality (Fig. 1B), TLow adjusted to 75% of peak expiratory flow produces VTs (Fig. 5B 6.2 mL/kg and Fig. 5C 9.2 mL/kg) that is proportional to CRS (Fig. 5B = CRS low; 5D = CRS high).
Applying APRV in the above manner requires continuous monitoring of intra-breath expiratory flow as well as the ability to control the termination of expiration. This precise method of setting the APRV mode using the SlopeEF is termed the time-controlled adaptive ventilation (TCAV) method (Fig. 7C). The TCAV method has been shown to enhance lung protection [63, 66, 68–83] by stabilizing alveoli [63] (Fig. 6A, B—APRV 75%) and progressively reopening recalcitrant regions of atelectasis (Fig. 5 B–D). TCAV rapidly pulls the lung out of the VILI vortex and then gradually reopens collapsed tissue (Fig. 2) [63, 70, 84].
The TCAV method has been extensively studied by several groups [63, 66, 68, 71–83, 85, 86]. It has been shown to recruit subpleural alveoli in a rat model of ARDS (Fig. 6, APRV 75%) [63, 71, 86] and reduce tissue damage compared to ARDSNet LVT strategy in a clinically applicable porcine sepsis and gut ischemia/reperfusion-induced ARDS model [72, 79]. The TCAV method has also been shown to reduce ARDS incidence and mortality in trauma patients [60]. These findings suggest the physiologic principles upon which TCAV is based represent a successful method to balancing the benefits of positive pressure ventilation against the harm it may cause to an already injured lung.
It must be noted that in patients with expiratory flow limitations (EFL) the TCAV method must be modified. Since flow/time is an integral of volume, airflow limitations are easily depicted with changes in peak expiratory flow rates and can be readily seen at the bedside with flow graphics using the TCAV method. The pattern of airflow limitations results in the following characteristics: (a) The peak expiratory flow rate decreases as expected in diseases with obstructed airways and (b) an uneven pattern of incomplete and sequential gas emptying that greatly increases the deceleration angle of the expiratory flow curve, which is a hallmark of obstructive lung disease. Once EFL is identified, the TCAV protocol is modified to increase the expiratory duration, since more time is needed for the same volume of gas to be exhaled. The TCAV protocol for patients with EFL is beyond the scope of this paper.
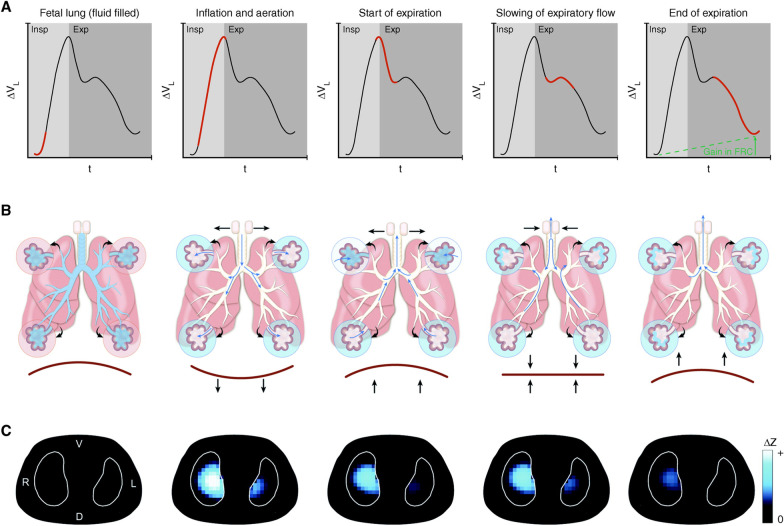
Insight into the mechanism by which the TCAV method opens collapsed lung tissue can be gained by analyzing the method the newborn infant uses to open their collapsed lung at birth (Fig. 8) [87]. Spontaneously breathing newborns recruit collapsed lung with a rapid inspiration (Fig. 8—Inflation and aeration) and prevent re-collapse of the newly opened tissue by partially closing the glottis to act as a ‘brake’ on expiratory flow (Fig. 8—Slowing of expiratory flow). Thus, the method by which nature opens the lung at birth is to inflate a small amount of tissue with each breath and then apply a ‘brake’ to prevent the newly opened tissue from re-collapsing. When a force causes a strain in one direction and some type of ‘brake’ prevents strain in the opposite direction it is termed a ratchet. Therefore, nature’s strategy is to ratchet open lung tissue with each breath until the lung is fully inflated.
Fig. 8.
Five phases of a newborn infant transitioning from a collapsed fluid-filled state to full aeration. A Lung volume/time curve representing a single ‘cry’ during inspiration (Insp) and expiration (Exp). B Diagram of airways and acini. C Electrical impedance tomography (EIT) images. (1) Fetal lung (fluid filled): The beginning of the newborn’s first inspiration (red line) with the lung still collapsed and fluid filled (blue in airways) and no gas in the lung measured with EIT (no blue). (2) Inflation and aeration: A rapid inspiration (red line) with the glottis fully open (horizontal arrows pointing out) and contraction of the diaphragm (downward arrows) as the newborn rapidly fills the lung with gas to begin a cry. This high velocity inflow of air moves liquid in the airways and alveoli (blue changing to white) into the interstitial space (small blue arrows). EIT shows gas entering the lung (light blue). (3) Start of expiration: Expiration is active with diaphragm contraction (arrows) forcing gas rapidly out of the lungs (red steep slope, volume/time curve). Intra-alveolar pressure falls, allowing fluid to refill into some alveoli (upper alveoli going from white to blue) and EIT shows a loss of lung volume. (4) Slowing of expiratory flow: To prevent further lung collapse and flooding, the glottis briefly ‘brakes’ expiratory gas flow (horizontal arrows pointing inward), re-pressurizing the lungs. Pendelluft (blue arrows) redistributes gas into partially flooded alveoli. (5) End of expiration: The remainder of expiration (red line on volume/time curve) occurs with a partially closed glottis to maintain a PEEP to preserve FRC. EIT demonstrates that FRC is preserved (blue areas). Thus, the newborn uses a rapid inspiration to open flooded tissue and partially closes the glottis as a brake to prevent re-collapse and flooding. This method of rapid inspiration to open lung and closing the glottis to ‘brake’ expiratory flow and maintain airway pressure to prevent re-collapse ratchets open small volumes of lung tissue with each breath until the lung is fully inflated. The TCAV method uses a similar ratchet approach to open collapsed and fluid-filled lung of the ARDS patient. When expiratory flow is terminated (Fig. 4B, Gas Flow/Time curve, red arrowhead), the lung is rapidly re-inflated until the set CPAP Phase pressure is reached. This is analogous to the rapid inspiration before a cry in the newborn (Inflation and aeration). The brief Release Phase (0.2–0.5 s) acts as an expiratory ‘brake’ (Fig. 4B, red arrowhead), which does not allow the lung to depressurize, maintaining a time-controlled PEEP (Fig. 4B, TC-PEEP). This is similar to the newborn using the glottis as a ‘brake’ to slow expiratory flow (Slowing of expiratory flow). To summarize, both the newborn and the TCAV method use a rapid inspiration to open a small volume of lung with each breath. A ‘brake’ in expiratory flow is used to prevent re-collapse. Combined this ratchet approach opens small volumes with each breath and over time fully recruits the collapsed lung
We postulate that the TCAV method uses the ratchet mechanism to open collapsed lung in the ARDS patient. There is a rapid lung inflation from the termination point of expiration (Fig. 4B, Gas Flow/Time curve) that recruits a small portion of collapsed lung, combined with a brief Release Phase to ‘brake’ expiratory flow preventing derecruitment during expiration. In addition, the prolonged CPAP Phase accelerates recruitment of alveoli with long opening time constants (Fig. 4B, CPAP Phase). Addition of CPAP has been shown to significantly increase FRC in premature infants [88].
If our hypothesis is correct, the ratchet approach is a novel and innovative method of lung recruitment. Unlike the OLA, which attempts to open the entire lung in a seconds or minutes [89], the TCAV method ratchets open small volumes of lung with each breath that will progressively open the entire lung over hours or days. As described above a ratchet is a device (ventilator) that causes an object to strain in one direction (alveolar recruitment during inspiration), while applying a ‘brake’ (very short expiratory duration) necessary to prevent strain in the opposite direction (prevent alveolar collapse during exhalation). An example of the ratcheting approach on progressive lung recruitment is shown in Fig. 5.
Conclusions
Low tidal volumes and airway pressures using the ARDSNet method can push the lung with mild ARDS into the VILI vortex. To prevent progressive lung collapse, the time-dependent nature of alveolar opening and collapse must be taken into account. The TCAV method to set APRV uses: (i) the ratchet approach combined with an extended inspiratory duration necessary to recruit alveoli and (ii) a brief expiratory duration to ‘brake’ the derecruitment of rapidly collapsing alveoli. The TCAV method is personalized and adaptable and has shown promising results in animal models of ARDS and has yielded positive clinical outcomes in the ICU. Whether TCAV, or a similar strategy, can significantly reduce ARDS-related mortality when implemented in a large-scale clinical trial remains to be seen, but it is clear that a new approach to MV in ARDS is needed. We propose that ventilating patients with ARDS in a manner that specifically addresses the time dependence of R/D is a logical strategy for interrupting and reversing the VILI vortex. Accordingly, we believe consideration should be given to the design of a clinical trial comparing the TCAV method to the current standard of care in ARDS patients.
Acknowledgements
Not applicable.
Author contributions
GN was involved in drafting of manuscript. MKS, HR, JS, SB, LAG, PA, AG, DWK, DG, JB, and NMH contributed to critical revisions of manuscript. All authors read and approved the final manuscript.
Funding
NIH R01HL142702—Support for Bates, Gaver, Nieman, Satalin, Blair. Office of the Assistant Secretary of Defense for Health Affairs, Peer Reviewed Medical Research Program, Award W81XWH-20-1-0696—Support for Nieman, Satalin, Blair, Kaczka, Habashi. Office of the Assistant Secretary of Defense for Health Affairs, Peer Reviewed Medical Research Program, Award W81XWH-20-1-0507—Support for Kaczka. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
MKS has received a Research Grant and GF an Unrestricted Educational Grant from Dräger Medical Systems, Inc. PLA, GFN, MKS, and NMH have presented and received honoraria and/or travel reimbursement at event(s) sponsored by Dräger Medical Systems, Inc., outside of the published work. PLA, GFN, MKS, and NMH have lectured for Intensive Care Online Network, Inc. (ICON). NMH is the founder of ICON, of which PLA is an employee. NMH holds patents on a method of initiating, managing and/or weaning airway pressure release ventilation, as well as controlling a ventilator in accordance with the same, but these patents are not commercialized, licensed, or royalty-producing. The authors maintain that industry had no role in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; nor the preparation, review, or approval of the manuscript. Dr. Kaczka is a co-founder and shareholder of OscillaVent, Inc., and is a co-inventor on a patent involving multi-frequency oscillatory ventilation. Dr. Kaczka also receives research support from ZOLL Medical Corporation and is a paid consultant for Lungpacer Medical, Inc. Dr. Bates is a consultant to and sharehold in OscillaVent, Inc., and has two patents pending in the field of MV.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Prim. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 3.Gattinoni L, Pesenti A. The concept of "baby lung". Intensive Care Med. 2005;31(6):776–784. doi: 10.1007/s00134-005-2627-z. [DOI] [PubMed] [Google Scholar]
- 4.Acute Respiratory Distress Syndrome N. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 5.Schiller HJ, McCann UG, 2nd, Carney DE, Gatto LA, Steinberg JM, Nieman GF. Altered alveolar mechanics in the acutely injured lung. Crit Care Med. 2001;29(5):1049–1055. doi: 10.1097/00003246-200105000-00036. [DOI] [PubMed] [Google Scholar]
- 6.Caser EB, Zandonade E, Pereira E, Gama AM, Barbas CS. Impact of distinct definitions of acute lung injury on its incidence and outcomes in Brazilian ICUs: prospective evaluation of 7133 patients*. Crit Care Med. 2014;42(3):574–582. doi: 10.1097/01.ccm.0000435676.68435.56. [DOI] [PubMed] [Google Scholar]
- 7.Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, Brochard L, Clarkson K, Esteban A, Gattinoni L, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42(12):1865–1876. doi: 10.1007/s00134-016-4571-5. [DOI] [PubMed] [Google Scholar]
- 8.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 9.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 10.Deans KJ, Minneci PC, Cui X, Banks SM, Natanson C, Eichacker PQ. Mechanical ventilation in ARDS: one size does not fit all. Crit Care Med. 2005;33(5):1141–1143. doi: 10.1097/01.ccm.0000162384.71993.a3. [DOI] [PubMed] [Google Scholar]
- 11.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 12.Yehya N, Hodgson CL, Amato MBP, Richard JC, Brochard LJ, Mercat A, Goligher EC. Response to ventilator adjustments for predicting acute respiratory distress syndrome mortality. Driving pressure versus oxygenation. Ann Am Thorac Soc. 2021;18(5):857–864. doi: 10.1513/AnnalsATS.202007-862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa ELV, Slutsky A, Brochard LJ, Brower R, Serpa-Neto A, Cavalcanti AB, Mercat A, Meade M, Morais CCA, Goligher E, et al. Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;204:303–311. doi: 10.1164/rccm.202009-3467OC. [DOI] [PubMed] [Google Scholar]
- 14.Aoyama H, Pettenuzzo T, Aoyama K, Pinto R, Englesakis M, Fan E. Association of driving pressure with mortality among ventilated patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2018;46(2):300–306. doi: 10.1097/CCM.0000000000002838. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Wei X, Liu G, Tai Q, Zheng D, Xie W, Chen L, Wang G, Sun JQ, Wang S, et al. Higher versus lower DP for ventilated patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Emerg Med Int. 2019;2019:4654705. doi: 10.1155/2019/4654705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goligher EC, Costa ELV, Yarnell CJ, Brochard LJ, Stewart TE, Tomlinson G, Brower RG, Slutsky AS, Amato MPB. Effect of lowering tidal volume on mortality in ARDS varies with respiratory system elastance. Am J Respir Crit Care Med. 2021;203:1378–1385. doi: 10.1164/rccm.202009-3536OC. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 18.Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, Rowan K, Cuthbertson BH, Group OS. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368(9):806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 19.Malhotra A, Drazen JM. High-frequency oscillatory ventilation on shaky ground. N Engl J Med. 2013;368(9):863–865. doi: 10.1056/NEJMe1300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrmann J, Lilitwat W, Tawhai MH, Kaczka DW. High-frequency oscillatory ventilation and ventilator-induced lung injury: size does matter. Crit Care Med. 2020;48(1):e66–e73. doi: 10.1097/CCM.0000000000004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaczka DW. Oscillatory ventilation redux: alternative perspectives on ventilator-induced lung injury in the acute respiratory distress syndrome. Curr Opin Physiol. 2021;21:36–43. doi: 10.1016/j.cophys.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Protti A, Andreis DT, Monti M, Santini A, Sparacino CC, Langer T, Votta E, Gatti S, Lombardi L, Leopardi O, et al. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med. 2013;41(4):1046–1055. doi: 10.1097/CCM.0b013e31827417a6. [DOI] [PubMed] [Google Scholar]
- 23.Protti A, Andreis DT, Iapichino GE, Monti M, Comini B, Milesi M, Zani L, Gatti S, Lombardi L, Gattinoni L. High positive end-expiratory pressure: only a dam against oedema formation? Crit Care. 2013;17(4):R131. doi: 10.1186/cc12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Protti A, Maraffi T, Milesi M, Votta E, Santini A, Pugni P, Andreis DT, Nicosia F, Zannin E, Gatti S, et al. Role of strain rate in the pathogenesis of ventilator-induced lung edema. Crit Care Med. 2016;44(9):e838–845. doi: 10.1097/CCM.0000000000001718. [DOI] [PubMed] [Google Scholar]
- 25.Jain SV, Kollisch-Singule M, Satalin J, Searles Q, Dombert L, Abdel-Razek O, Yepuri N, Leonard A, Gruessner A, Andrews P, et al. The role of high airway pressure and dynamic strain on ventilator-induced lung injury in a heterogeneous acute lung injury model. Intensive Care Med Exp. 2017;5(1):25. doi: 10.1186/s40635-017-0138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seah AS, Grant KA, Aliyeva M, Allen GB, Bates JHT. Quantifying the roles of tidal volume and PEEP in the pathogenesis of ventilator-induced lung injury. Ann Biomed Eng. 2011;39(5):1505–1516. doi: 10.1007/s10439-010-0237-6. [DOI] [PubMed] [Google Scholar]
- 27.Wilson MR, Patel BV, Takata M. Ventilation with "clinically relevant" high tidal volumes does not promote stretch-induced injury in the lungs of healthy mice. Crit Care Med. 2012;40(10):2850–2857. doi: 10.1097/CCM.0b013e31825b91ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Syring RS, Otto CM, Spivack RE, Markstaller K, Baumgardner JE. Maintenance of end-expiratory recruitment with increased respiratory rate after saline-lavage lung injury. J Appl Physiol. 2007;102(1):331–339. doi: 10.1152/japplphysiol.00002.2006. [DOI] [PubMed] [Google Scholar]
- 29.Hamlington KL, Smith BJ, Dunn CM, Charlebois CM, Roy GS, Bates JHT. Linking lung function to structural damage of alveolar epithelium in ventilator-induced lung injury. Respir Physiol Neurobiol. 2018;255:22–29. doi: 10.1016/j.resp.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otto CM, Markstaller K, Kajikawa O, Karmrodt J, Syring RS, Pfeiffer B, Good VP, Frevert CW, Baumgardner JE. Spatial and temporal heterogeneity of ventilator-associated lung injury after surfactant depletion. J Appl Physiol. 2008;104(5):1485–1494. doi: 10.1152/japplphysiol.01089.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cressoni M, Gotti M, Chiurazzi C, Massari D, Algieri I, Amini M, Cammaroto A, Brioni M, Montaruli C, Nikolla K, et al. Mechanical power and development of ventilator-induced lung injury. Anesthesiology. 2016;124(5):1100–1108. doi: 10.1097/ALN.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 32.Albert K, Krischer JM, Pfaffenroth A, Wilde S, Lopez-Rodriguez E, Braun A, Smith BJ, Knudsen L. Hidden microatelectases increase vulnerability to ventilation-induced lung injury. Front Physiol. 2020;11:530485. doi: 10.3389/fphys.2020.530485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cressoni M, Chiurazzi C, Gotti M, Amini M, Brioni M, Algieri I, Cammaroto A, Rovati C, Massari D, di Castiglione CB, et al. Lung inhomogeneities and time course of ventilator-induced mechanical injuries. Anesthesiology. 2015;123(3):618–627. doi: 10.1097/ALN.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 34.Cereda M, Xin Y, Meeder N, Zeng J, Jiang Y, Hamedani H, Profka H, Kadlecek S, Clapp J, Deshpande CG, et al. Visualizing the propagation of acute lung injury. Anesthesiology. 2016;124(1):121–131. doi: 10.1097/ALN.0000000000000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cereda M, Xin Y. Alveolar recruitment and lung injury: an issue of timing and location? Crit Care Med. 2013;41(12):2837–2838. doi: 10.1097/CCM.0b013e31829cb083. [DOI] [PubMed] [Google Scholar]
- 36.Cereda M, Emami K, Kadlecek S, Xin Y, Mongkolwisetwara P, Profka H, Barulic A, Pickup S, Mansson S, Wollmer P, et al. Quantitative imaging of alveolar recruitment with hyperpolarized gas MRI during mechanical ventilation. J Appl Physiol. 2011;110(2):499–511. doi: 10.1152/japplphysiol.00841.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaver DP, 3rd, Nieman GF, Gatto LA, Cereda M, Habashi NM, Bates JHT. The POOR Get POORer: a hypothesis for the pathogenesis of ventilator-induced lung injury. Am J Respir Crit Care Med. 2020;202(8):1081–1087. doi: 10.1164/rccm.202002-0453CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakahashi S, Imai H, Shimojo N, Magata Y, Einama T, Hayakawa M, Wada T, Morimoto Y, Gando S. Effects of the prone position on regional neutrophilic lung inflammation according to 18F-FDG pet in an experimental ventilator-induced lung injury model. Shock. 2022;57(2):298–308. doi: 10.1097/SHK.0000000000001818. [DOI] [PubMed] [Google Scholar]
- 39.Broche L, Pisa P, Porra L, Degrugilliers L, Bravin A, Pellegrini M, Borges JB, Perchiazzi G, Larsson A, Hedenstierna G, et al. Individual airway closure characterized in vivo by phase-contrast CT imaging in injured rabbit lung. Crit Care Med. 2019;47(9):e774–e781. doi: 10.1097/CCM.0000000000003838. [DOI] [PubMed] [Google Scholar]
- 40.Cereda M, Xin Y, Goffi A, Herrmann J, Kaczka DW, Kavanagh BP, Perchiazzi G, Yoshida T, Rizi RR. Imaging the injured lung: mechanisms of action and clinical use. Anesthesiology. 2019;131:716–749. doi: 10.1097/ALN.0000000000002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sang L, Zhao Z, Lin Z, Liu X, Zhong N, Li Y. A narrative review of electrical impedance tomography in lung diseases with flow limitation and hyperinflation: methodologies and applications. Ann Transl Med. 2020;8(24):1688. doi: 10.21037/atm-20-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaczka DW, Cao K, Christensen GE, Bates JH, Simon BA. Analysis of regional mechanics in canine lung injury using forced oscillations and 3D image registration. Ann Biomed Eng. 2011;39(3):1112–1124. doi: 10.1007/s10439-010-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrmann J, Gerard SE, Shao W, Hawley ML, Reinhardt JM, Christensen GE, Hoffman EA, Kaczka DW. Quantifying regional lung deformation using four-dimensional computed tomography: a comparison of conventional and oscillatory ventilation. Front Physiol. 2020;11:14. doi: 10.3389/fphys.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaczka DW, Hager DN, Hawley ML, Simon BA. Quantifying mechanical heterogeneity in canine acute lung injury: impact of mean airway pressure. Anesthesiology. 2005;103(2):306–317. doi: 10.1097/00000542-200508000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Marini JJ, Gattinoni L. Time course of evolving ventilator-induced lung injury: the "Shrinking Baby Lung". Crit Care Med. 2020;48(8):1203–1209. doi: 10.1097/CCM.0000000000004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossi S, Palumbo MM, Sverzellati N, Busana M, Malchiodi L, Bresciani P, Ceccarelli P, Sani E, Romitti F, Bonifazi M, et al. Mechanisms of oxygenation responses to proning and recruitment in COVID-19 pneumonia. Intensive Care Med. 2022;48(1):56–66. doi: 10.1007/s00134-021-06562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial I. Cavalcanti AB, Suzumura EA, Laranjeira LN, Paisani DM, Damiani LP, Guimaraes HP, Romano ER, Regenga MM, Taniguchi LNT, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) versus low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 49.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 50.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT, Natl Heart Lung Blood Inst AC Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 51.Hickling KG. The pressure–volume curve is greatly modified by recruitment. A mathematical model of ARDS lungs. Am J Respir Crit Care Med. 1998;158(1):194–202. doi: 10.1164/ajrccm.158.1.9708049. [DOI] [PubMed] [Google Scholar]
- 52.Hickling KG. Reinterpreting the pressure–volume curve in patients with acute respiratory distress syndrome. Curr Opin Crit Care. 2002;8:32–38. doi: 10.1097/00075198-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Gaver D, Samsel RW, Solway J. Effects of surface tension and viscosity on airway reopening. J Appl Physiol. 1990;69:74–85. doi: 10.1152/jappl.1990.69.1.74. [DOI] [PubMed] [Google Scholar]
- 54.Fraser RS, Paré PD. Fraser and Paré's diagnosis of diseases of the chest. 4. Philadelphia: W.B. Saunders; 1999. [Google Scholar]
- 55.Cassidy KJ, Halpern D, Ressler BG, Grotberg JB. Surfactant effects in model airway closure experiments. J Appl Physiol. 1999;87(1):415–427. doi: 10.1152/jappl.1999.87.1.415. [DOI] [PubMed] [Google Scholar]
- 56.Allen G, Lundblad LK, Parsons P, Bates JH. Transient mechanical benefits of a deep inflation in the injured mouse lung. J Appl Physiol. 2002;93(5):1709–1715. doi: 10.1152/japplphysiol.00473.2002. [DOI] [PubMed] [Google Scholar]
- 57.Allen G, Bates JH. Dynamic mechanical consequences of deep inflation in mice depend on type and degree of lung injury. J Appl Physiol. 2004;96(1):293–300. doi: 10.1152/japplphysiol.00270.2003. [DOI] [PubMed] [Google Scholar]
- 58.Massa CB, Allen GB, Bates JH. Modeling the dynamics of recruitment and derecruitment in mice with acute lung injury. J Appl Physiol. 2008;105(6):1813–1821. doi: 10.1152/japplphysiol.90806.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen GB, Leclair T, Cloutier M, Thompson-Figueroa J, Bates JH. The response to recruitment worsens with progression of lung injury and fibrin accumulation in a mouse model of acid aspiration. Am J Physiol. 2007;292(6):L1580–1589. doi: 10.1152/ajplung.00483.2006. [DOI] [PubMed] [Google Scholar]
- 60.Andrews PL, Shiber JR, Jaruga-Killeen E, Roy S, Sadowitz B, O'Toole RV, Gatto LA, Nieman GF, Scalea T, Habashi NM. Early application of airway pressure release ventilation may reduce mortality in high-risk trauma patients: a systematic review of observational trauma ARDS literature. J Trauma Acute Care Surg. 2013;75(4):635–641. doi: 10.1097/TA.0b013e31829d3504. [DOI] [PubMed] [Google Scholar]
- 61.Cereda M, Xin Y, Hamedani H, Bellani G, Kadlecek S, Clapp J, Guerra L, Meeder N, Rajaei J, Tustison NJ, et al. Tidal changes on CT and progression of ARDS. Thorax. 2017;72(11):981–989. doi: 10.1136/thoraxjnl-2016-209833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryans JM, Fujioka H, Gaver DP., 3rd Microscale to mesoscale analysis of parenchymal tethering: the effect of heterogeneous alveolar pressures on the pulmonary mechanics of compliant airways. J Appl Physiol. 2019;126(5):1204–1213. doi: 10.1152/japplphysiol.00178.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kollisch-Singule M, Emr B, Smith B, Roy S, Jain S, Satalin J, Snyder K, Andrews P, Habashi N, Bates J, et al. Mechanical breath profile of airway pressure release ventilation: the effect on alveolar recruitment and microstrain in acute lung injury. JAMA Surg. 2014;149(11):1138–1145. doi: 10.1001/jamasurg.2014.1829. [DOI] [PubMed] [Google Scholar]
- 64.Rylander C, Hogman M, Perchiazzi G, Magnusson A, Hedenstierna G. Functional residual capacity and respiratory mechanics as indicators of aeration and collapse in experimental lung injury. Anesth Analg. 2004;98(3):782–789, table of contents. doi: 10.1213/01.ane.0000096261.89531.90. [DOI] [PubMed] [Google Scholar]
- 65.Habashi NM. Other approaches to open-lung ventilation: airway pressure release ventilation. Crit Care Med. 2005;33(3 Suppl):S228–240. doi: 10.1097/01.ccm.0000155920.11893.37. [DOI] [PubMed] [Google Scholar]
- 66.Nieman GF, Al-Khalisy H, Kollisch-Singule M, Satalin J, Blair S, Trikha G, Andrews P, Madden M, Gatto LA, Habashi NM. A physiologically informed strategy to effectively open, stabilize, and protect the acutely injured lung. Front Physiol. 2020;11:227. doi: 10.3389/fphys.2020.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herrmann J, Gerard SE, Shao W, Xin Y, Cereda M, Reinhardt JM, Christensen GE, Hoffman EA, Kaczka DW. Effects of lung injury on regional aeration and expiratory time constants: insights from four-dimensional computed tomography image registration. Front Physiol. 2021;12:707119. doi: 10.3389/fphys.2021.707119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Albert SP, DiRocco J, Allen GB, Bates JH, Lafollette R, Kubiak BD, Fischer J, Maroney S, Nieman GF. The role of time and pressure on alveolar recruitment. J Appl Physiol. 2009;106(3):757–765. doi: 10.1152/japplphysiol.90735.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emr B, Gatto L, Roy S, Satalin J, Ghosh A, Snyder K, Andrews P, Habashi N, Marx W, Ge L, et al. Airway pressure release ventilation prevents ventilator-induced lung injury in normal lungs. JAMA Surg. 2013;148:1005–1012. doi: 10.1001/jamasurg.2013.3746. [DOI] [PubMed] [Google Scholar]
- 70.Kollisch-Singule M, Emr B, Smith B, Ruiz C, Roy S, Meng Q, Jain S, Satalin J, Snyder K, Ghosh A, et al. Airway pressure release ventilation reduces conducting airway micro-strain in lung injury. J Am Coll Surg. 2014;219(5):9. doi: 10.1016/j.jamcollsurg.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kollisch-Singule M, Jain S, Andrews P, Smith BJ, Hamlington-Smith KL, Roy S, DiStefano D, Nuss E, Satalin J, Meng Q, et al. Effect of airway pressure release ventilation on dynamic alveolar heterogeneity. JAMA Surg. 2015;151:1–9. doi: 10.1001/jamasurg.2015.2683. [DOI] [PubMed] [Google Scholar]
- 72.Kollisch-Singule M, Emr B, Jain SV, Andrews P, Satalin J, Liu J, Porcellio E, Kenyon V, Wang G, Marx W, et al. The effects of airway pressure release ventilation on respiratory mechanics in extrapulmonary lung injury. Intensive Care Med Exp. 2015;3(1):35. doi: 10.1186/s40635-015-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith BJ, Lundblad LK, Kollisch-Singule M, Satalin J, Nieman G, Habashi N, Bates JH. Predicting the response of the injured lung to the mechanical breath profile. J Appl Physiol. 2015;118(7):932–940. doi: 10.1152/japplphysiol.00902.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kollisch-Singule M, Jain SV, Satalin J, Andrews P, Searles Q, Liu Z, Zhou Y, Wang G, Meier AH, Gatto LA, et al. Limiting ventilator-associated lung injury in a preterm porcine neonatal model. J Pediatr Surg. 2017;52(1):50–55. doi: 10.1016/j.jpedsurg.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 75.Nieman GF, Satalin J, Kollisch-Singule M, Andrews P, Aiash H, Habashi NM, Gatto LA. Physiology in medicine: understanding dynamic alveolar physiology to minimize ventilator-induced lung injury. J Appl Physiol. 2017;122(6):1516–1522. doi: 10.1152/japplphysiol.00123.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nieman GF, Satalin J, Andrews P, Aiash H, Habashi NM, Gatto LA. Personalizing mechanical ventilation according to physiologic parameters to stabilize alveoli and minimize ventilator induced lung injury (VILI) Intensive Care Med Exp. 2017;5(1):8. doi: 10.1186/s40635-017-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nieman GF, Gatto LA, Habashi NM. Impact of mechanical ventilation on the pathophysiology of progressive acute lung injury. J Appl Physiol. 2015;119(11):1245–1261. doi: 10.1152/japplphysiol.00659.2015. [DOI] [PubMed] [Google Scholar]
- 78.Silva PL, Cruz FF, Samary CDS, Moraes L, de Magalhaes RF, Fernandes MVS, Bose R, Pelegati VB, Carvalho HF, Capelozzi VL, et al. Biological response to time-controlled adaptive ventilation depends on acute respiratory distress syndrome etiology. Crit Care Med. 2018;46(6):e609–e617. doi: 10.1097/CCM.0000000000003078. [DOI] [PubMed] [Google Scholar]
- 79.Roy S, Habashi N, Sadowitz B, Andrews P, Ge L, Wang G, Roy P, Ghosh A, Kuhn M, Satalin J, et al. Early airway pressure release ventilation prevents ARDS—a novel preventive approach to lung injury. Shock. 2013;39(1):28–38. doi: 10.1097/SHK.0b013e31827b47bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roy SK, Emr B, Sadowitz B, Gatto LA, Ghosh A, Satalin JM, Snyder KP, Ge L, Wang G, Marx W, et al. Preemptive application of airway pressure release ventilation prevents development of acute respiratory distress syndrome in a rat traumatic hemorrhagic shock model. Shock. 2013;40(3):210–216. doi: 10.1097/SHK.0b013e31829efb06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jain SV, Kollisch-Singule M, Sadowitz B, Dombert L, Satalin J, Andrews P, Gatto LA, Nieman GF, Habashi NM. The 30-year evolution of airway pressure release ventilation (APRV) Intensive Care Med Exp. 2016;4(1):11. doi: 10.1186/s40635-016-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nieman GF, Andrews P, Satalin J, Wilcox K, Kollisch-Singule M, Madden M, Aiash H, Blair SJ, Gatto LA, Habashi NM. Acute lung injury: how to stabilize a broken lung. Crit Care. 2018;22(1):136. doi: 10.1186/s13054-018-2051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kollisch-Singule M, Satalin J, Blair SJ, Andrews PL, Gatto LA, Nieman GF, Habashi NM. Mechanical ventilation lessons learned from alveolar micromechanics. Front Physiol. 2020;11:233. doi: 10.3389/fphys.2020.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kollisch-Singule M, Jain S, Andrews P, Smith BJ, Hamlington-Smith KL, Roy S, DiStefano D, Nuss E, Satalin J, Meng Q, et al. Effect of airway pressure release ventilation on dynamic alveolar heterogeneity. JAMA Surg. 2016;151(1):64–72. doi: 10.1001/jamasurg.2015.2683. [DOI] [PubMed] [Google Scholar]
- 85.Emr B, Gatto LA, Roy S, Satalin J, Ghosh A, Snyder K, Andrews P, Habashi N, Marx W, Ge L, et al. Airway pressure release ventilation prevents ventilator-induced lung injury in normal lungs. JAMA Surg. 2013;148(11):1005–1012. doi: 10.1001/jamasurg.2013.3746. [DOI] [PubMed] [Google Scholar]
- 86.Kollisch-Singule M, Emr B, Smith B, Ruiz C, Roy S, Meng Q, Jain S, Satalin J, Snyder K, Ghosh A, et al. Airway pressure release ventilation reduces conducting airway micro-strain in lung injury. J Am Coll Surg. 2014;219(5):968–976. doi: 10.1016/j.jamcollsurg.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tingay DG, Farrell O, Thomson J, Perkins EJ, Pereira-Fantini PM, Waldmann AD, Ruegger C, Adler A, Davis PG, Frerichs I. Imaging the respiratory transition at birth: unraveling the complexities of the first breaths of life. Am J Respir Crit Care Med. 2021;204(1):82–91. doi: 10.1164/rccm.202007-2997OC. [DOI] [PubMed] [Google Scholar]
- 88.Lam R, Schilling D, Scottoline B, Platteau A, Niederhausen M, Lund KC, Schelonka RL, MacDonald KD, McEvoy CT. The effect of extended continuous positive airway pressure on changes in lung volumes in stable premature infants: a randomized controlled trial. J Pediatr. 2020;217:66–72.e61. doi: 10.1016/j.jpeds.2019.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cui Y, Cao R, Wang Y, Li G. Lung recruitment maneuvers for ARDS patients: a systematic review and meta-analysis. Respiration. 2020;99(3):264–276. doi: 10.1159/000501045. [DOI] [PubMed] [Google Scholar]
- 90.Roy S, Sadowitz B, Andrews P, Gatto LA, Marx W, Ge L, Wang G, Lin X, Dean DA, Kuhn M, et al. Early stabilizing alveolar ventilation prevents acute respiratory distress syndrome: a novel timing-based ventilatory intervention to avert lung injury. J Trauma Acute Care Surg. 2012;73(2):391–400. doi: 10.1097/TA.0b013e31825c7a82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nieman GF, Gatto LA, Andrews P, Satalin J, Camporota L, Daxon B, Blair SJ, Al-Khalisy H, Madden M, Kollisch-Singule M, et al. Prevention and treatment of acute lung injury with time-controlled adaptive ventilation: physiologically informed modification of airway pressure release ventilation. Ann Intensive Care. 2020;10(1):3. doi: 10.1186/s13613-019-0619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.