Abstract
Fungal contamination of animal feed can severely affect the health of farm animals, and result in considerable economic losses. Certain filamentous fungi or molds produce toxic secondary metabolites known as mycotoxins, of which aflatoxins (AFTs) are considered the most critical dietary risk factor for both humans and animals. AFTs are ubiquitous in the environment, soil, and food crops, and aflatoxin B1(AFB1) has been identified by the World Health Organization (WHO) as one of the most potent natural group 1A carcinogen. We reviewed the literature on the toxic effects of AFB1 in humans and animals along with its toxicokinetic properties. The damage induced by AFB1 in cells and tissues is mainly achieved through cell cycle arrest and inhibition of cell proliferation, and the induction of apoptosis, oxidative stress, endoplasmic reticulum (ER) stress and autophagy. In addition, numerous coding genes and non-coding RNAs have been identified that regulate AFB1 toxicity. This review is a summary of the current research on the complexity of AFB1 toxicity, and provides insights into the molecular mechanisms as well as the phenotypic characteristics.
Keywords: Mycotoxins, Aflatoxin B1, Toxicities, Mechanisms, Regulation
Introduction
Due to global climate change, mycotoxin-producing fungal strains that were endemic in the tropical-subtropical climate zones have also appeared in the temperate zones. Mycotoxins are secondary metabolites produced by filamentous fungi or mold present in the soil, grain, forage, and silage. They are non-essential for fungal growth and reproduction but are capable of inducing biochemical, physiological and pathological changes in many species (Wang et al., 2018a). The Food and Agricultural Organization (FAO) estimates that around 25% of the global agricultural productions and derived food products are contaminated with mycotoxins, which render an estimated 50 million tons of food inedible, resulting in severe economic losses each year (Iheshiulor et al., 2011). However, the current detectable rate of mycotoxins is as high as 60–80%, which is considerably higher than the FAO estimate of 25% (Eskola et al., 2020). Aflatoxin (AFT) is mainly produced by Aspergillus flavus and A. parasiticus, and is a derivative of difuranoxano-naphtho-ketone consisting of a difuran ring and a coumarin ring. AFTs are produced at temperatures between 10–40 °C, pH 3.0–8.5, moisture content 18–20%, and water activity > 0.82 (Iqbal, Asi & Arino, 2013). Due to their stable structure, AFTs are heat-resistant and can only be degraded above 280 °C (Sun, 2017). In addition, AFTs are insoluble in water but soluble in methanol and other organic solvents. AFTs can contaminate agricultural produce during growth, harvest, transportation, and storage. Current food processing practices and conventional storage conditions cannot completely eliminate AFT contamination from the food supply chain. The products with longer storage, such as corn, soybean, wheat, peanuts, nuts, and dried fruits, are particularly susceptible to AFT contamination (Battilani et al., 2016). In addition, poor post-harvest management can lead to rapid deterioration of the nutritional quality or digestibility of the agricultural produce due to mold contamination, which causes discoloration and degradation of lipids and proteins. Molds also produce volatile metabolites that result in unpleasant odors, thereby affecting grain intake by livestock and poultry, eventually leading to feed refusal and emesis (Magan & Aldred, 2007).
According to their fluorescence, the AFTs are broadly classified into AFB1 and AFB2 (blue), and AFG1 and AFG2 (green) types (Dalvi, 1986). AFM1, AFM2, AFB2a, AFG2a, aflatoxicol (AFL), AFP1, AFQ1 and AFH1 are derivatives of AFTs. AFB1 is considered to be the most toxic AFT (Wong & Hsieh, 1976; Bondy & Pestka, 2000; Hernandez-Mendoza, Garcia & Steele, 2009), with 10-fold and 68-fold higher toxicity compared to that of potassium cyanide and arsenic respectively (Li, 2002), and is primarily responsible for food and feed contamination (Hernandez-Mendoza, Garcia & Steele, 2009). AFB1 has also been identified by the WHO and the International Agency for Research on Cancer, IARC (1993) as one of the most potent natural group 1A carcinogens (Heinrich, 2003). It is ubiquitous in the environment, soil, animal feed and food crops, and is highly toxic to human beings, livestock and poultry. Following ingestion of contaminated feed, AFB1 promotes inflammation and necrosis of liver cells and intestinal cells, impairs liver function (Monson et al., 2014; Zhang et al., 2016), decreases lymphocyte activity and increases thymocyte apoptosis. These changes eventually reduce livestock productivity (Theumer et al., 2003; Peng et al., 2016), and result in huge economic losses. Furthermore, AFB1 can easily enter the human food chain through contaminated meat, eggs, and milk (Kumar et al., 2017). Long-term exposure to sublethal doses of AFT can weaken immunity and lead to nutritional disorders, whereas the mortality rate due to acute exposure at high doses is close to 25% (Liu & Wu, 2010; Verheecke, Liboz & Mathieu, 2016). Death due to acute or chronic AFB1 poisoning has been reported in India, Kenya, and other developing countries (Krishnamachari et al., 1975; Probst, Njapau & Cotty, 2007). Studies also show that AFB1 can induce carcinogenesis in the liver, stomach, lung, kidney, rectal colon, breast, and gallbladder (Harrison, Carvajal & Garner, 1993; Wang & Groopman, 1999; Eom et al., 2013; Cui et al., 2015; Yip et al., 2017; Costa, Lima & Santos, 2021), and 4.6–28.2% of hepatocellular carcinoma (HCC) cases are the result of AFT exposure (Kucukcakan & Hayrulai-Musliu, 2015).
Despite reports of liver toxicity and immunotoxicity in humans and animals due to AFB1 poisoning, there is no systematic review on the hepatotoxicity, enterotoxicity, nephrotoxicity, immunotoxicity, neurotoxicity and reproductive toxicity of AFB1. In this review, we have discussed the mechanisms under the toxic effects of AFB1 in order to provide a reliable reference for further research in animal husbandry, mycotoxins, and the treatment of toxin-related diseases in humans, livestock and poultry.
Survey Methodology
PubMed and Web of Science databases were searched for relevant articles. A total of 7,715 articles appeared Pubmed database using “aflatoxin B1” as the search term and the date of publication from 1975/1/1 to 2022/3/1 . After narrowing the search with keywords including “toxicokinetics of aflatoxin B1”, “hepatotoxicity of aflatoxin B1”, “enterotoxicity of aflatoxin B1”, nephrotoxicity of aflatoxin B1, “neurotoxicity of aflatoxin B1”, “immunotoxicity of aflatoxin B1”, “reproductive toxicity of aflatoxin B1”, “aflatoxin B1 and COX2”, “aflatoxin B1 and Nrf2”, “aflatoxin B1 and p53”, “aflatoxin B1 and microRNA”, “aflatoxin B1 and lncRNA”, and “aflatoxin B1 and livestock”, 1,733 studies were obtained. With “aflatoxin B1” as the search term, 11,741 articles published between 1975–2022 appeared in the Web of Science database, of which 1,514 articles were selected as above. After removing duplicate articles and the articles with little relevance, 137 articles were selected for the review.
Toxicokinetics of AFB1
AFB1 exposure occurs through dietary intake, skin contact and inhalation of contaminated dust. More than 80% of the ingested AFB1 is absorbed in the duodenum and the jejunum through passive transport (Grenier & Applegate, 2013), and accumulates thereafter in the liver, kidney and spleen, although the main target organ of AFB1 is undoubtedly the liver. Toxic effects of AFB1 have been observed in the liver, gastrointestinal tract, nervous system, immune cells, and reproductive organs.
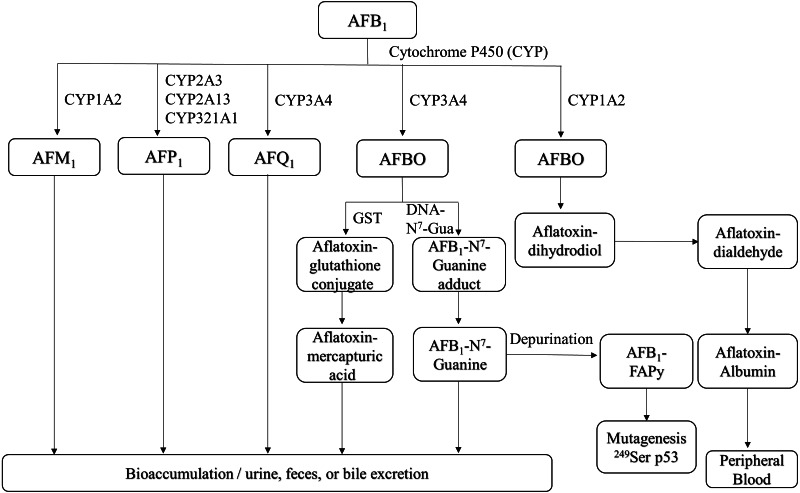
AFB1 is metabolized in the liver by P450 enzymes into the carcinogen AFB1-8,9-epoxide (AFBO), which includes the endo-8,9-epoxide (endo-AFBO) and exo-8,9-epoxide (exo-AFBO) isomers (Fig. 1). CYP1A2 and CYP3A4 enzymes play critical roles in the activation of AFB1 in human liver (Gallagher et al., 1994; Gallagher et al., 1996), and bovine hepatocytes (Kuilman, Maas & Fink-Gremmels, 2000). CYP1A2 oxidizes AFB1 into endo- and exo- AFBO and AFM1, whereas CYP3A4 catalyzes AFB1 oxidation into exo- AFBO and AFQ1. CYP2A13, CYP2A3 and CYP321A1 decompose AFB1 into AFP1 (Rushing & Selim, 2019). Metabolism of AFB1 by the supernatant fraction of liver homogenates from different species has shown that AFQ1 is the major metabolite produced in human, monkey and rat liver, and AFP1 is major metabolite produced in the human, monkey and mouse liver. On the other hand, duck liver homogenate metabolized AFB1 to AFL (Roebuck & Wogan, 1977). AFM1 and AFB1-dihydrodiol are the major metabolites in bovine hepatocytes (Kuilman, Maas & Fink-Gremmels, 2000). AFM1 was originally isolated and identified as an AFB1 metabolite in milk (Goto et al., 1986). AFP1 produces an oxidative metabolite of dihydroxyaflatoxin, which is excreted directly or as a glucuronic acid conjugate in bile (Eaton et al., 1988; Holeski et al., 1987). Furthermore, AFP1, AFM1, AFQ1 and AFL form glucuronide and sulfate conjugates (Coppock, Christian & Jacobsen, 2018), and AFP1, AFM1, AFQ1 and AFB1-N7-guanine have been detected in humans following AFB1 exposure (Groopman et al., 1985; Ross et al., 1992; Mykkanen et al., 2005). The different AFB1 metabolites are mainly expelled via feces and urine (Dohnal, Wu & Kuca, 2014). AFBO binds covalently to N7 on guanine to form AFB1-N7-guanine adducts in the DNA double helix (Iyer et al., 1994), resulting in point mutations that may drive carcinogenesis (Lin et al., 2014). The common point mutation caused by the AFB1-N7-guanine adduct is a G →T transversion (Schermerhorn & Delaney, 2014; Lin et al., 2016). Since exo-AFBO has a significantly higher affinity for guanine residues compared to endo-AFBO, it is considered to be the major carcinogenic metabolite. The AFB1-N7-guanine adduct forms an open ring structure under mild alkaline conditions, resulting in a stable AFB1-formamidopyridine adduct (AFB1-FAPy) (Smela et al., 2001) that is excreted with urine. Both isomers of AFBO are detoxified through glutathione (GSH) conjugation by glutathione-S-transferase. In addition, AFBO can be converted to AFT-mercapturic acid by GST, or to AFT-glucosiduronic acid by AFT-dihydropyridine, followed by the formation of GSH conjugates (Bryden, 2012). However, AFBO can also form adducts with serum albumin by covalently binding to the ɛ-amino group of lysine, which remains in circulation. Given its highly unstable nature, AFBO can spontaneously hydrolyze into AFB1 dihydrodiol, which can cause tissue damage, inflammation, and excessive cell proliferation by conjugating with different proteins, eventually promoting carcinogenesis. One of the most common AFB1-induced mutations in human hepatocytes is a G → T transversion in codon 249 of the p53 gene, which causes a 249Arg → 249Ser mutation in the encoded protein (Foster, Eisenstadt & Miller, 1983; Hollstein et al., 1991; Soini et al., 1996). In addition, AFB1 and AFBO can epigenetically increase the mutation rate of the p53 gene by methylating the CpG site in codon 248 (Narkwa, Blackbourn & Mutocheluh, 2017). The p53 gene is a tumor suppressor that is frequently mutated in human cancers, and the mutations promote tumor development by inhibiting apoptosis and increasing proliferation rates. AFBO can also induce mutations indirectly by binding to and damaging DNA repair enzymes (Weng et al., 2017). Furthermore, AFB1 metabolism by the P450 enzyme also generates reactive oxygen species (ROS), such as hydroxyl free radicals, hydrogen peroxide and other free radicals that damage cell membrane and macromolecules (Kucukcakan & Hayrulai-Musliu, 2015). The complex metabolic process of AFB1 is a major determinant of its potent toxicity. Since the formation of DNA adducts by AFB1, and its metabolites can activate proto-oncogenes, quantitative analysis of AFB1-DNA adducts is an important indicator of AFB1 toxicity.
Figure 1. Overview of AFB1 toxicokinetics.
AFB1 is metabolized in humans and animals by cytochrome P450 enzymes, and its metabolites include AFM1, AFP1, AFQ1 and AFBO. AFM1 accumulates in milk, whereas other metabolites are excreted through urine, feces, bile and can also enter the bloodstream. AFB1, aflatoxin B1; AFM1, aflatoxin M1; AFP1, aflatoxin P1; AFQ1, aflatoxin Q1; AFBO, aflatoxin B1-8,9-epoxide; GST, glutathione-S-transferase; AFB1-FAPy, AFB1-formamidopyridine adduct.
Effect of AFB1 on Livestock and Poultry
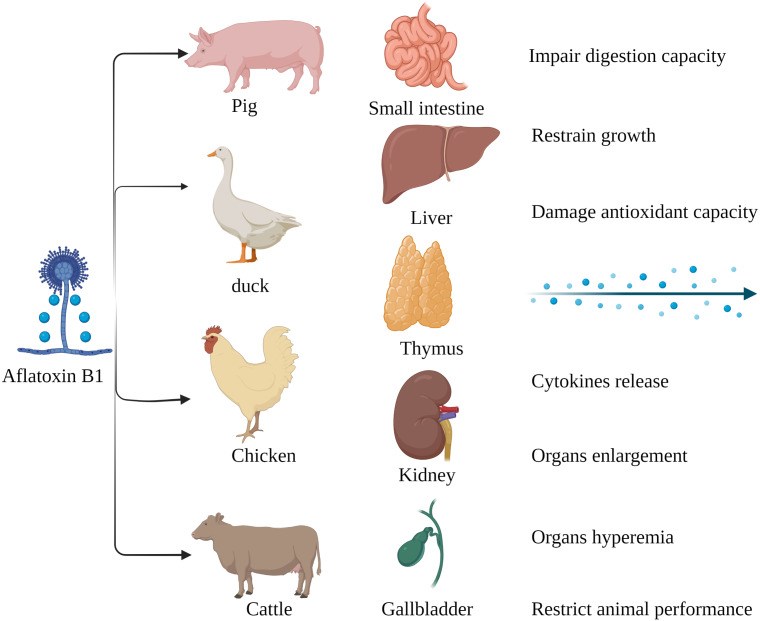
The susceptibility of livestock to AFB1 differs across species. Monogastric animals are more susceptible to AFB1 compared to the ruminants since the gut microbes in the latter can metabolize mycotoxins. Long-term exposure to low levels of AFB1 is more common in livestock as opposed to acute poisoning (Fig. 2). Pigs are especially susceptible AFB1 poisoning, and long-term exposure to low levels of dietary AFB1 can inhibit their growth, impair digestive function, and disrupt the intestinal barrier by decreasing SOD activity, and increasing production of pro-inflammatory cytokines such as TNFα, IL1 and TGFβ (Pu et al., 2021). Nevertheless, there are reports of AFB1 poisoning in ruminants as well. Cattle fed with AFB1-spiked fodder showed behavioral changes such as depression and anorexia. AFB1 significantly increased the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), serum creatinine (SCR), catalase (CAT) and malondialdehyde (MDA) in the affected cattle, and decreased that of total protein (TP), magnesium (Mg) and glutathione (GSH). Furthermore, autopsy of the poisoned cattle showed hepatomegaly, gallbladder enlargement, and intestinal and renal hyperemia (Elgioushy et al., 2020). AFB1 contamination of poultry feed can reduce reproductive capacity, hatching rate, chick weight, growth rate, production rate and quality of meat and eggs, and increase susceptibility to diseases and mortality rate (Pandey & Chauhan, 2007; Fouad et al., 2019). In addition, the younger broilers were more susceptible to AFB1 compared to older animals, which could be due to deficient detoxification mechanisms in the former (Wang et al., 2018b). In conclusion, exposure to AFB1can significantly affect the quality and productivity of livestock and poultry by altering their physiological and biochemical indices. Chronic exposure in particular leads to the accumulation of AFB1, and consumption of these contaminated products can adversely affect human health.
Figure 2. Harmful effects of AFB1 on livestock and poultry.
Pigs, ducks, chickens and cattle are particularly susceptible to AFB1, which mainly affects the small intestine, liver, thymus, kidney, gallbladder, etc. Made in ©BioRender (https://biorender.com/).
AFB1-Induced Toxicity
AFB1 toxicity depends the dose, exposure duration, administration mode, solvent, species, gender and target organs, and is summarized in Tables 1 and 2.
Table 1. The toxicities of AFB1 have been reported in vivo.
The hepatotoxicity, enterotoxicity, nephrotoxicity, immunotoxicity, neurotoxicity and reproductive toxicity of AFB1 have been reported in vivo.
| Type of toxicity | Doses | Time | Species | Gender | Route | Solvent | Signaling pathway/Cytokines/ potential target molecule | References |
|---|---|---|---|---|---|---|---|---|
| Hepatoxicity | 1 mg/kg (b.w.) | Every other day for 4 weeks | mice | – | Gavage (i.g.) | corn oil | COX2, p10 and IL1β↑ (protein) | Zhang et al. (2019a) |
| 1 mg/kg (b.w.) | Daily for 4, 6, 10 weeks | rats | male | Intraperitoneal (i.p.) | DMSO | TNFα, IL1α and PCNA ↑ (protein) | Singh, Maurya & Trigun (2015) | |
| 0.375, 0.75 and 1.5 mg/kg (b.w.) | Daily for 30 days | mice | male | Gavage (i.g.) | olive oil | Cyt-c, Bax, p53 and caspase-3/9 ↑ (protein & mRNA) | Xu et al. (2021) | |
| 250 µg/kg (b.w.) | 5 days per week over 4 weeks or 8 weeks | rats | female | Gavage (i.g.) | olive oil | – | Ali et al. (2021) | |
| 0.25, 0.75, and 1.5 mg/kg (b.w.) | Daily for 7 days | rats | male | Gavage (i.g.) | corn oil | p53 signaling pathway | Lu et al. (2013) | |
| 150 µg/kg feed | Daily for 14 days | rats | male | Feed | methanol | – | Qian et al. (2016) | |
| 0.32 mg/kg (b.w.) | Daily for 12 days | rats | male | Feed | – | – | Zhang et al. (2011) | |
| 0.25, 0.5 or 1.0 mg/kg (b.w.) | Daily for 7 days | rats | male | Gavage (i.g.) | olive oil | Ahr, Lipc and Lcat ↓ (protein & mRNA) , Scarb1 ↑ (protein & mRNA) | Rotimi et al. (2017) | |
| 1 mg/kg (b.w.) | Daily for 1, 4 or 7 days | rats | male | Gavage (i.g.) | olive oil | Nrf2, Hmgcoa, and Acc ↓ (mRNA) (day 1) | Rotimi et al. (2019) | |
| Enterotoxicity | 0.6 mg/kg feed | Daily for 21 days | broilers | – | Feed | methanol | FAS, FASL, TNFα, TNF-R1, GRP78/94, and caspase-3/8/10 ↑ (mRNA) | Zheng et al. (2017) |
| 0.3 mg/kg (b.w.) | Daily for 28 days | mice | male | Gavage (i.g.) | methanol | tight junction proteins (claudin-1, zonula occludens-1) ↓ (immunohistochemical staining) | Gao et al. (2021) | |
| 5, 25, 75 µg /kg (b.w.) | 5 days per week for 4 weeks | rats | male | Gavage (i.g.) | DMSO | – | Wang et al. (2016a) | |
| 5, 25, 75 µg /kg (b.w.) | 5 days per week for 5 weeks | rats | male | Gavage (i.g.) | DMSO | – | Zhou, Tang & Wang (2021) | |
| 5 mg/kg feed | Daily for 42 days | broilers | male | Feed | methanol | CYP1A1, CYP1A2, CYP2A6 and CYP3A4 ↑, GSTA3, GSTA4 and EPHX1 ↓ (mRNA) | Wang et al. (2018b) | |
| 22.02 ppb | – | broilers | male | Feed | – | – | Kana, Teguia & Tchoumboue (2010) | |
| 2.91 to 120.02 ug/kg feed | Daily for 0-14 days | ducks | male & female | Feed | Contaminated maize | – | Feng et al. (2017) | |
| 2.03 to 153.12 ug/kg feed | Daily for 15–35 days | ducks | male & female | Feed | Contaminated maize | – | Feng et al. (2017) | |
| 0.07, 0.75 mg/kg feed | Twice a day for 4 weeks | broilers | male | Feed | basal diet | – | Yunus et al. (2011) | |
| 1 ppm | Daily for 28 days | broilers | – | Feed | diet | – | Kumar & Balachandran (2009) | |
| 0.6 mg/kg feed | Daily for 21 days | broilers | – | Feed | methanol | TLR2-2, TLR-4, and TLR7 ↓ (mRNA) | Wang et al. (2018c) | |
| 0.6 mg/kg feed | Daily for 21 days | broilers | – | Feed | methanol | ATM, p53, Chk2 and p21 ↑, Mdm2, cdc25C, cdc2, cyclin B, MDM2 and PCNA ↓ (protein & mRNA) | Yin et al. (2016) | |
| Nephrotoxicity | 300 µg/kg (b.w.) | Daily for 30 days | mice | male | Gavage (i.g.) | DMSO | – | Huang et al. (2019) |
| 5, 7.5, 10 µg /kg (b.w.) | 8/16/24 h | carp | – | Gavage (i.g.) | feed | Keap1-Nrf2 pathway ↑ (mRNA) | Kövesi et al. (2020) | |
| 0.5 g/kg (b.w.) | Daily for 28 days | mice | male | Gavage (i.g.) | 1%DMSO | L-proline ↓ proline dehydrogenase (PRODH) and Bax, Caspase-3 ↑, Bcl-2 ↓ (protein & mRNA) | Li et al. (2018) | |
| 40 ppm | Daily for 8 weeks | rats | male | Feed | DMSO | GPx and GSH ↓, PER ↑ (activity) | Rotimi et al. (2018) | |
| Neurotoxicity | 300 µg/kg (b.w.) | Daily for 30 days | mice | male | Gavage (i.g.) | DMSO | – | Huang et al. (2020) |
| 0.025 mg/kg (b.w.) | Daily for 30 days, 60 days and 90 days | rats | male | Gavage (i.g.) | olive oil | CAT and SOD ↓, ACP, ALP, AST and LDH ↑ (activity) | Alsayyah et al. (2019) | |
| 0, 0.1, 0.5, 1, 2, 5, and 10 µM | 24 /48 h | 24/12 hpf zebrafish embryos | – | – | DMSO | gfap, mbp, and olig2 ↓ (protein), caspase 3a/8/9 and p53 ↑ (mRNA) | Park et al. (2020) | |
| 0, 0.25, 0.5, 1.0, and 2.0 µM | 24 h | 24, 28, 72, and 96 hpf zebrafish embryos | – | – | DMSO | Gly , Glu, and GABA ↑ (Metabolic Profiles) | Zuberi et al. (2019) | |
| 15.75 µg/kg (b.w.) | once weekly for 8 weeks | rats | female | Gavage (i.g.) | olive oil | – | Bahey, Abd Elaziz & Gadalla (2015) | |
| Immunotoxicity | 0.25 mg/kg (b.w.) | Daily for 15 days | mice | female | Gavage (i.g.) | ethanol | IFN-γ and TNFα↓, IL4 ↑ (mRNA) | Jebali et al. (2015) |
| 1 mg/kg (b.w.) | Daily for 4/6/10 weeks | rats | male | Intraperitoneal (i.p.) | DMSO | TNF-α, IL-1α and PCNA ↑ (mRNA) | Qin et al. (2016) | |
| 0.15, 0.3, and 0.6 mg /kg diet | Daily for 21 days | broilers | – | Feed | methanol | GSH-Px, GR, and GSH ↓, MDA ↑ (activity) | Chen et al. (2013) | |
| 385, 867 or 1807 µg/kg feed | Daily for 28 days | pigs | male | Feed | – | TNF-α, IL-1β, IL-6, IFN-γ and IL10 ↑ (mRNA) | Meissonnier et al. (2008) | |
| 0.6 mg/kg feed | Daily for 21 days | broilers | – | Feed | methanol | Bax, Bak, and cytC ↑, Bcl-2 and Bcl-xL ↓, FasL, Fas and FADD ↑ (mRNA) | Peng et al. (2016) | |
| 0.6 mg/kg feed | Daily for 21 days | broilers | – | Feed | methanol | Bax, Bak, cytC, caspase-9, Apaf-1, and caspase-3 ↑, Bcl-2 and Bcl-xL ↓, Grp78/Bip, Grp94 and CaM ↑ (mRNA) | Yuan et al. (2016) | |
| 0.6 mg/kg feed | Daily for 21 days | broilers | – | Feed | methanol | ATM-Chk2-cdc25-cyclin B/cdc2 pathway, ATM-Chk2-cdc25-cyclin D/CDK6 pathway and ATM-Chk2-p21-cyclin D/CDK6 pathway | Hu et al. (2018) | |
| 100 ppm | 120 min | rats | male | Intraperitoneal (i.p.) | – | TNF-α↑ (protein) | Mohammad et al. (2017) | |
| 5 mg/kg | 24 h | chicks | – | Gavage (i.g.) | – | MDA ↑, SOD and GSH-PX ↓ (activity), NLRP3, COX-2, iNOS, IL-6, IL-1β, TNF-α, caspase-1, caspase-3, and caspase-11 ↑ (mRNA) | Gao et al. (2022) | |
| Reproductive toxicity | 20 µg/kg (b.w.) | Daily for 7/14/21 days | mice | male | Intraperitoneal (i.p.) | corn oil and ethanol (95:5, v/v) | Bcl-2 ↓, Bax, p53, and caspase-3 ↑ (mRNA & protein) | Yasin, Mazdak & Mino (2018) |
| 0.375, 0.75, or 1.5 mg/kg (b.w.) | Daily for 30 days | mice | male | Gavage (i.g.) | corn oil | p38 MAPK signaling pathway, Occludin, N-cadherin, and Connexin 43 ↓, cyt-c and caspase-3 ↑ (protein) | Huang et al. (2021) | |
| 6 mg/kg | 6 h | mice | female | Intraperitoneal (i.p.) | DMSO | CYP1A2 and CYP3A4 ↑, GSTA1/2 ↓ (protein) | Sriwattanapong et al. (2017) | |
| 0.5 and 5 mg/kg feed | Daily for 8 weeks (only for mother) | rats | female | Feed | – | – | Rotimi et al. (2021) | |
| 1.0 mg/kg(b.w.) | 24 h | rams | male | Gavage (i.g.) | 4% ethanol | StAR, 3β-HSD, CYP11A1, and CYP17A1 ↑ (mRNA), Caspase3 ↓, Bax ↑ (mRNA) | Lin et al. (2022) | |
| 100 ppb, 200 ppb, and 400 ppb | Daily for 10 weeks | cockerels | male | Feed | – | IgM and IgG ↓ (antibody titers), LH, prolactin, and testosterone ↓ | Ashraf et al. (2022) |
Table 2. The toxicities of AFB1 have been reported in vitro.
The hepatotoxicity, enterotoxicity, nephrotoxicity, immunotoxicity, neurotoxicity and reproductive toxicity of AFB1 have been reported in vitro.
| Type of toxicity | Doses | Time | Cell type | Solvent | Signaling pathway/Cytokines/potential target molecule | References |
|---|---|---|---|---|---|---|
| Hepatoxicity | 0.05, 0.25 µM | 24 h | human liver HepaRG cells | corn oil | caspase 1/3, COX2, and IL-1β↑ (mRNA) | Zhang et al. (2019a) |
| 1 µM | 24 h | human liver HepaRG cells | corn oil | COX2 and IL1β↑ (protein) | Zhang et al. (2019a) | |
| 0.5, 1, 2.5 and 5 µmol/L | 6 /12 h | primary broiler hepatocytes | DMSO | Nrf2 ↑ (mRNA), caspase-3/9 ↑ (protein) | Liu & Wang (2016) | |
| Enterotoxicity | 0.5 and 4 µg/mL | 48 h | Caco-2 | DMSO | tight junction proteins (claudin-1/3/4, zonula occludens-1)↓ (mRNA & protein) | Gao et al. (2021) |
| Nephrotoxicity | 1, 2 and 6 µg/mL | 12 /24 /48 h | HEK-293T | DMSO | p21 ↑, PLK1, MYC, and PLD1 ↓ (mRNA & protein) | Huang et al. (2019) |
| 5, 10, 50, 100 mg/L | 48 h | HEK-293T | DMSO | PRODH and Bax, Caspase-3 ↑, Bcl-2 ↓ (protein & mRNA) | Li et al. (2018) | |
| 16.3, 32.60, 48.9 µM | 24 h | HEK-293T | complete culture medium | Nrf-2, SOD2, GPx, and Hsp70 ↑ (protein), OGG1 ↑ (mRNA), p53,NF-κb, c-IAP and Bax ↑ (protein), caspase 9/3/7↑ and caspase 8 ↓ (activity) | Dlamini et al. (2021) | |
| Neurotoxicity | 1, 5, 10, 20 µg/mL | 24 /48 h | IMR-32 | DMSO | – | Huang et al. (2020) |
| 2 µg/mL and 6 µg/mL | 24 h | IMR-32 | DMSO | NOX2 ↑, OXR1, SOD1 and SOD2 ↓, PARP1, BRCA2, and RAD51 ↓, CDKN1A, CDKN2C, and CDKN2D ↑, CASP3 and BAX ↑ (mRNA) | Huang et al. (2020) | |
| 1, 2, 5, 10, 20, or 50 µM; 0.3, 0.6, 1.5, 3.1, 6.2, or 15 µg/mL | 36 /48 h | NHA-SV40LT | DMSO | AKT and ERK1/2 signaling pathways, BAX, BAK, and cytochrome c ↑ (protein) | Park et al. (2020) | |
| Immunotoxicity | 20 ng/mL | 1/3/6/12/ 24/48 h | CHME5 | 96% ethanol | TLRs, MyD88, NFκB, and CxCr4 ↑ (mRNA), caspase-3/7 ↑ (activity), IFN-γ and GM-CSF ↑ (protein) | Mehrzad, Hosseinkhani & Malvandi (2018a) |
| 0.02, 0.04, 0.08 and 0.16 µg/mL | 48 h | 3D4/21 | DMSO | JAK2/STAT3 pathway, IL6 and TNFα↑ (mRNA), DNMT1/3a ↑ (mRNA & protein) | Zhou et al. (2019) | |
| 10 ng/mL | 2/12 h | MDDCs | 96% ethanol | cytochrome P450 (CYP) family, MyD88, NF-KB, TNF-α, TLR2, TLR4, COX-2, HLA-DR, CCR7, CD209, LFA3 and CD16 ↑,AhR, TGF-β, CD11c and CD64 ↓ (mRNA) | Mehrzad et al. (2018b) | |
| 0.01, 0.02, 0.04, 0.08, 0.16, 0.32, and 0.64 mg/mL | 24 /48 h | 3D4/21 | DMSO | NF-kB signaling pathway, IL6 and TNFα↑ (mRNA) | Hou et al. (2018) | |
| 0.16, 16, 1600, 160000 nmol/L | 24 /48 h | swine alveolar macrophages (SAM) | DMSO | – | Pang, Chiang & Chang (2020) | |
| 3.125, 6.25, 12.5, 25, 50 and 100 µm | 24 /48 h | RAW264.7 | DMSO | NOS2, TNF-α and CXCL2 ↑, CD86 ↓ (mRNA) | Ma et al. (2021) | |
| 0.4, 0.8, 1.6, and 3.2 µg/mL | 90 min | chicken heterophils | – | NADPH oxidase and p38 signaling pathways, glycolysis pathway | Gao et al. (2022) | |
| Reproductive toxicity | 0.1, 1, 10 and 100 µM | 2 /4 h | bull spermatozoa | DMSO | – | Komsky-Elbaz, Saktsier & Roth (2018) |
| 10 and 50 µM | 27 /44 h | porcine oocyte | DMSO | H3K27me3 and H3K4me2 ↓, H3K9me3 ↑ (fluorescence intensity), LC3 ↑ (protein), ATG3, ATG5 and ATG7 ↑ (mRNA), Bak, Bax, and Bcl-xl ↑ (mRNA) | Liu et al. (2015a) | |
| 0.01, 0.1, 1nM | 7 days | porcine embryos | DMSO | Bax and Casp3 ↑, Bcl2 and Bcl-xl ↓, Lc3 and Beclin1 ↑ (mRNA) | Shin et al. (2018) | |
| 0.01, 0.1, 1, 10 and 100 nM | 24 h | JEG-3 | DMSO | PKC-ERK signaling axis, COX2 ↑ (protein) | Zhu, Tan & Leung (2016) |
Hepatotoxicity
Liver is the main target organ for AFB1. Dietary supplementation of AFB1 in rats lead to irreversible liver damage in a dose-dependent manner (Qian et al., 2016; Lu et al., 2013) by inducing fat deposition, fatty acid oxidation (Zhang et al., 2011) and telomere shortening (Ali et al., 2021). Recent studies have also shown that AFB1 can trigger massive production of reactive oxygen species (ROS) in the liver cells, leading to oxidative stress, inflammation, and liver damage (Singh, Maurya & Trigun, 2015). Furthermore, AFB1 exposure enhanced apoptosis of liver cells, activated the resident Kupffer cells, and promoted an inflammatory response in the liver through dephosphorylated-cyclooxygenase-2 (COX2) (Zhang et al., 2019a). Prolonged exposure to AFB1 disrupted lipid and lipoprotein metabolism (Rotimi et al., 2017), and resulted in extensive damage to mitochondrial lipids and reduced antioxidant capacity in the rat liver (Rotimi et al., 2019). Likewise, mice exposed to AFB1 showed mitochondrial dysfunction and increased rates of mitochondria-dependent apoptosis in the liver (Xu et al., 2021). In one study, primary broiler hepatocytes (PBHs) treated with different concentrations of AFB1 showed mitochondrial dysfunction, oxidative stress, and ROS-dependent mitochondrial apoptosis through the nuclear factor-erythroid 2-related factor-2 (Nrf2) signaling pathway (Liu & Wang, 2016). Epidemiological studies have shown that AFB1 is one of the important risk factors of primary liver cancer (Kucukcakan & Hayrulai-Musliu, 2015). Furthermore, there is evidence that AFB1 and chronic hepatitis B virus can synergistically induce mutations in the p53 gene and initiate liver cancer (Liu et al., 2012). To summarize, AFB1 can induce liver damage and even liver cancer by inducing oxidative stress, inflammation, and mitochondrial dysfunction by targeting the p53, ROS, COX2, Nrf2 and other signaling pathways.
Enterotoxicity
The gut barrier function maintains the homeostasis between the resident immune cells and commensal microorganisms via the intestinal epithelial cells (IECs) (Peterson & Artis, 2014). Long-term exposure to AFB1 has been associated with chronic intestinal diseases. AFB1 caused intestinal mucosal injury and inhibited IECs proliferation in mice (Gaikwad & Pillai, 2004). In addition, AFB1 and AFM1 can damage the intestinal barrier in mice through clathrin-mediated endocytosis through synergistic and additive interactions (Gao et al., 2021). AFB1 also altered the composition of the intestinal microbiota of male F344 rats in a dose-dependent manner, and significantly decreased the abundance of the probiotic lactic acid bacteria (Wang et al., 2016a). The microbial-related metabolic changes in the gut microbiota of these AFB1-treated rats were analyzed by ion fragmentation spectroscopy. AFB1 significantly increased the number of inflammatory fecal liposomes, and altered intestinal microbiota-dependent biliary cholesterol metabolism, degradation of bilirubin and fatty acids, and glycolysis. The structural changes in the fecal microflora induced by AFB1 are similar to that observed in IBD (inflammatory bowel disease) patients. The combination of metabolic dysfunction, loss of IECs and glandular atrophy caused by AFB1 can lead to chronic intestinal diseases (Zhou, Tang & Wang, 2021).
The gastrointestinal system in poultry is especially sensitive to AFB1. Broilers fed with different doses of AFB1 exhibit severe damage to the intestinal villi characterized by lower density and absorption area (Yunus et al., 2011; Kana, Teguia & Tchoumboue, 2010), increased atrophy and shedding, and a significant reduction in height (Yin et al., 2016). Moreover, the jejunum of chickens exposed to AFB1 showed histopathological changes, increased apoptosis rates, and altered expression levels of death receptors, endoplasmic reticulum (ER) molecules and apoptotic factors (Zheng et al., 2017). Ingestion of AFB1-contaminated feed can also affect the absorption capacity of the small intestine and impair its innate immune function (Wang et al., 2018c). Furthermore, infiltration of inflammatory cells into the small intestine leads to muco-enteritis (Kumar & Balachandran, 2009). Ducks fed with AFB1-contaminated corns showed longer and wider jejunum villi, which was accompanied by lower average daily gain (ADG) and average daily feed intake (ADFI), resulting in reduced growth and development. Furthermore, the relative weight of the digestive organs, the activity of digestive enzymes and the biochemical indices of intestinal development were also altered (Feng et al., 2017). Thus, AFB1induced intestinal damage can restrict development, disturb the intestinal microflora, and lead to metabolic disorders or chronic intestinal diseases.
Nephrotoxicity
AFB1 is absorbed by the kidneys, and its accumulation in the renal tissues leads to the up-regulation of p21 by MYC, PLK1 and PLD1, resulting in S-phase cell cycle arrest and renal injury (Huang et al., 2019). HEK293 cells treated with AFB1 showed increased apoptosis and DNA fragmentation, which corresponded to the up-regulation of p53, Bax and caspases (Dlamini et al., 2021). AFB1 and AFM1 synergistically increased oxidative stress and the apoptosis pathway in renal cells by regulating the expression level of L-proline (Li et al., 2018). Furthermore, the combination of AFB1 exposure and low protein diet additively reduced weight gain and promoted renal dysfunction in rats, and exacerbated oxidative stress (Rotimi et al., 2018). Exposure to AFB1 and DON synergistically increased oxidative stress in the liver, kidney, and spleen of carp by upregulating Nrf2. Interestingly, ROS generation occurred earlier in the kidneys compared to the liver and spleen (Kövesi et al., 2020). In conclusion, the nephrotoxicity of AFB1 is mainly manifested as oxidative stress induced by p21, L-proline, Nrf2 and other genes. Moreover, AFB1 can have synergistic nephrotoxic effects along with nutrient level and other mycotoxins.
Neurotoxicity
Intragastric administration of AFB1 once weekly for 8 weeks significantly impaired brain function in rats by inducing pathological changes in the cerebral cortex and hippocampus (Bahey, Abd Elaziz & Gadalla, 2015). Long-term exposure to AFB1 may allow it to penetrate the blood–brain barrier, resulting in neurotoxic effects and even chronic neurodegeneration such as that observed in Alzheimer’s disease (Alsayyah et al., 2019). AFB1 inhibits proliferation of human astrocytes by inducing cell cycle arrest and mitochondria-dependent apoptosis (Park et al., 2020). Environmental AFB1 exposure may trigger neuroinflammatory responses by activating the microglia, and increase the susceptibility to neurodegenerative diseases (Mehrzad, Hosseinkhani & Malvandi, 2018a). The neurotoxic effects of AFB1 exposure have also been observed in zebrafish embryos by nuclear magnetic resonance (NMR) (Zuberi et al., 2019). AFB1 exposure decreased the survival rate of embryos by inhibiting oligodendrocyte development (Park et al., 2020). In addition, neuroblastoma cells (IMR-32 cell line) treated with AFB1 also presented intracellular ROS accumulation, DNA damage, S phase arrest and apoptosis (Huang et al., 2020). To summarize, AFB1 can inhibit neural cell development, promote apoptosis, disrupt the homeostasis of the nervous system, and increase the susceptibility to neurodegenerative diseases.
Immunotoxicity
Oxidative stress and apoptosis play key roles in AFB1-induced immunotoxicity (Chen et al., 2013). Oral administration of AFB1 downregulated IFN and TNF in the spleen of mice, increased IL4 levels, and damaged the thymus and spleen, eventually resulting in an impaired immune function (Jebali et al., 2015). AFB1 exposure in rats increased ROS generation and secretion of pro-inflammatory cytokines (TNFα) in the liver cells (Mohammad et al., 2017), which are conducive HCC development (Qin et al., 2016). AFB1 exposure affected transcription of key functional genes in human microglia cell line (CHME5) and human monocyte-derived dendritic cells (MDDCs), and increased apoptosis (Mehrzad, Hosseinkhani & Malvandi, 2018a; Mehrzad et al., 2018b). Similarly, AFB1 treatment decreased the viability of the mouse macrophage RAW264.7 cells in a dose- and time-dependent manner by increasing production of ROS and malondialdehyde (MDA) and decreasing GSH levels. These changes correlated with upregulation of NOS2, TNFα and CXCL2 mRNAs, and downregulation of CD86. AFB1-induced oxidative stress in macrophages also impaired the mitochondrial respiratory chain, leading to activation of the inflammatory response pathways (Ma et al., 2021). AFB1 also decreased the phagocytic capacity of 3D4/21 cells, and induced apoptosis, pro-inflammatory cytokine secretion, DNA damage and oxidative stress. In addition, 3D4/21 cells treated with AFB1 expressed high levels of DNA methyltransferase DNMT1 and DNMT3a, which led to the activation of the JAK2/STAT3 signaling pathway. Inhibition of p-JAK2 and p-STAT3 by blocking DNMT1 and DNMT3a alleviated AFB1-induced immunotoxicity (Zhou et al., 2019). The combination of AFB1 and ochratoxin A (OTA) increased production of TNFα and IL6 in these cells, and decreased lactate dehydrogenase secretion and the phagocytotic index in a concentration-dependent manner. In addition, the combination treatment significantly decreased the production of GSH, increased ROS levels, and promoted IκBa degradation, NF-κB phosphorylation and nuclear translocation of NF-κB. Thus, AFB1 and OTA can synergistically aggravate immunotoxicity by activating of the NF-κB signaling pathway (Hou et al., 2018). AFB1 also impaired the physiological functions of freshly isolated swine alveolar macrophages (SAM), and consumption of AFB1-contaminated feed increased the risk of secondary infections in pigs (Pang, Chiang & Chang, 2020). In addition, AFB1 activated the release of heterophil extracellular traps (HETs) in chicks, and induced the expression of TNFα, IL6 and IL1β, iNOS, COX2, NLRP3, caspase1, caspase3 and caspase11, which resulted in liver and kidney damage (Gao et al., 2022).
Studies show that intake of low doses of AFB1 in animals adversely affect immune organs, decrease antibody titers and complement activity, and cause lymphoid tissue damage. Monogastric animals, especially poultry and pigs, are more sensitive to AFB1-induced immunotoxicity. Broilers exposed to AFB1 showed increased apoptosis of thymocytes due to mitochondrial and death receptor-mediated signaling pathways, as well as DNA damage (Peng et al., 2016). AFB1 also induced tissue damage, cell cycle arrest (Hu et al., 2018) and apoptosis in the Bursa of Fabricius of broilers, which damaged their immune system (Yuan et al., 2016). Ingestion of AFB1-contaminated feed significantly decreased the body weight and lymphocyte activity of pigs, increased the levels of pro-inflammatory cytokines such as TNFα, IL6, IL1β and IFN γ in the spleen, and decreased that of the regulatory factor IL10 (Meissonnier et al., 2008). The immunotoxic effects of AFB1 in livestock may decrease the efficacy of vaccines and increase disease prevalence. These findings indicate that AFB1 affects immune cells and organs, and its immunotoxicity depends on the mitochondrial signaling, death receptor, endoplasmic reticulum, apoptosis, cell cycle and inflammatory pathways.
Reproductive toxicity
AFB1 can impair spermatogenesis through oxidative stress and mitochondria-dependent apoptosis. Mice exposed to AFB1 show impaired blood-testis barrier due to lower levels of the BTB-related junction protein, increased apoptosis in the testes, and the oxidative stress-mediated p38 MAPK signaling pathway, which ultimately affected spermatogenesis (Huang et al., 2021). In addition, AFB1 exposure decreased spermatogenesis in mice by inducing oxidative stress, decreasing mitochondrial content, and upregulating the pro-apoptotic Bax, p53 and Caspase 3 (Yasin, Mazdak & Mino, 2018). AFB1 also induced testicular damage and testicular dysfunction in Dorper rams (Lin et al., 2022), and impaired spermatogenesis in white leghorn cockerels (Ashraf et al., 2022). Exposure to low concentrations of AFB1 for several hours can decrease spermatozoa motility, hyperpolarize mitochondrial membranes, and increase DNA fragmentation (Komsky-Elbaz, Saktsier & Roth, 2018). In addition, AFB1 interferes with porcine oocyte maturation by inducing epigenetic modifications, oxidative stress, excessive autophagy, and apoptosis (Liu et al., 2015a), and affects early embryo development through oxidative stress, DNA damage, apoptosis, and autophagy (Shin et al., 2018). AFB1 can cross the placental barrier in humans (Wild et al., 1991), and AFB1 exposure during pregnancy or lactation can adversely affect the health of the mother as well as the infants. In addition, pregnancy in mice can modulate both phase I and II metabolism, and alter the biological potency of AFB1, thereby increasing liver damage (Sriwattanapong et al., 2017). Prenatal exposure to AFB1 reduces the body weight of neonatal rats, disrupts lipid and hormone levels, and affect the methylation levels of p53 and growth-regulator H19 in the liver and serum. The pathological changes may increase the risk of HCC in the offspring (Rotimi et al., 2021). These studies indicate that exposure of sexually mature animals to AFB1 affects gamete production, gamete quality, and gamete maturation, and AFB1 also disrupts embryonic development, posing a long-term health threat to both pregnant animal mothers and offspring. The reproductive toxicity of AFB1 depends on oxidative stress, DNA damage and repair, apoptosis, autophagy, and epigenetic modification.
Molecular Mechanism of AFB1 Toxicity
AFB1 and multi-omics analysis
Transcriptomic analyses have helped elucidate the mechanisms underlying the pathological effects of AFB1. A total of 1,452 differentially expressed genes (DEGs) have been identified in the liver tissues of AFB1-treated versus healthy mice. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis showed that the DEGs were enriched in functions such as cell adhesion, cell proliferation and cell cycle regulation (Xu et al., 2001). In addition, several lncRNAs involved in the regulation of genes related to apoptosis and DNA repair were upregulated following AFB1 exposure (Shi et al., 2016). Transcriptomic analysis of AFB1-exposed rat tissues indicated that the genes affected by AFB1 were mainly enriched in the p53 signaling pathway, bladder cancer-related signaling pathways, inflammatory response, antioxidant response, cell proliferation, and DNA repair. Metabolomic analysis showed that AFB1 dysregulated gluconeogenesis and lipid metabolism (Lu et al., 2013). AFB1 also induced transcriptomic changes in the genes related to cancer development, apoptosis, inflammation, biological activation, and detoxification in the bovine fetal hepatocyte-derived cell line (BFH12) (Pauletto et al., 2020). Finally, the transcriptomes of AFB1-exposed laying hens showed an upregulation of genes involved in hepatic fat deposition and hepatocyte apoptosis, including those related to the mTOR, FoxO, PPAR, fatty acid degradation and fatty acid metabolism pathways (Liu et al., 2020).
AFB1 and the encoding genes
AFB1 can alter the expression levels of oncogenes (such as ras and c-fos) and tumor suppressor genes (such as p53 and survivin) (Su et al., 2004; Duan et al., 2005), and induce genomic instability and mutations by forming DNA adducts, inhibiting DNA repair enzymes and increasing ROS production. It is bio-transformed to AFBO via cytochrome p450 enzymes, which then forms the carcinogenic adducts. In fact, the p53 gene is mutated in the majority of AFB1 induced HCC cases (Engin & Engin, 2019). In a recent study, transcriptomics and functional genomics identified p53 as the critical transcription factor driving the DNA damage response after exposure to benzo (A) pyrene and AFB1 (Smit et al., 2017).
The transcription factor Nrf2 regulates antioxidant/stress response genes and detoxification genes, and Nrf2 knockout rats are highly sensitive to AFB1 (Taguchi et al., 2016). Primary broiler hepatocytes (PBHs) and broiler cardiomyocytes (BCMs) showed a significant decrease in viability, increased mitochondrial dysfunction, ROS generation and high apoptosis rates following AFB1 treatment, all of which are mediated by the Nrf2 pathway (Liu & Wang, 2016; Wang et al., 2017).
Caveolin-1 (CAV1) is a key mediator of AFB1-induced hepatotoxicity. The human hepatocyte L02 cell line showed a marked decline in viability due to increased apoptosis and oxidative stress after AFB1 exposure, which was accompanied by increased expression of CAV1. The latter mediates AFB1-induced oxidative stress through its interaction with Nrf2, leading to the downregulation of cellular antioxidant enzymes and activation of apoptotic pathways. In addition, CAV1 regulates AFB1-induced autophagy via the EGFR/PI3K-AKT/mTOR signaling pathway. Taken together, CAV1 plays a crucial role in AFB1-induced hepatocyte apoptosis by regulating oxidative stress and autophagy, and is therefore a potential therapeutic target against AFB1-related hepatotoxicity (Xu et al., 2020).
AFB1 induces COX2 expression, promotes mitochondrial autophagy and impairs mitochondrial lipid metabolism in hepatocytes, leading to hepatic steatosis (Ren, Han & Meng, 2020). In addition, AFB1 can induce apoptosis and trigger an “eicosanoid and cytokine storm” in the liver, which can initiate tumor growth. Furthermore, AFB1-generated cellular debris can upregulate COX2, soluble epoxide hydrolase (sEH) and ER stress-response genes (BiP, CHOP and PDI) in macrophages (Fishbein et al., 2020). Meanwhile, the expression of COX2 during pregnancy is crucial, and exposure to AFB1 may induce physiological changes controlled by COX2 (Zhu, Tan & Leung, 2016).
AFB1 and non-coding RNAs
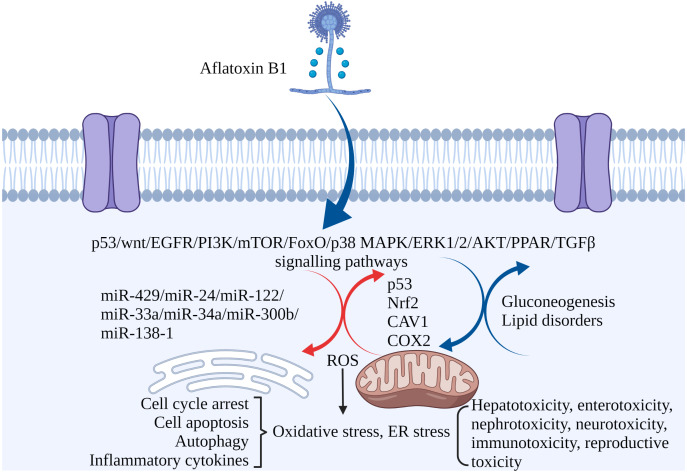
Non-coding RNAs play regulatory roles in mediating the toxic effects of AFB1. For instance, miR-429 and miR-24 are up-regulated in liver cancer tissues exposed to AFB1, and correlate to tumor size. Overexpression of both increased AFB1-DNA adducts in the cancer cells, inhibited apoptosis and promoted their proliferation. Thus, miR-429 and miR-24 are reliable biomarkers of AFB1-induced liver cancer (Huang et al., 2013; Liu et al., 2014). Other miRNAs significantly associated with HCC include hsa-miR-96-5p, hsa-miR-30a-3p, hsa-miR-34a-5p, hsa-miR-34b-5p, hsa-miR-222a-3p, hsa-miR-199a-3p and hsa-miR-4286 (Zhang et al., 2019b). Ectopic expression of miR-34a-5p in liver tumor-bearing rats led to cell cycle arrest by inhibiting MET, CCNE2 and CCND1, and promoted repair of AFB1-induced DNA damage in the liver (Liu et al., 2015b). High expression of miR-33a and miR-34a down-regulated the Wnt/β-catenin signaling pathway in AFB1-stimulated HCC cells (Fang et al., 2013; Zhu et al., 2015). In addition, overexpression of lncRNA H19 promoted HCC cell proliferation and infiltration (Lv et al., 2014). MiR-138-1* inhibits the proliferation, colony formation, migration, and invasion of P50 B-2A13 cells (immortalized human bronchial epithelial cells stably expressing CYP2A13), and mediates AFB1-induced malignant transformation by targeting 3-phosphoinositide-dependent protein kinase-1 (PDK1) (Wang et al., 2016b). MiRNA-429, miRNA-24, miRNA-122, miRNA-33a, miRNA-34a-5p, miRNA-300b-3p, miRNA-138-1 and miRNA-34a regulate AFB1-induced tumorigenesis via the GSK-3b–C/EBPa–miR-122–IGF-1R regulatory circuitry, p53 DNA repair axis, and the Wnt/β-catenin signaling pathway (Dai et al., 2017). AFB1-induced fat accumulation and apoptosis in the liver is regulated by the network of lncRNAs, miRNAs and protein-coding genes. Co-expression of lncRNA (TU45776), gga-miR-190a-3p and Bcl-6 gene induce apoptosis in liver cells, whereas co-expression of lncRNA (TU10057), gga-miR-301a-3p, gga-miR-301b-3p and PPARG gene cause fatty liver (Liu et al., 2020). Taken together, AFB1 regulates the expression of coding genes and non-coding RNAs involved in cell proliferation, apoptosis, and carcinogenesis (Fig. 3).
Figure 3. The molecular mechanisms of AFB1 toxicities.
Coding genes, non-coding RNAs and signaling pathways involved in AFB1-induced toxicity. AFB1 induces cell cycle arrest, apoptosis, oxidative stress, ER stress and autophagy through multiple genes, non-coding RNAs and signaling pathways. ROS, reactive oxygen species; ER stress, endoplasmic reticulum stress. Made in ©BioRender (https://biorender.com/).
Conclusions and Future Direction
The contaminated of agricultural produce with AFTs is practically unavoidable worldwide. The presence of AFTs in feeds may decrease feed intake, damage health and affect livestock productivity. In addition, the toxic residues in animal products (milk, meat, eggs) may have some adverse effects on human health. Contamination of plant and animal-derived food products by AFB1 is a major health concern. In this review, we summarized the hepatotoxic, enterotoxic, nephrotoxic, neurotoxic, immune-toxic and gonado-toxic effects of AFB1. In addition, the mechanisms underlying the toxicity of AFB1, including cell cycle arrest, apoptosis, oxidative stress, ER stress and autophagy, and the effector genes, non-coding RNAs and signaling pathways were also discussed.
The toxicity of AFB1 is very complex, and is closely related to the dose, exposure duration, administration mode, solvent, species, gender, age, target organs and so on. More comprehensive and systematic tests are needed to ascertain the acute and chronic AFB1 exposures doses in different species, develop standard dosing methods, and determine the quantified dose–response relationship in vitro and in vivo. In addition, the combined effects of AFB1 with other mycotoxins and with macronutrient deficiency, and well as the underlying mechanisms will be our future research concerns. The relationship between gut microorganisms and sensitivity to AFB1, and the impact of intestinal microbiota on AFB1 metabolism will also be an important research focus. Elucidating the molecular mechanisms underlying the toxicity of AFB1 can help mitigate or eliminate the toxic effects of AFB1 by genetic methods, which will also be worth investigating.
Supplemental Information
The number list of articles searched via databases.
Funding Statement
This work was supported by the Joint Funds of the National Natural Science Foundation of China (grant numbers: U2004156). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Congcong Li conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Xiangdong Liu conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Jiao Wu performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Xiangbo Ji performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Qiuliang Xu performed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Raw data are available in the Supplementary Files.
References
- Ali et al. (2021).Ali FAZ, Abdel-Maksoud FM, Abd Elaziz HO, Al-Brakati A, Elmahallawy EK. Descriptive histopathological and ultrastructural study of hepatocellular alterations induced by Aflatoxin B1 in rats. Animals. 2021;11:509. doi: 10.3390/ani11020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsayyah et al. (2019).Alsayyah A, ElMazoudy R, Al-Namshan M, Al-Jafary M, Alaqeel N. Chronic neurodegeneration by aflatoxin B1 depends on alterations of brain enzyme activity and immunoexpression of astrocyte in male rats. Ecotoxicology and Environmental Safety. 2019;182:109407. doi: 10.1016/j.ecoenv.2019.109407. [DOI] [PubMed] [Google Scholar]
- Ashraf et al. (2022).Ashraf A, Saleemi MK, Mohsin M, Gul ST, Zubair M, Muhammad F, Bhatti SA, Hameed MR, Imran M, Irshad H, Zaheer I, Ahmed I, Raza A, Qureshi AS, Khan A. Pathological effects of graded doses of aflatoxin B1 on the development of the testes in juvenile white leghorn males. Environmental Science and Pollution Research. 2022 doi: 10.1007/s11356-022-19324-6. Epub ahead of print 2022 12 March. [DOI] [PubMed] [Google Scholar]
- Bahey, Abd Elaziz & Gadalla (2015).Bahey NG, Abd Elaziz HO, Gadalla KK. Toxic effect of aflatoxin B1 and the role of recovery on the rat cerebral cortex and hippocampus. Tissue Cell. 2015;47:559–566. doi: 10.1016/j.tice.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Battilani et al. (2016).Battilani P, Toscano P, Vander Fels-Klerx HJ, Moretti A, Camardo Leggieri M, Brera C, Rortais A, Goumperis T, Robinson T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Scientific Reports. 2016;6:24328. doi: 10.1038/srep24328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy & Pestka (2000).Bondy GS, Pestka JJ. Immunomodulation by fungal toxins. Journal of Toxicology and Environmental Health - Part B: Critical Reviews. 2000;3:109–143. doi: 10.1080/109374000281113. [DOI] [PubMed] [Google Scholar]
- Bryden (2012).Bryden W. Mycotoxin contamination of the feed supply chain: implication of animal productivity and feed security. Animal Feed Science and Technology. 2012;173:134–158. doi: 10.1016/j.anifeedsci.2011.12.014. [DOI] [Google Scholar]
- Chen et al. (2013).Chen J, Chen K, Yuan S, Peng X, Fang J, Wang F, Cui H, Chen Z, Yuan J, Geng Y. Effects of aflatoxin B1 on oxidative stress markers and apoptosis of spleens in broilers. Toxicology and Industrial Health. 2013;32:278–284. doi: 10.1177/0748233713500819. [DOI] [PubMed] [Google Scholar]
- Coppock, Christian & Jacobsen (2018).Coppock RW, Christian RG, Jacobsen BJ. Aflatoxins. Veterinary Toxicology. (3rd edn) 2018;69:983–994. doi: 10.1016/B978-0-12-811410-0.00069-6. [DOI] [Google Scholar]
- Costa, Lima & Santos (2021).Costa J, Lima N, Santos C. An overview on possible links between aflatoxin B1 exposure and gallbladder cancer. Mycotoxin Research. 2021;37:205–214. doi: 10.1007/s12550-021-00431-1. [DOI] [PubMed] [Google Scholar]
- Cui et al. (2015).Cui A, Hua H, Shao T, Song P, Kong Q, Luo T, Jiang Y. Aflatoxin B1 induces Src phosphorylation and stimulates lung cancer cell migration. Tumor Biology. 2015;36:6507–6513. doi: 10.1007/s13277-015-3341-2. [DOI] [PubMed] [Google Scholar]
- Dai et al. (2017).Dai Y, Huang K, Zhang B, Zhu L, Xu W. Aflatoxin B1-induced epigenetic alterations: an overview. Food and Chemical Toxicology. 2017;109:683–689. doi: 10.1016/j.fct.2017.06.034. [DOI] [PubMed] [Google Scholar]
- Dalvi (1986).Dalvi RR. An overview of aflatoxicosis of poultry: its characteristics, prevention and reduction. Veterinary Medicine and Science. 1986;10:429–443. doi: 10.1007/BF02214006. [DOI] [PubMed] [Google Scholar]
- Dlamini et al. (2021).Dlamini NZ, Somboro AM, Amoako DG, Arhin I, Khumalo HM, Khan RB. Toxicogenicity and mechanistic pathways of aflatoxin B1 induced renal injury. Environmental Toxicology. 2021;36(9):1857–1872. doi: 10.1002/tox.23306. [DOI] [PubMed] [Google Scholar]
- Dohnal, Wu & Kuca (2014).Dohnal V, Wu Q, Kuca K. Metabolism of aflatoxins: key enzymes and interindividual as well as interspecies differences. Archives of Toxicology. 2014;88:1635–1644. doi: 10.1007/s00204-014-1312-9. [DOI] [PubMed] [Google Scholar]
- Duan et al. (2005).Duan XX, Ou JS, Li Y, Su JJ, Ou C, Yang C, Yue HF, Ban KC. Dynamic expression of apoptosis-related genes during development of laboratory hepatocellular carcinoma and its relation to apoptosis. World Journal of Gastroenterology. 2005;11:4740–4744. doi: 10.3748/wjg.v11.i30.4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton et al. (1988).Eaton DL, Monroe DH, Bellamy G, Kalman DA. Identification of a novel dihydroxy metabolite of aflatoxin B1 produced in vitro and in vivo in rats and mice. Chemical Research in Toxicology. 1988;1:108–114. doi: 10.1021/tx00002a004. [DOI] [PubMed] [Google Scholar]
- Elgioushy et al. (2020).Elgioushy MM, Elgaml SA, El-Adl MM, Hegazy AM, Hashish EA. Aflatoxicosis in cattle: clinical findings and biochemical alterations. Environmental Science and Pollution Research. 2020;27:35526–35534. doi: 10.1007/s11356-020-09489-3. [DOI] [PubMed] [Google Scholar]
- Engin & Engin (2019).Engin AB, Engin A. DNA damage checkpoint response to aflatoxin B1. Environmental Toxicology and Pharmacology. 2019;65:90–96. doi: 10.1016/j.etap.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Eom et al. (2013).Eom SY, Yim DH, Zhang Y, Yun JK, Moon SI, Yun HY, Song YJ, Youn SJ, Hyun T, Park JS, Kim BS, Lee JY, Kim YD, Kim H. Dietary aflatoxin B1 intake, genetic polymorphisms of CYP1A2, CYP2E1, EPHX1, GSTM1, and GSTT1, and gastric cancer risk in Korean. Cancer Causes Control. 2013;24:1963–1972. doi: 10.1007/s10552-013-0272-3. [DOI] [PubMed] [Google Scholar]
- Eskola et al. (2020).Eskola M, Kos G, Elliott CT, Hajslova J, Mayar S, Krska R. Worldwide contamination of food-crops with mycotoxins: validity of the widely cited ‘FAO estimate’ of 25. Critical Reviews in Food Science and Nutrition. 2020;60:2773–2789. doi: 10.1080/10408398.2019.1658570. [DOI] [PubMed] [Google Scholar]
- Fang et al. (2013).Fang Y, Feng Y, Wu T, Srinivas S, Yang W, Fan J, Yang C, Wang S. Aflatoxin B1 negatively regulates Wnt/beta-catenin signaling pathway through activating miR-33a. PLOS ONE. 2013;8:e73004. doi: 10.1371/journal.pone.0073004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng et al. (2017).Feng GD, He J, Ao X, Chen DW. Effects of maize naturally contaminated with aflatoxin B1 on growth performance, intestinal morphology, and digestive physiology in ducks. Poultry Science. 2017;96:1948–1955. doi: 10.3382/ps/pew420. [DOI] [PubMed] [Google Scholar]
- Fishbein et al. (2020).Fishbein A, Wang W, Yang H, Yang J, Hallisey VM, Deng J, Verheul SML, Hwang SH, Gartung A, Wang Y, Bielenberg DR, Huang S, Kieran MW, Hammock BD, Panigrahy D. Resolution of eicosanoid/cytokine storm prevents carcinogen and inflammation-initiated hepatocellular cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:21576–21587. doi: 10.1073/pnas.2007412117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, Eisenstadt & Miller (1983).Foster PL, Eisenstadt E, Miller JH. Base substitution mutations induced by metabolically activated aflatoxin B1. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:2695–2698. doi: 10.1073/pnas.80.9.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad et al. (2019).Fouad AM, Ruan D, El-Senousey HK, Chen W, Jiang S, Zheng C. Harmful effects and control strategies of aflatoxin B(1) produced by aspergillus flavus and aspergillus parasiticus strains on poultry: review. Toxins. 2019;11:176. doi: 10.3390/toxins11030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaikwad & Pillai (2004).Gaikwad SS, Pillai MM. Effect of aflatoxin B 1 in gastrointestine of mice. Journal of Ecophysiology and Occupational Health. 2004;4:153–159. [Google Scholar]
- Gallagher et al. (1996).Gallagher EP, Kunze KL, Stapleton PL, Eaton DL. The kinetics of aflatoxin B1 oxidation by human cDNA-expressed and human liver microsomal cytochromes P450 1A2 and 3A4. Toxicology and Applied Pharmacology. 1996;141:595–606. doi: 10.1006/taap.1996.0326. [DOI] [PubMed] [Google Scholar]
- Gallagher et al. (1994).Gallagher EP, Wienkers LC, Stapleton PL, Kunze KL, Eaton DL. Role of human microsomal and human complementary DNA-expressed cytochromes P4501A2 and P4503A4 in the bioactivation of aflatoxin B1. Cancer Research. 1994;54:101–108. [PubMed] [Google Scholar]
- Gao et al. (2021).Gao Y, Bao X, Meng L, Liu H, Wang J, Zheng N. Aflatoxin B1 and Aflatoxin M1 induce compromised intestinal integrity through clathrin-mediated endocytosis. Toxins. 2021;13:184. doi: 10.3390/toxins13030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al. (2022).Gao X, Jiang L, Xu J, Liu W, Li S, Huang W, Zhao H, Yang Z, Yu X, Wei Z. Aflatoxin B1-activated heterophil extracellular traps result in the immunotoxicity to liver and kidney in chickens. Developmental & Comparative Immunology. 2022;128:104325. doi: 10.1016/j.dci.2021.104325. [DOI] [PubMed] [Google Scholar]
- Goto et al. (1986).Goto T, Manabe M, Matsuura S, Hsieh DDH. Aflatoxin in milk. In: Richard JL, Thurston JR, editors. Diagnosis of mycotoxicoses. Springer Netherlands; Dordrecht: 1986. pp. 103–111. [Google Scholar]
- Grenier & Applegate (2013).Grenier B, Applegate TJ. Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals. Toxins. 2013;5:396–430. doi: 10.3390/toxins5020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman et al. (1985).Groopman JD, Donahue PR, Zhu JQ, Chen JS, Wogan GN. Aflatoxin metabolism in humans: detection of metabolites and nucleic acid adducts in urine by affinity chromatography. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:6492–6496. doi: 10.1073/pnas.82.19.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, Carvajal & Garner (1993).Harrison JC, Carvajal M, Garner RC. Does aflatoxin exposure in the United Kingdom constitute a cancer risk? Environmental Health Perspectives. 1993;99:99–105. doi: 10.1289/ehp.939999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich (2003).Heinrich M. IARC monographs on the evaluation of carcinogenic risks to humans: some traditional herbal medicines, some mycotoxins, naphthalene and styrene. Journal of Ethnopharmacology. 2003;88:299–300. doi: 10.1016/S0378-8741(03)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Mendoza, Garcia & Steele (2009).Hernandez-Mendoza A, Garcia HS, Steele JL. Screening of Lactobacillus casei strains for their ability to bind aflatoxin B1. Food and Chemical Toxicology. 2009;47:1064–1068. doi: 10.1016/j.fct.2009.01.042. [DOI] [PubMed] [Google Scholar]
- Holeski et al. (1987).Holeski CJ, Eaton DL, Monroe DH, Bellamy GM. Effects of phenobarbital on the biliary excretion of aflatoxin P1-glucuronide and aflatoxin B1-S-glutathione in the rat. Xenobiotica. 1987;17:139–153. doi: 10.3109/00498258709043924. [DOI] [PubMed] [Google Scholar]
- Hollstein et al. (1991).Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hou et al. (2018).Hou L, Gan F, Zhou X, Zhou Y, Qian G, Liu Z, Huang K. Immunotoxicity of ochratoxin A and aflatoxin B1 in combination is associated with the nuclear factor kappa B signaling pathway in 3D4/21cells. Chemosphere. 2018;199:718–727. doi: 10.1016/j.chemosphere.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2018).Hu P, Zuo Z, Li H, Wang F, Peng X, Fang J, Cui H, Gao C, Song H, Zhou Y, Chen Z. The molecular mechanism of cell cycle arrest in the Bursa of Fabricius in chick exposed to Aflatoxin B 1. Scientific Reports. 2018;8:1770. doi: 10.1038/s41598-018-20164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2020).Huang B, Chen Q, Wang L, Gao X, Zhu W, Mu P, Deng Y. Aflatoxin B1 induces neurotoxicity through reactive oxygen species generation, DNA damage, apoptosis, and S-phase cell cycle arrest. International Journal of Molecular Sciences. 2020;21:6517. doi: 10.3390/ijms21186517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2021).Huang W, Liu M, Xiao B, Zhang J, Song M, Li Y, Cao Z. Aflatoxin B1 disrupts blood-testis barrier integrity by reducing junction protein and promoting apoptosis in mice testes. Food and Chemical Toxicology. 2021;148:111972. doi: 10.1016/j.fct.2021.111972. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2019).Huang B, Mu P, Chen X, Tang S, Ye W, Zhu W, Deng Y. Aflatoxin B1 induces S phase arrest by upregulating the expression of p21 via MYC, PLK1 and PLD1. Biochemical Pharmacology. 2019;166:108–119. doi: 10.1016/j.bcp.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2013).Huang XY, Yao JG, Huang HD, Wang C, Ma Y, Xia Q, Long XD. MicroRNA-429 modulates hepatocellular carcinoma prognosis and tumorigenesis. Gastroenterology Research and Practice. 2013;2013:804128. doi: 10.1155/2013/804128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iheshiulor et al. (2011).Iheshiulor OOM, Esonu BO, Chuwuka OK, Omede AA, Okoli IC, Ogbuewu IP. Effects of mycotoxins in animal nutrition: a review. Asian Journal of Animal Sciences. 2011;5:19–33. doi: 10.3923/ajas.2011.19.33. [DOI] [Google Scholar]
- International Agency for Research on Cancer, IARC (1993).International Agency for Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 56. Lyon: International Agency for Research on Cancer; 1993. Some naturally occurring substances: food items and constituents, heterocyclic sromatic smines and mycotoxins. [Google Scholar]
- Iqbal, Asi & Arino (2013).Iqbal SZ, Asi MR, Arino A. Aflatoxins. In: Maloy S, Hughes K, editors. Brenner’s encyclopedia of genetics. 2nd edn. San Diego: Academic Press; 2013. pp. 43–47. [DOI] [Google Scholar]
- Iyer et al. (1994).Iyer RS, Coles BF, Raney KD, Thier R, Guengerich FP, Harris TM. DNA adduction by the potent carcinogen aflatoxin B1: mechanistic studies. Journal of the American Chemical Society. 1994;116:1603–1609. doi: 10.1021/ja00084a001. [DOI] [Google Scholar]
- Jebali et al. (2015).Jebali R, Abbes S, Salah-Abbes JB, Younes RB, Haous Z, Oueslati R. Ability of Lactobacillus plantarum MON03 to mitigate aflatoxins (B1 and M1) immunotoxicities in mice. Journal of Immunotoxicology. 2015;12:290–299. doi: 10.3109/1547691X.2014.973622. [DOI] [PubMed] [Google Scholar]
- Kana, Teguia & Tchoumboue (2010).Kana JR, Teguia A, Tchoumboue J. Effect of dietary plant charcoal from Canarium schweinfurthii England maize cob on aflatoxin B1 toxicosis in broiler chickens. Advances in Animal Biosciences. 2010;1:462–463. doi: 10.1017/S2040470010000841. [DOI] [Google Scholar]
- Komsky-Elbaz, Saktsier & Roth (2018).Komsky-Elbaz A, Saktsier M, Roth Z. Aflatoxin B1 impairs sperm quality and fertilization competence. Toxicology. 2018;393:42–50. doi: 10.1016/j.tox.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Kövesi et al. (2020).Kövesi B, Pelyhe C, Zándoki E, Mézes M, Balogh K. Combined effects of aflatoxin B1 and deoxynivalenol on the expression of glutathione redox system regulatory genes in common carp. Journal of Animal Physiology and Animal Nutrition. 2020;104:1531–1539. doi: 10.1111/jpn.13343. [DOI] [PubMed] [Google Scholar]
- Krishnamachari et al. (1975).Krishnamachari KA, Bhat RV, Nagarajan V, Tilak TB. Hepatitis due to aflatoxicosis. An outbreak in Western India. The Lancet. 1975;1:1061–1063. doi: 10.1016/s0140-6736(75)91829-2. [DOI] [PubMed] [Google Scholar]
- Kucukcakan & Hayrulai-Musliu (2015).Kucukcakan B, Hayrulai-Musliu Z. Challenging role of dietary Aflatoxin B1 exposure and hepatitis B infection on risk of hepatocellular carcinoma. Open Access Macedonian Journal of Medical Sciences. 2015;3:363–369. doi: 10.3889/oamjms.2015.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman, Maas & Fink-Gremmels (2000).Kuilman ME, Maas RF, Fink-Gremmels J. Cytochrome P450-mediated metabolism and cytotoxicity of aflatoxin B(1) in bovine hepatocytes. Toxicol In Vitro. 2000;14:321–327. doi: 10.1016/s0887-2333(00)00025-4. [DOI] [PubMed] [Google Scholar]
- Kumar et al. (2017).Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG. Aflatoxins: a global concern for food safety, human health and their management. Frontiers in Microbiology. 2017;7:2170. doi: 10.3389/fmicb.2016.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar & Balachandran (2009).Kumar R, Balachandran C. Histopathological changes in broiler chickens fed aflatoxin and cyclopiazonic acid. Veterinarski Arhiv. 2009;79:31–40. [Google Scholar]
- Li (2002).Li J. Hazardous material and detection. China Petrochemical Press; Beijing: 2002. [Google Scholar]
- Li et al. (2018).Li H, Xing L, Zhang M, Wang J, Zheng N. The toxic effects of Aflatoxin B1 and Aflatoxin M1 on kidney through regulating L-proline and downstream apoptosis. BioMed Research International. 2018;2018:9074861. doi: 10.1155/2018/9074861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2022).Lin LX, Cao QQ, Zhang CD, Xu TT, Yue K, Li Q, Liu F, Wang X, Dong HJ, Huang SC, Jian FC. Aflatoxin B1 causes oxidative stress and apoptosis in sheep testes associated with disrupting rumen microbiota. Ecotoxicology and Environmental Safety. 2022;232:113225. doi: 10.1016/j.ecoenv.2022.113225. [DOI] [PubMed] [Google Scholar]
- Lin et al. (2014).Lin YC, Li L, Makarova AV, Burgers PM, Stone MP, Lloyd RS. Molecular basis of aflatoxin-induced mutagenesis-role of the aflatoxin B1-formamidopyrimidine adduct. Carcinogenesis. 2014;35:1461–1468. doi: 10.1093/carcin/bgu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2016).Lin YC, Owen N, Minko IG, Lange SS, Tomida J, Li L, Stone MP, Wood RD, McCullough AK, Lloyd RS. DNA polymerase zeta limits chromosomal damage and promotes cell survival following aflatoxin exposure. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:13774–13779. doi: 10.1073/pnas.1609024113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2012).Liu Y, Chang CC, Marsh GM, Wu F. Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. European Journal of Cancer. 2012;48:2125–2136. doi: 10.1016/j.ejca.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2020).Liu X, Kumar Mishra S, Wang T, Xu Z, Zhao X, Wang Y, Yin H, Fan X, Zeng B, Yang M, Yang D, Ni Q, Li Y, Zhang M, Zhu Q, Chen F, Li D. AFB1 induced transcriptional regulation related to apoptosis and lipid metabolism in liver of chicken. Toxins. 2020;12:290. doi: 10.3390/toxins12050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2014).Liu YX, Long XD, Xi ZF, Ma Y, Huang XY, Yao JG, Wang C, Xing TY, Xia Q. MicroRNA-24 modulates aflatoxin B1-related hepatocellular carcinoma prognosis and tumorigenesis. BioMed Research International. 2014;2014:482926. doi: 10.1155/2014/482926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu & Wang (2016).Liu Y, Wang W. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes. Animal Science Journal. 2016;87:1490–1500. doi: 10.1111/asj.12550. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2015a).Liu J, Wang QC, Han J, Xiong B, Sun SC. Aflatoxin B1 is toxic to porcine oocyte maturation. Mutagenesis. 2015a;30:527–535. doi: 10.1093/mutage/gev015. [DOI] [PubMed] [Google Scholar]
- Liu & Wu (2010).Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environmental Health Perspectives. 2010;118:818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2015b).Liu C, Yu H, Zhang Y, Li D, Xing X, Chen L, Zeng X, Xu D, Fan Q, Xiao Y, Chen W, Wang Q. Upregulation of miR-34a-5p antagonizes AFB1-induced genotoxicity in F344 rat liver. Toxicon. 2015b;106:46–56. doi: 10.1016/j.toxicon.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2013).Lu X, Hu B, Shao L, Tian Y, Jin T, Jin Y, Ji S, Fan X. Integrated analysis of transcriptomics and metabonomics profiles in aflatoxin B1-induced hepatotoxicity in rat. Food and Chemical Toxicology. 2013;55:444–455. doi: 10.1016/j.fct.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Lv et al. (2014).Lv J, Yu YQ, Li SQ, Luo L, Wang Q. Aflatoxin B1 promotes cell growth and invasion in hepatocellular carcinoma HepG2 cells through H19 and E2F1. Asian Pacific Journal of Cancer Prevention. 2014;15:2565–2570. doi: 10.7314/apjcp.2014.15.6.2565. [DOI] [PubMed] [Google Scholar]
- Ma et al. (2021).Ma J, Liu Y, Guo Y, Ma Q, Ji C, Zhao L. Transcriptional profiling of Aflatoxin B1-induced oxidative stress and inflammatory response in macrophages. Toxins. 2021;13:401. doi: 10.3390/toxins13060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magan & Aldred (2007).Magan N, Aldred D. Post-harvest control strategies: minimizing mycotoxins in the food chain. International Journal of Food Microbiology. 2007;119:131–139. doi: 10.1016/j.ijfoodmicro.2007.07.034. [DOI] [PubMed] [Google Scholar]
- Mehrzad et al. (2018b).Mehrzad J, Bahari A, Bassami MR, Mahmoudi M, Dehghani H. Immunobiologically relevant level of aflatoxin B1 alters transcription of key functional immune genes, phagocytosis and survival of human dendritic cells. Immunology Letters. 2018b;197:44–52. doi: 10.1016/j.imlet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Mehrzad, Hosseinkhani & Malvandi (2018a).Mehrzad J, Hosseinkhani S, Malvandi AM. Human microglial cells undergo proapoptotic induction and inflammatory activation upon in vitro exposure to a naturally occurring level of Aflatoxin B1. Neuroimmunomodulation. 2018a;25:176–183. doi: 10.1159/000493528. [DOI] [PubMed] [Google Scholar]
- Meissonnier et al. (2008).Meissonnier GM, Pinton P, Laffitte J, Cossalter AM, Gong YY, Wild CP, Bertin G, Galtier P, Oswald IP. Immunotoxicity of aflatoxin B1: impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicology and Applied Pharmacology. 2008;231:142–149. doi: 10.1016/j.taap.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Mohammad et al. (2017).Mohammad KK, Ghazi-Khansari M, Hayati F, Staji H, Keywanloo M, Shahroozian E. The role of TNF-α in Aflatoxin B-1 induced hepatic toxicity in isolated perfused rat liver model. Acta Medica Iranica. 2017;55(7):416–421. [PubMed] [Google Scholar]
- Monson et al. (2014).Monson MS, Settlage RE, McMahon KW, Mendoza KM, Rawal S, El-Nezami HS, Coulombe RA, Reed KM. Response of the hepatic transcriptome to aflatoxin B1 in domestic turkey (Meleagris gallopavo) PLOS ONE. 2014;9:e100930. doi: 10.1371/journal.pone.0100930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykkanen et al. (2005).Mykkanen H, Zhu H, Salminen E, Juvonen RO, Ling W, Ma J, Polychronaki N, Kemilainen H, Mykkanen O, Salminen S, El-Nezami H. Fecal and urinary excretion of aflatoxin B1 metabolites (AFQ1, AFM1 and AFB-N7-guanine) in young Chinese males. International Journal of Cancer. 2005;115:879–884. doi: 10.1002/ijc.20951. [DOI] [PubMed] [Google Scholar]
- Narkwa, Blackbourn & Mutocheluh (2017).Narkwa PW, Blackbourn DJ, Mutocheluh M. Aflatoxin B1 inhibits the type 1 interferon response pathway via STAT1 suggesting another mechanism of hepatocellular carcinoma. Infectious Agents and Cancer. 2017;12:17. doi: 10.1186/s13027-017-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey & Chauhan (2007).Pandey I, Chauhan SS. Studies on production performance and toxin residues in tissues and eggs of layer chickens fed on diets with various concentrations of aflatoxin AFB1. British Poultry Science. 2007;48:713–723. doi: 10.1080/00071660701713534. [DOI] [PubMed] [Google Scholar]
- Pang, Chiang & Chang (2020).Pang VF, Chiang CF, Chang CC. The in vitro effects of aflatoxin B1 on physiological functions of swine alveolar macrophages. Veterinary Medicine and Science. 2020;6:919–925. doi: 10.1002/vms3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al. (2020).Park S, Lee JY, You S, Song G, Lim W. Neurotoxic effects of aflatoxin B1 on human astrocytes in vitro and on glial cell development in zebrafish in vivo. Journal of Hazardous Materials. 2020;386:121639. doi: 10.1016/j.jhazmat.2019.121639. [DOI] [PubMed] [Google Scholar]
- Pauletto et al. (2020).Pauletto M, Tolosi R, Giantin M, Guerra G, Barbarossa A, Zaghini A, Dacasto M. Insights into Aflatoxin B1 toxicity in cattle: an in vitro whole-transcriptomic approach. Toxins. 2020;12:429. doi: 10.3390/toxins12070429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng et al. (2016).Peng X, Yu Z, Liang N, Chi X, Li X, Jiang M, Fang J, Cui H, Lai W, Zhou Y, Zhou S. The mitochondrial and death receptor pathways involved in the thymocytes apoptosis induced by aflatoxin B1. Oncotarget. 2016;7:12222–12234. doi: 10.18632/oncotarget.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson & Artis (2014).Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nature Reviews Immunology. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- Probst, Njapau & Cotty (2007).Probst C, Njapau H, Cotty P. Outbreak of an acute aflatoxicosis in Kenya in 2004: identification of the causal agent. Applied and Environmental Microbiology. 2007;73:2762–2764. doi: 10.1128/AEM.02370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu et al. (2021).Pu J, Yuan Q, Yan H, Tian G, Chen D, He J, Zheng P, Yu J, Mao X, Huang Z, Luo J, Luo Y, Yu B. Effects of chronic exposure to low levels of dietary Aflatoxin B1 on growth performance, apparent total tract digestibility and intestinal health in pigs. Animals. 2021;11:336. doi: 10.3390/ani11020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian et al. (2016).Qian G, Tang L, Lin S, Xue KS, Mitchell NJ, Su J, Gelderblom WC, Riley RT, Phillips TD, Wang JS. Sequential dietary exposure to aflatoxin B1 and fumonisin B1 in F344 rats increases liver preneoplastic changes indicative of a synergistic interaction. Food and Chemical Toxicology. 2016;95:188–195. doi: 10.1016/j.fct.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin et al. (2016).Qin H, Li H, Zhou X, Peng C, Tan H, Wang M. Effect of superoxide and inflammatory factor on aflatoxin B1 triggered hepatocellular carcinoma. American Journal of Translational Research. 2016;8:4003–4008. [PMC free article] [PubMed] [Google Scholar]
- Ren, Han & Meng (2020).Ren XL, Han P, Meng Y. Aflatoxin B1-induced COX-2 expression promotes mitophagy and contributes to lipid accumulation in hepatocytes in vitro and in vivo. International Journal of Toxicology. 2020;39:594–604. doi: 10.1177/1091581820939081. [DOI] [PubMed] [Google Scholar]
- Roebuck & Wogan (1977).Roebuck BD, Wogan GN. Species comparison of in vitro metabolism of aflatoxin B1. Cancer Research. 1977;37:1649–1656. [PubMed] [Google Scholar]
- Ross et al. (1992).Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, Groopman JD, Gao YT, Henderson BE. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. The Lancet. 1992;339:943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- Rotimi et al. (2021).Rotimi OA, Onuzulu CD, Dewald AL, Ehlinger J, Adelani IB, Olasehinde OE, Rotimi SO, Goodrich JM. Early life exposure to Aflatoxin B1 in rats: alterations in lipids, hormones, and DNA methylation among the offspring. International Journal of Environmental Research and Public Health. 2021;18:589. doi: 10.3390/ijerph18020589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotimi et al. (2017).Rotimi OA, Rotimi SO, Duru CU, Ebebeinwe OJ, Abiodun AO, Oyeniyi BO, Faduyile FA. Acute aflatoxin B1 - induced hepatotoxicity alters gene expression and disrupts lipid and lipoprotein metabolism in rats. Toxicology Reports. 2017;4:408–414. doi: 10.1016/j.toxrep.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotimi et al. (2019).Rotimi OA, Rotimi SO, Goodrich JM, Adelani IB, Agbonihale E, Talabi G. Time-course effects of acute aflatoxin B1 exposure on hepatic mitochondrial lipids and oxidative stress in rats. Frontiers in Pharmacology. 2019;10:467. doi: 10.3389/fphar.2019.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotimi et al. (2018).Rotimi OA, Rotimi SO, Oluwafemi F, Ademuyiwa O, Balogun EA. Oxidative stress in extrahepatic tissues of rats co-exposed to Aflatoxin B1 and low protein diet. Toxicological Research. 2018;34:211–220. doi: 10.5487/TR.2018.34.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushing & Selim (2019).Rushing BR, Selim MI. Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food and Chemical Toxicology. 2019;124:81–100. doi: 10.1016/j.fct.2018.11.047. [DOI] [PubMed] [Google Scholar]
- Schermerhorn & Delaney (2014).Schermerhorn KM, Delaney S. A chemical and kinetic perspective on base excision repair of DNA. Accounts of Chemical Research. 2014;47:1238–1246. doi: 10.1021/ar400275a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2016).Shi J, He J, Lin J, Sun X, Sun F, Ou C, Jiang C. Distinct response of the hepatic transcriptome to Aflatoxin B1 induced hepatocellular carcinogenesis and resistance in rats. Scientific Reports. 2016;6:31898. doi: 10.1038/srep31898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin et al. (2018).Shin KT, Guo J, Niu YJ, Cui XS. The toxic effect of aflatoxin B1 on early porcine embryonic development. Theriogenology. 2018;118:157–163. doi: 10.1016/j.theriogenology.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Singh, Maurya & Trigun (2015).Singh KB, Maurya BK, Trigun SK. Activation of oxidative stress and inflammatory factors could account for histopathological progression of aflatoxin-B1 induced hepatocarcinogenesis in rat. Molecular and Cellular Biochemistry. 2015;401:185–196. doi: 10.1007/s11010-014-2306-x. [DOI] [PubMed] [Google Scholar]
- Smela et al. (2001).Smela ME, Currier SS, Bailey EA, Essigmann JM. The chemistry and biology of aflatoxin B(1): from mutational spectrometry to carcinogenesis. Carcinogenesis. 2001;22:535–545. doi: 10.1093/carcin/22.4.535. [DOI] [PubMed] [Google Scholar]
- Smit et al. (2017).Smit E, Souza T, Jennen DGJ, Kleinjans JCS, vandenBeucken T. Identification of essential transcription factors for adequate DNA damage response after benzo(a)pyrene and aflatoxin B1 exposure by combining transcriptomics with functional genomics. Toxicology. 2017;390:74–82. doi: 10.1016/j.tox.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Soini et al. (1996).Soini Y, Chia SC, Bennett WP, Groopman J, Wang J, De Benedetti VMG, Cawley H, Welsh J, Hansen C, Bergasa NV, Jones E, Di Bisceglie AM, Trivers G, Sandoval CA, Calderon IE, Espinosa L, Harris C. An aflatoxin-associated mutational hotspot at codon 249 in the p53 tumor suppressor gene occurs in hepatocellular carcinomas from Mexico. Carcinogenesis. 1996;17:1007–1012. doi: 10.1093/carcin/17.5.1007. [DOI] [PubMed] [Google Scholar]
- Sriwattanapong et al. (2017).Sriwattanapong K, Slocum SL, Chawanthayatham S, Fedeles BI, Egner PA, Groopman JD, Satayavivad J, Croy RG, Essigmann JM. Editor’s highlight: pregnancy alters aflatoxin b1 metabolism and increases DNA damage in mouse liver. Toxicological Sciences. 2017;160:173–179. doi: 10.1093/toxsci/kfx171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su et al. (2004).Su JJ, Ban KC, Li Y, Qin LL, Wang HY, Yang C, Ou C, Duan XX, Lee YL, Yang RQ. Alteration of p53 and p21 during hepatocarcinogenesis in tree shrews. World Journal of Gastroenterology. 2004;10:3559–3563. doi: 10.3748/wjg.v10.i24.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun (2017).Sun CH. Nutrition and food hygiene. 8th edn People’s Medical Publishing House; Beijing: 2017. pp. 307–308. [Google Scholar]
- Taguchi et al. (2016).Taguchi K, Takaku M, Egner PA, Morita M, Kaneko T, Mashimo T, Kensler TW, Yamamoto M. Generation of a new model rat: Nrf2 knockout rats are sensitive to Aflatoxin B1 toxicity. Toxicological Sciences. 2016;152:40–52. doi: 10.1093/toxsci/kfw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theumer et al. (2003).Theumer MG, Lopez AG, Masih DT, Chulze SN, Rubinstein HR. Immunobiological effects of AFB1 and AFB1-FB1 mixture in experimental subchronic mycotoxicoses in rats. Toxicology. 2003;186:159–170. doi: 10.1016/s0300-483x(02)00603-0. [DOI] [PubMed] [Google Scholar]
- Verheecke, Liboz & Mathieu (2016).Verheecke C, Liboz T, Mathieu F. Microbial degradation of aflatoxin B1: current status and future advances. International Journal of Food Microbiology. 2016;237:1–9. doi: 10.1016/j.ijfoodmicro.2016.07.028. [DOI] [PubMed] [Google Scholar]
- Wang & Groopman (1999).Wang JS, Groopman JD. DNA damage by mycotoxins. Mutation Research. 1999;424:167–181. doi: 10.1016/s0027-5107(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2018b).Wang H, Li W, Muhammad I, Sun X, Cui X, Cheng P, Qayum A, Zhang X. Biochemical basis for the age-related sensitivity of broilers to aflatoxin B1. Toxicology Mechanisms and Methods. 2018b;28:361–368. doi: 10.1080/15376516.2018.1428258. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2018a).Wang J, Mukhtar H, Ma L, Pang Q, Wang X. VHH Antibodies: reagents for mycotoxin detection in food products. Sensors. 2018a;18:485. doi: 10.3390/s18020485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2016a).Wang J, Tang L, Glenn TC, Wang JS. Aflatoxin B1 induced compositional changes in gut microbial communities of male F344 rats. Toxicological Sciences. 2016a;150:54–63. doi: 10.1093/toxsci/kfv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang WJ, Xu ZL, Yu C, Xu XH. Effects of aflatoxin B1 on mitochondrial respiration, ROS generation and apoptosis in broiler cardiomyocytes. Journal of Animal Science. 2017;88:1561–1568. doi: 10.1111/asj.12796. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2016b).Wang Y, Zhang Z, Wang H, Zhang Y, Ji M, Xu H, Wang C, Sun Z, Gao W, Wang SL. miR-138-1* regulates aflatoxin B1-induced malignant transformation of BEAS-2B cells by targeting PDK1. Archives of Toxicology. 2016b;90:1239–1249. doi: 10.1007/s00204-015-1551-4. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2018c).Wang F, Zuo Z, Chen K, Gao C, Yang Z, Zhao S, Li J, Song H, Peng X, Fang J, Cui H, Ouyang P, Zhou Y, Shu G, Jing B. Histopathological injuries, ultrastructural changes, and depressed TLR expression in the small intestine of broiler chickens with Aflatoxin B(1) Toxins. 2018c;10:131. doi: 10.3390/toxins10040131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng et al. (2017).Weng MW, Lee HW, Choi B, Wang HT, Hu Y, Mehta M, Desai D, Amin S, Zheng Y, Tang MS. AFB1 hepatocarcinogenesis is via lipid peroxidation that inhibits DNA repair, sensitizes mutation susceptibility and induces aldehyde-DNA adducts at p53 mutational hotspot codon 249. Oncotarget. 2017;8:18213–18226. doi: 10.18632/oncotarget.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild et al. (1991).Wild CP, Rasheed FN, Jawla MF, Hall AJ, Jansen LA, Montesano R. In-utero exposure to aflatoxin in west Africa. The Lancet. 1991;337:1602. doi: 10.1016/0140-6736(91)93295-k. [DOI] [PubMed] [Google Scholar]
- Wong & Hsieh (1976).Wong JJ, Hsieh DP. Mutagenicity of aflatoxins related to their metabolism and carcinogenic potential. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:2241–2244. doi: 10.1073/pnas.73.7.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2001).Xu XR, Huang J, Xu ZG, Qian BZ, Zhu ZD, Yan Q, Cai T, Zhang X, Xiao HS, Qu J, Liu F, Huang QH, Cheng ZH, Li NG, Du JJ, Hu W, Shen KT, Lu G, Fu G, Zhong M, Xu SH, Gu WY, Huang W, Zhao XT, Hu GX, Gu JR, Chen Z, Han ZG. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:15089–15094. doi: 10.1073/pnas.241522398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2021).Xu F, Li Y, Cao Z, Zhang J, Huang W. AFB1-induced mice liver injury involves mitochondrial dysfunction mediated by mitochondrial biogenesis inhibition. Ecotoxicology and Environmental Safety. 2021;216:112213. doi: 10.1016/j.ecoenv.2021.112213. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2020).Xu Q, Shi W, Lv P, Meng W, Mao G, Gong C, Chen Y, Wei Y, He X, Zhao J, Han H, Sun M, Xiao K. Critical role of caveolin-1 in aflatoxin B1-induced hepatotoxicity via the regulation of oxidation and autophagy. Cell Death & Disease. 2020;11:6. doi: 10.1038/s41419-019-2197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasin, Mazdak & Mino (2018).Yasin M, Mazdak R, Mino I. Aflatoxin B1 impairs spermatogenesis: an experimental study for crosslink between oxidative stress and mitochondria-dependent apoptosis. Environmental Toxicology. 2018;33:1204–1213. doi: 10.1002/tox.22627. [DOI] [PubMed] [Google Scholar]
- Yin et al. (2016).Yin H, Jiang M, Peng X, Cui H, Zhou Y, He M, Zuo Z, Ouyang P, Fan J, Fang J. The molecular mechanism of G2M cell cycle arrest induced by AFB1 in the jejunum. Oncotarget. 2016;7:35592–35606. doi: 10.18632/oncotarget.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip et al. (2017).Yip KY, Wan MLY, Wong AST, Korach KS, El-Nezami H. Combined low-dose zearalenone and aflatoxin B1 on cell growth and cell-cycle progression in breast cancer MCF-7 cells. Toxicology Letters. 2017;281:139–151. doi: 10.1016/j.toxlet.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan et al. (2016).Yuan S, Wu B, Yu Z, Fang J, Liang N, Zhou M, Huang C, Peng X. The mitochondrial and endoplasmic reticulum pathways involved in the apoptosis of bursa of Fabricius cells in broilers exposed to dietary aflatoxin B1. Oncotarget. 2016;7:65295–65306. doi: 10.18632/oncotarget.11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus et al. (2011).Yunus AW, Ghareeb K, Abd-El-Fattah AA, Twaruzek M, Bohm J. Gross intestinal adaptations in relation to broiler performance during chronic aflatoxin exposure. Poultry Science. 2011;90:1683–1689. doi: 10.3382/ps.2011-01448. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang NY, Qi M, Gao X, Zhao L, Liu J, Gu CQ, Song WJ, Krumm CS, Sun LH, Qi DS. Response of the hepatic transcriptome to aflatoxin B1 in ducklings. Toxicon. 2016;111:69–76. doi: 10.1016/j.toxicon.2015.12.022. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2019b).Zhang Z, Tang D, Wang B, Wang Z, Liu M. Analysis of miRNA-mRNA regulatory network revealed key genes induced by aflatoxin B1 exposure in primary human hepatocytes. Molecular Genetics & Genomic Medicine. 2019b;7:e971. doi: 10.1002/mgg3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2011).Zhang L, Ye Y, An Y, Tian Y, Wang Y, Tang H. Systems responses of rats to aflatoxin B1 exposure revealed with metabonomic changes in multiple biological matrices. Journal of Proteome Research. 2011;10:614–623. doi: 10.1021/pr100792q. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2019a).Zhang LY, Zhan DL, Chen YY, Wang WH, He CY, Lin Y, Lin YC, Lin ZN. Aflatoxin B1 enhances pyroptosis of hepatocytes and activation of Kupffer cells to promote liver inflammatory injury via dephosphorylation of cyclooxygenase-2: an in vitro, ex vivo and in vivo study. Archives of Toxicology. 2019a;93:3305–3320. doi: 10.1007/s00204-019-02572-w. [DOI] [PubMed] [Google Scholar]
- Zheng et al. (2017).Zheng Z, Zuo Z, Zhu P, Wang F, Yin H, Peng X, Fang J, Cui H, Gao C, Song H, Ouyang P, Zhou Y, Zhao S. A study on the expression of apoptotic molecules related to death receptor and endoplasmic reticulum pathways in the jejunum of AFB1-intoxicated chickens. Oncotarget. 2017;8:89655–89664. doi: 10.18632/oncotarget.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2019).Zhou X, Gan F, Hou L, Liu Z, Su J, Lin Z, Le G, Huang K. Aflatoxin B1 induces immunotoxicity through the DNA methyltransferase-mediated JAK2/STAT3 pathway in 3D4/21 cells. Journal of Agricultural and Food Chemistry. 2019;67:3772–3780. doi: 10.1021/acs.jafc.8b07309. [DOI] [PubMed] [Google Scholar]
- Zhou, Tang & Wang (2021).Zhou J, Tang L, Wang JS. Aflatoxin B1 induces gut-inflammation-associated fecal lipidome changes in F344 rats. Toxicological Sciences. 2021;183(2):363–377. doi: 10.1093/toxsci/kfab096. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2015).Zhu L, Gao J, Huang K, Luo Y, Zhang B, Xu W. miR-34a screened by miRNA profiling negatively regulates Wnt/beta-catenin signaling pathway in Aflatoxin B1 induced hepatotoxicity. Scientific Reports. 2015;5:16732. doi: 10.1038/srep16732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Tan & Leung (2016).Zhu Y, Tan YQ, Leung LK. Aflatoxin B1 disrupts transient receptor potential channel activity and increases COX-2 expression in JEG-3 placental cells. Chemico-Biological Interactions. 2016;260:84–90. doi: 10.1016/j.cbi.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Zuberi et al. (2019).Zuberi Z, Eeza MNH, Matysik J, Berry JP, Alia A. NMR-based metabolic profiles of intact zebrafish embryos exposed to Aflatoxin B1 recapitulates hepatotoxicity and supports possible neurotoxicity. Toxins. 2019;11(5):258. doi: 10.3390/toxins11050258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The number list of articles searched via databases.
Data Availability Statement
The following information was supplied regarding data availability:
Raw data are available in the Supplementary Files.