Abstract
Objective
To evaluate the rapid diagnostic accuracy of Mycobacterium tuberculosis RNA (TB-RNA) for pulmonary tuberculosis (PTB) in a large patient sample and to evaluate the difference in TB-RNA diagnostic accuracy in various respiratory specimens.
Methods
Patient medical records were retrospectively reviewed to determine the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) of the acid-fast bacillus (AFB) smear and TB-RNA to evaluate their diagnostic accuracy against final clinical diagnosis.
Results
Of the 2336 patients ultimately included, 1123 provided 1 sputum specimen each and 1213 provided 1 bronchoalveolar lavage fluid (BALF) specimen each. The overall sensitivity, specificity, PPV, NPV, and AUC of the AFB smear were 36.2%, 86.4%, 90.6%, 27.3%, and 0.61, respectively. The overall sensitivity, specificity, PPV, NPV, and AUC of TB-RNA for the rapid detection of PTB were 57.4%, 99.4%, 99.7%, 39.3%, and 0.78, respectively. When sputum and BALF specimens were used for AFB smear testing, the sensitivity, specificity, PPV, NPV, and AUC of the AFB smear were 44.5%, 81.5%, 87.5%, 33.5%, and 0.63; and 29.2%, 92.7%, 94.8%, 22.5%, and 0.61, respectively. The sensitivity, specificity, PPV, NPV, and AUC of TB-RNA for the rapid detection of PTB using sputum were 49.6%, 99.3%, 99.5%, 40.4%, and 0.74, respectively; whereas those of TB-RNA determined using BALF were 63.9%, 99.5%, 99.8%, 38.0%, and 0.82, respectively.
Conclusion
The diagnostic accuracy of TB-RNA for PTB was moderate and considerably better than that of the AFB smear. The diagnostic accuracy of TB-RNA for various respiratory specimens differed; the diagnostic accuracy of TB-RNA for BALF specimens was substantially better than that for sputum samples, and BALF specimens were more suitable for TB-RNA.
Keywords: tuberculosis RNA, pulmonary tuberculosis, bronchoalveolar lavage fluid, sputum, accuracy
Introduction
Tuberculosis (TB) is caused by Mycobacterium tuberculosis (Mtb), which infects humans and poses a major threat to human health;1 it is 1 of the top 10 causes of death from a single infectious disease worldwide.2 Based on whether Mtb infection involves the lungs, TB can be divided into pulmonary TB (PTB) and extrapulmonary TB (EPTB), of which the primary disease is PTB.3 PTB, because of the specificity of its infected organ (the lung is an open organ that is connected to the outside world through the airway), allows Mtb to be expelled from the lungs via coughing and the sputum, and its dissemination makes PTB the primary infectious source of TB transmission.4 Most EPTB cases are closed, with the exception of a few cases of Mtb dissemination due to factors such as abscess rupture. EPTB is also less infectious.5 Therefore, the control of TB is mainly dependent on the control of PTB.6 The first priority for PTB control is the early and rapid diagnosis of PTB so that effective antituberculosis treatment can be quickly administered to reduce the rate of transmission, thereby effectively controlling the source of infection and reducing disease transmission.7 The rapid diagnosis of PTB depends on the rapid detection of Mtb. Although a conventional AFB smear is the most widely utilized clinical means of Mtb detection,8 it has shortcomings: first, its diagnostic sensitivity is low, which can lead to misdiagnosis; second, although the method is simple and convenient, experienced microscopists are still the key to its diagnosis, and considering that many primary hospitals do not have specialized microscopists to conduct this test, it could result in reduced sensitivity.9,10 Although culture is another classic Mtb detection method, it is of limited use for the early and rapid diagnosis of TB because several weeks are required to obtain results using cultures; during this time, untreated patients with PTB could transmit the disease, which is thus not conducive to TB control.11
In recent years, with the development of molecular diagnostics, molecular tests have been widely used for the early and rapid diagnosis of TB,12 as well as for the rapid diagnosis of PTB.13 Although most current molecular assays target Mtb DNA,14,15 research on molecular assays that target Mtb RNA has been limited.16 Shanghai Rendu Biotechnology Co has developed a TB-RNA detection technology that involves simultaneous amplification and testing for the detection of Mtb RNA by employing isothermal 16S ribosomal RNA (rRNA) amplification.17 This TB-RNA assay has been used for both PTB and EPTB and has shown a relatively good diagnostic accuracy;18,19 however, the total number of studies remains limited. For the rapid diagnosis of PTB, most current studies have used only a single type of respiratory specimen (sputum or bronchoalveolar lavage fluid (BALF)) for evaluating a patient cohort of a limited size,20 and research on the differences in diagnostic efficacy of TB-RNA in various respiratory specimens is extremely limited. The diagnostic accuracy of the relevant tests in terms of different respiratory specimens may vary. Elucidating the differences in diagnostic accuracy of TB-RNA in various specimens can guide the choice of a practical clinical application to achieve optimal diagnostic efficacy and reduce the possibility of misdiagnosis. Therefore, this study aimed to further evaluate the rapid diagnostic accuracy of TB-RNA for PTB using a large patient sample and evaluate the differences in diagnostic accuracy of TB-RNA in various respiratory specimens to provide a reference for the selection of clinical specimens.
Materials and Methods
Study Design
This was a retrospective study conducted at the Zhejiang Tuberculosis Diagnosis and Treatment Center, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine. Patients with untreated suspected PTB who were hospitalized at our center from September 2018 to January 2020 were considered eligible for inclusion in this study, and such patients were screened by retrieving their relevant medical records from the electronic medical record system. Patients who met the following criteria were regarded as those with suspected PTB: 1) TB-related clinical symptoms such as cough and fever; 2) X-ray images indicating radiological changes such as lung tree-in-bud pattern and cavities; 3) positive results obtained for a tuberculin purified protein derivative (PPD) test and/or gamma interferon release test; and 4) no definite infection with other pathogens in the lungs.
Fresh respiratory specimens (BALF and sputum) were collected for the acid-fast bacillus (AFB) smear, culture, and TB-RNA assay. Patients who underwent all three tests simultaneously were included; those who did not undergo the relevant tests simultaneously or whose test results were unclear were excluded. BALF specimens were obtained by lavage of the lung lesion site through fiberoptic bronchoscopy. Written informed consent was obtained from all patients or their guardians and approval obtained from the Human Research Ethics Committee of the Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine. This study complied with the Declaration of Helsinki.
The final clinical diagnosis was utilized as the reference standard based on the Health Industry Standard of the People’s Republic of China — Diagnosis for pulmonary tuberculosis (WS 288–2017).21 A “confirmed” PTB diagnosis was based on the presence of Mtb in the respiratory specimens or in the lung tissue by employing Mycobacterium culture or molecular tests (such as Xpert MTB/RIF). A “probable” PTB diagnosis was made when typical imaging presentations were noted with a positive PPD test and/or gamma interferon release assay, or when PTB imaging presentations were combined with confirmed EPTB at other sites. A “non-PTB” diagnosis included the following: when there was no evidence of Mtb infection, other clear pathogenic infections, or if the patient had recovered from the disease without receiving anti-TB treatment. In this study, confirmed and probable PTB were considered a clinical diagnosis of PTB.
AFB Smear and Culture
Fresh BALF and sputum specimens were utilized for the AFB smear and culture. The specimens were digested and decontaminated with N-acetyl-l-cysteine–NaOH; they were then subjected to direct smear microscopy with auramine O, a fluorescent dye. Results were confirmed by Ziehl–Neelsen staining, based on the World Health Organization guidelines.22
Solid and liquid culture methods were used, wherein a solid Lowenstein–Jensen medium (Encode Medical Engineering Co., Ltd., China) was used for the solid culture and the BACTEC MGIT 960 Mycobacteria Culture System (BD Diagnostic Systems, Sparks, MD, USA) was used for the liquid culture.23
Mtb RNA
The TB-RNA assay (Shanghai Rendu Biotechnology, China) was performed according to the manufacturer’s instructions. The sputum and BALF specimens were digested and decontaminated with N-acetyl-l-cysteine–NaOH for 15 min, and the specimens were centrifuged at 13,000 × g for 5 min and the supernatant discarded. Approximately 50 µL dilution solution was added to the sediment and placed in a water bath sonicator (Shanghai Shengyan Ultrasound Machines, China) for 15 min. The mixture was centrifuged to obtain the supernatant, which was used for the simultaneous isothermal amplification of RNA. Approximately 2 µL of the supernatant was taken and 30 µL of reaction solution was added to it; this solution was added to a polymerase chain reaction (PCR) tube and pre-incubated at 60° for 10 min, followed by incubation at 42° for 5 min. Next, 2000 units of Moloney murine leukemia virus reverse transcriptase and 2000 units of T7 RNA polymerase (RD Bioscience, Inc., San Diego, CA, USA) were added to the pre-incubated mixture. A fluorescence quantitative real-time PCR instrument (SLAN-96S Real-Time PCR System ZEESAN Xiamen CN) was employed for 16S rRNA amplification, and the final mixed solution obtained previously was used for analysis. The test results were then interpreted according to the product manual.24
Data Processing and Statistical Analysis
SPSS 24.0 (IBM Corp., Armonk, NY, USA) and MedCalc statistical software v15.2.2 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org) were used as the primary software for the study’s data analysis. SPSS was used to calculate the mean, standard deviation, and frequency to obtain the four values for cross-tabulation of data obtained via the diagnostic tests: true positive, false positive, false negative, and true negative. MedCalc statistical software was used to calculate the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) with 95% confidence interval of the related tests. McNemar’s test was used for comparing paired data, the chi-squared test or Fisher’s exact test for comparing proportions, and the Z-test for comparing AUCs. Venn diagrams (http://www.bioinformatics.com.cn/static/others/jvenn/example.html) were generated through an interactive tool for comparing lists.25 P values <0.05 for differences between the two groups were considered statistically significant.
Results
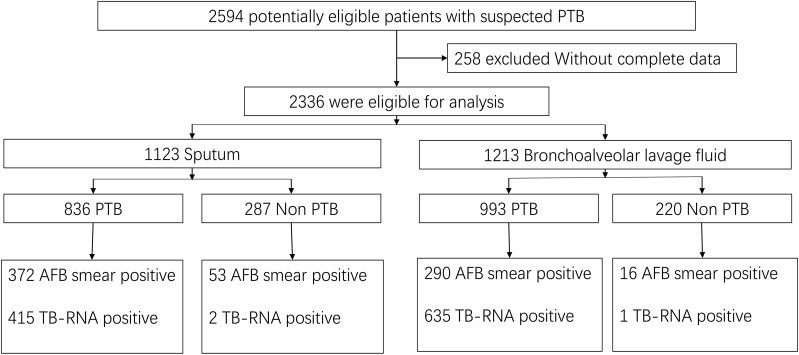
In total, 2594 potentially eligible patients with suspected PTB were screened initially. After excluding 258 patients with incomplete data, 2336 patients were ultimately included, of whom 1123 provided 1 sputum specimen each and 1213 provided 1 BALF specimen each (Figure 1). The mean age of all patients was 51.0±18.1 years, and 66.0% were men. The mean age of the patients who provided sputum specimens was 50.7±18.1 years, and 69.7% were men. The mean age of patients providing BALF specimens was 51.6±18.3 years, and 62.5% were men.
Figure 1.
Diagnostic classification of the included pulmonary tuberculosis in various respiratory specimens.
The diagnostic classification of the included patients is shown in Figure 1. Among the sputum specimens, 836 patients were diagnosed with PTB, 372 of whom had a positive AFB smear and 415 of whom had positive TB-RNA. Of the 287 patients with non-PTB, 53 had a positive AFB smear (the final culture was non-tuberculous mycobacteria (NTM)) and 2 had positive TB-RNA (no other evidence of TB, negative on retest, and recovered without antituberculosis treatment). Among the BALF specimens, 993 patients were diagnosed with PTB, 290 of whom had a positive AFB smear and 635 were positive for TB-RNA. Of the 220 patients with non-PTB, 16 had a positive AFB smear (the final culture was NTM) and 1 was positive for TB-RNA (no other evidence of TB, negative on retest, and recovered without antituberculosis treatment).
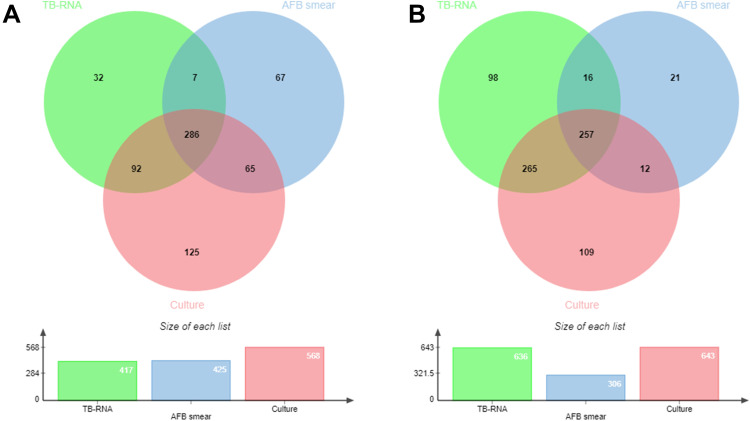
The distribution and overlap of positive AFB smear, culture, and TB-RNA in the sputum and BALF specimens are shown in Figure 2.
Figure 2.
Venn diagram of positive tests in the included pulmonary tuberculosis patient in various respiratory specimens. (A) sputum; (B) bronchoalveolar lavage fluid.
Diagnostic Accuracy of AFB Smear and TB-RNA
When clinical diagnosis was considered as the reference standard, the overall sensitivity, specificity, PPV, NPV, and AUC of the AFB smear for rapid PTB diagnosis were found to be 36.2% (34.0%–38.5%), 86.4% (83.1%–89.3%), 90.6% (88.2%–92.6%), 27.3% (25.1%–29.5%), and 0.61 (0.59–0.63), respectively; those for rapid TB-RNA diagnosis were 57.4% (55.1%–59.7%), 99.4% (98.3%–99.9%), 99.7% (99.2%–99.9%), 39.3% (36.6%–42.0%), and 0.78 (0.77–0.80), respectively. These results are summarized in detail in Table 1.
Table 1.
The Diagnostic Accuracy of AFB Smear and TB-RNA for Rapid Pulmonary Tuberculosis Diagnosis in Various Respiratory Specimens
| Test | Total PTB | Sputum | BALF | P value |
|---|---|---|---|---|
| AFB smear | ||||
| Sensitivity (%) | 36.2 (34.0–38.5) | 44.5 (41.1–47.9) | 29.2 (26.4–32.1) | < 0.001 |
| Specificity (%) | 86.4 (83.1–89.3) | 81.5 (76.6–85.9) | 92.7 (88.5–95.8) | < 0.001 |
| PPV (%) | 90.6 (88.2–92.6) | 87.5 (84.0–90.5) | 94.8 (91.7–97.0) | 0.001 |
| NPV (%) | 27.3 (25.1–29.5) | 33.5 (30.0–37.2) | 22.5 (19.8–25.4) | < 0.001 |
| AUC | 0.61 (0.59–0.63) | 0.63 (0.60–0.66) | 0.61 (0.58–0.64) | 0.445 |
| TB RNA | ||||
| Sensitivity (%) | 57.4 (55.1–59.7) | 49.6 (46.2–53.1) | 63.9 (60.9–66.9) | < 0.001 |
| Specificity (%) | 99.4 (98.3–99.9) | 99.3 (97.5–99.9) | 99.5 (97.5–100.0) | 0.724 |
| PPV (%) | 99.7 (99.2–99.9) | 99.5 (98.3–99.9) | 99.8 (99.1–100.0) | 0.337 |
| NPV (%) | 39.3 (36.6–42.0) | 40.4 (36.7–44.1) | 38.0 (34.0–42.1) | 0.379 |
| AUC | 0.78 (0.77–0.80)* | 0.74 (0.72–0.77)* | 0.82 (0.79–0.84)* | < 0.001 |
Notes: P values represent the difference between sputum and BALF. The difference between TB-RNA and AFB smear, *P < 0.001.
Abbreviations: PTB, pulmonary tuberculosis; BALF, bronchoalveolar lavage fluid; AFB, acid-fast bacillus; TB-RNA, Mycobacterium tuberculosis RNA; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve.
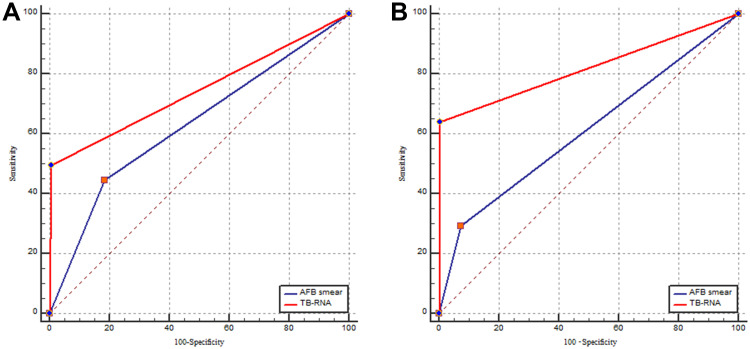
When sputum was used for the tests, the sensitivity, specificity, PPV, NPV, and AUC of the AFB smear were 44.5% (41.1%–47.9%), 81.5% (76.6%–85.9%), 87.5% (84.0%–90.5%), 33.5% (30.0%–37.2%), and 0.63 (0.60–0.66), respectively. Those values for TB-RNA were 49.6% (46.2%–54.3%), 99.3% (97.5%–99.9%), 99.5% (98.3%–99.9%), 40.4% (36.7%–44.1%), and 0.74 (0.72–0.77), respectively (Table 1). The receiver operating characteristic curves for the AFB smear and the TB-RNA assay using sputum and BALF specimens are shown in Figure 3.
Figure 3.
The receiver operating characteristic curves for the AFB smear and the TB-RNA in various respiratory specimens. (A) sputum; (B) bronchoalveolar lavage fluid.
When BALF was used for the tests, the sensitivity, specificity, PPV, NPV, and AUC of the AFB smear were 29.2% (26.4%–32.1%), 92.7% (88.5%–95.8%), 94.8% (91.7%–97.0%), 22.5% (19.8%–25.4%), and 0.61 (0.58–0.64), respectively; those for the TB-RNA were 63.9% (60.9%–66.9%), 99.5% (97.5%–100.0%), 99.8% (99.1%–100.0%), 38.0% (34.0%–42.1%), and 0.82 (0.79–0.84), respectively (Table 1).
For culture-negative PTB, the sensitivity, specificity, PPV, NPV, and AUC of the AFB smear were found to be 6.8% (4.9%–9.1%), 86.4% (83.1%–89.3%), 37.8% (28.8%–47.5%), 43.2% (40.1%–46.3%), and 0.47 (0.44–0.50), respectively; those for rapid TB-RNA diagnosis were 24.3% (20.9%–27.9%), 99.4% (98.3%–99.9%), 98.0% (94.4%–99.6%), 51.9% (48.7%–55.0%), and 0.62 (0.59–0.65), respectively.
Comparison of the Diagnostic Accuracy of the AFB Smear and TB-RNA
The accuracy of TB-RNA for the rapid diagnosis of PTB was significantly better than that of the AFB smear, regardless of the type of specimen used for testing (P < 0.05). When the same test was performed using various specimens, the diagnostic accuracy of the AFB smear using sputum and BALF was similar, with no statistically significant difference (P > 0.05, Table 1), whereas the accuracy of the TB-RNA using BALF was significantly better than that using sputum, with a statistically significant difference (P < 0.05).
Discussion
The early and rapid diagnosis of PTB remains challenging. Rapid PTB diagnosis allows for timely antituberculosis treatment, thereby reducing respiratory transmission, accelerating TB control, and improving the prognosis of patients with PTB.26 The most commonly used specimens for PTB diagnosis are sputum and BALF. Sputum can be obtained by coughing and is the most convenient specimen type that can be acquired in a noninvasive manner for PTB diagnosis. In patients with no sputum or scanty sputum, or for patients in whom presence of endobronchial lesions needs to be excluded despite a positive sputum AFB smear, fiberoptic bronchoscopy is typically performed to obtain a BALF specimen by lavage at the site of the lung lesion.27,28 Fiberoptic bronchoscopy is becoming increasingly important in the diagnosis of PTB, not only for lavage but also for biopsy.29 This approach is extremely safe (complication rate <0.1%–11% and mortality rate 0%–0.1%) and patients can cooperate positively,27 especially after introducing a painless approach for the same. It is generally accepted that BALF has some advantages over sputum: it may contain a higher bacterial content and be of better quality than sputum and is less susceptible to contamination.30 BALF could be a more reliable respiratory specimen, and the results of previous studies have shown that BALF has good diagnostic accuracy for PTB.31
The rapid diagnosis of PTB currently relies on AFB smear and molecular tests. The results of this study showed that the overall sensitivity, specificity, PPV, NPV, and AUC of AFB smear were 36.2%, 86.4%, 90.6%, 27.3%, and 0.61, respectively. The diagnostic accuracy of the AFB smear for the rapid diagnosis of PTB was shown to be extremely low, especially the sensitivity and the NPV. These results are consistent with those of previous related studies.32 An AFB smear cannot differentiate between Mtb and NTM infections because both bacteria show positive antacid staining.13 In this study, 69 patients were found to have a positive AFB smear; the final culture results showed the presence of NTM, suggesting a high probability of a positive AFB smear for Mtb infection. However, NTM infection cannot be excluded. If no other tests are available, anti-TB treatment is acceptable; but improving the accuracy of the diagnosis is still recommended using relevant molecular tests. The fact that all 69 patients tested negative for TB-RNA was indicative of an NTM infection, which required focused attention. Culture-negative PTB is one of the major problems in the diagnosis of this disease. Our study showed that the diagnostic efficacy of either AFB smear or TB-RNA assay for culture-negative PTB is still low, and more efficient detection methods for culture-negative PTB still need to be further explored.
When sputum and BALF specimens were used for AFB smear testing, the sensitivity, specificity, PPV, NPV, and AUC of the AFB smear were 44.5%, 81.5%, 87.5%, 33.5%, 0.63, and 29.2%, 92.7%, 94.8%, 22.5%, and 0.61, respectively. No significant differences were observed in the diagnosis of PTB by AFB smear in various respiratory specimens. This could be because the comparison of the two specimens in this study was not head-to-head and because both specimens were not obtained from the same patient for comparison. Fiberoptic bronchoscopy was mainly performed in patients without sputum or in patients who needed to exclude endobronchial tuberculosis. In addition, some patients with a positive sputum AFB smear did not undergo fiberoptic bronchoscopy, which could explain the low sensitivity of the AFB smear in BALF. The diagnostic accuracy of the AFB smear performed in either sputum or BALF was unsatisfactory and did not meet the standard required for rapid diagnosis.
The overall sensitivity, specificity, PPV, NPV, and AUC of TB-RNA for PTB rapid diagnosis were 57.4%, 99.4%, 99.7%, 39.3%, and 0.78, respectively. The overall diagnostic accuracy was moderate, which was similar to that noted in previous studies. The sensitivity, specificity, PPV, NPV, and AUC of TB-RNA detection using sputum were 49.6%, 99.3%, 99.5%, 40.4%, and 0.74, respectively; whereas these values for TB-RNA detection using BALF were 63.9%, 99.5%, 99.8%, 38.0%, and 0.82, respectively. Compared with sputum, TB-RNA testing using BALF showed a considerable improvement in diagnostic accuracy. Previous studies have shown good diagnostic efficacy for TB-RNA even in smear-negative PTB using BALF specimens, and the improved detection capability of TB-RNA by PCR amplification, coupled with the potentially higher bacterial BALF load, resulted in superior results for TB-RNA using BALF. TB-RNA studies applied to the rapid diagnosis of PTB showed results that were generally consistent with those of the present study; however, most previous studies utilized smaller cohort sizes and did not compare the differences in the diagnostic efficacy of TB-RNA in various respiratory specimens.17,33 The present study used a large sample size, which reduces bias and makes the results more robust and credible. The differences in TB-RNA in various specimens were also compared, which provides more information for clinical practice. Using BALF for TB-RNA testing was more appropriate and could provide better results.
The present study has some limitations. First, as a retrospective study, patient selection bias was inevitable Second, given that the comparison of the two types of respiratory specimens was not head-to-head, there could have been selection bias; thus, the results should be interpreted cautiously, and head-to-head studies are warranted to further confirm the results. Finally, the study was conducted in a high-TB burden region and application of the results to other regions could vary.
Conclusions
The diagnostic accuracy of TB-RNA for PTB was moderate and substantially better than that of the AFB smear. The diagnostic accuracy of TB-RNA in various respiratory specimens differed, and its accuracy in BALF was significantly better than that in sputum. BALF specimens were more suitable for TB-RNA testing, which could improve the detection rate.
Acknowledgments
We are grateful to all the patients and their families included in this study and also to our colleagues in this unit for their help.
Funding Statement
Xiaowei Qiu, 20191203B133, Hangzhou Science and Technology Bureau, http://kj.hangzhou.gov.cn. The founders did not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Sharing Statement
Data will be made available on reasonable request from corresponding authors.
Ethics Approval and Consent to Participate
All patients gave written informed consent and the study was approved by the Human Research Ethics Committee of Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine. This study complies with the Declaration of Helsinki.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
- 1.World Health Organization. Global Tuberculosis Report 2021. World Health Organization; 2021. [Google Scholar]
- 2.Ahmad M, Ibrahim WH, Sarafandi SA, et al. Diagnostic value of bronchoalveolar lavage in the subset of patients with negative sputum/smear and mycobacterial culture and a suspicion of pulmonary tuberculosis. Int J Infect Dis. 2019;82:96–101. [DOI] [PubMed] [Google Scholar]
- 3.Thangavelu K, Krishnakumariamma K, Pallam G, et al. Prevalence and speciation of non-tuberculous mycobacteria among pulmonary and extrapulmonary tuberculosis suspects in South India. J Infect Public Health. 2021;14(3):320–323. [DOI] [PubMed] [Google Scholar]
- 4.Malede A, Taye B, Legesse M, Debie A, Shibabaw A. Pulmonary tuberculosis preventive practices among Anibessa Bus users at Addis Ababa, Ethiopia: a cross-sectional study. BMC Res Notes. 2019;12(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang W, Yu J, Du J, et al. The epidemiology of extrapulmonary tuberculosis in China: a large-scale multi-center observational study. PLoS One. 2020;15(8):e0237753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biadglegne F, Schmidt JR, Engel KM, et al. Mycobacterium tuberculosis Affects Protein and Lipid Content of Circulating Exosomes in Infected Patients Depending on Tuberculosis Disease State. Biomedicines. 2022;10(4):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boardman NJ, Moore T, Freiman J, et al. Pulmonary Tuberculosis Disease Among Immigrant Detainees: rapid Disease Detection, High Prevalence of Asymptomatic Disease, and Implications for Tuberculosis Prevention. Clin Infect Dis. 2021;73(1):115–120. [DOI] [PubMed] [Google Scholar]
- 8.Luetkemeyer AF, Firnhaber C, Kendall MA, et al. Evaluation of Xpert MTB/RIF Versus AFB Smear and Culture to Identify Pulmonary Tuberculosis in Patients With Suspected Tuberculosis From Low and Higher Prevalence Settings. Clin Infect Dis. 2016;62(9):1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fachri M, Hatta M, Abadi S, et al. Comparison of acid fast bacilli (AFB) smear for Mycobacterium tuberculosis on adult pulmonary tuberculosis (TB) patients with type 2 diabetes mellitus (DM) and without type 2 DM. Respir Med Case Rep. 2018;23:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu G, Shen Y, Ye B, Chen D, Xu K. Comparison of CapitalBio™ Mycobacterium nucleic acid detection test and Xpert MTB/RIF assay for rapid diagnosis of extrapulmonary tuberculosis. J Microbiol Methods. 2020;168:105780. [DOI] [PubMed] [Google Scholar]
- 11.Sun L, Yao L, Fu G, Lin L, Zhu E, Huang J. A comparison of the accuracy of the CapitalBio Mycobacterium real-time polymerase chain reaction and the Xpert MTB/RIF assay for the diagnosis of tuberculous meningitis. Int J Infect Dis. 2021;104:92–96. [DOI] [PubMed] [Google Scholar]
- 12.Yu G, Wang X, Zhu P, Shen Y, Zhao W, Zhou L. Comparison of the efficacy of metagenomic next-generation sequencing and Xpert MTB/RIF in the diagnosis of tuberculous meningitis. J Microbiol Methods. 2021;180:106124. [DOI] [PubMed] [Google Scholar]
- 13.Shen Y, Fang L, Xu X, Ye B, Yu G. CapitalBio Mycobacterium real-time polymerase chain reaction detection test: rapid diagnosis of Mycobacterium tuberculosis and nontuberculous mycobacterial infection. Int J Infect Dis. 2020;98:1–5. [DOI] [PubMed] [Google Scholar]
- 14.Yu G, Shen Y, Ye B, Shi Y. Diagnostic accuracy of Mycobacterium tuberculosis cell-free DNA for tuberculosis: a systematic review and meta-analysis. PLoS One. 2021;16(6):e0253658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu G, Wang L, Shen Y, et al. Comparison of the Diagnostic Accuracy of Xpert MTB/RIF and CapitalBio Mycobacterium RT-PCR Detection Assay for Tuberculous Pericarditis. Infect Drug Resist. 2022;15:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Zhang L, Hong L, et al. Rapid diagnosis of pulmonary tuberculosis and detection of drug resistance by combined simultaneous amplification testing and reverse dot blot. J Clin Pathol. 2018;71(6):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li QH, Zhang Y, Zhao MM, et al. Simultaneous amplification and testing method for Mycobacterium tuberculosis rRNA to differentiate sputum-negative tuberculosis from sarcoidosis. Am J Physiol Lung Cell Mol Physiol. 2019;316(3):L519–l524. [DOI] [PubMed] [Google Scholar]
- 18.Li T, Shi T, Sun Y, et al. Application of real-time simultaneous amplification and testing method to accurately and rapidly detect extra-pulmonary tuberculosis. BMC Infect Dis. 2020;20(1):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu C, Pan N, Zhou Y, et al. Application Value of SAT-TB Combined with Acid-Fast Staining in the Diagnosis and Treatment of Pulmonary Tuberculosis. Biomed Res Int. 2020;2020:3620425. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Yan L, Zhang Q, Xiao H. Clinical diagnostic value of simultaneous amplification and testing for the diagnosis of sputum-scarce pulmonary tuberculosis. BMC Infect Dis. 2017;17(1):545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Health Commission of the People’s Republic of China. Health Industry Standard of the People’s Republic of China - Diagnosis for pulmonary tuberculosis (WS 288-2017). Chin J Infection Control. 2018;17:642–652. [Google Scholar]
- 22.Yu G, Zhong F, Zhao W, Ye B, Xu K, Chen G. Head-to-head comparison of the diagnostic value of five tests for constrictive tuberculous pericarditis: five tests for constrictive TBP. Int J Infect Dis. 2022;120:25–32. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y, Fang L, Ye B, Xu X, Yu G, Zhou L. The Role of Core Needle Biopsy Pathology Combined with Molecular Tests in the Diagnosis of Lymph Node Tuberculosis. Infect Drug Resist. 2022;15:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan L, Xiao H, Zhang Q. Using simultaneous amplification and testing method for evaluating the treatment outcome of pulmonary tuberculosis. BMC Infect Dis. 2018;18(1):512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinform. 2014;15(1):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebastian NM, Haveri SP, Nath AS. Delay in diagnosis of tuberculosis and related factors from a district in Kerala, India. Indian J Tuberc. 2021;68(1):59–64. [DOI] [PubMed] [Google Scholar]
- 27.Zheng H, Zhong F, Yu G, Shen Y. Comparison of the diagnostic efficacy of the CapitalBio Mycobacterium real-time polymerase chain reaction detection test and Xpert MTB/RIF in smear-negative pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis. 2021;40(5):969–977. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Chen Y, Ouyang H, et al. Tuberculosis Diagnosis by Metagenomic Next-generation Sequencing on Bronchoalveolar Lavage Fluid: a cross-sectional analysis. Int J Infect Dis. 2021;104:50–57. [DOI] [PubMed] [Google Scholar]
- 29.Mondoni M, Repossi A, Carlucci P, Centanni S, Sotgiu G. Bronchoscopic techniques in the management of patients with tuberculosis. Int J Infect Dis. 2017;64:27–37. [DOI] [PubMed] [Google Scholar]
- 30.Gowda NC, Ray A, Soneja M, Khanna A, Sinha S. Evaluation of Xpert(®)Mycobacterium tuberculosis/rifampin in sputum-smear negative and sputum-scarce patients with pulmonary tuberculosis using bronchoalveolar lavage fluid. Lung India. 2018;35(4):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu HC, Gao YL, Li DF, Zhao XY, Pan YQ, Zhu CT. Value of Xpert MTB/RIF Using Bronchoalveolar Lavage Fluid for the Diagnosis of Pulmonary Tuberculosis: a Systematic Review and Meta-analysis. J Clin Microbiol. 2021;59(4):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nsubuga R, Adrawa N, Okoboi S, Komuhangi A, Izudi J. Complete sputum smear monitoring among adults with pulmonary tuberculosis in central Uganda: evidence from a retrospective cohort study. BMC Infect Dis. 2022;22(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaikh A, Sriraman K, Vaswani S, Oswal V, Mistry N. Detection of Mycobacterium tuberculosis RNA in bioaerosols from pulmonary tuberculosis patients. Int J Infect Dis. 2019;86:5–11. [DOI] [PubMed] [Google Scholar]