Abstract
Background and Aims
Gastrointestinal (GI) symptoms are well-recognized manifestations of coronavirus disease 2019 (COVID-19). Our primary objective was to evaluate the association between GI symptoms and COVID-19 severity.
Methods
In this nationwide cohort of US veterans, we evaluated GI symptoms (nausea/vomiting/diarrhea) reported 30 days before and including the date of positive SARS-CoV-2 testing (March 1, 2020, to February 20, 2021). All patients had ≥1 year of prior baseline data and ≥60 days follow-up relative to the test date. We used propensity score (PS)-weighting to balance covariates in patients with vs without GI symptoms. The primary composite outcome was severe COVID-19, defined as hospital admission, intensive care unit admission, mechanical ventilation, or death within 60 days of positive testing.
Results
Of 218,045 SARS-CoV-2 positive patients, 29,257 (13.4%) had GI symptoms. After PS weighting, all covariates were balanced. In the PS-weighted cohort, patients with vs without GI symptoms had severe COVID-19 more often (29.0% vs 17.1%; P < .001). When restricted to hospitalized patients (14.9%; n=32,430), patients with GI symptoms had similar frequencies of intensive care unit admission and mechanical ventilation compared with patients without symptoms. There was a significant age interaction; among hospitalized patients aged ≥70 years, lower COVID-19–associated mortality was observed in patients with vs without GI symptoms, even after accounting for COVID-19–specific medical treatments.
Conclusion
In the largest integrated US health care system, SARS-CoV-2–positive patients with GI symptoms experienced severe COVID-19 outcomes more often than those without symptoms. Additional research on COVID-19–associated GI symptoms may inform preventive efforts and interventions to reduce severe COVID-19.
Keywords: COVID-19, Epidemiology, Infectious diseases, Outcomes, SARS-CoV-2
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection sparked an unprecedented global coronavirus disease 2019 (COVID-19) pandemic, infecting more than 511 million people and contributing to 6.2 million deaths, as of May 2022.1 With inadequate vaccination rates, high viral infectivity, treatment limitations, and iterative variants triggering new waves of disease, COVID-19 remains a leading cause of US deaths and continues to stress the health care system.2
Severe SARS-CoV-2 infection frequently presents with rapidly progressive respiratory and systemic symptoms. Yet, more than 20% of patients may present with gastrointestinal (GI) symptoms alone, including diarrhea, nausea, vomiting, and abdominal pain.3,4 A study from early in the pandemic identified that symptoms of diarrhea and nausea in patients testing positive for SARS-CoV-2 were significantly associated with an increased risk of hospitalization and mechanical ventilation.5 These early observations motivated investigations evaluating whether GI disease–focused phenotypes of SARS-CoV-2 infection might be associated with greater clinical severity. However, studies to date have yielded conflicting results.6, 7, 8, 9 A large population-based study could better inform the clinical management and triaging of patients presenting with GI symptoms and positive SARS-CoV-2 testing.
SARS-CoV-2 binds to the angiotensin-converting enzyme II receptor to infect cells. Angiotensin-converting enzyme II receptors are concentrated on type 2 alveolar cells and intestinal epithelial cells,6 with the small intestine exhibiting a 10-fold higher tissue concentration than the lungs.10 Viral replication occurs in the lung and GI tract; SARS-CoV-2 transmission is predominantly airborne and potentially via the fecal-oral route.7,11 SARS-CoV-2 replication within enterocytes12 may lead to a rapidly increased viral load, immune dysregulation, hyperacute inflammatory response, COVID-19–associated cytokine storm, and other modulating events, which may clinically manifest as severe COVID-19 disease outcomes (ie mechanical ventilation or death).13, 14, 15, 16 We hypothesized that the presenting GI symptoms of nausea, vomiting, or diarrhea at SARS-CoV-2 diagnosis may be associated with severe COVID-19 outcomes.
Methods
The Veterans Affairs Tennessee Valley Healthcare System Institutional Review Board and Research and Development Committees approved this study with a waiver of informed consent.
Data Sources and Cohort Construction
The Veterans Health Administration (VHA) is the largest integrated healthcare system in the US. For this study, we constructed a national retrospective veteran cohort that used the national VHA Observational Medical Outcomes Partnership (OMOP) database. OMOP integrates data from all VHA facilities, including veterans’ electronic health records, the Corporate Data Warehouse (CDW), the Veterans Affairs Informatics and Computing Infrastructure data warehouse, and pharmacy files. Using multiple patient identifiers, we linked these data to the VHA COVID-19 Shared Data Resource (SDR), which is a robust data domain collating longitudinal medical information of veterans who have been tested for SARS-CoV-2 by polymerase chain reaction (PCR) test, as previously described.17,18 The SDR is developed from VHA data sources that are routinely verified, validated, and updated. Through a centralized process, the SDR undergoes ongoing data refreshes and regular quality checks, including manual adjudication of case definitions, concept definitions, and data mappings to ensure accuracy. Phenotype algorithms, including for covariates, COVID-19 symptoms, and COVID-19–related outcomes, were validated before release in the COVID-19 SDR.
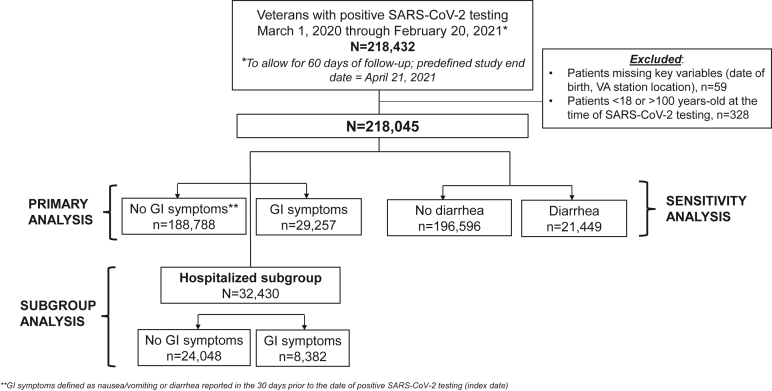
From these sources, we included veterans aged 18–100 years, who were receiving longitudinal care within the VHA, and who had at least one positive SARS-CoV-2 test between March 1, 2020, and February 20, 2021; patients diagnosed after this date were excluded to enable at least 60 days of follow-up to the study end date: April 21, 2021. We included the following patient-level data: demographics; clinical characteristics, including comorbidities; information related to outpatient and inpatient encounters, including dates, diagnostic tests, and procedures; diagnostic test results; details of filled inpatient and outpatient prescriptions; and death dates.
The index date was defined as the date of first positive SARS-CoV-2 test and indicated the start of follow-up. In patients with multiple positive tests, only the first positive test was considered. Patients with only negative SARS-CoV-2 PCR tests were excluded.
Primary Exposure: COVID-19 GI Symptoms
The predictor of interest was the presence of GI symptoms within 30 days before and including the index date of positive SARS-CoV-2 testing. GI symptoms were defined as the composite of nausea/vomiting or diarrhea. To assess the hypothesis that diarrhea may reflect GI viral replication (as opposed to nausea/vomiting in the absence of diarrhea), we also separately analyzed all patients with diarrhea, irrespective of concomitant nausea or vomiting. In the COVID-19 SDR, the presence vs absence of GI symptoms was ascertained using a phenotype that was validated centrally and leveraged the integration of International Classification of Diseases (ICD) versions 9 (ICD-9) and 10 (ICD-10) coding, natural language processing, and keyword text search of medical notes. As noted previously, all phenotype algorithms in the COVID-19 SDR undergo routine quality checks, including adjudication via manual chart review, before release for approved research.
Primary and Secondary Outcomes
Severe COVID-19 was the primary composite outcome, defined as hospitalization, admission to the intensive care unit (ICU), mechanical ventilation, or death within 60 days from the index date (positive SARS-CoV-2 PCR test). Because hospitalization and ICU admission may be driven by factors other than disease severity (eg, bed availability), we evaluated mechanical ventilation use or death as a secondary composite outcome. We also evaluated each component separately.
Covariates
We evaluated age, gender, race, and ethnicity as covariates. Additional covariates were defined within the 2 years before, but not including the index date: smoking status (current, former, never, unknown); body mass index (BMI); relevant comorbidities; Charlson comorbidity index; and select prescription fills, including renin-angiotensin-aldosterone system inhibitors (RAASi; ie angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers), given the mixed literature of RAASi and COVID-19–associated outcomes.19 Comorbidities were defined using a combination of ICD-9, ICD-10, and Current Procedural Terminology codes as previously described and further detailed in Table A1.18,20 VHA facility location was included as a covariate to account for variation in socioeconomic factors and disease prevalence across geographic regions. The integer calendar date of the index date was also included to account for the temporal evolution of COVID-19 trends with respect to epidemiology, clinical management, and other system-related factors.
For patients hospitalized with COVID-19, we also recorded whether or not patients received medical treatments authorized for COVID-19, specifically dexamethasone and remdesivir. Other therapies, including monoclonal antibody treatment, were either approved for COVID-19 treatment near the end of or outside of the study interval and thus were not included in this analysis.
Statistical Approach
We compared the means, standard deviations, and frequencies for continuous and categorical variables between patients according to the presence or absence of GI symptoms (nausea/vomiting or diarrhea in the primary analysis; diarrhea only in the sensitivity analysis as described below). Propensity score (PS) weighting was used to balance covariate distributions between patients with vs without GI symptoms. Inverse probability treatment weights were calculated using cross-validated logistic ridge regression models with optimal shrinkage parameters due to the large number of covariates. Weighting is more efficient than matching on PS in that it allows for sample size preservation by including all persons, but upweights or downweights them based on measured covariates. All aforementioned covariates and BMI were included in the PS models. COVID-19 treatments were not included in the PS model since they are determined after the index date (see mediation analysis below).
Except for BMI, no covariates had missing values. For BMI, a missingness indicator was included (not missing vs missing/out of range), and missing values were single imputed as the average of the values for nonmissing BMIs. Restricted cubic splines were applied to all continuous covariates to balance nonlinear associations. PS weights were scaled to preserve the cohort size, such that the unweighted and weighted samples were equal. We achieved sufficient overlap of PS weights between the groups without significant outliers or extreme weights (Figure A1). Balance was represented as standardized mean differences (SMDs). Smaller SMDs indicate better covariate balance between groups; SMD values <0.1 are considered optimal.
We compared the unweighted and weighted frequencies of each composite and individual component outcome within 60 days of the index date stratified by the presence vs absence of GI symptoms. We also performed an a priori subgroup analysis restricted to patients hospitalized with COVID-19. For this subgroup analysis, we generated a separate PS-weighted model using the approach described previously to balance covariate distributions between hospitalized patients with vs without GI symptoms (Figure).
Figure.
Flow diagram of cohort construction.
Based on the age distribution of COVID-19 cases, we also conducted a post hoc stratified analysis according to age group. To analyze the effect of COVID-19 treatments (dexamethasone, remdesivir) on the association between GI symptoms and COVID-19 outcomes, we conducted a post hoc exploratory mediation analysis.
Sensitivity Analysis: Diarrhea as Primary Exposure
We conducted a sensitivity analysis evaluating diarrhea as the only predictor of interest (Figure). We used the same approach described previously to recalculate PS weights, and sufficient overlap of PS weights between those with vs without diarrhea was achieved (Figure A1B). Severe COVID-19 outcomes were compared between the 2 groups using the same approach as described previously.
All analyses were performed in R 4.0.2, and the survey package was used to account for the PS weights.21 A 2-tailed P-value <.05 was considered statistically significant.
Results
Cohort Characteristics
A total of 218,045 SARS-CoV-2–positive patients had complete data available and were included in the cohort from March 1, 2020, to February 20, 2021 (Figure). Of these patients, 29,257 (13.4%) had GI symptoms in the 30 days up to or including their index date (15,558 [7.1%] nausea/vomiting and 21,449 (9.8%) diarrhea, nonmutually exclusive) in the unweighted cohort. We identified no differential reporting of GI symptoms during the study interval (Figure A2).
Reflective of the veteran population, the cohort was largely older non-Hispanic White men. In the unweighted cohort, patients with vs without GI symptoms were slightly younger (aged 58.4 vs 59.9 years on average), more often men (86.6% vs 84.4%), and non-Hispanic Black (23.4% vs 19.9%). They were more often current or former smokers and generally had higher frequencies of comorbidities and use of certain medications, including angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers (please refer to Table 1). Even so, most covariates in the unweighted cohort were balanced (SMD <0.1). After PS weighting, there was complete covariate balance, and no SMD exceeded 0.07 (Figure A3A). Complete covariate balance was also achieved for the separate PS-weighted models for the sensitivity analysis (diarrhea as the primary predictor) and subgroup analysis among hospitalized patients (Figures A3B and C).
Table 1.
Characteristics for the Full Cohort With Positive SARS-CoV-2 Testing, Stratified by No GI Symptoms vs Presence of GI Symptoms (Defined as Nausea, Vomiting, or Diarrhea)
| Covariates | Unweighted cohort |
Weighted cohort |
|||
|---|---|---|---|---|---|
| No GI symptoms (n = 188,788) | GI symptoms (n = 29,257) | No GI symptoms (n = 188,788) | GI symptoms (n = 29,257) | SMDa | |
| VHA facilityb | b | b | b | b | 0.070 |
| Age, mean years (SD) | 59.9 (16.7) | 58.4 (16.0) | 59.7 (16.6) | 59.7 (16.5) | 0.003 |
| Male sex, n (%) | 159,368 (84.4) | 25,342 (86.6) | 159,950 (84.7) | 25,034 (85.6) | 0.024 |
| Race/ethnicity, n (%) | 0.034 | ||||
| Non-Hispanic White | 103,311 (54.7) | 16,183 (55.3) | 103,487 (54.8) | 16,183 (55.3) | |
| Non-Hispanic Black | 37,577 (19.9) | 6855 (23.4) | 38,458 (20.4) | 6134 (21.0) | |
| Hispanic | 17,181 (9.1) | 3179 (10.9) | 17,627 (9.3) | 2758 (9.4) | |
| Non-Hispanic other or unknown | 30,719 (16.3) | 3040 (10.4) | 29,216 (15.5) | 4182 (14.3) | |
| Integer calendar date of index date, mean days (SD)c | 304.9 (84.8) | 304.5 (82.5) | 304.8 (84.6) | 304.2 (84.3) | 0.007 |
| Smoking status, n (%) | 0.034 | ||||
| Current smoker | 20,244 (10.7) | 3471 (11.9) | 20,555 (10.9) | 3259 (11.1) | |
| Former smoker | 72,282 (38.3) | 12,065 (41.2) | 73,022 (38.7) | 11,431 (39.1) | |
| Never smoker | 65,656 (34.8) | 11,157 (38.1) | 66,498 (35.2) | 10,462 (35.8) | |
| Unknown | 30,606 (16.2) | 2564 (8.8) | 28,713 (15.2) | 4105 (14.0) | |
| Comorbidities, n (%) | |||||
| Asthma | 11,470 (6.1) | 2248 (7.7) | 11,882 (6.3) | 1888 (6.5) | 0.007 |
| Coronary artery disease | 33,591 (17.8) | 5835 (19.9) | 34,149 (18.1) | 5349 (18.3) | 0.005 |
| Cancer | 33,451 (17.7) | 6189 (21.2) | 34,336 (18.2) | 5475 (18.7) | 0.014 |
| Cardiomyopathy | 5170 (2.7) | 1036 (3.5) | 5381 (2.9) | 861 (2.9) | 0.005 |
| Charlson comorbidity index, mean (SD) | 1.6 (2.1) | 1.9 (2.4) | 1.6 (2.2) | 1.7 (2.2) | 0.016 |
| Congestive heart failure | 12,606 (6.7) | 2451 (8.4) | 13,049 (6.9) | 2081 (7.1) | 0.008 |
| Chronic lung disease | 51,097 (27.1) | 9255 (31.6) | 52,266 (27.7) | 8203 (28.0) | 0.008 |
| Chronic neuromuscular disease | 6733 (3.6) | 925 (3.2) | 6632 (3.5) | 994 (3.4) | 0.006 |
| Chronic kidney disease | 23,163 (12.3) | 4350 (14.9) | 23,837 (12.6) | 3785 (12.9) | 0.009 |
| Chronic kidney failure | 2683 (1.4) | 619 (2.1) | 2865 (1.5) | 465 (1.6) | 0.006 |
| COPD | 26,542 (14.1) | 4673 (16.0) | 27,037 (14.3) | 4213 (14.4) | 0.002 |
| Central sleep apnea | 590 (0.3) | 112 (0.4) | 609 (0.3) | 103 (0.4) | 0.005 |
| Cardiovascular disease | 55,269 (29.3) | 9874 (33.7) | 56,403 (29.9) | 8849 (30.2) | 0.008 |
| Diabetes | 59,185 (31.3) | 10,250 (35.0) | 60,137 (31.9) | 9400 (32.1) | 0.006 |
| Drug dependency | 6689 (3.5) | 1541 (5.3) | 7121 (3.8) | 1121 (3.8) | 0.003 |
| Emphysema | 2710 (1.4) | 547 (1.9) | 2822 (1.5) | 454 (1.6) | 0.005 |
| Heart disease | 41,853 (22.2) | 7320 (25.0) | 42,587 (22.6) | 6666 (22.8) | 0.005 |
| Heart failure | 15,968 (8.5) | 3026 (10.3) | 16,458 (8.7) | 2600 (8.9) | 0.006 |
| Hypertension | 102,010 (54.0) | 17,416 (59.5) | 103,418 (54.8) | 16,302 (55.7) | 0.019 |
| Lower respiratory infection | 14,958 (7.9) | 3628 (12.4) | 16,094 (8.5) | 2585 (8.8) | 0.011 |
| Obstructive sleep apnea | 53,402 (28.3) | 9807 (33.5) | 54,730 (29.0) | 8600 (29.4) | 0.009 |
| Medications, n (%) | |||||
| ACEi | 46,551 (24.7) | 8485 (29.0) | 47,653 (25.2) | 7549 (25.8) | 0.013 |
| ARBs | 23,209 (12.3) | 4161 (14.2) | 23,711 (12.6) | 3765 (12.9) | 0.009 |
| NSAIDs | 98,782 (52.3) | 18,872 (64.5) | 101,863 (54.0) | 16,152 (55.2) | 0.025 |
| Statins | 83,770 (44.4) | 14,610 (49.9) | 85,208 (45.1) | 13,474 (46.1) | 0.018 |
| PPIs | 57,977 (30.7) | 11,687 (39.9) | 60,335 (32.0) | 9645 (33.0) | 0.022 |
| H2RAs | 13,059 (6.9) | 2646 (9.0) | 13,595 (7.2) | 2142 (7.3) | 0.005 |
ACEi, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II, receptor blockers; NSAIDs, nonsteroidal anti-inflammatory drugs; PPIs, proton-pump inhibitors; H2RAs, histamine 2 receptor blocker; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Only standardized mean differences (SMD) for the weighted cohort are provided in this table.
Each of the 127 VHA, facilities were included as covariates in this analysis; however, the proportion of patients at each station for each group is not listed here due to space considerations. SMD, plots are provided in Figure A3.
This variable represents the integer calendar date of the index date of SARS-CoV-2, testing, measured in days, to account for temporal differences. Please refer text for additional details.
Primary and Secondary Analyses
In the unweighted cohort, patients with GI symptoms had a significantly higher frequency of severe COVID-19 outcomes compared with those without GI symptoms. Those with GI symptoms experienced a higher frequency of hospitalization (28.6% vs 12.7%; P < .001), ICU admission (9.8% vs 4.6%; P < .001), and mechanical ventilation (3.5% vs 1.8%; P < .001) relative to patients without GI symptoms. No difference in the secondary outcomes of mechanical ventilation or death within 60 days postindex (6.9% vs 6.9%; P = .83) was observed in patients with vs without GI symptoms. Among patients hospitalized with COVID-19, patients with GI symptoms had lower mortality compared with those without GI symptoms (13.4% vs 17.0%, P < .001) and exhibited no difference in mechanical ventilation frequencies (11.7% vs 11.8%; P = .72; Table 2). Patients with GI symptoms more often received COVID-19 treatments in the unweighted hospitalized cohort (Table A2).
Table 2.
COVID-19–Related Disease Severity Outcomes According to Presence or Absence of GI Symptoms (Nausea, Vomiting, Diarrhea)
| Outcomes, n (%) | Weighted overall cohort |
Weighted hospitalized cohort |
||||
|---|---|---|---|---|---|---|
| No GI symptoms (n = 188,788) | GI symptoms (n = 29,257) | P-value | No GI symptoms (n = 24,048) | GI symptoms (n = 8382) | P-value | |
| Primary composite (hospitalization, ICU, mechanical ventilation, death) | 32,196 (17.1) | 8475 (29.0) | <.001 | N/A | N/A | N/A |
| Secondary composite (mechanical ventilation, death) | 12,925 (6.8) | 2071 (7.1) | .192 | 5121 (21.3) | 1628 (19.4) | .001 |
| Component outcomes | ||||||
| Death | 11,298 (6.0) | 1670 (5.7) | .104 | 3912 (16.3) | 1228 (14.6) | .001 |
| Mechanical ventilation | 3540 (1.9) | 972 (3.3) | <.001 | 2833 (11.8) | 986 (11.8) | .956 |
| ICU admission | 8830 (4.7) | 2716 (9.3) | <.001 | 8558 (35.6) | 2946 (35.2) | .489 |
| Hospitalization | 24,606 (13.0) | 7885 (26.9) | <.001 | N/A | N/A | N/A |
| Unweighted overall cohort | Unweighted hospitalized cohort | |||||
| Primary composite (hospitalization, ICU, mechanical ventilation, death) | 31,815 (16.9) | 8885 (30.4) | <.001 | N/A | N/A | N/A |
| Secondary composite (mechanical ventilation, death) | 13,045 (6.9) | 2032 (6.9) | .834 | 5278 (21.9) | 1529 (18.2) | <.001 |
| Component outcomes | ||||||
| Death | 11,461 (6.1) | 1592 (5.4) | <.001 | 4089 (17.0) | 1126 (13.4) | <.001 |
| Mechanical ventilation | 3472 (1.8) | 1038 (3.5) | <.001 | 2840 (11.8) | 977 (11.7) | .721 |
| ICU admission | 8615 (4.6) | 2876 (9.8) | <.001 | 8615 (35.8) | 2876 (34.3) | .013 |
| Hospitalization | 24,048 (12.7) | 8382 (28.6) | <.001 | N/A | N/A | N/A |
ICU, intensive care unit; N/A, not available.
After PS weighting, GI symptoms were more often associated with severe COVID-19 outcomes (primary composite, 29.0% vs 17.1%; P < .001) compared with those without GI symptoms. GI symptoms were associated with higher rates of hospitalization (26.9% vs 13.0%; P < .001), ICU admission (9.3% vs 4.7%; P < .001), and mechanical ventilation (3.3% vs 1.9%; P < .001), compared with those without GI symptoms. However, no difference in mortality was observed between the groups (5.7% vs 6.0%, P = .10). There was also no significant difference in the combined outcome of mechanical ventilation or death within 60 days (7.1% vs 6.8%; P = .19) in patients with vs without GI symptoms. In the PS-weighted hospitalized cohort, patients with GI symptoms exhibited a significantly lower frequency of death within 60 days (14.6% vs 16.3%; P = .001), yet no difference in mechanical ventilation was observed (11.8% vs 11.8%; P = .96), relative to patients without GI symptoms. In the PS-weighted hospitalized cohort, 54.0% and 46.1% of patients with GI symptoms received dexamethasone and/or remdesivir, respectively, compared with 48.5% and 39.7% of patients without GI symptoms (Table A2). In the exploratory mediation analysis, we found no evidence that remdesivir and dexamethasone mediated the protective association observed between GI symptoms and death. In the subset of hospitalized patients aged ≥70 years, the statistically significant association between GI symptoms and death remained effectively unchanged when adjusting for the therapies; specifically, the relative risk of death for those with GI symptoms was 0.86 (95% confidence interval, 0.80–0.93) without mediators (ie, dexamethasone, remdesivir) vs 0.84 (95% confidence interval, 0.78–0.90) with these mediators. Notably, in hospitalized patients aged <70 years, GI symptoms were not associated with COVID-19 mortality (Table A3).
There was a statistically significant interaction between age as a continuous variable and GI symptoms on the primary and secondary composite outcomes and the component outcomes of hospitalization and mortality (Figure A5). Because there was an inflection point for the secondary composite outcome and mortality near age 70 years old, we conducted an exploratory stratified analysis based on this age threshold (Table A3). As expected, patients aged ≥70 years more often experienced severe COVID-19 outcomes compared with patients aged <70 years. Irrespective of age grouping, patients with GI symptoms were more often hospitalized, admitted to the ICU, or needed mechanical ventilation compared with patients without GI symptoms; however, mortality trends differed. Among hospitalized patients aged <70 years, there was no statistically significant difference in COVID-19 severity outcomes between patients with vs without GI symptoms, although there was a trend toward higher COVID-19–related mortality in patients with vs without GI symptoms (8.0% vs 7.1%; P = .07) in the PS-weighted analysis. However, among patients aged ≥70 years hospitalized with COVID-19, GI symptoms were associated with significantly lower COVID-19–related mortality (20.4% vs 23.7%; P < .001; PS-weighted analysis); there was no difference in ICU admission nor need for mechanical ventilation (Table A3). As noted previously, accounting for remdesivir and dexamethasone using a mediation analysis did not alter these findings.
Sensitivity Analysis: Diarrhea as Primary Exposure
A total of 21,449 (9.8%) patients had diarrhea irrespective of concomitant symptoms of nausea/vomiting in the 30 days up to and including their positive SARS-CoV-2 test. Patients with diarrhea experienced severe COVID-19 outcomes significantly more often than those without diarrhea in the unweighted cohort (29.7% vs 17.5%; P < .001 for primary composite outcome). Patients with diarrhea had a higher frequency of hospitalization (27.9% vs 13.5%; P < .001), ICU admission (9.5% vs 4.8%; P < .001), and mechanical ventilation (3.7% vs 1.9%; P < .001), relative to patients without diarrhea. These findings were consistent in the PS-weighted cohort (Table A1), and all covariates were balanced between patients with vs without diarrhea (SMD <0.1) (Figure A3B). In the PS-weighted cohort, no differences in mortality (5.8% vs 6.0%; P = .38) nor the secondary composite outcome of mechanical ventilation or death within 60 days (7.1% vs 6.9%; P = .20) were noted in those with or without diarrhea.
Discussion
In this large national VHA cohort of over 218,000 SARS-CoV-2–positive patients, 1 in 8 experienced GI symptoms of nausea/vomiting or diarrhea. In the PS-weighted cohort, where complete covariate balance was achieved, patients with GI symptoms had a nearly 2-fold higher frequency of severe COVID-19 compared with patients without GI symptoms. Specifically, SARS-CoV-2–positive patients with GI symptoms exhibited approximately twice the frequency of hospitalization (26.9% vs 13.0%), ICU admission (9.3% vs 4.7%), and mechanical ventilation (3.3% vs 1.9%), relative to patients without GI symptoms, although there was no observed difference in mortality. Importantly, COVID-19 mortality in hospitalized cohort was approximately 15% overall, irrespective of GI symptoms. Although elderly age, not unexpectedly, was associated with a dramatic increase in the risk of death, a notable finding of this study was that elderly patients with GI symptoms had lower observed mortality compared with those without GI symptoms, even after PS weighting and mediation analysis accounting for COVID-19 treatments, which suggests that other (unmeasured) factors may be at play. Future studies that are specifically designed to identify these contributing factors are needed.
Our estimates of GI symptom prevalence align with those reported in a large systematic review.22 In a study by Han et al, patients presenting with GI symptoms exhibited a longer duration between symptom onset and viral clearance, as well as much higher rates of fecal SARS-CoV-2, compared with patients with respiratory symptoms.3 This higher GI viral load may contribute to potentiation of pro-inflammatory cytokines, immune dysregulation, hyperimmune response, and cytokine storm—a common pathway underlying severe COVID-19.14, 15, 16 Although smaller studies analyzing GI symptoms and the association with COVID-19 severity have generally yielded mixed results,23, 24, 25, 26 larger studies demonstrate more consistency. Similar to our large present study, Mao et al. found that GI symptoms were associated with increased COVID-19 severity relative to those without GI symptoms based on a meta-analysis of over 6600 SARS-CoV-2–positive patients.27 Two other meta-analyses reported no difference in COVID-19–associated mortality in patients with GI symptoms compared with those without symptoms.26,27 An earlier VHA cohort analysis of 10,131 veterans with SARS-CoV-2 test positivity and follow-up only through June 2020 similarly reported that patients with symptoms of nausea or diarrhea experienced significantly higher rates of hospitalization and mechanical ventilation compared with patients without nausea/diarrhea, but with no mortality difference.5 We significantly extend prior literature by also identifying that hospitalized patients with GI symptoms more often receive COVID-19–specific treatments, dexamethasone and remdesivir. Yet, although current data support reduced mortality associated with dexamethasone and remdesivir administration in patients with COVID-19, the results of our mediation analysis incorporating these treatments demonstrated that the observed association between GI symptoms and lower COVID-19 mortality in hospitalized patients aged ≥70 years was not significantly affected by whether or not patients received these treatments. This observation suggests that other unmeasured factors are relevant as explanations for the observed mortality difference.
Health system factors, such as bed availability, might impact the outcomes of hospitalization and ICU admission. For this reason, we analyzed each component of the composite outcomes separately and also analyzed mechanical ventilation or death as a secondary composite outcome. We also conducted analyses restricted to the subgroup of hospitalized patients. It is unlikely that the observed difference in COVID-19–related hospitalization among patients with GI symptoms, relative to those without GI symptoms, is fully attributable to health system factors, as supported by the separate PS-weighted subgroup analysis among hospitalized patients. Notwithstanding, irrespective of their accuracy as a surrogate for disease severity, COVID-19–associated hospitalizations stress the health care system and have substantial downstream consequences, including the well-documented impact on non–COVID-19–related acute care.28, 29, 30, 31
This is the largest US population–based study to analyze GI symptoms associated with SARS-CoV-2 infection and severe COVID-19 outcomes among all patients, as well as those hospitalized, observed for over 1 year of the pandemic timeframe. Our findings support the hypothesis that GI symptoms are associated with more severe COVID-19. Additional study strengths include the robust PS-weighted analysis (all SMDs below 0.1 threshold), as well as adjustment for geography and calendar date. We also note limitations. Given the retrospective design, we cannot account for unmeasured covariates. Second, reflective of the US veteran population, most patients in this cohort were older, White males with a high comorbidity burden; as such, it is possible that our findings may not reflect the general US population, especially with the disproportionate impact of COVID-19 on non-White racial and ethnic groups.32 Third, we are not able to reliably capture care outside of the VHA, which would systematically undercount the number of hospitalizations and bias our results toward the null; however, we do not expect VHA vs non-VHA hospitalization (or deaths) to be differential based on the presence vs absence of GI symptoms. Of note, although deaths that occur outside of the VHA are eventually registered in the VHA due to cessation of benefits, these are not immediately captured; however, our analysis restricted to the hospitalized cohort overcomes this potential underestimation because in-hospital deaths are readily captured. Reporting bias for GI symptoms may be a concern; however, the prevalence of GI symptoms did not vary substantially over time and argues against significant reporting bias. That said, given the retrospective nature of this study, there is still the potential for misclassification of the primary exposure, the presence vs absence of GI symptoms, because not all health care providers may ask or provide appropriate documentation, particularly if symptoms are mild, and also because we are not able to manually adjudicate all charts due to the very large sample size.
In conclusion, based on an analysis of a nationwide cohort of more than 218,000 patients with confirmed SARS-CoV-2 infection, the presence of GI symptoms was more often associated with severe COVID-19 outcomes, most notably hospitalization. Among patients hospitalized with COVID-19, GI symptoms were not associated with more severe diseases such as mechanical ventilation or death. Additional research should evaluate the relationship between GI symptoms and the associated increase in hospitalizations, as well as differences in COVID-19 treatment administration, to inform appropriate interventions.
Footnotes
Authors' Contributions: Shailja C. Shah contributed to study concept (lead), study design (equal), data analysis (supporting), data interpretation (equal), drafting manuscript (lead), and critical review of the article (lead). Andrew Canakis contributed to drafting of manuscript (equal) and critical review of the article (supporting). Alese Halvorson contributed to study design (equal), data analysis (lead), data interpretation (equal), and critical review of manuscript (supporting). Chad Dorn, Otis Wilson, and Jason Denton contributed to data acquisition (supporting). Christine Hunt contributed to drafting of manuscript (supporting) and critical review of the article (supporting). Richard Hauger, Ayako Suzuki, Michael E. Matheny, Edward Siew and Adriana Hung contributed to critical review of article (supporting). Robert A. Greevy contributed to study design (equal), data analysis (lead), data interpretation (equal), and critical review of article (supporting). Christianne L. Roumie contributed to study design (equal), data interpretation (supporting), and critical review of article (supporting).
Conflict of Interest: These authors disclose the following: C.M.H. is a consultant to Akebia Therapeutics, Inc. S.C.S. serves as an ad hoc consultant for Phathom Pharmaceuticals and RedHill Biopharma. The remaining authors disclose no conflicts.
Funding: S.C.S. is supported by a Veterans Affairs Career Development Award (ICX002027A) and an American Gastroenterological Association Research Scholar Award.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: The data will not be made publicly available. The analytic methods are provided in detail herein and the corresponding author will share any additional details regarding the methodological approach if requested.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2022.06.015.
Supplementary Materials
References
- 1.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int
- 2.Woolf S.H., Chapman D.A., Lee J.H. COVID-19 as the leading cause of death in the United States. JAMA. 2021;325(2):123–124. doi: 10.1001/jama.2020.24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han C., Duan C., Zhang S., et al. Digestive symptoms in COVID-19 patients with mild disease severity: clinical Presentation, Stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115(6):916–923. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmunzer B.J., Spitzer R.L., Foster L.D., et al. Digestive manifestations in patients hospitalized with coronavirus disease 2019. Clin Gastroenterol Hepatol. 2021;19(7):1355–1365.e4. doi: 10.1016/j.cgh.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioannou G.N., Locke E., Green P., et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9):e2022310. doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M.K., Yue H.Y., Cai J., et al. COVID-19 and the digestive system: a comprehensive review. World J Clin Cases. 2021;9(16):3796–3813. doi: 10.12998/wjcc.v9.i16.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao F., Tang M., Zheng X., et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin L., Jiang X., Zhang Z., et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 9.Luo S., Zhang X., Xu H. Don't overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19) Clin Gastroenterol Hepatol. 2020;18(7):1636–1637. doi: 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Concas G., Barone M., Francavilla R., et al. Twelve months with COVID-19: what gastroenterologists need to know. Dig Dis Sci. 2021;67(7):2771–2791. doi: 10.1007/s10620-021-07158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian Q., Fan L., Liu W., et al. Direct evidence of active SARS-CoV-2 replication in the intestine. Clin Infect Dis. 2021;73(3):361–366. doi: 10.1093/cid/ciaa925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M.Y., Li L., Zhang Y., et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeoh Y.K., Zuo T., Lui G.C.Y., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donowitz M., Tse C.M., Dokladny K., et al. SARS-COV-2 induced Diarrhea is inflammatory, Ca 2+ Dependent and involves activation of calcium activated Cl channels. Biorxiv. 2021 doi: 10.1101/2021.04.27.441695. [DOI] [Google Scholar]
- 15.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mönkemüller K., Fry L., Rickes S. COVID-19, coronavirus, SARS-CoV-2 and the small bowel. Rev Esp Enferm Dig. 2020;112(5):383–388. doi: 10.17235/reed.2020.7137/2020. [DOI] [PubMed] [Google Scholar]
- 17.Shah S., Halvorson A., McBay B., et al. Proton-pump inhibitor use is not associated with severe COVID-19-related outcomes: a propensity score-weighted analysis of a national veteran cohort. Gut. 2021;71(7):1447–1450. doi: 10.1136/gutjnl-2021-325701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birkelo B.C., Parr S.K., Perkins A.M., et al. Comparison of COVID-19 versus influenza on the incidence, features, and recovery from acute kidney injury in hospitalized United States Veterans. Kidney Int. 2021;100(4):894–905. doi: 10.1016/j.kint.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S.A., Park R., Yang J.H., et al. Increased risk of acute kidney injury in coronavirus disease patients with renin–angiotensin–aldosterone-system blockade use: a systematic review and meta-analysis. Sci Rep. 2021;11(1):13588. doi: 10.1038/s41598-021-92323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan H., Sundararajan V., Halfon P., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 21.Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9:1–19. [Google Scholar]
- 22.Sultan S., Altayar O., Siddique S.M., et al. AGA Institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159(1):320–334.e27. doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laszkowska M., Faye A.S., Kim J., et al. Disease course and outcomes of COVID-19 among hospitalized patients with gastrointestinal manifestations. Clin Gastroenterol Hepatol. 2021;19(7):1402–1409.e1. doi: 10.1016/j.cgh.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajifathalian K., Krisko T., Mehta A., et al. Gastrointestinal and hepatic manifestations of 2019 novel coronavirus disease in a large cohort of infected patients from New York: clinical implications. Gastroenterology. 2020;159(3):1137–1140.e2. doi: 10.1053/j.gastro.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramachandran P., Onukogu I., Ghanta S., et al. Gastrointestinal symptoms and outcomes in hospitalized coronavirus disease 2019 patients. Dig Dis. 2020;38(5):373–379. doi: 10.1159/000509774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tariq R., Saha S., Furqan F., et al. Prevalence and mortality of COVID-19 patients with gastrointestinal symptoms: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95(8):1632–1648. doi: 10.1016/j.mayocp.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao R., Qiu Y., He J.S., et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blecker S., Jones S.A., Petrilli C.M., et al. Hospitalizations for chronic disease and acute conditions in the time of COVID-19. JAMA Intern Med. 2021;181(2):269–271. doi: 10.1001/jamainternmed.2020.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Findling M.G., Blendon R.J., Benson J.M. Delayed care with harmful health consequences—reported experiences from national surveys during coronavirus disease 2019. JAMA Health Forum. 2020;1(12):e201463. doi: 10.1001/jamahealthforum.2020.1463. [DOI] [PubMed] [Google Scholar]
- 30.Bhambhvani H.P., Rodrigues A.J., Yu J.S., et al. Hospital volumes of 5 medical emergencies in the COVID-19 pandemic in 2 US medical centers. JAMA Intern Med. 2021;181(2):272–274. doi: 10.1001/jamainternmed.2020.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeJong C., Katz M.H., Covinsky K. Deferral of care for serious non-COVID-19 conditions: a hidden harm of COVID-19. JAMA Intern Med. 2021;181(2):274. doi: 10.1001/jamainternmed.2020.4016. [DOI] [PubMed] [Google Scholar]
- 32.Gu T., Mack J.A., Salvatore M., et al. Characteristics associated with racial/ethnic disparities in COVID-19 outcomes in an academic health care system. JAMA Netw Open. 2020;3(10):e2025197. doi: 10.1001/jamanetworkopen.2020.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.