Abstract
There has been a noted increase in the incidence of intracranial aspergillosis; this is often attributed to the wider use of antibiotics, corticosteroids, and immunosuppressants. Fungal cerebral aneurysms due to aspergillosis after neurosurgery remain extremely rare; in fact, only seven cases have been reported in the literature. In this study, we present a patient with an Aspergillus aneurysm that elicited subarachnoid hemorrhage after endoscopic endonasal surgery (EES) for craniopharyngioma. A 70-year-old woman with recurrent craniopharyngioma and steroid treatment underwent uneventful EES. On the 5th postoperative day, she suffered subarachnoid hemorrhage. As per her computed tomography angiography findings, an aneurysm was detected on the left internal carotid artery (ICA). Subsequent digital subtraction angiography showed occlusion of the ICA and an irregularly shaped wall. The diagnosis was pseudoaneurysm. We then performed craniotomy to place a left high-flow bypass and to trap the pseudoaneurysm. Despite continuous intensive care, she died on the 25th postoperative day of a huge, left cerebral infarct. The final diagnosis was made at autopsy; it revealed destruction of the ICA and Aspergillus invasion of the vessel wall, confirming the presence of a true fungal aneurysm. Perioperatively, patients with potential immunosuppression must be carefully managed. Advanced age is a risk factor. As surgery via the paranasal sinuses raises the risk for aspergillosis, fungal infection must be ruled out in patients whose postoperative course is deemed concerning.
Keywords: Aspergillus, craniopharyngioma, endoscopic endonasal surgery, fungal aneurysm, subarachnoid hemorrhage
Introduction
Aspergillosis of the central nervous system (CNS) is rare; however, due to the wider use of antibiotics, corticosteroids, and immunosuppressants, its incidence has increased. Vascular invasion by the fungus Aspergillus elicits intense inflammation of the arterial wall, leading to thrombosis, dilation, rupture, and fungal aneurysm formation. Fungal cerebral aneurysms due to aspergillosis after neurosurgery are extremely rare; in fact, only seven patients have been reported in the literature, wherein all seven patients died and the final diagnosis was made at autopsy.1-7)
In this study, we present a case of an elderly woman with a fungal aneurysm due to Aspergillus. She developed subacute subarachnoid hemorrhage (SAH) after endoscopic endonasal surgery (EES) for recurrent craniopharyngioma. Autopsy revealed Aspergillus invasion of the intima of the internal carotid artery (ICA). We then compared this case with the seven previously reported patients, presented the features of fungal cerebral aneurysms, and discussed their prevention. To our knowledge, ours is the first patient with fatal aspergillosis after ESS. We obtained her family's consent to report this case.
Case Report
A 70-year-old woman presented with vision failure. She had a history of hyperlipidemia but not of diabetes. As the diagnosis was cystic craniopharyngioma, she underwent cyst fenestration by microscopic transsphenoidal surgery (TSS) at another hospital and received hormone substitution therapy. On the day of that surgery, she was injected intravenously with 50 mg hydrocortisone every 6 hours. On the 1st postoperative day, she was injected with 50 mg hydrocortisone at 8-hour intervals. Because pituitary dysfunction was suspected, she received a daily average of 15 mg oral hydrocortisone perorally for 37 days. Her craniopharyngioma regrew 1 month after the operation, and she was transferred to our hospital with complaints of progressive visual deterioration.
Preoperative computed tomography (CT) and magnetic resonance imaging (MRI) studies performed at the other hospital had detected no paranasal sinus anomalies (Fig. 1A-D). Preoperative MRI performed at the time of craniopharyngioma recurrence showed a large sellar lesion with suprasellar extension that elevated the optic chiasm.
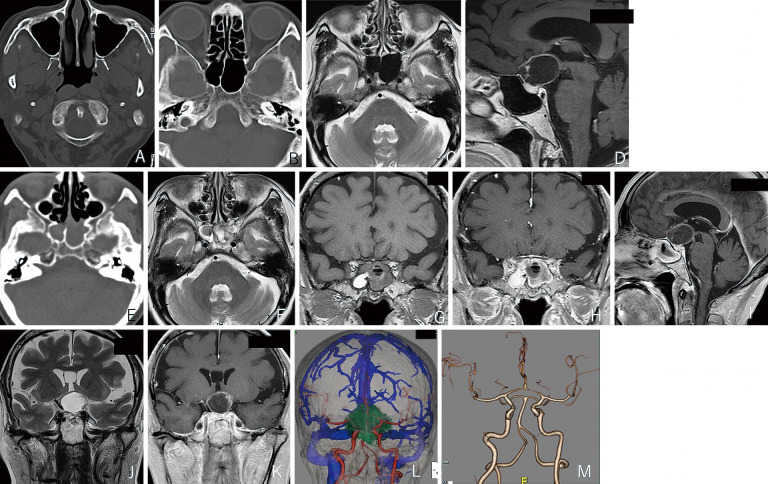
Fig. 1.
A–D Preoperative images obtained at the first hospital. The paranasal sinus. CT returned no abnormal finding at the maxillary (A) and the sphenoid sinus (B). MRI scans (C–D): T2 axial view (C), T1 sagittal view after contrast medium injection (D). Neither image shows abnormal finding at the sphenoid sinus.
E–M Preoperative images obtained at our hospital at the time of craniopharyngioma recurrence. CT shows an iso-dense lesion and no bone destruction at the sphenoid sinus (E). MRI (T2, axial view) (F) at the sphenoid sinus detected a mixed intensity lesion. MRI (T1, coronal view) (G) shows an iso- and high-density lesion. Contrast-enhanced T1 (coronal view) (H) and sagittal (I) images showing homogenous mucosal enhancement. MRI (T2 coronal view) (J) and the contrast-enhanced (T1 coronal view) (K) scans reveal a tumor encasing the left internal carotid artery. The fused image with venography (L) and the CTA scan (M) reveal no aneurysm at the laterally pushed left internal carotid artery.
Our examination revealed primary left optic atrophy and bilateral hemianopsia. The solid portion of the tumor and its cyst wall were enhanced by gadrinium. CT and MRI scans showed fluid collection without bone destruction; the mucosa at the sphenoid sinus was contrast-enhanced. These observations indicated postoperative changes (Fig. 1E-I). CT angiograms (CTA) and coronal MRI showed that the lesion encased the laterally deviating left ICA. No aneurysmal changes were noted (Fig. 1J-M).
At navigated EES, we punctured the cystic mass and dissected the cyst wall and the solid part of the cyst from the surrounding tissue that adhered tightly to the left optic nerve and slightly to the left ICA. Then, the tumor was carefully dissected, internally decompressed, and removed without active bleeding. The stalk, superior hypophyseal arteries, and the floor of the third ventricle were preserved. A small tumor portion that adhered to the left optic nerve remained in situ.
On the 1st postoperative day, she was fully conscious without neurological deficits except for left visual impairment. CT revealed no hemorrhage or new infarct. She was afebrile but complained of left eye pain on the 4th postoperative day. The next day, she suffered sudden-onset headache and emesis, and her condition deteriorated.
CT revealed massive SAH in the tumor cavity and the suprasellar, carotid, optic, and anterior interhemispheric cisterns. CTA showed an aneurysm on the left paraclinoid ICA, and subsequent intra-arterial digital subtraction angiography revealed an occluding left ICA pseudoaneurysm whose wall was irregular (Fig. 2). It was trapped, and a high-flow bypass from the left external to the left middle cerebral artery was placed using a radial artery graft. Thereafter, she remained unconscious and developed prolonged mild fever.
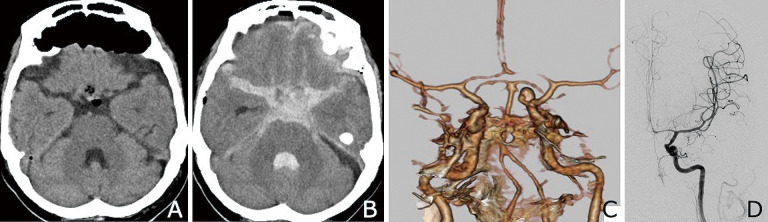
Fig. 2.
Post-endoscopic endonasal surgery images.
A CT performed on the 1st postoperative day shows no hemorrhage.
B CT scan obtained on the 5th postoperative day reveals massive SAH with intraventricular hemorrhage.
C CTA performed immediately shows a paraclinoid aneurysm on the left internal carotid artery.
D Digital subtraction angiogram acquired approximately 10 hours thereafter shows occlusion of the irregularly shaped aneurysm on the left internal carotid artery.
Analysis of her cerebrospinal fluid (CSF) performed on the 8th postoperative day showed an increase in CSF cells (180 cells/μL), a neutrophil/lymphocyte (N/L) ratio of 315:225, a high CSF protein (1205 mg/dL), and low glucose level (16 mg/dL). Based on these findings, we suspected meningitis.
We treated her prophylactically with cefazolin (3 g/day) during the 3 days preceding EES. After detecting her postoperative SAH, we switched to ciprofloxacin (600 mg/day) during the 3 days preceding SAH surgery. As CSF analysis had revealed mild pleocytosis, a decline in glucose level, and an increase in protein content, we treated her empirically with meropenem (6 g/day) for 17 days and vancomycin (1.5 g/day) for 4 days. As CSF cultures and blood tests showed no growths, we did not suspect fungal infection. We did not perform a β-D-glucan test.
Repeat CSF studies performed on the 21st postoperative day revealed a decrease in the number of CSF cells (22 cells/μL); the N/L ratio was 39:28, CSF protein fell to 105 mg/dL, and the glucose level was normalized (115 mg/dL). Although she remained under intensive care to monitor for post-SAH vasospasm, she developed pulmonary edema, respiratory failure, and a sudden, huge melena due to an anal ulcer. On the 25th day, we noted a huge left infarct and midline shift, and she died.
Autopsy revealed Aspergillus mycosis, and a final diagnosis of true fungal aneurysm was made. Aspergillus hyphae invaded the vessel wall; the internal elastic lamina was disrupted by inflammatory infiltrates. There was an invasion into the cerebrum by fungal colonies smaller than in the vessel wall, suggesting that Aspergillus hyphae had primarily invaded and then disrupted the vessels, leading to the spread of colonies into the cerebrum (Fig. 3). Pathologically, the tumor was diagnosed as a regrown adamantinomatous craniopharyngioma without malignant components. No Aspergillus hyphae were seen to surround the tumor.
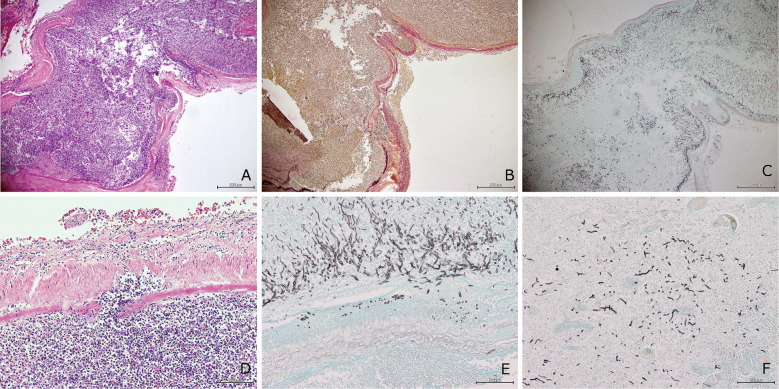
Fig. 3.
Images of the left internal carotid artery (ICA).
A–C The wall of the left ICA is disrupted.
A The H&E-stained section shows severe infiltration by acute and chronic inflammatory cells in all layers of the ICA (×40).
B EVG stain (×40). The internal elastic lamina is disrupted by inflammatory infiltrates.
C Grocott stain (×40). Mycotic colonies invading the vascular wall.
D H&E stain (×200). Note the severe infiltration of acute and chronic inflammatory cells in all ICA layers and the vascular lumen.
E Grocott stain (×200). Mycotic colonies invading the vascular wall. Characteristically branching Aspergillus hyphae suggest Aspergillus infection.
F Grocott stain (×200). Aspergillus hyphae invade the cerebrum. The invading colonies are smaller than in the ICA wall.
Discussion
Craniopharyngiomas are known to be rare, histologically benign epithelial tumors in the sellar/parasellar region.8) Their surgical resection presents significant challenges. The gross total removal (GTR) of primary craniopharyngiomas tends to be considered in all age groups.9,10) EES facilitates excellent midline access and visualization and allows for the GTR of craniopharyngiomas.10-13) However, CSF leakage can hamper the EES approach.12,14) Neuroendoscopes and skull base reconstruction have decreased the incidence of CSF leakage to 0-15%.10,13,15,16) The incidence of meningitis after CSF leakage also fell to 1.0-5.9%.13,15-17)
Recurrent, previously treated craniopharyngiomas develop scar tissue and adhesions and present surgical challenges. The rate of GTR in repeat surgeries is markedly lower than in primary surgeries, whereas the rate of perioperative morbidity and mortality is higher.9,13,16)
Only seven reported patients who had undergone neurosurgery followed by fungal aneurysm rupture attributable to Aspergillus have been reported (Table 1).1-7) They and our patient were diagnosed at autopsy. The basilar artery and the ICA were common sites of fungal aneurysm formation; in one patient, the anterior cerebellar artery was involved. The seven patients' primary diseases were ruptured aneurysms (n = 3), pituitary adenomas (n = 2), and craniopharyngioma or Rathke's cleft cyst (n = 1 each). The three ruptured aneurysms were treated transcranially, whereas the two pituitary adenomas and the Rathke's cleft cyst were addressed by TSS. The surgical procedure used in craniopharyngioma patients was not described. All were at risk for immunosuppression due to hypopituitarism, elevated growth hormone levels, prolonged treatment with corticosteroids and/or antibiotics, and their post-SAH status.
Table 1.
Summary of eight patients with ruptured fungal aneurysms attributable to Aspergillus after neurosurgery
| Authors, year | Age/
sex |
Primary disease | Site of aneurysm | Surgery | Risk factor | Postoperative meningitis | Date of SAH after surgery | Outcome |
|---|---|---|---|---|---|---|---|---|
| Visudihiphan et al. (1973) [3] | 13/M | Craniopharyngioma | BA | NA | Hypopituitarism | Confirmed | 6 weeks | Death |
| Mielke et al. (1981) [2] | 58/F | Pituitary adenoma | BA, ICA | TSS | GH elevation, alcohol application, irradiation | NA | 6 months | Death |
| Asari et al. (1988) [1] | 48/M | Ruptured AComA aneurysm | ACA | PA | Post-SAH, temporary clipping | NA | 4 days | Death |
| Piotrowski et al. (1990) [4] | 40/F | Ruptured paraclinoid ICA aneurysm | BA | PA | Post-SAH, prolonged administration of steroids and antibiotics | Suspected | 6 months | Death |
| Komatsu et al. (1991) [5] | 61/F | Rathke’s cleft cyst | ICA | TSS | Hypopituitarism, alcohol application | Confirmed | 27 days | Death |
| Endo et al. (2002) [6] | 50/F | Ruptured AComA aneurysm | BA | IHA | Post-SAH | Suspected | 26 days | Death |
| Radotra et al. (2015) [7] | 38/F | Pituitary adenoma | ICA | TSS | Hypopituitarism | Confirmed | 3 weeks | Death |
| Present case | 70/F | Craniopharyngioma | ICA | EES | Prolonged steroid treatment, reoperation | Suspected | 5 days | Death |
ACA, anterior cerebellar artery; AComA, anterior communicating artery; BA, basilar artery; EES, endoscopic endonasal surgery; GH, growth hormone; ICA, internal carotid artery; NA, not applicable; PA, pterional approach; SAH, subarachnoid hemorrhage; TSS, microscopic transsphenoidal surgery
As our patient was older than 70 years and had undergone repeat surgeries for recurrent craniopharyngioma and prolonged treatment with steroids, she was at risk for complications.
In some of the previously reported patients, the site of fungal aneurysm formation was suspected to be associated with direct damage to vessel structures elicited by the application of absolute alcohol in the tumor cavity, or associated with irradiation or temporary clipping.1,2,5) The interval between the first operation and rupture of the fungal aneurysm varied from 4 days to 6 months. According to Asari et al.,1) histology confirmed that the temporary clip application damaged vascular structures in their patient who suffered subacute SAH 4 days after the operation. Even minor iatrogenic injury to such structures may facilitate Aspergillus invasion through the vessel walls.1,5)
Most arterial injuries are followed immediately by SAH. Almefty et al.18) reported that in patients with delayed postoperative aneurysmal rupture, it was difficult to determine whether iatrogenic direct injury or infection was culpable. The unrecognized avulsion of small dural branches may result in the development of delayed pseudoaneurysms. Post-mortem studies confirmed that our patient had suffered fungal aneurysmal rupture. We cannot rule out the possibility that a fragile perforator had torn from the left ICA, thereby creating a site for the invasion of Aspergillus hyphae. Autopsy revealed that Aspergillus hyphae had primarily invaded vessels and that the inflammatory infiltrates disrupted and occluded vessels, resulting in large infarction that, in turn, led to her death. In earlier patients, autopsy revealed direct fungal invasion of the vessel wall, intravascular thrombi, and aneurysm formation that elicited ischemic stroke due to ICA occlusion.19)
Paranasal sinus infection with intracranial extension has been identified as the commonest pattern of aspergillosis. Radiological findings are often nonspecific and of little diagnostic value. However, low signal intensity on T2-weighted images and the presence of sinus disease with features of fungal sinusitis can be useful. On MRI scans of suspected aspergillosis sinusitis, lesions show hypointense signals on both T1-weighted and extremely T2-weighted images. After contrast administration, homogenous, heterogeneous, or ring enhancement of abnormal soft tissue may be seen.19-23) Cho et al.21) reported that CT images revealed bony erosion and sclerosis in patients with chronic invasive fungal sinusitis. The mean age of their patients exceeded 70 years, and many presented with hypertension, indicating that Aspergillus sinusitis can occur in elderly, and not only in immunosuppressed individuals.
Kawakami et al.20) also reported that individuals older than 65 years are at increased risk for invasive sino-orbital aspergillosis and that their prognosis may be poor. Among the seven patients (median age, 68 years) who have been reported with the disease, two were neither diabetic nor immunosuppressed. CT scans revealed bone destruction in five of the patients. In one patient, no CT or MRI abnormalities were detected initially. The initial symptoms of aspergillosis sinusitis were headache and periorbital and facial pain.20-23) Saini et al.19) reported that in 9 of 12 immunocompetent patients with Aspergillus fungal infection, there were no imaging data indicative of predisposing factors.
Although the risk for fungal infection is higher after TSS than after surgery via the transcranial route, we suspect that in half of the reported patients, the transcranial route, probably entering the frontal sinus, was applied.1-7) This approach raises the risk for fungal infection to the level seen after TSS. Although aspergillosis of paranasal sinus origin can be clinically benign and noninvasive, the invasive type can be rapidly progressive, and fulminant disease may develop in immunocompetent patients.19-21)
When SAH occurs after fungal aneurysmal rupture, the outcome is uniformly fatal irrespective of treatment modality.1-7) To prevent complications, we irrigate the sphenoid sinus cautiously and deliver an antifungal agent when there is the risk for Aspergillus colonization.
Especially in elderly patients, reoperation and corticosteroid delivery must be contemplated carefully. Considering our patient's age and the tumor site and pathology, we decided that EES was deemed appropriate. Her postoperative eye pain may have alerted us to the possibility of sino-orbital aspergillosis. The identification of the early stages of meningitis may improve the chance for survival.19,24) It remains difficult to diagnose CNS aspergillosis because in most patients, CSF cultures are negative, and CSF analysis yields only nonspecific findings such as pleocytosis and increased protein levels.6)
In conclusion, although intracranial Aspergillus aneurysmal rupture as a neurosurgical complication is extremely rare, the lack of specific findings on imaging and CSF studies renders a diagnosis difficult. Surgery that enters the paranasal sinuses may risk the development of aspergillosis. Especially immunosuppressed and elderly patients, those on steroid therapy, and patients scheduled for reoperation for recurrent craniopharyngioma must receive appropriate perioperative care, and the tumor must be carefully dissected from the vessels. An advanced age must be considered a risk factor, and fungal infection must be ruled out in patients whose postoperative course is unusual.
Funding
None
Abbreviations
CNS, central nervous system; CSF, cerebrospinal fluid; CT, computed tomography; EES, endoscopic endonasal surgery; GTR, gross total removal; ICA, internal carotid artery; MRI, magnetic resonance imaging; SAH, subarachnoid hemorrhage; TSS, microscopic transsphenoidal surgery
Conflicts of Interest Disclosure
None
Acknowledgments
The authors thank Mrs. Mariko Ogi and Mrs. Natsuki Abe for autopsy assistance and pathological research.
References
- 1). Asari S, Nishimoto A, Murakami M: [A rare case of cerebral aspergillus aneurysm at the site of temporary clip application]. No Shinkei Geka 16: 1079-1082, 1988 [PubMed] [Google Scholar]
- 2). Mielke B, Weir B, Oldring D, von Westarp C: Fungal aneurysm: Case report and review of the literature. Neurosurgery 9: 578-582, 1981 [DOI] [PubMed] [Google Scholar]
- 3). Visudhiphan P, Bunyaratavej S, Khantanaphar S: Cerebral aspergillosis. Report of three cases. J Neurosurg 38: 472-476, 1973 [DOI] [PubMed] [Google Scholar]
- 4). Piotrowski WP, Pilz P, Chuang IH: Subarachnoid hemorrhage caused by a fungal aneurysm of the vertebral artery as a complication of intracranial aneurysm clipping. Case report. J Neurosurg 73: 962-964, 1990 [DOI] [PubMed] [Google Scholar]
- 5). Komatsu Y, Narushima K, Kobayashi E, Tomono Y, Nose T: Aspergillus mycotic aneurysm--Case report. Neurol Med Chir (Tokyo) 31: 346-350, 1991 [DOI] [PubMed] [Google Scholar]
- 6). Endo T, Tominaga T, Konno H, Yoshimoto T: Fatal subarachnoid hemorrhage, with brainstem and cerebellar infarction, caused by Aspergillus infection after cerebral aneurysm surgery: Case report. Neurosurgery 50: 1147-1150; discussion 1150-1141, 2002 [DOI] [PubMed] [Google Scholar]
- 7). Radotra BD, Salunke P, Parthan G, Dutta P, Vyas S, Mukherjee KK: True mycotic aneurysm in a patient with gonadotropinoma after trans-sphenoidal surgery. Surg Neurol Int 6: 193, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM: The descriptive epidemiology of craniopharyngioma. J Neurosurg 89: 547-551, 1998 [DOI] [PubMed] [Google Scholar]
- 9). Morisako H, Goto T, Goto H, Bohoun CA, Tamrakar S, Ohata K: Aggressive surgery based on an anatomical subclassification of craniopharyngiomas. Neurosurg Focus 41: E10, 2016 [DOI] [PubMed] [Google Scholar]
- 10). Patel KS, Raza SM, McCoul ED, et al. : Long-term quality of life after endonasal endoscopic resection of adult craniopharyngiomas. J Neurosurg 123: 571-580, 2015 [DOI] [PubMed] [Google Scholar]
- 11). Cavallo LM, Prevedello DM, Solari D, et al. : Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. J Neurosurg 111: 578-589, 2009 [DOI] [PubMed] [Google Scholar]
- 12). Gardner PA, Prevedello DM, Kassam AB, Snyderman CH, Carrau RL, Mintz AH: The evolution of the endonasal approach for craniopharyngiomas. J Neurosurg 108: 1043-1047, 2008 [DOI] [PubMed] [Google Scholar]
- 13). Yamada S, Fukuhara N, Oyama K, et al. : Surgical outcome in 90 patients with craniopharyngioma: An evaluation of transsphenoidal surgery. World Neurosurg 74: 320-330, 2010 [DOI] [PubMed] [Google Scholar]
- 14). Kassam AB, Thomas A, Carrau RL, et al. : Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 63: ONS44-52; discussion ONS52-43, 2008 [DOI] [PubMed] [Google Scholar]
- 15). Cavallo LM, Frank G, Cappabianca P, et al. : The endoscopic endonasal approach for the management of craniopharyngiomas: A series of 103 patients. J Neurosurg 121: 100-113, 2014 [DOI] [PubMed] [Google Scholar]
- 16). Mou J, Wang X, Huo G, et al. : Endoscopic endonasal surgery for craniopharyngiomas: A series of 60 patients. World Neurosurg 124: e424-e430, 2019 [DOI] [PubMed] [Google Scholar]
- 17). Giese H, Haenig B, Haenig A, Unterberg A, Zweckberger K: Neurological and neuropsychological outcome after resection of craniopharyngiomas. J Neurosurg 132: 1425-1434, 2019 [DOI] [PubMed] [Google Scholar]
- 18). Almefty R, Dunn IF, Aziz-Sultan MA, Al-Mefty O: Delayed carotid pseudoaneurysms from iatrogenic clival meningeal branches avulsion: Recognition and proposed management. World Neurosurg 104: 736-744, 2017 [DOI] [PubMed] [Google Scholar]
- 19). Saini J, Gupta AK, Jolapara MB, et al. : Imaging findings in intracranial aspergillus infection in immunocompetent patients. World Neurosurg 74: 661-670, 2010 [DOI] [PubMed] [Google Scholar]
- 20). Kawakami H, Mochizuki K, Ishida K, Ohkusu K: Seven cases of localized invasive sino-orbital aspergillosis. Jpn J Ophthalmol 61: 179-188, 2017 [DOI] [PubMed] [Google Scholar]
- 21). Cho SJ, Choi YJ, Cho KJ, et al. : Image findings in patients with chronic invasive fungal infection of paranasal sinuses. J Neuroradiol 48: 325-330, 2021 [DOI] [PubMed] [Google Scholar]
- 22). Saffarian A, Derakhshan N, Taghipour M, et al. : Sphenoid aspergilloma with headache and acute vision loss. World Neurosurg 115: 159-161, 2018 [DOI] [PubMed] [Google Scholar]
- 23). Verma A, Jain KK, Mohan S, Phadke RV: Diffusion-weighted MR imaging in posterior ischemic optic neuropathy. AJNR Am J Neuroradiol 28: 1839-1840, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Ramos-Gabatin A, Jordan RM: Primary pituitary aspergillosis responding to transsphenoidal surgery and combined therapy with amphotericin-B and 5-fluorocytosine: Case report. J Neurosurg 54: 839-841, 1981 [DOI] [PubMed] [Google Scholar]