Abstract
Objective:
Nearly 25% of SLE patients are hospitalized yearly, often for outcomes that may have been avoided if patients had received sustained outpatient care. We examined acute care use for vaccine-preventable illnesses to determine sociodemographic contributors and modifiable predictors.
Methods:
Using U.S. Medicaid claims from 29 states (2000–2010), we identified adults (18–65 years) with prevalent SLE and 12 months of enrollment prior to the first SLE code (index date) to identify baseline data. We defined acute care use for vaccine-preventable illnesses as emergency department (ED) or hospital discharge diagnoses for influenza, pneumococcal disease, meningococcal disease, herpes zoster, high-grade cervical dysplasia/cervical cancer, and hepatitis B, after the index date. We estimated the incidence rate (IR) of vaccine-preventable illnesses and used Cox regression to assess risk (HR, 95% CI), by sociodemographic factors and healthcare utilization, adjusting for vaccinations, comorbidities and medications.
Results:
Among 45,654 Medicaid beneficiaries with SLE, less than 10% received vaccinations. There were 1,290 patients with ≥1 ED visit or hospitalization for a vaccine-preventable illness (6.6 per 1,000 person-years); 93% of events occurred in unvaccinated patients. Patients who were Black compared to White had 22% higher risk. Greater outpatient visits were associated with lower risk.
Conclusion:
Medicaid beneficiaries with SLE who are not vaccinated are at risk for potentially avoidable acute care use for vaccine-preventable illnesses. Racial disparities were noted with a higher risk among Black compared to White patients. Greater outpatient use was associated with reduced risk, suggesting that access to ambulatory care may reduce avoidable acute care use.
Background
Systemic lupus erythematosus (SLE) is a chronic, autoimmune disease that places patients at increased risk for adverse outcomes including serious infections, end-stage renal disease, cardiovascular disease and cerebrovascular disease. Almost one-fourth of patients with SLE are hospitalized each year in the U.S. and readmission rates are sixth among all chronic medical conditions resulting in high healthcare costs.(1) Nearly twenty years ago, the U.S. Agency for Healthcare Research and Quality defined a set of general conditions that are considered to be “ambulatory care sensitive” and may result in avoidable, costly acute care use (hospitalizations and emergency department (ED) visits).(2) Recently, this set of conditions was updated and tailored to SLE. The goal was to define a set of adverse outcomes for which high-quality sustained outpatient rheumatologic care could prevent the need for acute care use, or for which early intervention could minimize complications.(3) Vaccine-preventable illnesses, including influenza, pneumococcal disease, herpes zoster, Hepatitis B, high-grade cervical dysplasia/cervical cancer and meningococcal disease, were included as key ambulatory care-sensitive conditions.
Prior studies among individuals with SLE have demonstrated high rates of serious infection and substantial associated mortality.(4) Rates of hospitalization for herpes zoster in this population are especially high and may be rising.(5) In addition, individuals with SLE and other systemic rheumatic diseases may be at higher risk for high-grade cervical dysplasia and cervical cancer compared to the general population, which may be in part related to persistence of human papillomavirus infection. In general, vaccinations for these conditions have been found to be both safe and efficacious among individuals with SLE. While decreased immunogenicity has been suggested at times in the setting of immunosuppressive use, in most cases, the vaccines still appear to be efficacious.(6) Despite this, influenza, pneumococcal and human papillomavirus vaccination rates among individuals with systemic rheumatic diseases remain suboptimal.(7–9)
In this study, we investigated rates of potentially avoidable acute care use for vaccine-preventable illnesses among individuals enrolled in Medicaid, the largest U.S. public health insurance for low income individuals. We hypothesized that there may be disparities by race/ethnicity in these adverse outcomes that could be mitigated by improved access to preventive care services including vaccinations. We also hypothesized that regular ambulatory care use would be associated with reduced risk.
Methods
Study Population
We used the Medicaid Analytic eXtract (MAX), which includes demographic information, administrative billing claims and drug dispensing data for Medicaid beneficiaries. We included individuals age 18–65 years with prevalent SLE, defined as three or more ICD-9 codes of 710.0 separated by 30 or more days from 29 states between 2000–2010.(10) Individuals over age 65 were excluded because of likely dual enrollment in Medicare and the possibility of missing claims in MAX. We required at least 12 months of continuous enrollment in Medicaid prior to the third SLE ICD-9 code and defined date of the third code as the index date. We excluded individuals with billing codes during the 12 months prior to the index date (“baseline period”) for HIV/AIDS, hysterectomy, bone marrow or organ transplant, malignancy, Hepatitis B infection or cervical dysplasia.
Outcome of Interest
Our primary outcome of interest was discharge diagnosis codes in the first or second position from acute care use (hospitalizations or ED visits) for vaccine preventable illnesses. We defined these using ICD-9 codes for influenza, pneumococcal disease, herpes zoster, Hepatitis B, high-grade cervical dysplasia/cervical cancer and meningococcal disease (see Appendix for codes used). We examined this as a composite outcome for all women and removed high-grade cervical dysplasia/cervical cancer from the composite outcome for the full cohort. We assessed our outcome beginning the day after the index date.
Baseline Vaccine Uptake and Covariates
We assessed vaccine uptake during the 12 months prior to and including the index date. We defined this as one or more billing claim for influenza, varicella zoster, pneumococcal, hepatitis B, human papillomavirus or Neisseria meningitidis vaccine. We extracted baseline demographic factors from the index date including age, sex, race/ethnicity (Black, Asian, Hispanic, American Indian/Alaska Native and more than one race), and geographic region (North, South, Midwest, West). During the 12-months prior to an including the index date, we assessed lupus nephritis using a previously validated algorithm and the SLE risk adjustment index, which has been shown to be a better predictor of in-hospital mortality than the Charlson comorbidity index. (11) We included baseline period ever/never medication use including glucocorticoids, hydroxychloroquine and immunosuppressives, as well as number of medications on the index date. We also determined health care utilization (number of outpatient and ED visits and hospitalizations, and number of SLE-related laboratory tests) during the baseline period. For women, we also included procedure codes for ≥1 Papanicolaou (Pap) cervical cancer screening test.
Statistical Analyses
We first examined vaccine uptake during the 12-month baseline period. We then examined the incidence rate (IR, 95% CI) for our composite outcome (acute care use for vaccine-preventable illnesses) as well as for each individual vaccine-preventable condition. We stratified the IR by age less than or ≥50 years for herpes zoster given the availability of the vaccine for individuals ≥50 but the potential risk for infection across all age groups. We included all infections separated by 30 or more days to avoid counting readmissions for the same infection. Outcomes were assessed beginning the day following the index date until individuals were censored at disenrollment, death, or end of the study period (2010). We then examined the proportion of illnesses occurring among individuals who did not have documentation of vaccination during the baseline period. For influenza, we conducted an additional analysis where we updated vaccination status yearly. For herpes zoster vaccine uptake, we restricted our population to ≥50 years of age at the index date to ensure that they were eligible for vaccination. This included only the live attenuated vaccine, and not the recombinant vaccine as approval of the latter occurred following the dates of this study. For HPV vaccination, the first vaccine was approved in 2006 for individuals 26 and under. We therefore restricted this eligible population to females ≤27 at the index date between 2006–2010. We then used Cox proportional hazards models to assess risk (hazard ratio [HR] and 95% CI) of first acute care visit for a vaccine-preventable illness by baseline demographics and healthcare use, adjusting for calendar year, baseline vaccinations, SLE-related comorbidities and medications. For our models in the female-only cohort, we included cervical dysplasia/cervical cancer in our composite outcome and Pap Test as a covariate. We also examined yearly trends in overall acute care use for vaccine-preventable illnesses. All analyses were conducted using SAS 9.4 (Cary, NC). This study was approved by the BWH Institutional Review Board and data were obtained from the Centers for Medicare and Medicaid Services through a Data Use Agreement and per the agreement, cell sizes <11 are suppressed.
Results
We identified 45,654 individuals with prevalent SLE. The mean age was 41 (SD 12), 93% were female and the mean follow-up time was 4 (SD 3) years. Forty percent of individuals were Black, 38% White, 16% Hispanic, 3% Asian, 1% American Indian/Alaska Native and 3% more than one race. Twenty percent resided in the Midwest, 21% the Northeast, 38% the South and 21% the West.
During the 12-month baseline period, 3,288 individuals (7.2%) received ≥1 influenza vaccine, 542 (1.2%) received pneumococcal vaccine, 285 (0.6%) hepatitis B vaccine, and 17 (0.04%) meningococcal vaccine. Of the 10,698 individuals eligible for the herpes zoster vaccine (age ≥51 at the index date), 237 (2.2%) received it, and of the 6,717 individuals eligible for the HPV vaccine (age ≤27 at the index date), 75 (1.1%) received it.
Beginning at the index date, during the follow-up period, we identified 1,290 individuals with one or more instance of acute care use for a vaccine preventable illness (IR 6.6 per 1,000 person-years). There were 61 individuals who had more than one vaccine preventable illness (60 with 2 and one with three), and 1,352 ED visits and hospitalizations among the 1,290 individuals. Incidence rates for specific vaccine-preventable illness are shown in Table 1. For each, >90% occurred among individuals without documentation of the respective vaccination during the baseline period. For influenza, 95% occurred among individuals without vaccination during the baseline period; 92% when updated to include yearly vaccinations. For herpes zoster, 386 cases (IR 2.5, 95% 2.3–2.8) occurred among individuals under 50 and 90 cases (IR 1.9, 95% CI 1.5–2.5) among individuals 50 and greater; 97% of cases in both age groups were among those unvaccinated.
Table 1:
Incidence rates of acute care use* for vaccine-preventable illnesses among Medicaid beneficiaries with prevalent SLE (N=45,654)
| Number of patients | Person-years | Incidence Rate per 1,000 person-years (95% CI) | Percent of total events occurring among unvaccinated patients** | |
|---|---|---|---|---|
| Composite of vaccine-preventable illnesses + | 1290 | 196,171 | 6.6 (6.2–6.9) | 93% |
| Herpes zoster | 476# | 199,434 | 2.4 (2.2–2.6) | 97% |
| Influenza | 397 | 199,725 | 2.0 (1.8–2.2) | 95%++ |
| Pneumococcal disease | 310 | 200,351 | 1.5 (1.4–1.7) | NR |
| Hepatitis B | 86 | 201,241 | 0.4 (0.3–0.5) | 100% |
| High-grade cervical dysplasia/Cervical cancer | 58 | 190,309 | 0.3 (0.2–0.4) | 100% |
| Meningococcal disease | 25 | 201,434 | 0.1 (0.1–0.2) | 100% |
Acute care use includes emergency department visits or hospitalizations
61 SLE patients had more than one vaccine-preventable illness but were included once in the composite measure; there were 1,352 ED visits or hospitalizations among 1,290 patients
Vaccine claims during the 12-month baseline period
386 cases occurred in individuals <50 (IR 2.5, 95% CI 2.3–2.8) and 90 cases in individuals ≥50 (IR 1.9, 95% CI 1.6–2.4). In both groups, 97% of cases were among those unvaccinated.
Updating influenza vaccine yearly, 92% had events without vaccine claims in the preceding vaccination season
NR= Cell sizes >0 and <11 are not reported; percent of events among patients who received pneumococcal vaccine is <11
Results from multivariable-adjusted Cox proportional hazards models were similar for the full cohort with the composite outcome excluding high-grade cervical dysplasia/cervical cancer, and the female-only model including it (Table 2). We found a higher risk of acute care use for vaccine preventable illness among individuals who were Black (HR 1.22, 95% CI 1.06–1.39) compared to White. There was a higher risk in the South and Midwest compared to the Northeast. Individuals with more outpatient visits had a lower risk of acute care use for vaccine-preventable illnesses in a dose-dependent relationship; HR 0.74, 95% CI 0.61–0.91 for 6–10 visits compared to none, and HR 0.67 (95% CI 0.55–0.82) for >10 visits compare to none. Prior hospitalization and more ED visits were associated with significantly higher risk. Lupus nephritis was associated with 27% higher risk (p=0.009) and glucocorticoid use with 23% higher risk (p=0.002). Over the study period, examination of yearly IRs for acute care use for vaccine-preventable illnesses demonstrated decreased rates from 2001–2010, with a peak in 2003, which is in line with national estimates due to a more severe influenza season with predominate influenza A (H3N2) (Figure 1).
Table 2:
Multivariable Cox regression model for first episode of acute care use for vaccine-preventable illnesses* by demographic factors and baseline healthcare utilization among Medicaid beneficiaries with prevalent SLE
| Variables | Full Model (N=45,654) Hazard ratio (95% CI) | Female-only Model (N=42,606) Hazard ratio (95% CI) |
| Age category (ref=51–65 years) | ||
| 18–34 years | 1.10 (0.93–1.31) | 1.13 (0.95–1.34) |
| 35–50 years | 1.06 (0.91–1.25) | 1.08 (0.92–1.27) |
| Male (ref=Female) | 0.98 (0.77–1.26) | -- |
| Race/ethnicity (ref=White) | ||
| Black | 1.22 (1.06–1.39) | 1.21 (1.06–1.39) |
| Asian | 1.21 (0.83–1.77) | 1.19 (0.81–1.74) |
| Hispanic | 1.04 (0.87–1.25) | 1.00 (0.83–1.20) |
| American Indian/Alaska Native | 1.33 (0.81–2.20) | 1.30 (0.79–2.16) |
| More than one race | 1.40 (1.04–1.87) | 1.23 (0.90–1.67) |
| Geographic region (ref=Northeast) | ||
| Midwest | 1.28 (1.06–1.54) | 1.27 (1.06–1.53) |
| South | 1.31 (1.11–1.54) | 1.22 (1.04–1.44) |
| West | 1.22 (1.02–1.47) | 1.19 (0.99–1.43) |
| Outpatient visits (ref=0) | ||
| 1–5 visits | 0.88 (0.73–1.05) | 0.87 (0.73–1.04) |
| 6–10 visits | 0.74 (0.61–0.91) | 0.74 (0.60–0.90) |
| >10 visits | 0.67 (0.55–0.82) | 0.66 (0.53–0.80) |
| Hospitalization (ref=0) | 1.52 (1.34–1.72) | 1.52 (1.34–1.72) |
| Emergency department visits (ref=0) | ||
| 1–5 visits | 1.71 (1.49–1.96) | 1.73 (1.51–1.99) |
| >5 visits | 3.05 (2.49–3.74) | 3.02 (2.46–3.70) |
Model also adjusted for calendar year, SLE risk adjustment index, number of medications at index date, medication use (glucocorticoids, immunosuppressants, hydroxychloroquine), and vaccinations during the 12-month baseline period; bolded values are statistically significant (p<0.05). Composite outcome includes influenza, pneumococcal disease, meningococcal disease, herpes zoster and hepatitis B.
Female-only model additionally includes the outcome of high-grade cervical dysplasia/cervical cancer and adjusts for baseline Pap Test utilization
Figure 1.
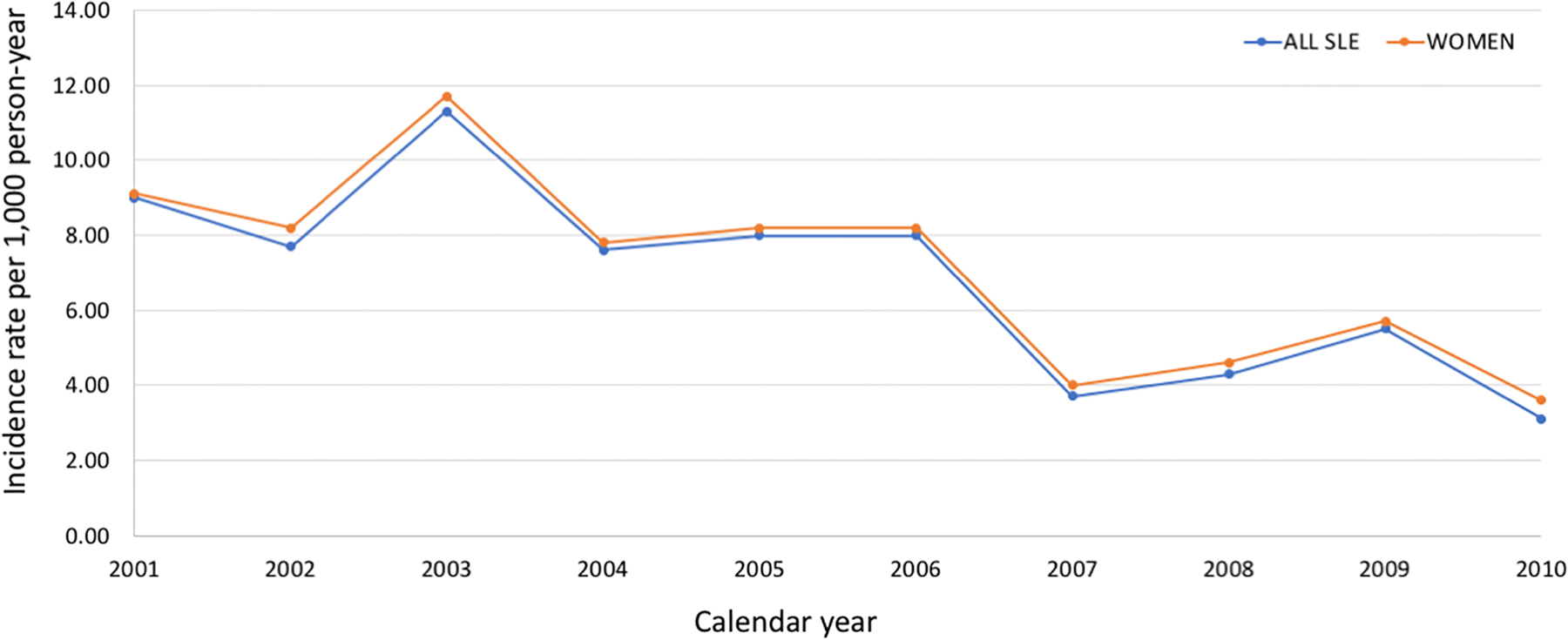
Trends in incidence rates of acute care use for vaccine-preventable illnesses
Discussion
In this population-based cohort of Medicaid beneficiaries with SLE, we identified low rates of vaccinations and a significant burden of potentially avoidable hospitalizations and ED visits for vaccine-preventable illnesses among individuals without documented vaccinations. We observed higher risk of these potentially avoidable hospitalizations and ED visits among individuals who were Black compared to those who were White and among those living in the South or Midwest compared to the Northeast. More frequent outpatient visits were associated with lower risk whereas more frequent acute care use at baseline was associated with a higher risk during follow-up.
We observed very low vaccine uptake in this population. A number of prior studies have reported suboptimal rates of influenza, pneumococcal, herpes zoster and HPV vaccine uptake among individuals with SLE and other systemic rheumatic diseases.(7–9, 12) The percentages of vaccine uptake in this cohort were on the lower end of the spectrum, which ranges from 10–70% depending on whether a quality improvement intervention was conducted, and how vaccine uptake was measured. The population included in this study was notably different from other studies. Prior studies that have similarly included Medicaid beneficiaries in their analyses have shown significantly lower vaccine uptake in this population, and in general among individuals with lower socioeconomic status. Prior studies examining individuals with SLE enrolled in Medicaid have demonstrated significantly poorer quality of care in multiple domains.(13) In addition, health providers are frequently reimbursed amounts below vaccination provision costs for Medicaid beneficiaries, which may provide less incentive for providers and thus reduce vaccine access for low-income individuals.(14) Vaccination rates have also been shown to be higher among individuals receiving care at a dedicated SLE clinic compared to a general rheumatology clinic and this population in particular has previously been shown to have more difficulty accessing subspecialty care.(15) Low rates observed in this study may also be attributed to lack of provider recommendations, which has been previously shown to be the main reason behind lack of pneumococcal and influenza vaccination in a cohort of SLE patients. (16) Low rates of the herpes zoster vaccine may also relate to issues of safety as it is a live vaccine that is not recommended in the setting of immunosuppression.
It is also possible the low rates we observed were at least in part due to underreporting for vaccinations that are less expensive or may have been administered at places of employment or as part of a local public health campaign, which would not be billed through insurance. We also only examined vaccine uptake during a 12-month period, and it is possible that individuals may have been vaccinated previously. It is also possible that patients with incident SLE may have different patterns. However, we examined both a cohort of prevalent SLE patients with a 24-month baseline period (N=36,248) and a cohort of patients with incident SLE (N=10,906) and both yielded findings similar to our primary analyses (See Supplemental Tables 1 and 2). The incidence rates of acute care use for vaccine-preventable illnesses were comparable between the incident and prevalent SLE populations. The incidence rates were slightly lower among prevalent SLE patients with a prolonged baseline period, possibly because of survival bias among those continuously enrolled in Medicaid, however the percentage occurring among unvaccinated patients was comparable to the primary analysis.
We observed a significant number of ED visits and hospitalizations for vaccine-preventable illnesses among individuals who did not have documentation of vaccination during the baseline period. There was a higher risk among Black individuals compared to White, which is in line with prior studies in this Medicaid SLE cohort that point to racial disparities in serious infection burden and in other adverse outcomes.(4) This finding remained after accounting for SLE disease severity as well as medication use. Access to high quality, subspecialty outpatient care and preventive services likely contributes significantly to this disparity. It is also possible that experiences of racial discrimination and provider implicit bias result in poorer access to vaccinations and in turn, a higher burden of vaccine-preventable illnesses. In a prior study, a higher number of outpatient physician visits was associated with higher likelihood of receipt of preventive care.(8) Here too, we found that more frequent outpatient visits was associated with lower risk of vaccine-preventable illnesses. More visits may be reflective of a sicker patient who requires more frequent follow-up, but it may also mean more opportunities for preventive care, including but not limited to vaccinations. Regional differences in quality of care have been demonstrated here as well as in prior studies in this population and may reflect issues of access or possibly, state-specific practices regarding subspecialty availability or preventive care use. We also identified similar if not slightly higher rates of herpes zoster among individuals under age 50 compared to those 50 and older. The zoster vaccine is only approved for individuals ≥50, yet the high rates among younger individuals with SLE suggest a missed opportunity for prevention.
While we did not have access to vaccine-preventable outcomes among non-SLE patients in this population, a previously published study examined the burden of vaccine-preventable illnesses from outpatient, ED and hospitalization claims among Medicaid beneficiaries from 2000–2006.(17) The IR of all utilization among individuals age 19–64 for influenza was 4.6 (95% CI 4.5–4.6) per 1,000 person-years and for pneumococcal disease, 0.64 (0.62–0.66) per 1,000 person years. For herpes zoster, among 50–64-year-olds in the general Medicaid population, the IR was 3.8 (95%CI 3.7–3.9) per 1,000 person-years. Trends over the time period in this general population suggest fluctuations in influenza, plateauing of pneumococcal disease and decreases in herpes zoster infections. To provide a comparison, when we examine estimates in this SLE population of Medicaid beneficiaries that include outpatient claims with ED visits and hospitalizations, the IR for influenza, pneumococcal disease and herpes zoster were 8.7 (95% CI 8.3–9.2, 4.6 (95% CI 4.3–4.9), and 8.6 (95% CI 8.2–9.0) per 1,000, respectively. This suggests, as previously demonstrated (4), that infection risk is extremely high among Medicaid beneficiaries with SLE, further emphasizing the importance of prevention in this population.
This study has several strengths. We included a racially/ethnically diverse population across the U.S. to understand the burden of costly acute care use for potentially avoidable conditions. By identifying populations at higher risk, strategies could be developed to improve vaccine uptake and access to outpatient care among the most vulnerable. We also demonstrated that while acute care use for vaccine-preventable illnesses has declined between 2000–2010, there are still a significant number of missed opportunities with this outcome occurring among unvaccinated individuals. This study also has limitations. As mentioned, vaccinations may be missed if patients paid out of pocket or were vaccinated outside of the baseline period window. There was also short follow-up for HPV vaccination, and the dates of this study preceded the approval of the new zoster vaccine that can be administered in the setting of immunosuppressive medications. We also limited our analyses to vaccine-preventable illnesses however there are other infections (e.g. Pneumocystis jiroveci pneumonia) that may be preventable by other means. Data that would differentiate between primary care and subspecialty outpatient care utilization were not available in this Medicaid data set limiting our ability to make inferences regarding where patients were receiving care. In addition, because of the small number of vaccinated individuals and data use restrictions, we are unable to present incidence rates of infections among those vaccinated vs. unvaccinated. However, we demonstrate that the vast majority (>90%) of acute care use episodes for vaccine-preventable illnesses occurred among individuals without claims for vaccines.
In this study, we identified a significant burden of potentially avoidable acute care use for vaccine-preventable illnesses with disparities by race/ethnicity and differences by U.S. region. While these preventable illnesses appeared to decrease over the study time period, these events present missed opportunities, particularly in a population of individuals who have heightened vulnerability to suboptimal care and adverse outcomes. Efforts should be made to ensure adequate provider reimbursement for vaccinations for Medicaid beneficiaries and outreach and care coordination programs should be implemented to improve access to preventive care.(14) Health system-wide efforts are also needed to augment the quality of outpatient and preventive care to reduce the risk of avoidable outcomes, and the associated costs of acute care use among vulnerable populations.
Supplementary Material
Significance and Innovation.
In this vulnerable population of SLE patients, we observed low rates of vaccinations (influenza 7.2%/year and other vaccines <3%/year) and avoidable acute care use for vaccine-preventable illnesses that predominately occurred among individuals without claims for vaccinations.
Sociodemographic factors, specifically Black race and residence in the South or Midwest, were associated with higher rates of acute care use for vaccine-preventable illnesses.
Greater outpatient use was associated with significantly reduced risk of acute care use for vaccine-preventable illnesses suggesting that engagement in sustained, high quality care may minimize avoidable adverse outcomes and costly healthcare utilization patterns.
Acknowledgements:
We would like to acknowledge Leah Santacroce’s programming support.
Funding:
This study was funded by NIH/NIAMS K23 AR071500 (Feldman) and NIH/NIAMS K24 AR066109 (Costenbader).
Disclosures:
Dr. Candace Feldman serves in unpaid positions on the Board of Directors of the American College of Rheumatology and on the Medical-Scientific Advisory Council of the Lupus Foundation of America. She receives research support from Pfizer Pharmaceuticals unrelated to this work.
References
- 1.Elixhauser A, Steiner C. Healthcare Cost and Utilization Project, Readmission to U.S. Hospitals by Diagnosis, 2010 Rockville, MD; 2010. [Google Scholar]
- 2.AHRQ Quality Indicators - Guide to Prevention Quality Indicators: Hospital Admission for Ambulatory Care Sensitive Conditions. In: Quality AfHRa, editor. Rockville, MD; 2001. [Google Scholar]
- 3.Feldman CH, Speyer C, Ashby R, Bermas B, Bhattacharyya S, Chakravarty E, et al. Development of a Set of Lupus-Specific Ambulatory Care Sensitive, Potentially Preventable Adverse Conditions: A Delphi Consensus Study. Arthritis Care Res (Hoboken). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman CH, Hiraki LT, Winkelmayer WC, Marty FM, Franklin JM, Kim SC, et al. Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol. 2015;67(6):1577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray SG, Schmajuk G, Trupin L, Gensler L, Katz PP, Yelin EH, et al. National Lupus Hospitalization Trends Reveal Rising Rates of Herpes Zoster and Declines in Pneumocystis Pneumonia. PLoS One. 2016;11(1):e0144918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao Z, Tang H, Xu X, Liang Y, Xiong Y, Ni J. Immunogenicity and Safety of Influenza Vaccination in Systemic Lupus Erythematosus Patients Compared with Healthy Controls: A Meta-Analysis. PLoS One. 2016;11(2):e0147856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman CH, Hiraki LT, Lii H, Seeger JD, Kim SC. Human papillomavirus vaccine uptake among individuals with systemic inflammatory diseases. PLoS One. 2015;10(2):e0117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yazdany J, Tonner C, Trupin L, Panopalis P, Gillis JZ, Hersh AO, et al. Provision of preventive health care in systemic lupus erythematosus: data from a large observational cohort study. Arthritis Res Ther. 2010;12(3):R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg S, Tsagaris K, Cozmuta R, Lipson A. Improving the Combination Pneumococcal Vaccination Rate in Systemic Lupus Erythematosus Patients at an Adult Rheumatology Practice. J Rheumatol. 2018;45(12):1656–62. [DOI] [PubMed] [Google Scholar]
- 10.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2013;65(3):753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward MM. Development and testing of a systemic lupus-specific risk adjustment index for in-hospital mortality. J Rheumatol. 2000;27(6):1408–13. [PubMed] [Google Scholar]
- 12.Sheth H, Moreland L, Peterson H, Aggarwal R. Improvement in Herpes Zoster Vaccination in Patients with Rheumatoid Arthritis: A Quality Improvement Project. J Rheumatol. 2017;44(1):11–7. [DOI] [PubMed] [Google Scholar]
- 13.Yazdany J, Feldman CH, Liu J, Ward MM, Fischer MA, Costenbader KH. Quality of care for incident lupus nephritis among medicaid beneficiaries in the united states. Arthritis Care Res (Hoboken). 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granade CJ, McCord RF, Bhatti AA, Lindley MC. State Policies on Access to Vaccination Services for Low-Income Adults. JAMA Netw Open. 2020;3(4):e203316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arora S, Nika A, Trupin L, Abraham H, Block J, Sequeira W, et al. Does Systemic Lupus Erythematosus Care Provided in a Lupus Clinic Result in Higher Quality of Care Than That Provided in a General Rheumatology Clinic? Arthritis Care Res (Hoboken). 2018;70(12):1771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawson EF, Trupin L, Yelin EH, Yazdany J. Reasons for failure to receive pneumococcal and influenza vaccinations among immunosuppressed patients with systemic lupus erythematosus. Semin Arthritis Rheum. 2015;44(6):666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnarajah G, Carroll C, Priest J, Arondekar B, Burstin S, Levin M. Burden of vaccine-preventable disease in adult Medicaid and commercially insured populations: analysis of claims-based databases, 2006–2010. Hum Vaccin Immunother. 2014;10(8):2460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


