Abstract
Using a federally compatible, naturalistic at-home administration procedure, the present study examined the acute effects of three cannabis flower chemovars with different tetrahydrocannabinol (THC) to cannabidiol (CBD) ratios, in order to test whether chemovars with a higher CBD content produce different effects. Participants were randomly assigned to ad libitum administration of one of three chemovars (THC-dominant: 24% THC, 1% CBD; THC+CBD: 9% THC, 10% CBD; CBD-dominant: 1% THC, 23% CBD); 159 regular cannabis users (male = 94, female = 65) were assessed in a mobile pharmacology lab before, immediately after, and 1 h after ad libitum administration of their assigned chemovar. Plasma cannabinoids as well as positive (e.g., high, elation) and negative (e.g., paranoia and anxiety) subjective effects were assessed at each time points. Participants who used the CBD-dominant and THC + CBD chemovars had significantly less THC and more CBD in plasma samples compared to participants who used the THC-dominant chemovar. Further, the THC + CBD chemovar was associated with similar levels of positive subjective effects, but significantly less paranoia and anxiety, as compared to the THC-dominant chemovar. This is one of the first studies to examine the differential effects of various THC to CBD ratios using chemovars that are widely available in state-regulated markets. Individuals using a THC + CBD chemovar had significantly lower plasma THC concentrations and reported less paranoia and anxiety while also reporting similar positive mood effects as compared to individuals using THC only, which is intriguing from a harm reduction perspective. Further research is needed to clarify the harm reduction potential of CBD in cannabis products.
Keywords: cannabidiol, cannabinoids, marijuana, subjective effects, tetrahydrocannabinol
1 |. INTRODUCTION
The literature on the acute effects of cannabis dates back to the 1970s. One of the primary methods used to understand the effects of delta-9-tetrahydrocannabinol (THC; the primary psychoactive constituent of the cannabis plant) involves standardized smoking of cannabis in a laboratory setting. Measures related to cognition, mood, and intoxication are typically collected before and after participants undergo controlled cannabis administration. With respect to self-report measures, considerable evidence demonstrates that cannabis acutely increases positive mood.1–4 Similarly, more recent studies report that acute cannabis use increases subjective effects such as “high” and “liking,”5,6 even when using a “balanced placebo” design.7 Thus, there is a long history of studies in the literature documenting that acute cannabis use produces changes in measures related to positive mood and reward.
Studies also suggest that cannabis, and specifically THC, has a number of unpleasant and negative subjective effects. For example, while THC may reduce anxiety at low doses, it has been found to increase anxiety at higher doses.8,9 In addition, a number of laboratory studies suggest that higher doses of THC produce psychotomimetic effects, including reports of paranoia, dissociation, and depersonalization,10–14 and these acute effects may be greater among individuals at risk for psychosis.15 There is also an association among long-term THC use and risk for psychosis, which is one of the most important health risks of increased access to cannabis, especially high potency products.16 Recent studies suggest that these negative effects may be mitigated by the coadministration of cannabidiol (CBD) (for reviews, see the literature17–22). Furthermore, mechanistic studies suggest that the psychotomimetic effects of THC are associated with the inhibitory effect of THC on glutamate in subcortical regions (for reviews, see previous works23,24), while administration of CBD may be associated with an increase in subcortical glutamate signaling,25,26 providing a pharmacological hypothesis regarding how CBD might alter the effects of THC.
While some studies suggest that CBD may diminish the effects of THC on positive mood and cognition,27,28 more recent data do not support this assertion (see Haney et al.29) and one study even suggests that CBD may increase the positive mood effects of THC.30 There appears to be more consistent support in the literature for the notion that CBD may mitigate the psychotomimetic effects of THC (for review, see Freeman et al.31). More broadly, discrepancies in the literature for both the main effects of THC on subjective effects as well its interaction with CBD may in part be explained by methodological differences in routes of administration (e.g., intravenous, inhaled, and versus oral THC) as well as what exactly is being administered (e.g., full plant derived cannabinoids and terpenes versus isolated and purified cannabinoids). As noted in recent papers,31,32 it is important for studies to examine products and routes of administration that are commonly used in order to more fully understand the potential for negative effects, or in the case of CBD, the potential to mitigate those effects.
Finally, it is also possible that CBD may influence how individuals self-titrate their use of THC, possibly by altering the subjective effects as noted above. A number of studies demonstrate that users self-titrate their use of cannabis to achieve a desired level of effect. For example, previous research on the effect of smoking cannabis on lung function indicates that individuals using higher potency cannabis inhale less,33 which is consistent with a more recent smoking topography study indicating that individuals inhale less when using higher potency cannabis.34 In addition, other studies demonstrate that THC concentrations are negatively associated with the amount used as individuals titrate to achieve certain subjective effects.35 Finally, two recent studies utilizing an ad libitum administration design also observed self-titration. One study allowed men and women to self-titrate to a desired effect and found that women displayed lower plasma THC concentrations yet achieved the same subjective effect as men.36 Likewise, another study allowed concentrate and flower users to self-titrate and found that concentrate users displayed greater plasma THC concentrations yet demonstrated the same subjective response as flower users.37 Empirical data thus suggest that users adapt to the type of cannabis used by self-titrating their use of THC to reach a desired effect. Thus, if CBD alters the subjective effects of cannabis, decreasing the negative effects and perhaps increasing the positive mood effects, it is also possible that it may alter self-titration of THC levels, such that higher or lower THC levels are required to reach the desired effect.
Notably, the existing literature does not reflect the strengths and concentrations of cannabis products widely available in state-regulated markets.38 Further, the drug administration approaches employed in controlled laboratory studies (e.g., standardized puffing procedures) do not reflect real-world cannabis consumption methods.39,40 Given the schedule 1 status of cannabis, federal restrictions prohibit researchers from studying state-regulated cannabis products in a controlled laboratory environment (for more information regarding federal regulations surrounding cannabis research, see Hutchison et al.40). As such, although controlled laboratory studies are critical to advancing our knowledge base, study designs that prioritize external validity are needed to understand the effects of cannabis that is currently distributed and used in the legal market.
Using a naturalistic at-home administration procedure in concert with a mobile pharmacology laboratory, we examined the effects of three different forms of cannabis flower with different ratios of THC to CBD (THC dominant: 24% THC, 1% CBD; THC ± CBD: 9% THC, 10% CBD; CBD dominant: 1% THC, 23% CBD) that are commonly available in state regulated dispensaries to determine if chemovars with higher CBD mitigate or enhance some of the effects of THC and alter self-titration (Note: This naturalistic design has been validated previously32,40). Specifically, we examined the acute effect of CBD on ad libitum administration and subsequent plasma concentrations of THC, positive subjective effects, and psychotomimetic effects (e.g., paranoia and anxiety). We hypothesized that the higher CBD chemovars would be associated with lower plasma levels of THC, less positive subjective effects, and lower anxiety and paranoia.
2 |. MATERIALS AND METHODS
2.1 |. Participants
The study was approved by the University of Colorado Boulder Institutional Review Board and was carried out in accordance with the Declaration of Helsinki. Participants were recruited using social media postings and mailed flyers. Participants were screened over the phone by research staff. Criteria for inclusion were (a) age between 21 and 70; (b) used cannabis flower at least 4 times in the past month; (c) endorsed prior use (at least once by self-report) of the highest potency of flower cannabis that could be assigned in the study (i.e., 24% THC); (d) no recreational drug use (other than cannabis) in the past 7 days which was confirmed with a urine toxicology screen; (e) no daily tobacco use; (f) drinking alcohol 3 times or less per week, and <5 (men)/<4 (women) drinks per drinking occasion; (g) not seeking treatment for drinking; (h) not pregnant (verified via pregnancy test), or trying to become pregnant; (i) not receiving treatment for/no reported diagnosis of psychotic disorder, bipolar disorder, or schizophrenia.
2.2 |. Appointments
Primary outcome measures (i.e., plasma cannabinoid concentrations, positive and negative subjective effects) were assessed at all four study time points: once during the baseline appointment (baseline) and three times during the mobile pharmacology laboratory appointment (pre-use, acute post-use, 1-h post-use).
2.2.1 |. Baseline appointment
Participants were instructed not to use cannabis for 24 h prior to their baseline visit. Following informed consent, participants were breathalyzed to ensure that they had no measurable level of blood alcohol. A urine toxicology screen was used to exclude individuals who demonstrated recent use of recreational drugs such as methamphetamine, opioids, benzodiazepines, and cocaine. Female participants were also given a pregnancy test to exclude women who were pregnant (none tested positive). Participants completed questionnaires on demographics, lifestyle, and medical history, as well as self-report measures of subjective drug effects, substance use, and other measures of mood.
At the end of the appointment, participants were randomly assigned to one of three chemovars of flower, each with a different ratio of THC to CBD: THC dominant (24% THC; 1% CBD), THC + CBD (9% THC; 10% CBD) or CBD dominant (1% THC; 23% CBD), using a random assignment table generated by the study staff. Participants were asked to purchase their assigned product at a local, study-partnered dispensary (The Farm; https://thefarmco.com/) and asked to purchase enough product to use for 5 days. Consistent with State of Colorado requirements, the THC and CBD potencies of each study product were on the label following testing in an International Organization of Standards (ISO) 17025 accredited laboratory. Thus, while researchers conducting all assessments were blind to condition, the participants themselves were not.
2.2.2 |. Mobile pharmacology laboratory appointment
Between the baseline and mobile pharmacology laboratory sessions, participants were asked to use the study cannabis product during a 5-day ad libitum use period leading up to their second appointment in order to familiarize themselves with their assigned product. On average, during the 5-day period, participants used their study product on 3.20 days (SD = 1.12). This did not differ significantly by condition (ps > .286). Participants were asked to abstain from using cannabis the day of their mobile pharmacology lab appointment. For the second appointment, two researchers traveled to the participant in our mobile pharmacology laboratory. At the first mobile laboratory assessment (pre-use), the participant completed the primary outcome measures (see below), then returned to their home to use their assigned cannabis chemovar ad libitum through their preferred mode of administration. Participants weighed their product before and after use on a study-provided scale in order to report how much of the product they used during the experimental session (see Table 1). After using their product, they returned to the mobile lab to complete the outcome measures while acutely intoxicated (acute post-use). They remained in the mobile lab until 1 h after using and then completed the measures a final time (1-h post-use).
TABLE 1.
Sample demographics and baseline characteristics by condition
| THC | THC ± CBD | CBD | |
|---|---|---|---|
| n = 57 | n = 51 | n = 51 | |
| Demographics | |||
| Age | 36.19 (15.27) | 35.80 (16.47) | 36.19 (15.95) |
| Gender (% female) | 38.60% | 43.14% | 41.18% |
| Marital status (% married) | 17.54% | 25.49% | 29.42% |
| Education (% BACHELORS OR HIGHER) | 47.37% | 45.10% | 58.82% |
| Employment (% full-time employed) | 49.12% | 31.37% | 49.02% |
| Race (% non-Hispanic White) | 82.46% | 76.47% | 84.31% |
| Body mass index (BMI) | 23.08 (3.38) | 25.03 (5.13) | 23.89 (5.20) |
| Cannabis history and current use | |||
| Age of onset of regular cannabis use | 19.86 (9.05) | 21.96 (10.42) | 20.28 (7.19) |
| Cannabis use disorder score | 2.56 (2.34) | 2.18 (2.64) | 2.65 (2.54) |
| Days of flower usea (past 30 days) | 18.18 (11.22) | 17.61 (10.98) | 17.39 (11.58) |
| Days of concentrate usea (past 30 days) | 5.53 (9.06) | 4.37 (8.17) | 6.25 (9.60) |
| Days of edible usea (past 30 days) | 3.46 (7.89) | 1.39 (5.48) | 2.90 (7.20) |
| Baseline plasma THC (ng/ml) | 4.90 (6.18) | 7.08 (15.09) | 4.13 (6.19) |
| Baseline plasma CBD (ng/ml) | .65 (1.61) | .53 (1.73) | .51 (2.06) |
| Baseline plasma THCV (ng/ml) | .00 (.00) | .06 (.27) | .00 (.00) |
| Baseline plasma CBN (ng/ml) | .00 (.00) | .12 (.59) | .02 (.16) |
| Baseline plasma CBG (ng/ml) | .02 (.11) | .10 (.48) | .00 (.00) |
| Baseline plasma CBC (ng/ml) | .21 (.51) | .36 (1.30) | .10 (.34) |
| Other substance use factors | |||
| Days of alcohol usea (past 30 days) | 8.25 (8.54) | 5.69 (5.89) | 5.55 (6.56) |
| Days of tobacco usea (past 30 days) | 1.88 (6.02) | 0.78 (4.27) | 1.73 (6.16) |
| AUDIT | 2.95 (1.93) | 2.18 (1.60) | 2.47 (1.96) |
| Cannabis use during experimental appt. | |||
| Grams usedb | .29 (.25) | .28 (.34) | .34 (.29) |
| Time away from mobile lab | 14.47 (7.02) | 15.56 (7.39) | 17.45 (9.65) |
| Mode of ad libitum administration | |||
| Joint | 20.75% | 10.42% | 25.53% |
| Bong | 30.19% | 18.75% | 14.89% |
| Pipe | 41.51% | 60.42% | 48.94% |
| Vaporizer | 7.54% | 10.42% | 10.64% |
Note: No significant differences in demographics, cannabis use history, current cannabis use, other substance use factors, or cannabis use during the experimental appointment emerged between conditions. Standard deviations are included in parentheses.
Abbreviations: MDS, Marijuana Dependence Scale, AUDIT, Alcohol Use Disorders Identification Test.
Using a 30-day timeline follow-back.
Participants brought our scale into their home to measure the amount of study cannabis used during the experimental appointment.
2.3 |. Primary outcome measures
2.3.1 |. Plasma cannabinoid concentrations
A certified phlebotomist collected ~50 ml of venous blood through venipuncture of a peripheral arm vein using standard, sterile phlebotomy techniques, which was stored on ice in the mobile laboratory. Upon return to the laboratory, plasma was separated from erythrocytes by centrifugation at 1000× g for 10 min, transferred to a fresh microcentrifuge tube for phytocannabinoid analysis and a separate microcentrifuge tube for endocannabinoid analysis, and stored at −80°C. Plasma samples were sent to the iC42 Lab at the Anschutz Medical Campus. In total, we quantified concentration of THC, THCV, CBN, CBG, CBD, and CBC using validated high-performance liquid chromatography/mass-spectroscopy (HPLC-MS/MS) (API5500) in MRM mode.41 The baseline and pre-use blood draws were used to confirm cannabis abstinence prior to the baseline and mobile pharmacology lab appointments.
2.3.2 |. Subjective high and mood effects
Multiple measures were employed to assess the subjective high and mood effects of cannabis. Three items assessed cannabis high: “feel high” (10-point Likert-type scale), “mentally stoned” (5-point Likert-type scale), and “physically stoned” (5-point Likert-type scale). These items were averaged to create a composite subjective high score (α = .69). A modified version of the Profile of Mood States (POMS) questionnaire42 was also administered. POMS items were rated on a 5-point Likert-type scale, with responses ranging from 1 (not at all) to 5 (extremely). Elation and anxiety/tension subscales of the POMS were retained as the two primary outcomes for the present study (α = .76; α = .82), consistent with our prior studies on the acute effects of alcohol and other drugs (e.g., Hutchison et al.40). Paranoia was measured using the “paranoia” item on the POMS. A single item from the Drug Effects Questionnaire (DEQ) was also used to assess drug liking (“do you like any of the effects you’re feeling?”) on a 5-point Likert-type scale with responses ranging from 1 (not at all) to (5) extremely.
2.4 |. Baseline substance use variables
2.4.1 |. Timeline follow-back (TLFB)
A research assistant administered a calendar-assisted TLFB43 in order to assess participants’ drug use over the past 30 days. The present study includes TLFB measures of cannabis flower use, cannabis concentrate use, orally ingested cannabis use, tobacco use, and alcohol use.
2.4.2 |. Cannabis use disorder scale (CUDS)
Cannabis Use Disorder symptoms were assessed using the 11-item CUDS44 which was developed based on cannabis dependence criteria included in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).
2.4.3 |. Alcohol use disorders identification test (AUDIT)
In order to further characterize the sample in terms of substance use, participants completed the self-report AUDIT.45 AUDIT scores range from 0–40, and a score of 8 or more is associated with harmful or hazardous drinking.
2.5 |. Planned analyses
Analyses of plasma cannabinoid levels (across three timepoints: pre-, acute post-, and 1-h post-use) were conducted separately using mixed-effects models estimating random intercepts for participant to determine if conditions were different in terms of their cannabinoid levels. Because we had data on the other major cannabinoids and because there is little published data on plasma levels of other cannabinoids, we included analyses of all of the measured cannabinoids. In each model, we included linear and quadratic change over time as fixed effects. Additionally, in each model we included a set of two orthogonal contrast codes as fixed effects to test for condition differences. For each outcome of interest, we ran three models varying the set of orthogonal contrast codes in order to examine three relevant condition differences in plasma cannabinoid levels: THC vs. THC + CBD, THC vs.CBD, and CBD vs. THC + CBD. This approach allowed us to test each of the condition differences without dropping any data. Lastly, interaction effects tested whether linear and quadratic change over time varied by condition. In models where both the linear and quadratic effect of time were significant, we focused on the higher-order quadratic effect. We conducted simple effects tests to determine condition differences at both the acute post-use and 1-h post-use assessment time points.
Analyses of subjective effects (across two time points: acute post- and 1-h post-use) were conducted separately using mixed-effects models estimating random intercepts for participant. In each model, we included linear change over time as a fixed effect. Additionally, to account for baseline differences in the subjective outcome of interest, the pre-use measure of the outcome variable was included as a covariate in each model (e.g., pre-use paranoia was included as a covariate in the model treating paranoia as the dependent variable). After observing the pattern of means and standard errors by condition (see Figure 3), in the analyses of positive subjective effects (i.e., high, elation, dug liking), we decided to supplement the overall analysis with comparisons of the THC and THC + CBD conditions, which did not differ, to the CBD condition. To do so, we included a contrast code comparing the THC and THC + CBD conditions to the CBD condition (CBD = −1, THC/THC + CBD = +1). In comparison, in the analyses of negative subjective effects (i.e., anxiety/tension, paranoia), we compared the CBD and THC + CBD conditions, which did not differ, to the THC condition. To do so, we included a contrast code comparing the THC condition to the CBD and THC + CBD conditions (CBD/THC + CBD = −1, THC = +1). Interaction effects tested whether linear change over time varied by condition. Lastly, we conducted simple effects tests to determine condition differences at both the acute post-use and 1-h post-use assessment time points.
FIGURE 3.
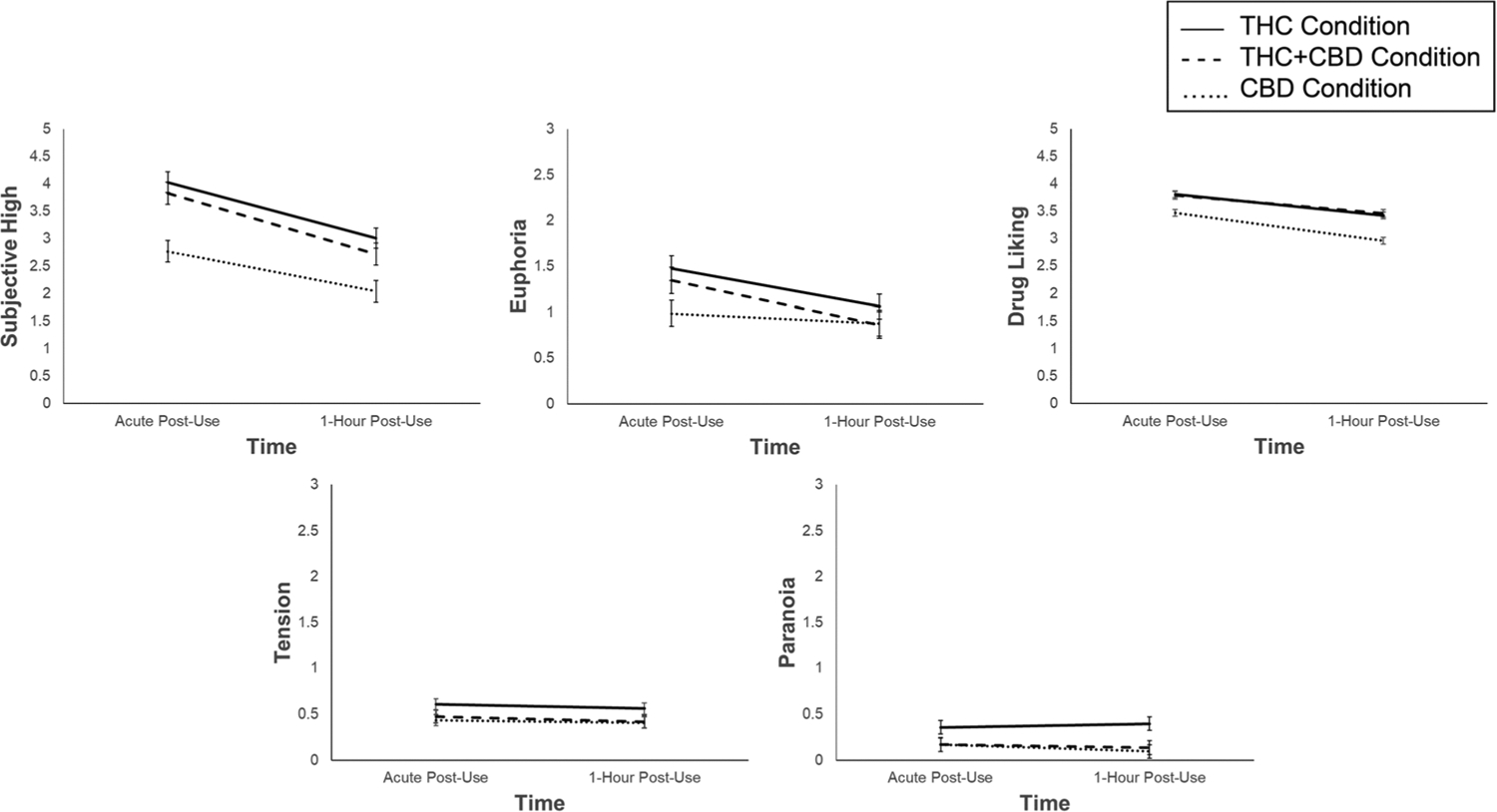
Adjusted mean subjective effects across the two post-use time points: acute and 1-h post-use (Positive subjective effects: At the acute post-use assessment, participants in the THC and THC + CBD conditions reported higher levels of subjective high, drug liking, and elation compared to those in the CBD condition. At the 1-h post-use assessment, participants in the THC and THC + CBD conditions reported higher levels of subjective high and drug liking compared to those in the CBD condition. Negative subjective effects: At the acute post-use and 1-h post-use assessments, participants in the THC condition reported higher levels of tension and paranoia compared to those in the CBD and THC + CBD conditions). Error bars are standard errors. All subjective effects were assessed on 5-point scales, with the exception of subjective high ratings which ranged from 1 to 6.67. Results for change in subjective effects from pre-use to acute post-use are presented in the supplementary materials
All analyses were conducted in R version 3.6.1 (http://www.rstudio.com) using the lme4 package version 1.1–25,46 which implements maximum likelihood (ML) estimation.
3 |. RESULTS
3.1 |. Demographics
One hundred eighty-four participants were recruited for the study, but 17 participants were dropped due to only completing their baseline appointment. An additional eight participants were excluded from the analysis due to acute post-use plasma cannabinoid concentrations that indicated that they did not use the correct chemovar (e.g., no CBD or high levels of THC detected in a participant assigned to use the CBD dominant chemovar). Thus, the final sample consisted of 159 (females = 65, males = 94) participants (see CONSORT flow diagram, Figure 1). Table 1 provides baseline characteristics of participants across the three flower conditions: THC, CBD, and THC + CBD. At baseline, there were no condition differences in circulating plasma cannabinoid levels. Grams of cannabis flower used during the experimental session did not differ across conditions.
FIGURE 1.
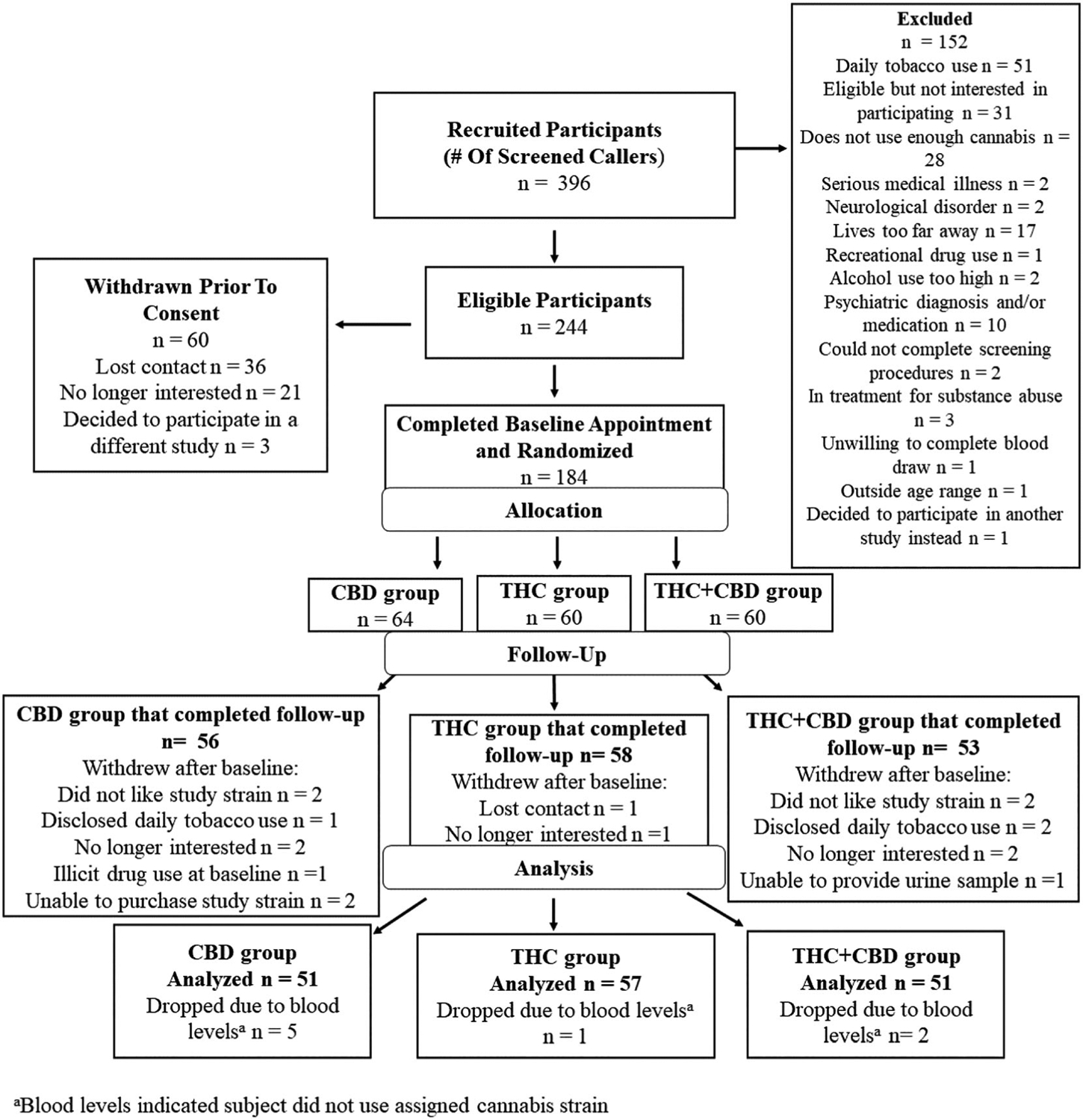
CONSORT flow diagram
3.2 |. Plasma cannabinoid concentrations over time
Plasma cannabinoid levels differed across the three assessment time points. See Figure 2 for plasma THC, CBD, CBC, THCV, CBN, and CBG levels across the three assessment time points (pre-use, acute post-use, 1-h post-use) by condition. Table 2 presents zero-order correlations between plasma cannabinoids and subjective effects at the acute post-use assessment time point (see Tables S1–S3 for correlations between plasma cannabinoids by cannabis chemovar condition). There were very few significant correlations between plasma cannabinoid concentrations and subjective effects at the acute post-use assessment time point (Note: This is in line with previous studies which have found that plasma cannabinoid levels are not always predictive of subjective drug effects).47,48 Results for change over time and condition differences in the minor cannabinoids (CBC, THCV, CBN, and CBG) are presented in the Supporting Information.
FIGURE 2.
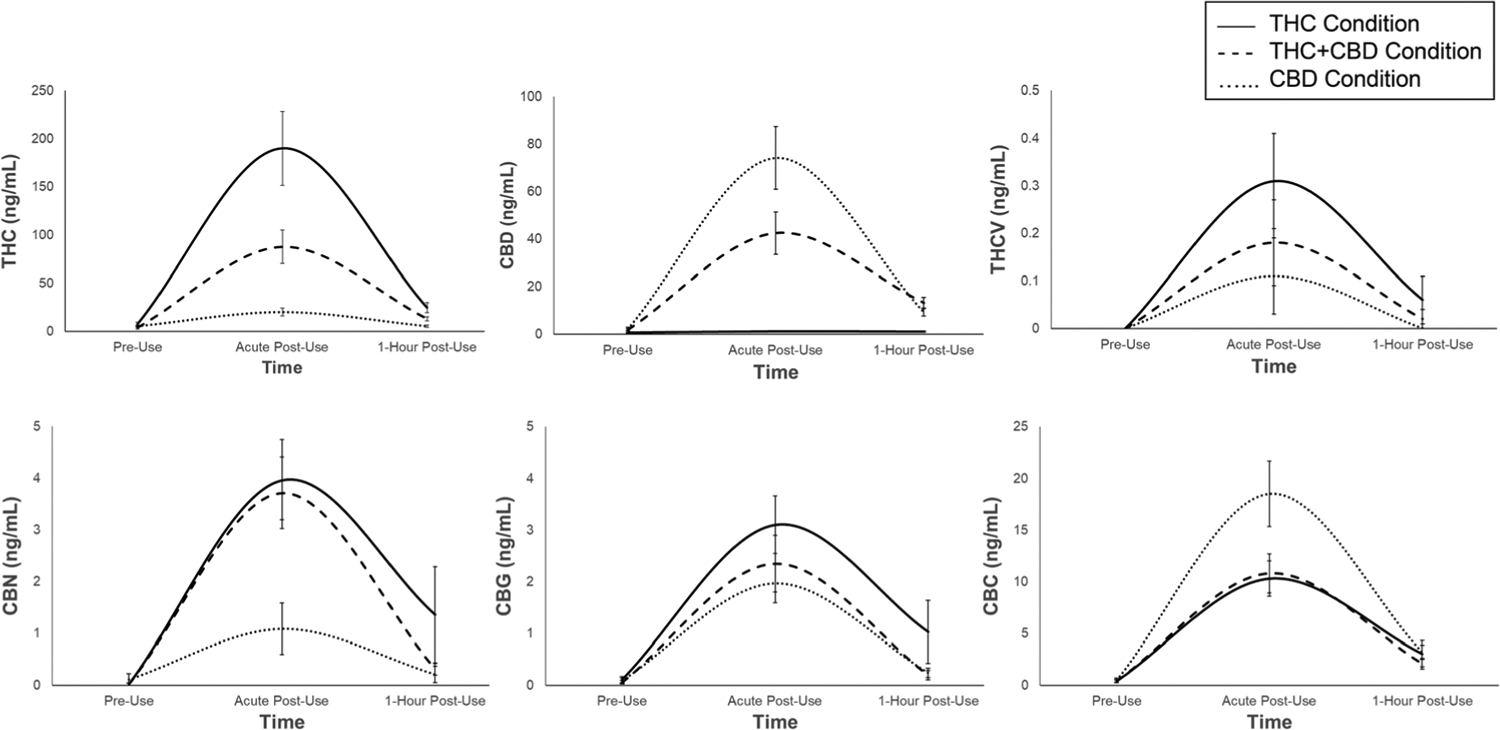
Unadjusted mean plasma cannabinoid levels (ng/ml) across the three assessment time points: Pre-use, acute post-use, and 1-h post-use. Error bars are standard errors
TABLE 2.
Means, standard deviations, and zero-order correlations between plasma cannabinoids and subjective effects at the acute post-use assessment time point
| Variable | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. THC (ng/ml) | 102.20 | 186.73 | ||||||||||
| 2. THCV (ng/ml) | 0.20 | 0.61 | .69*** | |||||||||
| 3. CBN (ng/ml) | 2.95 | 4.71 | .73*** | .59*** | ||||||||
| 4. CBG (ng/ml) | 2.49 | 3.44 | .77*** | .70*** | .73*** | |||||||
| 5. CBD (ng/ml) | 38.22 | 67.59 | .03 | .26** | .15 | .49*** | ||||||
| 6. CBC (ng/ml) | 13.16 | 16.10 | .43*** | .45*** | .43*** | .73*** | .77*** | |||||
| 7. Subjective high | 3.38 | 1.27 | .11 | .05 | .08 | −.02 | −.20* | −.16 | ||||
| 8. Elation | 1.16 | 1.07 | .04 | .01 | .08 | −.03 | −.15 | −.18* | .35*** | |||
| 9. Drug liking | 3.70 | 0.95 | .07 | .08 | .10 | .07 | .01 | −.01 | .38*** | .33*** | ||
| 10. Anxiety/tension | 0.36 | 0.45 | −.02 | −.11 | −.16 | −.07 | .01 | .05 | .14 | −.04 | −.12 | |
| 11. Paranoia | 0.21 | 0.51 | .02 | −.03 | −.08 | −.13 | −.16 | −.12 | .20* | .03 | −.05 | .61*** |
Note: M and SD are used to represent mean and standard deviation, respectively.
p < .05.
p < .01.
p < .001.
3.2.1 |. THC
THC exhibited a significant quadratic effect of time, such that plasma THC levels peaked at the acute post-use assessment and dropped an hour after use (B = 29.90 SE = 3.41, p < .001). All condition × quadratic time interactions were significant (ps < .013). Simple effects tests indicated that at the acute post-use assessment, participants in the THC condition had higher THC levels compared to those in the THC + CBD (p < .001) and CBD (p < .001) conditions, and participants in the THC + CBD condition had higher THC levels compared to those in the CBD condition (p = .024). There were no condition differences in THC levels 1-h post-use (ps > .487).
3.2.2 |. CBD
CBD exhibited a significant quadratic effect of time (B = 11.36, SE = 1.18, p < .001). All condition × quadratic time interactions were significant (ps < .002). Simple effects tests indicated that at the acute post-use assessment, participants in the CBD condition had higher CBD levels compared to those in the THC (p < .001) and THC + CBD (p < .001) conditions, and participants in the THC + CBD condition had higher CBD levels compared to those in the THC condition (p < .004). At the 1-h post-use assessment, participants in the CBD condition had marginally higher CBD levels compared to those in the THC condition (p = .064). No other condition differences emerged (ps > .338).
3.3 |. Subjective effects over time
See Figure 3 for subjective effects across the two post-use assessment time points (acute post-use, 1-h post-use), adjusted for pre-use ratings of the subjective effect of interest. Pre-use values of subjective effects by condition are presented in Table S4. Results for change in subjective effects from pre-use to acute post-use are presented in the Supporting Information.
3.3.1 |. Subjective high
Controlling for pre-use ratings of high, there was a significant decrease in feeling high from acute post-use to 1-h post-use (B = −0.48, SE = 0.03, p < .001) across all three groups. There was a significant time × condition interaction (B = −0.08, SE = 0.03, p = .018), such that participants in the THC and THC + CBD conditions reported a steeper decrease in subjective high from acute post-use to 1-h post-use relative to participants in the CBD condition. Simple effects tests indicated that participants in the THC and THC + CBD conditions reported feeling higher than those in the CBD condition at both the acute post-use assessment (p < .001) and the 1-h post-use assessment (p < .001).
3.3.2 |. Elation
Controlling for pre-use ratings of elation, there was a significant decrease in elation from acute post-use to 1-h post-use (B = −0.17, SE = 0.03, p < .001). There was a significant time × condition interaction (B = −0.09, SE = 0.04, p = .023); although participants in the THC and THC + CBD conditions reported a significant decrease in elation from acute post-use to 1-h post-use (p < .001), participants in the CBD condition did not experience a significant change in elation across the two time points (p = .389). Simple effects tests indicated that at the acute post-use assessment, participants in the THC and THC + CBD conditions experienced more elation than those in the CBD condition (p = .012)*. Elation levels were similar 1-h post-use (p = .614).
3.3.3 |. Drug liking
Drug liking significantly decreased from acute post-use to 1-h post-use (B = −0.21, SE = 0.03, p < .001). There was not a significant time × condition interaction (B = −0.04, SE = 0.04, p = .333). Simple effects tests indicated that participants in the THC and THC + CBD conditions reported greater drug liking compared to those in the CBD condition at both the acute post-use assessment (p = .052) and the 1-h post-use assessment (p = .006).
3.3.4 |. Anxiety/tension
Controlling for pre-use ratings of anxiety, anxiety levels marginally decreased from acute post-use to 1-h post-use (B = −0.02, SE = 0.01, p = .091). There was not a significant time × condition interaction (B = −0.002, SE = 0.01, p = .903). Simple effects tests indicated that participants in the THC condition reported higher levels of anxiety compared to those in the CBD and THC + CBD conditions at both the acute post-use assessment (p = .032) and the 1-h post-use assessment (p = .040).
3.3.5 |. Paranoia
Controlling for pre-use ratings of paranoia, paranoia levels did not differ from acute post-use to 1-h post-use (B = −0.01, SE = 0.02, p = .627). There was not a significant time × condition interaction (B = 0.02, SE = 0.02, p = .352). Simple effects tests indicated that participants in the THC condition reported higher levels of paranoia compared to those in the CBD and THC + CBD conditions at both the acute post-use assessment (p = .034) and the 1-h post-use assessment (p = .002).
4 |. DISCUSSION
The shifting policy, legal, and cultural landscape surrounding cannabis use in the United States has led to increased concern regarding public health risks associated with cannabis use. This is one of the first studies to examine the differential effects of various THC to CBD ratios using cannabis flower chemovars that are widely available in state-regulated markets. The present findings suggest that CBD may be associated with an overall reduction of THC exposure and may mitigate the negative psychotomimetic effects of THC without diminishing the effects of THC that individuals report liking. This study is an important step in identifying cannabinoid ratios that may alter risks for the user, thus highlighting a critical avenue in harm reduction research.
The first set of analyses examined how plasma cannabinoid levels differed across the chemovars after acute ad libitum administration. As expected, plasma levels of THC were significantly higher among individuals who were using the THC chemovar, significantly lower among those using the THC + CBD chemovar, and lowest among those using the CBD chemovar. Further, there were no condition differences in grams of cannabis flower used during the mobile pharmacology lab appointment. In effect, there was no evidence that individuals were titrating up their use of the THC + CBD or CBD chemovars in order to achieve higher THC levels. CBD levels mirrored the THC levels, such that the CBD group was the highest, followed by the THC + CBD group, and THC group. It is important to note that few, if any, large studies to date have examined plasma cannabinoids other than THC and CBD. The present study revealed significant differences across the chemovars in other cannabinoids, including THCV, CBN, CBC, and CBG. Because these cannabinoids are highly correlated with plasma levels of THC and CBD, it is not possible to know the degree to which they uniquely contribute to the results observed in this study. However, given the overall low levels of these cannabinoids relative to THC and CBD, it seems likely that the effects of CBD and THC explain the majority of effects. Nonetheless, the minor cannabinoid results highlight an important issue that is not commonly presented in research on cannabis products (i.e., that there is variation in terms of cannabinoids other than THC and CBD in the products and in plasma samples of people who are using the products) that could influence the effects of those products.
With respect to the effects of the chemovars on positive subjective states (e.g., high and elation), analyses suggested that the THC and THC + CBD chemovars were associated with almost identical increases in high and elation, despite the fact that the plasma level analyses indicated that those in the THC + CBD condition had significantly less (i.e., approximately 50% less) THC in their plasma. Consistent with this finding, the analysis of how much participants reported “liking” the chemovar indicated that participants liked the THC + CBD chemovar as much as the THC chemovar. Overall, these results suggest that the combination of THC + CBD was no different than THC with respect to positive subjective effects. Given the similar effects, individuals using a THC + CBD chemovar would not have a reason to increase their cannabis use to achieve higher levels of THC. The positive mood findings are consistent with other studies that have suggested that CBD does not diminish the effects of THC on positive mood29 (or may actually increase the positive mood effects of THC49).
One of the key questions in the literature is whether CBD mitigates the psychotomimetic effects of THC on paranoia and anxiety. In contrast to the analyses of positive states, analyses of the negative states (paranoia, anxiety) indicated that individuals in the THC condition reported greater paranoia and anxiety than individuals in the THC + CBD condition and the CBD condition, which were similar to one another. The measurable level of paranoia in the THC condition in this study was somewhat surprising, in that the current study involved the ad libitum use of THC in a highly experienced sample. Previous studies that have demonstrated an effect for THC on psychotomimetic measures directly infused synthetic THC in inexperienced users and found strong psychotomimetic and anxiogenic effects.11 Despite studying common products used ad libitum in frequent users, participants in the THC condition still showed statistically significantly higher levels of paranoia in comparison to the CBD and THC + CBD conditions. Thus, the present study suggests that chemovars with greater CBD and less THC are associated with differential effects on paranoia and anxiety among experienced users during ad libitum use, which is consistent with laboratory studies.31,50
While it is possible that the presence of CBD is having a direct effect on anxiety and paranoia, another possible interpretation of differences in anxiety and paranoia might be related to the effect of CBD on positive mood and self-titration. In other words, if CBD is enhancing the positive mood effects which leads to a reduction in THC consumption, one would also expect a reduction in anxiety and paranoia with or without any direct effects of CBD on these measures. This interpretation is consistent with a recently published paper that examined the effect of vaporizing THC with different levels of CBD.30 In that study, THC was compared to THC + CBD and other conditions using a tightly controlled vaporized administration procedure. Plasma levels of THC were similar in the two conditions due to the tight control over administration, but the THC + CBD condition was associated with a greater degree of subjective high, suggesting that CBD enhanced the effect of THC.30 Thus, in both studies, it is possible that CBD is enhancing the positive mood effects of THC. In the present study, in which participants were allowed to self-titrate, the enhancement led to similar levels of positive affect despite less THC exposure, which in turn may be the reason for less paranoia and anxiety. Alternatively, it is possible that these findings are the result of a ceiling effect of THC on the positive subjective effects but not the negative subjective effects of cannabis.
Although naturalistic administration procedures are needed to examine the effects of commercially available cannabis products, federal cannabis regulations led to several methodological shortcomings, including the lack of control over participants’ dosing and other aspects of cannabis administration (e.g., smoking topography), as well as the lack of a placebo control condition. In addition, as participants are legally prohibited from using commercially available cannabis products in a laboratory setting, they administered their products ad libitum in their own residences which precluded researchers from objectively verifying their cannabis use during the experimental session (i.e., grams used, mode of administration). Further, participants were not blind to the product they were using, and thus, expectancy effects may have influenced subjective outcomes. Further data are needed to explore the effects of different cannabis chemovars consumed in different formulations (e.g., orally administered vs. vaporized, cannabis flower vs. cannabis concentrate, etc.) on plasma cannabinoid levels and subjective effects. Future studies should also explore the many potential interactions between cannabinoid ratios, potencies, and individual factors (e.g., modality of consumption, smoking topography, expectancies) in predicting objective and subjective cannabis use outcomes. Lastly, it is important to note that the concentration of THC was greater in the THC-dominant chemovar (24%) relative to the THC + CBD chemovar (9%). Given this, we are unable to rule out the possibility that the diminished negative effects observed in the THC + CBD (vs. THC) condition are a result of less THC exposure, rather than the addition of CBD. Future naturalistic studies that match THC and THC + CBD chemovars on THC potency are needed to establish whether these findings are driven by (a) the inclusion of CBD or (b) differences in THC exposure.
In conclusion, the present study indicates that cannabis chemovars containing CBD in addition to THC may reduce some of the negative effects associated with THC consumption and should be tested as a potential harm reduction tool for cannabis users in future studies. Specifically, the results of the present study suggest that participants using the THC + CBD chemovar had significantly lower plasma THC levels and reported less paranoia and anxiety as compared to participants using the THC dominant chemovar. Importantly, despite these differences, participants in both the THC + CBD and THC conditions reported similar positive subjective effects. That participants in the THC + CBD condition displayed lower levels of plasma THC during the acute administration session while still reporting similar positive mood effects are intriguing from a harm reduction perspective. The harm reduction implication of these findings is that cannabis chemovars containing CBD may result in less overall exposure to THC and subsequently less potential for harm, particularly with respect to the psychotomimetic effects of THC.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by the National Institute on Drug Abuse (DA039707 to KEH).
Funding information
National Institute on Drug Abuse, Grant/Award Number: DA039707
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Men reported significantly greater elation at the acute post-use assessment time point relative to women (p = .028). No other significant gender differences in subjective effects or plasma cannabinoid concentrations emerged (ps > .602).
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The study protocol and de-identified participant data collected during the trial is available upon request. Data will be available beginning 3 months and ending 5 years following article publication. Data will be shared with researchers who provide a methodologically sound proposal. Proposals should be directed to kent.hutchison@colorado.edu. To gain access, data requesters will need to sign a data access agreement.
REFERENCES
- 1.Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96(7):1023–1034. 10.1080/09652140120053084 [DOI] [PubMed] [Google Scholar]
- 2.Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav. 1990;37(3):561–565. 10.1016/0091-3057(90)90028-G [DOI] [PubMed] [Google Scholar]
- 3.Chait LD, Zacny JP. Reinforcing and subjective effects of oral Δ9-THC and smoked marijuana in humans. Psychopharmacology (Berl). 1992;107(2–3):255–262. 10.1007/BF02245145 [DOI] [PubMed] [Google Scholar]
- 4.Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology (Berl). 1988;94(2): 206–212. 10.1007/BF00176846 [DOI] [PubMed] [Google Scholar]
- 5.Schacht JP, Selling RE, Hutchison KE. Intermediate cannabis dependence phenotypes and the FAAH C385A variant: an exploratory analysis. Psychopharmacology (Berl). 2009;203(3):511–517. 10.1007/s00213-008-1397-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper ZD, Haney M. Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor. Addict Biol. 2008;13(2):188–195. 10.1111/j.1369-1600.2007.00095.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metrik J, Kahler CW, Reynolds B, et al. Balanced placebo design with marijuana: pharmacological and expectancy effects on impulsivity and risk taking. Psychopharmacology (Berl). 2012;223(4):489–499. 10.1007/s00213-012-2740-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol Clin Exp. 2009; 24(7):515–523. 10.1002/hup.1048 [DOI] [PubMed] [Google Scholar]
- 9.Hunault CC, Böcker KBE, Stellato RK, Kenemans JL, de Vries I, Meulenbelt J. Acute subjective effects after smoking joints containing up to 69 mg Δ9-tetrahydrocannabinol in recreational users: a randomized, crossover clinical trial. Psychopharmacology (Berl). 2014;231(24):4723–4733. 10.1007/s00213-014-3630-2 [DOI] [PubMed] [Google Scholar]
- 10.Sagar KA, Gruber SA. Marijuana matters: reviewing the impact of marijuana on cognition, brain structure and function, & exploring policy implications and barriers to research. Int Rev Psychiatry. 2018; 30(3):251–267. 10.1080/09540261.2018.1460334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004; 29(8):1558–1572. 10.1038/sj.npp.1300496 [DOI] [PubMed] [Google Scholar]
- 12.Morrison PD, Nottage J, Stone JM, et al. Disruption of frontal theta coherence by Δ9-tetrahydrocannabinol is associated with positive psychotic symptoms. Neuropsychopharmacology. 2011;36(4):827–836. 10.1038/npp.2010.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison PD, Zois V, McKeown DA, et al. The acute effects of synthetic intravenous Δ9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39(10):1607–1616. 10.1017/S0033291709005522 [DOI] [PubMed] [Google Scholar]
- 14.Colizzi M, McGuire P, Giampietro V, Williams S, Brammer M, Bhattacharyya S. Modulation of acute effects of delta-9-tetrahydrocannabinol on psychotomimetic effects, cognition and brain function by previous cannabis exposure. Eur Neuropsychopharmacol. 2018;28(7):850–862. 10.1016/J.EURONEURO.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Mason O, Morgan CJA, Dhiman SK, et al. Acute cannabis use causes increased psychotomimetic experiences in individuals prone to psychosis. Psychol Med. 2009;39(6):951–956. 10.1017/S0033291708004741 [DOI] [PubMed] [Google Scholar]
- 16.Hasan A, von Keller R, Friemel CM, et al. Cannabis use and psychosis: a review of reviews. Eur Arch Psychiatry Clin Neurosci. 2019;270(4):1–10. 10.1007/s00406-019-01068-z [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of Δ−9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35(3):764–774. 10.1038/npp.2009.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machado Bergamaschi M, Helena Costa Queiroz R, Waldo Zuardi A, Crippa AS. Safety and side effects of cannabidiol, a cannabis sativa constituent. Curr Drug Saf. 2011;6(4):237–249. 10.2174/157488611798280924 [DOI] [PubMed] [Google Scholar]
- 19.Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36(6):1219–1226. 10.1038/npp.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94–e94. 10.1038/tp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niesink RJM, van Laar MW. Does cannabidiol protect against adverse psychological effects of THC? Front Psych. 2013;4:130. 10.3389/fpsyt.2013.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crippa JAS, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25(1):121–130. 10.1177/0269881110379283 [DOI] [PubMed] [Google Scholar]
- 23.Colizzi M, Weltens N, McGuire P, et al. Delta-9-tetrahydrocannabinol increases striatal glutamate levels in healthy individuals: implications for psychosis. Mol Psychiatry. 2019;25(12):1–10. 10.1038/s41380-019-0374-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colizzi M, McGuire P, Pertwee RG, Bhattacharyya S. Effect of cannabis on glutamate signalling in the brain: a systematic review of human and animal evidence. Neurosci Biobehav Rev. 2016;64:359–381. 10.1016/j.neubiorev.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 25.Linge R, Jiménez-Sánchez L, Campa L, et al. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology. 2016;103:16–26. 10.1016/J.NEUROPHARM.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 26.Pretzsch CM, Freyberg J, Voinescu B, et al. Effects of cannabidiol on brain excitation and inhibition systems: a randomised placebo-controlled single dose trial during magnetic resonance spectroscopy in adults with and without autism spectrum disorder. Neuropsychopharmacology. 2019;44(8):1398–1405. 10.1038/s41386-019-0333-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan CJA, Freeman TP, Schafer GL, Curran HV. Cannabidiol attenuates the appetitive effects of Δ9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology. 2010;35(9):1879–1885. 10.1038/npp.2010.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27(1):19–27. 10.1177/0269881112460109 [DOI] [PubMed] [Google Scholar]
- 29.Haney M, Malcolm RJ, Babalonis S, et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology. 2016;41(8):1974–1982. 10.1038/npp.2015.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solowij N, Broyd S, Greenwood LM, et al. A randomised controlled trial of vaporised Δ9-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: acute intoxication effects. Eur Arch Psychiatry Clin Neurosci. 2019;269(1): 17–35. 10.1007/s00406-019-00978-2 [DOI] [PubMed] [Google Scholar]
- 31.Freeman AM, Petrilli K, Lees R, et al. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neurosci Biobehav Rev. 2019;107:696–712. 10.1016/j.neubiorev.2019.09.036 [DOI] [PubMed] [Google Scholar]
- 32.Bidwell LC, Ellingson JM, Karoly HC, et al. Naturalistic administration of cannabis flower and concentrates: cannabinoid blood levels, subjective intoxication, cognition and motor function. JAMA Psychiat. 2020;77(8):787–796. 10.1001/jamapsychiatry.2020.0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthias P, Tashkin DP, Marques-Magallanes JA, Wilkins JN, Simmons MS. Effects of varying marijuana potency on deposition of tar and Δ9-THC in the lung during smoking. Pharmacol Biochem Behav. 1997;58(4):1145–1150. [DOI] [PubMed] [Google Scholar]
- 34.van der Pol P, Liebregts N, Brunt T, et al. Cross-sectional and prospective relation of cannabis potency, dosing and smoking behaviour with cannabis dependence: an ecological study. Addiction. 2014; 109(7):1101–1109. 10.1111/add.12508 [DOI] [PubMed] [Google Scholar]
- 35.Korf DJ, Benschop A, Wouters M. Differential responses to cannabis potency: a typology of users based on self-reported consumption behaviour. Int J Drug Policy. 2007;18(3):168–176. [DOI] [PubMed] [Google Scholar]
- 36.Matheson J, Sproule B, Di Ciano P, et al. Sex differences in the acute effects of smoked cannabis: evidence from a human laboratory study of young adults. Psychopharmacology (Berl). 2020;237(2):305–316. 10.1007/s00213-019-05369-y [DOI] [PubMed] [Google Scholar]
- 37.Bidwell LC, Ellingson JM, Karoly HC, et al. Association of naturalistic administration of cannabis flower and concentrates with intoxication and impairment. JAMA Psychiat. 2020;77(8):787–796. 10.1001/JAMAPSYCHIATRY.2020.0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vergara D, Bidwell LC, Gaudino R, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Sci Rep. 2017;7(1):1–8. 10.1038/srep46528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bidwell LC, Mueller R, YorkWilliams SL, et al. A novel observational method for assessing acute responses to cannabis: preliminary validation using legal market strains. Cannabis Cannabinoid Res. 2018;3(1): 35–44. 10.1089/can.2017.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutchison KE, Bidwell LC, Ellingson JM, Bryan AD. Cannabis and health research: rapid progress requires innovative research designs. Value Health. 2019;22(11):1289–1294. 10.1016/j.jval.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 41.Klawitter J, Sempio C, Mörlein S, et al. An atmospheric pressure chemical ionization MS/MS assay using online extraction for the analysis of 11 cannabinoids and metabolites in human plasma and urine. Ther Drug Monit. 2017;39(5):556–564. 10.1097/FTD.0000000000000427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 43.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Measuring Alcohol Consumption. Humana Press; 1992:41–72. 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- 44.Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68(5): 898–908. 10.1037/0022-006X.68.5.898 [DOI] [PubMed] [Google Scholar]
- 45.Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88(6):791–804. 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 46.Bates D, Sarkar D, Bates MD, Matrix L. The lme4 package. R Packag version. 2007;2(1):74. [Google Scholar]
- 47.Gibson LP, Gust CJ, Ellingson JM, et al. Investigating sex differences in acute intoxication and verbal memory errors after ad libitum cannabis concentrate use. Drug Alcohol Depend. 2021;223:108718. 10.1016/j.drugalcdep.2021.108718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spindle TR, Cone EJ, Schlienz NJ, et al. Acute pharmacokinetic profile of smoked and vaporized cannabis in human blood and oral fluid. J Anal Toxicol. 2019;43(4):233–258. 10.1093/jat/bky104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solowij N, Broyd SJ, Beale C, et al. Therapeutic effects of prolonged cannabidiol treatment on psychological symptoms and cognitive function in regular cannabis users: a pragmatic open-label clinical trial. Cannabis Cannabinoid Res. 2018;3(1):21–34. 10.1089/can.2017.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M. Clinical and preclinical evidence for functional interactions of cannabidiol and delta-9-tetrahydrocannabinol. Neuropsychopharmacology. 2018; 43(1):142–154. 10.1038/npp.2017.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol and de-identified participant data collected during the trial is available upon request. Data will be available beginning 3 months and ending 5 years following article publication. Data will be shared with researchers who provide a methodologically sound proposal. Proposals should be directed to kent.hutchison@colorado.edu. To gain access, data requesters will need to sign a data access agreement.


