Central Illustration
Key Words: cardiac transplantation, gene editing, xenotransplantation
Abbreviations and Acronyms: CRISPR/Cas9, clustered regularly interspaced short palindromic repeats–associated RNA-guided DNA endonuclease Cas9; Gal, α-galactose 1,3-galactose; GH, growth hormone; HF, heart failure; HTx, heart transplantation; NHP, nonhuman primate; PERV, porcine endogenous retrovirus; XTx, xenotransplantation
Highlights
-
•
Cross-species experiments heralded progress in clinical cardiac xenotransplantation.
-
•
Immune, physiological, socio-economic and ethical barriers need to be overcome.
-
•
A framework for clinical trials is needed to advance clinical xenotransplantation.
Summary
The increased need for heart transplantation in patients with advanced heart failure has introduced demand for a greater supply of donor hearts. Progress in cross-species experimental models has led to promise for ushering in the clinical use of xenotransplantation (XTx) as a potential solution to the organ shortage worldwide. In this review, the authors first highlight the historical advances that led to the first pig-to-human heart transplantation, a landmark moment in the field of advanced heart failure. The authors discuss immunologic, infectious, and physiological challenges for implementation of XTx, as well as innovations in the science of genetic manipulation that allowed clinical translation of this therapy. The authors consider ongoing barriers that affect ongoing translation of this technology into clinical care in the current era. Finally, the authors propose a framework for advancing clinical application of XTx.
An estimated 1 in 20 patients living with chronic heart failure (HF) progresses to advanced stage disease each year, and this proportion is anticipated to increase as foundational pharmacologic therapy provides further prolongation of life.1 However, once patients transition to advanced HF, they exhibit refractoriness to pharmacologic therapy and treatment designed to address heart rhythm disorders.2 In such cases, permanent left ventricular assist devices or heart transplantation (HTx) is required to improve quality and duration of life. The use of HTx is challenged by a shortage of suitable donor organs, requiring strict criteria for the use of this limited resource. It is this growing need that has ignited interest in the field of xenotransplantation (XTx), using organs from animal sources that are available without limits. Yet after decades of failure in experimental animal models and limited success, the ability to genetically modify animal organs allowed the performance of the first porcine-to-human HTx on January 7, 2022. In this state-of-the-art review, we first highlight the historical advances and discuss scientific innovations that allowed clinical human translation of XTx. We consider ongoing barriers that affect ongoing translation of this technology into clinical care in the current era (Central Illustration). Finally, we propose a framework for advancing clinical application of XTx.
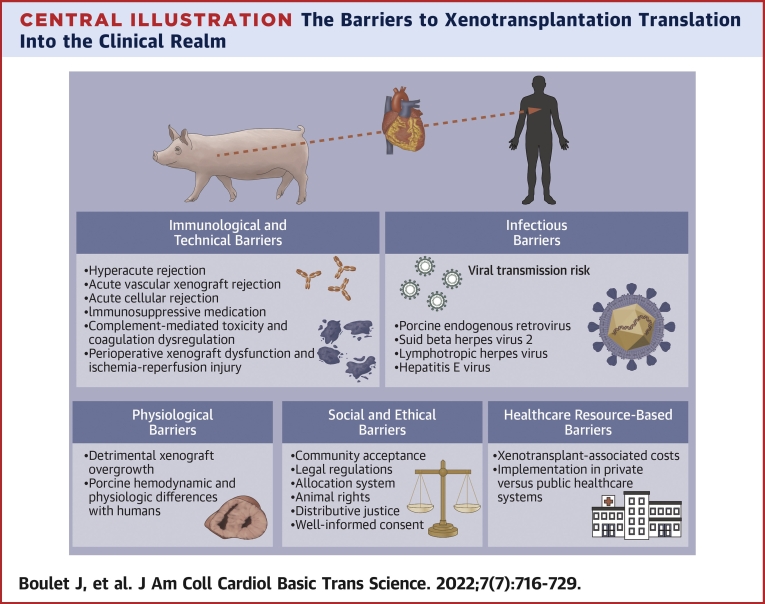
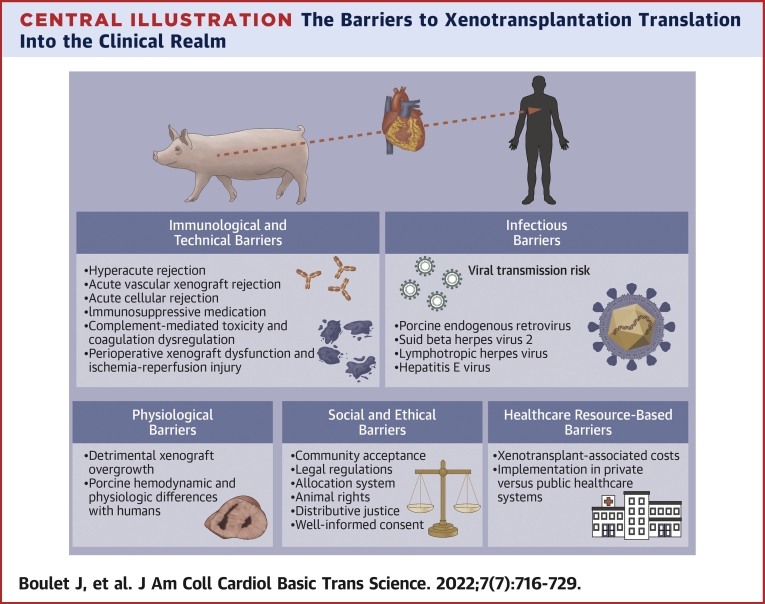
Central Illustration.
The Barriers to Xenotransplantation Translation Into the Clinical Realm
Historical Background of Human XTx
The concept of human XTx first emerged in 1667, when Jean-Baptiste Denys performed farm animal–to–human blood transfusions,3 evolving to skin xenografts from rabbits, dogs, and even pigeons in the 19th century,4 to insertion of rabbit kidney slices as well as porcine and goat renal heterografts in patients with renal insufficiency.5,6 In the early 1960s, Keith Reemtsma performed 13 chimpanzee-to-human kidney transplantations while at Tulane University; 1 patient survived for 9 months. Nonhuman primates (NHPs) were chosen for their close evolutionary relationship to humans, but most patients died days to a few months following surgery, highlighting incompatibility between donors and recipients.7 Similar outcomes were observed after primate-to-human liver transplantation, despite the use of cyclosporine.8 James Hardy performed a heart XTx in 1964 using a chimpanzee donor, but the heart was too small to support the patient’s circulation, and graft loss occurred in 2 hours because of antibody-mediated rejection.9,10 Subsequent attempts at heart XTx were unsuccessful (Table 1) until 1984, when Leonard Bailey at Loma Linda University performed an orthotopic baboon HTx into an infant girl born with hypoplastic left heart syndrome, referred to as Baby Fae.11 The patient survived for 21 days, and death ensued because of rejection.11,12 The main challenge of hyperacute rejection could not be surmounted, leading to <24-hour survival in subsequent experimental attempts.
Table 1.
Historical Background of Clinical Heart Xenotransplantation
| Year | Surgeon | Institution | Donor | Type of Transplantation | Outcome | Survival | Ref. # |
|---|---|---|---|---|---|---|---|
| 1964 | Hardy | University of Mississippi | Chimpanzee | OHT | Heart too small to support human circulation | 2 h | 90 |
| 1968 | Ross | National Heart Hospital, United Kingdom | Pig | HHT | Rejection | 4 min | 91,92 |
| 1968 | Ross | National Heart Hospital, United Kingdom | Pig | Perfused with human blood (not transplanted) | Rejection | Immediate death | 91,92 |
| 1968 | Cooley | Texas Heart Institute | Sheep | OHT | Rejection | Immediate death | 93,94 |
| 1969 | Marion | Lyon, France | Chimpanzee | OHT | High pulmonary vascular resistance | Rapid death | 95,96 |
| 1977 | Barnard | University of Cape Town, South Africa | Baboon | HHT | Heart too small to support human circulation | 5 h | 95 |
| 1977 | Barnard | University of Cape Town, South Africa | Chimpanzee | HHT | Rejection | 4 d | 95 |
| 1984 | Bailey | Loma Linda University | Baboon | OHT | Rejection | 20 d | 97 |
| 1992 | Religa | Silesian Academy of Medicine, Poland | Pig | OHT | Unknown cause of death | <24 h | 98 |
| 1997 | Baruah | India | Pig | OHT | Unknown cause of death | <24 h | 99 |
| 2022 | Griffith | University of Maryland Medical Center | Pig | OHT | Unknown cause of death | 2 mo | 18,85 |
HHT = heterotopic heart transplantation; OHT = orthotopic heart transplantation.
Evolution of Cardiac XTx
Following the unsuccessful use of NHP hearts for HTx, pigs have emerged as the most promising donor species for several reasons, including similar heart size and anatomy, the feasibility of precise genetic modifications, favorable breeding characteristics, and relative low risk for infection. However, because of the large evolutionary differences between pigs and primates, porcine organs transplanted into NHPs and humans undergo hyperacute rejection. One of the major reasons for the hyperacute rejection of transplanted pig organs in humans is that humans possess preformed natural antibodies against carbohydrate antigens that are expressed on the surface of porcine cells. A central breakthrough to avoid hyperacute rejection was the identification of the α-galactose 1,3-galactose (Gal) carbohydrate linkage as the major xenoepitope responsible for hyperacute rejection.13 This was followed by the creation of genetically modified α-1,3-galactosyltransferase gene-knockout pigs, and early experimental successes ushered an era of genetically modified pigs that could be fit for XTx.13,14 Additional porcine gene knockouts for immune and thrombosis regulation pathways allowed the XTx field to progress, and by 2012 the discovery of CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats–associated RNA-guided DNA endonuclease Cas9) gene editing tools facilitated the removal of porcine endogenous retroviruses (PERVs).15,16
On January 7, 2022, a genetically engineered porcine heart (United Therapeutics) was successfully transplanted into a 57-year-old patient with HF at the University of Maryland Medical Center, who had been deemed ineligible for available advanced HF therapies.17 The patient died on March 8, 2022.18 In the following review, we discuss the details surrounding the clinical course of the first patient who was transplanted with a genetically modified pig heart, as well as the social and scientific and barriers that will need to be overcome before cardiac XTx can be adopted into clinical use.
Immunologic and Technical Barriers
As shown in Figure 1, overcoming hyperacute, acute humoral, and acute cellular xenograft rejection is central to the success of cardiac XTx, as well as overcoming the problems of complement-mediated toxicity, dysregulated coagulation, perioperative cardiac xenograft dysfunction and the need for novel immunosuppressive medication. Each of these is discussed in more detail in the following sections.
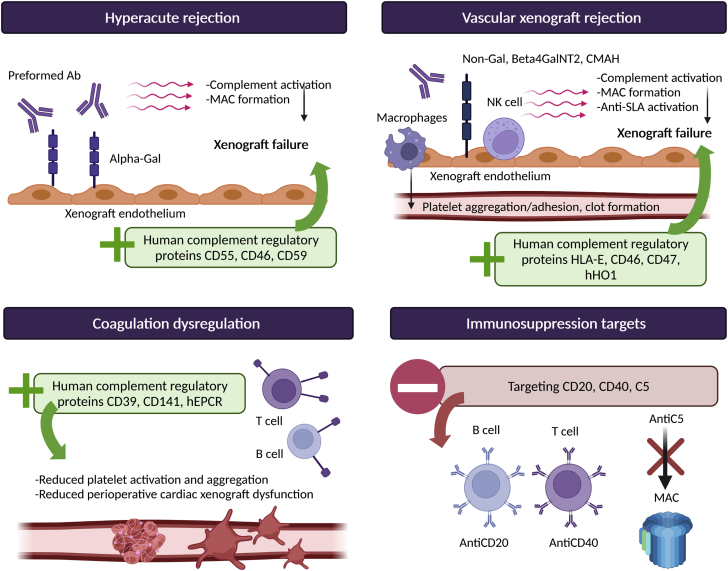
Figure 1.
Clusters of Differentiation and Inflammation in Xenotransplantation
Ab = antibody; alpha-Gal = α-1,3-galactosyltransfer; anti-SLA = anti–swine leukocyte antigen antibody; Beta4GalNT2 = β1,4-N-acetylgalactosyltransferase; CMAH = CMP-N-acetylneuraminic acid hydroxylase; hEPCR = human endothelial protein C receptor; hHO1 = human hemeoxygenase-1; HLA-E = human leukocyte antigen E; hTM = human thrombomodulin; MAC = membrane attack complex; NK = natural killer.
Prevention of hyperacute rejection
After successful vascular anastomoses, natural antibodies in primates react with surface antigens present on porcine cells, leading to hyperacute rejection within minutes to hours, a process originating in the endothelium of small arteries.19 Natural antibodies in the innate immune system represent one among many immunologic barriers to xenograft survival and arise without exposure to their known ligands. A key natural antibody, the anti-Gal antibody, constitutes up to 4% of circulating human immunoglobulins and targets the Gal carbohydrate moiety (Gal alpha[1,3]Gal, formed by alpha 1,3-galactosyl transferase), which places a terminal galactose residue in an alpha linkage to another galactose, is present on several cell surface glycoproteins and glycolipids of different mammals, including pigs.19,20 Gal is not expressed in humans and NHPs, as it was lost during evolution after a frame-shift genetic alteration in α-1,3-galactosyltransferase.21 The xenoreactive immunoglobulin anti-Gal has been shown to mediate complement activation early in the course of hyperacute rejection,22 but initial attempts for removal of anti-Gal antibodies in pig-to-baboon kidney transplantation models did not improve long-term graft survival despite absence of hyperacute rejection.23 In the early 21st century, a nuclear transfer technology removing Gal epitopes and the creation of Gal-deficient pigs led to the prevention of hyperacute rejection in pig-to-baboons heart XTx.24, 25, 26 Additionally, other natural antibodies were identified as mediators of delayed acute vascular xenograft rejection, which occurred in days to weeks. These other carbohydrate natural antibodies included β1,4-N-acetylgalactosyltransferase, responsible for Sda synthesis; a red blood cell antigen-like terminal carbohydrate; and CMP-N-acetylneuraminic acid hydroxylase, responsible for Neu5Gc synthesis, highly expressed on endothelial cells of all mammals except humans.27,28 The markedly extended survival of xenografts observed with the genetic modification of the 3 carbohydrate xenoantigens was significantly greater than what was seen with the very first genetic modifications performed in pigs, which did not successfully prevent hyperacute rejection. These modifications targeted complement regulatory proteins, as hyperacute rejection initially involved activation of the complement pathway and consisted in the introduction of transgenic human complement regulatory proteins, namely, CD55, CD46, and CD59, into the porcine genome.29,30
Genome engineering with transcription activator–like effector nucleases (zinc finger nucleases, Talens) and somatic cell nuclear transfer using CRISPR/Cas9 have significantly improved the feasibility and success of XTx.31 Research identified several microbial genomic loci consisting of an interspaced repeat array, which were uniformly called CRISPR in the early 2000s,32 their biological significance later determined from their extrachromosomal and phage-associated origins.33 The CRISPR loci was found to play a role in microbial immunity, more specifically in the adaptive immune system.33,34 CRISPR research accelerated in subsequent years, leading to experimental evidence allowing the engineering of a simple RNA-programmable DNA endonuclease.35,36 The CRISPR/Cas9 technique allows editing of multiple distinct DNA targets in parallel within the endogenous genome in any desired organism.34,35
Prevention of acute vascular xenograft rejection
Macrophages, natural killer cells, and anti-swine leukocyte antigen antibodies are involved in the immune reaction that occurs days to weeks following XTx, known as acute vascular rejection.37 Acute vascular xenograft rejection begins with the recipient’s native antibodies reacting with donor’s antigens at the surface of vascular endothelial cells of small arteries, which leads to adhesion of natural killer cells and macrophages, leading to platelet aggregation and adhesion with subsequent formation of clots in the lumen of small arteries.38,39 Strategies to prevent acute vascular xenograft rejection have included genetic modifications targeting major pathways. Modification of class I major histocompatibility complex molecules by disabling the β-2-microglobulin activity encoded by the β-2-microglobulin gene, an indispensable player for the assembly of major histocompatibility complex I surface receptors, led to extended skin xenograft survival of β-2-microglobulin–deficient pigs.40 Additionally, to reduce the cytotoxicity of natural killer cells, human leukocyte antigen E and human CD46 have been added on the surface of porcine cells as regulators.39,41 To interfere with the role of macrophages in acute vascular xenograft rejection, modification in the porcine genome leading to the expression of human CD47 on the surface of porcine cells inhibited phagocytosis from xenograft cells by the recipient’s macrophages.42,43
Prevention of acute cellular rejection
Acute cellular rejection has rarely been seen in pig-to-NHP XTx compared with hyperacute rejection and acute vascular xenograft rejection, which have significantly affected graft survival in XTx research. The major contributors to acute cellular rejection are the recipient’s CD8+ and CD4+ T cells’ interacting with the donor’s class I and II swine leukocyte antigen, which necessitates binding between T cell receptors and the major histocompatibility complex on antigen-presenting cells.44,45 Other players involved, but to a lesser extent, in acute cellular rejection include B cells, macrophages, and natural killer cells. Attempts have been made to produce transgenic pigs expressing cytotoxic T cell antigen 4, responsible for inhibiting T-cell activity, but the genetically modified pigs died prematurely from other infection or vascular rejection causes.44,46 The production of class I major histocompatibility complex knockout pigs using guide RNAs and the Cas9 gene editing technique, as well as reducing the expression of class II swine leukocyte antigen through expression of the human dominant-negative mutant class II transactivator transgene, have been tried.47,48 The initial results of these approaches were promising, as the genetically engineered pigs exhibited normal development despite reduced levels of CD8+ and CD4+ T cells.
Use of immunosuppressive medication
Whether the use of conventional immunosuppression regimen used for HTx patients will maintain long-term rejection-free survival of xenografts remains to be established. Studies using porcine-to-NHP islet cell transplantation have demonstrated that “real-world” immunosuppression may not completely overcome the cytotoxicity and cytokine production from activation of T cells and innate cytotoxic cells.49,50 Yamamoto et al51 compared 2 different maintenance immunosuppressive regimen in pig-to-baboon kidney transplantations using α-1,3-galactosyltransferase gene-knockout pigs expressing human CD46, among other gene modifications performed. All transplanted baboons received induction therapy with antithymocyte globulin and anti-CD20mAb. The 2 maintenance immunosuppression regimens have consisted of either CTLA4-Ig and/or tacrolimus combined with rapamycin or mycophenolate mofetil or anti-CD40mAb combined with rapamycin. The regimen based on anti-CD40mAb provided substantially longer graft survival. Despite these results suggesting that calcineurin inhibitor–based regimens may not be as effective as CD40-based regimens, little is known about the mechanisms of graft failure in NHPs, and these may not directly apply to XTx in humans. In the first clinical-grade porcine kidney XTx using a human decedent model, Porrett et al52 opted for a calcineurin inhibitor-based regimen using antithymocyte globulin and anti-CD20 for induction immunosuppression and tacrolimus, mycophenolate mofetil, and prednisone for maintenance immunosuppression.52 The study was terminated after 77 hours after reperfusion and 8 days after declaration of brain death secondary to severe coagulopathy causing exsanguinating hemorrhage. Additional pharmacologic interventions may be necessary to improve xenograft survival in humans as understanding of the underlying mechanisms of long-term graft failure is improved.
Mitigating complement-mediated toxicity and dysregulation of coagulation
Xenograft cells are more vulnerable to membrane attack complex and cell lysis from complement activation, as they are less susceptible to the inhibitory action of complement regulatory proteins.53 Complement regulatory proteins are surface proteins inhibiting complement activation at different stages of the complement cascade, and they also play a role in xenograft accommodation.54, 55, 56 These complement inhibitors have been early targets of genetic modifications to reduce complement-mediated antibody-dependent toxicity.53 Genetic engineering leading to the expression of human complement regulatory proteins including CD46, CD55, and CD59 on transgenic porcine cells increased resistance to hyperacute rejection and complement-mediated damage.57 Additional pharmacologic interventions might be helpful to prevent further complement-mediated cytotoxicity such as the anti-C5 antibody tesidolumab, which has recently demonstrated benefits in NHP models by reducing early antibody-mediated rejection and prolonging survival in renal XTx.58,59
The coagulation pathway is disrupted in the setting of XTx because the recipient’s hematologic system and the donor’s clotting pathway inhibitors present on the endothelial surface interact poorly together and lead to disordered coagulation.60 The mechanisms underlying the observed disseminated coagulation and thrombotic microangiopathy seen with pig-to-baboon kidney and cardiac xenograft rejection are not completely understood and escape the genetic engineering described thus far in this review, which counteracts natural antibodies, complement activation, and as T cell– and B cell–mediated activity. Other studies evaluating the role of the clotting system in the immune reactions that underlie xenograft failure targeted 2 potential human molecules to be expressed in swine models, thrombomodulin or CD141 and the endothelial cell protein C receptor.44 The expression of human thrombomodulin in pig-to-baboon heart XTx resulted in extended graft survival,61,62 while other studies have demonstrated recipient reduced platelet aggregation and donor delay in blood clotting with reduced prothrombinase activity.63,64 Platelet aggregation was also found to be reduced in in vitro studies from transgenic pigs expressing endothelial cell protein C receptor, a protein that mediates cytoprotective, anticoagulant, and anti-inflammatory signaling.64 Expression of human CD39 has also been considered for the prevention of thrombotic microangiopathy development, but attempts conducted in pig-to-baboon kidney and lung XTx have yielded mixed results.65 CD39 possesses anti-inflammatory and thromboregulatory effects, and its expression on genetically modified pigs leads to reduced platelet sequestration with partial reduction of platelet activation.66,67 Thrombotic microangiopathy remains a significant barrier to the success of XTx and to the prolongation of xenograft survival.
Perioperative cardiac xenograft dysfunction
In the first 48 hours after surgery, perioperative cardiac xenograft dysfunction has been frequently observed in NHPs with orthotopic XTx.68,69 The process appears to be reversible within the first 2 weeks after transplantation, from follow-up echocardiographic studies.69 Histologic findings of perioperative cardiac xenograft dysfunction differ from what is seen with hyperacute rejection and include a preserved myocardium in the presence of vascular antibody deposition, resembling cardiac stunning and ischemic reperfusion injury. Techniques to minimize early post-XTx inflammation and ischemia reperfusion injury include improved organ preservation, such as cold nonischemic perfusion and transgenic expression of human CD39 in pigs.70,71 Cold nonischemic perfusion with an oxygenated albumin-containing hyperoncotic cardioplegic solution is superior to cold static ischemic cardioplegia in preserving systolic graft function.70 The production of transgenic pigs expressing human CD39, which has a role to play in cardiovascular protective and antithrombotic pathways, has been shown to reduce infarct size following myocardial ischemia reperfusion injury in baboons.71
Infectious Barriers
There is a concern for transmission of viruses from nonhuman species to xenograft recipients, and the greatest apprehension comes from the possibility of PERV transmission to humans.44,72 The 3 subtypes of the PERV viruses, PERV A, PERV B, and PERV C, are integrated into the porcine genome. In vitro but not in vivo studies have demonstrated the transfer of recombinant PERV A and PERV B subtypes into human cells. Other potential threats in the context of pig-to-human XTx include suid beta herpes virus 2 (also known as porcine cytomegalovirus), lymphotropic herpes virus, and hepatitis E virus.73 Importantly, all preclinical studies and clinical studies using pig cells, tissues, and organs for the purpose of XTx did not show any evidence that PERV is transmitted to humans.72 Some strategies have been developed to prevent transmission of PERV from pigs to humans, including the production of low-virus-producing pigs, vaccines, antiretroviral treatment, therapies interfering with RNA, and inactivation of PERV activity in porcine cell lines or creating PERV-knockout animals using the CRISPR/Cas9 technique to target the PERV polymerase gene.74 The transgenic 10-gene-edited pigs developed for humans previously are tested for the presence of porcine viruses, including suid beta herpes virus 2 and PERV, every 3 months, following rigorous maintenance standards of potential donor pigs.52 The first clinical-grade porcine kidney XTx using a human decedent model did not show any evidence of PERV transmission or peripheral chimerism in the decedent.52 Peripheral chimerism testing to detect the presence of expression of the gene for a porcine ribosomal protein, pRPL4, was absent at any point. Genetic manipulations do not reduce the transmission of suid beta herpes virus 2 from the mother to swine offspring, which may be prevented using cesarean section delivery and cautious isolation of donor animals.75 The possibility of infection transmission or zoonoses needs to be very carefully addressed to avoid potential risks for reduced xenograft survival and introduction of major public health risks.
Physiological Barriers
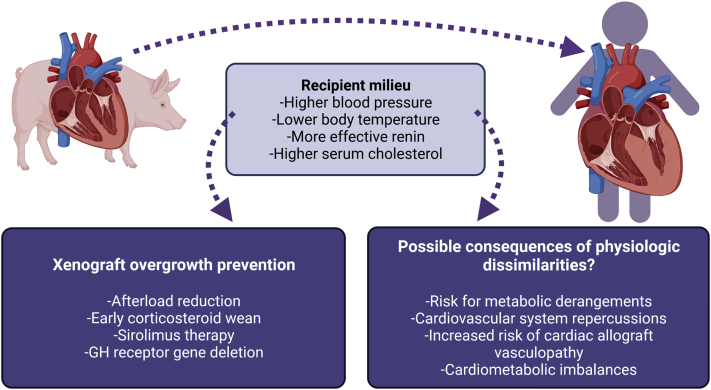
Prevention of detrimental xenograft overgrowth
Detrimental xenograft overgrowth has been observed with pig-to-baboon kidney and cardiac XTx.76,77 The mechanisms underlying the observed doubled porcine heart sizes, or the 4-fold increase in swine kidney volume seen in transplanted baboons are not fully explained but are thought to be genetically determined with intrinsic factors at play leading the overgrowth of donor xenografts.76,77 Consequences of such significant and rapid cardiac xenograft lead to diastolic pump failure, lung compression, and restriction, leading to pulmonary edema and oxygen desaturation. Different approaches have been used to successfully prevent xenograft recipients from experiencing detrimental organ overgrowth (Figure 2). Decreasing the afterload and blood pressure of baboons closer to the pig’s natural blood pressure level is one such strategy, as relative hypertension may contribute to postoperative hypertrophy.77,78 Other strategies consist of early weaning of corticosteroid therapy and use of the sirolimus prodrug temsirolimus, which blocks the mechanistic target of rapamycin signaling pathway, inhibiting the effect of growth hormone (GH).77,79 Finally, gene editing performed with the CRISPR/Cas9 technology can be used to delete the GH receptor gene, which has been demonstrated through the mutation of exon 3 of the GH receptor gene in porcine zygotes.80 The produced GH receptor–knockout pigs demonstrated normal birth weight with growth retardation at 5 weeks and 60% body weight reduction at 6 months.
Figure 2.
Physiological Adaptations in Xenotransplantation
GH = growth hormone.
Selecting pigs as donors for human XTx
A lot of effort has been put into overcoming the immunologic barriers for transplantation between different species, but only a few studies have addressed whether porcine organs would function adequately in human hosts from a physiological perspective. Some of the main reasons why pigs were chosen as better organ donors than primates for human XTx were their organ size; similar anatomy, structure, and physiology to humans; and shorter gestational periods with larger litter sizes of 5 to 10 offspring.19 Additionally, pigs rapidly grow to their adult size, reaching sexual maturity at 5 months, enabling more rapid genetic engineering compared with primates.19 With respect to their similarities with human tissues and organs, they do have comparable hearts and circulatory system but also kidneys, lungs, liver, pancreas, and skin.81 As the research in XTx progresses and porcine xenograft survival in NHPs and humans is extended, there are hemodynamic and physiological dissimilarities that will likely be uncovered. As an example, which complicates thoracic xenografts, the blood pressure difference between pigs and primates leads to detrimental xenograft overgrowth. Not only blood pressure but also body temperature differs between pigs and humans, and a decrease of 2°C could potentially induce metabolic derangements in porcine xenografts once transplanted into humans.82 Another important dissimilarity that has been uncovered with the use of porcine kidney xenografts is the demonstration of less effective heterologous renin to elicit the release of angiotensin compared with homologous human renin, which may have direct consequences on the cardiovascular system.83 Additionally, serum cholesterol in humans is 3 times higher than what is seen in pigs, which could potentially increase the risk for cardiac graft vasculopathy in the xenograft.82 Other uncertainties include whether the genetically engineered porcine heart will adapt to cardiometabolic stress during effort and exercise and whether change of blood flow orientation to the lungs from a horizontal to an upright position matters, which might alter quality-of-life gains in humans. Meeting the physiological demands of recipients with transplanted heart xenografts that will function cooperatively with other organs is central to the success of clinical XTx application, and genetic manipulation using the CRISPR/Cas9 technique might be one solution to overcoming such physiological barriers. It is unknown whether other physiological differences between pigs and humans could lead to organ incompatibility.
Combining Strategies to Extend Porcine Xenograft Survival
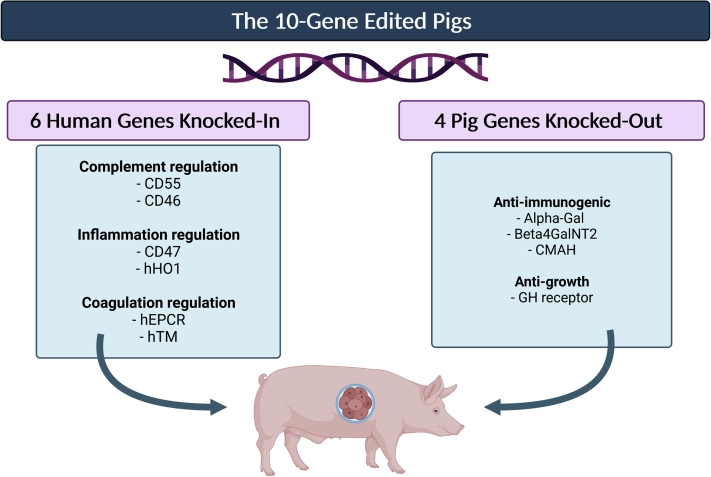
The combination of the aforementioned strategies to produce transgenic pigs using somatic cell nuclear transfer CRISPR/Cas9 significantly helped progression of the XTx field. As laid out by Cooper et al,84 a total of 9 genetic modifications that have currently been identified may be performed to create genetically engineered donors that will survive once transplanted in their hosts for extended periods of time. These 9 mutations include the deletion of 3 genes responsible for the carbohydrate xenoantigens described previously (triple-knockout pigs), expression of 2 human complementary-regulatory antigens (CD46 and CD55), and 2 human coagulation regulatory proteins (thrombomodulin and endothelial cell protein C receptor). Additionally, the expression of immunomodulatory genes in the porcine genome, including human hemeoxygenase-1, which confers anti-inflammatory and antiapoptotic properties, as well as human CD47, suppresses in part macrophages and T-cell immune activity as described earlier.
These 9 modification and deletion of the GH receptor gene that inhibits organ growth were integrated into the 10-gene-edited pigs (Figure 3) used in the recent first pig-to-human heart XTx performed in January 2022.52,85 One major genetic difference consisted of the deletion of genes responsible for red blood cell antigens at the surface of red blood cells in the porcine genome, making the pigs universal donors with respect to their blood type. This transgenic porcine heart transplanted into a human survived for 2 months.
Figure 3.
10-Gene-Edited Pig Used in the First Pig-to-Human Heart Transplantation
Alpha-Gal = α-1,3-galactosyltransferase; Beta4GalNT2 = β1,4-N-acetylgalactosyltransferase; CMAH = CMP-N-acetylneuraminic acid hydroxylase; GH = growth hormone; hEPCR = human endothelial protein C receptor; hHO1 = human hemeoxygenase-1.
The First Pig-to-Human Heart XTX
The details regarding the patient’s clinical course post-XTx and the cause of his death have not been published as of May 4, 2022, but have already been provided by personal communication with Bartley Griffith, the cardiac surgeon who oversaw this sentinel procedure. Preoperatively, the patient had been hospitalized for 61 days and supported on extracorporeal membrane oxygenation support for 48 days. There was severe sarcopenia and bone marrow suppression with significant leukopenia and thrombocytopenia. The induction and maintenance regimen used included KPL-404 (an anti-CD40 monoclonal antibody), rituximab, rabbit antithymocyte globulin, a C-1 esterase inhibitor, mycophenolate mofetil (subsequently replaced by low-dose tacrolimus), and corticosteroids.
The postoperative course was initially promising, with a well-functioning xenograft producing a cardiac output of up to 7 L/min in the absence of any inotropic support (and even required antihypertensive therapy initially). Three weeks after surgery, the patient developed ischemic bowel with suppurative peritonitis, requiring surgical intervention and reduction of immunosuppression. Intravenous immunoglobulin was administered, as the patient was noted to have severe hypogammaglobulinemia. A few days later, manifestations of xenograft dysfunction became apparent, with elevated troponin levels, low cardiac output with restrictive filling, and evidence of myocardial edema, necessitating up-titration of immunosuppression and return to extracorporeal membrane oxygenation support. The decision was made to withdraw care 2 months after implantation.
Pathologic examination of the xenograft revealed multiple competing issues that may have contributed to graft failure and the patient’s demise. Areas of infarction with marked edema were present in the context of diffuse capillary destruction and endothelialitis observed on electron microscopy. There was no evidence of vessel thrombosis or other typical markers of cellular rejection. Areas of infarction were associated with complement deposition, which did not involve the vascular beds, arguing against a traditional form of antibody-mediated rejection. In situ hybridization histochemistry revealed the presence of suid beta herpes virus 2 intracellularly, which was transmitted from the host, having escaped detection by polymerase chain reaction testing prior to transplantation.
These findings raise significant interest and speculation surrounding potentially unaccounted for pathways that could have contributed to graft failure. Potential causes of the patient’s demise include: 1) the initial severe sepsis syndrome with associated cytokine up-regulation and deleterious effects on the xenograft; 2) the use of intravenous immunoglobulin, which could have transmitted antiporcine antibodies (although ex vivo assessments did not show these antibodies to be cytotoxic in nature); 3) a distinct form of rejection that may have activated yet unidentified complement pathways (in the presence of severe leukopenia); and 4) the suid beta herpes virus 2, which may have been responsible for the reduced graft survival as observed in prior studies of pig-to-baboon heart and kidney XTx.75,86
The outcome of this clinical experiment raise important questions about what additional gene modifications are required to target other rejection pathways contributing to short-term and longer-term graft failure but also what other interventions can be performed to prevent porcine-t- human viral transmission. Additionally, the preoperative state of patients for future XTx attempts will be a significant consideration in improving postoperative xenograft outcomes. All in all, this case shows promise for pig-to-human XTx and has set the table for future attempts at heart XTx. Importantly, there is a need to remain vigilant for immunologic, infectious, and physiological barriers and important social, ethical, and economic challenges to address for the acceptance and application of XTx.
Social and Ethical Barriers
Bioethicist and professor of philosophy Bernard E. Rollin used Frankenstein’s monster as a symbol of society’s view of biotechnology and the dilemma associated with genetic engineering of animals.87 The ethical framework that is currently in place for clinical application and decision making might need modifications for XTx to be ethically, socially, and legally accepted. Other questions arise with organ access by purchase and impact on systems of fair allocation of organs for HTx. There is currently no guideline in place to dictate legal regulations of pig-to-human heart XTx, and uniform social acceptance of the practice is unlikely. Major differences in cultural and religious beliefs will make it particularly challenging to build consensus guidelines on XTx.
One major ethical consideration in XTx is the concern for animal rights and the anthropocentric view of putting humans above all other animal species.88 The welfare of animals being bred in laboratories with living conditions that significantly differ from their usual environments will need to be defined.89 Other challenges include the fairness of resource allocation and distributive justice, in an era when there is already much confusion in patients with respect to the current organ allocation system. Another major aspect will be appropriately educating clinicians and patients, particularly with respect to the challenge of obtaining well-informed consent in an area with a high degree of uncertainty. These considerable ethical, social, and legal challenges will require careful consideration and discourse in a manner concurrent with scientific advances in the field.
Health Care Resource-Based Barriers
The annual costs of HTx in the United States represent a relatively small proportion of total annual health care expenditures, which is likely due in part to the relatively small number of transplantations perform yearly.90 With XTx, the availability of organs would be expected to increase, likely leading to a significant rise in annual expenditures attributable to transplantation. The current exorbitant costs of XTx will assuredly have to reduce over time to allow this organ donor source to meet its goal of universal use. Aspects to be considered when forecasting potential XTx costs include breeding and genetic engineering of the animals; the surgery, follow-up patient care, and management of complications; higher costs of immunosuppressive medications; and expenses specific to organ retrieval. This should be contrasted with economic and social costs of reducing the burden of HF in wait-listed patients and those who never make it to the wait list. Cost-effectiveness will need to be established for private and subsequent public payer approval, and one may also wonder if this strategy will ultimately lead to a reduction in overall expenditures by avoiding a subsequent therapy or increase costs if it serves as a bridge. These uncertainties are crucial to consider, and efforts should be made to address them as innovations in XTx progress toward human clinical trials.
Framework for Future Clinical Testing
Given the complexity of between species transplantation and the recent advances in the XTx field, a comprehensive framework for future clinical trials is needed. In 2000, an advisory committee of the International Society for Heart and Lung Transplantation proposed efficacy recommendations for the cardiac applications of future XTx.82 Although proposed benchmarks in animal experiments have been met, and disease transmission from pigs to NHPs and humans is considered minimal, the question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case. As the history of evolution of cardiac allotransplantation or mechanical circulatory support attests, advances occur incrementally rather than in a transformative manner. It is critical that the most appropriate patient be matched to the technology while exercising vigilance for novel challenges that will nevertheless surface. The ideal next few cases will be those patients with advanced HF who are optionless for either cardiac allotransplantation or destination-therapy durable left ventricular assist devices (eg, a patient with cancer able to survive to at least 1 year but with biventricular failure unable to be supported by a left ventricular assist pump because of a small chamber size). Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology. A critical facet will include the development of sensitive and specific laboratory testing for the detection of zoonotic transmission as well as to monitor xenograft function from an immunologic standpoint, as the immunotherapy required will be distinct from that used in allotransplantation. The safety of XTx in early feasibility phase I clinical trials will be required with survival that meets or exceeds a greater duration than that achieved in the sentinel case before clinical trials can be initiated more broadly across different populations. Over time, we believe that XTx will likely find application as a bridge to allotransplantation, an alternative to destination therapy in those with persistent right HF or even in patients in need of a second transplantation because of first transplantation failure, in whom ethical challenges exist with the use of an allograft because of a lesser expected outcome.
Conclusions
The potential for XTx to allow an unlimited donor supply and resolve the organ shortage is now closer than ever. Gene editing has driven success in transplanting porcine hearts into NHPs. Key innovations have included eliminating porcine antigens, addition of human isoforms of key coagulation and complement regulatory proteins, and control of organ size. Translation of these breakthroughs to human recipients poses new scientific, regulatory, ethical, and financial challenges. Although XTx offers great potential, careful clinical studies will be needed to assess whether long-term outcomes rival currently available alternatives, including mechanical circulatory support devices and marginal or currently discarded human donor hearts.
Funding Support and Author Disclosures
Dr Mehra has received payments to his institution from Abbott for consulting; has received consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and is a scientific advisory board member for NuPulseCV, Leviticus, and FineHeart. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Kalogeropoulos A.P., Samman-Tahhan A., Hedley J.S., et al. Progression to stage D heart failure among outpatients with stage C heart failure and reduced ejection fraction. J Am Coll Cardiol HF. 2017;5(7):528–537. doi: 10.1016/j.jchf.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Crespo-Leiro M.G., Metra M., Lund L.H., et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(11):1505–1535. doi: 10.1002/ejhf.1236. [DOI] [PubMed] [Google Scholar]
- 3.Fastag E., Varon J., Sternbach G. Richard Lower: the origins of blood transfusion. J Emerg Med. 2013;44(6):1146–1150. doi: 10.1016/j.jemermed.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Gibson T. Zoografting: a curious chapter in the history of plastic surgery. Br J Plast Surg. 1955;8(3):234–242. doi: 10.1016/s0007-1226(55)80040-9. [DOI] [PubMed] [Google Scholar]
- 5.Princeteau M. Greffe rénale. J Méd Bordeaux. 1905;26:549. [Google Scholar]
- 6.Jaboulay M. Greffe de reins au pli du coude par soudures artérielles et veineuses. Lyon Méd. 1906;107:575–577. [Google Scholar]
- 7.Reemtsma K., McCracken B.H., Schlegel J.U., et al. Renal heterotransplantation in man. Ann Surg. 1964;160(3):384–410. doi: 10.1097/00000658-196409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl T.E., Marchioro T.L., Faris T.D., McCardle R.J., Iwaski Y. Avenues of future research in homotransplantation of the liver with particular reference to hepatic supportive procedures, antilymphocyte serum, and tissue typing. Am J Surg. 1966;112(3):391–400. doi: 10.1016/0002-9610(66)90209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose A.G., editor. Histopathology of Cardiac Xenograft Rejection. Springer; Heidelberg, Germany: 1991. [Google Scholar]
- 10.Hardy J.D., Kurrus F.D., Chavez C.M., et al. Heart transplantation in man. developmental studies and report of a case. JAMA. 1964;188:1132–1140. [PubMed] [Google Scholar]
- 11.Bailey L.L., Nehlsen-Cannarella S.L., Concepcion W., Jolley W.B. Baboon-to-human cardiac xenotransplantation in a neonate. JAMA. 1985;254(23):3321–3329. [PubMed] [Google Scholar]
- 12.Murthy R., Bajona P., Bhama J.K., Cooper D.K. Heart xenotransplantation: historical background, experimental progress, and clinical prospects. Ann Thorac Surg. 2016;101(4):1605–1613. doi: 10.1016/j.athoracsur.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Milland J., Christiansen D., Sandrin M.S. Alpha1,3-galactosyltransferase knockout pigs are available for xenotransplantation: are glycosyltransferases still relevant? Immunol Cell Biol. 2005;83(6):687–693. doi: 10.1111/j.1440-1711.2005.01398.x. [DOI] [PubMed] [Google Scholar]
- 14.Perkel J.M. Xenotransplantation makes a comeback. Nat Biotechnol. 2016;34(1):3–4. doi: 10.1038/nbt0116-3. [DOI] [PubMed] [Google Scholar]
- 15.Mohiuddin M.M., Singh A.K., Corcoran P.C., et al. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J Thorac Cardiovasc Surg. 2014;148(3):1106–1113. doi: 10.1016/j.jtcvs.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu D., Wei H.J., Lin L., et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017;357(6357):1303–1307. doi: 10.1126/science.aan4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reardon S. First pig-to-human heart transplant: what can scientists learn? Nature News. https://www.nature.com/articles/d41586-022-00111-9 [DOI] [PubMed]
- 18.Rabin R.C. Patient in groundbreaking heart transplant dies. The New York Times. https://www.nytimes.com/2022/03/09/health/heart-transplant-pig-bennett.html
- 19.Sykes M., Sachs D.H. Transplanting organs from pigs to humans. Sci Immunol. 2019;4(41) doi: 10.1126/sciimmunol.aau6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker W., Bruno D., Holzknecht Z.E., Platt J.L. Characterization and affinity isolation of xenoreactive human natural antibodies. J Immunol. 1994;153(8):3791. [PubMed] [Google Scholar]
- 21.Galili U., Shohet S.B., Kobrin E., Stults C.L., Macher B.A. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263(33):17755–17762. [PubMed] [Google Scholar]
- 22.Platt J.L., Lindman B.J., Geller R.L., et al. The role of natural antibodies in the activation of xenogenic endothelial cells. Transplantation. 1991;52(6):1037–1043. doi: 10.1097/00007890-199112000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Kozlowski T., Fuchimoto Y., Monroy R., et al. Apheresis and column absorption for specific removal of Gal-alpha-1,3 Gal natural antibodies in a pig-to-baboon model. Transplant Proc. 1997;29(1-2):961. doi: 10.1016/s0041-1345(96)00299-0. [DOI] [PubMed] [Google Scholar]
- 24.Kuwaki K., Tseng Y.L., Dor F.J., et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 25.Phelps C.J., Koike C., Vaught T.D., et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada K., Yazawa K., Shimizu A., et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 27.Zhu A., Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9(6):376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 28.Miwa Y., Kobayashi T., Nagasaka T., et al. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation. 2004;11(3):247–253. doi: 10.1111/j.1399-3089.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 29.Niemann H., Rath D. Progress in reproductive biotechnology in swine. Theriogenology. 2001;56(8):1291–1304. doi: 10.1016/s0093-691x(01)00630-6. [DOI] [PubMed] [Google Scholar]
- 30.Bühler L., Friedman T., Iacomini J., Cooper D.K. Xenotransplantation—state of the art—update 1999. Front Biosci. 1999;4:D416–D432. doi: 10.2741/A438. [DOI] [PubMed] [Google Scholar]
- 31.Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansen R., Embden J.D., Gaastra W., Schouls L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol. 2002;43(6):1565–1575. doi: 10.1046/j.1365-2958.2002.02839.x. [DOI] [PubMed] [Google Scholar]
- 33.Mojica F.J., Díez-Villaseñor C., García-Martínez J., Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60(2):174–182. doi: 10.1007/s00239-004-0046-3. [DOI] [PubMed] [Google Scholar]
- 34.Barrangou R., Fremaux C., Deveau H., et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315(5819):1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 35.Garneau J.E., Dupuis M., Villion M., et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468(7320):67–71. doi: 10.1038/nature09523. [DOI] [PubMed] [Google Scholar]
- 36.Deltcheva E., Chylinski K., Sharma C.M., Gonzales K., Chao Y., Pirzada Z.A., et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471(7340):602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer K., Rieblinger B., Hein R., et al. Viable pigs after simultaneous inactivation of porcine MHC class I and three xenoreactive antigen genes GGTA1, CMAH and B4GALNT2. Xenotransplantation. 2020;27(1) doi: 10.1111/xen.12560. [DOI] [PubMed] [Google Scholar]
- 38.Satyananda V., Hara H., Ezzelarab M.B., Phelps C., Ayares D., Cooper D.K.C. New concepts of immune modulation in xenotransplantation. Transplantation. 2013;96(11):937–945. doi: 10.1097/TP.0b013e31829bbcb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsieh C.L., Obara H., Ogura Y., Martinez O.M., Krams S.M. NK cells and transplantation. Transpl Immunol. 2002;9(2-4):111–114. doi: 10.1016/s0966-3274(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., Du Y., Zhou X., Wang L., Li J., Wang F., et al. Efficient generation of B2m-null pigs via injection of zygote with TALENs. Sci Rep. 2016;6:38854. doi: 10.1038/srep38854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Längin M., Mayr T., Reichart B., Michel S., Buchholz S., Guethoff S., et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564(7736):430–433. doi: 10.1038/s41586-018-0765-z. [DOI] [PubMed] [Google Scholar]
- 42.Barclay A.N., Brown M.H. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6(6):457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 43.Okazawa H., Motegi S., Ohyama N., et al. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol. 2005;174(4):2004–2011. doi: 10.4049/jimmunol.174.4.2004. [DOI] [PubMed] [Google Scholar]
- 44.Ryczek N., Hryhorowicz M., Zeyland J., Lipiński D., Słomski R. CRISPR/Cas technology in pig-to-human xenotransplantation research. Int J Mol Sci. 2021;22(6):3196. doi: 10.3390/ijms22063196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J., Ezzelarab M.B., Ayares D., Cooper D.K. The potential role of genetically-modified pig mesenchymal stromal cells in xenotransplantation. Stem Cell Rev Rep. 2014;10(1):79–85. doi: 10.1007/s12015-013-9478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song Y.-W., Pan Z.-Q. Reducing porcine corneal graft rejection, with an emphasis on porcine endogenous retrovirus transmission safety: a review. Int J Ophthalmol. 2019;12(2):324–332. doi: 10.18240/ijo.2019.02.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hara H., Witt W., Crossley T., et al. Human dominant-negative class II transactivator transgenic pigs—effect on the human anti-pig T-cell immune response and immune status. Immunology. 2013;140(1):39–46. doi: 10.1111/imm.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reyes L.M., Estrada J.L., Wang Z.Y., et al. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol. 2014;193(11):5751–5757. doi: 10.4049/jimmunol.1402059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hering B.J., Wijkstrom M., Graham M.L., Hårdstedt M., Aasheim T.C., Jie T., et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 50.Cardona K., Korbutt G.S., Milas Z., Lyon J., Cano J., Jiang W., et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto T., Hara H., Foote J., et al. Life-supporting kidney xenotransplantation from genetically engineered pigs in baboons: a comparison of two immunosuppressive regimens. Transplantation. 2019;103(10):2090–2104. doi: 10.1097/TP.0000000000002796. [DOI] [PubMed] [Google Scholar]
- 52.Porrett P.M., Orandi B.J., Kumar V., et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am J Transplant. 2022;22(4):1037–1053. doi: 10.1111/ajt.16930. [DOI] [PubMed] [Google Scholar]
- 53.Griesemer A., Yamada K., Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol Rev. 2014;258(1):241–258. doi: 10.1111/imr.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griesemer A.D., Okumi M., Shimizu A., et al. Upregulation of CD59: potential mechanism of accommodation in a large animal model. Transplantation. 2009;87(9):1308–1317. doi: 10.1097/TP.0b013e3181a19afc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding J.W., Zhou T., Ma L., et al. Expression of complement regulatory proteins in accommodated xenografts induced by anti-alpha-Gal IgG1 in a rat-to-mouse model. Am J Transplant. 2008;8(1):32–40. doi: 10.1111/j.1600-6143.2007.02016.x. [DOI] [PubMed] [Google Scholar]
- 56.Kim D.D., Song W.C. Membrane complement regulatory proteins. Clin Immunol. 2006;118(2-3):127–136. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 57.Klymiuk N., Aigner B., Brem G., Wolf E. Genetic modification of pigs as organ donors for xenotransplantation. Mol Reprod Dev. 2010;77(3):209–221. doi: 10.1002/mrd.21127. [DOI] [PubMed] [Google Scholar]
- 58.Hillmen P., Young N.S., Schubert J., et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 59.Adams A.B., Lovasik B.P., Faber D.A., et al. Anti-C5 antibody tesidolumab reduces early antibody-mediated rejection and prolongs survival in renal xenotransplantation. Ann Surg. 2021;274(3):473–480. doi: 10.1097/SLA.0000000000004996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robson S.C., Cooper D.K., d’Apice A.J. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 2000;7(3):166–176. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 61.Mohiuddin M.M., Singh A.K., Corcoran P.C., et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. doi: 10.1038/ncomms11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh A.K., Chan J.L., DiChiacchio L., et al. Cardiac xenografts show reduced survival in the absence of transgenic human thrombomodulin expression in donor pigs. Xenotransplantation. 2019;26(2) doi: 10.1111/xen.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iwase H., Ekser B., Hara H., et al. Regulation of human platelet aggregation by genetically modified pig endothelial cells and thrombin inhibition. Xenotransplantation. 2014;21(1):72–83. doi: 10.1111/xen.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miwa Y., Yamamoto K., Onishi A., et al. Potential value of human thrombomodulin and DAF expression for coagulation control in pig-to-human xenotransplantation. Xenotransplantation. 2010;17(1):26–37. doi: 10.1111/j.1399-3089.2009.00555.x. [DOI] [PubMed] [Google Scholar]
- 65.Le Bas-Bernardet S., Tillou X., Poirier N., et al. Xenotransplantation of galactosyl-transferase knockout, CD55, CD59, CD39, and fucosyl-transferase transgenic pig kidneys into baboons. Transplant Proc. 2011;43(9):3426–3430. doi: 10.1016/j.transproceed.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 66.Dwyer K.M., Mysore T.B., Crikis S., et al. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation. 2006;82(3):428–432. doi: 10.1097/01.tp.0000229023.38873.c0. [DOI] [PubMed] [Google Scholar]
- 67.Crikis S., Lu B., Murray-Segal L.M., et al. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant. 2010;10(12):2586–2595. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohiuddin M.M., Reichart B., Byrne G.W., McGregor C.G.A. Current status of pig heart xenotransplantation. Int J Surg. 2015;23(Pt B):234–239. doi: 10.1016/j.ijsu.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Byrne G.W., McGregor C.G. Cardiac xenotransplantation: progress and challenges. Curr Opin Organ Transplant. 2012;17(2):148–154. doi: 10.1097/MOT.0b013e3283509120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Längin M., Reichart B., Steen S., et al. Cold non-ischemic heart preservation with continuous perfusion prevents early graft failure in orthotopic pig-to-baboon xenotransplantation. Xenotransplantation. 2021;28(1) doi: 10.1111/xen.12636. [DOI] [PubMed] [Google Scholar]
- 71.Wheeler D.G., Joseph M.E., Mahamud S.D., et al. Transgenic swine: expression of human CD39 protects against myocardial injury. J Mol Cell Cardiol. 2012;52(5):958–961. doi: 10.1016/j.yjmcc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fishman J.A. Infectious disease risks in xenotransplantation. Am J Transplant. 2018;18(8):1857–1864. doi: 10.1111/ajt.14725. [DOI] [PubMed] [Google Scholar]
- 73.Morozov V.A., Plotzki E., Rotem A., Barkai U., Denner J. Extended microbiological characterization of Göttingen minipigs: porcine cytomegalovirus and other viruses. Xenotransplantation. 2016;23(6):490–496. doi: 10.1111/xen.12265. [DOI] [PubMed] [Google Scholar]
- 74.Denner J. Recent progress in xenotransplantation, with emphasis on virological safety. Ann Transplant. 2016;21:717–727. doi: 10.12659/aot.900531. [DOI] [PubMed] [Google Scholar]
- 75.Yamada K., Tasaki M., Sekijima M., et al. Porcine cytomegalovirus infection is associated with early rejection of kidney grafts in a pig to baboon xenotransplantation model. Transplantation. 2014;98(4):411–418. doi: 10.1097/TP.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanabe T., Watanabe H., Shah J.A., et al. Role of intrinsic (graft) versus extrinsic (host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am J Transplant. 2017;17(7):1778–1790. doi: 10.1111/ajt.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reichart B., Längin M. On the way (my way) to clinical xenogeneic heart transplantation. Xenotransplantation. 2020;27(6) doi: 10.1111/xen.12637. [DOI] [PubMed] [Google Scholar]
- 78.Längin M., Konrad M., Reichart B., et al. Hemodynamic evaluation of anesthetized baboons and piglets by transpulmonary thermodilution: normal values and interspecies differences with respect to xenotransplantation. Xenotransplantation. 2020;27(5) doi: 10.1111/xen.12576. [DOI] [PubMed] [Google Scholar]
- 79.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hinrichs A., Kessler B., Kurome M., et al. Growth hormone receptor-deficient pigs resemble the pathophysiology of human Laron syndrome and reveal altered activation of signaling cascades in the liver. Mol Metab. 2018;11:113–128. doi: 10.1016/j.molmet.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cooper D.K., Ekser B., Burlak C., et al. Clinical lung xenotransplantation—what donor genetic modifications may be necessary? Xenotransplantation. 2012;19(3):144–158. doi: 10.1111/j.1399-3089.2012.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cooper D.K., Keogh A.M., Brink J., et al. Report of the Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation: the present status of xenotransplantation and its potential role in the treatment of end-stage cardiac and pulmonary diseases. J Heart Lung Transplant. 2000;19(12):1125–1165. doi: 10.1016/s1053-2498(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 83.Sen S., Hirawawa K., Smeby R.R., Bumpus F.M. Measurement of plasma renin substrate using homologous and heterologous renin. Am J Physiol. 1971;221(5):1476–1480. doi: 10.1152/ajplegacy.1971.221.5.1476. [DOI] [PubMed] [Google Scholar]
- 84.Cooper D.K.C., Hara H., Iwase H., et al. Justification of specific genetic modifications in pigs for clinical organ xenotransplantation. Xenotransplantation. 2019;26(4) doi: 10.1111/xen.12516. Am J Transplant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.University of Maryland Medical Center University of Maryland School of Medicine faculty scientists and clinicians perform historic transplant of porcine heart into adult human with end-stage heart disease. https://www.umms.org/ummc/news/2022/first-successful-transplant-of-porcine-heart-into-adult-human-heart
- 86.Morozov V.A., Abicht J.-M., Reichart B., Mayr T., Guethoff S., Denner J. Active replication of porcine cytomegalovirus (PCMV) following transplantation of a pig heart into a baboon despite undetected virus in the donor pig. Robert Koch-Institut. https://edoc.rki.de/handle/176904/2426
- 87.Rollin B.E. Cambridge University Press; Cambridge and New York: 1995. The Frankenstein syndrome: ethical and social issues in the genetic engineering of animals, Bernard E. Rollin. 241 pp. ISBN 0-521-47230-X. [Google Scholar]
- 88.Cengiz N., Wareham C.S. Ethical considerations in xenotransplantation: a review. Curr Opin Organ Transplant. 2020;25(5):483–488. doi: 10.1097/MOT.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 89.Rollin B.E. Ethical and societal issues occasioned by xenotransplantation. Animals (Basel) 2020;10(9):1695. doi: 10.3390/ani10091695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Institute of Medicine (US) National Academies Press; Washington, District of Columbia: 1996. Committee on Xenograft Transplantation: Ethical Issues and Public Policy. Xenotransplantation: Science, Ethics, and Public Policy. [PubMed] [Google Scholar]
- 91.Organisation for Economic Co-operation and Development . Organisation for Economic Co-operation and Development; Paris, France: 1996. Advances in Transplantation Biotechnology: Animal to Human Organ Transplants (Xenotransplantation) [Google Scholar]
- 92.UK Advisory Group on the Ethics of Xenotransplantation . Her Majesty’s Stationary Office; Norwich, United Kingdom: 1997. Animal Tissues Into Humans. [Google Scholar]
- 93.U.S. Department of Health and Human Services, Public Health Service Draft guidelines on infectious disease issues in xenotransplantation. Fed Reg. 1996;61:49920–49932. [PubMed] [Google Scholar]
- 94.UK Department of Health . Her Majesty’s Stationary Office; London, United Kingdom: 1997. The Government Response to Animal Tissues Into Humans. [Google Scholar]
- 95.Xenotransplantation. Encyclopedia of Bioethics. Encyclopedia.com. Accessed June 2, 2022. https://www.encyclopedia.com
- 96.Ethics Committee of the Transplantation Society The Transplantation Society and xenotransplantation (draft guidelines) Transplant Soc Bull. 1997;6:11–14. [Google Scholar]
- 97.World Health Organization . World Health Organization; Geneva, Switzerland: 1997. Division of Emerging and Other Communicable Disease Surveillance and Control. Draft Recommendations on Xenotransplantation and Infectious Disease Prevention and Management. [Google Scholar]
- 98.Health Canada . Health Canada; Ottawa, Ontario, Canada: 1997. Xenotransplantation: Clinical, Ethical, and Regulatory Issues. Report of the National Forum. [Google Scholar]
- 99.Mudur G. Indian surgeon challenges ban on xenotransplantation. BMJ. 1999;318:79. doi: 10.1136/bmj.318.7176.79a. [DOI] [PMC free article] [PubMed] [Google Scholar]