Abstract
Introduction
This study evaluated patient characteristics and treatment patterns according to weight in pediatric patients with psoriasis in a real-world setting.
Methods
Primary care and specialist physicians treating pediatric patients with psoriasis aged 6–17 years in five European countries were surveyed in the 2019–2020 Adelphi Real World Pediatric Psoriasis Disease Specific Programme. At least two patients with current or previous biologic use were included per physician. Patient characteristics and treatment patterns were analyzed overall and for patients weighing 25–50 kg or more than 50 kg.
Results
Data from 772 patients weighing 25–50 kg and 1147 weighing more than 50 kg were analyzed. Median age at diagnosis was significantly less in lighter than heavier patients (10.0 vs. 14.0 years; p < 0.001), as was median disease duration (2.2 vs. 3.0 years; p < 0.001). Topical treatments were prescribed in 59.0% of patients overall (70.3% of lighter and 51.4% of heavier patients; p < 0.001), and were used to treat mild rather than moderate-to-severe psoriasis. Conventional systemic use was low (10.8% of patients overall) and predominantly for moderate-to-severe psoriasis. In this biologic-enriched sample, most biologics (78.2%) were prescribed in older (> 13 years) patients. Biologic use increased with line of therapy (6.6% of first-line, 18.0% of second-line, 33.7% of third-line, 44.7% of fourth-line treatments).
Conclusion
Biologics are predominantly prescribed in older (> 13 years) and heavier (> 50 kg) patients, with little first- or second-line use. The low use of biologics in European pediatric patients with psoriasis may represent an unmet treatment need, as topical or conventional systemic agents remain the main treatment option for moderate or severe psoriasis in these patients through the treatment pathway.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00761-7.
Keywords: Biologic treatment, Body weight, Observational study, Pediatrics, Population characteristics, Psoriasis, Treatment patterns
Plain Language Summary
This study looked into types of treatments according to body weight in children with psoriasis, since approved dosing regimens for some treatments are based on body weight. Primary care and specialist physicians treating children with psoriasis aged 6–17 years in five European countries completed a survey. Patient information for those receiving specific types of psoriasis treatments were collected. Of the children included, 772 weighed 25–50 kg and 1147 weighed more than 50 kg. Most children received treatments applied to the skin, such as creams and ointments; this occurred in 70% of lighter patients and in 51% of heavier patients. Conventional treatments taken via the mouth were prescribed in a few patients (11% [overall]), while newer biologic drugs were taken to a greater extent in heavier (30%) than lighter (16%) patients. Most biologics (78%) were prescribed in older (> 13 years) patients. Biologic use increased with the number of failed previous treatments, comprising 7%, 18%, 34%, and 45% of first, second, third, and fourth treatments, respectively. We conclude that children with psoriasis who are treated with biologic drugs are predominantly older and heavier, and have more severe psoriasis. Prescriptions for biologics are given after many other treatments have been tried.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00761-7.
Key Summary Points
| Why carry out this study? |
| Dosing for recently approved psoriasis treatments in children is primarily based on body weight, but there are limited published real-world data on patient characteristics and treatment patterns according to weight in pediatric patients with psoriasis. |
| This study evaluated patient characteristics and treatment patterns by weight in pediatric patients with psoriasis in a real-world setting. |
| What was learned from this study? |
| Biologic treatments for pediatric patients with psoriasis in Europe are predominantly prescribed in older, heavier patients. |
| Low use of biologics may represent an unmet treatment need, as topical or conventional systemic agents remain the main treatment option for moderate or severe psoriasis in pediatric patients through the treatment pathway. |
Introduction
Psoriasis is a chronic, inflammatory, immune-mediated skin disease characterized by scaly plaques on the skin and often requires lifelong treatment [1]. Psoriasis affects 2–3% of the global population [2, 3] and its prevalence gradually increases with age [4]. For one-third of those affected, psoriasis starts before the age of 18 years [5, 6], with an estimated prevalence of approximately 1% in children under 18 years of age [7, 8]. Psoriasis in children can severely impact quality of life, affecting self-esteem, family and social relationships, and school life [4–6]. Early diagnosis and treatment may reduce the impact of psoriasis on these parameters [6, 9–13].
The management of pediatric psoriasis is challenging and may require a multidisciplinary approach involving dermatologists, pediatricians, and potentially rheumatologists [14]. Guidelines for the management of pediatric psoriasis have recently been published [8, 15–17]; these provide an advance on previous approaches to treatment, which were based on recommendations for adults, expert opinion [18–21], or physician personal experience. In addition, physicians often used off-label treatments [22, 23]. It is therefore important to obtain an updated overview of treatment patterns for pediatric psoriasis in Europe.
Although pediatric patients with psoriasis typically respond well to treatment with topical products or phototherapy, a substantial proportion (around one-quarter) experience inadequate disease control with such treatment, requiring systemic treatment with immunosuppressants, such as methotrexate and cyclosporine [24]. These are off-label treatments for pediatric psoriasis [23], but may be considered for the treatment of moderate-to-severe or recalcitrant disease [17, 20, 25–28]. A number of biologic agents, including the newer biologics ixekizumab and secukinumab, are now approved in Europe for moderate-to-severe pediatric psoriasis (Table 1) [8, 16, 17, 20, 29–35], and other agents are being investigated, including brodalumab [36], guselkumab [37], and risankizumab [38].
Table 1.
Biologics approved for the treatment of plaque psoriasis in pediatric/adolescent patients [30, 31, 33–35]
| Biologic | Year of EMA approval for use in children | Indication | Recommended dose |
|---|---|---|---|
| Etanercept | 2009 | Children aged ≥ 6 years with long-term severe disease inadequately controlled by other systemic therapies | 0.8 mg/kg up to a maximum of 50 mg/dose once weekly for up to 24 weeks |
| Adalimumab | 2015 | Children aged ≥ 4 years with long-term severe disease |
Initial dose then Q2W starting 1 week after the initial dose: 15 to < 30 kg: 20 mg ≥ 30 kg: 40 mg |
| Ustekinumab | 2015 | Children aged ≥ 6 years with moderate-to-severe disease |
Initial dose, dosing after 4 weeks, then Q12W: < 60 kg: 0.75 mg/kg 60–100 kg: 45 mg > 100 kg: 90 mg |
| Ixekizumab | 2020 | Children aged ≥ 6 years weighing ≥ 25 kg with moderate-to-severe disease |
25–50 kg: initial dose 80 mg, then 40 mg Q4W > 50 kg: initial dose 160 mg, then 80 mg Q4W |
| Secukinumab | 2020 | Children aged ≥ 6 years with moderate-to-severe disease |
Once weekly for 4 weeks, then Q4W: < 25 kg: 75 mg 25 to < 50 kg: 75 mg ≥ 50 kg: 150 mg (may be increased to 300 mg) |
QXW every X weeks, EMA European Medicines Agency
Dosing in children for recently approved psoriasis treatments is primarily based on body weight [30, 31]. However, there are limited published data on patient characteristics and treatment patterns according to age and weight in pediatric patients receiving treatment for psoriasis in a real-world setting. The aim of this study was to describe patient demographics, disease, and treatment characteristics, with a focus on biologic drug use, in two weight groups of pediatric patients (25–50 kg and > 50 kg) with psoriasis from five European countries. The two weight groups were chosen to reflect approved dosage regimens for biologics in psoriasis [30, 31].
Methods
Study Design, Data Source, and Population
This study used physician survey data from the Adelphi Real World Pediatric Psoriasis Disease Specific Programme (DSP™). Adelphi DSPs are large, multinational, point-in-time surveys that collect cross-sectional real-world data through patient and physician surveys [39]. For the current study, primary care or specialist physicians treating pediatric patients with psoriasis in France, Germany, Italy, Spain, and the UK were surveyed between December 2019 and June 2020. Third-party fieldwork agencies identified, contacted, and recruited physicians on the basis of public lists of healthcare professionals. Thirty dermatologists, 15 pediatricians, and 10 primary care physicians (PCPs) were recruited per country. In the UK, the fieldwork agency advised that pediatricians would be difficult to recruit, which proved to be the case; consequently, pediatricians were replaced with dermatologists. In all countries, physicians were compensated for their participation according to fair market research rates consistent with the time involved. Care was taken to ensure that the sample of surveyed physicians was geographically representative of each country.
Each physician was required to be actively managing pediatric patients with psoriasis of any severity and to see at least five such patients in a typical month (three or more in the UK). They were asked to recruit their next 10 consulting pediatric patients with psoriasis into the study. Dermatologists were requested to include two patients who were currently receiving or had received a biologic in the last 12 months. Patients had to be at least 6 but less than 18 years of age, and to be receiving treatment for any-severity plaque psoriasis (including nail, scalp, inverse/flexural, and palmoplantar).
Physicians completed a patient record form (PRF) for each patient online, covering demographics, disease severity, symptoms, treatment history, patient management, and physician satisfaction with disease control. Information was collected from the time of diagnosis until the time of data collection. Disease severity was captured at diagnosis, immediately prior to initiation of the current treatment and at the time of data collection, and was rated as mild, moderate, or severe on the basis of the physicians’ own judgment. Psoriasis Area and Severity Index (PASI) scores within a range of 0–72 were also provided if known. Physicians were asked to provide a treatment history for each patient, working backwards from current to first regimen. A change in the line of treatment was defined as the initiation of new treatment, switch, or discontinuation of treatment. Treatment discontinuation was defined as discontinuation of a particular product (excluding topical treatment, for which data were only collected at the class level). Treatment breaks and treatment duration were not captured. First-line treatment was defined as the first treatment regimen the patient received, second-line treatment as the second treatment regimen, and so on. For each treatment line, patients could receive more than one type of drug category. Data were captured on up to 10 previous treatment lines. In addition, physicians completed a workload form and questions about the type and number of patients treated, and what drove the treatment choice.
Statistical Analysis
Patient and treatment characteristics were compared between those weighing 25–50 kg and those weighing more than 50 kg. Continuous variables were compared using Student’s t test; ordinal and categorical variables were compared using chi-squared or Fisher’s exact test. All other data were analyzed descriptively (no adjustments) overall and by country. Data on treatment characteristics were also analyzed by type of prescriber, as this is likely to be of clinical interest given that pediatric patients consult different medical specialties at different stages of their disease and depending on disease severity. Data were analyzed using the software package SPSS® Version 15 (SPSS Inc., Chicago, IL, USA). Continuous variables were summarized as mean ± standard deviation and median with interquartile range, while categorical variables were summarized as the number and percentage of patients. No allowance was made for missing data (e.g., by multiple imputation); where data were missing for specific variables (e.g., sometimes patients did not answer all questions), patients were excluded from the analyses for that variable.
Ethical Considerations
The study was approved by the WCG IRB (tracking number 20193181). All participants provided consent to participate in the survey. Physician responses were anonymized and pseudonymized, and data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy. The study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments, Good Pharmacoepidemiology Practices, and applicable laws and regulations of the participating countries.
Results
A total of 239 physicians participated in the survey, including 138 dermatologists, 45 pediatricians, and 56 PCPs (Supplementary Material, Fig. S1). PRFs were completed for 1919 patients, of whom 772 (40.2%) weighed 25–50 kg and 1147 (59.8%) weighed more than 50 kg. The proportion of patients weighing 25–50 kg was lower in Germany, Spain, and the UK (29.3–36.8%) than in France and Italy (47.3%) (Supplementary Material, Table S1).
Patient Characteristics at Diagnosis and at the Time of Data Collection
Patient characteristics at diagnosis and time of data collection, overall and by weight category, are shown in Table 2. Some characteristics were significantly different between the two weight groups, including age, the proportion of male patients, body mass index (BMI), age at diagnosis, time since diagnosis, and disease severity at diagnosis. Median patient age at diagnosis was 12.0 years overall, 10.0 years in patients weighing 25–50 kg, and 14.0 years in those weighing more than 50 kg (p < 0.001). Overall, 56.9% of patients were male. Median current BMI was 21.0 overall, 19.0 in patients weighing 25–50 kg, and 22.4 in those weighing more than 50 kg (p < 0.001). Similar patterns were seen across countries for age, sex, and BMI (Supplementary Material, Table S2). The majority of patients (90.4%) had plaque psoriasis. Overall, 4.3% of patients had psoriatic arthritis (PsA).
Table 2.
Patient characteristics and history of psoriasis at the time of data collection, overall and by weight category
| Parametera | Overall | Weighing 25–50 kg | Weighing > 50 kg | p valueb |
|---|---|---|---|---|
| Number and percentage of patients | 1919 (100.0%) | 772 (40.2%) | 1147 (59.8%) | |
| Current age (years) | ||||
| Mean ± SD | 13.9 ± 2.9 | 11.5 ± 2.8 | 15.5 ± 1.6 | < 0.001 |
| Median [IQR] | 15.0 [12.0, 16.0] | 11.0 [9.0, 14.0] | 16.0 [15.0, 17.0] | |
| Male | 1091 (56.9%) | 352 (45.6%) | 739 (64.4%) | < 0.001 |
| Current body mass index | ||||
| Mean ± SD | 21.9 ± 7.3 | 19.8 ± 6.8 | 23.3 ± 7.2 | < 0.001 |
| Median [IQR] | 21.0 [19.0, 23.0] | 19.0 [18.0, 20.0] | 22.4 [21.0, 24.0] | |
| Type of psoriasis | ||||
| Plaque | 1734 (90.4%) | 694 (89.9%) | 1040 (90.7%) | 0.294 |
| Other | 185 (9.6%) | 78 (10.1%) | 107 (9.3%) | |
| Age at diagnosis (years) | ||||
| Mean ± SD | 11.4 ± 3.5 | 9.5 ± 2.9 | 12.8 ± 3.1 | < 0.001 |
| Median [IQR] | 12.0 [9.0, 14.0] | 10.0 [8.0, 11.0] | 14.0 [11.0, 15.0] | |
| Time since diagnosis (years) | ||||
| Mean ± SD | 2.7 ± 2.7 | 2.2 ± 2.4 | 3.0 ± 2.9 | < 0.001 |
| Median [IQR] | 1.8 [0.8, 3.6] | 1.4 [0.5, 3.1] | 2.0 [1.1, 4.1] | |
| Severity of psoriasis at diagnosisc | ||||
| Mild | 641 (33.4%) | 295 (38.2%) | 346 (30.2%) | < 0.001 |
| Moderate/severe | 1278 (66.6%) | 477 (61.8%) | 801 (69.8%) | |
| BSA affected at diagnosis (%) | ||||
| Mean ± SD | 15.2 ± 14.9 | 15.0 ± 15.9 | 15.4 ± 14.1 | 0.655 |
| Median [IQR] | 10.0 [6.0, 20.0] | 10.0 [5.0, 18.0] | 10.0 [6.0, 20.0] | |
| Family history of psoriasis | 899 (46.8%) | 353 (45.7%) | 546 (47.6%) | 0.249 |
| Diagnosis of psoriatic arthritis | 82 (4.3%) | 28 (3.6%) | 54 (4.7%) | 0.300 |
| Diagnosed using CASPAR criteria | 42 (51.2%)d | 11 (39.3%)d | 31 (57.4%)d | 0.143 |
Values are shown as mean ± standard deviation and median [IQR] or n (%) unless otherwise indicated
BSA body surface area, CASPAR Classification Criteria for Psoriatic Arthritis, IQR interquartile range, SD standard deviation
aNumber of patients was lower for some parameters
bFor the comparison between the two patient weight groups. Continuous variables were compared using Student’s t test; ordinal and categorical variables were compared using chi-squared or Fisher’s exact test
cDisease severity was based upon the physicians’ own judgment
dOf those with a diagnosis of psoriatic arthritis
Disease Severity and Symptoms
Overall, 66.6% of patients had moderate or severe psoriasis at diagnosis, and this was similar for the two weight categories (Table 2). Immediately before starting the current/most recent treatment regimen (topical and/or systemic), most patients (71.8%) had moderate or severe psoriasis (Table 3). While receiving current treatment, most patients (80.0%) had mild psoriasis. Dermatologists treated more patients with moderate or severe psoriasis (as defined immediately prior to the current/most recent treatment regimen) than pediatricians and PCPs. The three types of prescribers were uniform in rating current disease severity (Supplementary Material, Table S3).
Table 3.
Current treatment characteristics overall and by weight category
| Treatmenta | Overall (N = 1919) | Weighing 25–50 kg (N = 772) | Weighing > 50 kg (N = 1147) | p valueb |
|---|---|---|---|---|
| Severity of psoriasis immediately prior to current/most recent treatment regimen | < 0.001 | |||
| Mild | 541 (28.2%) | 261 (33.8%) | 280 (24.4%) | |
| Moderate/severe | 1378 (71.8%) | 511 (66.2%) | 867 (75.6%) | |
| Current severity of psoriasis | 1.000 | |||
| Mild | 1536 (80.0%) | 618 (80.1%) | 918 (80.0%) | |
| Moderate/severe | 383 (20.0%) | 154 (19.9%) | 229 (20.0%) | |
| Topical only | 1133 (59.0%) | 543 (70.3%) | 590 (51.4%) | < 0.001 |
| Topical corticosteroidc | 648 (33.8%) | 300 (38.9%) | 348 (30.3%) | < 0.001 |
| Topical non-corticosteroidc | 569 (29.7%) | 266 (34.5%) | 303 (26.4%) | < 0.001 |
| Topical combination productc,d | 498 (26.0%) | 194 (25.1%) | 304 (26.5%) | 0.490 |
| Phototherapy (PUVA, UVB)c | 139 (7.2%) | 57 (7.4%) | 82 (7.1%) | 0.858 |
| Conventional systemics | 208 (10.8%) | 63 (8.2%) | 145 (12.6%) | 0.002 |
| Methotrexatec | 30 (6.8%) | 43 (5.6%) | 87 (7.6%) | 0.095 |
| Cyclosporinec | 50 (2.6%) | 15 (1.9%) | 35 (3.1%) | 0.146 |
| Acitretinc | 25 (1.3%) | 6 (0.8%) | 19 (1.7%) | 0.104 |
| Fumaratec | 5 (0.3%) | 1 (0.1%) | 4 (0.3%) | 0.654 |
| Biologicse | 466 (24.3%) | 121 (15.7%) | 345 (30.1%) | < 0.001 |
| Etanerceptc | 80 (4.2%) | 27 (3.5%) | 53 (4.6%) | 0.245 |
| Etanercept or biosimilarc | 107 (5.6%) | 38 (4.9%) | 69 (6.0%) | 0.361 |
| Adalimumabc | 221 (11.5%) | 60 (7.8%) | 161 (14.0%) | < 0.001 |
| Adalimumab or biosimilarc | 264 (13.8%) | 66 (8.5%) | 198 (17.3%) | < 0.001 |
| Ustekinumabc | 95 (5.0%) | 17 (2.2%) | 78 (6.8%) | < 0.001 |
| Otherf | 9 (0.5%) | 3 (0.4%) | 6 (0.5%) | 0.747 |
| Ever received a biologic | 501 (26.1%) | 135 (17.5%) | 366 (31.9%) | < 0.001 |
Values are shown as n (%)
PUVA psoralen and ultraviolet A, UVB ultraviolet B
aNumber of patients was lower for some parameters
bFor the comparison between the two patient weight groups (Fisher’s exact test)
cPercentages based on the number of patients with a treatment history: 1898 overall, 765 for patients weighing 25–50 kg and 1133 for patients weighing > 50 kg
dFor example, calcipotriol + betamethasone dipropionate
eNo data on off-label biologic use were collected
fIncluded urea, tacrolimus, calcipotriol/betamethasone, and emollients
Mean PASI prior to the initiation of current treatment was 7.0 ± 8.4 for patients with mild psoriasis, 12.8 ± 9.9 for those with moderate disease, and 22.5 ± 14.7 for those with severe disease. For patients weighing 25–50 kg, mean PASI prior to the initiation of current treatment was 21.5 ± 14.7 for biologic-treated patients and 10.2 ± 11.3 for patients treated with non-biologics. Corresponding values for patients weighing more than 50 kg were 20.7 ± 12.6 and 11.4 ± 10.8, respectively.
The most common current symptoms overall were scaling/flaking (55.5% of patients), red inflamed skin (49.6%), itching (48.0%), redness/discoloration (28.6%), cracked skin (26.0%), and burning (18.4%). A similar pattern was seen across the weight categories and countries.
Overall Treatment
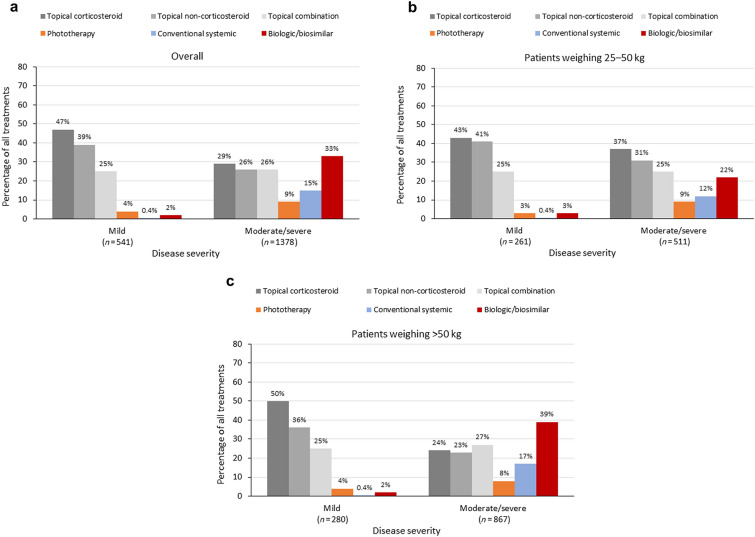
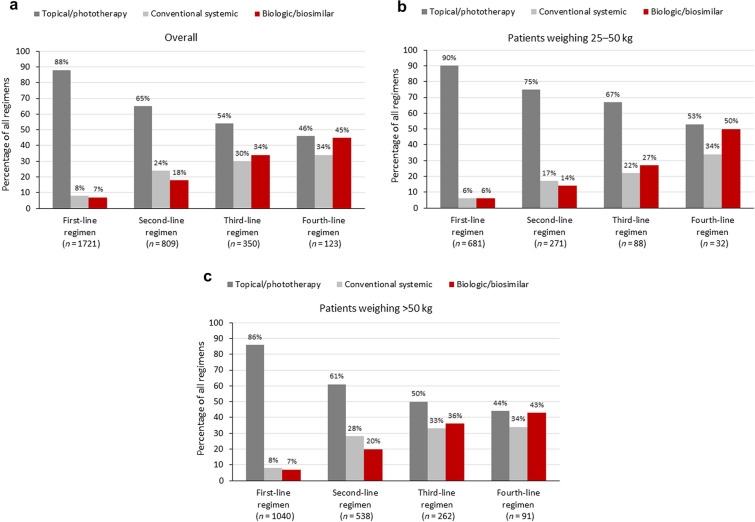
Most patients (N = 1919) across both weight categories were prescribed topical treatments (Table 3). These were more likely to be used to treat mild rather than moderate-to-severe psoriasis, while conventional systemics and biologics were more likely to be used for moderate-to-severe rather than mild psoriasis (Fig. 1). A similar pattern was seen across countries (Supplementary Material, Fig. S2). A complete treatment history was available for 1721 patients, all of whom had received a first-line regimen, 809 a second-line regimen, 350 a third-line regimen, and 123 a fourth-line regimen (Fig. 2).
Fig. 1.
Treatments received according to disease severity prior to the initiation of current treatment in a the overall patient population, b patients weighing 25–50 kg, and c patients weighing more than 50 kg. Patients could receive multiple treatments
Fig. 2.
Treatments by line of therapy for patients with a complete treatment history for a the overall patient population, b patients weighing 25–50 kg, and c patients weighing more than 50 kg. Data were collected at the class level only. Percentages for each regimen line add up to more than 100% as patients could receive multiple treatments for each regimen line
Topical and Conventional Systemic Treatments
Topical therapy alone was the most prescribed current treatment (59.0% of patients overall), with significantly greater use in patients weighing 25–50 kg (70.3%) than in those weighing more than 50 kg (51.4%; p < 0.001) (Table 3). Topical therapy was more likely to be prescribed by pediatricians (75.4%) or PCPs (87.7%) than dermatologists (43.4%) (Supplementary Material, Table S3). The use of topical therapy alone decreased with regimen line, but was consistently higher in patients in the lower-weight category. The exception was for first-line regimens, where usage was similar between the two weight categories (Fig. 2).
Conventional systemics, including methotrexate, cyclosporine, acitretin, and fumarate, were prescribed in 10.8% of patients overall. The use of these agents was higher in patients weighing more than 50 kg than in those weighing 25–50 kg (p = 0.002; Table 3). A similar pattern was seen across countries, apart from Spain, where their use was similar between the two weight categories (Supplementary Material, Table S4). Conventional systemics were more likely to be prescribed by dermatologists (14.5%) than pediatricians (7.0%) or PCPs (4.1%) (Supplementary Material, Table S3). The use of conventional systemics was low for first-line regimens (7.6% overall), but increased for later regimen lines (Fig. 2).
Methotrexate was the most prescribed conventional agent overall (6.8% of patients with a treatment history, 19.3% of patients prescribed systemic treatments [conventional systemics and biologics], and 62.5% of patients prescribed conventional systemics) (Table 3) and across all countries apart from Italy (Supplementary Material, Table S4). In Italy, cyclosporine was the most prescribed conventional agent (67.5% of patients receiving conventional systemics), followed by methotrexate (37.5% of patients prescribed conventional systemics).
Biologic Treatments
Overall, in our biologic user-enriched population, 466 of 1919 patients (24.3%) were currently receiving biologic therapy, while 501 patients (26.1%) had ever received a biologic (patients who received a biologic at some point in their psoriasis treatment pathway, and including those currently receiving any-line biologic therapy). Biologic therapy was significantly more likely to be prescribed in patients weighing more than 50 kg (74.0% of patients prescribed a biologic) than in those weighing 25–50 kg (26.0% of patients prescribed a biologic; p < 0.001) (Table 3). A similar pattern was seen across countries (Supplementary Material, Table S4). Biologics were also more likely to be prescribed in patients with moderate-to-severe disease (32.9%) than in those with mild disease (2.4%; Fig. 1 and Supplementary Material, Fig. S2), in patients with comorbid PsA (69.5% were prescribed a biologic), and, in line with inclusion criteria, by dermatologists (35.4%) than pediatricians (13.4%) or PCPs (3.4%) (Supplementary Material, Table S3).
For patients currently receiving a biologic, the median age at biologic initiation was 16.0 years overall (Supplementary Material, Fig. S3). Biologic use started to increase at the age of 13.0 years, and most biologics (78.2%) were prescribed to patients older than this. A similar pattern was seen across countries (Supplementary Material, Fig. S3). The current median weight of patients receiving biologics was 60.0 kg, and most biologics (approx. 75%) were prescribed to patients weighing more than 50 kg and those with a BMI of 20–24, although differences between countries were observed (Supplementary Material, Fig. S4).
As expected, across all patients with complete treatment history data (n = 1721), biologic use increased with line of therapy, from 6.6% of all first-line regimens to 18.0% as a second-line regimen, 33.7% as a third-line regimen, and 44.7% as a fourth-line regimen (Fig. 2). A similar pattern was observed across countries, except that the use of biologics was much lower in the UK for earlier regimen lines (Supplementary Material, Fig. S5).
The most prescribed biologic was adalimumab or a biosimilar (13.8% of all patients, 56.7% of patients prescribed a biologic; Table 3), and use of this agent or a biosimilar was significantly greater among patients weighing more than 50 kg than among those weighing 25–50 kg (17.3% vs. 8.5%; p < 0.001). A similar pattern was seen across countries (Supplementary Material, Table S4). The use of etanercept or a biosimilar was relatively consistent across treatment lines, whereas the use of adalimumab or a biosimilar and ustekinumab increased with treatment line, both overall and in the two weight categories.
The mean length of time on biologic treatment for patients currently receiving a biologic was 32.9 ± 27.9 weeks overall; this was similar for the two weight categories (33.8 ± 26.3 weeks for those weighing 25–50 kg and 32.5 ± 28.4 weeks for those weighing more than 50 kg). A similar pattern was seen in Germany and the UK, while treatment was shorter in patients in the lower-weight category in France, but longer in patients in the lower-weight category in Italy and Spain (Supplementary Material, Table S5).
Of the 466 patients who were prescribed a biologic, approximately one-third (36.5% [n = 170]) were taking concomitant treatment: 4.7% (n = 22) were also using a topical or phototherapy, and 31.8% (n = 148) were also taking a conventional systemic.
Factors Influencing Biologic Prescribing
The main factors physicians considered from a pre-selected list before prescribing a biologic were previous treatments received (68.4% of physicians), the patient’s PASI (65.8%), the presence/absence of PsA and/or joint pain (46.0%), the patient’s age (> 12 years; 43.9%), and the impact of treatment on patients’ normal daily activities (43.5%) and emotional well-being (42.6%). Specific clinical measures (PASI, body surface area [BSA] affected) were more of an influence on dermatologists’ decision to prescribe a biologic than for pediatricians and PCPs, whereas pediatricians and PCPs were more concerned about the number and size of plaques than dermatologists.
The main factors influencing physician choice of the current biologic (n = 466) were the likelihood of clearing psoriatic lesions through achievement of at least 75% improvement in PASI (50.9%), control of flares (45.1%), long-term efficacy (44.6%), likelihood of clearing psoriatic lesions through achievement of at least 90% improvement in PASI (44.4%), relief of itching (43.1%), improved/maintained patient quality of life (41.8%), and a reduction in the BSA affected (41.6%). Thirty-one percent (31.1%) of physicians considered the biologic’s general and long-term safety profile, and 21.0% considered the likelihood of serious adverse events when choosing a biologic. Findings were generally similar across countries.
Physician Satisfaction with Disease Control
For patients with moderate or severe disease, 41.3% of physicians were satisfied with current control of the disease, 15.1% were not satisfied but believed this was the best control that could realistically be achieved, and 43.6% were not satisfied and believed better control could be achieved. The main reasons for dissatisfaction with current disease control (any treatment) were incomplete skin clearance (35.6%), slow onset of efficacy (27.1%), loss of response over time (22.7%), and dissatisfied patient (20.4%). For patients with mild psoriasis, 89.0% of physicians were satisfied with current disease control, 3.8% were not satisfied but believed this was the best control that could realistically be achieved, and 7.1% were not satisfied and believed better control could be achieved.
Discussion
This study investigated treatment patterns and patient characteristics by weight in a biologic-enriched sample of pediatric patients with psoriasis in a large real-world sample of pediatric patients aged 6 years or older, both overall and by country. Results showed that the use of topical agents was high across all treatment lines. There was little use of biologics as first- or second-line therapy. Biologic use started to increase at the age of 13 years, and most pediatric patients receiving these drugs were older (≥ 16 years) and heavier (> 50 kg). For patients with moderate-to-severe psoriasis, the main reason for physician dissatisfaction with current treatment was incomplete skin clearance.
The finding that pediatric patients with psoriasis were most likely to be prescribed topical treatments was supported by the results of a survey of 92 German pediatricians who were treating patients with psoriasis [40]. In this survey, 53% of German pediatricians would prescribe topical treatment for confirmed psoriasis and 10% would recommend conventional systemics. Similarly, a survey of 384 French physicians (PCPs, pediatricians, and dermatologists) showed that topical corticosteroids were the most prescribed agents (by 88% of physicians) for children with psoriasis, while the prescribing of systemic treatments was limited [41]. In our study, the use of topical treatments was greater among lower- than higher-weight patients, possibly reflecting physician reluctance to prescribe systemic treatments in lower-weight patients who are also likely to be younger.
Methotrexate was the preferred conventional systemic overall in the current study (62.5% of patients taking these drugs) and in all countries apart from Italy, where cyclosporine was the preferred conventional systemic, prescribed in 67.5% of patients receiving systemic treatment. A similar pattern was observed in a study of systemic treatment patterns in 58 Italian children and adolescents with moderate-to-severe psoriasis [42]. Cyclosporine was the preferred first-line treatment, prescribed in 53% of patients. However, in contrast to our study, acitretin was prescribed in 22% of patients (compared with none in Italy in our study) and methotrexate in 7% of patients (compared with 37.5% in Italy in our study). In line with our study, a retrospective medical record review of 234 pediatric patients with psoriasis treated with methotrexate and/or biologics in Europe and North America found that 70% of patients were treated exclusively with methotrexate [43].
As a result of the biologic-enriched sample included, one-quarter of pediatric patients in our study were prescribed biologics at some point in their treatment pathway; although not necessarily representative of prescribing patterns, this enabled meaningful characterization of these biologic-treated patients and their associated treatment patterns. In the study by Bronckers et al. [43], 20% of patients were treated exclusively with biologics, while 10% received methotrexate and biologics sequentially. The previously mentioned survey of French physicians found that 5.2% had prescribed etanercept to their pediatric patients with psoriasis, similar to the 7.9% in France in our study [41], while the survey of German pediatricians mentioned above found that none had prescribed a biologic in children with psoriasis, even in those with severe disease [40]. By comparison, 8.7% of patients prescribed a biologic in Germany had received their prescription from a pediatrician in our study.
In the current study, country differences were seen in the distribution of BMI among patients receiving biologics. In France, children with psoriasis are more likely to be obese than those without psoriasis [44, 45]. However, in the current study, the distribution of BMI in France matched that seen overall, with most patients receiving biologics having a BMI of 20–24, and fewer patients with a BMI above 25 receiving these agents. This difference was most likely due to the majority of patients (61.0%) having a BMI of 20–24 and a smaller number (18.1%) having a BMI of greater than 25.
The main factors physicians considered from a pre-selected list before prescribing a biologic were previous treatments received, the patient’s PASI, the presence of psoriatic arthritis, and the patient’s age. Likewise, in a French retrospective observational study of biologic drug survival in 134 pediatric patients with psoriasis, factors significantly associated with the choice of first-line biologic were age at onset of psoriasis, age at biologic initiation, PASI, and physician global assessment [46]. Conversely, a survey of 384 French physicians treating children with psoriasis showed that severity scores were underused, with only 4% of PCPs and pediatricians and 24% of dermatologists reporting the use of severity measures [41].
Strengths of our study are that Adelphi DSPs contribute data from large international databases providing country-specific real-world information on disease characteristics, management, and outcomes; and the use of standardized data collection tools allowing comparisons to be made between countries. Study limitations are that the sample was not truly random as the next 10 consulting patients who were suitable were included, creating a convenience sample. Consequently, patients who consulted more frequently were more likely to be included in the sample; it is possible that such patients had more active, advanced, or complex disease, which may have accounted for the high proportion of patients with moderate-to-severe disease in our study. In addition, patients receiving biologics were oversampled in the study, and, as dermatologists were most frequently surveyed, this could explain the high proportion of patients with moderate-to-severe disease. Also, only around half of patients on biologics were taking concomitant therapy, which is lower than expected. Therefore, our sample may not represent the overall population of pediatric patients with psoriasis.
Furthermore, the diagnosis of psoriasis was based primarily on the judgment and diagnostic skills of the physician rather than a formal diagnostic checklist, although patients were managed in accordance with routine diagnostic procedures reflecting those in clinical practice. Nevertheless, it is possible that some patients were misclassified as having mild disease but actually had moderate disease, as 6% of patients with mild disease had BSA involvement of greater than 10%, which objectively may be considered moderate disease [47]. Physician inclusion was likely influenced by willingness to participate, which may introduce selection bias; data quality was dependent on the accurate reporting of information by physicians and may have been subject to recall bias; and finally, as data collection in the DSPs is cross-sectional, longitudinal treatment use cannot be tracked and the data cannot be used to demonstrate cause and effect.
Conclusions
The use of topical agents is high across all treatment lines in European pediatric patients with psoriasis aged 6–17 years. There is little first- or second-line use of biologic agents and, when prescribed, biologics tend to be used in older patients and those weighing more than 50 kg. Physicians report dissatisfaction with current treatments for moderate-to-severe psoriasis. The low use of biologics may represent an unmet treatment need, as topical or conventional systemic agents remain the main treatment option for moderate or severe psoriasis in pediatric patients through the treatment pathway.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Funding
Eli Lilly and Company provided financial support for the conduct of this research, preparation of the article, and funding of the journal’s Rapid Service Fee.
Medical Writing, Editorial, and Other Assistance
The authors would like to acknowledge Susanne Hartz, Julie Hill, Serena Losi, Erika Lorenzo Vivas, and Lill-Brith von Arx (Eli Lilly and Company) for their valuable contributions to the manuscript, and James Hetherington (Adelphi Real World, Bollington, Macclesfield, Cheshire, UK) for data extraction, analysis, and related activities. They would also like to acknowledge Dr. Sue Chambers and Greg Plosker (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript (funded by Eli Lilly and Company), plus the physicians, and the patients and their families who participated in the surveys.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Catherine Reed and James Lucas contributed to the study conception and design. Material preparation, data collection, and analysis were performed by James Lucas. All authors were involved in interpretation of data for the work. The first draft of the manuscript was written by Catherine Reed, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Michael Sticherling has participated on advisory boards for AbbVie, Amgen, BMS, Celgene, Janssen Cilag, Leo, Lilly, Pfizer, Novartis, Sanofi, and UCB; he is on speaker boards for AbbVie, Celgene, Janssen Cilag, Leo, MSD, Novartis, and Pfizer; and has participated in clinical studies for AbbVie, Amgen, BMS, Boehringer Ingelheim, Celgene, Galderma, GSK, Janssen Cilag, Leo, Novartis, Pfizer, Regeneron, and Sanofi. Tess McPherson has participated in pediatric dermatology advisory boards for Abbvie and Sanofi and received support for conference attendance/teaching from Leo, Sanofi, Janssen, and Novartis. Raúl de Lucas Laguna has declared no conflicts. Antonio Costanzo served as advisory board member and consultant, and has received fees and speaker's honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, Leo Pharma, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. Catherine Reed, Esther Artime, Camille Robert, and Christopher Schuster are employees of Eli Lilly and Company. James Lucas is an employee of Adelphi Real-World. Emmanuel Mahé has undertaken paid activities as a consultant, advisor, or speaker for AbbVie, Amgen, Celgene, Janssen, Leo Pharma, Lilly, and Novartis.
Compliance with Ethics Guidelines
The study was approved by the WCG IRB (tracking number 20193181). All participants provided consent to participate in the survey. Physician responses were anonymized and pseudonymized, and data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy. The study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments, Good Pharmacoepidemiology Practices, and applicable laws and regulations of the participating countries.
Data Availability
All data generated or analyzed during this study are included in this published article and the supplementary information files.
Contributor Information
Michael Sticherling, Email: michael.sticherling@uk-erlangen.de.
Tess McPherson, Email: tess.mcpherson@msd.ox.ac.uk.
Raúl de Lucas Laguna, Email: rauldelucas@gmail.com.
Antonio Costanzo, Email: acostanzo2000@gmail.com.
Catherine Reed, Email: reed_catherine@lilly.com.
Esther Artime, Email: artime_esther@lilly.com.
Camille Robert, Email: robert_camille@lilly.com.
James Lucas, Email: James.Lucas@adelphigroup.com.
Christopher Schuster, Email: schuster_christopher@lilly.com.
Emmanuel Mahé, Email: emmanuel.mahe@ch-argenteuil.fr.
References
- 1.Parisi R, Symmons D, Griffiths C, Ashcroft D. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 2.Lewis-Beck C, Abouzaid S, Xie L, et al. Analysis of the relationship between psoriasis symptom severity and quality of life, work productivity, and activity impairment among patients with moderate-to-severe psoriasis using structural equation modeling. Patient Prefer Adher. 2013;7:199–205. doi: 10.2147/PPA.S39887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082. doi: 10.1038/nrdp.2016.82. [DOI] [PubMed] [Google Scholar]
- 4.Eichenfield LF, Paller AS, Tom WL, et al. Pediatric psoriasis: evolving perspectives. Pediatr Dermatol. 2018;35:170–181. doi: 10.1111/pde.13382. [DOI] [PubMed] [Google Scholar]
- 5.Bronckers IMGJ, Paller AS, Van Geel MJ, Van de Kerkhof PCM, Seyger MMB. Psoriasis in children and adolescents: diagnosis, management and comorbidities. Paediatr Drugs. 2015;17:373–384. doi: 10.1007/s40272-015-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronckers I, van Geel MJ, van de Kerkhof PCM, et al. A cross-sectional study in young adults with psoriasis: potential determining factors in quality of life, life course and work productivity. J Dermatol Treat. 2019;30:208–215. doi: 10.1080/09546634.2018.1506077. [DOI] [PubMed] [Google Scholar]
- 7.Augustin M, Glaeske G, Radtke MA, et al. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162:633–636. doi: 10.1111/j.1365-2133.2009.09593.x. [DOI] [PubMed] [Google Scholar]
- 8.Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161–201. doi: 10.1016/j.jaad.2019.08.049. [DOI] [PubMed] [Google Scholar]
- 9.Warren RB, Kleyn CE, Gulliver WP. Cumulative life course impairment in psoriasis: patient perception of disease-related impairment throughout the life course. Br J Dermatol. 2011;164(Suppl. 1):1–14. doi: 10.1111/j.1365-2133.2011.10280.x. [DOI] [PubMed] [Google Scholar]
- 10.Mahé E, Maccari F, Beauchet A, et al. Childhood-onset psoriasis: association with future cardiovascular and metabolic comorbidities. Br J Dermatol. 2013;169:889–95. doi: 10.1111/bjd.12441. [DOI] [PubMed] [Google Scholar]
- 11.Mattei PL, Corey KC, Kimball AB. Cumulative life course impairment: evidence for psoriasis. Curr Probl Dermatol. 2013;44:82–90. doi: 10.1159/000350008. [DOI] [PubMed] [Google Scholar]
- 12.Ros S, Puig L, Carrascosa JM. Cumulative life course impairment: the imprint of psoriasis on the patient’s life. Acta Dermosifiliogr. 2014;105:128–134. doi: 10.1016/j.ad.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Herbert AA, Bobonich MA, Rodriguez Capriles C, et al. Higher rates of skin clearance and efficacy in challenging body areas are associated with better health-related quality of life following ixekizumab maintenance treatment in pediatric patients with plaque psoriasis. Pediatr Dermatol. 2022;39:55–60. doi: 10.1111/pde.14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Megna M, Napolitano M, Balato A, et al. Psoriasis in children: a review. Curr Ped Rev. 2015;11:10–26. doi: 10.2174/1573400511666150504125456. [DOI] [PubMed] [Google Scholar]
- 15.Eisert L, Augustin M, Bach S, et al. S2k guidelines for the treatment of psoriasis in children and adolescents—short version part 1. J Dtsch Dermatol Ges. 2019;17:856–870. doi: 10.1111/ddg.13907. [DOI] [PubMed] [Google Scholar]
- 16.Eisert L, Augustin M, Bach S, et al. S2k guidelines for the treatment of psoriasis in children and adolescents—short version part 2. J Dtsch Dermatol Ges. 2019;17:959–973. doi: 10.1111/ddg.13936. [DOI] [PubMed] [Google Scholar]
- 17.Lansang P, Bergman JN, Fiorillo L, et al. Management of pediatric plaque psoriasis using biologics. J Am Acad Dermatol. 2020;82:213–221. doi: 10.1016/j.jaad.2019.05.056. [DOI] [PubMed] [Google Scholar]
- 18.Sticherling M, Augustin M, Boehncke WH, et al. Therapy of psoriasis in childhood and adolescence—a German expert consensus. J Dtsch Dermatol Ges. 2011;9:815–823. doi: 10.1111/j.1610-0387.2011.07668.x. [DOI] [PubMed] [Google Scholar]
- 19.Ståhle M, Atakan N, Boehncke WH, et al. Juvenile psoriasis and its clinical management: a European expert group consensus. J Dtsch Dermatol Ges. 2010;8:812–818. doi: 10.1111/j.1610-0387.2010.07507.x. [DOI] [PubMed] [Google Scholar]
- 20.Belloni Fortina A, Bardazzi F, Berti S, et al. Treatment of severe psoriasis in children: recommendations of an Italian expert group. Eur J Pediatr. 2017;176:1339–1354. doi: 10.1007/s00431-017-2985-x. [DOI] [PubMed] [Google Scholar]
- 21.Kravas G, Gholam K. Use of topical therapies for pediatric psoriasis: a systematic review. Pediatr Dermatol. 2018;35:296–302. doi: 10.1111/pde.13422. [DOI] [PubMed] [Google Scholar]
- 22.Napolitano M, Megna M, Balato A, et al. Systemic treatment of pediatric psoriasis: a review. Dermatol Ther. 2016;6:125–142. doi: 10.1007/s13555-016-0117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahé E, Corgibet F, Maccari F, et al. Off-label drugs in childhood psoriasis. Ann Dermatol Venereol. 2020;147:429–438. doi: 10.1016/j.annder.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Bruins FM, Bronckers IMGJ, Cai R, et al. Treatment persistence in paediatric and adolescent psoriasis patients followed into young adulthood. From topical to systemic treatment: a prospective, longitudinal, observational cohort study of 448 patients. Br J Dermatol. 2021;184:464–472. doi: 10.1111/bjd.19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright NA, Piggott CDS, Eichenfield LF. The role of biologics and other systemic agents in the treatment of pediatric psoriasis. Semin Cutan Med Surg. 2010;29:20–27. doi: 10.1016/j.sder.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Van Geel MJ, Mul K, de Jager ME, et al. Systemic treatments in paediatric psoriasis: a systematic evidence-based update. J Eur Acad Dermatol Venereol. J Eur Acad Dermatol Venereol. 2015;29:425–437. doi: 10.1111/jdv.12749. [DOI] [PubMed] [Google Scholar]
- 27.Van Geel MJ, Oostveen AM, Hoppenreijs EPAH, et al. Methotrexate in pediatric plaque-type psoriasis: long-term daily clinical practice results from the Child-CAPTURE registry. J Dermatol Treat. 2015;26:406–412. doi: 10.3109/09546634.2014.996515. [DOI] [PubMed] [Google Scholar]
- 28.Torrelo A. The use of biologics for childhood psoriasis. J Eur Acad Dermatol Venereol. 2019;33:1816. doi: 10.1111/jdv.15855. [DOI] [PubMed] [Google Scholar]
- 29.Sun HY, Phan K, Paller AS, Sebaratnam DF. Biologics for pediatric psoriasis: a systematic review and meta-analysis. Pediatr Dermatol. 2022;39:42–48. doi: 10.1111/pde.14870. [DOI] [PubMed] [Google Scholar]
- 30.European Medicines Agency. Taltz. European Public Assessment Report. July 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/taltz Accessed 10 December 2020.
- 31.European Medicines Agency. Cosentyx. European Public Assessment Report. November 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/cosentyx. Accessed 29 January 2021.
- 32.Bodemer C, Kaszuba A, Kingo K, et al. Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a phase 3 double-blind randomised, controlled trial. J Eur Acad Dermatol Venereol. 2021;35:938–947. doi: 10.1111/jdv.17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.European Medicines Agency. Enbrel. Summary of Product Characteristics. November 2021. https://www.ema.europa.eu/en/documents/product-information/enbrel-epar-product-information_en.pdf. Accessed 23 April 2022.
- 34.European Medicines Agency. Humira. Summary of Product Characteristics. September 2021. https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf. Accessed 23 April 2022.
- 35.Janssen Pharmaceutical Companies. Stelara Prescribing Information. November 2019. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/STELARA-pi.pdf. Accessed 23 April 2022.
- 36.European Medicines Agency. Brodalumab paediatric investigation plan. November 2018. https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-001089-pip02-13-m01. Accessed 29 January 2021.
- 37.European Medicines Agency. Guselkumab paediatric investigation plan. April 2019. https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-001523-pip03-18. Accessed 29 January 2021.
- 38.ClinicalTrials.gov. A study of subcutaneous risankizumab injection for pediatric participants with moderate to severe plaque psoriasis to assess change in disease symptoms. NCT04435600. https://clinicaltrials.gov/ct2/show/NCT04435600. Accessed 12 May 2021.
- 39.Anderson P, Benford M, Harris N, et al. Real-world physician and patient behaviour across countries: disease-specific programmes—a means to understand. Curr Med Res Opin. 2008;24:3063–3072. doi: 10.1185/03007990802457040. [DOI] [PubMed] [Google Scholar]
- 40.Pinter A, Mielke N, Malisiewicz B, et al. Management of paediatric psoriasis by paediatricians: a questionnaire-based survey. Dermatol Ther. 2020;10:671–680. doi: 10.1007/s13555-020-00390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahé E, Bursztejn AC, Phan A, Corgibet F, Beauchet A. Management of childhood psoriasis in France. A national survey among general practitioners, pediatricians, and dermatologists. Dermatol Ther. 2018;31:e12567. doi: 10.1111/dth.12567. [DOI] [PubMed] [Google Scholar]
- 42.Di Lernia V, Neri I, Pinton PC, et al. Treatment patterns with systemic antipsoriatic agents in childhood psoriasis: an Italian database analysis. G Ital Dermatol Venereol. 2017;152:327–332. doi: 10.23736/S0392-0488.16.05287-X. [DOI] [PubMed] [Google Scholar]
- 43.Bronckers IMGJ, Paller AS, West DP, et al. Psoriasis Investigator Group the Pediatric Dermatology Research Alliance and the European Working Group on Pediatric Psoriasis. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384–392. doi: 10.1001/jamadermatol.2019.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahé E, Beauchet A, Bodemer C, et al. Psoriasis and obesity in French children: a case-control, multicentre study. Br J Dermatol. 2015;172:1593–1600. doi: 10.1111/bjd.13507. [DOI] [PubMed] [Google Scholar]
- 45.Mahé E, Maccari F, Ruer-Mulard M, et al. Psoriasis de l’enfant vu en milieu libéral: les aspects cliniques et épidémiologiques diffèrent des données habituellement publiées. Ann Dermatol Venereol. 2019;146:354–362. doi: 10.1016/j.annder.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 46.Phan C, Beauchet A, Burztejn AC, et al. Biological treatments for paediatric psoriasis: a retrospective observational study on biological drug survival in daily practice in childhood psoriasis. J Eur Acad Dermatol Venereol. 2019;33:1984–1992. doi: 10.1111/jdv.15579. [DOI] [PubMed] [Google Scholar]
- 47.Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol. 2005;152:861–867. doi: 10.1111/j.1365-2133.2005.06502.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and the supplementary information files.