Abstract
Introduction
Keratosis pilaris (KP) is a disfiguring disease and is resistant to treatment. Several treatment methods are available, but the efficacy is limited. This prospective, rater-blinded, split-body comparative study investigated the efficacy and safety of long-pulsed 755-nm alexandrite laser in the treatment of KP.
Methods
Twenty-two patients with KP of bilateral arms were enrolled in this study. All participants were randomized and treated with a long-pulsed 755-nm alexandrite laser on the left or right arm in four sessions held 3 weeks apart. The unified moisturizing lotion was applied on both left and right arms once a day. Physicians’ assessment scores and patients’ self-assessment scores were recorded, and skin imaging changes in dermoscopy, high-frequency ultrasound, and skin biopsy were obtained at baseline and 4 weeks after the fourth treatment.
Results
Of the 21 patients who completed the study, 15 were women and 6 were men. At 4 weeks after the fourth treatment, the laser side showed significantly lower total (2.0 versus 4.5), roughness (1.0 versus 2.0), and redness (1.0 versus 2.0) scores according to physicians’ assessment (all P < 0.05). Furthermore, the laser side showed significantly lower total (2.0 versus 4.0), roughness (1.0 versus 2.0), and redness scores (1.0 versus 2.0) according to the patients’ self-assessment (all P < 0.05). The proportions of patients who achieved dermoscopically and ultrasonographically showed excellent improvements in follicular plugs (57.1% versus 14.3%), perifollicular erythema (52.4% versus 9.5%), perifollicular hyperpigmentation (47.6% versus 14.3%), and the number of epidermal bulges (57.1% versus 19.1%) in the laser side was significantly higher than those who achieved such improvements in the control side (all P < 0.05). Histopathology showed that the follicular plugs and inflammatory cell infiltration were improved at the final visit. Three patients exhibited reversible postinflammatory hyperpigmentation.
Conclusion
Long-pulsed 755-nm alexandrite laser treatment is effective and safe in treating both skin roughness and redness in KP.
Trial Registration Number: ChiCTR2100054489.
Keywords: Keratosis pilaris, 755-nm alexandrite laser, Efficacy, Safety
Key Summary Points
| Why carry out this study? |
| In KP, vellus hair is the center of abnormal keratinization and inflammatory response. The long-pulsed 755-nm alexandrite laser has been demonstrated to be effective in both vellus hair removal and the treatment of vascular diseases |
| This study aimed to evaluate the efficacy and safety of a long-pulsed 755-nm alexandrite laser for the treatment of KP |
| What was learned from the study? |
| Improvements in objectives and subjective outcome measurements were recorded in 21 patients after four treatment sessions conducted at 3-week intervals |
| Long-pulsed 755-nm alexandrite laser treatment was found to be effective and safe against skin roughness and redness from KP |
Introduction
Keratosis pilaris (KP) is a common autosomal dominant disorder characterized by the occurrence of follicular plugs with varying degrees of perifollicular erythema and hyperpigmentation. The lesions in KP usually improve during the summer and may benefit from treatment with emollients, exfoliants, intense pulsed light, and laser therapy [1]. Vascular devices, such as 532-nm, 585-nm, and 595-nm [2] lasers, have shown modest success in reducing the redness in KP [1, 3]. However, a highly effective or long-term treatment is not available. Vellus hair is the center of abnormal keratinization, and there is a secondary inflammatory response around it in the histopathology of KP. Recently, the use of an 810-nm diode laser as a hair removal technique has been proven to be beneficial in treating skin roughness and pigmentation in KP [4]. However, the method has little efficacy in the case of erythema. Long-pulsed 755-nm alexandrite laser is also an effective laser technique for vellus hair removal. Furthermore, sequential therapy with long-pulsed 755-nm alexandrite laser and a 595-nm laser is a safe and effective approach for relatively deep or thick hemangioma [5]. On the basis of the above findings, it is speculated that a 755-nm alexandrite laser may be effective in both vellus hair removal and erythema. In this study, the efficacy and safety of long-pulsed 755-nm alexandrite laser in KP were evaluated using physicians’ assessment and patients’ self-assessment scores, dermoscopic examination, a novel noninvasive ultrasonographic (high-frequency ultrasound, HFUS) examination, and skin biopsy to optimize the treatment for KP.
Methods
This was a prospective, rater-blinded, split-body, randomized, comparative clinical trial (ChiCTR2100054489), and the study protocol was approved by the Ethical Review Committee of Beijing Friendship Hospital (2021-P2-316-01). All participants provided their signed informed consent. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Patient Selection
The patients were recruited from the Dermatology Department of Beijing Friendship Hospital during the enrollment time from 1 to 31 December 2021. The study inclusion criteria were an age of 18–65 years, in good health, and a diagnosis of KP on both arms. The KP lesions on both arms were consistent in severity. Patients who received any systemic or topical medication, any laser treatment within 12 months before recruitment, with a concurrent diagnosis of another skin condition or malignancy, with a tan or sunburn on the arm within 1 month before recruitment, with open ulcers or infection on any skin site, and pregnant or breastfeeding women were excluded from the study.
Randomization
Each participant was assigned a number according to a random number table created by computer software. If the number was odd, the left upper arm was used as the laser side and the right upper arm as the control side. If the number was even, the right upper arm was used as the laser side and the left upper arm as the control side.
Study Procedures and Follow-up
The laser side arms received long-pulsed 755-nm alexandrite laser treatment (GentleMax Pro, Candela, America) for a total of four times, at an interval of 3 weeks. The control side arms were not treated. Topical emollients (Moisturising Lotion, CeraVe, America) were applied once a day to both arms. At the baseline and 4 weeks after the fourth treatment, the clinical photographs, dermoscopic images, and high-frequency ultrasonographic images of all patients’ lesions were recorded in the same clinic room, and histological examination (1.2-mm trephine) was performed on three patients. For clinical photographs, the camera system used was the Canon EOS M100 and the lens used was EF-M15-45 mm f/3.5–6.3 IS STM. The camera parameters settings were Automatic White Balance (AWB) and Automatic Exposure (AE) without a built-in flashlight, and the photographs were obtained at a consistent room light intensity. A dermoscopy system (FotoFinder bodystudio ATBM, FotoFinder Systems GmbH, Germany) was used to obtain dermoscopic images of the target zone under 20× magnification. Ultrasound Biomicroscopy (UBM) at 50 MHz (MD-310S, MEDA Co., Ltd. Tianjin, China) was used to obtain the ultrasonographic images. The examination was performed using a specially designed cup (26-mm diameter) that formed a water bath environment of 3-cm height, after which the ultrasonographic images were obtained on the UBM screen. The roughness and redness scores at the baseline and 4 weeks after the fourth treatment were evaluated by the patients themselves and two blinded physicians.
Laser Treatments
Hairs on the upper arms were removed by shaving with a razor before treatment, followed by the application of a long-pulsed 755-nm alexandrite laser on the laser side under the following parameters: initial energy parameters 14–16 J/cm2, spot size 15 mm, pulse duration 3 ms, 1 Hz. Each treatment session entailed two non-overlapping passes separated by a 1-min delay. The energy parameters of the subsequent treatments were set according to the response of the skin lesions to laser treatment, and the treatment endpoints were mild perifollicular edema and erythema. KP patients received four treatments at a 3-week interval.
Evaluation and Outcome Measures
Two blinded physicians and the patients themselves rated the redness and roughness on each arm using a scale of 0 (least severe) to 3 (most severe) for a total maximum score of 6 per patient per arm at the baseline and 4 weeks after the last treatment. To correlate the scores with clinical improvement of KP, we set the following criterion on the basis of the mean change of physicians’ assessment scores before and after the treatment: score 0, 0–25% improvement (mild); score 0.5–1, 26–50% improvement (moderate); score 1.5–2, 51–75% improvement (marked); score 2.5–3, ≥ 76% improvement (excellent). Dermoscopic (including the number of follicular plugs, the extent of perifollicular erythema, and hyperpigmentation) and ultrasonographic improvement (the proportion of epidermal bulges reduced) were evaluated as follows: 0–25% improvement (mild), 26–50% improvement (moderate), 51–75% improvement (marked), > 75% improvement (excellent).
The primary endpoint was the differences in the average physicians’ assessment scores (including the roughness, redness, and total scores) before and 4 weeks after the fourth treatment. The secondary endpoints were the patients’ self-assessment scores, skin images, and histological changes. The skin images included standardized digital clinical photographs, dermoscopic and high-frequency ultrasonographic images.
Power Analysis and Sample Size
According to the previous study, the assessment scores of the treatment and control sides were 3.0 and 4.0, respectively. Using the PASS11.0 software, 18 patients (18 lesions on each side) were required to obtain 80% power (power = 1 − β) at a level of α = 0.05 (bilateral) to detect differences between the two sides, and 22 patients needed to be enrolled eventually according to a 20% drop-out rate.
Statistical Analysis
All statistical analyses were performed using SPSS 26.0, and the paired test of the ranked data was performed using Wilcoxon signed-rank test. For comparing the categorical data, chi-square (χ2) test was performed. An exact test was performed when the expected frequency was less than 5, and P < 0.05 was considered significant statistically.
Results
Demographic Characteristics
A total of 22 patients with KP who had left-to-right symmetrical lesions were enrolled, 95.5% (21/22) of whom completed the study. The demographic characteristics of the patients are presented in Table 1. Of the 21 patients, 15 were women and 6 were men. It was observed that 16 (76.2%) patients developed KP in their second decade of life. Furthermore, 4 patients (19.1%) were of Fitzpatrick skin type II, 12 patients (61.9%) were of Fitzpatrick skin type III, and 4 patients (19.1%) were of Fitzpatrick skin type IV. Most patients (20/21, 95.2%) were asymptomatic. A positive family history of KP was noted in 15 patients (71.4%), and 5 patients (23.8%) had associated ichthyosis. For all the patients, the initial energy parameters were set to 14–16 J/cm2, and the energy parameters for subsequent treatments in most of the patients were set to 16–18 J/cm2. There were two special cases: one patient (skin type IV) whose initial energy parameter was set to 14 J/cm2 and then gradually decreased to 10 J/cm2 owing to post-treatment hyperpigmentation, and another patient (skin type II) whose initial energy parameter was set to 14 J/cm2 and then gradually increased to 20 J/cm2 after three sessions of the treatment.
Table 1.
Demographics of the enrolled study patients
| Characteristics | No./total no. (%) |
|---|---|
| Completed study | 21/22(95.5) |
| Dropped out | 1/22(4.5) |
| Sexa | |
| Male | 6/21(28.6) |
| Female | 15/21(71.4) |
| Age on seta (years) | |
| 0–9 | 2/21(9.5) |
| 10–19 | 16/21(76.2) |
| 20–29 | 2/21(9.5) |
| 30–39 | 1/21(4.8) |
| Fitzpatrick skin typea | |
| II | 4/21(19.1) |
| III | 13/21(61.9) |
| IV | 4/21(19.1) |
| Itcha | |
| Yes | 1/21(4.8) |
| No | 20/21(95.2) |
| Family historya | |
| Yes | 15/21(71.4) |
| No | 6/21(28.6) |
| Ichthyosisa | |
| Yes | 5/21(23.8) |
| No | 16/21(76.2) |
aIncludes patients who completed the study
Physicians’ Assessment Scores
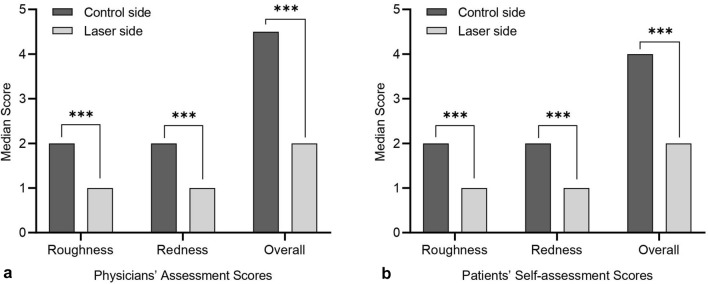
At baseline, the physicians’ assessment showed that the median total scores for the laser and control sides were both 5.0 (interquartile range, IQR, 4.0–5.0) and the median roughness and redness scores for both sides were the same without statistically significant difference. At the last follow-up visit, the roughness, redness, and overall scores on the laser side were significantly lower than those on the control side. The median roughness score was 1.0 (IQR, 1.0–1.5) on the laser side and 2.0 (IQR, 2.0–2.25) on the control side (P = 0.000086), and the median redness score was 1.0 (IQR, 1.0–1.0) on the laser side and 2.0 (IQR, 2.0–3.0) on the control side (P = 0.000074). The median total score on the laser side was 2.0 (IQR, 2.0–2.5), and on the control side it was 4.5 (IQR, 4.0–5.0), with a difference of 2.5 (P = 0.000081) (Fig. 1a).
Fig. 1.
The comparison of the outcome measures for the laser and control sides at the last follow-up visit. a Physicians’ assessment scores. The roughness, redness, and overall scores on the laser side decreased significantly compared with those on the control side at the last follow-up visit (P = 0.000086 for roughness scores, P = 0.000074 for redness scores, P = 0.000081 for overall scores, all P < 0.05). b Patients’ self-assessment scores. The roughness, redness, and overall scores on the laser side decreased significantly more than those on the control side at the last follow-up visit (P = 0.00007 for roughness scores, P = 0.000386 for redness scores, P = 0.000085 for overall scores, all P < 0.05). ***P < 0.001
Comparison between the two sides revealed significant differences in the improvement of both roughness and erythema (Fig. 2). With regard to roughness, the laser side showed the efficacy of > 25% improvement in 20/21 (95.2%) patients, but the control side demonstrated this improvement in only 2/21 (9.5%) patients. In regard to the redness, the laser side showed an efficacy of 51–75% improvement in 13/21 (61.9%) patients, whereas none achieved > 50% improvement on the control side. An overall improvement of 26–75% was observed in 19/21 (90.5%) patients on the laser side but only in 1/21 patients on the control side (Table 2).
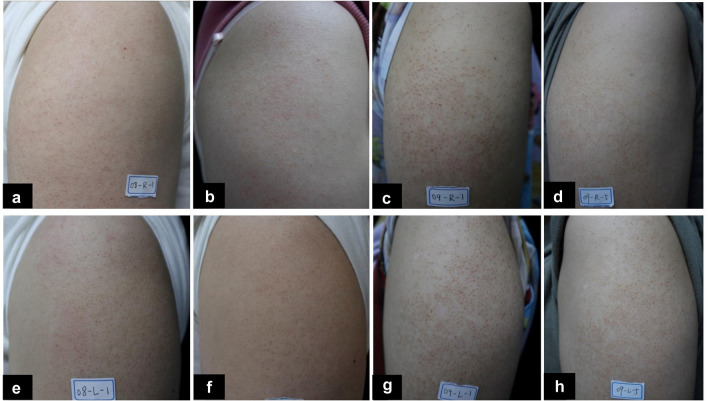
Fig. 2.
The comparison of clinical photographs at the baseline and at the last follow-up visit. a, b, e, f A mild-moderate KP patient’s photographs at the baseline (a, e) and at the last follow-up visit (b, f) for the laser side (a, b) and the control side (e, f). c, d, g, h A severe KP patient’s photographs at the baseline (c, g) and at the last follow-up visit (d, h) for the laser side (c, d) and for the control side (g, h)
Table 2.
Comparison of clinical improvement between laser and control sides
| Roughness | Redness | Total | ||||
|---|---|---|---|---|---|---|
| 755 | Control | 755 | Control | 755 | Control | |
| Grade | ||||||
| 1, < 25% improvement | 1 (4.8) | 19 (90.5) | 0 | 18 (85.7) | 2 (9.5) | 20 (95.2) |
| 2, 26–50% improvement | 13 (61.9) | 2 (9.5) | 8 (38.1) | 3 (14.3) | 16 (76.2) | 1 (4.8) |
| 3, 51–75% improvement | 7 (33.3) | 0 | 13 (61.9) | 0 | 3 (14.3) | 0 |
| 4, 76–100% improvement | 0 | 0 | 0 | 0 | 0 | 0 |
| χ2 | 33.909 | 33.273 | 33.285 | |||
| P value | < 0.001 | < 0.001 | < 0.001 | |||
Patients’ Self-assessment Scores
At baseline, the median roughness/redness/total scores of the patients themselves for the laser and control sides were respectively equal, with no significant difference. At the last follow-up visit, the scores of patients treated with 755-nm alexandrite laser were significantly lower compared with the control side. The self-reported median roughness scores for laser and control sides were 1.0 (IQR 1.0–1.0) and 2.0 (IQR 2.0–2.0), respectively, P = 0.00007. The median erythema score for the laser side was 1.0 (IQR 1.0–1.75) and for the control side was 2.0 (IQR 2.0–2.5), P = 0.000386. The median overall score on the laser side was 2.0 (IQR 2.0–3.0) and on the control side was 4.0 (IQR 4.0–4.0), with a difference of 2.0, P = 0.000085 (Fig. 1b).
Skin Imaging
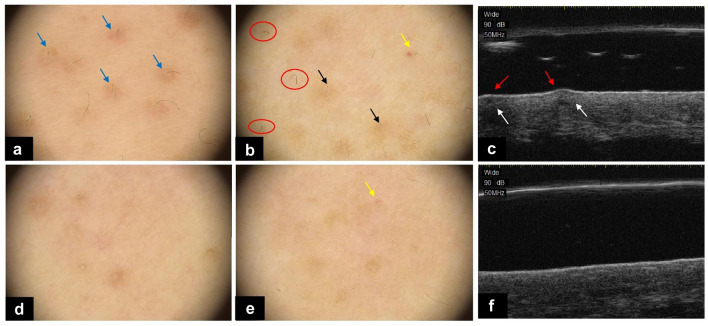
The proportions of patients who achieved dermoscopically > 50% improvement in the number of follicular plugs (57.1% versus 14.3%), the extent of perifollicular erythema (52.4% versus 9.5%), and hyperpigmentation (47.6% versus 14.3%) on the laser side were all significantly higher than those on the control side (all P < 0.05). As regards HFUS, the proportion of patients who achieved > 50% improvement in the number of epidermal bulges (57.1% versus 19.1%) on the laser side was significantly higher than that on the control side (P = 0.011 < 0.05) (Fig. 3).
Fig. 3.
Dermoscopic image (20×) of KP at the baseline (a, b) and at the final visit (d, e). Perifollicular erythema (blue arrows) and hyperpigmentation (black arrows), follicular plugs (blue arrows), and cherry hemangioma (yellow arrows) were all improved at the final visit. The number of vellus twisted hairs (red circles) reduced or disappeared at the final visit (d, e). Ultrasonographic images detected by a 50-MHz HFUS probe at baseline (c) and at the final visit (f). The epidermal bulges (red arrows) were flattened and echo heterogeneity (which means the signal is not uniform, indicated by white arrows) improved at the last follow-up visit
Histopathologic Examination
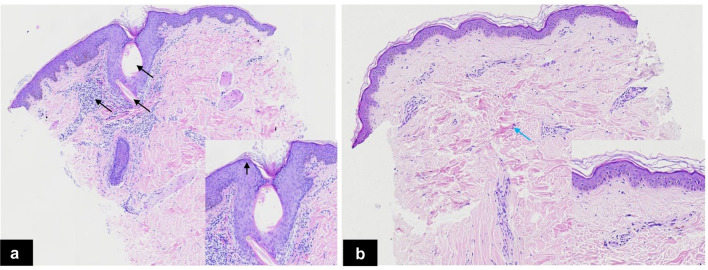
Histopathology revealed hyperkeratosis, slight acanthosis, hypergranulosis, marked follicular plug, and moderate lymphocytic infiltration before treatment. All pathological manifestations improved at the final visit, and the collagen fibers were locally thickened (Fig. 4).
Fig. 4.
Histopathologic changes. a Before treatment: dilated vellus hair follicle containing keratin and coiled vellus hair with moderate perifollicular mononuclear cell infiltration (black arrows). b The last follow-up visit: All pathological manifestations mentioned above had improved, and the collagen fibers were locally thickened (blue arrows)
Adverse Effects
No severe side effects were reported during the study. Three patients experienced postinflammatory hyperpigmentation (PIH). The patients were instructed to apply the same emollients containing the ceramide component to the affected arm, and there was almost 30% improvement in the hyperpigmentation 1 month after the final laser treatment.
Discussion
KP is a hereditary disease that typically presents during childhood [6]. Our data showed that the age of onset was within the first and second decades of life in 85.7% of the patients. Moreover, most of the patients participating in our study, especially women, felt embarrassed to wear T-shirts in summer owing to the ugly appearance of the exposed upper arm. Although KP spontaneously improves after adolescence [7], it is disfiguring and causes psychological burden to the affected patients.
Recent studies have shown that the most supported method for KP treatment is laser therapy, particularly the Q-switched Nd: YAG (1064-nm) laser and the 810-nm diode laser [1]. However, the above laser techniques have the limitations of high incidence of adverse effects [8, 9] and low efficacy in treating erythema. Therefore, this study aimed to explore a kind of laser that would be safer and more effective in treating patients with KP.
It is believed that KP is not a primary disorder of keratinocytes, but rather a hair shaft disease [10, 11]. 755-nm alexandrite laser penetrates deeply and may be more effective on twisted hair shaft, hyperpigmentation (melanin), and erythema (hemoglobin) [5, 12] than 695-nm and 810-nm diode lasers. Previous studies used 595-nm pulsed dye laser combined with long-pulsed 755-nm alexandrite laser and microdermabrasion technique to treat KP [13, 14], and the results showed that 75.9% (22/29) of the lesions had ≥ 25% improvement [13]. However, this study was not a randomized controlled trial, and this combined treatment method affected the patients’ lives because of the high frequency (1–2 weeks) of hospital visits. Our randomized self-controlled study investigated the efficacy of 755-nm alexandrite laser alone in treating KP. After four treatments spaced 3 weeks apart, an overall improvement of 26–75% was observed in 19/21 (90.48%) patients on the laser side, and the patients visited the hospital once every 3 weeks.
The 810-nm diode laser is one of the most supported lasers in treating KP, and it has been reported that patients treated using this method showed significant improvements in skin texture and roughness. However, the baseline erythema did not improve [4]. In our study, the laser side showed an efficacy of 51–75% improvement in redness in 13/21 (61.9%) patients. This finding signifies that the 755-nm alexandrite laser offers an advantage in redness improvement. This conclusion can also be drawn from the prominent improvements in perifollicular erythema and upper arm cherry hemangioma under dermoscopic examination.
Our study found that the 755-nm alexandrite laser treatment was relatively safe and did not cause serious or unexpected adverse effects. Although both fractional CO2 and Q-switched Nd: YAG (1064-nm) laser techniques have been proven to be safe and effective in the treatment of KP, some patients complained of a stinging sensation during treatment with Q-switched Nd: YAG (1064-nm) laser [8], and the skin lesions treated with fractional CO2 took a long time to repair [9]. Compared with the above two laser techniques, the 755-nm alexandrite laser is nonablative and is equipped with a cryogen spray cooling device, which greatly reduces the feeling of discomfort during treatment. In our study, 3 out of the 21 patients developed reversible hyperpigmentation, and the phototypes of these patients were 2 patients with type IV and 1 patient with type III. Therefore, we speculate that hyperpigmentation was caused by excessive laser energy in dark-skinned people. Hence, caution should be exercised during energy selection when using 755-nm alexandrite laser therapy in dark-skinned people.
Dermoscopy has been used to monitor the treatment outcome of KP in previous studies [15, 16]. Apart from dermoscopy that measures the horizontal and superficial structures, HFUS was used as a monitoring method for detecting vertical and deep structures during KP treatment in our study. HFUS is a noninvasive method that is mainly employed for the detection of neoplastic (such as melanoma and basal cell carcinoma) and inflammatory (such as sclerosing disorders and psoriasis) diseases [17]. Our study confirmed the feasibility of using HFUS for the evaluation of clinical efficacy in hair follicle and keratotic diseases. Our findings increase the application of HFUS in skin diseases and might help future studies on KP.
The dermoscopic and ultrasonographic findings agreed with the histologic manifestations. Marked follicular plugs and inflammatory cell infiltration were histologically in accord with the twisted vellus hairs and perifollicular erythema under dermoscopy and epidermal bulges and echo heterogeneity under HFUS before treatment. The reduced follicular plugs and perifollicular inflammation were histologically in line with the disappeared or reduced hair shafts and erythema under dermoscopy and the flattened epidermal bulges and homogeneous dermis under HFUS. Moreover, histopathological examination revealed thickened collagen and reduced inflammatory cell infiltration in the dermis, thereby establishing that a 755-nm alexandrite laser could penetrate the mid-dermis to induce regeneration and relieve the inflammation.
The limitations of our study are the small sample size and the lack of long-term follow-up. Therefore, further studies are needed for a better understanding of the effect of long-pulsed 755-nm alexandrite laser in the treatment of KP. The inconsistent color temperature of the photographs before and after the final treatments might have resulted from the setting of AWB in the camera parameters. Hence, a fixed white balance should be set in future studies.
Conclusions
Long-pulsed 755-nm alexandrite laser treatment is effective and safe in treating skin roughness and redness in KP. Noninvasive dermoscopic and ultrasonographic examinations appear to be the methods of choice to monitor the treatment outcome. Further research is needed to examine the long-term efficacy of long-pulsed 755-nm alexandrite laser in treating KP.
Acknowledgements
Funding
This research was supported by the Beijing Natural Science Foundation (No. 7222040) and Beijing Friendship Hospital, Capital Medical University (Contract grant number: Seed Project YYZZ202128). The authors funded the Rapid Service Fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Man Li, Yue Bai, Zhixuan Duan, Ruofei Yuan, Xiaoduo Liu, Yi Liu, Xuelei Liang, and Haixuan Wu. Fenglin Zhuo had full access to all the data in the study and takes responsibility for the integrity of data and the accuracy of the data analysis. The first draft of the manuscript was written by Man Li and Yue Bai. All authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Medical Writing, Editorial, and Other Assistance
The authors thank Mr. Tao Huang, Dr. Dongdong Liu, and Dr. Jianlan Xie for their assistance in managing the database.
Disclosures
Man Li, Yue Bai, Zhixuan Duan, Ruofei Yuan, Xiaoduo Liu, Yi Liu, Xuelei Liang, Haixuan Wu, and Fenglin Zhuo have nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by the ethics committee of the Beijing Friendship Hospital (2021-P2-316-01). Written informed consent was obtained for the publication and the use of all patients’ images before their enrollment in the study. This study was performed in accordance with the Helsinki Declaration of 1964 and its subsequent amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on a reasonable request.
Thanking Patient Participant(s)
We thank the patients who participated in the study.
Footnotes
Man Li and Yue Bai contributed equally to this study.
References
- 1.Maghfour J, Ly S, Haidari W, et al. Treatment of keratosis pilaris and its variants: a systematic review. J Dermatolog Treat. 2020;2020:1–12. doi: 10.1080/09546634.2020.1818678. [DOI] [PubMed] [Google Scholar]
- 2.Lanigan S. Reduction of pain in the treatment of vascular lesions with a pulsed dye laser and pneumatic skin flattening. Lasers Med Sci. 2009;24(4):617–620. doi: 10.1007/s10103-008-0632-5. [DOI] [PubMed] [Google Scholar]
- 3.Kechichian E, Jabbour S, el Hachem L, et al. Light and laser treatments for keratosis pilaris: a systematic review. Dermatol Surg. 2020;46(11):1397–1402. doi: 10.1097/DSS.0000000000002441. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim O, Khan M, Bolotin D, et al. Treatment of keratosis pilaris with 810-nm diode laser: a randomized clinical trial. JAMA Dermatol. 2015;151(2):187–191. doi: 10.1001/jamadermatol.2014.2211. [DOI] [PubMed] [Google Scholar]
- 5.Jin WW, Tong Y, Wu JM, et al. Observation on the effects of 595- nm pulsed dye laser and 755- nm long-pulsed alexandrite laser on sequential therapy of infantile hemangioma. J Cosmet Laser Ther. 2020;22(3):159–164. doi: 10.1080/14764172.2020.1783452. [DOI] [PubMed] [Google Scholar]
- 6.Wang JF, Orlow SJ. Keratosis pilaris and its subtypes: associations, new molecular and pharmacologic etiologies, and therapeutic options. Am J Clin Dermatol. 2018;19(5):733–757. doi: 10.1007/s40257-018-0368-3. [DOI] [PubMed] [Google Scholar]
- 7.Poskitt L, Wilkinson JD. Natural history of keratosis pilaris. Br J Dermatol. 1994;130(6):711–713. doi: 10.1111/j.1365-2133.1994.tb03406.x. [DOI] [PubMed] [Google Scholar]
- 8.Park J, Kim BJ, Kim MN, et al. A pilot study of Q-switched 1064-nm Nd:YAG laser treatment in the keratosis pilaris. Ann Dermatol. 2011;23(3):293–298. doi: 10.5021/ad.2011.23.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sobhi RM, Adawy NAH, Zaky IS. Comparative study between the efficacy of fractional CO2 laser, Q-switched Nd:YAG laser (1064 nm), and both types in treatment of keratosis pilaris. Lasers Med Sci. 2020;35(6):1367–1376. doi: 10.1007/s10103-020-02956-w. [DOI] [PubMed] [Google Scholar]
- 10.Schneider MR, Paus R. Deciphering the functions of the hair follicle infundibulum in skin physiology and disease. Cell Tissue Res. 2014;358(3):697–704. doi: 10.1007/s00441-014-1999-1. [DOI] [PubMed] [Google Scholar]
- 11.Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichol. 2012;4(4):255–258. doi: 10.4103/0974-7753.111215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uyar B, Saklamaz A. Effects of the 755-nm alexandrite laser on fine dark facial hair: review of 90 cases. J Dermatol. 2012;39(5):430–432. doi: 10.1111/j.1346-8138.2011.01480.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Choi MJ, Zheng Z, et al. Combination of 595-nm pulsed dye laser, long-pulsed 755-nm alexandrite laser, and microdermabrasion treatment for keratosis pilaris: retrospective analysis of 26 Korean patients. J Cosmet Laser Ther. 2013;15(3):150–154. doi: 10.3109/14764172.2013.769276. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Chung WS, Kim J, et al. Combination of 595-nm pulsed dye laser, long-pulsed 755-nm alexandrite laser and microdermabrasion treatment for keratosis pilaris. J Dermatol. 2012;39(5):479–480. doi: 10.1111/j.1346-8138.2011.01374.x. [DOI] [PubMed] [Google Scholar]
- 15.Ismail S, Omar SS. Clinical and dermoscopic evaluation of fractional carbon dioxide laser in management of keratosis pilaris in Egyptian type skin. J Cosmet Dermatol. 2020;19(5):1110–1120. doi: 10.1111/jocd.13140. [DOI] [PubMed] [Google Scholar]
- 16.Panchaprateep R, Tanus A, Tosti A. Clinical, dermoscopic, and histopathologic features of body hair disorders. J Am Acad Dermatol. 2015;72(5):890–900. doi: 10.1016/j.jaad.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Levy J, Barrett DL, Harris N, et al. High-frequency ultrasound in clinical dermatology: a review. Ultrasound J. 2021;13(1):24. doi: 10.1186/s13089-021-00222-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on a reasonable request.