Abstract
Introduction
Achievement of remission in psoriatic arthritis is a key goal for patients and clinicians, yet definitions of remission may vary. Previous efforts have utilized multidomain measures such as minimal disease activity that assess the status of joints, skin, and function to determine current level of psoriatic arthritis (PsA) disease activity. The goal of this study is to identify factors associated with patient-reported psoriatic arthritis remission.
Methods
The National Psoriasis Foundation conducted a cross-sectional study using an online survey of a random stratified sample of 1570 individuals with psoriatic disease in the USA. Participants were asked about a provider diagnosis of psoriasis and/or psoriatic arthritis, comorbid conditions, and psoriatic arthritis impact and disease activity, and demographic questions. All participants reporting a physician-given diagnosis of psoriatic arthritis were asked if they felt their psoriatic arthritis was in remission (“Do you feel your psoriatic arthritis is in remission?” Yes/No/Unsure) and, if so, length of remission. Individuals with psoriasis and psoriatic arthritis reporting a body surface area impacted by psoriasis 3% or less were asked if they felt their psoriasis was in remission. Psoriatic arthritis disease activity and impact was assessed using the nine-question Psoriatic Arthritis Impact of Disease (PsAID-9) instrument and a global PsA-related quality of life question. PsAID-9 scores ≤ 4 were used to indicate acceptable disease state. Multivariate logistic regression was used to identify factors associated with patient-perceived PsA remission.
Results
Of 834 participants with PsA, including 76 (4.8%) with PsA without skin involvement ever, 144 (17.3%) felt their psoriatic arthritis was in remission, with an average remission duration of 43 months. Of those in remission, 116 (78.4%) reported currently using a treatment for their PsA, with most (75.7%) reporting using a biologic therapy for their PsA in the past 12 months. Multivariate logistic regression revealed that patient-perceived psoriatic arthritis remission was independently associated with experiencing acceptable disease state (PsAID-9 ≤ 4), perception of psoriasis remission, lower impact of PsA on global quality of life, and non-white race. Age, sex, body mass index, or biologic use in the last 12 months were not associated with patient-reported PsA remission.
Conclusion
Overall, patient perception of PsA remission was most strongly associated with patient-reported psoriasis remission.
Keywords: Psoriatic arthritis, Psoriasis, Remission, Treat-to-target, Quality of life
Key Summary Points
| Defining remission for psoriatic arthritis is challenging. |
| Understanding how patients with psoriatic arthritis define remission can inform efforts to define remission for psoriatic arthritis. |
| This study sought to understand patient-defined remission for psoriatic arthritis and identify factors associated with patient-reported psoriatic arthritis remission. |
| Study results suggest that patient perceived psoriatic arthritis remission is experiencing acceptable disease state (PsAID-9 ≤ 4), perception of psoriasis remission, lower impact of PsA on global quality of life, and non-white race. |
| Our study highlights that psoriatic arthritis remission is multifactorial and patient-dependent. |
| Patient-defined psoriatic arthritis remission is linked with patient perception of skin disease and psoriatic arthritis impact on quality of life. |
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory joint disease associated with psoriasis [1]. The clinical presentation of PsA is heterogeneous and may involve peripheral joint arthritis, dactylitis, enthesitis, and skin and nail disease [2]. Unlike rheumatoid arthritis, patients with PsA commonly have asymmetric disease. The wide variety of impacted health domains and the multidimensional nature of PsA makes defining remission status complex [1]. Although some have proposed clinical guidelines to facilitate treatment goals and define treatment success, there is currently no single, universally accepted definition of PsA remission. One “treat-to-target” guideline suggests that the goal for PsA treatment is to achieve minimal disease activity (MDA), which is defined as low disease activity across several domains of joints, skin, and other patient-reported outcomes, including quality of life [3]. However, the difference between remission and low disease activity remains ill defined.
Given the complex nature of PsA, patients should also be involved in defining their treatment goals, as every patient may have different perspectives on the target for therapy and the means to achieve it. [3]
Evaluating the course of the patient’s disease activity can lead to objective measures to assess PsA remission. However, since patients often perceive remission in terms of the impact on their quality of life and physical limitations, objective measures have poor correlation with patient-reported remission [4]. Furthermore, it is difficult to expect the same level of remission across patients with different disease manifestations. For example, a complete absence of disease activity may be achievable in patients with early signs of PsA and limited joint damage but may not be realistic in patients with a more established disease with irreversible damage. There may also be a substantial pain component that does not track with improvement in inflammation and that activates central sensitization pathways [5]. Thus, allowing patients to define remission of their disease becomes more important.
To further explore remission from a patient-centered view, the National Psoriasis Foundation aimed to identify demographic and clinical factors associated with patient-reported remission of psoriatic arthritis.
Methods
The National Psoriasis Foundation conducted a cross-sectional study using an online and telephone-based survey of a random, stratified sample of individuals with psoriasis in October and November 2019. The survey was approved by the Genetic Alliance Institutional Review Board. Participants included individuals living in the USA with a physician-given diagnoses of psoriasis and/or psoriatic arthritis, 18 years of age or older, who contacted the foundation between 2017 and 2019. Participants reported physician-diagnosed psoriasis and/or psoriatic arthritis. All participants who reported having psoriatic arthritis were asked questions about their perspective on whether their psoriatic arthritis is in remission. Quality of life was assessed using a single-question global PsA-related quality of life measure and the nine-question Psoriasis Impact of Disease (PsAID-9), a validated patient-reported outcome measure that assesses impact of PsA on patients’ lives [6]. PsAID-9 score ≤ 4 is considered to represent patient-acceptable symptom state (PASS) and PsAID-9 score > 4 represents non-PASS. The global PsA-related quality of life question asked participants the following: “[i]n all the ways your psoriatic arthritis affects you, how would you rate the way your felt over the past week” on a scale of 0 to 10, with 0 being “excellent” and 10 being “poor.” The survey included questions about current and past treatments, physician-diagnosed comorbidities (Table 1), and participant demographics, including race, biologic sex, height, and weight. Participant-reported height and weight were used to calculate body mass index (BMI) using the standard formula: weight (lb)/height (in)2 × 703.
Table 1.
Comorbidities among participants with PsA
| Question: Has a doctor ever told you that you have any of the following conditions? | |||||
|---|---|---|---|---|---|
| Cardiovascular disease | 9.6% (71) | Dyslipidemia | 1.5% (11) | COPD | 5.1% (38) |
| Stroke | 2.0% (15) | Depression | 41.2% (305) | Uveitis | 3.6% (27) |
| Hypertension | 21.8% (161) | Anxiety | 42.6% (315) | Liver disease | 1.9% (14) |
| High blood pressure | 48.0% (355) | Sexual dysfunction | 7.7% (57) | Fatty liver disease | 13.1% (97) |
| Heart attack | 3.6% (27) | Crohn’s disease | 2.0% (15) | Rheumatoid arthritis | 9.3% (69) |
| Adult-onset diabetes (type 2 diabetes) | 17.3% (128) | Ulcerative colitis | 2.2% (16) | Gout | 7.4% (55) |
| Metabolic syndrome | 5.8% (43) | Cancer | 10.7% (79) | Osteoarthritis | 28.6% (212) |
| High triglyceride levels | 19.3% (143) | Cutaneous T-cell lymphoma | 0.3% (2) | Fibromyalgia | 17.3% (128) |
| High cholesterol | 39.6% (293) | Chronic kidney disease | 3.6% (27) | Thyroid disease | 19.1% (141) |
| Coronary artery disease | 4.6% (34) | Sleep apnea | 26.2% (194) | Other (specify) | 13.9% (116) |
| Atherosclerosis | 3.0% (22) | Hardening/narrowing of arteries | 3.1% (23) | None of the above | 8.8% (73) |
Participants with psoriasis were asked to self-report severity of their psoriasis at the time of data collection using the Patient Report of Extent of Psoriasis Involvement (PREPI) [7]. The PREPI is a validated patient-reported outcome measure to assess percentage of body surface area affected by psoriasis. Participants who reported a body surface area (BSA) of 3% or less were asked questions about their perspective on whether their psoriasis is in remission. All participants with PsA were asked if the question, “Do you feel your psoriatic arthritis is in remission?” To more closely reflect patient perspective on remission, participants were not given a definition of remission for psoriasis or PsA or any explanation of potential indicators for remission. Individuals who perceived their PsA to be in remission were asked if they were currently taking therapies to treat their PsA. Separately, participants were asked if they used any of the following therapy types in the last 12 months: biologics, phototherapy, topical medications, prescribed oral systemic medications, or over-the-counter products, as well as if they are not currently on any treatment or a treatment other than those listed.
Descriptive statistics were used to report number and percentage of respondents who feel like their psoriatic arthritis is in remission. For univariate analyses, chi-square tests of independence were conducted to explore associations with patient perception of psoriatic arthritis remission. Independent-samples t-test was used to assess if global psoriatic arthritis quality of life differed on the basis of perception of remission. Results from univariate analyses informed logistic regression models, which were used to assess factors related to patient perception of psoriatic arthritis remission. For the logistic regression models, the continuous variable for age was used, race was collapsed into a dichotomous “white” and “non-white” variable owing to the lack of racial diversity among participants, and a continuous variable for BSA was used for disease severity.
Results
A total of 834 individuals with psoriatic arthritis completed the survey (Table 2) and were asked if they felt their PsA was in remission. Of these, 144 (21.6%), felt that their PsA was in remission (Table 3). Of participants who felt their PsA was in remission, most are currently taking a treatment for the PsA (76.7%) (Table 4) and the average length of remission was 25.3 months (Table 3). Patient perception of PsA remission did not differ on the basis of presence of psoriasis (17.7%—without skin involvement ever; 22.4% PsA and psoriasis) (Table 3). Overall, most participants with PsA (59.5%) reported not experiencing PASS (Table 3).
Table 2.
Participant demographics
| Characteristic | n (%) |
|---|---|
| Disease | |
| Psoriatic arthritis | 76 (9.1%) |
| Psoriatic arthritis and psoriasis | 758 (90.9%) |
| Race | |
| White or Caucasian | 732 (89.3%) |
| Asian or Asian American | 19 (2.3%) |
| Black or African American | 20 (2.4%) |
| American Indian or Alaskan Native | 6 (0.7%) |
| Native Hawaiian or other Pacific Island | 2 (.2%) |
| Two or more races | 20 (2.4%) |
| Other | 13 (1.6%) |
| Unsure | 8 (1.0%) |
| Biologic sex | |
| Male | 310 (37.4%) |
| Female | 520 (62.6%) |
| Age | |
| 18–35 years | 59 (7.3%) |
| 36–50 years | 219 (27.1%) |
| 51–65 years | 377 (46.7%) |
| Older than 65 years | 152 (18.8%) |
| BMI categories | |
| Underweight | 10 (1.3%) |
| Normal weight | 174 (22.4%) |
| Overweight | 251 (32.3%) |
| Obese | 342 (44.0%) |
| Mean number of comorbidities | 3.70 (± 2.64) |
Table 3.
Descriptive statistics
| Response | n (%) |
|---|---|
| Perception of remission | |
| PsA not in remission | 521 (78.4%) |
| PsA in remission | 144 (21.6%) |
| Length of remission | |
| Number of months (mean, SD) | 25.6 (± 23.8) |
| PsA activity and impact | |
| Unacceptable (PsAID > 4) | 477 (59.5%) |
| Acceptable (PsAID ≤ 4) | 324 (40.5%) |
| Biologic use | |
| No | 250 (30.6%) |
| Yes | 567 (69.4%) |
Table 4.
Chi-square tests of independence
| Response | Psoriatic arthritis not in remission | Psoriatic arthritis in remission | p value |
|---|---|---|---|
| Disease diagnosed | |||
| Psoriatic arthritis without skin involvement | 51 (82.3%) | 11 (17.7%) | p = 0.431 |
| Psoriatic arthritis and psoriasis | 470 (90.2%) | 133 (22.4%) | |
| PsAID-9 score | |||
| PsAID-9 > 4 | 375 (95.9%) | 20 (5.1%) | p < 0.001 |
| PsAID-9 ≤ 4 | 128 (52.2%) | 117 (47.8%) | |
| Biologic use | |||
| Not biologic user | 159 (82.4%) | 34 (17.6%) | p = 0.108 |
| Biologic user | 349 (76.7%) | 106 (23.3%) | |
| Race* | |||
| Non-white | 45 (67.2%) | 22 (32.8%) | p < 0.05 |
| White | 465 (79.3%) | 121 (10.6%) | |
| Biologic sex | |||
| Female | 330 (80.5%) | 80 (19.5%) | p = 0.096 |
| Male | 189 (75.0%) | 63 (25.0%) | |
| Obese or overweight** | |||
| Normal or underweight | 102 (68.5%) | 47 (31.5%) | p = 0.001 |
| Obese or overweight | 383 (78.1%) | 89 (18.9%) | |
| Psoriasis in remission*** | |||
| No | 137 (90.1%) | 15 (9.9%) | p < 0.001 |
| Yes | 123 (59.4%) | 84 (40.6%) | |
| Currently using therapies to treat psoriatic arthritis | |||
| Yes | – | 102 (76.7%) | p = 0.378 |
| No | – | 31 (23.3%) | |
| Number of therapies** | |||
| Only one therapy | 161 (73.5%) | 58 (26.5%) | p < 0.05 |
| More than one therapy | 299 (81.9%) | 66 (18.1%) | |
| Number of comorbidities | |||
| None | 39 (7.6%) | 13 (9.4%) | p < 0.05 |
| 1 | 55 (10.7%) | 27 (19.6%) | |
| 2 | 73 (14.2%) | 28 (20.3%) | |
| 3 | 89 (17.3%) | 18 (13.0%) | |
| 4 | 79 (15.4%) | 20 (14.5%) | |
| 5 | 58 (11.3%) | 10 (7.3%) | |
| 6 or more | 121 (23.5% | 22 (15.9%) | |
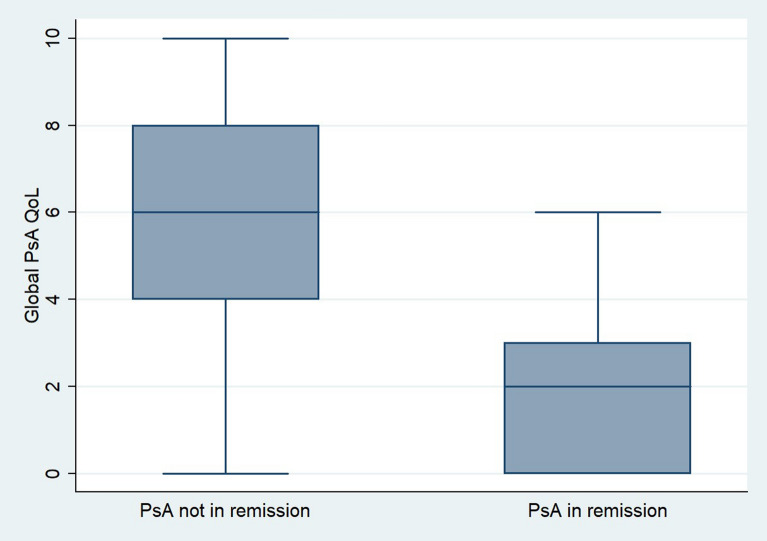
Univariate analysis results suggest that PASS, non-white race, BMI < 25.0, using one therapy to treat psoriatic disease, and perceiving psoriasis to be in remission were independently associated with patient-reported psoriatic arthritis remission (Table 4). Compared with individuals with non-PASS (5.1%), a higher percentage of participants with PASS (47.8%) felt their psoriatic arthritis was in remission (p < 0.001). Patient-reported psoriatic arthritis remission was higher among non-white participants (32.8%) compared with white participants (10.6%; p < 0.05) and among non-overweight versus obese participants (31.5% versus 18.9%, p = 0.001). A higher percentage of those who felt their psoriasis was in remission reported using only one therapy (26.5%) compared with those using more than one therapy (18.1%, p < 0.05) to treat their psoriatic disease. Patient-reported psoriatic arthritis remission was higher among individuals who felt their psoriasis was in remission (40.6%) compared with those who did not feel their psoriasis is in remission (9.9%). Individuals who do not perceive their PsA as being in remission experience higher impact of PsA on their quality of life than those who feel their PsA is in remission (M = 5.90, SD 2.53 versus M = 1.92, SD 1.65) (Fig. 1).
Fig. 1.
Boxplot—PsA Global QoL by Perception of Remission
Factors associated with patient-reported PsA remission were explored using logistic regression. Results from multivariate logistic regression, controlling for BMI, sex, age, and type and number of therapies used, suggest that a PsAID-9 score ≤ 4 [odds ratio (OR) 4.58, 95% confidence interval (CI) 2.65–13.43, p < 0.05], perceiving psoriasis to be in remission (OR 4.51, 95% CI 1.84–11.02, p < 0.05), global quality of life score (OR 0.55, 95% CI 0.43–0.69, p < 0.001), and white race (OR 0.28, 95% CI 0.28–1.00, p < 0.05) are associated with patient-reported PsA remission (Table 5). Individuals achieving PASS were 358% more likely to report their PsA is in remission (p < 0.05). As impact of PsA on quality of life increased, the likelihood of perceiving PsA as in remission decreased by 45%. Individuals who felt that their psoriasis was in remission were 351% more likely to report their PsA was in remission (p < 0.05). White race was associated with an 82% decrease in likelihood of reporting PsA was in remission compared with non-white race (p < 0.05).
Table 5.
Logistic regression models
| Psoriatic arthritis in remission | ||||
|---|---|---|---|---|
| Odds ratio | SE | 95% CI | p value | |
| Model 1 | ||||
| PsA acceptable disease activity | 17.13 | 4.50 | 10.24–28.68 | < 0.0001 |
| Model 2 | ||||
| PsA acceptable disease activity | 3.56 | 1.17 | 1.86–6.80 | < 0.0001 |
| PsA Global QoL | 0.55 | 0.40 | 0.48–0.64 | < 0.0001 |
| Model 3 | ||||
| PsA acceptable disease activity | 4.68 | 2.04 | 1.99–11.00 | < 0.0001 |
| PsA Global QoL | 0.58 | 0.05 | 0.48–0.70 | < 0.0001 |
| Psoriasis in remission | 7.07 | 2.78 | 3.27–15.27 | < 0.0001 |
| Model 4 | ||||
| PsA acceptable disease activity | 4.70 | 2.10 | 1.96–11.24 | 0.01 |
| PsA Global QoL | 0.57 | 0.05 | 0.47–0.69 | < 0.0001 |
| Psoriasis in remission | 6.80 | 2.68 | 3.14–14.74 | < 0.0001 |
| White | 0.28 | 0.17 | 0.08–0.92 | 0.05 |
| Model 5 | ||||
| PsA acceptable disease activity | 5.10 | 2.74 | 1.78–14.61 | 0.01 |
| PsA Global QoL | 0.54 | 0.06 | 0.43–0.69 | < 0.0001 |
| Psoriasis in remission | 4.84 | 2.18 | 2.00–11.69 | < 0.0001 |
| White | 0.28 | 0.18 | 0.08–1.00 | 0.051 |
| Multiple therapy use | 0.62 | 0.24 | 0.28–1.34 | 0.225 |
| Obese/overweight | 1.06 | 0.47 | 0.45–2.52 | 0.888 |
| Male | 0.78 | 0.33 | 0.34–1.78 | 0.561 |
| Biologic user | 0.97 | 0.50 | 0.35–2.69 | 0.953 |
| Model 6 | ||||
| PsA acceptable disease activity | 4.58 | 2.51 | 1.56–13.43 | 0.01 |
| PsA Global QoL | 0.55 | 0.06 | 0.43–0.69 | < 0.0001 |
| Psoriasis in remission | 4.51 | 2.05 | 1.84–11.02 | 0.01 |
| White | 0.28 | 0.18 | 0.08–1.00 | 0.05 |
| Multiple therapy use | 0.61 | 0.24 | 0.28–1.33 | 0.215 |
| Obese/overweight | 1.04 | 0.46 | 0.44–2.46 | 0.935 |
| Male | 0.82 | 0.36 | 0.35–1.93 | 0.665 |
| Biologic user | 0.91 | 0.48 | 0.32–2.57 | 0.853 |
| Age | 0.99 | 0.01 | 0.96–1.02 | 0.725 |
| Number of comorbidities | 1.05 | 0.09 | 0.88–1.25 | 0.567 |
Discussion
This study highlights several clinical and demographic factors associated with PsA remission from the patient perspective.
Patients with low psoriatic arthritis disease activity and impact, as determined by a PsAID-9 ≤ 4, were more likely to report remission. The PsAID-9 was developed by Gossec et al. to specifically assess quality of life and physical function in PsA patients [6]. The PsAID-9 uses a series of numeric rating scale (NRS) items that range from 0 to 10 and integrates up to 12 different domains of health. PsAID-12 provides questions that are most relevant to clinical management and patient care over a long period of time, whereas PsAID-9 uses questions that are more relevant for clinical trials [6]. Several studies have shown that the PsAID is a reliable method of measuring patient-reported outcomes that can differentiate PsA disease activities across patients [6, 8–10]. The results in our study strengthen the relationship between patient-reported outcome measures and remission status. It also highlights the importance of understanding the patient’s perspective on not only the physical burden, but also the psychological impact of PsA when defining remission.
Patients who perceived their psoriasis to be in remission were also more likely to report PsA remission. This highlights that, although PsA involves numerous health domains, skin involvement is an important factor associated with patient-perceived remission, or that reduced skin activity tracks closely with the use of especially efficacious therapies that also alleviate PsA symptoms. A recent study demonstrated that patients with PsA involving both joint and skin symptoms have worse patient-reported outcomes and more severe disease [11]. Moreover, a second study demonstrated that improvement in health-related quality of life in patients with psoriatic arthritis was dependent on successfully treating both skin and joint symptoms [12]. While studies have shown that skin symptoms largely impact the course of psoriatic arthritis, our study further highlights that skin involvement independently contributes to patients’ perception of PsA remission.
Lower global PsA quality of life score was also found to be significantly associated with remission status even when joint disease activity was controlled. This questionnaire was measured on a scale of 0–10, with 0 indicating the least impact on the overall quality of life due to psoriatic arthritis. Patients with PsA were found to have significantly reduced health-related quality of life compared with the general population even after 5 years from disease onset [13]. This suggests that long-term reductions in quality of life may be associated with PsA disease, which explains why improvement in quality of life is associated with patient-reported disease remission.
Non-white patients were also more likely to report remission than white patients. The role of race in psoriatic arthritis remission is unclear; however, studies have demonstrated the prevalence and disease characteristics of PsA may vary between races. A cross-sectional US claims database study found that PsA prevalence is highest among white patients compared with other racial and ethnic groups [14]. Another study found that PsA was also estimated to occur twice as frequently in white patients compared with Black patients [15]. The severity of psoriatic arthritis was found to be higher in individuals of Indian ethnicity compared with Chinese ethnicity, suggesting genetic or environmental factors may play a role [16]. Future studies are needed to evaluate the role of race in psoriatic arthritis severity, disease characteristics, and treatment to understand their impact on PsA remission. A notable limitation of our study is the predominance of white race subjects.
This study has some limitations. First, using an online survey is a potential weakness of the study. Online surveys require access to the internet and a certain level of computer skills to navigate. Additionally, age and race also affect response to web-based surveys. Individuals who contact the National Psoriasis Foundation differ from the general population with psoriasis in several ways that could influence study results. Members of the National Psoriasis Foundation tend to be older and more affluent, have more significant disease, and be more knowledgeable about treating the psoriatic disease compared with the general public.
Conclusions
PsA is a heterogeneous, inflammatory disease with multiple domains of importance. We evaluated the clinical and demographic factors associated with patient-perceived PsA remission through the use of a cross-sectional survey. Low disease activity and impact (low PSAID score) was associated with remission status. Furthermore, perceived psoriasis remission, quality of life, and non-white race were also significantly associated with patient-reported PsA remission. Ultimately, our study highlights that PsA remission status is multifactorial and patient-dependent.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
George Gondo and Stacie J. Bell collected the data. George Gondo, Megan Mosca, Julie Hong, Tina Bhutani, and Wilson Liao performed data analysis. George Gondo, Megan Mosca, Julie Hong, Emanual Maverakis, Joseph F. Merola, April W. Armstrong, Tina Bhutani, and Wilson Liao drafted, reviewed, and edited the manuscript.
Disclosures
April W. Armstrong serves as a research investigator and/or scientific advisor to AbbVie, Almirall, Arcutis, ASLAN, BI, BMS, EPI, Incyte, Leo, UCB, Janssen, Lilly, Nimbus, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, Pfizer, and Modmed. George Gondo is an employee of the National Psoriasis Foundation and Stacie J. Bell is a former employee of the National Psoriasis Foundation. Wilson Liao has received research grant funding from Abbvie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Joseph F. Merola serves as a consultant and/or investigator for Amgen, Bristol-Myers Squibb, Abbvie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Sanofi, Regeneron, Sun Pharma, Biogen, Pfizer and Leo Pharma Tina Bhutani is a principal investigator for trials sponsored by Abbvie, Castle, CorEvitas, Dermavant, Galderma, Mindera, and Pfizer. She has received research grant funding from Novartis and Regeneron. She has been an advisor for Abbvie, Arcutis, Boehringer-Ingelheim, Bristol Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, Sun, and UCB. Megan Mosca, Julie Hong, and Emanual Maverakis state no conflict of interest.
Compliance with Ethics Guidelines
This study was approved by the Genetic Alliance Institutional Review Board (Protocol # NPF-ASP-2019) and performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Study participants consented to the study electronically.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol Hoboken NJ. 2019;71(1):5–32. doi: 10.1002/art.40726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69(1):48–53. doi: 10.1136/ard.2008.102053. [DOI] [PubMed] [Google Scholar]
- 3.Coates LC, Moverley AR, McParland L, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet Lond Engl. 2015;386(10012):2489–2498. doi: 10.1016/S0140-6736(15)00347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson K, Ye JY, Chandran V, Gladman DD. A novel role for the psoriatic arthritis impact of disease (PsAID) questionnaire. Semin Arthritis Rheum. 2019;49(2):241–245. doi: 10.1016/j.semarthrit.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Adami G, Gerratana E, Atzeni F, et al. Is central sensitization an important determinant of functional disability in patients with chronic inflammatory arthritides? Ther Adv Musculoskelet Dis. 2021;13:1759720X21993252. doi: 10.1177/1759720X21993252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73(6):1012–1019. doi: 10.1136/annrheumdis-2014-205207. [DOI] [PubMed] [Google Scholar]
- 7.Dommasch ED, Shin DB, Troxel AB, Margolis DJ, Gelfand JM. Reliability, validity and responsiveness to change of the Patient Report of Extent of Psoriasis Involvement (PREPI) for measuring body surface area affected by psoriasis. Br J Dermatol. 2010;162(4):835–842. doi: 10.1111/j.1365-2133.2009.09589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holland R, Tillett W, Korendowych E, et al. Validation of the Psoriatic Arthritis Impact of Disease (PsAID) Questionnaire and its potential as a single-item outcome measure in clinical practice. Ann Rheum Dis. 2018;77(3):343–347. doi: 10.1136/annrheumdis-2017-211996. [DOI] [PubMed] [Google Scholar]
- 9.da Cruz Ribeiro E Souza E, da Silva Carneiro SC, Yazbek MA, et al. Validation and clinical interpretability of PsAID—psoriatic arthritis impact of disease. Adv Rheumatol Lond Engl. 2020;60(1):49. doi: 10.1186/s42358-020-00149-1. [DOI] [PubMed] [Google Scholar]
- 10.Di Carlo M, Becciolini A, Lato V, Crotti C, Favalli EG, Salaffi F. The 12-item psoriatic arthritis impact of disease questionnaire: construct validity, reliability, and interpretability in a clinical setting. J Rheumatol. 2017;44(3):279–285. doi: 10.3899/jrheum.160924. [DOI] [PubMed] [Google Scholar]
- 11.de Vlam K, Merola JF, Birt JA, et al. Skin involvement in psoriatic arthritis worsens overall disease activity, patient-reported outcomes, and increases healthcare resource utilization: an observational, cross-sectional study. Rheumatol Ther. 2018;5(2):423–436. doi: 10.1007/s40744-018-0120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kavanaugh A, Gottlieb A, Morita A, et al. The contribution of joint and skin improvements to the health-related quality of life of patients with psoriatic arthritis: a post hoc analysis of two randomised controlled studies. Ann Rheum Dis. 2019;78(9):1215–1219. doi: 10.1136/annrheumdis-2018-215003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geijer M, Alenius GM, André L, et al. Health-related quality of life in early psoriatic arthritis compared with early rheumatoid arthritis and a general population. Semin Arthritis Rheum. 2021;51(1):246–252. doi: 10.1016/j.semarthrit.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Ogdie A, Matthias W, Thielen RJ, Chin D, Saffore CD. Racial differences in prevalence and treatment for psoriatic arthritis and ankylosing spondylitis by insurance coverage in the USA. Rheumatol Ther. 2021;8(4):1725–1739. doi: 10.1007/s40744-021-00370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34(10):1753–1759. doi: 10.1007/s10067-014-2763-3. [DOI] [PubMed] [Google Scholar]
- 16.Leung YY, Fong W, Lui NL, Thumboo J. Effect of ethnicity on disease activity and physical function in psoriatic arthritis in a multiethnic Asian population. Clin Rheumatol. 2017;36(1):125–131. doi: 10.1007/s10067-016-3460-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.