Abstract
Enterobacter ludwigii is an oral growing bacteria responsible for teeth blackening. It can form biofilm. The exopolysaccharide (EPS) cluster associated with biofilm formation was isolated using ethanol precipitation and the formaldehyde–sodium hydroxide method. The chemical characterization of EPS was done using UV spectroscopy, Fourier transforms infrared spectroscopy, and gas chromatography–mass spectrometry. Energy-dispersive X-ray spectroscopy (EDS) analysis of EPS has revealed the presence of carbon > boron > nitrogen > phosphorous > calcium > sulfur > iron > potassium > magnesium. The carbon content was quite high (72.72–77.63%) in the EPS due to polysaccharide composition. The study showed the presence of different monosaccharides glucose (16.91%), galactose (4.25%), mannose (4.04%), and xylose (8.06%) as the major components of EPS. It appears such as thin filaments with three-dimensional structure, compact, irregular lumps and stacked flakes of polysaccharides. The EPS was also examined using different 1D, 2D Nuclear Magnetic Resonance (NMR) spectroscopy techniques (1H NMR, 13C NMR, 1H–1H COSY, 1H–13C HSQC, 1H–13C HMBC) with different deuterated solvents (Protic and aprotic solvents for exchangeable protons), which showed eight distinguished monomers (seven confirmed by HSQC spectrum and one from 1H spectrum). Semi-crystalline nature and thermal stability were confirmed by X-ray diffractogram and differential scanning calorimetry analysis, respectively. The EPS further shows antioxidant potential in a concentration-dependent manner. It can form a stable emulsion against different edible oil that makes it promising alternative for use in food, and pharmaceutical industries.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03279-z.
Keywords: Bacteria, Exopolysaccharide, Enterobacter, Emulsifier, Monosaccharide
Introduction
The mouth is the vital part of the gastrointestinal tract, which acts as a storehouse for various bacteria. Some bacteria have a positive effect, whereas others can harm human health. The bacteria with good impact are known as probiotics and can affect oral health and helps in the digestion of food in the gastrointestinal tract (Sahu et al. 2022). More than 700 bacterial strains grow within the oral cavity (Aas et al. 2005). Some members of Enterobacteriaceae family can survive in gastrointestinal conditions, although the survival rate differs from genus to genus (Balkan et al. 2014). Enterobacter colonies are mucous-like substances that help in aggregation and biofilm formation (Pompermayer and Gaylarde 2000). The type and time of biofilm formation vary from species to species and are associated with quorum sensing (Miller and Bassler 2001).
EPS is not an essential component for bacterial growth. However, it provides metabolic stability to bacterial cells (Decho and Gutierrez 2017). The EPS structure, such as the branching of polysaccharides, linkage type, and degree of polymerization, allows it to be used in the various pharmaceutical industry (Varki et al. 2015; Wang et al. 2015). The chemical nature of EPS can impart emulsifying, stabilizing, and gelling properties to many food-related products (Das 2021; Laws et al. 2001). Thus, it is used in pharmaceuticals, cosmetics, printing, and textiles (Arvidson et al. 2006). The physical properties of EPS, such as viscosity, can affect the functional properties of EPS, since the viscosity depends on the substituents, linkage, monomer type, and molecular mass (Laws et al. 2001).
EPS are gaining importance in the industry, since it is biocompatible and generally regarded as safe for human usage (Nwodo et al. 2012). EPS matrix has a 3-dimensional structure with carbohydrate, protein, lipid, humic substances, uronic substances, and a small amount of DNA (Decho 2000). The major monosaccharide of EPS includes glucose, mannose, fucose, galactose, and rhamnose (Bales et al. 2013; Rani et al. 2018). The EPS from aerobic, acidogenic, and methanogenic bacteria has been already reported (Liu and Fang 2002). Thus, various extraction processes are designed to obtain pure EPS. The physical method of EPS extraction includes centrifugation, ultra-sonication, cation exchange resin associated with sonication, and heating. The chemical method of EPS extraction includes the use of alkaline sodium hydroxide, ethylenediaminetetraacetic acid (EDTA), cation, or anion exchange resin. Among all the methods formaldehyde–NaOH method has the upper hand, since it enhances the yield and the solubility of EPS in water (Liu and Fang 2002).
Bacterial EPS are classified on the basis of linkage bond, and chemical structure. Based on the chemical nature, homoexopolysaccharides are neutral, but heteroexopolysaccharides are polyanionic (Czaczyk and Wojciechowska 2003). The linkage bonds are 1,3-α/β, 1,4-α/β, or 1,6-α/β. Homoexopolysaccharides are composed of a single type of sugar monosaccharide but heteroexopolysaccharides are composed of two or more different monosaccharides. Homo and heteropolysaccharide differs in linkage bonds. Determination of the monosaccharide composition of EPS requires the cleavage of linkage by acid hydrolysis. Glycosidic linkage is very common in bacterial EPS (More et al. 2021). Bacterial EPS have repeated structural units with 2–7 sugar residues and their sequences could be determined by NMR. Sometimes, the sequence of the repeated motif cannot be determined from NMR spectra due to poor resolution. In such cases, a more authentic structure can be derived from the analysis of the oligosaccharide sample. The oligosaccharide sample could be prepared from the polysaccharide by acid hydrolysis or enzymatic degradation. Chemical analysis of different linkage between monosaccharides is determined by GC–MS analysis of methylated derivative/alditol acetate derived from the methylation of polysaccharides followed by acid hydrolysis, acetylation, and reduction (Paramonov et al. 2015; Sletmoen et al. 2003).
Enterobacter species are human pathogens. They are motile bacteria mainly found in gut microbiota. Among Enterobacter species, E. ludwigii is an oral growing bacteria that may survive at intestinal pH. The EPS produced by E. ludwigii may help to emulsify the fatty meal. By keeping this fact in mind, this study emphasizes the gastrointestinal survivability of bacteria and emulsifying potential of EPS produced by the bacteria. This study has reported the EPS from E. ludwigii, which was isolated from the oral cavity (Ashe et al. 2017). Microbial production of EPS guarantees fast production due to high growth rates. EPS is produced by many members of the family Enterobacteriaceae (Freitas et al. 2011). EPS derived from Enterobacter species, i.e., Enterobacter strain A47 DSM 23,139 (Freitas et al. 2011), Enterobacter sp. YG4 (Nagaraj et al. 2016), Enterobacter cloacae strain P2B (Naik et al. 2012), Enterobacter cloacae Z0206 (Wang et al. 2013) has been reported earlier. EPS produced by E. cloacae is composed of glucose and galactose, which exhibit the emulsifying activity with n-hexadecane (Hua et al. 2010), mineral oil, and vegetable oil (Iyer et al. 2006). Enterobacter produces the EPS composed of galactose, glucose, and fucose (Alves et al. 2010). In this research work, EPS characterization was done for its physicochemical and biological properties. This research paved the way for formulating a new emulsifying agent.
Materials and methods
Material required
Pepsin was purchased from Sigma Aldrich. Hydrochloric acid, pancreatin, peptone, bile salt, sodium chloride, potassium chloride, sodium hydroxide, glucose, sodium bicarbonate, sulfuric acid, and phenol were obtained from Himedia Laboratories Pvt. Ltd. Mumbai, India. For bacterial culture, Luria Bertani broth and Luria Bertani agar were obtained from HiMedia Laboratories Pvt. Ltd. (India). Skimmed milk was purchased from the local market (Rourkela, India).
Bacterial culture conditions
The bacteria E. ludwigii (NCBI Genbank assigned accession number KT371488) used in the current study was reported by Ashe et al. (Ashe et al. 2017). The strain was grown using the LB broth. Freshly cultured bacteria were centrifuged, and cell pellets were used for a gastrointestinal tolerance study.
Gastrointestinal tolerance study
The gastrointestinal tolerance of the bacteria was checked as per Zarate et al. (Zárate et al. 2000). Briefly, the bacteria were inoculated in the culture media with 2% v/v of the selected bacterial strain and incubated at 37 °C for 24 h. Next, the culture was centrifuged at 20,000xg, 10 min, and 4 °C to get the bacterial cell pellet. The cell was suspended with the artificial gastric juice composed of sodium chloride, sodium bicarbonate, pepsin (3 g/L), and hydrochloric acid. The pH of the artificial gastric juice was adjusted with 2 N sodium hydroxide (NaOH) and HCl solution to get final pH of 2, 3, 4, and 7. The bacterial suspension was incubated at 37 °C at varying time intervals of 0, 30, 60, and 180 min with artificial gastric juice at a constant agitation of 120 rpm to provide the ambient condition for bacterial growth. Next, the survivability of the bacteria was checked using the colony counting method or by measuring optical density at 560 nm using the ELISA plate reader (Biobase-EL10A Elisa reader, Shandong). The same experiment was performed by adding skimmed milk to the artificial gastric juices, and the survivability of the bacteria was checked. After the gastric tolerance study, the bacterial suspension was suspended in artificial intestinal juice with pH 8 and composed of 0.15% W/V bile salt and 0.1% W/V pancreatin in water. The bacterial suspension was incubated in artificial intestinal fluid at varying time intervals of 0, 30, 60, and 180 min, and the survivability of the bacteria was checked.
Characterization of biofilm
Biofilm formation was checked based on its ability to adhere to 96 well plates. LB media was used in bacterial cell culture (200 µl per well). The overnight grown stationary phase cells were diluted (100 times) with fresh medium and grown again in 96 well plates. The inoculated sample was incubated for 24 h at 37 °C followed by rinsing the plate with deionized water and dried under laminar flow for half an hour. 200 µl of 0.4% crystal violet solution was added to the well and then again incubated for 30 min. The dye was removed from crystal violet stained biofilm and mixed with 300 µl of 70% ethanol. The mixture was transferred to a fresh plate, and absorbance was taken at 590 nm.
Microscopy analysis of biofilm-forming cells
500 µl of bacterial cell suspension (E. ludwigii) was transferred into 50 ml of LB broth in a beaker that contains the glass slide placed in a slanting position with the wall of the beaker. It was grown at 37 °C for 24 h/48 h/72 h. The slide was removed carefully with the help of sterile forceps, followed by washing two times with deionized water, and dried under a laminar hood at room temperature. The cells were stained with DAPI (5 µg/ml) for 15 min. It is reported that DAPI is specific for DNA (bind to AT region) in both viable as well as non-viable cells (Saby et al. 1997). The cell viability of the formed biofilm can be assessed by staining the cells directly without any fixation. In brief, biofilm-containing slides were washed with distilled water. After that DAPI solution was poured on the slide without any fixation process. It was covered with a coverslip and kept in the dark condition for half an hour. The slide was immediately observed under a fluorescence microscope.
Exopolysaccharide extraction
EPS isolation and purification from E. ludwigii were done using the ethanol precipitation method and the Formaldehyde sodium hydroxide method (D’Abzac et al. 2010; Feng et al. 2019; Liu and Fang 2002) with some modifications. Briefly, the bacterial culture was centrifuged (20000xg, 10 min, 4 °C), and the supernatant was mixed with a threefold volume of absolute alcohol and stored at 4 °C overnight. Next, the precipitate was separated by centrifugation at 4000xg for 15 min. To increase the yield of EPS, formaldehyde–sodium hydroxide method was employed. Briefly, it is treated with formaldehyde (36.5%) at 40C for 1 hour. Next, it is treated with 1 N NaOH at 4 °C for 3 h which is followed by centrifugation at 20000xg at 4 °C for 20 min. Its purification step involves the evaporation of excess solvent by air drying. The collected raw EPS was taken to prepare the 0.1% W/V aqueous solution and adjusted to pH 3 with 10% trichloroacetic acid which is followed by stirring at 4 °C for 2 h. The sample is again centrifuged at 30,000xg for 30 min, at 4 °C, and the supernatant was precipitated with 4 volumes of absolute ethanol from the aqueous phase and resolved again in distilled water. It was repeated four times to purify the EPS. Finally, it was dialyzed with dialysis membrane-60 (Himedia) against deionized water at 4 °C for 48 h followed by filtering and lyophilization at – 50 °C for 48 h. The collected sample was characterized by biochemical estimation for carbohydrates, protein, and lipid (SI). The physical form of EPS was analyzed by XRD and DSC analysis (SI).
UV–Vis, FTIR spectroscopic analysis of EPS
The UV–Vis spectra of EPS were recorded between 200 and 600 nm using a spectrophotometer (Shimadzu Corporation, Japan/UV-2450). EPS extracted by the different methods were characterized using FTIR spectroscopic technique to identify the functional groups. FTIR spectra of the EPS sample were recorded on ATR–FTIR, Bruker-α (alpha), and platinum-ATR. The measurements were recorded between 4000 and 400 cm−1.
Monosaccharide composition analysis
EPS derivatization was done by following the protocol published earlier (Wang et al. 2010). 20 mg of EPS were hydrolyzed with trifluoroacetic acid 2 Molar (2 ml) at 120 °C for 2 h. Hydrolysis is followed by the removal of TFA by the decompression evaporation method. Derivatized product is then subjected to GC–MS analysis (Agilent GC: 7980B Agilent MS: 5977A with auto-sampler) for determination of monosaccharide.
The operating condition is as follows (Fooladi et al. 2019): Carrier gas used was helium and the column had a flow rate of 1 ml/min. The initial temperature was 100 °C for 5 min in the beginning, further which is enhanced at the rate of 5 °C/min to 150 °C, which is held at 150 °C for 5 min, again increased at the rate of 5 °C/min to 240 °C and held at 240 °C for 2 min. The temperature of the injector and detector were kept at 250 °C. Monosaccharide was identified by making the comparison with the standard glucose, fructose, mannose, galactose, rhamnose, xylose, and arabitol.
Nuclear magnetic resonance (NMR) spectra of EPS
The 1H NMR and 13C NMR spectra were recorded in either DMSO-d6 or D2O (1H 400.15 MHz; 13C 100.62 MHz) using Bruker Avance 400. The chemical shifts are reported in ppm relative to residual solvent in DMSO-d6 (δ 2.50 ppm for DMSO), D2O (δ 4.79 ppm for H2O) for 1H NMR. For the 13C NMR spectra, the DMSO-d6 (δ 39.52 ppm) was used as the internal standard. 1H NMR spectra have been reported as peaks with chemical shifts (δ in ppm), multiplicities, coupling constants (Hz), and their relative integration values. Data for 13C NMR spectra have been reported as peaks with chemical shifts (δ in ppm).
Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) analysis of EPS
Electron microscopic structure and surface properties of EPS were analyzed by SEM, and element detection was done by EDS analysis. 5 mg of extracted EPS were mounted on carbon tape with gold sputtering, and then the image was obtained using a scanning electron microscope (JSM-M6480-LV) at an accelerating voltage of 15 kV.
Emulsifying properties of EPS
The emulsifying ability of EPS was detected using the cooper and Goldenberg method (Cooper and Goldenberg 1987; Freitas et al. 2009a, 2009b) and Pearce and Kinsella method (Pearce and Kinsella 1978) with some modification. The emulsion was prepared with different oil, such as sunflower oil, hydrocarbon (hexane), castor oil, coconut oil, sesame oil, and mustard oil. Oil in water emulsion (2:3) was prepared with different oil, water, and EPS as an emulsifying agent. The method to prepare emulsion was adopted from Thaiphanit et al. (Thaiphanit and Anprung 2014). The prepared emulsion was characterized by an analysis of particle size and zeta potential. Emulsifying activity index (EAI) and emulsion stability index (ESI) of the prepared emulsion were calculated using the turbidimetric method developed by Pearce and Kinsella (Pearce and Kinsella 1978). The thoroughly homogenized emulsion was taken at 0, and 10 min and 50-fold dilutions were done with 0.1% w/v SDS. The absorbance of the finally diluted emulsion was measured at 500 nm with a UV spectrophotometer. EAI and ESI were calculated using the equation (Thaiphanit and Anprung 2016) given below:
A0 represents the absorbance of emulsion at 0 min. ϕ is the oil volume fraction in a prepared emulsion. C is the concentration of EPS in gram per ml before the emulsion preparation. is the change in absorbance between 0 and 10 min. t is the time interval (10 min).
Results and Discussion
Simulated gastrointestinal (GI) tolerance study of E. ludwigii
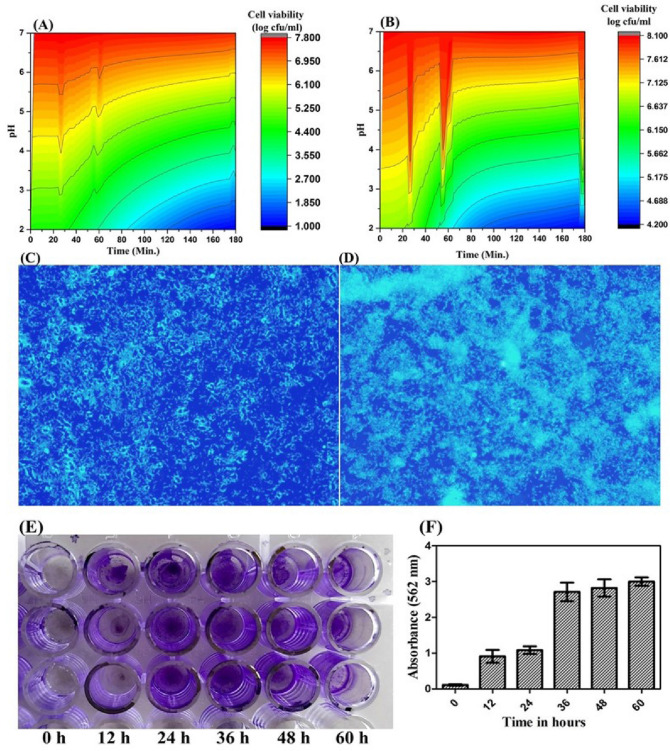
Biological properties of E. ludwigii were studied which reveals information regarding the gastrointestinal tolerance of bacteria in a simulated gastric environment. The simulation model of the gastrointestinal tract in a bioreactor system is an excellent alternative to check the tolerance of bacteria within the gastrointestinal environment. In this study, the gastric/intestinal condition was created in a conical flask using different salt and acid. The survival rate of E. ludwigii was represented through a contour plot (Fig. 1A, B). Survivability in gastric conditions provides easy accessibility to the intestine. The bacteria which survive in gastric condition has the probability to get access to the intestine. In intestine, these bacteria can help in emulsification of fatty meal due to their high biofilm-forming capacity. It is known that the biofilm composed of EPS. The emulsification capacity of bacterial EPS is discussed in later part of the manuscript. Survivability of E. Ludwigii suspended in sodium chloride (NaCl) solution in conjunction with the chemically simulated gastric environment is quite less in comparison to E. ludwigii suspended in milk (taken as food matrix). Milk provides a favorable condition for the growth of E. Ludwigii; therefore, the survival rate is high in the presence of milk. Bacterial survivability also varies with varying time and pH of the chemically simulated environment. At low pH (2–3) we found that the survivability of E. ludwigii is less (1.8 log cfu/ml) but in presence of milk, it increases up to 4.6 log cfu/ml at the same pH. At pH 3 to 5 the survival rate increases. At pH 7 the survivability of E. ludwigii reached to maximum (7.8 log cfu/ml) without the food matrix but in presence of milk, it increased to 8.1 log cfu/ml. Bacterial growth kinetics shows alteration in presence of milk. The acidic condition reduces the survival of E. ludwigii in a time-dependent manner, but a further clinical investigation is needed to confirm the acid tolerance. Survivability in chemically simulated intestinal juice (which contains pancreatin and bile salt) is relatively less at pH 8 to 9, only a few viable cells were observed at the end of the incubation period.
Fig. 1.
Biological characteristics; Gastric resistance of E. ludwigii A without skimmed milk B with skimmed milk as a food matrix, under chemically simulated gastric condition. The X-axis represented the time (In min) and Y-axis represented the pH value, the bacterial survivability (log cfu/ml) is shown in color as the scale shown on the right-hand side of the graph. Microscopic visualization of E. ludwigii cells by staining with DAPI and observed under fluorescence microscopy. C Biofilm formation after 2-day incubation of E. ludwigii cells. D biofilm formation after 3 day incubation of E. ludwigii cells. E Crystal violet staining of biofilm. F Quantification of biofilm by colorimetric assay
Similarly, the growth of E. ludwigii is also inhibited in presence of simulated pancreatic juices. Bile salt is known to alter the morphology of E. ludwigii by rupturing the bacterial cell membrane and causing cell death resulting in fewer cells in intestinal simulated conditions. Bacterial survivability under simulated gastric juice doesn’t confirm the survivability in a human body or physiological condition, because some other factor in the human body may alter its survivability.
Biofilm formation by E. ludwigii
E. ludwigii is a gram-negative species and human pathogen which possesses the general features of the Enterobacter genus. E. ludwigii secrete highly viscous EPS which has a crucial role in teeth blackening (Ashe et al. 2017; Konar et al. 2019). It has a biofilm-forming capacity, DAPI staining confirms the presence of bacteria in the E. ludwigii biofilm (Fig. 1C, D). It can easily adhere to the abiotic surface at temperatures of 28–37 °C. It can form biofilm in LB broth but the planktonic cells are less in number as compared to adherent cells. The biofilm formed on an abiotic surface, such as glass slides or 96 well plates, around the air–liquid interfaces. A biofilm contains the bacterial population that is enclosed in an adherent matrix that is composed of extracellular polymeric substances. The viability of cells in the biofilm was assessed by DAPI staining. DAPI stained both live as well as fixed cells (Saby et al. 1997). Biofilm formed by the E. ludwigii was stained with DAPI and observed under a fluorescence microscope (Fig. 1C, D). DAPI stained the bacterial cells by penetrating the cell membrane and it allows the microscopic analysis of biofilm-forming bacteria. Biofilm formation was further confirmed by crystal violet staining (Fig. 1E, F). It suggests that the biofilm formation was started after 12 h of incubation of cells. After 48 h of incubation a thick mass of biofilm was observed.
UV–Vis and FTIR Spectroscopic analysis of EPS
EPS are a long chain of monosaccharides generally linked by a glycosidic bond. Monosaccharide has the hydroxyl group that interacts with other groups in a different configuration to form a polysaccharide. The orbital transition may be used for the analysis of polysaccharides with other supporting data, such as FTIR, and NMR (Morris et al. 1975).
The UV spectrum of the EPS sample share similarity with the garlic straw polysaccharide (Kallel et al. 2015) which showed a strong absorption peak at 200–240 nm. There was a small absorption peak at 260–280 nm which indicates that the extracted EPS contains a very small amount of protein. UV spectroscopic analysis of EPS has shown the wavelength maxima at region 200–240 nm (Fig. 2A) due to n–σ* and/or π–π* transition (Yun and Park 2003). FTIR analyses were performed to characterize the presence of different functional groups and covalent bonding in EPS (Fig. 2B). Branching patterns and chemical composition affect the structure of EPS. The peak observed between 3500 to 3100 cm−1 is mainly attributed to the stretching vibration of O–H. The band observed at 3448 cm−1 is characteristic of the stretching vibration of a hydroxyl group (Bremer and Geesey 1991) which is responsible for the water solubility of EPS. Low band intensity between 3600 and 3200 cm−1 can contribute to the hydroxyl stretching vibration of polysaccharides (Bremer and Geesey 1991). The peak observed from 3000 to 2900 cm−1 is responsible for C–H stretching and bending (Shi et al. 2016). The intense peak at 1660 cm−1 contributes to the C = O stretch of amide I. The neighbor peak at 1410 cm−1 is assigned to COO− symmetric stretching (Hebbar et al. 1992; Wolpert and Hellwig 2006). The peak observed between 1200 and 800 cm−1 is attributed to the C–O–C stretch, C–O stretch, and vibration in polysaccharides (Naumann et al. 1991; Synytsya et al. 2003) which is also a characteristics feature of EPS (Čopíková et al. 2006). The absorption band at 1080 cm−1 represents the sugar structure has a galactopyranose configuration (Shao et al. 2014).
Fig. 2.
Chemical Properties; Chemical characterization of EPS A UV spectrum B FTIR spectra of EPS. C and D is the GC–MS analysis of derivatized standard monosaccharide and EPS, respectively
GC–MS analysis of monosaccharide composition in EPS
The chemical analysis of EPS involves the identification of monosaccharide/sugar residue repeating unit formed by chain group constituents i.e. acyl and phosphate group. Due to diversity in monosaccharide composition, a wide range of molecular structures are possible. Moreover, the variation in glycosyl linkage is also possible. The possible combination of monomer along with glycosidic linkage leads to a complex chemical structure which may be linear homopolysaccharide or branched heteropolysaccharide. The linkage type is analyzed by methylation of the free hydroxyl group followed by hydrolysis to monosaccharides which are analyzed by GC–MS. The monosaccharide composition of EPS is species/strain-dependent. The growth media and environmental conditions (pH, humidity, and temperature) also influence monosaccharide composition of EPS (Ayyash et al. 2020; Zhou et al. 2019). Detailed information on the linkage and monosaccharide composition of the hydrolyzed product is presented in Table 1 and Fig. S1.4
Table 1.
Different sugar derivatives were identified in GC–MS spectra of EPS after its derivatization
| Sugar derivative identified | Retention time | Mass charge ratio (m/z) |
|---|---|---|
| Methyl-D-lyxofuranoside | 9.744 | 45, 57, 73, 86, 103, 115, 133, 164 |
| Methyl-β-D-ribofuranoside | 9.744 | 45, 57, 73, 87, 103, 115, 133, 146, 164 |
| 3-Methylmannoside | 9.770 | 45, 73, 91, 116, 133, 163, 194 |
| 1,5-Anhydro-D-altriol | 9.796 | 43, 73, 103, 133, 164 |
| Methyl 6-O-[1-methylpropyl]-β-D-galactopyranoside | 9.848 | 45, 57, 73, 87, 99, 117, 129, 143, 159, 171, 189, 203, 219, 250 |
| Methyl-D-glycero-β-D-gluco-heptoside | 9.848 | 43, 60, 73, 87, 103, 115, 128, 143, 157, 174, 193, 206, 224 |
| D-Glucoheptose | 10.045 | 18, 43, 73, 103, 131, 163, 193 |
| D-Tagatose | 10.045 | 15, 31, 57, 73, 103, 131, 149 |
| β-D-Glucosyloxyazoxymethane | 10.060 | 45, 60, 73, 86, 103, 119, 144, 163, 185 |
| α-D-Xylofuranoside, methyl | 16.230 | 45, 73, 104, 133 |
| β-D-Mannofuranoside, 1-O-(10-Undecenyl) | 16.250 | 27, 41, 55, 73, 87, 101, 116, 131, 145, 163, 181, 207, 225 |
GC–MS analysis was done to study the monosaccharide composition of EPS derived from E. ludwigii. EPS extract was subjected to derivatization, which is followed by GC–MS analysis. Identification of the monosaccharide was done by comparing the mass/charge ratio of different sugar derivatives with the mass/charge ratio of sugar found in EPS. GC–MS analysis suggests the presence of Methyl-D-lyxofuranoside, Methyl-β-D-ribofuranoside, and different other sugar derivatives listed in Table 1. Analysis of the GC–MS spectra of derivatized EPS has shown the similarity with the GC–MS spectra of the standard monosaccharide (Fig. 2C, D). This study reported the presence of glucose (16.91%), galactose (4.25%), mannose (4.04%), and xylose (8.06%) as major components in the EPS derived from E. ludwigii. Our results suggest that the purified EPS is a heteropolysaccharide (Table 1). It is already known that arabinose, xylose, mannose, galactose, and glucose are the main constituent of EPS, identified in the Enterobacter genus (Enterobacter cloacae) (Naik et al. 2012; Wang et al. 2013).
NMR analysis of EPS
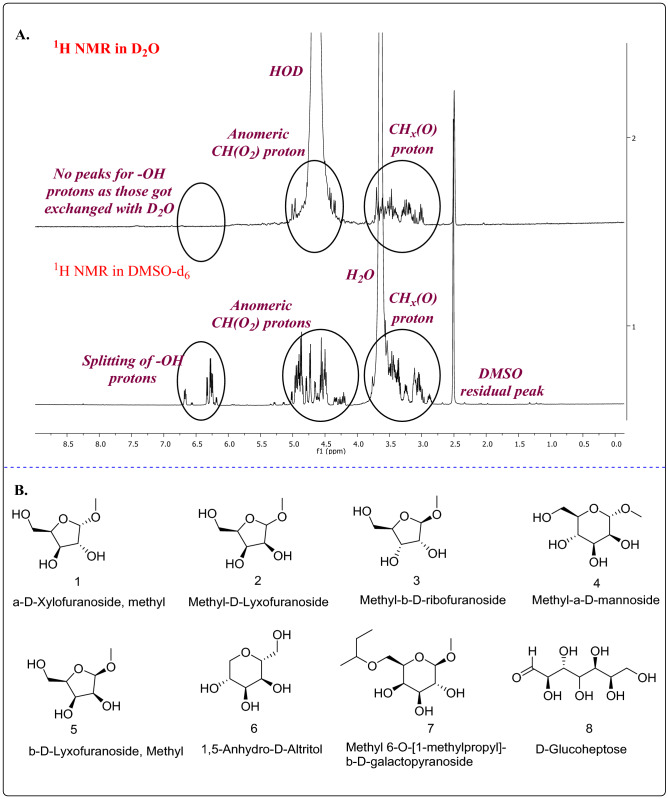
The structural repeating units (monosaccharide constituents) and the presence of functional groups were elucidated using NMR spectroscopic technique. Initially, the assignment of the proton signals in D2O was difficult as the anomeric region δ 4.50–5.50 ppm was overlapped with the huge HOD peak at δ 4.79 ppm (Which is obtained by the exchange of the hydroxyl groups of the monosaccharide units with D2O). According to the literature (Kielak et al. 2017), by increasing the temperature, the resonance of the HOD signal can be moved further toward the upfield region (δ 4.50 ppm at 70 °C). However, by changing the solvent to DMSO-d6, the moisture peak shifted to δ 3.30 ppm and the proton signals of the anomeric region (4.00 to 5.50 ppm) were relatively clear (Fig. 3A). An earlier report (Wang et al. 2013) also suggests that 1H NMR of the hydrolyzed EPS derivative showed anomeric protons (δ 4.50–5.50 ppm). The peaks in the range δ 6.15–6.70 ppm could be assigned to hydroxyl protons (-OH groups) as these signals were absent in the 1H NMR in D2O due to H/D exchange. Possible/expected monosaccharide units in the EPS are shown in figure (Fig. 3B).
Fig. 3.
Nuclear magnetic resonance analysis. A Comparison of 1H NMR in D2O and DMSO-d6, which showed the coupling of –OH protons. B Probable monosaccharide units of EPS
Analysis of 1H NMR spectroscopy (Fig. 4A)
Fig. 4.
Nuclear magnetic resonance analysis. A 1H NMR spectrum of hydrolyzed EPS derivative in DMSO-d6 (with zoomed regions for the respective peaks). B 13C NMR of the hydrolyzed EPS derivative in DMSO-d6
The 1H NMR spectroscopy taken in DMSO-d6, was analyzed and the results obtained are as follows; (i) The spectral region δ 0.00 to 2.00 ppm does not have any signal related to the saturated hydrocarbons (Fig. 4A), which indicates the absence of pyruvic acid (9) and fucose (10) monosaccharides units.
In addition, the spectrum showed proton signals belonging to –CH/-CH2/-CH3 groups connected to ‘O’ atom via a single bond (HxC-O–H/R, x = 1,2,3) between δ 2.75 and 3.75 ppm which overlap with the moisture peak in DMSO-d6, but are distinctly visible in the spectrum in D2O. As presumed, a large number of peaks were observed in the anomeric region (δ 4.50–5.50 ppm). It was difficult to assign the anomeric protons by analyzing the 1H spectrum alone. The number of anomers could be confirmed by looking into the 2D-spectroscopy method HSQC (Fig. 5A). The spectral region δ 6.20–6.80 ppm attributes to the presence of the hydroxyl protons, present in the EPS. Furthermore, a singlet at δ 8.25 ppm points to an aldehyde group (–HC = O) which might be correlated to an acyclic carbohydrate moiety. The signals present at 4.21 ppm and 4.27 ppm bearing coupling constants (J1,2) in the range 7.6–7.8 Hz were corresponding to β-anomeric protons, whereas the signal at 4.56 ppm bearing coupling constants (J1,2) in the range 4.0 Hz consists of the α-anomeric protons. Other anomeric peaks were overlapped, so coupling constants couldn’t be found.
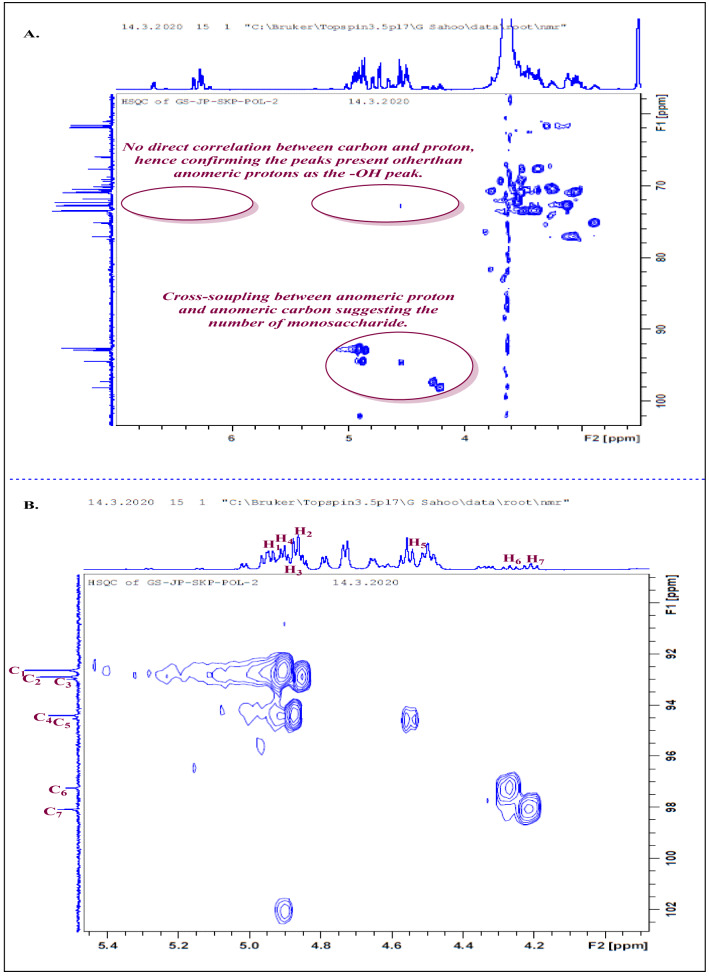
Fig. 5.
Nuclear magnetic resonance analysis. A 1H–13C HSQC correlation and the identification of anomers of the EPS derivative. B 1H–13C HSQC correlation anomeric protons with their corresponding carbons
Analysis of 13C NMR spectroscopy (Fig. 4B)
A total number of seven peaks appeared in the anomeric region (δ 90–110 ppm) of the 13C spectrum. This suggests that at least seven anomeric carbons (if not merged peaks) were present in the EPS. The carbon corresponding to the anomers appeared at δ 92.6, 92.89, 92.98, 94.4, 94.5, 97.2, and 98.0 ppm. The spectrum also showed the other carbons as follows; (i) The peaks present in the region δ 60–78 ppm attributed to the carbons connected to the –O atom except for the anomeric carbons. In literature, the absence of peaks in the region δ 79–84 ppm could be attributed to the absence of glycosidic linkages (Gottlieb et al. 1997). (ii) Peaks for “C–O–H/R” (the carbon attached to hydroxyl or alkoxy group) of furanose and mannose were present at δ 61.0, δ 61.6, and δ 61.9 ppm (Gottlieb et al. 1997). (iii) The absence of the peaks in the region (δ 10–30 ppm) ascertained the absence of saturated hydrocarbon moieties in the EPS.
2D spectral analysis: analysis of 1H–13C HSQC spectrum (Fig. 5B)
To distinguish/exclude the hydroxyl protons (OH) from proton connected to carbon atom which is further linked to hydroxyl/alkoxy group(s) (-CH–O and O–CH–O) in the region 2.75–5.50 ppm, 1H–13C HSQC spectrum was taken as hydroxyl protons (OH) will have no correlation peaks. Moreover, the overlapped anomeric peaks will be distinct in case there would be any merged peaks. Analysis showed cross-couplings between corresponding anomeric protons with the attached anomeric carbon (1JHC). Therefore, this concludes the presence of seven anomers corresponding to seven monosaccharide units. Conclusions from the HSQC spectrum are as follows: (i) Peaks from 6.20 to 8.00 ppm in 1H NMR: These peaks have no correlation peaks in the 1H–13C HSQC spectrum, suggesting these as hydroxyl protons (OH), as there is no C directly attached to the protons. (ii) Peaks from 4.20 to 5.50 ppm in 1H NMR: This region contains anomeric–CH peaks along with hydroxyl peaks. The analysis showed a correlation between the protons (δ 4.50–5.50 ppm) and the carbons (δ 90.0–100.0 ppm), which confirms that the peaks present except the hydroxyl protons are anomeric protons. (iii) Peaks from 2.75 to 4.00 ppm in 1H NMR: The corresponding carbons (62.0–78.0 ppm) showed correlation peaks with the protons present at δ 2.75–4.00 ppm, representing the carbons connected to ‘O’ atom via a single bond (HxC–O–H/R, x = 1,2,3) present in the monosaccharide units (Fig. 4A). The enlarged spectra of 1H–13C HSQC showed the correlation of the anomeric carbons with their corresponding protons from the anomeric proton region (4.5–5.5 ppm). The carbon assigned C1 at δ 92.6 ppm showed a correlation with proton H1 at 4.90 ppm. Similarly, other correlations can be assigned as (C2, 92.89 ppm: H2, 4.85 ppm), (C3, 92.98 ppm: H3, 4.88 ppm), (C4, 94.4 ppm: H4, 4.87 ppm), (C5, 94.5 ppm: H5, 4.56 ppm), (C6, 97.2 ppm: H6, 4.26 ppm), (C7, 98.0 ppm: H7, 4.20 ppm).
Analysis of 1H–.1H COSY spectrum (Fig. 6A)
Fig. 6.
Nuclear magnetic resonance analysis. A 1H–1H cosy spectrum. B 1H–13C HMBC spectrum of EPS derivative
1H–1H COSY spectrum provides information regarding the neighboring vicinal protons. Thus the anomeric protons, as well as the hydroxyl protons, showed correlation peaks with their neighboring protons.
Analysis of 1H–13C HMBC spectrum (Fig. 6B)
1H–13C HMBC correlation spectra showed C–H correlation through two bonds (2JHC) and three bonds (3JHC) of the monosaccharides (Fig. 6B). The anomeric carbons showed correlation with the hydroxyl protons present in the region δ 4.50–5.50 ppm. Subsequently, the anomeric carbons showed a correlation with the protons connected to ‘O’ atom via a single bond of (HxC–O–H/R, x = 1,2,3) in the region δ 2.75–4.00 ppm through three bonds (3JHC) coupling. The carbons corresponding to (HxC–O–H/R, x = 1,2,3) also showed a correlation with the protons present at two bonds and three bond distances.
Scanning electron microscopic study and energy dispersive X-ray spectroscopic analysis of EPS
SEM studies (Fig. 7A, B) were done to elucidate the surface morphology of EPS for physical characterization. E. Ludwigii derived EPS has thin filaments like structure with three-dimensional structure have a compact, irregular lumps and stacked flakes of polysaccharide. Similar kinds of flakes structure were reported in L. gasseri and L. plantarum (Rani et al. 2018; Wang et al. 2015, 2010). The smooth and glossy surface of polysaccharides is the characteristic feature that imparts film-making property to EPS. Some irregular spherical structures were also observed. EDS analysis of EPS (Table S1 and Fig. 7C) has revealed the presence of carbon > boron > nitrogen > phosphorous > calcium > sulfur > iron > potassium > magnesium. The carbon content was quite high (72.72–77.63%) in the EPS due to polysaccharide composition. Along with carbon content, it also has a small amount of nitrogen and oxygen due to amino linkage in between the sugar moiety. Potassium, sulfur, phosphorous may be bound to the carboxyl and hydroxyl group of monosaccharides. Boron is also found in the EPS matrix. Cell–cell communication (quorum sensing) in bacteria is accomplished by an autoinducer agent that acts as a signaling molecule, and this autoinducer contains boron (Chen et al. 2002).
Fig. 7.
Physicochemical properties, A and B electron micrograph of crude EPS. C Element analysis of crude EPS by energy dispersive X-ray analysis. D DSC thermogram E X-ray diffractogram of EPS
Differential scanning calorimetry analysis of EPS
The industrial applicability of EPS is dependent on the thermal stability and its flow property. DSC analysis (Fig. 7D) of EPS showed an endothermic peak with a heat flow ranging from 10 °C to 300 °C. Glass transition temperature (40 °C) suggests that the EPS has an amorphous structure that transforms into a crystalline structure upon heating. At the beginning of a cycle, we observed a significant change in baseline (endothermic changes) due to variation in heat capacity of EPS and reference pan. Apparent melting at the glass transition temperature was also observed. The heating of EPS through its glass transition temperature leads to the release of inter-particle stress in the material. During this process, molecular changes occur in the EPS due to endothermic transition, which is very close to a glass transition temperature and it induces molecular relaxation. Molecular relaxation at glass transition temperature appeared as the endothermic melting peak. DSC analysis of EPS shows the thermal transition as the endothermic melting peak at 97.74 °C. EPS studied in our work has near about the same melting point as compared to the EPS obtained from L. kefiranofaciens ZW3 and L. plantarum KF5 which have the melting point of 97.38 °C and 86.35 °C, respectively (Wang et al. 2008, 2010) and it indicates the thermal stability of the product at solid-state. Endothermic peak and absence of exothermic peak were found due to conformational changes in polysaccharide at varying temperatures. Exothermic or/and endothermic reaction during the DSC is associated with the physical transformation of EPS, such as shape deformation and melting. The endothermic enthalpy change of EPS from this study is 275.328 J/g which is sufficient to melt 1 g of EPS. The thermal properties of different EPS derived from different bacteria vary. Our result entails that the EPS is semi-crystalline and it is further confirmed by XRD analysis.
XRD analysis of EPS
XRD patterns of the EPS were shown in the figure (Fig. 7E). It reveals information about the crystallinity or amorphous nature of the substances. The crystallinity of the polysaccharide was confirmed by the strong, narrow, and sharp peaks on the other hand small and broad peaks are associated with the amorphous component in EPS (Park et al. 2010; Rani et al. 2018). Overall we can say that EPS is semi-crystalline. EPS derived from E. ludwigii showed the diffraction peak at 2θ value (34.46°, 36.47°, 38.73°, 40.17°, 56.37°). XRD pattern has confirmed the presence of a crystalline component in the EPS with a crystallinity index of 0.559. It is observed that 55.9% of total EPS contains the crystalline domain which could be responsible for providing thermodynamic stability.
Application and properties of EPS
Emulsifying properties of EPS
The effect of EPS on emulsifying activity was analyzed by measuring the emulsifying activity index (EAI) and emulsion stability index (ESI) Table S2. EAI of EPS was determined by preparing emulsion with different oil (coconut oil, sunflower oil, mustard oil, hexane as hydrocarbon) using EPS as an emulsifier. EAI is the interfacial area of the prepared emulsion with the per unit weight of EPS (m2/g). It describes the emulsifying potency of EPS by avoiding the creaming, flocculation, and coalescence of emulsion at pH 7. EAI determines the ability of an EPS to form a stable emulsion, on the other hand, ESI determines the stability period of an emulsion. The emulsion is a thermodynamically unstable system, because their stability breakdown over some time at a certain temperature. EPS tends to reduce the interfacial tension between the two immiscible liquids (oil and water).
The effect of EPS on emulsifying activity and stability was determined in terms of EAI and ESI (Fig. 8 A, B, respectively). EPS showed a quite similar emulsifying activity with different oil but its stability showed wide variation with different oil, in the case of castor oil-in-water emulsion it has shown the EAI 11.454 ± 2 m2/g but it was stable only for 21 ± 10 min. In the case of a sunflower oil-in-water emulsion, it has an EAI of 8.165 ± 1 m2/g and it showed good stability lasting for 473 ± 10 min. When two large droplets of varying size approach each other they collapse and shape deformation occurs which causes the formation of interfacial thin film around the droplet. A reduction in interfacial tension induced by the EPS could be the possible reason behind the emulsifying activity. The ESI of sunflower-oil-in-water emulsion with EPS was significantly higher than that for castor oil-in-water emulsion and the white milky emulsion was obtained in both cases. EPS has the ability to form the stable emulsion against different edible oil that makes it promising alternative for use in food, and pharmaceuticals. Emulsifying activity of EPS is comparatively less as compared to commercially available emulsifiers but the surface modification strategies could be adopted in future to enhance the emulsifying capacity of EPS. It can improve the rheological properties of oil in water emulsion due to its good water binding capacity that allow them to form the viscous emulsion. It forms the interfacial layers which prevents the droplets flocculation.
Fig. 8.
It depicts the A emulsifying activity index (EAI) and B emulsion stability index (ESI) of the prepared emulsion with different oil and hydrocarbon at pH 7, using EPS as an emulsifying agent. C Determination of the antioxidant activity of EPS, using ascorbic acid as a reference sample by DPPH free radical scavenging assay. Data are represented as the mean ± SD obtained from three independent replicates
Zeta potential of oil in water emulsion
Zeta potential is the electrokinetic property of the colloidal system (either emulsion or suspension). It is the electric potential around the dispersed particles in a continuous phase. Emulsions having high zeta potential (either negative or positive) provide physical stability to an emulsion. Electrostatic charge distribution around the droplets of emulsion showed the zeta potential of around ± 30 mV. Zeta potential > ± 30 mV signifies the stability of an emulsion. Variation in zeta potential in the different emulsions is due to the change in surface composition at the interfacial layer. If a small amount of protein presents along with the EPS, it can change the surface potential due to the amphiphilic nature of the protein. These protein molecules tend to adsorb in the interfacial layer between the oil droplets and water molecules to avoid coalescence. A total charge of the adsorb EPS molecules decides the zeta potential of the emulsion (Jain et al. 2015) (Geyik et al. 2016). From the surface engineering point of view, EPS has the properties, such as adsorption and biodegradability (Wang et al. 2014). The zeta potential of prepared emulsion with different oil and hydrocarbon is shown in Fig. S1.5 and Table 2. The stability of the emulsion could be enhanced by increasing the concentration of EPS for industrial applicability.
Table 2.
Emulsion characterization by measuring the particle size and zeta potential of the prepared emulsion
| S.N | Emulsion | Particle size (dilution with 1% SDS) | Zeta potential (dilution with 1% SDS) | Particle size (dilution with PBS) | Zeta potential (dilution with PBS) |
|---|---|---|---|---|---|
| 1 | Castor oil emulsion | 0.6 µm | – 49.8 mV | 1.1 µm | -39.1 mV |
| 2 | Coconut oil emulsion | 0.04 µm | – 65.8 mV | 0.58 µm | – 49.8 mV |
| 3 | Hexane emulsion | 0.65 µm | – 73.5 mV | 0.44 µm | – 9.98 mV |
| 4 | Mustard oil emulsion | 0.8 µm | – 95.6 mV | 0.7 µm | – 21.8 mV |
| 5 | Sesame oil emulsion | 0.08 µm | – 98.5 mV | 0.07 µm | 51.4 mV |
| 6 | Sunflower oil emulsion | 1.6 µm | – 21.6 mV | 0.4 µm | – 7.15 mV |
Particle size distribution in the oil in water emulsion
The particle size of the prepared emulsion determined by the zeta sizer is shown in Fig. S1.5 and Table 2. The emulsifying potential of EPS also depends on the balance between hydrophilic and lipophilic portions, which provides stability to the emulsion with particle size varying from 1 µm to 5 µm. Flocculation of a particle affects the stability of an emulsion but it doesn’t have any direct correlation with the particle size, because no droplet merged in flocculation.
Antioxidant property of EPS
The generation of free radicals is harmful to any living organism, by keeping that in mind this study determined the antioxidant potential of crude EPS. Free radicals may cause severe damage by reacting with biomacromolecules. This study compared the free radical scavenging activity of EPS with standard ascorbic acid. The free radical scavenging potential of EPS is dependent on concentration (Fig. 8C). This study demonstrated the antioxidant activity of EPS from 1 mg/ml to 10 mg/ml which suggests that its antioxidant potency is less but the isolated crude EPS can be further purified to find out the active constituent responsible for the antioxidant property.
Conclusions
The current study involves the isolation and characterization of crude EPS followed by an investigation of the biological, physicochemical, and functional properties. The EPS is hetero-polysaccharide in nature having a molecular weight of ≥ 12 k Da. The proximate investigation of EPS showed the presence of carbohydrates with a small amount of protein and lipid. It showed a good emulsification index in the oil/water emulsion system. The stability of emulsion varied with a different oil. EPS shows good emulsifying activity with a different oil. The concentration of EPS also affects the stability of the prepared emulsion. SEM image and XRD pattern revealed that the EPS derived from E. ludwigii has a three-dimensional structure and is semi-crystalline. Its semi-crystalline nature at varying temperature and thermal stability was observed in DSC analysis. Chemical characterization of EPS done by FTIR, GC–MS, and NMR analysis hints at the presence of different monosaccharides (glucose, galactose, mannose, xylose) as a chief component of the EPS. The hydrolyzed derivative of the EPS was subjected to different analysis techniques which showed a total of 8 monomer units [7 confirmed from HSQC spectrum and another one from the 1H spectrum (Aldehyde peak)]. The most probable structures of these monosaccharides were characterized based on the GC–MS data. It has good emulsifying and antioxidant properties. The concentration of EPS could be enhanced for good emulsifying activity (> 2 mg/ml). This study also suggests that the E. ludwigii can grow in simulated gastric conditions and EPS produced by the bacteria have the emulsifying potential for the emulsification of fatty contents. The survivability of the bacteria in the gastric condition entails that the bacteria might be able to pass through the intestine and colonize there to emulsify the fatty meal in the intestine. The characterized EPS can be used in various industries, such as food, pharmaceutical, or biomedicine, and the bacteria can be grown on a pilot scale for maximum production of EPS.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
SKP is thankful to the Ministry of Human Resources and Development, New Delhi, India for financial assistance. We are grateful to the central instrument facility of NIT, Rourkela, Odisha, India for providing the instruments. We would like to thank Mrs. Swagatika Tripathi and Subrat Pradhan for their kind support.
Authors contribution
MM, GS, JP and SKP conceptualized and designed the research. SKP and JP conducted experiments. GS contributed new reagents or analytical tools. MM, SKP and GS, JP analyzed the data. SKP and JP wrote the original manuscript which was further revised by all co-authors. All authors read and approved the manuscript.
Funding
MM lab is supported by Grant No. BT/PR21857/NNT/28/1238/2017, EMR/2017/003054, Odisha DBT 3325/ST(BIO)-02/2017 Government of India. JP thanks SERB, DST, India (Project code—ECRA/2016/001975) for the research fellowship, and GS thanks SERB, DST, India (Project code—(ECRA/2016/001975) and Science and Technology Department, Odisha for the research funding (Project code—27562800512017/201296ST).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethical approval
This article does not contain any studies with animals.
Footnotes
Sanjeev Kumar Paikra and Jeetendra Panda authors are equally contributed to this work.
Contributor Information
Sanjeev Kumar Paikra, Email: sanjeevpaikra01@gmail.com.
Jeetendra Panda, Email: jeetendrapanda1993@gmail.com.
Gokarneswar Sahoo, Email: sahoog@nitrkl.ac.in.
Monalisa Mishra, Email: mishramo@nitrkl.ac.in.
References
- Arvidson SA, Rinehart BT, Gadala-Maria F. Concentration regimes of solutions of levan polysaccharide from Bacillus sp. Carbohyd Polym. 2006;65(2):144–149. [Google Scholar]
- Ashe S, Agasti S, Lakkoji S, Rauta PR, Sahoo H, Mishra M, Nayak B. Novel chromogenic bacteria characterized and their probable treatment options using herbal products and reagents to restrict biofilm formation. J Appl Biomed. 2017;15(4):291–298. [Google Scholar]
- Ayyash M, Abu-Jdayil B, Olaimat A, Esposito G, Itsaranuwat P, Osaili T, Obaid R, Kizhakkayil J, Liu S-Q. Physicochemical, bioactive and rheological properties of an exopolysaccharide produced by a probiotic Pediococcus pentosaceus M41. Carbohyd Polym. 2020;229:115462. doi: 10.1016/j.carbpol.2019.115462. [DOI] [PubMed] [Google Scholar]
- Bales PM, Renke EM, May SL, Shen Y, Nelson DC. Purification and characterization of biofilm-associated EPS exopolysaccharides from ESKAPE organisms and other pathogens. PLoS ONE. 2013;8(6):e67950. doi: 10.1371/journal.pone.0067950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkan II, Aygün G, Aydın S, Mutcalı SI, Kara Z, Kuşkucu M, Midilli K, Şemen V, Aras Ş, Yemişen M. Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: treatment and survival. Int J Infect Dis. 2014;26:51–56. doi: 10.1016/j.ijid.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Bremer PJ, Geesey GG. An evaluation of biofilm development utilizing non-destructive attenuated total reflectance Fourier transform infrared spectroscopy. Biofouling. 1991;3(2):89–100. [Google Scholar]
- Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415(6871):545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- Cooper DG, Goldenberg BG. Surface-active agents from two Bacillus species. Appl Environ Microbiol. 1987;53(2):224–229. doi: 10.1128/aem.53.2.224-229.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čopíková J, Barros AS, Šmídová I, Černá M, Teixeira DH, Delgadillo I, Synytsya A, Coimbra MA. Influence of hydration of food additive polysaccharides on FT-IR spectra distinction. Carbohyd Polym. 2006;63(3):355–359. [Google Scholar]
- Czaczyk K, Wojciechowska K. Formation of bacterial bioiilms-the essence of the matter andmechanisms of interactions. Biotechnologia. 2003;62(3):180–192. [Google Scholar]
- D’Abzac P, Bordas F, Van Hullebusch E, Lens PN, Guibaud G. Extraction of extracellular polymeric substances (EPS) from anaerobic granular sludges: comparison of chemical and physical extraction protocols. Appl Microbiol Biotechnol. 2010;85(5):1589–1599. doi: 10.1007/s00253-009-2288-x. [DOI] [PubMed] [Google Scholar]
- Das S. Structural and mechanical characterization of biofilm-associated bacterial polymer in the emulsification of petroleum hydrocarbon. 3 Biotech. 2021;11(5):1–15. doi: 10.1007/s13205-021-02795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decho AW. Microbial biofilms in intertidal systems: an overview. Cont Shelf Res. 2000;20(10–11):1257–1273. [Google Scholar]
- Decho AW, Gutierrez T. Microbial extracellular polymeric substances (EPSs) in ocean systems. Front Microbiol. 2017;8:922. doi: 10.3389/fmicb.2017.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Lotti T, Lin Y, Malpei F. Extracellular polymeric substances extraction and recovery from anammox granules: Evaluation of methods and protocol development. Chem Eng J. 2019;374:112–122. [Google Scholar]
- Fooladi T, Soudi MR, Alimadadi N, Savedoroudi P, Heravi MM. Bioactive exopolysaccharide from Neopestalotiopsis sp. strain SKE15: Production, characterization and optimization. Int J Biol Macromol. 2019;129:127–139. doi: 10.1016/j.ijbiomac.2019.01.203. [DOI] [PubMed] [Google Scholar]
- Freitas F, Alves VD, Carvalheira M, Costa N, Oliveira R, Reis MA. Emulsifying behaviour and rheological properties of the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohyd Polym. 2009;78(3):549–556. [Google Scholar]
- Freitas F, Alves VD, Pais J, Costa N, Oliveira C, Mafra L, Hilliou L, Oliveira R, Reis MA. Characterization of an extracellular polysaccharide produced by a Pseudomonas strain grown on glycerol. Biores Technol. 2009;100(2):859–865. doi: 10.1016/j.biortech.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Freitas F, Alves VD, Torres CA, Cruz M, Sousa I, Melo MJ, Ramos AM, Reis MA. Fucose-containing exopolysaccharide produced by the newly isolated Enterobacter strain A47 DSM 23139. Carbohyd Polym. 2011;83(1):159–165. [Google Scholar]
- Geyik AG, Kılıç B, Çeçen F. Extracellular polymeric substances (EPS) and surface properties of activated sludges: effect of organic carbon sources. Environ Sci Pollut Res. 2016;23(2):1653–1663. doi: 10.1007/s11356-015-5347-0. [DOI] [PubMed] [Google Scholar]
- Gottlieb HE, Kotlyar V, Nudelman A. NMR chemical shifts of common laboratory solvents as trace impurities. J Org Chem. 1997;62(21):7512–7515. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- Hebbar K, Gueniot B, Heyraud A, Colin-Morel P, Heulin T, Balandreau J, Rinaudo M. Characterization of exopolysaccharides produced by rhizobacteria. Appl Microbiol Biotechnol. 1992;38(2):248–253. [Google Scholar]
- Hua X, Wu Z, Zhang H, Lu D, Wang M, Liu Y, Liu Z. Degradation of hexadecane by Enterobacter cloacae strain TU that secretes an exopolysaccharide as a bioemulsifier. Chemosphere. 2010;80(8):951–956. doi: 10.1016/j.chemosphere.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Iyer A, Mody K, Jha B. Emulsifying properties of a marine bacterial exopolysaccharide. Enzyme Microb Technol. 2006;38(1–2):220–222. [Google Scholar]
- Jain R, Jordan N, Weiss S, Foerstendorf H, Heim K, Kacker R, Hübner R, Kramer H, van Hullebusch ED, Farges F. Extracellular polymeric substances govern the surface charge of biogenic elemental selenium nanoparticles. Environ Sci Technol. 2015;49(3):1713–1720. doi: 10.1021/es5043063. [DOI] [PubMed] [Google Scholar]
- Kallel F, Driss D, Bouaziz F, Belghith L, Zouari-Ellouzi S, Haddar A, Chaabouni SE, Ghorbel R. Polysaccharide from garlic straw: extraction, structural data, biological properties and application to beef meat preservation. RSC Adv. 2015;5(9):6728–6741. [Google Scholar]
- Kielak AM, Castellane TC, Campanharo JC, Colnago LA, Costa OY, Da Silva MLC, Van Veen JA, Lemos EG, Kuramae EE. Characterization of novel Acidobacteria exopolysaccharides with potential industrial and ecological applications. Sci Rep. 2017;7(1):1–11. doi: 10.1038/srep41193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konar M, Nayak N, Priyadarsini S, Mishra M, Sahoo H. Antimicrobial activity of nanoparticle-based dental fillers on novel chromogenic bacteria Enterobacter ludwigii. Mater Res Exp. 2019;6(8):085407. [Google Scholar]
- Laws A, Gu Y, Marshall V. Biosynthesis, characterisation, and design of bacterial exopolysaccharides from lactic acid bacteria. Biotechnol Adv. 2001;19(8):597–625. doi: 10.1016/s0734-9750(01)00084-2. [DOI] [PubMed] [Google Scholar]
- Liu H, Fang HH. Extraction of extracellular polymeric substances (EPS) of sludges. J Biotechnol. 2002;95(3):249–256. doi: 10.1016/s0168-1656(02)00025-1. [DOI] [PubMed] [Google Scholar]
- Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55(1):165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- More VS, Ebinesar A, Prakruthi A, Praveen P, Fasim A, Rao A, Zameer F, Anantharaju K, More S (2021) Isolation and purification of microbial exopolysaccharides and their industrial application. In: Vaishnav A, Choudhary DK (eds) Microbial polymers. Springer, Singapore, pp 69–86. 10.1007/978-981-16-0045-6_3
- Morris ER, Rees DA, Sanderson GR, Thom D. Conformation and circular dichroism of uronic acid residues in glycosides and polysaccharides. J Chem Soc Perkin Trans. 1975;2(13):1418–1425. [Google Scholar]
- Nagaraj K, Devasya RP, Bhagwath AA. Exopolysaccharide produced by Enterobacter sp. YG4 reduces uranium induced nephrotoxicity. Int J Biol Macromol. 2016;82:557–561. doi: 10.1016/j.ijbiomac.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Naik MM, Pandey A, Dubey SK. Biological characterization of lead-enhanced exopolysaccharide produced by a lead resistant Enterobacter cloacae strain P2B. Biodegradation. 2012;23(5):775–783. doi: 10.1007/s10532-012-9552-y. [DOI] [PubMed] [Google Scholar]
- Naumann D, Helm D, Labischinski H. Microbiological characterizations by FT-IR spectroscopy. Nature. 1991;351(6321):81–82. doi: 10.1038/351081a0. [DOI] [PubMed] [Google Scholar]
- Nwodo UU, Green E, Okoh AI. Bacterial exopolysaccharides: functionality and prospects. Int J Mol Sci. 2012;13(11):14002–14015. doi: 10.3390/ijms131114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramonov N, Aduse-Opoku J, Hashim A, Rangarajan M, Curtis MA. Identification of the linkage between A-polysaccharide and the core in the A-lipopolysaccharide of Porphyromonas gingivalis W50. J Bacteriol. 2015;197(10):1735–1746. doi: 10.1128/JB.02562-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK. Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels. 2010;3(1):10. doi: 10.1186/1754-6834-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce KN, Kinsella JE. Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem. 1978;26(3):716–723. [Google Scholar]
- Pompermayer DM, Gaylarde CC. The influence of temperature on the adhesion of mixed cultures of Staphylococcus aureus and Escherichia coli to polypropylene. Food Microbiol. 2000;17(4):361–365. [Google Scholar]
- Rani RP, Anandharaj M, Ravindran AD. Characterization of a novel exopolysaccharide produced by Lactobacillus gasseri FR4 and demonstration of its in vitro biological properties. Int J Biol Macromol. 2018;109:772–783. doi: 10.1016/j.ijbiomac.2017.11.062. [DOI] [PubMed] [Google Scholar]
- Saby S, Sibille I, Mathieu L, Paquin J, Block J. Influence of water chlorination on the counting of bacteria with DAPI (4', 6-diamidino-2-phenylindole) Appl Environ Microbiol. 1997;63(4):1564–1569. doi: 10.1128/aem.63.4.1564-1569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu S, Jaysingh P, Mishra M (2022) As an in vivo model for the investigation of host-microbiota interaction. In: Prebiotics, probiotics and nutraceuticals. Springer, Singapore, pp 275–300
- Shao P, Chen X, Sun P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohyd Polym. 2014;105:260–269. doi: 10.1016/j.carbpol.2014.01.073. [DOI] [PubMed] [Google Scholar]
- Shi J-J, Zhang J-G, Sun Y-H, Qu J, Li L, Prasad C, Wei Z-J. Physicochemical properties and antioxidant activities of polysaccharides sequentially extracted from peony seed dreg. Int J Biol Macromol. 2016;91:23–30. doi: 10.1016/j.ijbiomac.2016.05.082. [DOI] [PubMed] [Google Scholar]
- Sletmoen M, Maurstad G, Sikorski P, Paulsen BS, Stokke BT. Characterisation of bacterial polysaccharides: steps towards single-molecular studies. Carbohyd Res. 2003;338(23):2459–2475. doi: 10.1016/j.carres.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Synytsya A, Čopíková J, Matějka P, Machovič V, Fourier transform Raman and infrared spectroscopy of pectins. Carbohyd Polym. 2003;54(1):97–106. [Google Scholar]
- Thaiphanit S, Anprung P .2014 Increasing the utilization of coconut ( Cocos nucifera L.) wet processing waste: physicochemical and functional properties of coconut protein powder. In: The Proceedings of the 1st Joint ACS AGFD-ACS ICSCT Symposium on Agricultural and Food Chemistry 2014(4);207e215
- Thaiphanit S, Anprung P. Physicochemical and emulsion properties of edible protein concentrate from coconut (Cocos nucifera L.) processing by-products and the influence of heat treatment. Food Hydrocoll. 2016;52:756–765. [Google Scholar]
- Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH (2015) Essentials of glycobiology, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring, Harbor, NY, pp 66–84 [PubMed]
- Wang F, Yang H, Wang Y. Structure characterization of a fucose-containing exopolysaccharide produced by Enterobacter cloacae Z0206. Carbohyd Polym. 2013;92(1):503–509. doi: 10.1016/j.carbpol.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Wang J, Li Q, Li M-M, Chen T-H, Zhou Y-F, Yue Z-B. Competitive adsorption of heavy metal by extracellular polymeric substances (EPS) extracted from sulfate reducing bacteria. Biores Technol. 2014;163:374–376. doi: 10.1016/j.biortech.2014.04.073. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhao X, Yang Y, Zhao A, Yang Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int J Biol Macromol. 2015;74:119–126. doi: 10.1016/j.ijbiomac.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ahmed Z, Feng W, Li C, Song S. Physicochemical properties of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir. Int J Biol Macromol. 2008;43(3):283–288. doi: 10.1016/j.ijbiomac.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li C, Liu P, Ahmed Z, Xiao P, Bai X. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohyd Polym. 2010;82(3):895–903. [Google Scholar]
- Wolpert M, Hellwig P. Infrared spectra and molar absorption coefficients of the 20 alpha amino acids in aqueous solutions in the spectral range from 1800 to 500 cm− 1. Spectrochim Acta Part A Mol Biomol Spectrosc. 2006;64(4):987–1001. doi: 10.1016/j.saa.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Yun U, Park H. Physical properties of an extracellular polysaccharide produced by Bacillus sp CP912. Lett Appl Microbiol. 2003;36(5):282–287. doi: 10.1046/j.1472-765x.2003.01309.x. [DOI] [PubMed] [Google Scholar]
- Zárate G, Chaia AP, González S, Oliver G. Viability and β-galactosidase activity of dairy propionibacteria subjected to digestion by artificial gastric and intestinal fluids. J Food Prot. 2000;63(9):1214–1221. doi: 10.4315/0362-028x-63.9.1214. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cui Y, Qu X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: a review. Carbohyd Polym. 2019;207:317–332. doi: 10.1016/j.carbpol.2018.11.093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.