Abstract
Several epidemiological studies have suggested that Epstein–Barr virus (EBV) lytic infection is essential for the development of nasopharyngeal carcinoma (NPC), as the elevation of antibody titers against EBV lytic proteins is a common feature of NPC. Although ZEBRA protein is a key trigger for the initiation of lytic infection, whether its expression affects the prognosis and pathogenesis of NPC remains unclear. In this study, 64 NPC biopsy specimens were analyzed using immunohistochemistry. We found that ZEBRA was significantly associated with a worsening of progression‐free survival in NPC (adjusted hazard ratio, 3.58; 95% confidence interval, 1.08–11.87; p = 0.037). Moreover, ZEBRA expression positively correlated with key endocrinological proteins, estrogen receptor α, and aromatase. The transcriptional level of ZEBRA is activated by estrogen in an estrogen receptor α‐dependent manner, resulting in an increase in structural gene expression levels and extracellular virus DNA copy number in NPC cell lines, reminiscent of lytic infection. Interestingly, it did not suppress cellular proliferation or increase apoptosis, in contrast with cells treated with 12‐O‐tetradecanoylphorbol‐13‐acetate and sodium butyrate, indicating that viral production induced by estrogen is not a cell lytic phenomenon. Our results suggest that intratumoral estrogen overproduced by aromatase could induce ZEBRA expression and EBV reactivation, contributing to the progression of NPC.
Keywords: BZLF1, estrogen, lytic infection, nasopharyngeal carcinoma, ZEBRA
We have revealed that EBV lytic protein ZEBRA is significantly associated with poor prognosis of nasopharyngeal carcinoma. Moreover, our study suggests that locally expressed intratumoral estrogen induces EBV lytic protein ZEBRA expression from the gene expression level. We expect of the development of novel treatment modalities such as anti‐hormonal treatment.
Abbreviations
- CPS
combined positive score
- E2
estradiol
- EBV
Epstein–Barr virus
- ER
estrogen receptor
- ERα
estrogen receptor α
- FPKM
fragments per kilobase of exon per million mapped sequence reads
- GEO
Gene Expression Omnibus
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LC–MS
liquid chromatography–mass spectrometry
- LMP‐1
latent membrane protein 1
- neo r
neomycin resistant
- NPC
nasopharyngeal carcinoma
- OS
overall survival
- PD‐L1
programmed death ligand 1
- PFS
progression‐free survival
- SB
sodium butyrate
- TPS
tumor proportion score
- Zp
BZLF1 promoter
1. INTRODUCTION
Epstein–Barr virus is a human gamma‐herpes virus associated with several types of malignancies, including NPC. Recent advances in radiation therapy and chemotherapy have reduced the mortality rate of patients with NPC. However, some NPC populations continue to exhibit poor prognoses, even in the early stages of cancer. Therefore, more sensitive biological markers must be developed that reflect the poor prognosis of patients with NPC.
In most EBV‐associated tumors, EBV infection is predominantly latent. The immediate–early BZLF1 gene product of EBV, ZEBRA, activates the promoters of EBV lytic genes, induces a cascade of viral gene expression, and switches from latent to lytic infection. Recently, several studies have revealed the contribution of lytic infection to the oncogenesis of NPC. 1 , 2 Some studies have reported that serological anti‐ZEBRA titers correlate with diagnosis and prognosis after radiation therapy for NPC. 3 , 4 , 5 , 6 We previously demonstrated that ZEBRA‐positive NPC is a risk factor for advanced lymph node metastasis using immunohistochemistry. 7 ZEBRA expression initiates lytic infection triggered by biological or chemical substances in vitro, including TPA, sodium butyrate (SB), calcium ionophores, and anti‐Ig. 8 Nonetheless, the physiological stimuli that trigger ZEBRA expression and lytic infection in vivo have not been identified. 8 In addition, whether its expression affects the prognosis remains to be determined.
Estrogen is a risk factor for various tumors, including breast, colon, and lung cancers. 9 , 10 , 11 , 12 Circulating estrogen levels are strongly associated with an increased risk of breast cancer. 9 In addition, some meta‐analyses have reported that 20%–30% of breast cancer tissues carry the EBV genome and express viral genes. 13 , 14 BZLF1 expression was also detected in EBV‐positive breast cancer tissues, and anti‐ZEBRA titers were correlated with a worse prognosis in these cases. 15 Moreover, aromatase, a cytochrome P450 enzyme complex that mediates estrogen synthesis, is ectopically expressed. 10 , 11 , 16 , 17 , 18 Estrogen is activated by binding to the ER, a member of the nuclear receptor family, located in the cytoplasm. 19 Activated ER acts as a transcription factor and influences various biological activities including carcinogenesis.
In this study, we hypothesized that EBV‐infected nasopharyngeal cells are exposed to high estrogen levels due to aromatase expression during tumorigenesis. Indeed, we observed that the expression of ZEBRA in NPC was associated with an increase in the expression levels of aromatase and ERα, as well as with the worsening of PFS. In in vitro models, ZEBRA expression is induced by estrogen in an ERα‐dependent manner and contributes to invasion and migration. Interestingly, we observed that the estrogen‐ZEBRA axis resulted in an increase in viral production but not in cell death, in a manner distinct from that of conventional TPA + SB‐treated lytic cells.
2. MATERIAL AND METHODS
2.1. Patient characteristics and ZEBRA detection
The present study included Japanese patients with NPC who were diagnosed in the Department of Otolaryngology, Head and Neck Surgery at Kanazawa University Hospital between January 2000 and December 2019. Unavailable tissue specimens were excluded. Finally, 64 patients were examined (Table 1). Based on the immunohistochemical results of ZEBRA, we divided the patients into ZEBRA‐positive and ZEBRA‐negative groups using a 15% positive cutoff value (median value of expression score) and compared the clinical outcomes. The primary outcomes were OS and PFS rates. This study was approved by the Ethics Committee of Kanazawa University (IRB#2016‐033), and written informed consent was obtained from all the patients before enrollment.
TABLE 1.
Association between ZEBRA status and clinicopathological parameters in 64 nasopharyngeal carcinomas
| Value | ZEBRA status | ||
|---|---|---|---|
| Positive (n = 32) | Negative (n = 32) | p‐Value | |
| Age (years ± SD) * | 58.2 ± 13.6 | 59.0 ± 13.7 | 0.81 |
| Sex (%) | |||
| Male | 28 (88) | 26 (81) | 0.73 |
| Female | 4 (12) | 6 (19) | |
| Smoking (%) | |||
| Never | 9 (28) | 14 (43) | 0.30 |
| Past and present | 23 (72) | 18 (57) | |
| Alcohol (%) | |||
| Never | 20 (63) | 17 (53) | 0.61 |
| Past and present | 12 (37) | 15 (47) | |
| T (%) | |||
| 1–2 | 18 (56) | 15 (47) | 0.78 |
| 3–4 | 14 (44) | 17 (53) | |
| N (%) | |||
| 0–1 | 14 (44) | 18 (56) | 0.32 |
| 2–3 | 18 (56) | 14 (44) | |
| M (%) | |||
| 0 | 30 (94) | 30 (94) | 1 |
| 1 | 2 (6) | 2 (6) | |
| Stage (%) | |||
| I–II | 7 (22) | 8 (25) | 0.86 |
| III–IV | 25 (78) | 24 (75) | |
| WHO classification (%) | |||
| 2 | 20 (63) | 22 (69) | 0.79 |
| 3 | 12 (37) | 10 (31) | |
| Treatment (%) | |||
| CRT | 28 (88) | 30 (94) | 0.67 |
| RT | 3 (9) | 2 (6) | |
| Chemotherapy | 1 (3) | 0 (0) | |
| ERα (%) | |||
| Negative | 3 (9) | 27 (84) | <1 × 10 −8 |
| Positive | 29 (91) | 5 (16) | |
| Aromatase (%) | |||
| Negative | 10 (31) | 23 (72) | <0.01 |
| Positive | 22 (69) | 9 (28) | |
Note: The ZEBRA, ER⍺, and aromatase status was evaluated by immunohistochemistry (IHC), and the cutoff values were 15%, 10%, and 20%, respectively, determined from the median value of IHC of each positivity. All other values represent the number of cases and were evaluated cross‐table using the chi‐squared test. A p‐value <0.05 is considered significant and shown in boldface.
Abbreviations: CRT, chemoradiotherapy; ERα, estrogen receptor α; RT, radiotherapy; SD, standard deviation.
Data are presented as mean and SD and were evaluated by t‐test.
2.2. Immunohistochemical analysis
In total, 64 NPC biopsy specimens were examined to determine the expression levels of ZEBRA, aromatase, ERα, and PD‐L1. Tumor fixation and immunohistochemical analyses were performed as described previously. 20 The antibodies used in this study are listed in Table S1. The stained sections were independently evaluated by two investigators (HD and SK) who were blinded to clinical data. Aromatase expression was considered positive when cytoplasmic staining was diffused in the absence of nuclear staining, as described previously. 21 ERα and ZEBRA expression was considered positive if there was nuclear‐specific dark brown staining, as described previously. 7 , 22 , 23 Specimens with at least 10% ERα‐, 15% ZEBRA‐, and 20% aromatase‐positive tumor cells were considered positive. Each cutoff value was determined using the median value of each expression score for immunohistochemical positivity. PD‐L1 expression was assessed using the TPS and CPS. TPS is the percentage of PD‐L1‐positive tumor cells in the total number of viable tumor cells, whereas CPS is the percentage of PD‐L1‐positive cells (tumor cells, lymphocytes, and macrophages) in the total number of viable tumor cells. Moreover, five of the 32 primary NPC specimens were used for dual fluorescence immunostaining of PD‐L1 and ZEBRA, and ERα and ZEBRA. Paraffin sections were deparaffinized, treated with 3% hydrogen peroxide, and incubated with a protein blocker (Dako). The sections were incubated overnight at 4°C with primary antibodies. Next, the sections were incubated with goat anti‐mouse Alexa Fluor 594 and anti‐rabbit Alexa Fluor 488 IgG secondary antibodies (1:500; Thermo Fisher Scientific) after washing with PBS. The sections were counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI; Thermo Fisher Scientific, catalog no. P36962).
2.3. Reagents and cell culture
HK1 NPC cells (kindly gifted by Dr. George Tsao, Hong Kong University) and C666‐1 NPC cells (kindly gifted by Dr. Dolly P. Huang, Hong Kong University) were maintained in RPMI 1640 medium with 10% FBS. Akata‐EBV‐eGFP and Akata‐EBV‐NeoR cells were purchased from JCRB and cultured in RPMI 1640 supplemented with 800 μg/ml G‐418 (Nacalai Tesque, catalog no. 09380) and 10% FBS. TW01 NPC cells 24 were maintained in minimal essential medium with 10% FBS.
2.4. Plasmids and retroviral infection
The BZLF1 expression vector pcDNA3‐BZLF1 was constructed as described previously. 25 From the plasmid, the cDNA of the pcDNA3‐BZLF1 fragment digested with BamHl and XhoI was introduced into the pFB‐Neo vector (Addgene, catalog no. 69767), and the plasmid was named pFB‐BZLF1. Retroviruses were prepared as described previously. 26 HK1 cells were infected with the retroviruses in RPMI 1640 medium containing polybrene. Stable transfected cell lines were selected using G418.
2.5. EBV infection
EBV‐negative NPC cell lines, HK1 and TW01, were reinfected with EBV using neo r EBV‐infected Akata cells and green fluorescent protein (eGFP) neo r EBV‐infected Akata cells, respectively, as described previously. 27 HK1‐EBV‐NeoR and NPC‐TW01‐EBV‐NeoR cells were prepared using neo r EBV‐infected Akata cells. HK1‐EBV‐eGFP and NPC‐TW01‐EBV‐eGFP cells were prepared using eGFP‐neo r EBV‐infected Akata cells.
2.6. Western blotting
SDS‐PAGE and western blotting were performed as described previously. 28 The antibodies used are listed in Table S1.
2.7. RNA extraction and qRT‐PCR
RNA extraction and reverse transcription were performed on cell samples as described previously. 20 qRT‐PCR was performed using PCR mixtures of each sample with Thunderbird SYBR qPCR mix (Toyobo Life Science; catalog no. QPS‐201). The forward and reverse primers for BZLF1 (forward: 5′‐CGGGGGATAATGGAGTCAAC‐3′; reverse: 5′‐GATTTTTGTCCGCAGGTGGC‐3′), 29 and GAPDH (forward: 5′‐TGCACCACCAACTGCTTAGC‐3′; reverse: 5′‐GGCATGGACTGTGGTCATGAG‐3′) were used as housekeeping genes.
2.8. Transfection and luciferase assay
To examine the activity of the Zp, we prepared pZp‐luc and its derivatives, as described previously and shown in Figure 4A. 30 These plasmid DNAs were transfected into HK‐1 cells using FuGENE 6 Transfection Reagent (Promega, catalog no. E2691). After transfection, the cells were treated with DMSO or 10 nM E2 for 48 h. Proteins were extracted using a dual‐luciferase reporter assay system (Promega, catalog no. E1910), and luciferase activity was examined using a Turner BioSystems Luminometer Model TD‐20/20 (Promega). The activity of firefly luciferase was divided into that of Renilla luciferase.
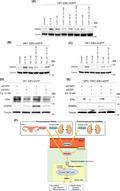
FIGURE 4.
BZLF1 promoter of nasopharyngeal carcinoma (NPC) cells is activated via estrogen treatment. (A) Alignment of wild‐type (wt) and mutated sequences of the EBV minimal BZLF1 promoter. The cis‐regulatory element sequences are indicated in capital letters. Mutations are boxed and indicated in capital letters. (B) Effect of estradiol (E2) exposure on the reporter plasmid BZLF1 promoter (pZp)‐luc (wt). (C) Effect of the derivatives of pZp‐luc on DMSO. pZp‐luc containing ZII mutations was commonly inactivated compared with pZp‐luc (wt) in HK1 cells. (D) Effect of E2 exposure on each derivative of pZp‐luc. Only pZp‐luc containing ZII mutations was not activated by E2 exposure in HK1 cells. The data are expressed as the mean ± SD. NS, not significant; *p < 0.01
2.9. DNA extraction from the cells and medium
DNA was extracted from the cells using the QIAamp DNA Mini Kit (QIAGEN, catalog no. 51304). DNA from the medium was concentrated using a PEG‐it Virus Precipitation Solution (System Biosciences, catalog no. LV810A‐1), whereas DNA from the aliquot was extracted using the QIAamp Min Elute Media kit (QIAGEN, catalog no. 57414). qPCR analysis of EBV DNA from the cells and medium was performed as described previously. 31
2.10. Cell proliferation assay
In total, 2.0 × 103 cells were seeded into a 96‐well plate, and cell proliferation was performed using a CellTiter 96 Aqueous One Solution Cell Proliferation Kit (Promega, catalog no. G3580) as described previously. 32
2.11. Statistical analysis
Statistical significance was set at p < 0.05. All reported p‐values were two‐sided. All in vitro experiments were performed in triplicate unless otherwise stated. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University), a graphical user interface for R (R Foundation for Statistical Computing). 33
Detailed information on the materials and methods is available in the supporting information (Appendix S1).
3. RESULTS
3.1. Aromatase expression in palatine and pharyngeal tonsil samples
To examine whether aromatase is expressed in normal tonsil tissues under physiological conditions, we first performed immunohistochemical staining of aromatase in 10 palatine and pharyngeal tonsil samples (Figure 1A,B). Aromatase expression was considered positive when cytoplasmic staining was diffuse in the absence of nuclear staining (Figure 1B). The pharyngeal tonsils (Figure 1A) had significantly higher aromatase expression than the palatine tonsils (Figure 1B) according to the chi‐squared test (Table S2, p = 0.02).
FIGURE 1.
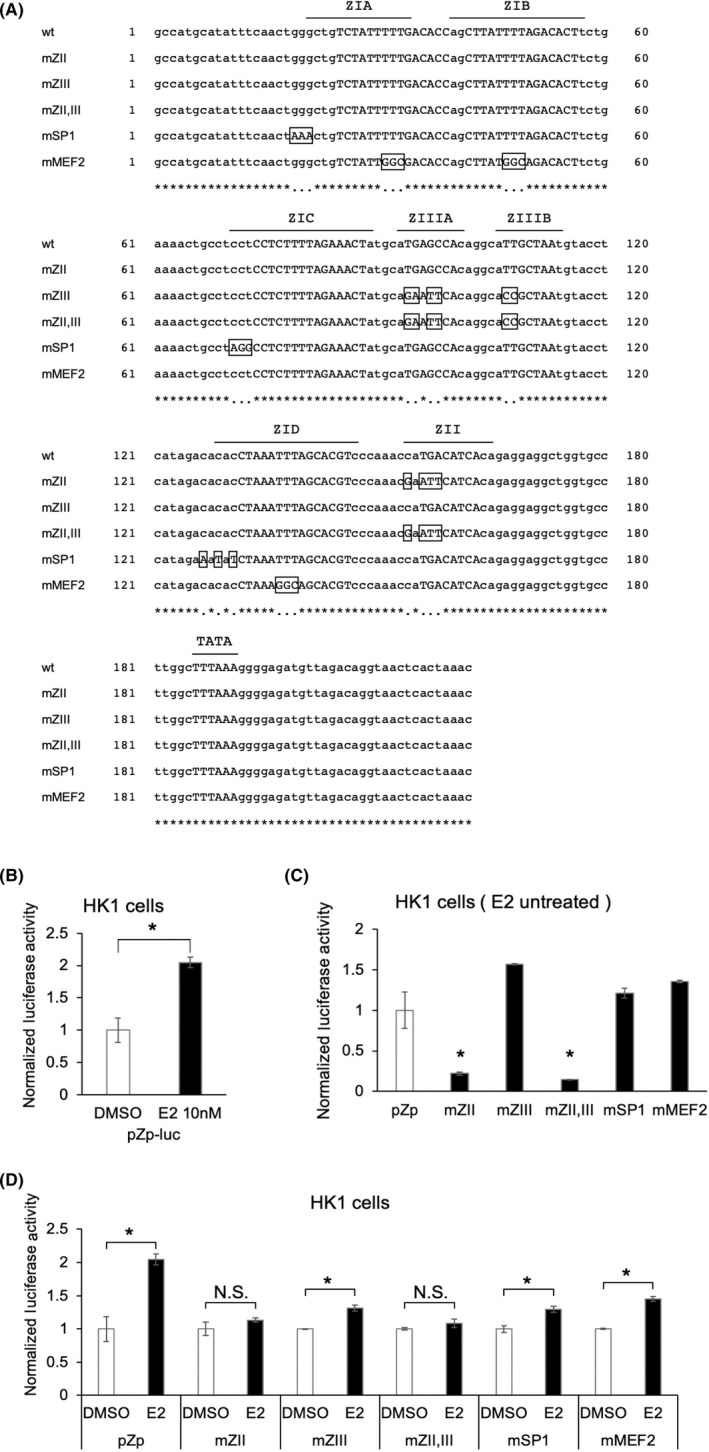
(A–D) Immunostaining of aromatase, estrogen receptor α (ERα), and ZEBRA in normal tonsils and nasopharyngeal carcinoma (NPC) samples. (magnification ×100, scale bar shows 100 μm; lower right rectangle ×400, scale bar shows 50 μm). (A, B) Representative examples of aromatase staining of normal palatine and pharyngeal tonsils. (A) Normal palatine tonsil is negative, and normal (B) pharyngeal tonsil is positive for the expression of aromatase. (C) Positive immunostaining for aromatase, ERα, and ZEBRA in NPC is obtained from the same patient, and (D) negative immunostaining from the other patient. (E) Correlation between the expression levels of ZEBRA and ERα in NPC. The expression scores of ZEBRA and ERα for 64 cases are plotted. Spearman correlation coefficient indicates that the expression of ZEBRA demonstrates a significant correlation with that of ERα (r = 0.782, p = 1 × 10−14). (F) Correlation between the expression levels of ERα and aromatase in NPC (r = 0.486, p < 0.0001). (G) Correlation between the expression levels of ZEBRA and aromatase in NPC (r = 0.417, p < 0.001). (H) Dual fluorescence immunostaining of ERα, ZEBRA, and DAPI in NPC tissue samples. A merged image of ERα and ZEBRA indicates co‐expression of ERα and ZEBRA. Original magnifications: ×400. Kaplan–Meier estimates of overall survival (I) and progression‐free survival (J) according to the ZEBRA status of 64 patients with NPC in the immunohistochemical study
3.2. Expression of ZEBRA is positively associated with the expression levels of ERα and aromatase in NPC
The present study included 64 patients with NPC for immunohistochemical analysis. We assessed whether the expression levels of ERα and aromatase were related to ZEBRA expression (Figure 1C,D). Nucleus‐specific dark brown staining of tumor cells was considered positive for ERα and ZEBRA (Figure 1C). 23 Pearson's correlation analysis of ERα and ZEBRA expression in NPC samples showed a marked association (r = 0.78, p = 1 × 10−14; Figure 1E), suggesting that estrogen levels might be related to the mechanism underlying ZEBRA expression. A weak association was observed between the expression levels of ERα and aromatase (r = 0.49, p < 0.0001; Figure 1F) and aromatase and ZEBRA (r = 0.42, p < 0.001; Figure 1G). Next, we performed dual fluorescence immunostaining for the expression of ERα and ZEBRA in the same tissue sample. Several cells co‐expressed both ERα and ZEBRA in NPC tissue samples (Figure 1H).
No association was found between the expression levels of PD‐L1 (TPS and CPS) and ZEBRA when observed by immunohistochemical analysis (Figure S1A,B). In dual fluorescence immunostaining of PD‐L1 and ZEBRA, the expression of ZEBRA was not correlated with the expression of PD‐L1 (Figure S1C).
3.3. Role of aromatase in female patients with NPC
To verify the significance of aromatase expression in women, we examined its association with other clinical factors. In our cohort of 64 patients with NPC, 10 were women. The average age of the female patients with NPC was significantly higher than that of the male patients (68.8 years [female] vs. 56.7 years [male], p < 0.01; Figure S2A). Moreover, nine of the 10 female patients were aged over 55 years, indicating that most female patients were postmenopausal. We did not find any difference in aromatase expression levels between male and female patients with NPC (p = 0.85; Figure S2B). Therefore, intratumoral local aromatase expression in women, particularly those who were postmenopausal, was as important as that in men in this study.
3.4. ZEBRA can predict progression‐free survival in NPC
Next, we examined whether ZEBRA affects NPC prognosis. The baseline characteristics of the analyzed population of the ZEBRA‐positive (n = 32) and ZEBRA‐negative (n = 32) groups, according to immunohistochemical analyses, are summarized in Table 1. The baseline characteristics of the patients were similar between the groups. Kaplan–Meier curves for OS and PFS are shown in Figure 1I,J, respectively. The log‐rank test revealed that the PFS of ZEBRA‐positive patients with NPC was poorer than that of ZEBRA‐negative patients with NPC (p = 0.01, Figure 1J); however, the OS was not (p = 0.24; Figure 1I). Therefore, we performed a Cox proportional hazard regression analysis of PFS to determine whether ZEBRA positivity remained a significant variable. Multivariate analysis showed that ZEBRA positivity in NPC was a significant risk factor for poor PFS (adjusted hazard ratio 3.58, 95% confidence interval 1.08–11.87, p = 0.037; Table 2). These analyses indicate that ZEBRA expression is associated with poor prognosis in NPC. In total, 13 cases of recurrence were observed following first‐line treatment, and nine of these cases were treated with chemotherapy or immune checkpoint inhibitors. As described in Figure S3, NPC is susceptible to second‐line treatments including chemotherapy and immune checkpoint inhibitors.
TABLE 2.
Cox proportional hazard regression analysis of progression‐free survival of 64 nasopharyngeal carcinoma patients
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p‐Value | HR (95% CI) | p‐Value | |
| Age a | 0.99 (0.95–1.03) | 0.68 | ||
| Sex (men vs. women) | 0.99 (0.22–4.44) | 0.99 | ||
| Stage a | 2.86 (1.22–6.71) | 0.016 * | 2.60 (1.10–6.14) | 0.030 |
| Histologic type (WHO classification 3 vs. others) | 0.62 (0.19–1.99) | 0.42 | ||
| Therapy (CRT vs. others) | 1.47 (0.41–5.30) | 0.56 | ||
| ER⍺ (+ vs. –) b | 3.16 (0.96–10.38) | 0.058 | ||
| Aromatase (+ vs. –) b | 2.58 (0.86–7.77) | 0.092 | ||
| ZEBRA (+ vs. –) b | 4.25 (1.28–14.13) | 0.018 * | 3.58 (1.08–11.87) | 0.037 |
Note: Statistically significant values (p < 0.05) are in boldface.
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval.
Data were evaluated as continuous value.
ER⍺, aromatase, and ZEBRA status was evaluated by immunohistochemistry (IHC), and the cutoff values were 10%, 20%, and 20%, respectively, determined from the median value of IHC of each positivity.
Data were considered significant in univariate analysis and examined in multivariate analysis.
3.5. BZLF1 promotes cell invasion and migration
To further clarify whether ZEBRA expression conferred malignant cellular properties, we established a stable NPC cell line expressing ZEBRA. ZEBRA expression was detected by western blotting in HK1‐pFB‐BZLF1 cells; however, not in HK1‐pFB‐Neo cells (Figure 2A).
FIGURE 2.
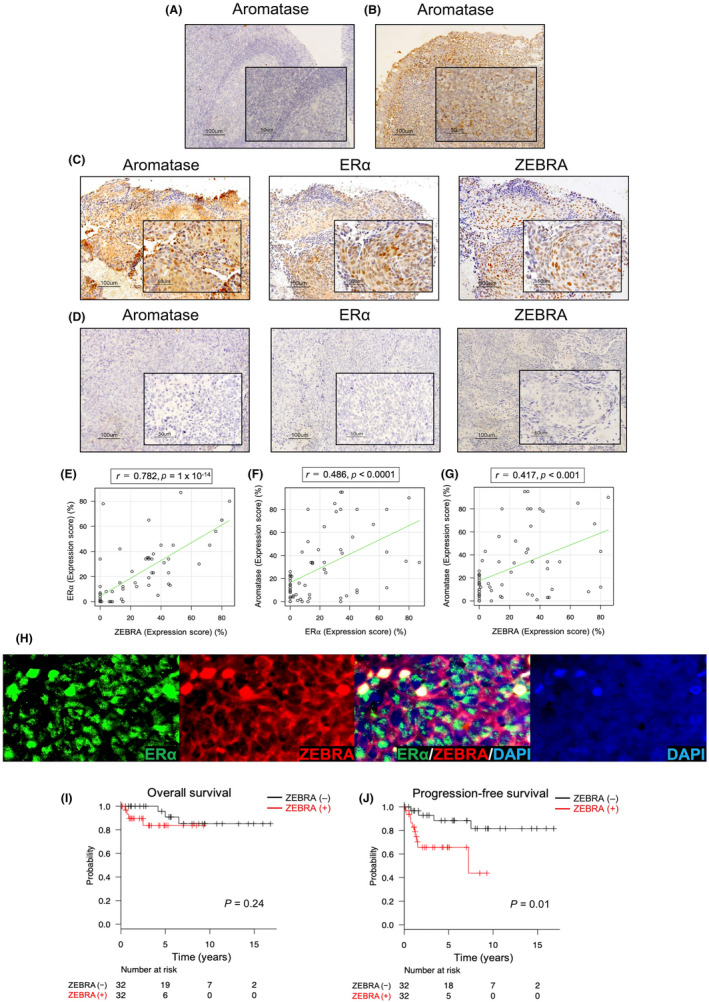
Malignant cellular properties of ZEBRA and public RNA‐seq NPC data analysis. (A) HK1 cells expressing pFB‐Neo or pFB‐BZLF1 are assessed using western blotting. (B) Absorbance generated in cell‐mediated MTS assays of HK1 cells expressing pFB‐Neo or pFB‐BZLF1. (C) Representative images of transwell invasion assay in HK1 cells expressing pFB‐Neo or pFB‐BZLF1. (D) Comparison of the invaded cell counts in HK1‐pFB‐Neo or HK1‐pFB‐BZLF1. (E) Optical microscopy of the migration assay of HK1‐pFB‐Neo or HK1‐pFB‐BZLF1 at 0 and 10 h. (F) Comparison of the cell migration in HK1‐pFB‐Neo or HK1‐pFB‐BZLF1. (G) The ESR1 mRNA expression level in BZLF1‐negative group (n = 61) and positive group (n = 59). (B, D, F) The data are expressed as the mean ± standard deviation (SD). *p < 0.0001. (G) A t‐test is performed to determine the difference between the groups. The box plots represent the median, first, and third quartile
To analyze the effects of BZLF1 on cells, cell growth kinetics were examined. The growth ratio of HK1‐pFB‐BZLF1 cells was not significantly increased compared with that of HK1‐pFB‐Neo cells (Figure 2B). However, according to Matrigel‐coated Transwell and wound scratch migration assays, HK1‐pFB‐BZLF1 cells had a significantly higher number of invasive cells (p < 0.0001; Figure 2C,D) and showed an increase in the rate of migration (p < 0.0001; Figure 2E,F) compared with HK1‐pFB‐Neo cells. These data suggest that ZEBRA expression upregulates the invasion and migration of tumor cells.
3.6. Transcriptomic analysis of publicly available NPC data
To further validate the immunohistochemical results, RNA‐seq analysis was performed using four publicly available data sets (Table S3). The flow of sample selection for the NPC GEO data is shown in Figure S4. We excluded 10 EBER‐negative NPC samples from the 131 NPC samples from the GEO database.
3.6.1. Association between BZLF1 and ESR1 expression
In total, 121 NPC data points were divided into BZLF1‐positive (n = 59) and BZLF1‐negative (n = 62) groups. The expression level of ESR1 mRNA in the BZLF1‐positive group was significantly higher than that in the BZLF1‐negative group (p = 0.016; Figure 2G). These results support our hypothesis that ERα induces ZEBRA expression in NPC.
3.6.2. Association between BZLF1 expression and prognosis
Time‐to‐event data for PFS were obtained for 87 of 121 NPC samples. In total, 87 NPC cases were divided into BZLF1‐positive (n = 45) and BZLF1‐negative (n = 42) groups. A trend toward worse PFS was observed in the BZLF1‐positive group, although the difference was not statistically significant (p = 0.18; Figure S5A). Additionally, we examined the PFS for morphologically undifferentiated NPCs only (n = 36), and a similar trend was observed (p = 0.24; Figure S5B). Although we could not detect a significant difference, we hypothesized that the short follow‐up duration of patients (average 2.0 ± 0.9 years) in this study could be attributed to the lack of statistical power in this analysis. Nevertheless, it was notable that there was a trend of a relationship between worse prognosis and BZLF1 expression observed in the transcriptomic analysis, comparable with the results of our immunohistochemical analysis.
3.7. Intratumoral estradiol in NPC
ZEBRA expression was found to be associated with ERα expression through immunohistochemical and RNA‐seq analyses using publicly available data sets (Figures 1C–H and 2G). Therefore, we hypothesized that E2 was present in NPCs and stimulated the expression of ERα and ZEBRA. Therefore, to evaluate the presence of E2 and related metabolites in NPC, we performed LC–MS. For this purpose, we analyzed the E2 standard using LC–tandem MS (LC–MS/MS). LC–MS/MS analysis using a compound discoverer revealed the presence of several metabolites in the samples. Although E2 itself was not detected in any of the three NPC samples, estradiol undecylate was detected specifically in one of the three NPC samples. As shown in Figure S6A, the corresponding peak is detected at a retention time of ~16.4 min. The peak area of the NPC sample is ~10 times higher than that of the background. The protonated ion at m/z 441.33 was observed in the MS spectra (Figure S6B,C). The predicted composition of the compound discoverer software predicted the chemical formula of the molecule to be C29H44O3, among some candidates (Table S4A). Among the many candidates with C29H44O3, estradiol undecylate showed the highest score (mzlogic score) (Table S4B). Endogenous esterases convert the estradiol undecylate present in the human body into pharmacologically active E2; therefore, estradiol undecylate is considered a natural form of estrogen. 34 Moreover, the sample containing estradiol undecylate showed a high expression of ERα and ZEBRA. These results suggest that estrogen can be expressed intratumorally to induce ERα and ZEBRA expression in NPC.
3.8. Estrogen induces BZLF1 transcription in NPC cells
To clarify the causal relationship between ZEBRA and ERα upregulation, we treated EBV‐positive NPC cell lines with E2. E2 significantly induced ZEBRA expression in HK1‐EBV‐eGFP cells exposed to E2 (Figure 3A), C666‐1 cells (Figure 3B), and NPC‐TW01‐EBV‐eGFP cells (Figure 3C). E2 also induced ERα expression in all cell lines (Figures 3A–C). This result suggested that E2 stimulation in NPC cells upregulated ER, and this upregulation might be related to the induction of the ZEBRA protein. Although TPA + SB induced the expression of LMP‐1 and PD‐L1, E2 did not induce the expression of LMP‐1 or PD‐L1 (Figure S7).
FIGURE 3.
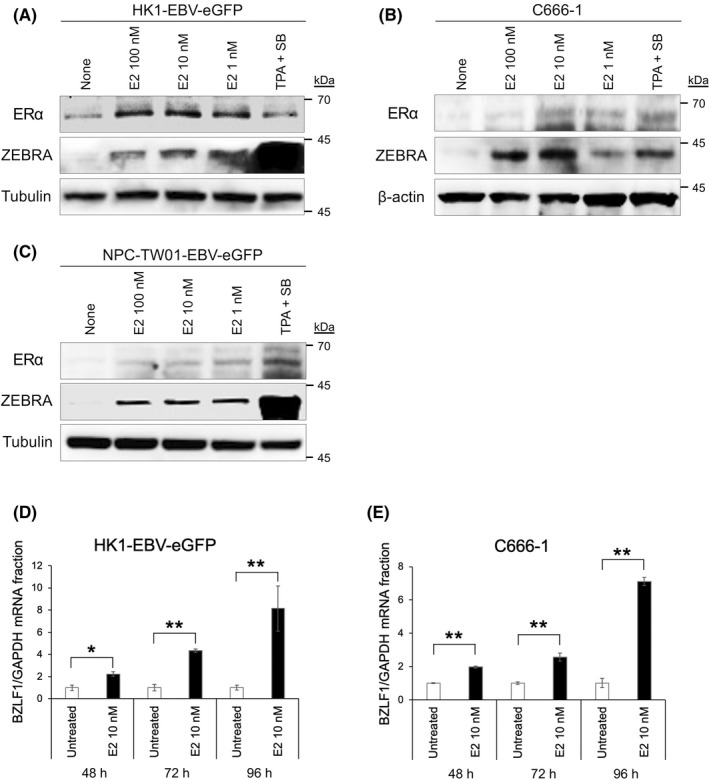
Estrogen induced the expression of ZEBRA and BZLF1 mRNA in EBV‐positive nasopharyngeal carcinoma cells. (A) Western blotting showing the ZEBRA protein levels following treatment with E2 in HK1‐EBV‐eGFP, (B) C666‐1, and (C) NPC‐TW01‐EBV‐eGFP. The quantity of the BZLF1 mRNA of (D) HK1‐EBV‐eGFP and (E) C666‐1 cells, which are untreated or treated with E2 (10 nM), are assessed using qRT‐PCR. The BZLF1 mRNA is normalized with that of GAPDH mRNA. The data are expressed as the mean ± standard error (SE). *p < 0.05; **p < 0.01
To test whether E2 could also induce the transcription of BZLF1, HK1‐EBV‐eGFP and C666‐1 cells were treated with E2 (10 nM) and subjected to qRT‐PCR analysis. Significant induction was observed in the mRNA expression levels of ZEBRA in HK1‐EBV‐eGFP (Figure 3D) and C666‐1 (Figure 3E) cells exposed to E2 (10 nM) for 48, 72, and 96 h. Additionally, as shown in Figure S8, double immunofluorescence staining for ZEBRA and ERα showed dominant ERα staining in the nuclei of each NPC cell line treated with E2 (10 nM). Moreover, ZEBRA staining occurred predominantly around the nucleus of cells treated with E2 (10 nM), which was consistent with the localization of ERα staining. These results suggest that E2 induces ERα and ZEBRA expression in EBV‐infected cells.
3.9. Estrogen activates the BZLF1 promoter, and the ZII cis‐element in BZLF1 promoter is essential for the estrogen effect of BZLF1 expression
We demonstrated that ZEBRA protein synthesis and BZLF1 mRNA production were induced by E2. These results prompted us to examine whether Zp was activated by E2 stimulation using the reporter plasmid Zp (pZp)‐luc and its derivatives, as shown in Figure 4A. E2 significantly induced WT‐Zp luciferase activity in HK1 cells (p < 0.001; Figure 4B). Next, to determine which part of the Zp requires the activation of Zp by E2, we further tested reporter vectors with mutated bZIP‐binding motifs (Figure 4C). In the absence of E2, the luciferase activities of the derivatives containing the ZII mutation (pZp‐luc mZII and pZp‐luc mZII+III) were significantly lower than those of the others, suggesting that ZII of Zp is important for Zp activation in NPC cells (Figure 4C). Moreover, E2 had little effect on the luciferase activity of the derivatives containing the ZII mutation, including pZp‐luc mZII and pZp‐luc mZII+III (Figure 4D). These results demonstrated that ZII is essential for the transcriptional activation of Zp by E2.
3.10. Interfering with estrogen and ER pathway decreases ZEBRA expression under estrogen treatment
Through immunohistochemical and RNA‐seq data analyses using publicly available data sets and E2 exposure experiments in NPC cells, we found that the pathway of E2 and ERα pathways, which are induced by E2, were important for the expression of ZEBRA. To ensure that the E2 and ERα pathways were essential for ZEBRA expression, we examined the effect of modulation and downregulation of the E2 and ERα pathways under E2 treatment on NPC cells.
HK1‐EBV‐eGFP cells were treated with selective ER modulators, including 4‐hydroxytamoxifen (4‐OHT), raloxifene, and the selective ER down regulator ICI182780, under E2 exposure. 4‐OHT, raloxifene, and ICI182780 are competitive ER antagonists that bind to ER and affect its transcriptional activity. Treatment with E2 and anti‐estrogen agents, including 4‐OHT (Figure 5A), raloxifene (Figure 5B), and ICI182780 (Figure 5C), decreased ZEBRA expression compared with that in cells treated with only E2.
FIGURE 5.
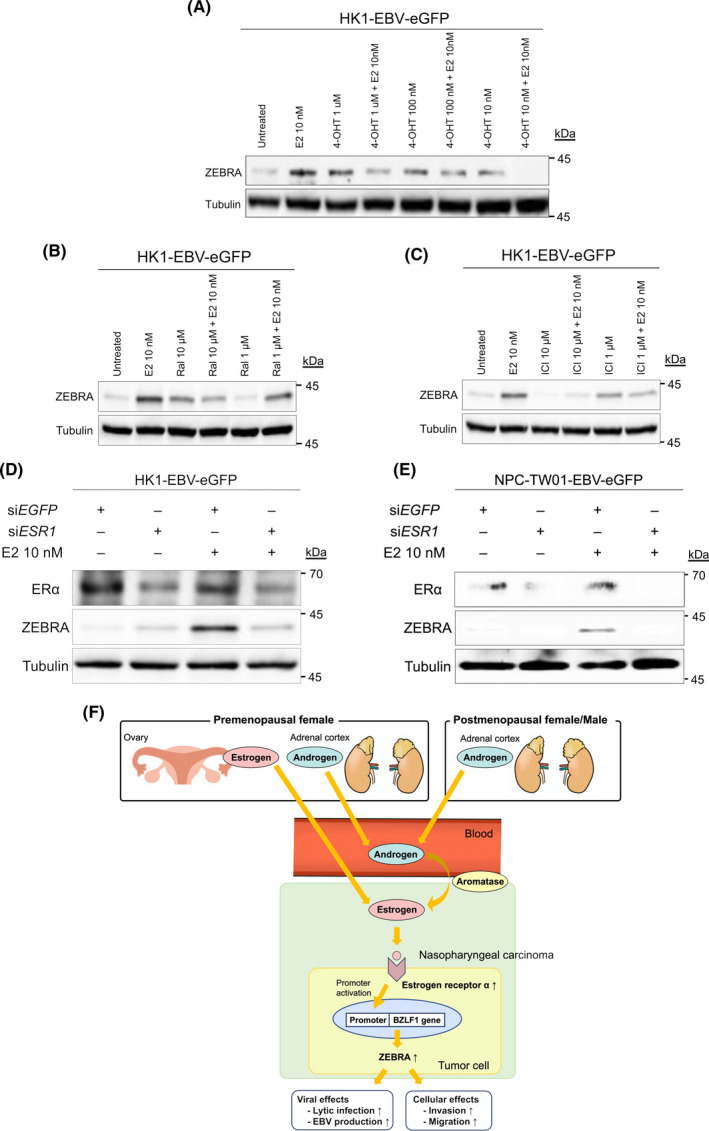
Blocking of estrogen and estrogen receptor pathway decreased ZEBRA expression under estrogen treatment. Effect of the selective estrogen receptor modulators, 4‐hydroxytamoxifen (4‐OHT), raloxifene (Ral), and the selective estrogen receptor down regulator, ICI182780 (ICI), on the expression levels of ZEBRA in HK1‐EBV‐eGFP. HK1‐EBV‐eGFP cells treated with (A) 4‐OHT, (B) Ral, and (C) ICI, in the presence of estradiol (E2). All the selective estrogen receptor modulators and the down regulator impaired the effect of E2, which induced ZEBRA expression, compared with only E2 treatment. HK1‐EBV‐eGFP (D) and NPC‐TW01‐EBV‐eGFP (E) cells were investigated to determine whether E2 could induce the expression of ZEBRA in NPC cells under the suppression of estrogen receptor α (ERα) via the ERα‐short interfering RNA (siESR1) assessed using western blotting. (F) A predictive model for the mechanism by which aromatase and estrogen induce the expression of ZEBRA and activate the lytic cycle showing the difference between men and women and between the state of premenopausal and postmenopausal
3.11. Estrogen affects ZEBRA expression via the estrogen receptor‐dependent signaling pathway
Based on the results of luciferase reporter assays of Zp and anti‐E2 treatment, we hypothesized that the activation of ERα, which is a transcription factor, is the key to Zp activation. Therefore, we verified whether ERα was necessary for E2‐mediated induction of ZEBRA using HK1‐EBV‐eGFP (Figure 5D) and NPC‐TW01‐EBV‐NeoR cells (Figure 5E) transfected with ERα‐short interfering RNA (ERα‐siESR1). Combined treatment with DMSO and ERα‐siRNA inhibited ERα protein expression; however, it did not affect ZEBRA protein expression levels. Combined treatment with E2 and ERα‐siRNA inhibited the protein expression levels of both ERα and ZEBRA to a greater extent than EGFP‐siRNA‐transfected controls (Figure 5D,E). These results suggest that the ERα pathway is essential for ZEBRA expression in response to E2 treatment.
Based on these results (Figures 1, 2, 3, 4, 5), we hypothesized a predictive model for the mechanism by which aromatase and estrogen induce ZEBRA expression (Figure 5F). In postmenopausal women and men with NPC, intratumoral aromatase converts androgens in the blood into estrogen, following which intratumorally produced estrogen is exposed to the tumor. Conversely, in premenopausal women with NPC, we hypothesized that there are two pathways of estrogen exposure to the tumor. One pathway involves the production of estrogen in the ovary, which is exposed to the tumor via the blood, and the other pathway, in which the estrogen is locally produced via aromatase, is described above. Estrogen, which is supplied intratumorally through these production pathways, activates ERα in the membranes of NPC cells. Activated ERα moves into the nucleus and activates Zp either directly or indirectly by activating other transcription factors related to Zp, which warrants further investigation.
3.12. NPC cells produce and release EBV DNA extracellularly in an estrogen dependent manner
One definition for activating the EBV lytic cycle is that progeny virus particles are produced. As shown in Figure S9, E2 also induces the expression of p18 and gp350/220, which are essential for the production of progeny viruses. To investigate whether E2 activates the EBV lytic cycle completely, we further evaluated the copy number of EBV in cells and medium to determine whether E2 produced EBV DNA and released it extracellularly from NPC cells. There was a significant increase in the quantity of EBV DNA in cells (p < 0.01; Figure 6A) and medium (p < 0.01; Figure 6B) in HK1‐EBV‐eGFP cells treated with E2 compared with that in untreated cells.
FIGURE 6.
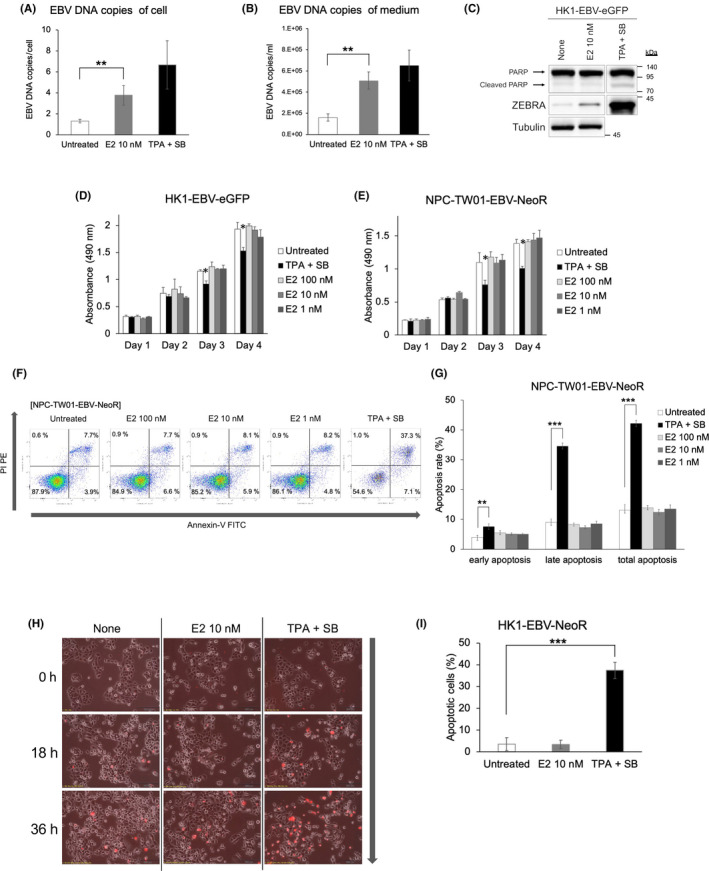
Nasopharyngeal carcinoma (NPC) cells produced EBV and extracellularly released it via E2 stimulation, and E2 did not affect cell apoptosis. EBV copy number in HK1‐EBV‐eGFP cells (A) and medium (B). The quantity of the EBV copy number in HK1‐EBV‐eGFP cells is normalized to that of the HMBS gene. (C) Western blotting showing PARP, ZEBRA, and tubulin protein levels following treatment with E2 (10 nM) in HK1‐EBV‐eGFP. Absorbance generated in the cell‐mediated MTS assays of HK1‐EBV‐eGFP (D) and NPC‐TW01‐EBV‐NeoR (E). Each cell line is untreated, treated with E2 (100, 10, and 1 nM), or treated with TPA + SB. TPA + SB was added on day 2. (F) Apoptosis assay using flow cytometry on NPC‐TW01‐EBV‐NeoR cells. Cells are labeled with annexin‐V FITC and propidium iodide (PI) PE after no treatment, E2 (100, 10, and 1 nM), or TPA + SB as positive controls. (G) Quantification of the average of three independent flow cytometry experiments in NPC‐TW01‐EBV‐NeoR cells. (H) Time‐lapse microscopy of HK1‐EBV‐NeoR cell proliferation at 0, 18, and 36 h with no treatment, E2 (10 nM), or TPA + SB. (I) Quantification of the average number of PI (red)‐positive cells, indicating apoptosis. (A, B, D, E, G, I) The data are expressed as the mean ± SD. * p < 0.05; ** p < 0.01; *** p < 0.001
3.13. Estrogen did not decrease the NPC cell viability compared with lytic inducers
Lytic inducers, such as TPA + SB, reportedly induce apoptosis. 34 , 35 , 36 Consistently, when EBV‐negative NPC‐TW01 and NPC‐TW01‐EBV‐NeoR cells were treated with TPA + SB, EBV‐infected TW01 cells showed a significant increase in apoptosis compared with that in uninfected cells (Figure S10). This suggests that EBV infection sensitizes cells to apoptosis induced by TPA + SB.
In contrast, western blotting for HK1‐EBV‐eGFP (Figure 6C) revealed that E2 did not induce the expression of PARP, an apoptotic marker. When we assessed the effect of E2 on cellular proliferation, the growth kinetics of neither HK1‐EBV‐eGFP nor NPC‐TW01‐EBV‐eGFP cells were affected by E2 treatment, whereas treatment with TPA + SB affected the growth kinetics (p < 0.05; Figure 6D,E). Consistent with these results, flow cytometry also revealed an increase in apoptotic cells in NPC‐TW01‐EBV‐NeoR cells upon TPA + SB treatment, but not with E2 (Figure 6F,G). Moreover, HK1‐EBV‐eGFP cells were treated with E2 (10 nM) or TPA + SB, and untreated cells were observed over time using time‐lapse microscopy with propidium iodide (PI) staining (Figure 6H). Although exposure to TPA + SB significantly increased apoptosis, as detected by PI staining, exposure to E2 did not increase the number of apoptotic cells (Figure 6I). These results suggest that E2 increased viral production, but it did not affect cellular proliferation or apoptosis, unlike conventional lytic inducers such as TPA + SB.
3.14. Estrogen does not interfere with the cell cycle progression of EBV‐positive tumor cells
To identify the mechanism by which the E2‐induced lytic cycle does not induce apoptosis, we performed RNA‐seq on HK1‐EBV‐eGFP cells treated with E2 or TPA + SB and subjected them to GO enrichment analysis. In TPA + SB‐treated cells, GO terms for the “mitotic cell cycle process” (GTSE1, MKI67, and CDCA5) were downregulated (Figure S11A). Moreover, several GO terms related to cell cycle progression, such as “regulation of cell cycle process,” “DNA replication,” and “positive regulation of cell cycle process” were downregulated in TPA + SB (Figure S11A). In TPA + SB‐treated cells, GO terms for the “extracellular matrix” (ANGPTL6, LRRC24, and AZGP1) were upregulated (Figure S11B). In contrast, there was no significant enrichment of GO terms in E2‐treated cells that were related to the cell cycle (Figure S11C,D). KEGG pathway analysis demonstrated that downregulated genes of HK1‐EBV‐eGFP cells treated with TPA + SB were significantly involved in the “cell cycle,” “Fanconi anemia pathway,” and “progesterone‐mediated oocyte maturation” (Figure S12A–C). The KEGG map of the cell cycle signaling pathways is shown in Figure S12D. In the KEGG map of the cell cycle, 15 genes were significantly downregulated in HK1‐EBV‐eGFP cells treated with TPA + SB. These results suggest that TPA + SB induces G1 arrest and apoptosis, which are disadvantageous for cell survival and viral replication; however, E2 can successfully circumvent these two events.
4. DISCUSSION
ZEBRA is a transcriptional activator, and its expression alone is adequate for the initiation of the entire lytic cycle. 3 Although previous studies have focused on the latency of EBV in the development of EBV‐associated malignancies, recent evidence has demonstrated the contribution of lytic infection to EBV‐mediated oncogenesis induced by ZEBRA. 1 , 2 , 37 However, the involvement of ZEBRA in NPC prognosis has not been verified. The present immunohistochemical study revealed that high expression levels of ZEBRA were significantly associated with worse PFS in patients with NPC. Although ZEBRA expression did not significantly affect the OS of patients with NPC, there was a trend for worse OS in ZEBRA‐positive patients, particularly within 5 years. As shown in Figure S3, NPC is generally susceptible to second‐line treatment, including chemotherapy and immune checkpoint inhibitors. Such second‐line treatments may have contributed to prolonging the survival of patients with recurrent NPC. Moreover, the lower number of overall deaths than anticipated might be attributable to the lack of power to detect the survival risk of NPC in this study. Additionally, there is a trend for poor prognostic value of BZLF1 expression as observed in the public RNA‐seq data analysis, although not statistically significant, which is comparable with the immunohistochemical analysis. Further studies with larger cohorts are required to verify the significance of these results.
We also discovered an association between the expression of ERα and the EBV lytic protein, ZEBRA, by assessing immunohistochemical and RNA‐seq data. Moreover, E2 induced ERα and ZEBRA expression, and lytic infection in EBV‐positive NPC cells. In lytic infections, tumor cells produce more EBV progeny. EBV production contributes to oncogenesis. 2 A complete lytic infection is essential for the generation of EBV particles. However, conventional lytic replication leads to cell death, which is a disadvantage for tumor cells. 38 Conversely, some cells adopt an incomplete (abortive) lytic infection, 39 in which they express early lytic genes that facilitate cellular proliferation and prevent cell death. However, these cells do not express late lytic genes or produce new viral particles, which is disadvantageous for EBV proliferation. Here, we found a distinct type of lytic replication in which tumor cells stimulated by estrogen express viral late lytic genes and produce virions but do not interfere with G1 arrest and apoptosis, allowing both tumor cells and viruses to coexist and proliferate. Estrogen‐induced lytic replication appears to be more advantageous for both cellular proliferation and viral production than conventional methods.
Plasma EBV‐DNA levels reportedly correlate with tumor volume and stage of EBV‐positive NPC, which is associated with poor treatment outcome. 37 , 40 , 41 , 42 , 43 Taken together with our findings that E2 induces ZEBRA and lytic replication, increasing evidence supports the speculation that elevated serum EBV‐DNA levels reflect ZEBRA expression in NPC. In an alternative and not mutually exclusive manner, it may simply reflect the volume of EBV‐positive tumors. Considering that ZEBRA expression increases invasion and migration, elevated serum EBV DNA may serve as a marker of poor prognosis. Indeed, serum EBV DNA indicates lytic infection and EBV production, 44 and elevated IgA against the EBV capsid antigen is a strong indicator for predicting NPC development in a Taiwanese cohort, 1 suggesting the importance of active EBV production for NPC development. Additionally, anti‐estrogen treatments, including aromatase inhibitors, are promising therapies for breast cancer, and this study raises the possibility that they can be applied to NPC treatment by targeting the estrogen–ERα–ZEBRA axis.
ZEBRA is also involved in the invasion and migration of tumor cells, which explains its association with the poor prognosis of NPC. Others have reported that ZEBRA induces the expression of MMP1, MMP3, and MMP9, which are associated with angiogenesis and extracellular matrix degradation. 2 , 7 , 45 , 46 Moreover, the ZEBRA protein reportedly contributes to tumorigenesis via other mechanisms such as genome instability, tumor‐promoting inflammation, and immune evasion. 47 The causal relationship and contribution of each mechanism to invasion, migration, metastasis, and survival remains to be determined.
Tissue‐specific expression of estrogen is induced by the conversion of androgens in the blood to estrogen and is mediated by the tissue‐specific expression of aromatase. Recently, the carcinogenic properties associated with estrogen overexpression, mediated by tissue‐specific expression of aromatase, have gained worldwide attention. Aromatase activity has been detected not only in gonadal tissues but also in some extragonadal tissues, including adipose tissues, skin fibroblasts, liver, and brain. 48 Local expression of aromatase is a risk factor for colon and lung cancers. 11 , 18 Moreover, we demonstrated that aromatase was expressed in the normal pharyngeal tonsil, and the expression levels of ZEBRA, ERα, and aromatase were correlated in NPC. Collectively, our study proposes a noncanonical, tumorigenic role for paracrine/intracrine estrogen, not limited to breast and uterine tumors, developed by endocrine estrogen. 49
In conclusion, we demonstrated the significance of ZEBRA expression, induced by local estrogen, in NPC development. Further studies are required to investigate the detailed mechanism of tumorigenesis and to establish novel strategies targeting the aromatase‐estrogen‐ZEBRA axis, such as anti‐hormonal medications.
AUTHOR CONTRIBUTIONS
HD, SK, and TY conceived and designed the project. HD, SK, MF, THH, MT, TN, HM, and MMK performed the experiments. HD, SK, EK, NH, TK, TU, YN, MH, KE, HS, NW, and TY contributed to sample preparation and the interpretation of the results. HD wrote the paper. SK, MF, KW, TN, AK, MM, and TY reviewed and revised the manuscript. TM, AN, KW, WPJ, NW, and SHJ were involved in planning and supervised the work. All authors reviewed the results and approved the final version of the manuscript.
DISCLOSURE
Atsushi Kaneda is currently an editorial board member in Cancer Science. The authors declare no conflicts of interest associated with this manuscript.
Supporting information
Appendix S1
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Figure S6
Figure S7
Figure S8
Figure S9
Figure S10
Figure S11
Figure S12
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
We thank Dr. Naoto Uramaru (Nihon Pharmaceutical University, Saitama, Japan) for his generous help and Dr. Mariko Murata (Mie University, Mie, Japan) and Dr. Sai‐Wah Tsao (University of Hong Kong, China) for their kind gift of cell lines. We also thank the Center for Biomedical Research and Education at Kanazawa University for use of their facilities. This work was supported by Grants‐in‐Aid from the Ministry of Education, Culture, Sports, Science and Technology (16H05480 to SK, 17H01590 to TY). We would like to thank Editage (www.editage.com) and Wiley Editing Services (www.wileyeditingservices.com) for English language editing.
Dochi H, Kondo S, Murata T, et al. Estrogen induces the expression of EBV lytic protein ZEBRA, a marker of poor prognosis in nasopharyngeal carcinoma. Cancer Sci. 2022;113:2862‐2877. doi: 10.1111/cas.15440
Division of Otolaryngology‐Head and Neck Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920‐8640, Japan: The institution at which the work was primarily conducted.
DATA AVAILABILITY STATEMENT
The accession number for the RNA‐seq data reported in this study is NCBI GEO GSE190360.
REFERENCES
- 1. Chien Y‐C, Chen J‐Y, Liu M‐Y, et al. Serologic markers of Epstein–Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345:1877‐1882. [DOI] [PubMed] [Google Scholar]
- 2. Rosemarie Q, Sugden B. Epstein–Barr virus: how its lytic phase contributes to oncogenesis. Microorganisms. 2020;8:1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joab I, Nicolas JC, Schwaab G, et al. Detection of anti–Epstein–Barr–virus transactivator (ZEBRA) antibodies in sera from patients with nasopharyngeal carcinoma. Int J Cancer. 1991;48:647‐649. [DOI] [PubMed] [Google Scholar]
- 4. Dardari R, Khyatti M, Benider A, et al. Antibodies to the Epstein‐Barr virus transactivator protein (ZEBRA) as a valuable biomarker in young patients with nasopharyngeal carcinoma. Int J Cancer. 2000;86:71‐75. [DOI] [PubMed] [Google Scholar]
- 5. Yip T‐T, Ngan R‐K, Lau W‐H, et al. A possible prognostic role of immunoglobulin‐G antibody against recombinant Epstein‐Barr virus BZLF‐1 transactivator protein ZEBRA in patients with nasopharyngeal carcinoma. Cancer. 1994;74:2414‐2424. [DOI] [PubMed] [Google Scholar]
- 6. Yoshizaki T, Miwa H, Takeshita H, Sato H, Furukawa M. Elevation of antibody against Epstein‐Barr virus genes BRLF1 and BZLF1 in nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2000;126:69‐73. [PubMed] [Google Scholar]
- 7. Yoshizaki T, Sato H, Murono S, Pagano JS, Furukawa M. Matrix metalloproteinase 9 is induced by the Epstein–Barr virus BZLF1 transactivator. Clin Exp Metastasis. 1999;17:431‐436. [DOI] [PubMed] [Google Scholar]
- 8. Murata T. Regulation of Epstein‐Barr virus reactivation from latency. Microbiol Immunol. 2014;58:307‐317. [DOI] [PubMed] [Google Scholar]
- 9. Travis RC, Key TJ. Oestrogen exposure and breast cancer risk. Breast Cancer Res. 2003;5:239‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kazmi N, Márquez‐Garbán DC, Aivazyan L, et al. The role of estrogen, progesterone and aromatase in human non–small–cell lung cancer. Lung Cancer Manag. 2012;1:259‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sato R, Suzuki T, Katayose Y, et al. Aromatase in colon carcinoma. Anticancer Res. 2012;32:3069‐3075. [PubMed] [Google Scholar]
- 12. Caiazza F, Ryan EJ, Doherty G, Winter DC, Sheahan K. Estrogen receptors and their implications in colorectal carcinogenesis. Front Oncol. 2015;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farahmand M, Monavari SH, Shoja Z, Ghaffari H, Tavakoli M, Tavakoli A. Epstein–Barr virus and risk of breast cancer: a systematic review and meta‐analysis. Future Oncol. 2019;15:28730‐28785. [DOI] [PubMed] [Google Scholar]
- 14. Jin Q, Su J, Yan D, et al. Epstein‐Barr virus infection and increased sporadic breast carcinoma risk: a meta‐analysis. Med Princ Pract. 2020;29:195‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xue SA, Lampert IA, Haldane JS, Bridger JE, Griffin BE. Epstein–Barr virus gene expression in human breast cancer: protagonist or passenger? Br J Cancer. 2003;89:113‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sabnis G, Brodie A. Aromatase and its inhibitors. In: Schwab M, ed. Encyclopedia of Cancer. Springer; 2015:6. [Google Scholar]
- 17. English MA, Kane KF, Cruickshank N, Langman MJS, Stewart PM, Hewison M. Loss of estrogen inactivation in colonic cancer. J Clin Endocrinol Metab. 1999;84:2080‐2085. [DOI] [PubMed] [Google Scholar]
- 18. Weinberg OK, Marquez‐Garban DC, Fishbein MC, et al. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65:11287‐11291. [DOI] [PubMed] [Google Scholar]
- 19. Lee H‐R, Kim T‐H, Choi K‐C. Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Lab Anim Res. 2012;28:71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kano M, Kondo S, Wakisaka N, et al. Expression of estrogen receptor alpha is associated with pathogenesis and prognosis of human papillomavirus–positive oropharyngeal cancer. Int J Cancer. 2019;145:1547‐1557. [DOI] [PubMed] [Google Scholar]
- 21. Miki Y, Suzuki T, Tazawa C, et al. Aromatase localization in human breast cancer tissues: possible interactions between intratumoral stromal and parenchymal cells. Cancer Res. 2007;67:3945‐3954. [DOI] [PubMed] [Google Scholar]
- 22. Regan MM, Viale G, Mastropasqua MG, et al. Re–evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays. J Natl Cancer Inst. 2006;98:1571‐1581. [DOI] [PubMed] [Google Scholar]
- 23. Chu P‐G, Chang K‐L, Chen Y‐Y, Chen WG, Weiss LM. No significant Association of Epstein‐Barr Virus Infection with invasive breast carcinoma. Am J Pathol. 2001;159:571‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uramaru N, Chang E‐C, Yen W‐P, Yeh MY, Juang SH, Wong FF. Synthesis and evaluation of in vitro bioactivity for polysubstituted N‐arylpyrazole derivatives. Arab J Chem. 2019;12:1908‐1917. [Google Scholar]
- 25. Murata T, Sato Y, Nakayama S, et al. TORC2, a coactivator of cAMP‐response element‐binding protein, promotes Epstein‐Barr virus reactivation from latency through interaction with viral BZLF1 protein. J Biol Chem. 2009;284:8033‐8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nitta T, Chiba A, Yamamoto N, et al. Lack of cytotoxic property in a variant of Epstein–Barr virus latent membrane protein‐1 isolated from nasopharyngeal carcinoma. Cell Signal. 2004;16:1071‐1081. [DOI] [PubMed] [Google Scholar]
- 27. Imai S, Nishikawa J, Takada K. Cell‐to‐cell contact as an efficient mode of Epstein‐Barr virus infection of diverse human epithelial cells. J Virol. 1998;72:4371‐4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kitamura K, Wang Z, Chowdhury S, Simadu M, Koura M, Muramatsu M. Uracil DNA glycosylase counteracts APOBEC3G–induced hypermutation of hepatitis B viral genomes: excision repair of covalently closed circular DNA. PLoS Pathog. 2013;9:e1003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsusaka K, Kaneda A, Nagae G, et al. Classification of Epstein–Barr virus–positive gastric cancers by definition of DNA methylation epigenotypes. Cancer Res. 2011;71:7187‐7197. [DOI] [PubMed] [Google Scholar]
- 30. Murata T, Narita Y, Sugimoto A, Kawashima D, Kanda T, Tsurumi T. Contribution of myocyte enhancer factor 2 family transcription factors to BZLF1 expression in Epstein‐Barr virus reactivation from latency. J Virol. 2013;87:10148‐10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seishima N, Kondo S, Wakisaka N, et al. EBV infection is prevalent in the adenoid and palatine tonsils in adults. J Med Virol. 2017;89:1088‐1095. [DOI] [PubMed] [Google Scholar]
- 32. Zen Y, Fujii T, Yoshikawa S, et al. Histological and culture studies with respect to ABCG2 expression support the existence of a cancer cell hierarchy in human hepatocellular carcinoma. Am J Pathol. 2007;170:1750‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanda Y. Investigation of the freely available easy–to–use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Michael O, Ekkehard S. Estrogens and Antiestrogens II. Springer; 1999. [Google Scholar]
- 35. Inman GJ, Binné UK, Parker GA, et al. Activators of the Epstein‐Barr virus lytic program concomitantly induce apoptosis, but lytic gene expression protects from cell death. J Virol. 2001;75:2400‐2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsurumi T, Fujita M, Kudoh A. Latent and lytic Epstein‐Barr virus replication strategies. Rev Med Virol. 2005;15:3‐15. [DOI] [PubMed] [Google Scholar]
- 37. Li H, Liu S, Hu J, et al. Epstein‐Barr virus lytic reactivation regulation and its pathogenic role in carcinogenesis. Int J Biol Sci. 2016;12:1309‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fitzsimmons L, Kelly GL. EBV and apoptosis: the viral master regulator of cell fate? Viruses. 2017;9:1‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shannon‐Lowe C, Adland E, Bell AI, Delecluse HJ, Rickinson AB, Rowe M. Features distinguishing Epstein‐Barr virus infections of epithelial cells and B cells: viral genome expression, genome maintenance, and genome amplification. J Virol. 2009;83:7749‐7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lertbutsayanukul C, Kannarunimit D, Prayongrat A, Chakkabat C, Kitpanit S, Hansasuta P. Prognostic value of plasma EBV DNA for nasopharyngeal cancer patients during treatment with intensity–modulated radiation therapy and concurrent chemotherapy. Radiol Oncol. 2018;52:195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu W, Chen G, Gong X, et al. The diagnostic value of EBV–DNA and EBV–related antibodies detection for nasopharyngeal carcinoma: a meta–analysis. Cancer Cell Int. 2021;21:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen Q, Hu W, Xiong H, et al. Changes in plasma EBV–DNA and immune status in patients with nasopharyngeal carcinoma after treatment with intensity–modulated radiotherapy. Diagn Pathol. 2019;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lo YM, Chan LY, Lo KW, et al. Quantitative analysis of cell‐free Epstein‐Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 1999;59:1188‐1191. [PubMed] [Google Scholar]
- 44. Shotelersuk K, Khorprasert C, Sakdikul S, Pornthanakasem W, Voravud N, Mutirangura A. Epstein‐Barr virus DNA in serum/plasma as a tumor marker for nasopharyngeal cancer. Clin Cancer Res. 2000;6:1046‐1051. [PubMed] [Google Scholar]
- 45. Lu J, Chua H‐H, Chen S‐Y, Chen JY, Tsai CH. Regulation of matrix metalloproteinase‐1 by Epstein‐Barr virus proteins. Cancer Res. 2003;63:256‐262. [PubMed] [Google Scholar]
- 46. Lan Y‐Y, Yeh T‐H, Lin W‐H, et al. Epstein‐Barr virus Zta upregulates matrix metalloproteinases 3 and 9 that synergistically promote cell invasion in vitro. PLoS One. 2013;8:e56121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Germini D, Sall FB, Shmakova A, et al. Oncogenic properties of the EBV ZEBRA protein. Cancers (Basel). 2020;12:1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harada N, Utsumi T, Takagi Y. Tissue–specific expression of the human aromatase cytochrome P–450 gene by alternative use of multiple exons 1 and promoters, and switching of tissue–specific exons 1 in carcinogenesis. Proc Natl Acad Sci USA. 1993;90:11312‐11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McNamara KM, Guestini F, Sakurai M, et al. How far have we come in terms of estrogens in breast cancer? Endocr J. 2016;63:413‐424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Figure S6
Figure S7
Figure S8
Figure S9
Figure S10
Figure S11
Figure S12
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
The accession number for the RNA‐seq data reported in this study is NCBI GEO GSE190360.


