Abstract
The KEYNOTE‐659 study evaluated the efficacy and safety of first‐line pembrolizumab plus S‐1 and oxaliplatin (SOX) (cohort 1) or S‐1 and cisplatin (SP) (cohort 2) for advanced gastric/gastroesophageal junction (G/GEJ) cancer in Japan. Herein, we update the results of cohort 1 and describe the results of cohort 2. This open‐label phase IIb study enrolled patients with advanced programmed death‐ligand 1 (PD‐L1)‐positive (combined positive score ≥ 1) human epidermal growth factor receptor 2 (HER2)‐negative G/GEJ adenocarcinoma. The primary end‐point was the objective response rate (ORR). Other end‐points were duration of response (DOR), disease control rate (DCR), progression‐free survival (PFS), overall survival (OS), and safety. One hundred patients were enrolled. In cohorts 1 and 2, median follow‐up time was 16.9 and 17.1 months; ORR (central review), 72.2% and 80.4%; DOR, 10.6 and 9.5 months; DCR (central review), 96.3% and 97.8%; median PFS (central review), 9.4 and 8.3 months; and median OS, 16.9 and 17.1 months, respectively. Treatment‐related adverse events (TRAEs) occurred in all patients, including peripheral sensory neuropathy (94.4%, cohort 1), decreased neutrophil count (82.6%, cohort 2), nausea (59.3% and 60.9% in cohorts 1 and 2), and decreased appetite (61.1% and 60.9% in cohorts 1 and 2). Grade 3 or higher TRAEs were reported by 59.3% (cohort 1) and 78.3% (cohort 2), including decreased platelet count (14.8%, cohort 1) and decreased neutrophil count (52.2%, cohort 2). Pembrolizumab in combination with SOX or SP showed favorable efficacy and safety in patients with PD‐L1‐positive, HER2‐negative G/GEJ adenocarcinoma.
Keywords: cisplatin, gastric cancer, oxaliplatin, pembrolizumab, S‐1
The KEYNOTE‐659 study was a multicenter, open‐label phase IIb study that evaluated the efficacy and safety of first‐line pembrolizumab with S‐1 + oxaliplatin (cohort 1) or S‐1 + cisplatin (cohort 2) for advanced gastric/gastroesophageal junction cancer in Japan. In cohorts 1 and 2, the median follow‐up times were 16.9 and 17.1 months, respectively; the objective response rates (primary endpoint) were 72.2% and 80.4%, respectively; and treatment‐related adverse events occurred in all patients, including peripheral sensory neuropathy (94.4%, cohort 1), decreased neutrophil count (82.6%, cohort 2), nausea (59.3% and 60.9% in cohorts 1 and 2) and decreased appetite (61.1% and 60.9% in cohorts 1 and 2). First‐line pembrolizumab with S‐1 + oxaliplatin or S‐1 + cisplatin showed favorable efficacy and manageable safety in PD‐L1‐positive, HER2‐negative advanced gastric or gastroesophageal junction adenocarcinoma in Japanese patients.

Abbreviations
- 5‐FU
5‐fluorouracil
- AE
adverse event
- ASaT
all subjects as treated
- BSA
body surface area
- CI
confidence interval
- CPS
combined positive score
- CR
complete response
- DCR
disease control rate
- DOR
duration of response
- G/GEJ
gastric/gastroesophageal junction
- HER2
human epidermal growth factor receptor 2
- KM
Kaplan–Meier
- NE
not estimable
- ORR
objective response rate
- OS
overall survival
- PD‐1
programmed cell death 1
- PD‐L
programmed death‐ligand
- PFS
progression‐free survival
- PR
partial response
- S‐1
tegafur–gimeracil–oteracil potassium
- SOX
S‐1 and oxaliplatin
- SP
S‐1 and cisplatin
- SP3
SP every 3 weeks
- TRAE
treatment‐related adverse event
- TTR
time to response
1. INTRODUCTION
Gastric cancer is the fifth most frequently diagnosed cancer worldwide and the second most common cancer in Japan. 1 , 2 In Japan, the standard first‐line chemotherapy regimen for HER2‐negative advanced G/GEJ cancer is combination therapy with platinum and fluoropyrimidine agents. 3 , 4 , 5 , 6 , 7 Of note, immune checkpoint blockade with Abs targeting the PD‐1 pathway has therapeutic benefits in several cancers, including gastric cancer. 8
Pembrolizumab (MK‐3475) is a potent, highly specific, humanized IgG4/kappa‐isotype mAb that directly inhibits the binding of PD‐1 and its ligands PD‐L1 and PD‐L2. Programmed cell death‐L1 expression or PD‐L1 CPS ≥ 1, calculated as the number of PD‐L1‐positive cells (tumor cells, macrophages, and lymphocytes) divided by the total number of cells multiplied by 100, is detected in 67%–82% of gastric cancer patients. 9 , 10 The relationship between CPS and efficacy has been shown with pembrolizumab monotherapy. 11
The KEYNOTE‐059 study evaluated the safety and efficacy of pembrolizumab plus fluoropyrimidine (5‐FU) or capecitabine and cisplatin in chemotherapy‐naïve patients with advanced gastric cancer. 12 The ORR, median PFS, and median OS were 60% (95% CI, 39%–79%), 6.6 months (95% CI, 5.9–10.6 months), and 13.8 months (95% CI, 8.6 months–not estimable), respectively. 12
In the subsequent KEYNOTE‐062 phase III study in patients with HER2‐negative advanced gastric or GEJ adenocarcinoma with PD‐L1 CPS ≥1, OS with pembrolizumab alone as first‐line therapy was noninferior to chemotherapy with 5‐FU and cisplatin or capecitabine and cisplatin. However, OS with pembrolizumab plus 5‐FU and cisplatin or capecitabine and cisplatin was not superior to 5‐FU and cisplatin or capecitabine and cisplatin in patients with PD‐L1 CPS ≥1 or PD‐L1 CPS ≥10. 11
The current study (KEYNOTE‐659) investigated the efficacy and safety of pembrolizumab in combination with S‐1 (tegafur–gimeracil–oteracil potassium) and oxaliplatin or S‐1 and cisplatin as first‐line therapy in Japanese patients with advanced G/GEJ adenocarcinoma. The results from cohort 1 (patients treated with pembrolizumab plus SOX) of this study have been reported previously and showed an ORR of 72.2% (95% CI, 58.4%–83.5%), DCR of 96.3% (95% CI, 87.3%–99.5%), PFS of 9.4 months (95% CI, 6.6 months–not evaluable), and the median OS was not reached (cut‐off June 21). 13 In this article, we report the updated results for cohort 1 (patients treated with pembrolizumab plus SOX), as well as the efficacy and safety results for cohort 2 (patients treated with pembrolizumab plus SP).
2. MATERIALS AND METHODS
2.1. Study design and patients
Details of the study design have been previously published. 13 Briefly, this nonrandomized, multicenter, open‐label, phase IIb trial (KEYNOTE‐659, NCT03382600/JapicCTI‐183829) targeted patients with advanced G/GEJ adenocarcinoma that was PD‐L1 CPS‐positive and HER2‐negative and evaluated the efficacy and safety of SOX (cohort 1) or SP (cohort 2) with pembrolizumab as first‐line treatment. Enrollment of cohort 2 commenced following completion of enrollment of cohort 1.
For cohorts 1 and 2, the key eligibility criteria were as follows: age 18 to ≤75 years; no previous chemotherapy for a histologically or cytologically confirmed advanced G/GEJ adenocarcinoma; no neoadjuvant or adjuvant therapy within 6 months of enrollment (patients who completed therapy at least 6 months prior to enrollment were eligible); CPS of ≥1 by immunohistochemistry using 22C3 PharmDx assay by central laboratory; ECOG performance status of 0 or 1; at least one measurable lesion assessed per RECIST version 1.1 by the investigator; and adequate organ function.
Main exclusion criteria included: squamous cell or undifferentiated gastric cancer; HER2‐positive status; radiotherapy within 14 days of enrollment; active central nervous system metastases and/or carcinomatous meningitis; an active autoimmune disease requiring systemic treatment in the past 2 years, excluding replacement therapy such as thyroxine, insulin, or physiologic corticosteroid replacement therapy for adrenal or pituitary insufficiency; history of (noninfectious) pneumonitis that required steroids or current pneumonitis; active infection requiring systemic therapy; and grade 2 or higher peripheral sensory neuropathy.
2.2. Treatment
Pembrolizumab 200 mg (a 30 min i.v. infusion) and oxaliplatin (130 mg/m2 i.v.) or cisplatin (60 mg/m2 i.v.) were given on day 1 of each 3‐week cycle and S‐1 was given orally twice daily for the first 2 weeks of each 3‐week cycle at a dose of 40 mg for a BSA < 1.25 m2, 50 mg for a BSA from 1.25 to <1.5 m2, and 60 mg for a BSA ≥1.5 m2. As prophylactic treatment for nausea and vomiting, patients were managed with palonosetron plus aprepitant or fosaprepitant. Additionally, the use of steroids was allowed for oxaliplatin or cisplatin‐associated antiemetic purposes.
Treatment with pembrolizumab plus chemotherapy was continued until disease progression, unacceptable AEs, withdrawal of consent, or until the patient had received 35 treatments with pembrolizumab.
Patients who discontinued SOX (cohort 1), SP (cohort 2), or pembrolizumab could continue receiving pembrolizumab monotherapy, S‐1 monotherapy, oxaliplatin (cohort 1), or cisplatin (cohort 2), respectively, if they had not met the discontinuation criteria.
2.3. Assessments
Computed tomography was carried out at baseline within 21 days prior to enrollment. Subsequent tumor imaging was carried out every 6 weeks (42 days ± 7 days) or more frequently if clinically indicated.
Tumor response was assessed according to RECIST version 1.1 by central review as well as by the investigator. Tumor imaging to confirm PR or CR was carried out at least 4 weeks after the first indication of response was observed. In clinically stable patients, disease progression was confirmed based on iRECIST between 4 and 8 weeks after the first progression. 14
2.4. End‐points
The primary end‐point was the ORR assessed by central review according to RECIST version 1.1. The secondary end‐points were DOR, DCR, TTR, PFS, OS, and safety. The DOR was defined as the time from the date of first response (CR or PR) to the date of disease progression or death, TTR as the time from the date of enrollment to the first date of CR or PR, PFS as the time from the date of enrollment to the first documented disease progression or death due to any cause, whichever occurred first, and OS as the time from the date of enrollment to death due to any cause. For safety, AEs were monitored throughout the trial and graded in severity according to the guidelines outlined in the NCI’s Common Terminology Criteria for Adverse Events version 4.0. Subgroup analyses of OS and event rate according to PD‐L1 CPS status (<10 and ≥10) were included as exploratory end‐points.
2.5. Statistical analysis
The target sample size was 90 patients, comprising 45 patients in cohort 1 and 45 patients in cohort 2, to account for a drop‐off rate before treatment of 10%. No specific hypotheses were tested in this study. However, the planned sample sizes were considered appropriate to allow the estimation of the effect of pembrolizumab with SOX or SP according to the point estimate and 95% CI by the exact binomial method: if 28 of 40 patients achieved an objective response, the ORR was estimated to be 70.0% with a CI of 53.5%–83.4%.
The ASaT population, which consisted of all patients who received at least one dose of study treatment, was used to analyze ORR, DCR, TTR, PFS, OS, and safety. A total of 80 patients (pembrolizumab plus SOX group [cohort 1], 40 patients; pembrolizumab plus SP group [cohort 2], 40 patients) were planned to be included in the ASaT population. The analysis population for DOR consisted of responders with a best response of CR or PR in the ASaT population. For the primary end‐point (ORR), 95% CIs were calculated using the exact binomial method proposed by Clopper and Pearson. 15 For DOR, PFS, TTR, and OS, KM curves and median estimates from the KM curves were provided as appropriate. All statistical analyses were undertaken using SAS version 9.4 or later (SAS Institute Inc.). Follow‐up time was defined as the number of days from enrollment to the last survival follow‐up date for censored subjects or the number of days from the date of enrollment to the date of death.
3. RESULTS
3.1. Patient characteristics
First, 54 patients were enrolled in cohort 1 from April 2018 to September 2018. Subsequently, 46 patients were enrolled in cohort 2 from September 2018 to June 2019. All patients received study treatment and were included in the ASaT population. The data cut‐off date for safety and efficacy analyses was May 30, 2021, with a median follow‐up time of 16.9 months in cohort 1 and 17.1 months in cohort 2.
In cohort 1, 42 patients were discontinued due to disease progression (40 had radiological disease progression, and two had clinical disease progression). Three patients discontinued due to TRAEs (enteritis, pancreatitis/pancreatic pseudocyst, and cardiac arrest). Additionally, nine patients discontinued for other reasons, including six patients who completed 35 pembrolizumab treatments and two patients who underwent surgery for curative intent. Only one patient was lost to follow‐up. In cohort 2, 35 patients discontinued due to disease progression: 31 had radiological disease progression, and four had clinical disease progression. Three patients discontinued due to an AE: two had treatment‐related rash, and one had pancreatitis. One patient withdrew from treatment. Seven patients discontinued for other reasons, including three patients who completed 35 pembrolizumab treatments and three patients who underwent surgery for curative intent.
The baseline demographic and clinical characteristics of patients are shown in Table 1. More than half of the population were men (79.6% in cohort 1, 60.9% in cohort 2), the median age was 66.0 and 65.0 years in cohorts 1 and 2, respectively, and CPS was ≥10 in 31 (57.4%) and 27 (58.7%) patients in cohorts 1 and 2, respectively.
TABLE 1.
Baseline demographic and clinical characteristics of Japanese patients with gastric cancer treated with first‐line pembrolizumab with S‐1 + oxaliplatin (cohort 1) or S‐1 + cisplatin (cohort 2) (all subjects as treated population)
| Characteristic | Cohort 1 (N = 54) | Cohort 2 (N = 46) |
|---|---|---|
| Age, years, median (range) | 66.0 (32–75) | 65.0 (30–75) |
| Sex | ||
| Male | 43 (79.6) | 28 (60.9) |
| Female | 11 (20.4) | 18 (39.1) |
| ECOG PS (screening phase) | ||
| 0 | 46 (85.2) | 35 (76.1) |
| 1 | 8 (14.8) | 11 (23.9) |
| Metastatic disease (at initial diagnosis) | ||
| No | 5 (9.3) | 7 a (15.2) |
| Yes | 49 (90.7) | 39 (84.8) |
| Primary location | ||
| Gastric | 46 (85.2) | 40 (87.0) |
| Gastroesophageal junction | 8 (14.8) | 6 (13.0) |
| Histological type | ||
| Intestinal | 21 (38.9) | 20 (43.5) |
| Diffuse | 32 (59.3) | 24 (52.2) |
| Unknown | 1 (1.9) | 2 (4.3) |
| Number of metastatic sites (central review) | ||
| <3 | 37 (68.5) | 33 (71.7) |
| ≥3 | 17 (31.5) | 13 (28.3) |
| History of surgery | ||
| No | 49 (90.7) | 35 (76.1) |
| Yes | 5 (9.3) | 11 (23.9) |
| CPS | ||
| 1–9 | 23 (42.6) | 19 (41.3) |
| ≥10 | 31 (57.4) | 27 (58.7) |
Note: Unless otherwise stated, all data are n (%). Data cut‐off: May 30, 2021.
Abbreviations: CPS, combined positive score; PS, performance status.
Includes one patient for whom the presence of metastasis was unknown at initial diagnosis.
3.2. Treatment delivery
In cohorts 1 and 2, the median treatment duration of pembrolizumab was 6.0 and 5.1 months, respectively. The median treatment duration of SOX (cohort 1) was 4.9 months and the median treatment duration of SP (cohort 2) was 4.4 months. The actual and relative dose intensities for each drug, along with the cumulative doses, are shown in Table 2. In cohort 1, 35 (64.8%) patients underwent a dose reduction of S‐1, 47 (87.0%) patients underwent a dose reduction of oxaliplatin, 44 (81.5%) patients had a dose interruption of S‐1, and 31 (57.4%) patients had a dose interruption of oxaliplatin. In cohort 2, 33 (71.7%) patients underwent a dose reduction of S‐1, 43 (93.5%) patients underwent a dose reduction of cisplatin, 29 (63.0%) patients had a dose interruption of S‐1, and 22 (47.8%) patients had a dose interruption of cisplatin. In cohorts 1 and 2, 72.2% and 84.8% of patients, respectively, received subsequent chemotherapy. The common regimens were paclitaxel‐based regimen (63.0% in cohort 1, 69.6% in cohort 2), irinotecan‐based regimen (27.8% in cohort 1, 19.6% in cohort 2), trifluridine and tipiracil (22.2% in cohort 1, 19.6% in cohort 2), and nivolumab (22.2% in cohort 1, 10.9% in cohort 2).
TABLE 2.
Cumulative dose and dose intensity for each drug (cohort 1: pembrolizumab with S‐1 + oxaliplatin; cohort 2: pembrolizumab with S‐1 + cisplatin) among Japanese patients with gastric cancer (all subjects as treated population)
| Cohort 1 (N = 54) | Cohort 2 (N = 46) | |
|---|---|---|
| Pembrolizumab | ||
| Cumulative dose (mg) | ||
| Median (range) | 1700.0 (400–7000) | 1600.0 (400–7000) |
| Actual dose intensity (mg/week) a | ||
| Mean (SD) | 57.8 (9.1) | 55.7 (10.8) |
| Median | 59.8 | 57.4 |
| Relative dose intensity (%)b | ||
| Mean (SD) | 86.8 (13.6) | 83.5 (16.2) |
| Median | 89.7 | 86.1 |
| S‐1 | ||
| Cumulative dose (mg) | ||
| Median (range) | 11130.0 (2180–55,380) | 9940.0 (1500–40,460) |
| Actual dose intensity (mg/m2/week) a | ||
| Mean (SD) | 234.5 (51.6) | 223.7 (72.4) |
| Median | 240.8 | 229.4 |
| Relative dose intensity (%) b | ||
| Mean (SD) | 71.6 (17.1) | 67.6 (22.3) |
| Median | 72.2 | 68.4 |
| Oxaliplatin | ||
| Cumulative dose (mg/m2) | ||
| Median (range) | 688.6 (230–1633) | NA |
| Actual dose intensity (mg/m2/week) a | ||
| Mean (SD) | 24.2 (10.5) | NA |
| Median | 25.8 | NA |
| Relative dose intensity (%) b | ||
| Mean (SD) | 56.0 (24.2) | NA |
| Median | 59.6 | NA |
| Cisplatin | ||
| Cumulative dose (mg/m2) | ||
| Median (range) | NA | 289.5 (109–719) |
| Actual dose intensity (mg/m2/week) a | ||
| Mean (SD) | NA | 11.2 (5.4) |
| Median | NA | 11.4 |
| Relative dose intensity (%) b | ||
| Mean (SD) | NA | 56.1 (27.2) |
| Median | NA | 57.0 |
Abbreviations: BSA, body surface area; NA, not applicable.
Actual dose intensity calculated as:
Pembrolizumab (mg/week): cumulative dose (mg)/(total treatment duration [weeks]).
S‐1 (mg/m2/week): (cumulative dose during the study (mg)/BSA (at baseline) (m2))/(total treatment duration [weeks]).
Oxaliplatin/cisplatin (mg/m2/week): (cumulative dose during the study (mg)/BSA (at baseline) (m2))/(total treatment duration [weeks]).
Relative dose intensity (%) calculated as:
Pembrolizumab: dose intensity (mg/week)/(first amount of actual dose (mg)/3) × 100.
S‐1: dose intensity (mg/m2/week)/((first amount of actual dose (mg) × 2 × 14/BSA at baseline (m2))/3) × 100.
Oxaliplatin/cisplatin: dose intensity (mg/m2/week)/((first amount of actual dose (mg)/BSA at baseline (m2))/3) × 100.
3.3. Efficacy
The ORR assessed by central review was 72.2% (95% CI, 58.4%–83.5%; 39/54 patients) in cohort 1, and 80.4% (95% CI, 66.1%–90.6%; 37/46 patients) in cohort 2 (Table 3). Overall, 52 (96.3%) of 54 patients in cohort 1 and 44 (95.7%) of 46 patients in cohort 2 showed any tumor shrinkage from baseline (Figure 1). DCR, as assessed by central review, was 96.3% (95% CI, 87.3%–99.5%; 52 of 54 patients) in cohort 1 and 97.8% (95% CI, 88.5%–99.9%; 45 of 46 patients) in cohort 2 (Table 3).
TABLE 3.
Best overall response and survival results among Japanese patients with gastric cancer treated with first‐line pembrolizumab with S‐1 + oxaliplatin (cohort 1) or S‐1 + cisplatin (cohort 2) (all subjects as treated population), assessed by central review per RECIST version 1.1
| Category | Cohort 1 (N = 54) | Cohort 2 (N = 46) | ||
|---|---|---|---|---|
| n | (95% CI) | n | (95% CI) | |
| Objective response rate (CR + PR) | 39 | 72.2% (58.4%–83.5%) | 37 | 80.4% (66.1%–90.6%) |
| Disease control rate (CR + PR + SD) | 52 | 96.3% (87.3%–99.5%) | 45 | 97.8% (88.5%–99.9%) |
| Best overall response, n (%) | ||||
| Complete response | 2 (3.7) | 4 (8.7) | ||
| Partial response | 37 (68.5) | 33 (71.7) | ||
| Stable disease | 13 (24.1) | 8 (17.4) | ||
| Progressive disease | 2 (3.7) | 1 (2.2) | ||
| Progression‐free survival, months, median | 9.4 (6.6–12.6) | 8.3 (5.8–15.3) | ||
| Overall survival, months, median | 16.9 (13.4–20.0) | 17.1 (12.6–23.1) | ||
| Time to response, months, median | 1.5 | 1.5 | ||
| Duration of response, months, median | 10.6 | (5.6–NE) | 9.5 | (4.7–15.3) |
Note: Data cut‐off: May 30, 2021.
Abbreviations: CI, confidence interval; CR, complete response; NE, not estimable; PR, partial response; SD, stable disease.
FIGURE 1.
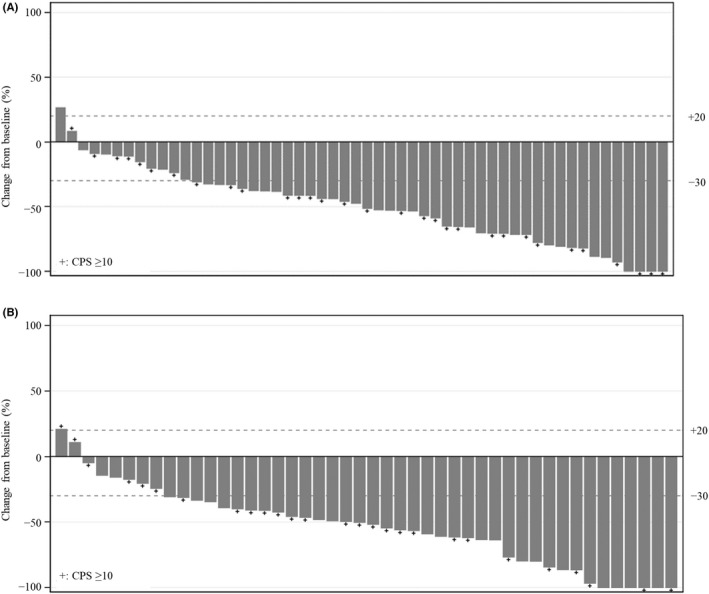
Overall tumor response in Japanese patients with gastric cancer treated with (A) first‐line pembrolizumab with S‐1 + oxaliplatin (cohort 1) or (B) first‐line pembrolizumab with S‐1 + cisplatin (cohort 2), assessed by central review according to RECIST version 1.1. Best change from baseline in the sum of the longest target lesion diameter per patient by programmed cell death‐ligand 1 expression. CPS, combined positive score
In cohorts 1 and 2, respectively, the median PFS assessed by central review was 9.4 (95% CI, 6.6–12.6) months and 8.3 (95% CI, 5.8–15.3) months (Figure 2). Median OS was 16.9 (95% CI, 13.4–20.0) months in cohort 1 and 17.1 (95% CI, 12.6–23.1) months in cohort 2 (Figure 3). Median DOR was 10.6 (95% CI, 5.6–NE) months in cohort 1 and 9.5 (95% CI, 4.7–15.3) months in cohort 2, and median TTR was 1.5 months in both cohorts (Table 3). The updated results in cohort 1 for ORR, DCR, median TTR, and PFS (by central review) did not differ from the previously reported results. 13 At the previous data cut‐off (June 21, 2019), the median DOR and OS were not reached.
FIGURE 2.
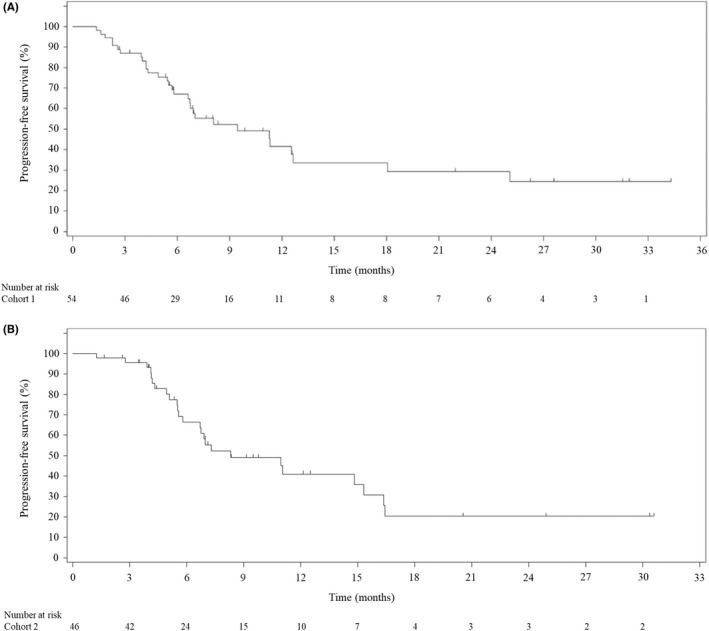
Kaplan–Meier estimates of progression‐free survival in Japanese patients with gastric cancer treated with (A) first‐line pembrolizumab with S‐1 + oxaliplatin (cohort 1) or (B) first‐line pembrolizumab with S‐1 + cisplatin (cohort 2), assessed by central review
FIGURE 3.

Kaplan–Meier estimates of overall survival in Japanese patients with gastric cancer treated with (A) first‐line pembrolizumab with S‐1 + oxaliplatin (cohort 1) or (B) first‐line pembrolizumab with S‐1 + cisplatin (cohort 2)
Tumor response assessed by the investigator is shown in Table 4. In cohort 1, ORR was 72.2% (95% CI, 58.4%–83.5%), DCR was 94.4% (95% CI, 84.6%–98.8%), median PFS was 6.9 (5.6–8.3) months, and there was no difference from previously reported results. In cohort 2, ORR was 63.0% (95% CI, 47.5%–76.8%), DCR was 97.8% (95% CI, 88.5%–99.9%), and median PFS was 6.7 (5.3–8.4) months.
TABLE 4.
Best overall response and survival results among Japanese patients with gastric cancer treated with first‐line pembrolizumab with S‐1 + oxaliplatin (cohort 1) or S‐1 + cisplatin (cohort 2) (all subjects as treated population), assessed by investigator per RECIST version 1.1
| Category | Cohort 1 (N = 54) | Cohort 2 (N = 46) | ||
|---|---|---|---|---|
| n | (95% CI) | n | (95% CI) | |
| Objective response rate (CR + PR) | 39 | 72.2% (58.4%–83.5%) | 29 | 63.0% (47.5%–76.8%) |
| Disease control rate (CR + PR + SD) | 51 | 94.4% (84.6%–98.8%) | 45 | 97.8% (88.5%–99.9%) |
| Best overall response, n (%) | ||||
| Complete response | 2 (3.7) | – | 1 (2.2) | – |
| Partial response | 37 (68.5) | – | 28 (60.9) | – |
| Stable disease | 12 (22.2) | – | 16 (34.8) | – |
| Progressive disease | 3 (5.6) | – | 1 (2.2) | – |
| Progression‐free survival, months, median | – | 6.9 (5.6–8.3) | – | 6.7 (5.3–8.4) |
| Overall survival, months, median | – | 16.9 (13.4–20.0) | – | 17.1 (12.6–23.1) |
Note: Data cut‐off: May 30, 2021.
Abbreviations: CI, confidence interval; CR, complete response; PR, partial response; SD, stable disease.
In an exploratory analysis of OS according to CPS status, OS in cohort 1 was 14.9 (95% CI, 9.5–19.1) months in patients with a CPS ≥10 and 17.7 (95% CI, 13.4–23.7) months in those with a CPS <10. In cohort 2, OS was 15.5 (95% CI, 11.5–22.9) months in patients with a CPS ≥10 and 21.7 (95% CI, 8.3–NE) months in those with a CPS <10. Similarly, the median PFS in cohort 1 was 8.1 (95% CI, 5.5–12.6) months in patients with a CPS ≥10 and 12.6 (95% CI, 6.6–NE) months in those with a CPS <10. The median PFS in cohort 2 was 7.0 (95% CI, 5.1–NE) months in patients with a CPS ≥10 and 14.8 (95% CI, 5.8–16.4) months in those with a CPS <10. Kaplan–Meier event rates according to CPS are shown in Figures 4 and 5.
FIGURE 4.

Kaplan–Meier estimates of progression‐free survival in Japanese patients with gastric cancer treated with (A) first‐line pembrolizumab with S‐1 + oxaliplatin (cohort 1) or (B) first‐line pembrolizumab with S‐1 + cisplatin (cohort 2) according to combined positive score (CPS), assessed by central review
FIGURE 5.
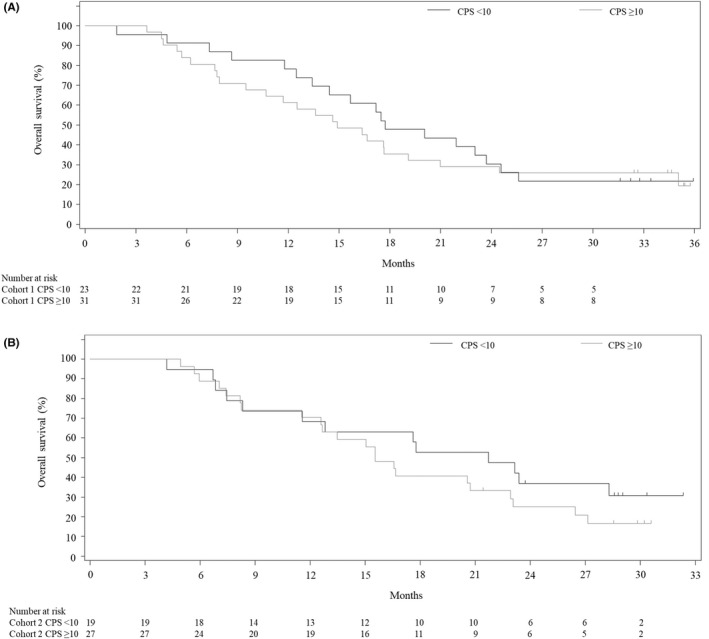
Kaplan–Meier estimates of overall survival in Japanese patients with gastric cancer treated with (A) first‐line pembrolizumab with S‐1 + oxaliplatin (cohort 1) or (B) first‐line pembrolizumab with S‐1 + cisplatin (cohort 2) according to combined positive score (CPS)
3.4. Safety
Overall, TRAEs of any grade occurred in all patients in this study. The incidence of TRAEs that occurred in at least 10% of patients in this study are presented in Table 5; the most common were peripheral sensory neuropathy (51 [94.4%] in cohort 1), decreased neutrophil count (38 [82.6%] in cohort 2), nausea (32 [59.3%] in cohort 1 and 28 [60.9%] in cohort 2), and decreased appetite (33 [61.1%] in cohort 1 and 28 [60.9%] in cohort 2). Grade 3 or higher TRAEs were reported in 32 (59.3%) patients in cohort 1, and 36 (78.3%) patients in cohort 2 (Table 5). Decreased neutrophil count was the most frequently reported TRAE of grade 3 or higher, with 8 (14.8%) patients in cohort 1 and 24 (52.2%) in cohort 2. The most common reason for SP dose reduction was decreased neutrophil count. Serious TRAEs were reported in 18 (33.3%) patients in cohort 1 and 17 (37.0%) patients in cohort 2. The most common serious TRAE was adrenal insufficiency (3 [5.6%] in cohort 1 and 3 [6.5%] in cohort 2). One patient had a cardiac arrest in cohort 1, and this event was considered a treatment‐related death.
TABLE 5.
Incidence of treatment‐related adverse events (TRAEs) occurring in at least 10% of patients and TRAEs of interest among Japanese patients with gastric cancer treated with first‐line pembrolizumab with S‐1 + oxaliplatin (cohort 1) or S‐1 + cisplatin (cohort 2) (all subjects as treated population)
| TRAE, n (%) | Cohort 1 (N = 54) | Cohort 2 (N = 46) | ||
|---|---|---|---|---|
| Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | |
| Any event | 54 (100.0) | 32 (59.3) | 46 (100.0) | 36 (78.3) |
| Neutrophil count decreased a | 24 (44.4) | 8 (14.8) | 38 (82.6) | 24 (52.2) |
| Decreased appetite | 33 (61.1) | 1 (1.9) | 28 (60.9) | 4 (8.7) |
| Nausea | 32 (59.3) | 0 (0.0) | 28 (60.9) | 1 (2.2) |
| Constipation | 10 (18.5) | 0 (0.0) | 22 (47.8) | 0 (0.0) |
| Diarrhea | 21 (38.9) | 2 (3.7) | 20 (43.5) | 0 (0.0) |
| Anemia | 9 (16.7) | 3 (5.6) | 15 (32.6) | 7 (15.2) |
| Stomatitis | 14 (25.9) | 0 (0.0) | 15 (32.6) | 0 (0.0) |
| Dysgeusia | 19 (35.2) | 0 (0.0) | 14 (30.4) | 0 (0.0) |
| Malaise | 20 (37.0) | 0 (0.0) | 13 (28.3) | 0 (0.0) |
| Peripheral sensory neuropathy | 51 (94.4) | 2 (3.7) | 12 (26.1) | 0 (0.0) |
| Platelet count decreased b | 29 (53.7) | 8 (14.8) | 11 (23.9) | 1 (2.2) |
| Fatigue | 11 (20.4) | 0 (0.0) | 11 (23.9) | 1 (2.2) |
| Vomiting | 9 (16.7) | 1 (1.9) | 10 (21.7) | 0 (0.0) |
| White blood cell count decreased | 7 (13.0) | 1 (1.9) | 9 (19.6) | 5 (10.9) |
| Rash c | 16 (29.6) | 0 (0.0) | 11 (23.9) | 2 (4.3) |
| Aspartate aminotransferase increased | 9 (16.7) | 0 (0.0) | 7 (15.2) | 1 (2.2) |
| Hiccups | 2 (3.7) | 0 (0.0) | 7 (15.2) | 0 (0.0) |
| Hyponatremia | 0 (0.0) | 0 (0.0) | 6 (13.0) | 4 (8.7) |
| Blood creatinine increased | 1 (1.9) | 0 (0.0) | 6 (13.0) | 0 (0.0) |
| Skin hyperpigmentation | 3 (5.6) | 0 (0.0) | 6 (13.0) | 0 (0.0) |
| Edema peripheral | 4 (7.4) | 0 (0.0) | 5 (10.9) | 0 (0.0) |
| TRAEs of interest, n (%) | ||||
| Adrenal insufficiency | 3 (5.6) | 3 (5.6) | 3 (6.5) | 2 (4.3) |
| Hypothyroidism | 5 (9.3) | 0 (0.0) | 3 (6.5) | 0 (0.0) |
| Hyperthyroidism | 2 (3.7) | 0 (0.0) | 2 (4.3) | 0 (0.0) |
| Colitis d | 6 (11.1) | 4 (7.4) | 1 (2.2) | 0 (0.0) |
| Pneumonitis e | 4 (7.4) | 1 (1.9) | 1 (2.2) | 0 (0.0) |
| Type 1 diabetes mellitus | 1 (1.9) | 1 (1.9) | 1 (2.2) | 1 (2.2) |
Note: Data cut‐off: May 30, 2021.
Includes neutropenia.
Includes thrombocytopenia.
Includes rash maculopapular, butterfly rash, and rash papular.
Includes autoimmune colitis, enterocolitis, and enteritis.
Includes pneumonia, interstitial lung disease, and autoimmune lung disease.
4. DISCUSSION
In this study, the efficacy and safety of combination therapy with first‐line pembrolizumab plus SOX or SP was similar in both cohorts of patients with advanced G/GEJ adenocarcinoma. KEYNOTE‐659 is the first study to evaluate the anti‐PD‐1 Ab and SP combination. In patients who received SP3 in the S‐1 Optimal Schedule (SOS) study, the ORR was 59.7% (114 of 191 patients), median PFS was 5.5 (95% CI, 4.7–6.6) months, and median OS was 14.1 (95% CI, 11.4–15.8) months. 16 In this study, the ORR (80.4% [95% CI, 66.1%–90.6%]), median PFS (8.3 [range, 5.8–15.3] months), and median OS (17.1 [range, 12.6–23.1] months) with pembrolizumab plus SP3. Although cross‐study comparisons should be interpreted with caution, pembrolizumab in combination with SP is expected to show promising antitumor effects compared with SP due to possible add‐on antitumor effects of pembrolizumab for patients with PD‐L1 expression.
The updated results (data cut‐off date: May 30, 2021) for cohort 1 (pembrolizumab plus SOX: ORR of 72.2%, DCR of 96.3%, median PFS of 9.4 months by central review, median OS of 16.9 months, with a median follow‐up of 16.9 months) showed no major differences from the results of cohort 2. For OS, no major differences were observed in the two cohorts even after considerable follow‐up results were collected.
Regarding the safety profile of pembrolizumab in combination with SP, decreased neutrophil count (grade ≥ 3) was more frequently reported in cohort 2 (52.2%) than in cohort 1 (14.8%) (Table 5). Overall, the most frequently reported TRAEs in cohort 2 were anemia (32.6%), diarrhea (43.5%), nausea (60.9%), and fatigue (23.9%), and the respective incidences in cohort 1 were 16.7%, 38.9%, 59.3%, and 20.4%. By contrast, grade ≥3 platelet count decreased (including thrombocytopenia, 14.8%) and peripheral sensory neuropathy (3.7%) were more frequently reported in cohort 1 than in cohort 2 (2.2% and 0.0%, respectively). Study discontinuation because of rash was reported in two patients in this study, but treatment discontinuation due to rash was not reported in cohort 1. 13 Grade 3 colitis was observed in three patients (5.6%) in cohort 1, 13 but no grade ≥3 colitis was observed in cohort 2. The other TRAEs of interest were not notably different from those reported with pembrolizumab monotherapy. 10 , 11
Although SP is usually given every 5 weeks in Japan, in this study, SP3 treatment matched the timing of pembrolizumab treatment (every 3 weeks). In addition, the efficacy and safety of SP3 have previously been confirmed in the SOS study, a phase III study in Japan and Korea that investigated SP3 as first‐line therapy in patients with advanced gastric cancer. 16 The incidence and profile of AEs in this study are consistent with the SP3 safety profile reported in the SOS study, showing no novel safety concern with the combination of SP3 with pembrolizumab. 10 , 11
Although the initial dose of oxaliplatin (130 mg/m2) was higher than that in the G‐SOX study (100 mg/m2), an increase of hematological toxicities was not observed in this study. The relative dose intensity for oxaliplatin in this study (59.6%) was lower than that in the G‐SOX study (79.0%). 17 These toxicities could be managed by appropriate dose reduction or interruption. For first‐line therapy in gastric cancer, the main platinum agents used are cisplatin and oxaliplatin. It has been suggested that oxaliplatin increases the effect of immune checkpoint inhibitors by induction of immunogenic cell death. 18 , 19 In our study, regimens that included two different platinum agents were investigated, but no difference was suggested between cohorts by platinum agent. Although there are some differences in the safety profiles of the platinum agents, both SOX and SP could be candidates for use in pembrolizumab combination regimens.
In the KEYNOTE‐062 study, no statistically significant benefit of the combination of pembrolizumab and 5‐FU and cisplatin or capecitabine and cisplatin was shown among patients with PD‐L1 CPS ≥1. 11 However, nivolumab, another anti‐PD‐1 Ab, showed superiority in OS to FOLFOX (leucovorin, fluorouracil, and oxaliplatin) or CAPOX (capecitabine and oxaliplatin) in the CheckMate 649 study. 9 Similarly, the ORIENT‐16 study, which evaluated sintilimab and chemotherapy as first‐line treatment for advanced gastric cancer in China, reported statistically significant OS benefits with sintilimab in combination with chemotherapy in participants with CPS ≥5, and in all randomized participants. 20 In contrast, in Asia, the combination of nivolumab plus SOX or capecitabine and oxaliplatin was evaluated for all‐comers in the ATTRACTION‐4 study, 21 and prolonged PFS, but not OS, was observed with nivolumab.
The present study findings cannot be directly compared because the results were affected not only by the differences in platinum agents but also by differences in target populations, including PD‐L1 expression status, post‐study treatment, and statistical analysis methods. The target population of this study consisted of patients with PD‐L1 CPS ≥1. Exploratory analyses did not show enrichment of efficacy in CPS ≥10, although it is difficult to assess the survival benefit of pembrolizumab because of the single‐arm study design. Similar results were observed in this study to those of KEYNOTE‐062, in which no difference by CPS status was observed in the cohort that received combination therapy. 11 In contrast, superiority of OS was shown in patients with CPS ≥1 and CPS ≥5, but not with CPS <1 or CPS <5 in the CheckMate 649 study, a global study with a larger sample size using 28‐8 PharmDx PD‐L1 assays. 9 The survival benefit of pembrolizumab and the impact of CPS will be further evaluated in the ongoing trial, KEYNOTE‐859 (NCT03675737), a randomized, multicenter, double‐blind study of first‐line pembrolizumab plus chemotherapy 5‐FU plus cisplatin or capecitabine plus oxaliplatin versus placebo plus chemotherapy for patients with advanced G/GEJ cancer.
The present study had several limitations. It was a single‐arm phase II study, and we were unable to confirm the additional effects of pembrolizumab on SOX or SP. Therefore, further evaluation is required to confirm the results of combination therapy with pembrolizumab plus chemotherapy as a first‐line treatment in patients with gastric cancer.
In conclusion, the combination of pembrolizumab plus SOX or SP showed favorable efficacy and manageable safety as first‐line treatment in Japanese patients with PD‐L1‐positive, HER2‐negative G/GEJ adenocarcinoma.
DISCLOSURE
KY declares research grants received by their institution from Taiho Pharma, Yakult Honsha, and Ono Pharma., and has personally received honoraria from Taiho Pharma, Daiichi Sankyo, Eli Lilly Japan, Ono Pharma, and Bristol Myers Squibb. DS declares research grants received by their institution from Chugai Pharmaceutical Co., Ltd, Yakult Honsha Co., Ltd, Ono Pharmaceutical Co., Ltd, and Eli Lilly and Co., and has personally received honoraria from Chugai Pharmaceutical Co., Ltd, and Daiichi Sankyo Co., Ltd. TN has received honoraria from Ono Pharma, Taiho Pharma, Chugai Pharma, Daiichi Sankyo, and Bristol Myers Squibb. YO has received grants from Ono Pharmaceutical, Five Prime, Daiichi Sankyo, Astellas, and BeiGene. HK has received grants from Chugai Pharmaceutical Co., Ltd, Bristol‐Myers Squibb Co. Ltd, Kobayashi Pharmaceutical Co., Ltd, and Eisai Co., Ltd, and consulting fees from Daiichi Sankyo Co., Ltd, Bristol Myers Squibb Co. Ltd, MSD K.K., Eli Lilly Japan K.K., Ono Pharmaceutical Co., Ltd, and Taiho Pharmaceutical Co., Ltd, and honoraria from Bristol Myers Squibb Co. Ltd, Daiichi Sankyo Co., Ltd, Ono Pharmaceutical Co., Ltd, MSD K.K., Chugai Pharmaceutical Co., Ltd, Taiho Pharmaceutical Co., Ltd, Merck Biopharma Co., Ltd, Takeda Pharmaceutical Co., Ltd, Yakult Pharmaceutical Industry, GlaxoSmithKline K.K., and Bayer Yakuhin Ltd. TE declares research grants received by their institution from MSD, Novartis, Sumitomo Dainippon, Ono, Daiichi Sankyo, Astellas, Astellas Amgen Biopharma, IQVIA, Quintiles, Parexel, and Merck Serono, and has personally received honoraria from Taiho, Chugai, Ono, Eli Lilly, MSD, Daiichi Sankyo, Sanofi, Bayer, Merck Serono, and Takeda. NS has received honoraria from Chugai, Taiho, and Eli Lilly. KKa has received consulting fees from Ono, Roche, BMS, Bayer, BeiGene/Novartis, and AstraZeneca, honoraria from Ono and BMS, and has participated in advisory meetings for Ono, AstraZeneca, MSD, and BMS. KA has received grants from MSD, Nippon Zoki Pharmaceutical, Taiho Pharmaceutical, Hisamitsu Pharmaceutical, Daiichi Sankyo. TTsu has received honoraria from Takeda, Taiho Pharmaceutical, Ono Pharmaceutical, Eli Lilly Japan, Yakult Honsha, and MSD. KS declares research grants received by their institution from Astellas Pharma, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharma, Merck Pharmaceutical, Mediscience, Amgen, and Eisai, has personally received honoraria from Bristol Myers Squibb and Takeda Pharmaceutical, and is on monitoring/advisory boards for Eli Lilly and Company, Bristol Myers Squibb, Takeda Pharmaceutical, Pfizer Inc., Ono Pharmaceutical, Novartis, AbbVie Inc., Daiichi Sankyo, Taiho Pharmaceutical, Merck Pharmaceutical, GlaxoSmithKline, Amgen, Boehringer Ingelheim, and Janssen. SH, SS, and TTa are employees of MSD K.K, Tokyo, Japan. KM, MT, SI, TO, HH, KKo, HY, YN, and KI have no conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol by an institutional review board: This study was approved by the institutional review board/independent ethics committee at each of the 25 study centers. It was carried out in accordance with the ethical principles outlined in the Declaration of Helsinki, International Conference on Harmonization Technical Requirements for Registration of Pharmaceuticals for Human Use – Good Clinical Practice Guidelines, and all applicable federal, state, and local laws, rules, and regulations relating to the conduct of the clinical study.
CONSENT
All patients provided written informed consent before enrollment
ACKNOWLEDGMENTS
The authors thank the patients, their families, and caregivers for participating in this study, and the medical advisor for this study, Professor Takako Eguchi Nakajima of Kyoto University Graduate School of Medicine. Medical writing assistance was provided by Nila Bhana, MSc (Hons), of Edanz (www.edanz.com), which was funded by Taiho Pharmaceutical, in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Yamaguchi K, Minashi K, Sakai D, et al. Phase IIb study of pembrolizumab combined with S‐1 + oxaliplatin or S‐1 + cisplatin as first‐line chemotherapy for gastric cancer. Cancer Sci. 2022;113:2814‐2827. doi: 10.1111/cas.15462
Funding information
This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Taiho Pharmaceutical Co., Ltd, Tokyo, Japan, provided the S‐1 and collaborated in the development of this study.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov (NCT03382600)/JAPIC Clinical Trials (JapicCTI‐183829).
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Cancer Information Service, National Cancer Center, Japan . Cancer registry and statistics. 2018. (in Japanese). https://ganjoho.jp/reg_stat/statistics/stat/summary.html. Accessed August 30, 2021.
- 3. National Comprehensive Cancer Network . NCCN clinical practice guidelines in Oncology (NCCN Guidelines). Gastric Cancer Version 4. 2020. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed September 15, 2021.
- 4. Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;275:v38‐v49. [DOI] [PubMed] [Google Scholar]
- 5. Muro K, Van Cutsem E, Narita Y, et al. Pan‐Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic gastric cancer: a JSMO–ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:19‐33. [DOI] [PubMed] [Google Scholar]
- 6. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2018, 5th edition. Gastric Cancer. 2020;24:1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sawaki A, Yamada Y, Yamaguchi K, et al. Regional differences in advanced gastric cancer: exploratory analyses of the AVAGAST placebo arm. Gastric Cancer. 2018;21:429‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janjigian YY, Shitara K, Moehler M, et al. First‐line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro‐oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open‐label, phase 3 trial. Lancet. 2021;398:27‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro‐oesophageal junction cancer (KEYNOTE‐061): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2018;392:123‐133. [DOI] [PubMed] [Google Scholar]
- 11. Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first‐line, advanced gastric cancer: the KEYNOTE‐062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bang YJ, Kang YK, Catenacci DV, et al. Pembrolizumab alone or in combination with chemotherapy as first line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II non‐randomized KEYNOTE‐059 study. Gastric Cancer. 2019;22:828‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawazoe A, Yamaguchi K, Yasui H, et al. Safety and efficacy of pembrolizumab in combination with S‐1 plus oxaliplatin as a first‐line treatment in patients with advanced gastric/gastroesophageal junction cancer: cohort 1 data from the KEYNOTE‐659 phase IIb study. Eur J Cancer. 2020;129:97‐106. [DOI] [PubMed] [Google Scholar]
- 14. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143‐e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404‐416. [Google Scholar]
- 16. Ryu MH, Baba E, Lee KH, et al. Comparison of two different S‐1 plus cisplatin dosing schedules as first‐line chemotherapy for metastatic and/or recurrent gastric cancer: a multicenter, randomized phase III trial (SOS). Ann Oncol. 2015;26:2097‐2101. [DOI] [PubMed] [Google Scholar]
- 17. Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S‐1 with cisplatin plus S‐1 in chemotherapy‐naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141‐148. [DOI] [PubMed] [Google Scholar]
- 18. Zhu H, Shan Y, Ge K, Lu J, Kong W, Jia C. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol (Dordr). 2020;43:1203‐1214. [DOI] [PubMed] [Google Scholar]
- 19. Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482‐491. [DOI] [PubMed] [Google Scholar]
- 20. Xu J, Jiang H, Pan Y, et al. Sintilimab plus chemotherapy (chemo) versus chemo as first‐line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT‐16): first results of a randomized, double‐blind, phase III study. Ann Oncol. 2021;32:S1283‐S1346. [Google Scholar]
- 21. Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2‐negative, untreated, unresectable advanced or recurrent gastric or gastro‐oesophageal junction cancer (ATTRACTION‐4): a randomised, multicentre, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234‐247. [DOI] [PubMed] [Google Scholar]


