Abstract
Elevated adenosine generated by CD73 (ecto‐5′‐nucleotidase; NT5E) could boost immunosuppressive responses and promote immune evasion in the tumor microenvironment. However, despite the immune response, CD73 could also promote tumor progression in a variety of cancers, and the nonimmunologic role and corresponding molecular mechanism of CD73 involved in head and neck squamous cell carcinoma (HNSCC) progression are not well characterized. Here, we demonstrated that CD73/NT5E is overexpressed in HNSCC tissues and predicts poor prognosis. Suppression of CD73 inhibited the proliferation, migration, and invasion of HNSCC cell lines (CAL27 and HN4) in vitro and in vivo. Gene set variation analysis (GSVA) and gene set enrichment analysis (GSEA) predicted that CD73 may be involved in invadopodia formation and MAPK signaling activation. As expected, knockdown of CD73 inhibited the MAPK signaling pathway, and the suppressive effect of CD73 knockdown on proliferation, migration, invasion, and invadopodia formation was reversed by a MAPK signaling activator. Our results suggest that CD73 could promote the proliferation, migration, invasion, and invadopodia formation of HNSCC via the MAPK signaling pathway and provide new mechanistic insights into the nonimmunological role of CD73 in HNSCC.
Keywords: CD73, EMT, HNSCC, invadopodia, MAPK signaling pathway
Despite immunomodulating role, corresponding molecular mechanism of CD73 involved in HNSCC progression are not well characterized. In this work, We found that CD73 was up‐regulated in HNSCC tissues and could serve as independent prognostic factor. Based on the result of GSEA and GSVA, we found that CD73 take part in the invadopodia formation of HNSCC and activation of MAPK signaling pathway may account for the tumor promoting role of CD73.

Abbreviations
- ECM
extracellular matrix
- EMT
epithelial‐mesenchymal‐transition
- ERKs
extracellular signal–regulated kinases
- GPI
glycosylphosphatidylinositol
- GSEA
gene set enrichment analysis
- GSVA
gene set variation analysis
- HNSCC
head and neck squamous cell carcinoma
- HOK
human oral keratinocytes
- HR
hazard ratio
- IHC
immunohistochemistry
- JKLOD
Jiangsu Key Laboratory of Oral Disease
- JNKs
Jun amino terminal kinases
- MAPK
mitogen‐activated protein kinase
- MDSCs
myeloid‐derived suppressor cells
- NT5E
ecto‐5′‐nucleotidase
- p38/SAPKs
stress‐activated protein kinases
- TME
tumor microenvironment
- Treg
regulatory T cells
1. INTRODUCTION
Head and neck cancer (HNC) was the world's seventh most common cancer in 2018, with most cases being squamous cell carcinoma arising from mucosal surfaces of four major anatomical sites: the oral cavity, sinonasal cavity, larynx, and pharynx. 1 Head and neck squamous cell carcinoma (HNSCC) is a global health burden due to its high incidence and poor prognosis. There are approximately 600,000 new cases worldwide, with 40‐50% mortality. 2 , 3 Current primary modalities for the treatment of HNSCC are surgery, radiotherapy, and concurrent chemoradiation. Accounting for distinct anatomical subgroups, invasive properties, and cervical lymph node metastasis of the tumors, the prognosis of this malignancy is still frustrating despite employment of combined treatment. 4 , 5 Therefore, further investigation into the underlying mechanism is of great help to improve the prognosis of HNSCC patients.
Ecto‐5′‐nucleotidase (CD73), encoded by the NT5E gene, is a 70‐kD glycosylphosphatidylinositol (GPI) protein anchored on the cell surface that can function in enzymatic or nonenzymatic pathways. 6 , 7 As an AMP hydrolyzing enzyme, CD73 is involved in the conversion of AMP into phosphate and adenosine, which play important roles in immunomodulation in the tumor microenvironment (TME). 7 , 8 In addition to enzymatic function, CD73 interacts directly with extracellular matrix (ECM) components and is engaged in T‐cell signal transduction and cell adhesion. 9 CD73 has been reported to influence cancer progression in both enzymatic and nonenzymatic pathways. 7 Ample evidence has shown that CD73 is overexpressed in many cancers, such as breast cancer, non–small cell lung cancer, gastric cancer, colorectal cancer, ovarian cancer, and gallbladder cancer. Furthermore, CD73 has been found to be linked to the clinical characteristics and prognosis of cancer patients and plays a critical role during tumor progression. 6 , 10 , 11 , 12 , 13 Recently, it was reported that the expression of CD73 is upregulated in clinical HNSCC tissues and that CD73 expression in HNSCC correlated positively with tumor stage and poor prognosis. However, whether CD73 could facilitate HNSCC progression and the underlying molecular mechanism still needs further elucidation. 10 , 14
Metastasis is the major reason for cancer‐related death. 15 Invasion and penetration of ECM, or other tissue barriers, is a prerequisite in cancer metastasis. 16 Invadopodia, dynamic actin‐rich protrusions in cancer cells, specialize in degrading ECM, thus facilitating tumor metastasis. 15 , 17 Signaling, such as growth factor and integrins, could stimulate F‐actin assembly, which is modulated by structural proteins, such as N‐WASP, TKS4, TKS5, and cortactin, priming the formation of invadopodia. Proteases, such as MT1‐MMP, MMP2, and MMP9, are especially enriched in invadopodia and released to drill the ECM. 17
Mitogen‐activated protein kinase (MAPK) signaling pathways constitute a complex and delicate network modulating cellular biological processes such as proliferation, differentiation, and cell survival. 18 , 19 As a result, aberrant MAPK signal transduction is involved in a variety of human diseases, especially cancer. 20 The MAPK cascade mainly consists of three crucial kinases, MAPK3K, MAPKK, and MAPK, which work through sequential phosphorylation. 20 There are three distinctive cascades of MAPKs: extracellular signal–regulated kinases (ERKs), Jun amino terminal kinases (JNKs), and stress‐activated protein kinases (p38/SAPKs). ERKs tend to be activated by growth factors or mitogens and are responsible for proliferation, survival, differentiation, metabolism, nervous system development, and immune response, while p38 MAPKs can respond to multiple inflammatory and stressful factors and participate in inflammation, proliferation, differentiation, apoptosis, and invasion. Similarly, JNKs are usually activated by stress factors such as oxidative stress and radiation and are responsible for inflammation, apoptosis, cytokine secretion, and metabolism. 21 , 22 , 23
In this study, we analyzed the clinical implication of CD73 in HNSCC. Additionally, we performed GSEA and GSVA for CD73 to predict the biological function of CD73 and the corresponding signaling pathway, which was further verified by in vitro and in vivo experiments.
2. MATERIALS AND METHODS
The materials and methods are described in Appendix S1.
3. RESULTS
3.1. CD73/NT5E expression was significantly upregulated in HNSCC and predicted poor prognosis
To explore the expression difference of CD73/NT5E between HNSCC tissues and adjacent normal tissues, we first extracted the mRNA quantification of NT5E in the transcriptomic sequence dataset of our JKLOD cohort, including 81 pairs of oral squamous cell carcinoma tissues and adjacent normal tissues. The results showed that NT5E was significantly upregulated in oral squamous cell carcinoma tissues (Figure 1A, p < 0.0001). The high expression of NT5E in HNSCC tissue was also confirmed in the TCGA‐HNSCC cohort, which included 502 HNSCC tissues and 44 adjacent normal tissues (Figure 1B p < 0.0001). In addition to transcriptome analysis, we also found that the protein level of CD73 was robustly upregulated in HNSCC tissues in 10 pairs of normal and tumor tissues (Figure 1C), which was further verified by IHC (Figure 1D).
FIGURE 1.

CD73/NT5E is overexpressed and predicts poor prognosis in head and neck squamous cell carcinoma (HNSCC). A, Expression of NT5E in HNSCC tissues (n = 81) compared with paired adjacent normal tissues (n = 81) in the Jiangsu Key Laboratory of Oral Disease (JKLOD) cohort and the significance of difference was evaluated by paired t test. B, Expression of NT5E in HNSCC tissues (n = 502) compared with adjacent normal tissues (n = 44) in the TCGA‐HNSCC cohort and the significance of difference was evaluated by unpaired t test. C, Western blot detecting the expression of CD73 in 10 pairs of HNSCC tissues and adjacent normal tissues. D, Immunohistochemistry (IHC) staining of CD73 in HNSCC tissues and adjacent normal tissues. E, F, Kaplan‐Meier curves for the overall survival and relapse‐free survival of 502 patients in the TCGA‐HNSCC cohort divided into NT5E_high and NT5E_low groups according to NT5E expression. HR, hazard ratio. G, H, Kaplan‐Meier curves for the overall survival and disease‐free survival of 122 patients divided into NT5E_high and NT5E_low groups according to CD73 expression by IHC staining. I, Multivariate Cox regression analysis of CD73 by IHC score along with clinical prognostic parameters for 122 HNSCC patients. ns, not significant; *p < 0.05, **p < 0.01, and ***p < 0.001
Furthermore, Kaplan=Meier survival analysis was employed to determine the prognostic value of NT5E in patients with HNSCC. At the transcriptome level, the expression of NT5E was correlated with poor overall survival probability (Figure 1E, HR = 1.53; log‐rank p = 0.0017) and short relapse‐free survival time (Figure 1F, HR = 1.66; log‐rank p = 0.0079) in the TCGA‐HNSCC cohort. In addition, we scored CD73 expression for IHC of 122 HNSCC tissues with prognostic information in the JKLOD cohort. Similarly, the protein level of CD73 could also predict poor overall survival time (Figure 1G, HR = 3.16; log‐rank p = 2.4 × 10−5) and short progression‐free survival (Figure 1H, HR = 1.66; log‐rank p = 2.4 × 10−5). The clinical feature of CD73 was also investigated in the JKLOD cohort. It turned out that higher CD73 expression is associated with later pathological staging and it may be correlated with later T staging or N staging, while the sample size could not make the difference significant (Figure S1). Multivariate Cox regression analysis involving clinical features further indicated that CD73/NT5E is an independent prognostic factor for HNSCC (Figure 1I, HR = 3.01, p < 0.001).
3.2. CD73 promotes the proliferation, migration, and invasion of HNSCC cells
To investigate the functional role of CD73 in HNSCC progression, first we detected the expression level of CD73 in a normal human oral keratinocytes (HOK) and four other HNSCC cell lines (HSC3, FADU, HN4, CAL27). The results showed that the expression of CD73 was upregulated in all four HNSCC cell lines compared with that in HOK cells and was highest in HN4 cells (Figure 2A). Then, HN4, a human laryngeal squamous cell carcinoma cell line, and CAL27, a commonly used human tongue squamous cell carcinoma cell line, were chosen for further study. Both cell lines were transfected with three candidate small interfering RNAs (siRNAs) targeting CD73/NT5E and a negative control siRNA. Two siRNAs with higher interfering efficiency, named si‐CD73‐01 and si‐CD73‐02, were selected (Figure 2B).
FIGURE 2.

Depletion of CD73 inhibits the proliferation, migration, and invasion of head and neck squamous cell carcinoma (HNSCC) cells. A, Western blot detecting the expression of CD73 in HNSCC cell lines and HOK cells. B, Western blot detecting the expression of CD73 in CAL27 and HN4 cells transfected with si‐NC and si‐CD73. C, D, CCK‐8 assay estimating the viability of CAL27 and HN4 cells after transfection with si‐NC and si‐CD73. E, F, EdU assay estimating the proliferation ratio of CAL27 and HN4 cells after transfection with si‐NC and si‐CD73. G, Colony‐formation assay estimating the colony‐formation ability of CAL27 and HN4 cells after transfection with si‐NC and si‐CD73. H, J, Wound‐healing assay estimating the migration ability of CAL27 and HN4 cells after transfection with si‐NC and si‐CD73. I, K, Transwell migration and invasion assays estimating the migration and invasion ability of CAL27 and HN4 cells after transfection with si‐NC and si‐CD73. L, Western blot detecting the expression of EMT‐related and proliferation‐related markers in CAL27 and HN4 cells after transfection with si‐NC and si‐CD73. ns, not significant; *p < 0.05, **p < 0.01, and ***p < 0.001
To determine the role of CD73 in HNSCC progression, we first performed a CCK‐8 assay. Downregulating the expression of CD73 significantly suppressed the viability of HN4 and CAL27 cells (Figure 2C and D). Meanwhile, the EdU assay showed that CD73‐knockdown HNSCC cell lines exhibited a reduced proliferation ratio (Figure 2E,F), and the colony formation experiment showed that depletion of CD73 significantly decreased the number of colonies formed (Figure 2G). These results strongly indicated that CD73 could promote the proliferation of HNSCC cells. Furthermore, wound‐healing assays (Figure 2H,J) and transwell assays (Figure 2I,K, Figure S2A,B) showed that depletion of CD73 also impaired the aggressive potential of HNSCC cells in migration and invasion. Finally, Western blot detection of proliferation markers (PCNA) and the epithelial‐mesenchymal‐transition (EMT) markers (E‐cadherin, N‐cadherin, vimentin, and Snail) in CD73‐depleted CAL27 and HN4 cells also demonstrated compromised proliferation and EMT phenotypes (Figure 2L). Meanwhile, we also overexpressed CD73 in CAL27 and HN4 and repeated all the above phenotype experiment. As expected, overexpression of CD73/NT5E significantly increased proliferation, migration, and invasion of CAL27 and HN4 (Figure S3).
Taken together, these data strongly indicated that CD73 could promote the proliferation, migration, and invasion of HNSCC cells.
3.3. CD73 promotes invadopodia formation in HNSCC cells
With the role of CD73 in HNSCC cell malignancy confirmed, we next investigated the specific biological processes in which CD73 is involved. First, a transcriptome sequence dataset of 502 tumor tissues in the TCGA‐HNSCC cohort was downloaded. Utilizing the GSVA algorithm, we scored 9996 gene sets of biological processes for each sample. Then, Spearman correlation analysis was employed to calculate the correlation between NT5E/CD73 expression and the score of each biological process. The results showed that “invadopodium” and “invadopodium membrane” were among the top 10 correlated biological processes (Figure 3A). In addition, we divided the 502 samples into two groups based on the expression of NT5E/CD73 and analyzed the biological processes enriched in the group with high NT5E/CD73 expression. Invadopodium‐ and cytoskeletal regulation–related biological processes were highly enriched (Figure 3B).
FIGURE 3.

Depletion of CD73 inhibits invadopodia formation in head and neck squamous cell carcinoma (HNSCC) cells. A, Correlation analysis between NT5E expression and gene set variation analysis (GSVA) scores of biological processes. The top 10 correlated biological processes with Spearman correlation coefficients are shown in the left panel, and the correlation dot plot of NT5E and GSVA score of “invadopodium” is shown in the right panel. B, Gene set enrichment analysis (GSEA) results of invadopodia‐related biological processes. C, Western blot detecting the expression of invadopodia‐related markers in CAL27 and HN4 cells transfected with si‐NC and si‐CD73. D, Double fluorescence staining with phalloidin to label F‐actin and anti‐cortactin antibody for si‐NC– or si‐CD73–transfected CAL27 and HN4 cells. E, Fluorescent gelatin degradation assay evaluating the invasion ability of invadopodia in si‐NC– or si‐CD73–transfected CAL27 and HN4 cells. ns, not significant; *p < 0.05, **p < 0.01, and ***p < 0.001
To test the correlation between CD73 and invadopodia formation, we performed Western blotting to detect the changes in key proteins involved in invadopodia formation upon CD73 knockdown, including WASP, NWASP, and MMP14 (Figure 3C). 17 Interestingly, all invadopodia‐related markers were downregulated after suppression of CD73 in CAL27 and HN4 cells. Then, we performed double fluorescence staining with phalloidin to label F‐actin and anti‐cortactin antibody for si‐NC– or si‐CD73–transfected CAL27 and HN4 cells. Colocalization of intensive phalloidin staining and cortactin, which could regulate dynamic actin assembly, represents pseudopodia formation (Figure 3D). The colocalization region of phalloidin and anti‐cortactin antibody evidently decreased in si‐CD73–transfected CAL27 and HN4 cells compared with those transfected with si‐NC, indicating decreased pseudopodia formation by CD73 knockdown. Furthermore, to quantify the influence of CD73 knockdown on the invasion ability of HNSCC cells, we performed a fluorescent‐gelatin degradation assay as previously described. In brief, CAL27 or HN4 cells were seeded on cell culture plates coated with Oregon Green 488–conjugated gelatin. The fluorescence intensity of gelatin was measured after 24 hours of incubation. The proteolysis region of the gelatin invaded by CAL27 and HN4 fused into pieces, and the invaded gelatin measured by fluorescence intensity was significantly reduced after CD73 knockdown (Figure 3E). Also, we repeated the above experiments after overexpression of CD73 in CAL27 and HN4. It turned out that CAL27 and HN4 with higher CD73 expression showed even more vigorous invadopodia (Figure S4).
These results further indicated that CD73 may boost HNSCC malignancy by facilitating invadopodia formation.
3.4. CD73 could activate the MAPK signaling pathway
Our results support that CD73 can promote HNSCC progression in many ways; however, the potential molecular mechanism is largely unknown. First, bioinformatic analysis was employed to predict the possible relevant pathways. RNA sequencing data of the TCGA‐HNSCC cohort were divided into two groups according to the mRNA expression of CD73/NT5E. KEGG pathways enriched in the NT5E_high group were calculated utilizing the GSEA algorithm. The results showed that “Pathways in cancer” and “MAPK signaling pathway” were significantly enriched (Figure 4A,B). In addition, genes related to the MAPK signaling pathway showed differential expression between the NT5E_high and NT5E_low groups (Figure 4C).
FIGURE 4.
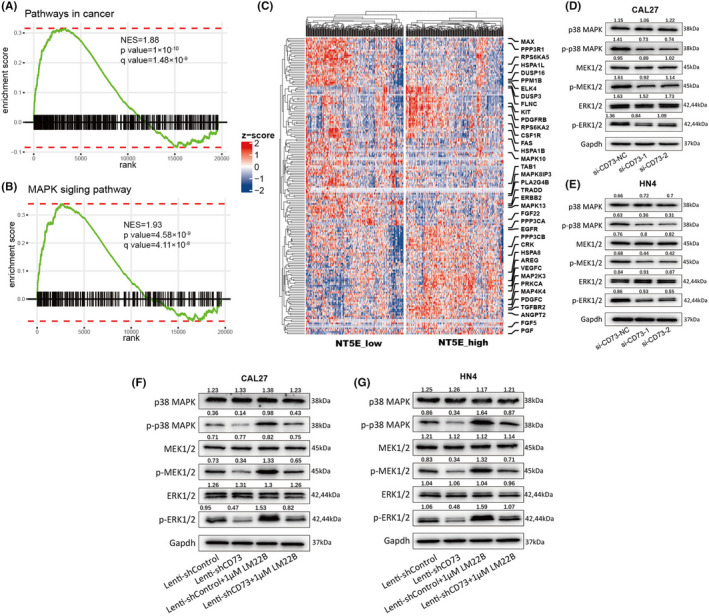
CD73 activates MAPK signaling pathway. A, B, Gene set enrichment analysis (GSEA) plots showing that “pathway in cancer” and “MAPK signaling pathway” were highly enriched in the NT5E_high groups. C, Heatmap showing the expression of genes in the MAPK signaling pathway in the NT5E_high and NT5E_low groups. D, E, Western blot analysis of the expression of markers in the MAPK signaling pathway in CAL27 and HN4 cells transfected with si‐NC and si‐CD73. F, G, Western blot detecting the expression of markers in the MAPK signaling pathway in CAL27 and HN4 cells with combined treatment of CD73 knockdown and LM22B
Then, we detected by immunoblotting the expression of key proteins in the MAPK signaling pathway affected by CD73 interference. Our results showed that knockdown of CD73 could inhibit the phosphorylated forms of p38 MAPK, MEK1/2, and ERK1/2 but barely influence the expression level of total p38 MAPK, MEK1/2, and ERK1/2 in CAL27 and HN4 cells (Figure 4D,E). Additionally, the changes in these markers of the MAPK signaling pathway could be reversed by LM22B, which could activate the MAPK signaling pathway. Therefore, we concluded that CD73 could activate the MAPK signaling pathway (Figure 4F,G).
3.5. CD73 boosts HNSCC malignancy via the MAPK signaling pathway
As the MAPK signaling pathway could be activated by CD73, we wondered whether it is responsible for the phenotypic change caused by CD73. First, we constructed Lenti‐shControl and Lenti‐shCD73 cell lines for CAL27 and HN4 with control lentivirus and lentivirus targeting CD73/NT5E. The lenti‐shCD73 cell line showed a decreased number of colonies, and the MAPK signaling pathway activator compared with the lenti‐shControl cell line LM22B reversed the effect of CD73 knockdown on colony formation (Figure 5A,B). LM22B also reversed the compromised migration and invasion ability of CAL27 and HN4 cell lines by lenti‐shCD73, which was verified via wound‐healing assay and transwell invasion assay (Figure 5C–F, Figure S2E,F). Additionally, activation of the MAPK signaling pathway with LM22B promoted invadopodia formation and reversed the inhibitory effect on invadopodia by lenti‐shCD73 in both CAL27 and HN4 cell lines (Figure 5G,H). Finally, the immunoblotting assay was employed to detect the change in specific markers upon Lenti‐shControl or Lenti‐shCD73 transfection and LM22B treatment. Lenti‐shCD73, similar to si‐CD73, increased the expression of E‐cadherin and decreased the expression of N‐cadherin, vimentin, Snail, Ki67, PCNA, WASP, NWASP, and MP‐MMP, indicating compromised EMT phenotype, proliferation ratio, and invadopodia formation, while these markers showed opposite changes upon LM22B treatment compared with Lenti‐shCD73 transfection. Furthermore, LM22B treatment reversed the change in these markers caused by lenti‐shCD73 (Figure 5I,J). In view of the above, we concluded that CD73 could boost HNSCC malignancy via the MAPK signaling pathway.
FIGURE 5.
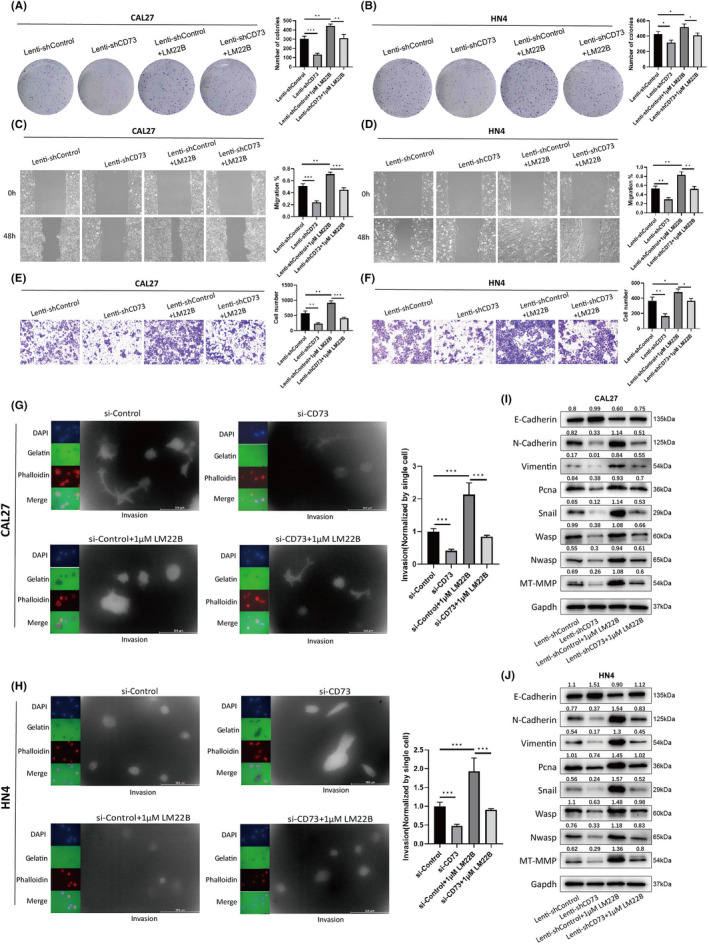
CD73 promotes proliferation, migration, invasion, and invadopodia formation via the MAPK signaling pathway. A, B, Colony‐formation assay estimating the colony‐formation ability of CAL27 and HN4 cells with combined treatment of CD73 knockdown and LM22B. C, D,) Wound‐healing assay estimating the migration ability of CAL27 and HN4 cells with combined treatment of CD73 knockdown and LM22B. E, F,) Transwell migration and invasion assays estimating the migration and invasion ability of CAL27 and HN4 cells with combined treatment of CD73 knockdown and LM22B. G, H, Fluorescent gelatin degradation assay evaluating the invasion ability of invadopodia in CAL27 and HN4 cells with combined treatment of CD73 knockdown and LM22B. I, J, Western blot detecting the expression of markers related to EMT phenotype, proliferation ratio, and invadopodia formation in CAL27 and HN4 cells with combined treatment of CD73 knockdown and LM22B. ns, not significant; *p < 0.05, **p < 0.01, and ***p < 0.001
3.6. Knockdown of CD73 inhibits tumor growth and lung metastasis in vivo
To determine the effect of CD73 in vivo, we established xenograft and lung metastasis models with Lenti‐shControl and Lenti‐shCD73 cell lines. Three weeks after subcutaneous injection, the xenograft model was sacrificed, and the volume and weight of the tumor were measured. Tumors in the CD73 knockdown group showed decreased tumor size and tumor volume compared with those in the control group, indicating that CD73 may promote the proliferation of HNSCC cells in vivo (Figure 6A–C). For the lung metastasis model, mice were sacrificed 8 weeks after tail intravenous injection. Then, we counted the number of lung metastatic lesions as detected by H&E staining and measured the weight of the lung for both the Lenti‐shControl and Lenti‐shCD73 groups. Knockdown of CD73 significantly decreased the number of lung metastatic lesions and the total weight of the lung compared with the control group, indicating that CD73 may promote the metastatic ability of HNSCC cells in vivo (Figure 6D–F). Finally, we detected the expression of E‐cadherin, vimentin, Ki67, and NWASP in tumors of the xenograft model via IHC staining. Similar to the in vitro experiment, knockdown of CD73 increased the expression of E‐cadherin and decreased the expression of vimentin, Ki67, and NWASP (Figure 6G,H).
FIGURE 6.

Depletion of CD73 inhibits the proliferation and metastasis of head and neck squamous cell carcinoma (HNSCC) cells in vivo. A, General images of subcutaneous tumors of lenti‐shNC– or lenti‐shCD73–transfected CAL27 cells from a xenograft nude mouse model. B, Volume of tumors in lenti‐shNC or lenti‐shCD73 groups at indicated time. E, The weights of the tumors are measured. D‐F, White light and H&E staining images of pulmonary metastasis of lenti‐shNC– or lenti‐shCD73–transfected CAL27 cells from a lung metastasis model. The number of metastases and weight of the metastatic lung were measured. G, Immunohistochemistry (IHC) staining of Ki67, E‐cadherin, vimentin, and NWASP. H, The protein expression of Ki67, E‐cadherin, vimentin, and NWASP was measured. ns, not significant; *p < 0.05, **p < 0.01, and ***p < 0.001
4. DISCUSSION
CD73, as an ectoenzyme for ATP to form adenosine, is involved in cellular homeostasis, physiological adaptation, multiple disease development, and the progression of various cancers. 24 , 25 Upon exposure to persistent hypoxia and inflammation, elevated adenosine generated by CD73 could boost immunosuppressive responses and promote immune evasion in the TME by activating regulatory T cells (Tregs) and myeloid‐derived suppressor cells (MDSCs). 26 , 27 , 28 Despite the immune response, CD73 could promote tumor metastasis in a wide range of cancer types, such as breast cancer, 29 , 30 hepatocellular carcinoma, 31 and gastric cancers. 32 Evidence has shown that the expression of CD73 in both primary and metastatic HNSCC lesions is upregulated and correlated with poor prognosis. 10 , 11 Blockade of CD73‐related purinergic signaling in HNSCC could significantly reverse the immunosuppressive TME. 33 , 34 , 35 However, the nonimmunologic role and molecular mechanism of CD73 involved in HNSCC progression are not well characterized. Here, we demonstrated that the expression of CD73/NT5E is upregulated in HNSCC tissues at both the transcriptomic and protein levels. Meanwhile, the mRNA expression of CD73 by RNA sequencing and the protein expression by IHC both predict poor prognosis of HNSCC patients. These clinical findings were in accordance with previous findings and further confirmed their potential as biomarkers for diagnosis and prognosis prediction in HNSCC.
Then, despite the immunomodulatory role of CD73, we investigated the biological functions of CD73 in HNSCC cancer cell lines. In vitro and in vivo experiments demonstrated that silencing CD73 suppresses the proliferation, migration, and invasion of HNSCC cell lines. With Western blot, we detected that E‐cadherin was upregulated and N‐cadherin, vimentin and Snail were downregulated by knockdown of CD73, which are EMT‐related markers. EMT is the key process by which tumor cells gain migratory and invasive properties, during which loss of E‐cadherin is the iconic event. 36 , 37 Our results suggested that CD73 may promote the motility and invasion of HNSCC cells by activating EMT.
Invadopodia are long protrusions with proteolytic properties and are a hallmark of cancer cells undergoing invasion and metastasis. 17 To predict the specific biological processes in which CD73 is involved, we analyzed the correlation between the expression of CD73 and the GSVA score of all the biological processes. Interestingly, “invadopodium” was among the top 10 correlated biological processes according to the Spearman correlation coefficient, which was also confirmed by GSEA. There is evidence that CD73 is involved in cytoskeletal regulation and could influence the invasion properties of gastric cancer cells. 32 WASP and NWASP are key proteins regulating pseudopodia and invadopodia assembly, and MT1‐MMP denotes the maturation and invasion properties of invadopodia. Western blot analysis showed that WASP, NWAP, and MT1‐MMP were significantly downregulated after CD73 knockdown. Double fluorescence staining with phalloidin to label F‐actin and anti‐cortactin showed decreased pseudopodia, and a fluorescent gelatin degradation assay showed decreased invaded gelatin by invadopodia after CD73 suppression. Thus, we conclude that CD73 is involved in invadopodia formation in HNSCC.
Furthermore, we investigated the potential molecular mechanism through which CD73 boosts the malignancy of HNSCC. GSEA revealed that the “MAPK signaling pathway” was significantly upregulated in tumor tissues with higher NT5E expression. Previous studies showed that CD73 could activate the MEK/ERK and p38 MAPK signaling pathways, which are two major branches of the MAPK signaling pathway. 38 , 39 Interestingly, MAPK signaling could also promote the expression of CD73 via transcriptional regulation. 40 Additionally, ERK signaling is responsible for invadopodia formation. 14 , 41 , 42 As expected, the phosphorylated forms of p38 MAPK, MEK1/2, and ERK1/2 were inhibited by knockdown of CD73. Furthermore, functional recovery experiments indicated that activating the MAPK signaling pathway could reverse the effect of CD73 knockdown on cell proliferation, migration, invasion, and invadopodia formation. Thus, we speculated that CD73 could promote the proliferation, migration, invasion and invadopodia formation of HNSCC cells, while the specific mechanisms still need to be clarified further.
In summary, CD73/NT5E was highly expressed in HNSCC tissues and predicted poor prognosis, indicating its potential as a diagnostic and prognostic biomarker. It could also promote proliferation, migration, invasion, and invadopodia formation via the MAPK signaling pathway. Moreover, CD73 depletion of HNSCC cells effectively suppressed proliferation and metastasis in a xenograft or lung metastasis nude mouse model, suggesting CD73 as a promising therapeutic target through nonimmune pathways.
AUTHOR CONTRIBUTIONS
H.Y. and Y.Y. provided the direction and guidance of this study. F.X. and R.W. wrote the manuscript. F.X., T.W. and H.S. performed the experiments. H.F. and G.F. collected the tissue samples and analyzed data. All authors have read and approved the final manuscript.
DISCLOSURE
The authors have declared that no competing interests exist.
ETHICS STATEMENT
The study was authorized by the research ethics committee of Stomatological Hospital of Jiangsu Province.
INFORMED CONSENT
All the subjects signed informed consent on sample acquisition and privacy protection.
ANIMAL STUDIES
The animal studies were implemented according to the guidelines of the Committee of Nanjing Medical University for Animal Resources (Approval ID 2102024).
Supporting information
Appendix S1
Figure S1
Figure S2
Figure S3
Figure S4
ACKNOWLEDGMENTS
This work was supported in part by National Natural Science Foundation of China [81672678], Open Project from Jiangsu Key Laboratory of Oral Diseases, Nanjing Medical University (JSKLOD‐KF‐2105), Key Project of Health Commission of Jiangsu Province [ZDB202001], Project of Health Commission of Jiangsu Province [H2019034], Priority Academic Program Development of Jiangsu Higher Education Institutions [PAPD, 2018–87] and sponsored by LIUGEYI Project [LGY2019088].
Xue F, Wang T, Shi H, et al. CD73 facilitates invadopodia formation and boosts malignancy of head and neck squamous cell carcinoma via the MAPK signaling pathway. Cancer Sci. 2022;113:2704‐2715. doi: 10.1111/cas.15452
Feifei Xue, Tianxiao Wang, and Hao Shi contributed equally to this work.
Contributor Information
Yao Yao, Email: graceindeed@163.com.
Hua Yuan, Email: yuanhua@njmu.edu.cn.
REFERENCES
- 1. Chow LQM. Head and neck cancer. N Engl J Med. 2020;382:60‐72. [DOI] [PubMed] [Google Scholar]
- 2. Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18:269‐282. [DOI] [PubMed] [Google Scholar]
- 3. Budach V, Tinhofer I. Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: a systematic review. Lancet Oncol. 2019;20:e313‐e326. [DOI] [PubMed] [Google Scholar]
- 4. Rischin D, Ferris RL, Le Q‐T. Overview of advances in head and neck cancer. J Clin Oncol. 2015;33:3225‐3226. [DOI] [PubMed] [Google Scholar]
- 5. Kaidar‐Person O, Gil Z, Billan S. Precision medicine in head and neck cancer. Drug Resist Updat. 2018;40:13‐16. [DOI] [PubMed] [Google Scholar]
- 6. Zhu J, Zeng Y, Li W, et al. CD73/NT5E is a target of miR‐30a‐5p and plays an important role in the pathogenesis of non‐small cell lung cancer. Mol Cancer. 2017;16:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao Z‐w, Dong K, Zhang H‐z. The roles of CD73 in cancer. Biomed Res Int. 2014;2014:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghiringhelli F, Bruchard M, Chalmin F, Rébé C. Production of adenosine by Ectonucleotidases: a key factor in tumor Immunoescape. J Biomed Biotechnol. 2012;2012:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346‐5358. [DOI] [PubMed] [Google Scholar]
- 10. Ren Z‐H, Yuan Y‐X, Ji T, Zhang C‐P. CD73 as a novel marker for poor prognosis of oral squamous cell carcinoma. Oncol Lett. 2016;12:556‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mandapathil M, Boduc M, Netzer C, et al. CD73 expression in lymph node metastases in patients with head and neck cancer. Acta Otolaryngol. 2018;138:180‐184. [DOI] [PubMed] [Google Scholar]
- 12. Xiong L, Wen Y, Miao X, Yang Z. NT5E and FcGBP as key regulators of TGF‐1‐induced epithelial–mesenchymal transition (EMT) are associated with tumor progression and survival of patients with gallbladder cancer. Cell Tissue Res. 2014;355:365‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang J, Liao X, Yu J, Zhou P. Role of CD73 in disease: promising prognostic indicator and therapeutic target. Curr Med Chem. 2018;25:2260‐2271. [DOI] [PubMed] [Google Scholar]
- 14. Qi S, Perrino S, Miao X, Lamarche‐Vane N, Brodt P. The chemokine CCL7 regulates invadopodia maturation and MMP‐9 mediated collagen degradation in liver‐metastatic carcinoma cells. Cancer Lett. 2020;483:98‐113. [DOI] [PubMed] [Google Scholar]
- 15. Williams KC, Cepeda MA, Javed S, et al. Invadopodia are chemosensing protrusions that guide cancer cell extravasation to promote brain tropism in metastasis. Oncogene. 2019;38:3598‐3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mak AS. p53 in cell invasion, podosomes, and invadopodia. Cell Adhes Migr. 2014;8:205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eddy RJ, Weidmann MD, Sharma VP, Condeelis JS. Tumor cell Invadopodia: invasive protrusions that orchestrate metastasis. Trends Cell Biol. 2017;27:595‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rezatabar S, Karimian A, Rameshknia V, et al. RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. J Cell Physiol. 2019;234:14951‐14965. [DOI] [PubMed] [Google Scholar]
- 19. Liu F, Yang X, Geng M, Huang M. Targeting ERK, an Achilles' heel of the MAPK pathway, in cancer therapy. Acta Pharm Sin B. 2018;8:552‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marampon F, Ciccarelli C, Zani BM. Biological rationale for targeting MEK/ERK pathways in anti‐cancer therapy and to potentiate tumour responses to radiation. Int J Mol Sci. 2019;20:2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donohoe F, Wilkinson M, Baxter E, Brennan DJ. Mitogen‐activated protein kinase (MAPK) and obesity‐related cancer. Int J Mol Sci. 2020;21:1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang T, Xia Y, Lv J, et al. A novel protein encoded by circMAPK1 inhibits progression of gastric cancer by suppressing activation of MAPK signaling. Mol Cancer. 2021;20:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yong H‐Y, Koh M‐S, Moon A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin Investig Drugs. 2009;18:1893‐1905. [DOI] [PubMed] [Google Scholar]
- 24. Minor M, Alcedo KP, Battaglia RA, Snider NT. Cell type‐ and tissue‐specific functions of ecto‐5′‐nucleotidase (CD73). Am J Phys Cell Phys. 2019;317:C1079‐C1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiarella AM, Ryu YK, Manji GA, Rustgi AK. Extracellular ATP and adenosine in cancer pathogenesis and treatment. Trends Cancer. 2021;7:731‐750. [DOI] [PubMed] [Google Scholar]
- 26. Alcedo KP, Bowser JL, Snider NT. The elegant complexity of mammalian ecto‐5′‐nucleotidase (CD73). Trends Cell Biol. 2021;31:829‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lan J, Lu H, Samanta D, Salman S, Lu Y, Semenza GL. Hypoxia‐inducible factor 1‐dependent expression of adenosine receptor 2B promotes breast cancer stem cell enrichment. Proc Natl Acad Sci USA. 2018;115:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hatfield SM, Kjaergaard J, Lukashev D, et al. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1α‐dependent and extracellular adenosine‐mediated tumor protection. J Mol Med. 2014;92:1283‐1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stagg J, Divisekera U, McLaughlin N, et al. Anti‐CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci USA. 2010;107:1547‐1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang L, Zhou X, Zhou T, et al. Ecto‐5′‐nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol. 2008;134:365‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma X‐L, Shen M‐N, Hu B, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1‐mediated membrane localization of P110β and predicts poor prognosis. J Hematol Oncol. 2019;12:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu Z, Gu C, Yao X, et al. CD73 promotes tumor metastasis by modulating RICS/RhoA signaling and EMT in gastric cancer. Cell Death Dis. 2020;11:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma S‐R, Deng W‐W, Liu J‐F, et al. Blockade of adenosine A2A receptor enhances CD8+ T cells response and decreases regulatory T cells in head and neck squamous cell carcinoma. Mol Cancer. 2017;16:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Du X, Moore J, Blank BR, et al. Orally bioavailable small‐molecule CD73 inhibitor (OP‐5244) reverses immunosuppression through blockade of adenosine production. J Med Chem. 2020;63:10433‐10459. [DOI] [PubMed] [Google Scholar]
- 35. Deng WW, Li YC, Ma SR, et al. Specific blockade CD 73 alters the “exhausted” phenotype of T cells in head and neck squamous cell carcinoma. Int J Cancer. 2018;143:1494‐1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huber MA, Kraut N, Beug H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548‐558. [DOI] [PubMed] [Google Scholar]
- 37. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69‐84. [DOI] [PubMed] [Google Scholar]
- 38. Zhou L, Jia S, Chen Y, et al. The distinct role of CD73 in the progression of pancreatic cancer. J Mol Med. 2019;97:803‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu S, Zhu W, Shao M, et al. Ecto‐5′‐nucleotidase (CD73) attenuates inflammation after spinal cord injury by promoting macrophages/microglia M2 polarization in mice. J Neuroinflammation. 2018;15:2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reinhardt J, Landsberg J, Schmid‐Burgk JL, et al. MAPK signaling and inflammation link melanoma phenotype switching to induction of CD73 during immunotherapy. Cancer Res. 2017;77:4697‐4709. [DOI] [PubMed] [Google Scholar]
- 41. Valenzuela‐Iglesias A, Burks HE, Arnette CR, et al. Desmoglein 1 regulates Invadopodia by suppressing EGFR/Erk signaling in an Erbin‐dependent manner. Mol Cancer Res. 2019;17:1195‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Noi M, Mukaisho K‐I, Yoshida S, et al. ERK phosphorylation functions in invadopodia formation in tongue cancer cells in a novel silicate fibre‐based 3D cell culture system. Int J Oral Sci. 2018;10:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Figure S1
Figure S2
Figure S3
Figure S4


