Abstract
This multicenter, prospective phase IIb trial evaluating the efficacy and safety of tucidinostat (HBI‐8000) in patients with relapsed or refractory (R/R) adult T‐cell leukemia/lymphoma (ATLL) was undertaken in Japan. Eligible patients had R/R ATLL and had failed standard of care treatment with chemotherapy and with mogamulizumab. Twenty‐three patients received tucidinostat 40 mg orally twice per week and were included in efficacy and safety analyses. The primary end‐point was objective response rate (ORR) assessed by an independent committee. The ORR was 30.4% (95% confidence interval [CI], 13.2, 52.9]. Median progression‐free survival was 1.7 months (95% CI, 0.8, 7.4), median duration of response was 9.2 months (95% CI, 2.6, not reached), and median overall survival was 7.9 months (95% CI, 2.3, 18.0). All patients experienced adverse events (AEs), which were predominantly hematologic and gastrointestinal. Incidence of grade 3 or higher AEs was 78.3%; most were laboratory abnormalities (decreases in platelets, neutrophils, white blood cells, and hemoglobin). Tucidinostat was well tolerated with AEs that could be mostly managed with supportive care and dose modifications. Tucidinostat is a meaningful treatment option for R/R ATLL patients; further investigation is warranted.
Keywords: ATLL, HBI‐8000, HDAC inhibitor, ORR, tucidinostat
Tucidinostat is an oral novel benzamide HDACi of HDAC isoenzymes 1, 2, 3 and 10 selectively. 23 patients with relapsed/refractory ATLL were treated with tucidinostat 40mg orally twice a week. Out of 23 patients, 7 showed objective responses including 1 CR, and ORR was 30.4%.
Abbreviations
- AE
adverse event
- allo‐HSCT
allogeneic hematopoietic stem cell transplantation
- ATLL
adult T‐cell leukemia/lymphoma
- CI
confidence interval
- CR
complete response
- DOR
duration of response
- HDAC
histone deacetylase
- HDACi
histone deacetylase inhibitor
- IOERC
Independent Overall Efficacy Review Committee
- NHL
non‐Hodgkin's lymphoma
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- PR
partial response
- PTCL
peripheral T‐cell lymphoma
- R/R
relapsed or refractory
- SAE
serious adverse event
- SD
stable disease
- TEAE
treatment‐emergent adverse event
1. INTRODUCTION
Adult T‐cell leukemia/lymphoma caused by latent infection of HTLV‐1 is a mature T‐cell malignancy that is highly aggressive and difficult to treat. 1 Worldwide, approximately 15–20 million people are estimated to be infected with HTLV‐1. 2 Infection with HTLV‐1 is endemic in several regions, including southwestern Japan, sub‐Saharan Africa, the Caribbean, South America, parts of the Middle East and Australo‐Melanesia, 3 and also in some areas of Europe. 4 Japan has one million HTLV‐1 carriers. 5
Worldwide, at least 3000 new cases of ATLL are diagnosed each year. 6 A rising incidence of ATLL has been reported in nonendemic regions such as the United States, 7 especially in urban communities with Caribbean and Latin American populations. 8 The median age of ATLL patients in the United States is 54 years, 9 whereas in Japan it is around 68 years. 10
Adult T‐cell leukemia/lymphoma generally has a poor prognosis, with shorter OS than other common subtypes of PTCL. 11 Aggressive types of ATLL are associated with a particularly poor prognosis (median OS approximately 8–10 months); the median OS of indolent types is approximately 5 years. 12 In Japan, approximately 1000 people die from ATLL each year. 13
In Japan, the Practical Guidelines for Hematological Malignancies 2018 recommended multidrug combination chemotherapy for aggressive ATLL (acute type, lymphoma type, and unfavorable chronic type) that progresses rapidly. 14 For aggressive ATLL, chemotherapy with the multiagent protocol modified VCAP‐AMP‐VECP regimen (mLSG15) has been recommended as first‐line treatment in Japan based on the results of a phase III trial, however, the prognosis after treatment is still poor. 14 In the phase III study JCOG9801, the PFS rate at 1 year with mLSG15 was 28% and survival at 3 years was 24%, with median survival of 13 months. 15 Mogamulizumab, a defucosylated anti‐CC chemokine receptor 4 Ab, is approved as first‐line therapy in combination with chemotherapy for untreated aggressive ATLL. 16 For R/R ATLL, mogamulizumab monotherapy and lenalidomide monotherapy have been approved in Japan. 16 , 17
As the response to intensive chemotherapy is not generally durable, and cumulative toxicities discourage the use of long‐term intensive chemotherapy, early allo‐HSCT is recommended after response to first‐line therapy. 18 Allogeneic HSCT has the potential to cure some patients with aggressive ATLL, but carries the risk of transplant‐related mortality, 19 and the clinical outcome of allo‐HSCT after PD is poor. 20 Patient age is a key factor in determining the eligibility of allo‐HSCT and the type of conditioning regimen, 21 and allo‐HSCT is less likely to be an option for elderly patients. As the age of ATLL patients in Japan at diagnosis has been increasing, 10 treatment options for elderly patients are needed.
Especially challenging is the management of patients with R/R aggressive ATLL. 1 The prognosis for these patients is dismal, although the use of lenalidomide or mogamulizumab has produced some encouraging results. 22 Options for treatment of R/R ATLL are very limited and might include a clinical trial, best supportive care, or an alternative therapy not previously used. 23 , 24 The low survival rates in patients with ATLL and the lack of curative therapy present an unmet medical need for which we should explore new targeted therapies. 22
T‐cell lymphomas have been responsive to HDACi, 25 and several HDACi have achieved marketing approval, including tucidinostat (HBI‐8000; chidamide in China). Tucidinostat is a benzamide HDACi of HDAC isoenzymes 1, 2, 3, and 10 selectively. There are three distinct mechanisms of action associated with tucidinostat: direct tumor suppression, immunomodulation, and epigenetic modification of cellular functions. 26 , 27 Histone deacetylase inhibitors cause apoptosis of ATLL cells, 28 precancerous lymphocytes infected with HTLV‐1, or HTLV‐1 transfected cell lines. Therefore, tucidinostat presents a novel mechanism of action that does not overlap with approved drugs to treat R/R ATLL.
The antitumor effect of tucidinostat in ATLL was investigated using cell lines derived from Japanese patients with ATLL and primary tumor cells obtained directly from patients with ATLL. In most cases tucidinostat induced apoptosis in both cell lines and primary ATLL cells. 29 Tucidinostat stimulates accumulation of acetylated histones H3 and H4 in tumor cells, which can increase the gene expression of various tumor suppressors, such as p53 and p21. Tucidinostat inhibits proliferation and cell viability in both ATLL‐derived cell lines and primary ATLL cells freshly obtained from ATLL patients. 29 These preclinical observations support the hypothesis that tucidinostat will be efficacious in ATLL.
Clinical studies of tucidinostat have been undertaken mainly in patients with T‐cell lymphomas. 30 , 31 A phase I study in Japanese patients with R/R NHL, including ATLL patients, showed a manageable safety profile, and 40 mg twice per week was selected for subsequent clinical development. An encouraging efficacy signal was observed in patients with ATLL (three of four patients with ATLL achieved PR), along with an acceptable tolerability/safety profile. 31
The aim of the current phase IIb study was to evaluate the efficacy and safety of tucidinostat in patients with R/R ATLL. This is the first study for patients with R/R aggressive ATLL with a treatment history of mogamulizumab. The recommended dose of tucidinostat was selected based on the previous Japanese phase I study in R/R NHL, which is slightly higher than the chidamide (HBI‐8000) approved dose for PTCL in China. The data described herein led to the approval of tucidinostat in 2021 by Japan's Ministry of Health, Labour and Welfare for R/R aggressive ATLL.
This paper reports the results of the final analysis when all patients had completed follow‐up assessments.
2. MATERIALS AND METHODS
2.1. Patients
The study cohort included Japanese adult patients with R/R aggressive ATLL (acute, lymphoma or unfavorable chronic types, histologically or cytologically diagnosed) after receiving prior therapy with mogamulizumab, or at least one systemic therapy with cytotoxic chemotherapy if intolerance/contraindication for mogamulizumab was observed, and for whom no other standard treatment could be considered appropriate. Patients had to have at least one evaluable ATLL lesion, ECOG performance status 32 of 0–2, estimated life expectancy of more than 3 months, no previous organ transplantation, no previous allo‐HSCT, no autologous HSCT within 12 weeks of starting the study drug, and no active infection or heart abnormalities/arrhythmias.
2.2. Study design
This was a phase IIb, open‐label, single‐arm, nonrandomized study to evaluate the efficacy and safety of tucidinostat in patients with R/R ATLL (registered at ClinicalTrials.gov as NCT02955589). It was performed in accordance with International Council for Harmonization Good Clinical Practice Guideline 33 and the Declaration of Helsinki, 34 and was approved by the institutional review board/independent ethics committee at each study site.
After informed consent, eligible patients were to ingest 40 mg tucidinostat approximately 30 min after a meal twice per week. A treatment cycle was defined as 28 consecutive days. Tucidinostat treatment was to be continued until PD or the occurrence of unacceptable toxicities despite appropriate dose reduction, treatment interruption, and/or supportive care.
The primary efficacy end‐point of the study was ORR, which was analyzed in the per‐protocol set. Secondary end‐points included PFS, DOR, and ORR in each ATLL subtype.
2.3. Assessments
Response to treatment and progression of ATLL was evaluated according to modified criteria of the International Consensus Meeting. 23 Best responses were determined from the efficacy assessments conducted at end of the 4th week, the 8th week after first study dose, and then every 8 weeks from the 8th week through the study period. An IOERC of hematologic oncologists reviewed all data related to disease status, including evaluation of imaging studies by Independent Radiology Review, a modification of the Severity‐Weighted Assessment Tool score for skin lesions, 35 peripheral blood findings (abnormal lymphocytes), and applicable clinical observations.
Safety and tolerability were evaluated by the occurrence of AEs, clinical laboratory tests, vital signs, physical examination, and electrocardiograms. Treatment‐emergent AEs were graded according to the NCI Common Terminology Criteria for Adverse Events version 4.03, 36 and a Data Safety Monitoring Board reviewed data on safety signals. Survival status was followed until death or end of study, 12 months after the last dose of study drug of the last patient.
2.4. Statistical analyses
The target sample size was 22 patients to have at least 18 evaluable patients for efficacy analysis. The target ORR for this study was 30%. To show a response rate of more than 5% in 18 subjects, the power was 80% at a 5% significance level and a two‐sided alpha.
First and final analyses were planned. The first analysis was for the purpose of regulatory submission and planned to be carried out when all patients completed end of treatment assessment. The final analysis was planned to be carried out when all patients completed follow‐up assessments.
3. RESULTS
The study was conducted from November 2016 to November 2019.
3.1. Patients
A total of 23 eligible Japanese patients from 18 study sites were treated with at least one dose of tucidinostat and included in analyses.
All patients had advanced ATLL with rapidly progressing disease. All but one patient had ECOG performance status 0–1. The median time from initial diagnosis was 1.5 years. The median time from the last treatment before the first dose of the study drug was 89 days (range, 30–496 days; mean, 131 days). All patients had previously received chemotherapy and mogamulizumab in combination or sequentially. In fact, 73.9% (17/23) already had at least two lines of treatment. Furthermore, 13.0% (3/23) had five or more regimens. Overall, 21.7% (5/23) of patients had disease progression during their last treatment and were thus classified as refractory (Table 1). The median number of tucidinostat treatment cycles was 1.3 (range, 0.3–13.9 cycles).
TABLE 1.
Demographic data and disease history of 23 Japanese patients with relapsed or refractory adult T‐cell leukemia/lymphoma (ATLL)
| Characteristic (unit) | Statistic | (N = 23) |
|---|---|---|
| Age (years) | Median (range) | 72.0 (60–89) |
| Sex | ||
| Male | n (%) | 15 (65.2) |
| Female | n (%) | 8 (34.8) |
| ECOG performance status | ||
| 0 | n (%) | 12 (52.2) |
| 1 | n (%) | 10 (43.5) |
| 2 | n (%) | 1 (4.3) |
| ATLL disease subtype | ||
| Acute | n (%) | 13 (56.5) |
| Lymphoma | n (%) | 8 (34.8) |
| Unfavorable chronic | n (%) | 2 (8.7) |
| Duration since initial diagnosis (years) | Median (range) | 1.50 (0.31–21.95) |
| ATLL subset based on response to last previous treatment a | ||
| Relapsed | n (%) | 5 (21.7) |
| Recurrent | n (%) | 13 (56.5) |
| Refractory | n (%) | 5 (21.7) |
| Number of patients with mogamulizumab therapy | n | 23 |
| Chemotherapy plus mogamulizumab therapy | n (%) | 11 (47.8) |
| Mogamulizumab monotherapy | n (%) | 12 (52.2) |
| Number of days from last previous treatment | Median (range) | 89.0 (30–496) |
| Number of previous chemotherapy regimens including target therapy | Median (range) | 2.0 (1–5) |
| 5 or more | n (%) | 3 (13.0) |
| 2 or more | n (%) | 17 (73.9) |
Abbreviations: n, number of patients; N, number of patients in analysis set.
Relapsed, response to last previous treatment was complete response and unconfirmed complete response; Recurrent, response to last previous treatment was partial response and stable disease; Refractory: response to last previous treatment was progressive disease.
3.2. Efficacy
The primary end‐point of ORR was determined to be 30.4% (7/23), confirmed independently by the IOERC. Of the seven patients with a response, one had CR and six reached PR. Results were similar when based on the investigator response assessment (34.8%). Disease control (CR, PR, or SD) was reported in 12 of the 23 patients (52.2%) (Table 2).
TABLE 2.
Tumor response overall and by adult T‐cell leukemia/lymphoma (ATLL) subtype and ATLL disease status in Japanese patients treated with tucidinostat (N = 23)
| Best overall response | n (%) | |
|---|---|---|
| Objective response | (CR or PR) | 7 (30.4%) (95% CI, 13.2, 52.9) |
| Best response | CR | 1 (4.3) |
| PR | 6 (26.1) | |
| SD | 5 (21.7) | |
| PD | 11 (47.8) | |
| Acute ATLL | 13 | |
| ORR (CR or PR) | 6 (46.2) | |
| CR | 1 (7.7) | |
| PR | 5 (38.5) | |
| SD | 2 (15.4) | |
| PD | 5 (38.5) | |
| Lymphoma ATLL | 8 | |
| ORR (CR or PR) | 1 (12.5) | |
| PR | 1 (12.5) | |
| SD | 2 (25.0) | |
| PD | 5 (62.5) | |
| Unfavorable chronic ATLL | 2 | |
| ORR (CR or PR) | 0 (0.0) | |
| SD | 1 (50.0) | |
| PD | 1 (50.0) | |
| Relapsed ATLL | 5 | |
| ORR (CR or PR) | 4 (80.0) | |
| CR | 1 (20.0) | |
| PR | 3 (60.0) | |
| SD | 1 (20.0) | |
| Recurrent ATLL | 13 | |
| ORR (CR or PR) | 3 (23.1) | |
| PR | 3 (23.1) | |
| SD | 2 (15.4) | |
| PD | 8 (61.5) | |
| Refractory ATLL | 5 | |
| ORR (CR or PR) | 0 (0.0) | |
| SD | 2 (40.0) | |
| PD | 3 (60.0) | |
Abbreviations: CI, confidence interval; CR, complete response; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
When analyzed by ATLL subtype, ORR in acute ATLL was 46.2%, in lymphoma ATLL ORR was 12.5%, and there was no response in the two patients with unfavorable chronic ATLL (Table 2).
A tumor response (CR or PR) was observed in nodal and extranodal target lesions in 30.0% (6/20), in skin lesions in 62.5% (5/8), and in peripheral blood (abnormal lymphocytes) in 60.0% (3/5) (Table 3).
TABLE 3.
Response to tucidinostat treatment by target lesion type in Japanese patients with adult T‐cell leukemia/lymphoma (N = 23)
| ORR (%) (n/N, 95% CI) | Response of target lesions % (n/N, 95% CI) | ||
|---|---|---|---|
| Nodal/extranodal | Skin | Peripheral blood | |
| 30.4 (7/23; 3.2, 52.9) | 30.0 (6/20; 11.9, 54.3) | 62.5 (5/8; 24.5, 91.5) | 60.0 (3/5; 14.7, 94.7) |
Abbreviations: CI, confidence interval; ORR, overall response rate.
Subgroup analysis showed a tendency for higher ORR in patients with acute type disease, of female gender, younger than 75 years, who had undergone fewer than three prior regimens, and prior mogamulizumab combination therapy (Figure 1).
FIGURE 1.
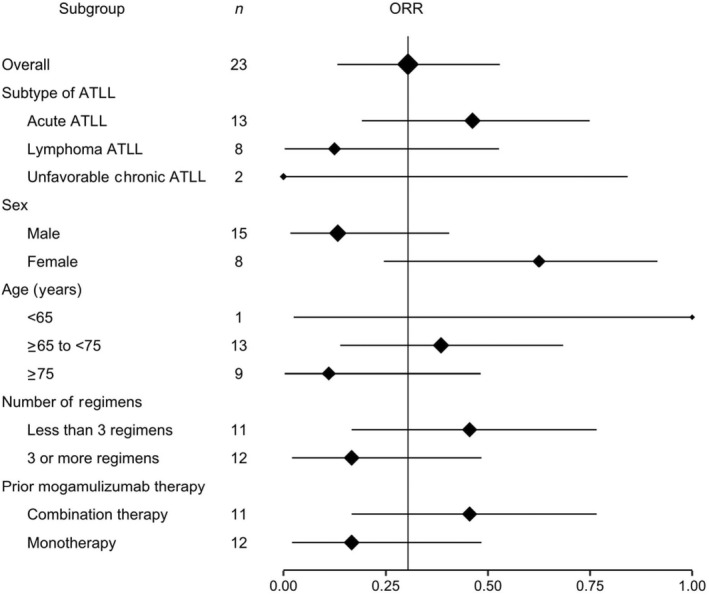
Forest plot of objective response rates (ORR) by subgroups among 23 Japanese patients with relapsed or refractory adult T‐cell leukemia/lymphoma (ATLL) treated with tucidinostat
The ORR was higher in patients who were classified with relapsed ATLL (80.0%; 4/5) compared with recurrent ATLL (23.1%; 3/13); patients with refractory ATLL had no response.
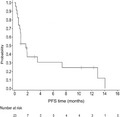
Secondary end‐points were confirmed by the IOERC. Median PFS was 1.7 months (95% CI, 0.8, 7.4) (Figure 2).
FIGURE 2.
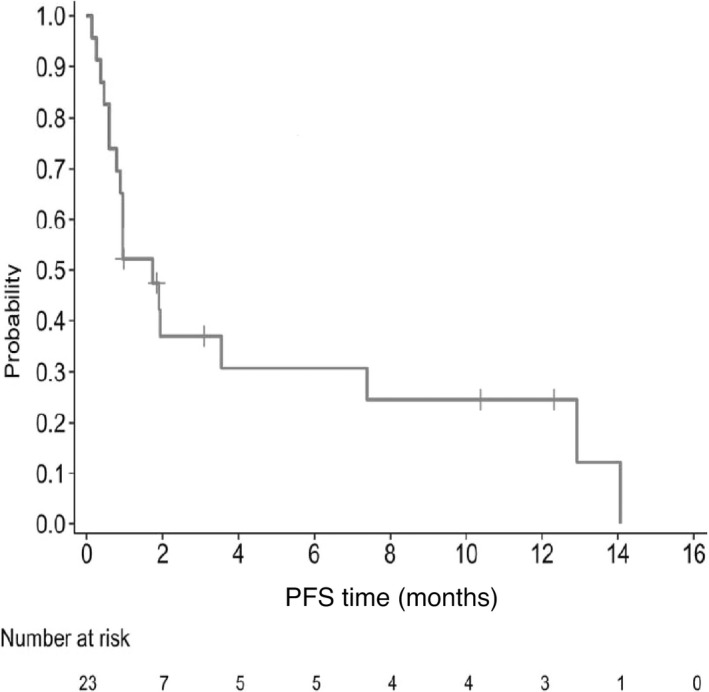
Progression‐free survival (PFS) among 23 Japanese patients with relapsed or refractory adult T‐cell leukemia/lymphoma treated with tucidinostat. Kaplan–Meier plot of PFS probability over time (months), with numbers of patients at risk
Median DOR was 9.2 months (95% CI, 2.6, not reached); four patients had PD and three were censored (Figure 3).
FIGURE 3.
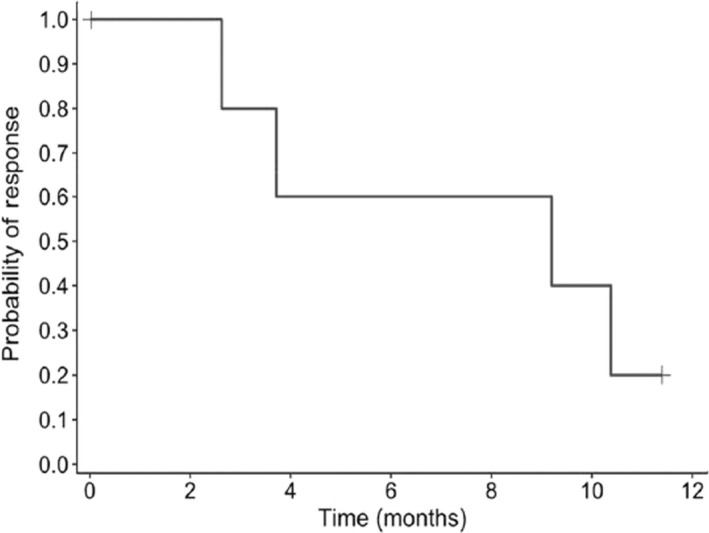
Duration of response among 23 Japanese patients with relapsed or refractory adult T‐cell leukemia/lymphoma treated with tucidinostat. Kaplan–Meier plot of duration of response probability over time (months)
Exploratory end‐points included median time to treatment response of 3.1 months. Median OS was 7.9 months (95% CI, 2.3, 18.0); 16 (69.6%) patients died and seven (30.4%) were censored at the last date confirmed alive (Figure 4). Nineteen of 23 patients had other anti‐ATLL treatments such as lenalidomide and mogamulizumab after the completion of this study treatment.
FIGURE 4.
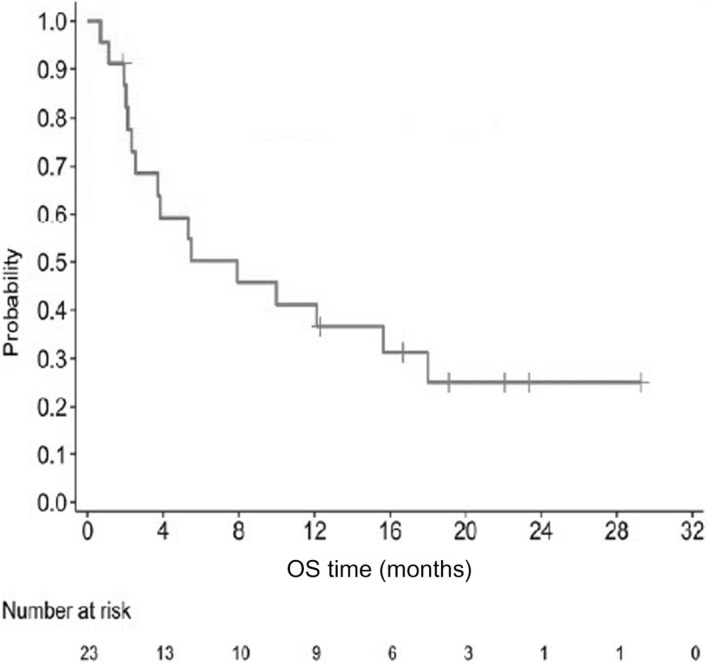
Overall survival (OS) among 23 Japanese patients with relapsed or refractory adult T‐cell leukemia/lymphoma treated with tucidinostat. Kaplan–Meier plot of OS probability over time (months), with numbers of patients at risk
3.3. Safety
All 23 patients had TEAEs that were considered related to study drug; most frequently hematologic (mainly platelet count decreased, neutrophil count decreased, white blood cell count decreased, and anemia) and gastrointestinal (mainly decreased appetite, malaise, and diarrhea). The incidence of grade 3 or higher TEAEs was 78.3%; most were laboratory abnormalities, the majority of which were decreases in platelets, neutrophils, and white blood cells, and anemia (Table 4). Changes in hematologic parameters of platelet count, white blood cell count, neutrophils, and hemoglobin were, in most cases, improved by study drug interruption or reduction and patients resumed dosing.
TABLE 4.
Treatment‐emergent adverse events (TEAEs) related to tucidinostat in more than 10% of study patients with adult T‐cell leukemia/lymphoma, and any TEAEs grade 3 or higher (N = 23)
| Preferred term | Any grade, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|
| Number of patients with at least one TEAE | 23 (100.0) | 9 (39.1) | 9 (39.1) |
| Platelet count decreased | 15 (65.2) | 4 (17.4) | 5 (21.7) |
| Neutrophil count decreased | 11 (47.8) | 7 (30.4) | 2 (8.7) |
| White blood cell count decreased | 9 (39.1) | 7 (30.4) | 0 (0.0) |
| Anemia | 8 (34.8) | 4 (17.4) | 0 (0.0) |
| Decreased appetite | 8 (34.8) | 0 (0.0) | 0 (0.0) |
| Malaise | 7 (30.4) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 5 (21.7) | 0 (0.0) | 0 (0.0) |
| Weight decreased | 4 (17.4) | 1 (4.3) | 0 (0.0) |
| Dysgeusia | 4 (17.4) | 0 | 0 (0.0) |
| Thrombocytopenia | 3 (13.0) | 2 (8.7) | 1 (4.3) |
| Nausea | 3 (13.0) | 0 (0.0) | 0 (0.0) |
| Hypoalbuminemia | 3 (13.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 2 (8.7) | 2 (8.7) | 0 (0.0) |
| Lymphocyte count decreased | 2 (8.7) | 1 (4.3) | 0 (0.0) |
| Gamma‐glutamyltransferase increased | 2 (8.7) | 1 (4.3) | 0 (0.0) |
| Hyponatremia | 2 (8.7) | 0 (0.0) | 1 (4.3) |
| Neutropenia | 1 (4.3) | 0 (0.0) | 1 (4.3) |
| Febrile neutropenia | 1 (4.3) | 1 (4.3) | 0 (0.0) |
| Hemoglobin decreased | 1 (4.3) | 1 (4.3) | 0 (0.0) |
| Device‐related infection | 1 (4.3) | 1 (4.3) | 0 (0.0) |
| Pneumocystis jirovecii pneumonia | 1 (4.3) | 1 (4.3) | 0 (0.0) |
| Urinary tract infection | 1 (4.3) | 1 (4.3) | 0 (0.0) |
| Blood alkaline phosphatase increased | 1 (4.3) | 1 (4.3) | 0 (0.0) |
| Lipase increased | 1 (4.3) | 0 (0.0) | 1 (4.3) |
| Hypophosphatemia | 1 (4.3) | 1 (4.3) | 0 (0.0) |
| Interstitial lung disease | 1 (4.3) | 0 (0.0) | 1 (4.3) |
| Hypertension | 1 (4.3) | 1 (4.3) | 0 (0.0) |
Seven patients (30.4%) experienced SAEs (urinary tract infection, Pneumocystis jirovecii pneumonia, palpitations, platelet count decreased [in two patients], acute respiratory failure, interstitial lung disease, neutrophil count decreased); all but acute respiratory failure were considered to be related to study drug. All SAEs resolved except for the case of grade 1 palpitations. Nine patients (39.1%) discontinued the study drug due to a TEAE. Four patients discontinued because grade 3 or 4 platelet count decreased or thrombocytopenia did not recover to grade 1 within 2 weeks as required in the study protocol. One patient discontinued because grade 3 or higher neutrophil count decrease recurred at 20 mg, the lowest dose defined in the study protocol. The remaining four patients discontinued due to grade 3 or higher nonhematological AEs (interstitial lung disease, P. jirovecii pneumonia, fatigue, γ‐glutamyltransferase/alkaline phosphatase increased). The most frequent TEAEs leading to dose reduction/interruption and/or drug discontinuation were neutrophil count decreased and platelet count decreased. No patient died as a result of an AE.
4. DISCUSSION
This is the first prospective study for the treatment of patients with R/R aggressive ATLL who had previously been treated with chemotherapy and mogamulizumab and patients with refractory ATLL. Tucidinostat 40 mg twice per week, given orally, showed an ORR of 30.4% by the IOERC and 34.8% by investigator assessment. As a post hoc analysis, the ORR in patients with relapsed or recurrent ATLL was 38.9% (7/18). This result is comparable to phase II studies of the currently available treatments in Japan. Mogamulizumab was reported to produce an ORR of 50% in patients with relapsed ATLL, 37 and an ORR of mogamulizumab in a study of mogamulizumab versus investigator's choice of chemotherapy in patients who were refractory or relapsed after at least one prior systemic therapy was 34% by investigator assessment and 28% by independent review. 38 Lenalidomide was reported to produce an ORR of 42% in patients with relapsed or recurrent ATLL. 17 In our study, 78.3% of patients had two or more prior treatment regimens, and 17.4% had five or more; five (22%) of the enrolled patients were refractory to their previous chemotherapy. In addition, the median time since last ATLL treatment was 89.0 days in our study, but 234.5 days in the lenalidomide study, 17 suggesting that patients with more aggressive ATLL were enrolled in our study.
Tucidinostat showed an acceptable safety profile. The most common AEs were hematological, such as thrombocytopenia, neutropenia, leukopenia, and anemia, and these laboratory abnormalities also constituted the grade 3 or higher TEAEs. Some patients discontinued the study drug due to hematological AEs according to the treatment discontinuation guidelines. As patients were treated with multiple cancer treatments before entering this study, previous treatments might have increased the hematological toxicities necessitating discontinuation of the study drug. The most of hematological AEs are manageable with dosing interruption or reduction in a timely manner in conjunction with preemptive supportive care.
The hematological toxicities of HDACi are well known, and AEs reported in the present study were similar to those in other HDACi studies. A phase II study of romidepsin in patients with PTCL showed the most common grade 3 or higher TEAEs to be lymphopenia (74%), neutropenia (54%), leukopenia (46%), and thrombocytopenia (38%). 39 A phase II study of vorinostat in patients with NHL or mantle cell lymphoma showed that 80% of patients experienced grade 3/4 AEs, most frequently thrombocytopenia and neutropenia, all of whom recovered after dose reduction, interruption, or discontinuation and adequate supportive measures. 40 A previous study of tucidinostat in China showed that most AEs of grade 3 or higher were thrombocytopenia (22%), leukopenia (13%), and neutropenia (11%). 30 The typical AEs of HDACi such as thrombocytopenia, neutropenia, and anemia have been reported as transient and reversible, 41 which were also observed in our study.
Few other chemotherapeutic drugs offer durable disease control in ATLL patients; therefore, new treatment options are needed. Mogamulizumab was approved based on a phase II study in patients who had relapsed after their last anti‐ATLL therapy, 37 therefore efficacy of mogamulizumab in refractory ATLL was not examined in the phase II study. Mogamulizumab in combination with intensive chemotherapy mLSG15 improves CR but was found to be potentially associated with a less favorable safety profile, particularly for infectious and skin‐related events. 16 A postmarketing survey of mogamulizumab 42 showed a shorter median survival (5.5 months) than in the prospective clinical study reported by Ishida et al. 37 as well as safety management challenges with regard to infusion reactions and skin disorders. 42 Lenalidomide was approved based on the results of a phase II study in patients with “relapsed or recurrent” ATLL whether or not they had previously received mogamulizumab, and showed two responders among 11 patients pretreated with mogamulizumab. Patients who did not respond to their last previous treatment were not included, thus the efficacy of lenalidomide in patients with refractory ATLL was not evaluated. In that study, the median PFS was 3.8 months. 17 In the present tucidinostat study, five refractory ATLL patients received tucidinostat, and two patients were observed with SD, but there was no responder. The median PFS observed in this study appeared to be shorter than in mogamulizumab and lenalidomide studies in which refractory patients were excluded. It is likely that the extent of advanced disease of the study cohort and the limitation of sample sizes attributed to the difference in observed efficacy.
In conclusion, tucidinostat monotherapy showed clinically meaningful antitumor activity for patients with R/R aggressive ATLL, with an acceptable and manageable safety profile. Tucidinostat targets different pathways than current treatments, thus it could further expand the number of available treatment options and improve outcomes for patients with R/R aggressive ATLL.
DISCLOSURE
A. Utsunomiya has consultant or advisory relationships (Meiji Seika, JIMRO, Otsuka Medical Devices). K. Izutsu receives research funding (AstraZeneca, AbbVie, Incyte, Celgene, Bristol Myers Squibb, Novartis, Bayer, Pfizer, Janssen, Yakult, Kyowa Kirin, Ono Pharmaceutical, Daiichi Sankyo, Chugai, Beigene, Genmab, Otsuka) and honoraria (Janssen, Ono Pharmaceutical). S. Yoshida receives research funding (Bayer, HUYABIO). K. Tsukasaki receives research funding (Chugai). K. Ando receives research funding (Solasia, Novartis, Janssen, Otsuka, IQVIA, Zenyaku, Chugai, Astellas) and honoraria (Kyowa Kirin, Takeda, Chugai, Meiji Seika, Eisai, Mochida). Y. Imaizumi receives honoraria (Kyowa Kirin, Celgene, BMS, Eisai, Sanofi, Meiji Seika). K. Kato has consultant or advisory relationships (AbbVie, AstraZeneca, Celgene, Chugai, Eisai, Janssen, Novartis, Daiichi Sankyo, Ono, Takeda, MSD, Kyowa Kirin, Mundy, Sumitomo Dainippon, BMS) and receives research funding (Chugai, Takeda, Kyowa Kirin, AbbVie, Eisai, Janssen, Celgene, Ono, Novartis, Daiichi Sankyo). S. Kusumoto has consultant or advisory relationships (Chugai). H. Shibayama has consultant or advisory relationships (Takeda, Novartis, Celgene, Janssen, Chugai, Kyowa Kirin, Ono, Sanofi) and receives research funding (Janssen, Takeda, Ono, Novartis, AbbVie, HUYABIO, AstraZeneca, Eisai, Chugai, PharmaEssentia, GSK) and honoraria (Celgene, Ono, Astellas, Teijin, Shionogi, Taiho, Eisai). K. Shimoda has consultant or advisory relationships (Novartis, Takeda, BMS, Celgene) and receives research funding (Perseus Proteomics, PharmaEssentia, AbbVie, Astellas, MSD, Chugai, Kyowa Kirin, Pfizer, Novartis, Otsuka, Asahikasei). Y. Takamatsu has consultant or advisory relationships (Takeda) and receives honoraria (Takeda, Chugai, Kyowa Kirin, Taiho, Ono). K. Yonekura has received honoraria (AbbVie, Celgene, Daiichi Sankyo, Eisai, Eli Lilly Japan, Janssen, Kaken, Kyowa Kirin, Maruho, Minophagen, Novartis, Sanofi, Taiho, Torii, UCB). S. Makita received honoraria (Celgene/BMS, Takeda). K. Tobinai has consultant or advisory relationships (Zenyaku, Eisai, Takeda, Mundipharma, HUYABIO, Kyowa Kirin, Celgene, Chugai, Ono, Yakult, Daiichi Sankyo, Solasia). M. Gillings and H. Onogi are employees of HUYABIO International LLC. The other authors have no conflict of interest.
ACKNOWLEDGMENTS
The authors would like to thank the patients, families, other investigators (Dr. Kenichi Ishizawa, Dr. Norifumi Tsukamoto, Dr. Yasuhito Terui, Dr. Hideaki Nitta, Dr. Itaru Matsumura, Dr. Yoshinobu Maeda, Dr. Toshihiko Ando, Dr. Kenji Ishitsuka, Dr. Takero Shindo, Dr. Kisato Nosaka, Dr. Yukiyoshi Moriuchi, Dr. Takashi Ishida, Dr. Nobuhito Ohno, Dr. Junnosuke Uchihara, Dr. Taizo Shimomura, and Dr. Ki‐Ryang Koh) and all staff in each hospital who supported the present study. We would also like to thank Dr. Kazuhito Yamamoto, Dr. Yosuke Minami, and Noriko Fukuhara (members of the IOERC) and Dr. Noriko Usui, Dr. Yoshinobu Kanda, Dr. Keita Kirito, and Dr. Yasuo Ohashi (members of the Data Safety Monitoring Board). Karen Rittweger provided medical writing assistance, funded by HUYABIO International. This study was supported by HUYABIO International.
Utsunomiya A, Izutsu K, Jo T, et al. Oral histone deacetylase inhibitor tucidinostat (HBI‐8000) in patients with relapsed or refractory adult T‐cell leukemia/lymphoma: Phase IIb results. Cancer Sci. 2022;113:2778‐2787. doi: 10.1111/cas.15431
ClinicalTrials.gov Identifier: NCT02955589.
REFERENCES
- 1. Hermine O, Ramos JC, Tobinai K. A review of new findings in adult T‐cell leukemia‐lymphoma: a focus on current and emerging treatment strategies. Adv Ther. 2018;35(2):135‐152. doi: 10.1007/s12325-018-0658-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Proietti FA, Carneiro‐Proietti AB, Catalan‐Soares BC, Murphy EL. Global epidemiology of HTLV‐I infection and associated diseases. Oncogene. 2005;24(39):6058‐6068. doi: 10.1038/sj.onc.1208968 [DOI] [PubMed] [Google Scholar]
- 3. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV‐1 infection. Front Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verdonck K, González E, Dooren SV, Vandamme AM, Vanham G, Gotuzzo E. Human T‐lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7(4):266‐281. doi: 10.1016/S1473-3099(07)70081-6 [DOI] [PubMed] [Google Scholar]
- 5. Satake M, Yamaguchi K, Tadokoro K. Current prevalence of HTLV‐1 in Japan as determined by screening of blood donors. J Med Virol. 2012;84(2):327‐335. doi: 10.1002/jmv.23181 [DOI] [PubMed] [Google Scholar]
- 6. Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceshi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609‐e616. doi: 10.1016/S2214-109X(16)30143-7 [DOI] [PubMed] [Google Scholar]
- 7. Malpica L, Pimentel A, Reis IM, et al. Epidemiology, clinical features, and outcome of HTLV‐1–related ATLL in an area of prevalence in the United States. Blood Adv. 2018;2(6):607‐620. doi: 10.1182/bloodadvances.2017011106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phillips AA, Shapira I, Willim RD, et al. A critical analysis of prognostic factors in north American patients with human T‐cell lymphotropic virus type‐1‐associated adult t‐cell leukemia/lymphoma. Cancer. 2010;116:3438‐3446. doi: 10.1002/cncr.25147 [DOI] [PubMed] [Google Scholar]
- 9. Zell M, Assal A, Derman O, et al. Adult T‐cell leukemia/lymphoma in the Caribbean cohort is a distinct clinical entity with dismal response to conventional chemotherapy. Oncotarget. 2016;7(32):51981‐51990. doi: 10.18632/oncotarget.10223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nosaka K, Iwanaga M, Imaizumi Y, et al. Epidemiological and clinical features of adult T‐cell leukemia‐lymphoma in Japan, 2010–2011: a nationwide survey. Cancer Sci. 2017;108(12):2478‐2486. doi: 10.1111/cas.13398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. International T‐Cell Lymphoma Project . International peripheral T‐cell and natural killer/T‐cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124‐4130. doi: 10.1200/JCO.2008.16.4558 [DOI] [PubMed] [Google Scholar]
- 12. Imaizumi Y, Iwanaga M, Nosaka K, et al. Prognosis of patients with adult T‐cell leukemia/lymphoma in Japan: a nationwide hospital‐based study. Cancer Sci. 2020;111:4567‐4580. doi: 10.1111/cas.14658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamada Y, Atogami S, Hasegawa H, et al. Nationwide survey of adult T‐cell leukemia/lymphoma (ATL) in Japan. Rinsho Ketsueki. 2011;52(11):1765‐1771. doi: 10.11406/rinketsu.52.1765 [DOI] [PubMed] [Google Scholar]
- 14. Tsukasaki K, Fukushima T. JSH practical guidelines for hematological malignancies, 2018: II. Lymphoma‐8. Adult T‐cell leukemia‐lymphoma. Int J Hematol. 2019;109:249‐259. doi: 10.1007/s12185-018-02588-5 [DOI] [PubMed] [Google Scholar]
- 15. Tsukasaki K, Utsunomiya A, Fukuda H, et al. VCAP‐AMP‐VECP compared with biweekly CHOP for adult T‐cell leukemia‐lymphoma: Japan clinical oncology group study JCOG9801. J Clin Oncol. 2007;25(34):5458‐5464. doi: 10.1200/JCO.2007.11.9958 [DOI] [PubMed] [Google Scholar]
- 16. Ishida T, Jo T, Takemoto S, et al. Dose‐intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T‐cell leukaemia‐lymphoma: a randomized phase II study. Br J Haematol. 2015;169(5):672‐682. doi: 10.1111/bjh.13338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishida T, Fujiwara H, Nosaka K, et al. Multicenter phase II study of lenalidomide in relapsed or recurrent adult T‐cell leukemia/lymphoma: ATLL‐002. J Clin Oncol. 2016;34(34):4086‐4093. doi: 10.1200/JCO.2016.67.7732 [DOI] [PubMed] [Google Scholar]
- 18. Fuji S, Fujiwara H, Nakano N, et al. Early application of related SCT might improve clinical outcome in adult T‐cell leukemia/lymphoma. Bone Marrow Transplant. 2016;51:205‐211. doi: 10.1038/bmt.2015.265 [DOI] [PubMed] [Google Scholar]
- 19. Hishizawa M, Kanda J, Utsunomiya A, et al. Transplantation of allogeneic hematopoietic stem cells for adult T‐cell leukemia:a nationwide retrospective study. Blood. 2010;116(8):1369‐1376. doi: 10.1182/blood-2009-10-247,510 [DOI] [PubMed] [Google Scholar]
- 20. Fujiwara H, Fuji S, Wake A, et al. Dismal outcome of allogeneic hematopoietic stem cell transplantation for relapsed adult T‐cell leukemia/lymphoma, a Japanese nation‐wide study. Bone Marrow Transplant. 2017;52(3):484‐488. doi: 10.1038/bmt.2016.313 [DOI] [PubMed] [Google Scholar]
- 21. Katsuya H, Ishitsuka K, Utsunomiya A, et al. Treatment and survival among 1594 patients with ATL. Blood. 2015;126(24):2570‐2577. doi: 10.1182/blood-2015-03-632,489 [DOI] [PubMed] [Google Scholar]
- 22. El Hajj H, Tsukasaki K, Cheminant M, Bazarbachi A, Watanabe T, Hermine O. Novel treatments of adult T cell leukemia lymphoma. Front Microbiol. 2020;11:1062. doi: 10.3389/fmicb.2020.01062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T‐cell leukemia‐lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27(3):453‐459. doi: 10.1200/JCO.2008.18.2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) . Non‐Hodgkin's Lymphomas, version 2. 2015. https://www.tri‐kobe.org
- 25. Duan H, Heckman CA, Boxer LM. Histone deacetylase inhibitors down‐regulate bcl‐2 expression and induce apoptosis in t(14;18) lymphomas. Mol Cell Biol. 2005;25(5):1608‐1619. doi: 10.1128/MCB.25.5.1608-1619.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ning ZQ, Li ZB, Newman MJ, et al. Chidamide (CS055/HBI‐8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol. 2012;69(4):901‐909. doi: 10.1007/s00280-011-1766-x [DOI] [PubMed] [Google Scholar]
- 27. Pan DS, Yang QJ, Fu X, et al. Discovery of an orally active subtype‐selective HDAC inhibitor, chidamide, as an epigenetic modulator for cancer treatment. Med Chem Commun. 2014;5:1789‐1796. doi: 10.1039/C4MD00350K [DOI] [Google Scholar]
- 28. Hasegawa H, Yamada Y, Tsukasaki K, et al. LBH589, a deacetylase inhibitor, induces apoptosis in adult T‐cell leukemia/lymphoma cells via activation of a novel RAIDD‐caspase‐2 pathway. Leukemia. 2011;25:575‐587. doi: 10.1038/leu.2010.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hasegawa H, Bissonnette RP, Gillings M, et al. Induction of apoptosis by HBI‐8000 in adult T‐cell leukemia/lymphoma is associated with activation of Bim and NLRP3. Cancer Sci. 2016;107(8):1124‐1133. doi: 10.1111/cas.12971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi Y, Dong M, Hong X, et al. Results from a multicenter, open‐label, pivotal phase II study of chidamide in relapsed or refractory peripheral T‐cell lymphoma. Ann Oncol. 2015;26(8):1766‐1771. doi: 10.1093/annonc/mdv237 [DOI] [PubMed] [Google Scholar]
- 31. Onizuka M, Ando K, Yoshimitsu M, et al. Oral HDAC inhibitor HBI‐8000 in Japanese patients with non‐Hodgkin lymphoma (NHL): phase I safety and efficacy results. Blood. 2016;128(22):1827. doi: 10.1182/blood.V128.22.1827.1827 [DOI] [Google Scholar]
- 32. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982. Dec;5(6):649‐655. [PubMed] [Google Scholar]
- 33. ICH Working Group . International council for harmonization (ICH) good clinical practice (GCP) guideline. https://www.ich.org/page/ich‐guidelines
- 34. World Medical Association . WMA declaration of helsinki – ethical principles for medical research involving human subjects. https://www.wma.net/policies‐post/wma‐declaration‐of‐helsinki‐ethical‐principles‐for‐medical‐research‐involving‐human‐subjects/ [PubMed]
- 35. Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the international society for cutaneous lymphomas, the United States cutaneous lymphoma consortium, and the cutaneous lymphoma task force of the European organization for research and treatment of cancer. J Clin Oncol. 2011;29(18):2598‐2607. doi: 10.1200/JCO.2010.32.0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. National Institutes of Health National Cancer Institute . CTCAE v4.03 Index of /ftp1/CTCAE/CTCAE_4.03 (nih.gov). https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010‐06‐14_QuickReference_5x7.pdf
- 37. Ishida T, Joh T, Uike N, et al. Defucosylated anti‐CCR4 monoclonal antibody (KW‐0761) for relapsed adult T‐cell leukemia‐lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837‐842. doi: 10.1200/JCO.2011.37.3472 [DOI] [PubMed] [Google Scholar]
- 38. Phillips AA, Fields PA, Hermine O, Ramos JC, Beltran BE, Pereira J. Mogamulizumab versus investigator's choice of chemotherapy regimen in relapsed/ refractory adult T‐cell leukemia/lymphoma. Haematologica. 2019;104(5):993‐1003. doi: 10.3324/haematol.2018.205096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maruyama D, Tobinai K, Ogura M, et al. Romidepsin in Japanese patients with relapsed or refractory peripheral T‐cell lymphoma: a phase I/II and pharmacokinetics study. Int J Hematol. 2017;106:655‐665. doi: 10.1007/s12185-017-2286-1 [DOI] [PubMed] [Google Scholar]
- 40. Ogura M, Ando K, Suzuki T, et al. A multicentre phase II study of vorinostat in patients with relapsed or refractory indolent B‐cell non‐Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol. 2014;165(6):768‐776. doi: 10.1111/bjh.12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Subramanian S, Bates SE, Wright JJ, Espinoza‐Delgado I, Piekarz RL. Clinical toxicities of histone deacetylase inhibitors. Pharmaceuticals. 2010;3(9):2751‐2767. doi: 10.3390/ph3092751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ishitsuka K, Yurimoto S, Tsuji Y, Iwabuchi M, Takahashi T, Tobinai K. Safety and effectiveness of mogamulizumab in relapsed or refractory adult T‐cell leukemia‐lymphoma. Eur J Haematol. 2019;102(5):407‐415. doi: 10.1111/ejh.13220 [DOI] [PMC free article] [PubMed] [Google Scholar]


