Abstract
In Saccharomyces cerevisiae the FUR4-encoded uracil permease catalyzes the first step of the pyrimidine salvage pathway. The availability of uracil has a negative regulatory effect upon its own transport. Uracil causes a decrease in the level of uracil permease, partly by decreasing the FUR4 mRNA level in a promoter-independent fashion, probably by increasing its instability. Uracil entry also triggers more rapid degradation of the existing permease by promoting high efficiency of ubiquitination of the permease that signals its internalization. A direct binding of intracellular uracil to the permease is possibly involved in this feedback regulation, as the behavior of the permease is similar in mutant cells unable to convert intracellular uracil into UMP. We used cells impaired in the ubiquitination step to show that the addition of uracil produces rapid inhibition of uracil transport. This may be the first response prior to the removal of the permease from the plasma membrane. Similar down-regulation of uracil uptake, involving several processes, was observed under adverse conditions mainly corresponding to a decrease in the cellular content of ribosomes. These results suggest that uracil of exogenous or catabolic origin down-regulates the cognate permease to prevent buildup of excess intracellular uracil-derived nucleotides.
Pyrimidine nucleotides are precursors for the synthesis of nucleic acids, are involved in postranslational modification of proteins, such as glycosylation, and are precursors for phospholipids. With the exception of some parasites, cells display a capacity for de novo pyrimidine nucleotide biosynthesis by a well-conserved metabolic pathway that starts with the formation of carbamoyl phosphate. Cells can also convert free pyrimidine bases or nucleosides to nucleotides, although this process differs in different organisms. The free bases which originate from the environment or from the catabolic breakdown of RNA are salvaged, and hence this other route is known as the pyrimidine salvage pathway. Both pathways provide UMP as first pyrimidine nucleotide from which all others are derived.
In the yeast Saccharomyces cerevisiae, the de novo pyrimidine nucleotide biosynthesis has been elucidated by genetic and biochemical studies. The key regulation of the pathway involves the URA2-encoded multifunctional protein that is feedback inhibited by UTP, the final product of the pathway (20, 29, 38). The highly efficient salvage pathway in yeast involves the uptake of uracil, cytosine, and uridine, mediated by specific permeases (13, 23). Intracellular cytosine is then quantitatively converted to uracil by deamination, and uracil gives UMP in a single step catalyzed by the FUR1-encoded uracil phosphoribosyltransferase (26). Uridine is directly converted into UMP by a specific kinase. The salvage pathway is able to quench de novo pyrimidine biosynthesis. The presence of uracil in the growth medium indeed decreases the transcription of the URA2 gene (38). The intracellular level of uracil is the result of a balance between its entry catalyzed by the FUR4-encoded uracil permease characterized as a proton symport (4, 17), and its excretion is catalyzed by another energy-dependent carrier which has not been characterized at the molecular level (7, 22).
As uracil permease catalyzes one of the first steps of the salvage pathway, it is a candidate for control of the pathway. A mutation named dhu1, not linked to the FUR4 gene, results in an enhanced synthesis of FUR4 transcript and hence in more uracil permease (4). The half-life of the uracil permease is decreased by various adverse metabolic conditions, including nutritional starvation and mild heat shock. Internalization by endocytosis is the first step in the degradation of the permease that occurs in the vacuole (49). A PEST-like sequence in the N terminus of the protein mediates phosphorylation of several serine residues. This, in turn, is required for production of ubiquitin-permease conjugates that signal the endocytosis of the permease (9, 32).
Many yeast transporters are regulated by the availability of their substrate or alternate preferred nutrient. Both the synthesis and half-lives of these proteins are subject to negative and positive controls. For example, the synthesis of the galactose permease is induced by its substrate, and glucose triggers its inactivation (18). Similarly, the maltose permease undergoes glucose-triggered catabolite degradation (33, 39). This phenomenon is not restricted to sugar transporters. Expression of the general amino acid Gap1 permease is blocked, and preexisting Gap1p is submitted to catabolite inactivation when cells grown on a poor nitrogen source are provided with ammonium (reference 45 and references within). In contrast, some other transporters appear to be negatively controlled by their own substrate. Thus, the presence of inositol in the growth medium promotes inactivation of the inositol permease and repression of its synthesis (30, 31). Copper uptake, mediated by Ctr1p, is highly responsive to copper availability, being induced by copper deprivation and decreased by an excess of copper (5, 36). Similarly, transcription of the ZRT1 gene, encoding the high-affinity zinc transporter, is repressed in cells replete with zinc, and endocytosis of the transporter is triggered by the exposure of cells to high levels of zinc (11). The regulation is more sophisticated when a single nutrient such as glucose can be transported by a family of homologous transporters (1). In the latter case, two members of the family, the SNF3 and RGT2 gene products, are involved in the nutrient-induced expression of some other members and thereby act as glucose sensors (37). One member of a family of amino acid transporters also appears to act as a sensor for external amino acids (6, 19).
Here we investigated the effect of exogenous pyrimidines on the uracil permease which is the sole transporter involved in uracil uptake (24). We show that uracil down-regulates its own transport by acting at several levels, increasing the turnover rate of the cognate permease and probably also that of its transcript. The presence of uracil and other environmental changes trigger an inactivation and an enhanced ubiquitination of uracil permease which signals its endocytosis.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The yeast strains and plasmids used in this study are listed in Table 1. Yeast strains were transformed according to the method described by Gietz et al. (10). Cells were grown at 30°C (or 24°C for act1-3-thermosensitive cells) in minimal medium that contained 0.67% yeast nitrogen base without amino acids, supplemented with 0.05% Casamino Acids. Unless otherwise indicated, the carbon source was 2% glucose or 4% galactose plus 0.02% glucose. act1-3 cells grown at 24°C were heat shocked by the addition of an equal volume of the same medium previously warmed to 48°C, immediately resulting in the restrictive temperature 36°C.
TABLE 1.
Yeast strains and plasmids used in this study
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| Strains | ||
| FL200 | MATα his4 | F. Lacroute |
| NC122sp6 | MATaleu2 fur4Δ | 24 |
| NC217-5C | MATα dhu1-1 | 4 |
| 23344C | MATa ura3 | 14 |
| 23344Cfur1Δ | MATa ura3 fur1Δ::KanMX4 | This study |
| 27038a | MATa ura3 npi1 | 14 |
| NY279 | MATα ura3-52 act1-3 | 43 |
| W303-1B | MATα ade2-1 ura3-1 his3-11 leu2-3,112 trp1-1 can1-100 | 46 |
| Plasmids | ||
| pfF | 2μm LEU2 FUR4 | 44 |
| pfFK272E | 2μm LEU2 fur4K272E | 47 |
| pgF | 2μm LEU2 gal10-FUR4 | 44 |
| p195gF | 2μm URA3 gal10-FUR4 | 49 |
| p195Δ5′gF | 2μm URA3 gal10-FUR4 | This study |
| pFL38gF | CEN URA3 gal10-FUR4 | This study |
| pflacZ | 2μm LEU2 fur4-lacZ | This study |
Disruption of the FUR1 locus.
A replacement cassette with long flanking homology regions (50) was used to disrupt the FUR1 gene in strain 23344C. PCR amplification performed with Pwo polymerase (Boehringer Mannheim), from wild-type genomic DNA with the oligonucleotide primers L1 (5′-GACATGCTTTCTCATGACTGCC-3′) and L2 (5′-GGGGATCCGTCGACCTGCAGCGTACCGGGTTCATGGTTCAAGAAG-3′) and L3 (5′-AACGAGCTCGAATTCATCGATGATATAAATAAATCACACCCGAACACC-3′) and L4 (5′-GATTGGCTAGAGGACAGTACCCG-3′) generated two DNA products corresponding to the FUR1 promoter and terminator, respectively (26), with 25-bp extensions (underlined) homologous to the KanMX4 marker containing the geneticin resistance gene (51). In a second PCR amplification, one strand of each of these molecules was used as a long primer, with KanMX4 as the template. The resulting linear fragment was used to transform 23344C cells. Correct integration at the FUR1 locus in geneticin-resistant cells was confirmed by whole-cell PCR.
Plasmid construction.
The plasmid pFL38gF, containing the FUR4 gene on the CEN vector pFL38 (2), was constructed by subcloning a KpnI-PstI fragment, containing the FUR4 gene under the control of the GAL10 promoter, derived from plasmid p195gF (Table 1). A plasmid p195Δ5′gF containing no FUR4 5′ untranslated region (UTR) was constructed from p195gF. The first step was the insertion of the missing 14 bp downstream of the GAL10 promoter and a 3′ PstI restriction site at 57 bp upstream from the start codon by site-directed mutagenesis with the Stratagene Chameleon double-stranded DNA site-directed mutagenesis kit. Then the FUR4 5′ UTR region was deleted by replacing a 2,434-bp PstI-PstI fragment in the construct by a 2,375-bp PstI-PstI fragment (amplified by PCR with the oligonucleotides 5′-GCTATGACCATGATTACGCCAAGC-3′ and 5′-CGAGCTGCAGATAATGCCAGACAATCTATC-3′) containing only 4 bp upstream of the initiating ATG codon (underlined). For construction of a FUR4-lacZ reporter plasmid, the promoter region of FUR4 was PCR amplified from yeast genomic DNA with forward 5′-GCTCTAGACAGATTTTAGTAGACAAGCGCGAGG-3′ and reverse 5′-GCTCTAGAATCATTATTCCCTCCTATTCTTATTATGCGTAGG-3′ primers containing sequences for XbaI restriction sites (underlined), 350 nucleotides of the 5′ UTR region, and the initiating ATG (in bold in the reverse primer). This fragment was ligated to the lacZ gene of the 2μm-based plasmid YEp368 (35).
RNA isolation and Northern analysis.
Total yeast RNA, isolated as described previously (42), was electrophoresed on agarose-formaldehyde gels and transferred to nylon membranes by vacuum blotting. 32P-labeled probes were made with the random primer DNA labeling system (Boehringer Mannheim). The FUR4 probe was derived from a 1.15-kb BglII-PvuII fragment of the coding sequence isolated from plasmid pfF. The ACT1 probe consisted of a 1.1-kb XhoI-HindIII fragment. The FCY2 probe was a 1.033-kb fragment generated by PCR amplification from wild-type genomic DNA with primers 5′-GACTTGGAGAAGAGAGATCTCCCTG-3′ and 5′-CCGTTCAGAGAGTTAGGAACCAG-3′. Membranes were stripped and rehybridized with another probe when required by standard procedures. Northern blot signals were quantified with a PhosphorImaging analyzer and ImageQuant software from Molecular Dynamics. The values reported are the averages of duplicate determinations from at least two independent experiments.
Measurement of mRNA half-life.
The half-life of FUR4 mRNA was measured by blocking transcription by glucose repression in NC122sp6 cells transformed with the pgF plasmid containing the FUR4 gene under the control of the GAL10 promoter. Cells were grown in minimal medium with 1% galactose as a carbon source to an A600 of 0.5, and 2% glucose was added. Before and after transcription arrest, aliquots (20 ml) were filtered, and filters were immediately frozen in liquid nitrogen. RNA samples were then prepared and analyzed as described above.
Yeast cell extracts and Western immunoblotting.
Yeast cell extracts were prepared, and aliquots, corresponding roughly to 5.106 cells, were electrophoresed in 10% polyacrylamide sodium dodecyl sulfate-Tricine gels. The separated proteins were transferred to a nitrocellulose membrane and probed with an antiserum recognizing the last 10 residues of the permease (kindly provided by R. Jund and M. R. Chevallier [44]), used without further purification. Bound primary antibodies were detected with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G and chemiluminescence (Boehringer Mannheim).
β-Galactosidase assay.
β-Galactosidase activity was measured on chloroform-permeabilized cells that were grown to early exponential phase (41). Assays were performed in triplicate on two separate cultures, and activities are expressed as Miller units (A420 × 1000/min/A600).
Measurement of uracil uptake.
Uracil uptake was measured in exponentially-growing cells as previously described (44). One milliliter of yeast culture was incubated with 5 mM [14C]uracil (NEN Life Science Products) for 20 s at 30°C and then quickly filtered through Whatman GF/C filters, which were washed twice with ice-cold water and assessed for radioactivity.
RESULTS
Exogenous pyrimidines decrease the uracil permease level.
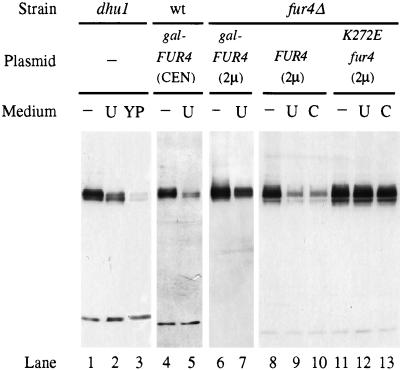
To assess whether the steady-state level of the yeast uracil permease depends upon the availability of uracil, we analyzed crude extracts of cells grown in the presence or absence of uracil. Western blots were probed with an antiserum raised against the last 10 residues of uracil permease (Fig. 1). The chromosome-encoded uracil permease, normally produced in very small amounts, is not detectable in extracts of wild-type cells (48). dhu1 mutant cells that overproduce uracil permease (4) were thus used. The presence of uracil in a synthetic minimal growth medium decreased the amount of uracil permease in cells, and the decrease was greater in dhu1 cells grown in rich medium (lanes 1 to 3). As estimated by Western analysis of serial dilutions of extracts, the concentration of permease was two- and fivefold lower in cells grown in the presence of uracil or in rich medium, respectively. The effect of rich medium was presumably due to the fact that the yeast extract contained substantial amounts of uracil (and other pyrimidine bases). In wild-type cells transformed with a CEN-based plasmid bearing the FUR4 gene under the control of the GAL10 promoter, the permease level was also decreased two- to threefold by the presence of uracil in the growth medium (Fig. 1, lanes 4 to 5). Phosphorylation of the permease results in the appearance of several bands on immunoblots. Analysis of the effect of alkaline phosphatase treatment and the banding pattern of a less-phosphorylated mutant permease indicate that the faster-running bands correspond to lower levels of phosphorylation (32, 48). The presence of uracil resulted in a banding pattern which corresponds to an enrichment in less-phosphorylated permease species (Fig. 1, lanes 1 to 5).
FIG. 1.
The level of uracil permease responds to exogenous pyrimidines. NC217-5C cells (dhu1) (lanes 1 to 3), 23344C cells (wild type [wt]) (lanes 4 to 5) transformed with pFL38gF (gal-FUR4 CEN), and NC122sp6 cells (fur4Δ) (lanes 6 to 13) transformed with either pgF (gal-FUR4 2μ), pfF (FUR4 2μ), or pfFK272 E (fur4 K272E 2μ) were grown on minimal medium without (−) or with 40 μg of either uracil (U) or cytosine (C)/ml or on rich medium (YP). The carbon source was glucose (lanes 1 to 3 and 8 to 13) or galactose (lanes 4 to 7). Protein extracts were prepared from cells in mid-exponential phase, and aliquots were analyzed for uracil permease by Western immunoblotting. Red Ponceau staining and/or detection of an unrelated low-molecular-weight species allowed us to ensure that the lanes within a panel were equally loaded.
We next examined the steady-state level of permease produced from a 2μm-based plasmid, driven either by the inducible GAL10 or by the FUR4 promoter. In both cases, the presence of uracil decreased the steady-state level of permease by two- to threefold (lanes 6 to 9). To determine whether uracil entry into the cell was necessary to decrease the uracil permease level, cells that produce an inactive permease were tested. The K272E mutant permease has an abnormally low affinity for uracil and impaired transport activity (47). The permease level in cells transformed with a multicopy plasmid carrying the K272E mutant allele was not sensitive to added uracil (lanes 11 to 12). As uracil was added to the medium at a concentration high enough to allow its binding to this low-affinity mutant permease, it appears that uracil must be taken up into cells to trigger down-regulation of the permease. The uracil-induced difference in the phosphorylation pattern, described above, was not seen in the NC122sp6 genetic background. The banding pattern of the different phosphorylated forms is extremely sensitive to minor alterations in electrophoresis conditions, and the extent of the modification of the pattern depended upon both the carbon source and the genetic background. However, any change in the presence of uracil was toward underphosphorylated permease.
Other pyrimidines were also tested for their effects on the level of uracil permease. Uridine had no effect (data not shown). The addition of cytosine to the growth medium resulted in a decrease in the level of uracil permease, the extent of which was similar to that caused by uracil (compare lanes 9 to 10 with lane 8). Although cytosine is not a substrate for the uracil permease, its effect was not unexpected. Cytosine is indeed transported by the FCY2-encoded cytosine permease, which is much more efficient than the uracil permease (13, 22). Once inside the cells, it is quantitatively transformed into uracil by the cytosine deaminase. The resulting intracellular uracil might then downregulate its cognate permease. Surprisingly, exogenous cytosine failed to decrease significantly the level of the inactive permease (compare lanes 12 to 13 with lane 11). As uracil can be excreted, the negative effect of cytosine may involve the reimportation of excreted uracil. Therefore, providing cells with uracil or cytosine, the utilization of which passes through uracil, resulted in a decrease in uracil permease.
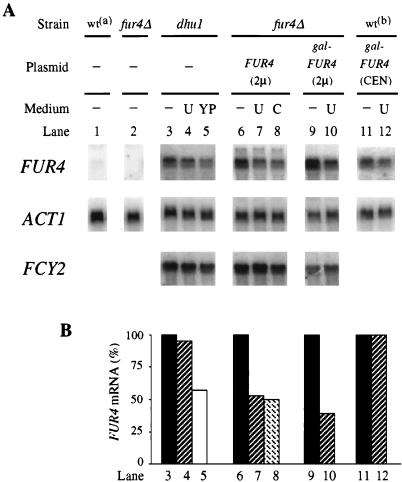
FUR4 mRNA steady-state levels respond to uracil entry.
To investigate the mechanism by which uracil exerts negative control on the level of permease, Northern blot analysis quantified by phosphorimagery (Fig. 2) was performed to determine whether the abundance of FUR4 mRNA was sensitive to the availability of uracil. The single genomic copy of FUR4 produced a barely detectable transcript (compare lanes 1 and 2). As previously described (4), the transcript was produced in dhu1 mutant cells at six- to eightfold above the wild-type chromosomal level. The presence of uracil in minimal medium did not significantly change the amount of mRNA, whereas growth on rich medium resulted in a 40% decrease of FUR4 mRNA. These results are consistent with the steady-state level of protein, which was also more sensitive to growth in rich medium than to the presence of uracil (Fig. 1, lanes 1 to 3). The steady-state level of the FUR4 transcript was approximately 40- to 50-fold higher in cells transformed with a 2 μm-based plasmid than in wild-type untransformed cells. There was approximately half as much FUR4 mRNA in cells replete with uracil (by adding uracil or cytosine to the growth medium) than in uracil-starved cells (Fig. 2, lanes 6 to 8). A negative effect of uracil was also obtained with cells that harbored the FUR4 gene on a multicopy plasmid under the control of the GAL10 promoter (lanes 9 and 10). With weaker FUR4 overexpression, from a centromeric plasmid, the mRNA level was not sensitive to the presence of uracil in the medium (lanes 11 and 12). Therefore, negative control of the steady-state level of the FUR4 transcript appears to require a high level of uracil entry. In contrast to the varation in the level of FUR4 mRNA, that of FCY2 mRNA (normalized to ACT1 mRNA) remained similar under all these conditions. Thus, there is no feedback effect of cytosine on the FCY2-encoded purine-cytosine permease, and the down-regulation of FUR4 mRNA due to uracil is a specific effect. As uracil exerted its negative control in the absence of the endogenous FUR4 promoter, it most probably acts on mRNA stability and not on transcription.
FIG. 2.
Effect of exogenous pyrimidines upon the FUR4 transcript level. Total RNA was prepared from FL200 cells (wta) (lane 1), NC122sp6 cells (fur4Δ) untransformed (lane 2) or transformed with either pfF (FUR4 2μ) (lanes 6 to 8) or pgF (gal-FUR4 2μ) (lanes 9 and 10), NC217-5C cells (dhu1) (lanes 3 to 5), and and 23344C cells (wtb) transformed with pFL38gF (gal-FUR4 CEN) (lanes 11 and 12). Cells were grown to an A600 of 0.5 on minimal medium without (−) or with 40 μg of either uracil (U) or cytosine (C)/ml or on rich medium (YP). The carbon source was glucose (lanes 1 to 8) or galactose (lanes 9 to 12). Ten micrograms of RNA was separated on a formaldehyde gel, blotted, and probed with a fragment from the FUR4 gene, the FCY2 gene, and the ACT1 gene. (A) Signals obtained with the PhosphorImager are presented. (B) Signals were quantified, and values for FUR4 mRNA, normalized to ACT1 as an internal standard, are plotted as percentages of the value obtained for the same cells grown in the absence of pyrimidine.
To check that transcription of the FUR4 gene is truly independent of the availability of uracil, we used a FUR4-lacZ fusion expressing β-galactosidase under the control of the FUR4 promoter. W303 cells were cotransformed with the multicopy plasmid pfZ bearing the FUR4-lacZ fusion and p195gF overproducing uracil permease from the GAL10 promoter. Cells were grown in minimal medium, with galactose as a carbon source, to logarithmic phase and were then assayed for β-galactosidase activity. The level of β-galactosidase activity (75 ± 8 units) was consistent with low expression of the FUR4 gene. Under conditions that resulted in a twofold decrease in the FUR4 mRNA level, i.e., growth in the presence of uracil (40 μg/ml), there was no significant change in FUR4-driven β-galactosidase activity. We also checked that β-galactosidase activity was not derepressed when cells grown overnight in the presence of uracil were transferred to medium without uracil and grown for 4 more h. Therefore, uracil has no effect on the transcription of the FUR4 gene and presumably acts at a posttranscriptional step.
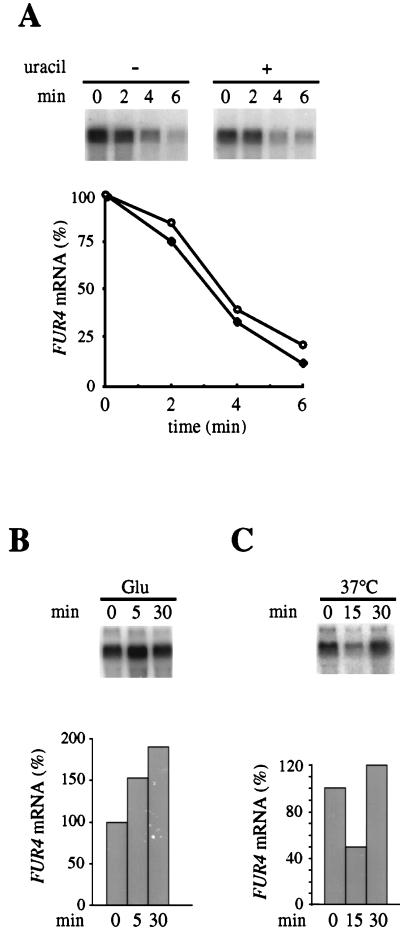
To test directly the influence of uracil on mRNA stability, the kinetics of decay of FUR4 mRNA were studied. Cells disrupted for the chromosomal copy of FUR4 but expressing FUR4 under the control of the GAL10 promoter were used. The loss of preexisting mRNA was followed by Northern blot analysis after glucose arrest of FUR4 transcription, quantified, and normalized to ACT1 mRNA levels (Fig. 3A). Under these conditions, the half-life of FUR4 mRNA was 2 to 3 min, consistent with the value previously determined by another method, for the transcript produced from the endogenous promoter (4). It is, however, much shorter than the average half-life (20 min) of yeast mRNA (15). The experiment was repeated with cells fed with uracil for 1 h: the FUR4 mRNA level was half that in uracil-starved cells at time zero, but the decay observed after the addition of glucose was the same (Fig. 3A). Varying the preincubation time with uracil did not affect the decay, and no difference in the stability of the transcript in the absence or presence of uracil was detected. Failure to detect a uracil-induced increase in the transcript turnover rate may have occurred because a transient upshift due to glucose overrides the effect of uracil. The addition of glucose to cells growing on a less-efficient carbon source produces numerous changes, some of which are transient (reference 28 and references within). There is some evidence for this type of upregulation of the FUR4 transcript. The transcript produced from the native promoter is similarly abundant in cells grown in minimal medium containing either galactose or glucose as a carbon source (data not shown). However, the addition of glucose to galactose-grown cells resulted in a rapid increase of the transcript level (Fig. 3B). Conversely, the FUR4 transcript level was transiently decreased in wild-type cells after a mild temperature shock from 24 to 37°C (Fig. 3C). This latter transient decrease is similar to that of the ribosomal protein mRNAs whose transient increase in the rate of degradation is the main reason for the overall decline in mRNA content following mild heat shock (16).
FIG. 3.
Northern blot analysis of FUR4 mRNA level. (A) To measure mRNA decay, NC122sp6 cells expressing the FUR4 gene from plasmid pgF were subjected to a glucose arrest of galactose-induced transcription in the absence (●) or presence (○) of 40 μg of uracil per ml. RNA was extracted at the indicated times as described in Materials and Methods, and quantitative Northern blotting was performed as shown in Fig. 2. Values of FUR4 mRNA, normalized to ACT1 as an internal standard, are plotted as percentages of the initial value at point zero. (B) NC122sp6 cells transformed with plasmid pfF were grown with galactose as the carbon source, glucose was added, and the FUR4 mRNA level was assayed at the indicated times as described for panel A. (C) NC122sp6 cells transformed with plasmid pfF were grown at 24°C and then subjected to a mild temperature shock to 36°C, and FUR4 mRNA was assayed at the indicated times as described for panel A.
In conclusion, the abundance of the FUR4 transcript, a short-lived species, is acutely sensitive to environmental changes, whether they are global, such as mild heat shock and carbon source modification, or specific, such as the presence of uracil or cytosine. The mechanism of this control is very likely the modulation of the stability of the mRNA. We tested whether the 57 nucleotides upstream from the initiator ATG of FUR4 and present in plasmid pgF might contain instability elements responsive to uracil. This sequence was thus deleted, and the FUR4 coding sequence was fused directly to the GAL10 promoter sequence. The FUR4 mRNA level was determined in cells harboring the new plasmid grown in the absence or presence of uracil. Uracil led to a twofold reduction in the steady-state level of the FUR4 transcript, i.e., strictly to the same extent as that described above (data not shown), suggesting that cis-acting instability determinants are located within the coding or 3′ region of the FUR4 gene and not in the 5′ region. This finding contrasts with several cases in which 5′ cis sequences are required for the control of changes in mRNA half-life in response to environmental changes (3, 12).
Uracil-induced degradation of uracil permease.
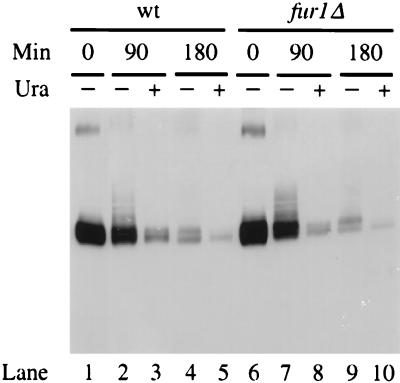
Uracil permease undergoes endocytosis at a basal rate under normal growth conditions and more rapidly under adverse conditions (49). Under both conditions, ubiquitination mediated by the Npi1 ubiquitin-protein ligase is required for the internalization step of endocytosis (9). We wanted to determine whether exogenous uracil affects the turnover rate of the permease. The level of uracil permease was monitored in wild-type cells producing the permease under the control of the GAL10 promoter, after repression by glucose of new permease synthesis. Protein extracts were prepared at various times and analyzed by Western immunoblotting. Exogenous uracil indeed resulted in an acceleration of the permease turnover rate (Fig. 4, lanes 1 to 5). Uracil-induced degradation also involved ubiquitination, since the permease was equally stabilized with or without added uracil in npi1 mutant cells (data not shown). It was shown above that uracil must enter the cells to trigger faster turnover of its cognate permease. We determined whether this was due to intracellular uracil itself or to a metabolite. Disruption of the FUR1 gene renders cells unable to utilize exogenous uracil and cytosine (26). The level of the permease produced under the control of the GAL10 promoter was examined in FUR1-disrupted cells shifted to a glucose medium that contained or did not contain uracil. The permease was degraded more rapidly in the presence of uracil (Fig. 4, lanes 6 to 10). Therefore, the transport of uracil into cells was sufficient to trigger increased turnover of the permease. The direct binding of intracellular uracil to the permease may increase the efficiency of permease internalization, and this uracil-induced event may be regulated by the phosphorylation level of the permease, as uracil favored underphosphorylated species (Fig. 1 and 4).
FIG. 4.
Degradation of the permease upon exposure of cells to uracil. Strains 23344C (wild type [wt]) and 23344Cfur1Δ (fur1Δ) transformed with plasmid p195gF were grown at 30°C to an A600 of 0.5, with galactose as the carbon source. Glucose was then added, and growth was followed in the absence (−) or presence (+) of 40 μg of uracil/ml. Protein extracts were prepared at the times indicated after the addition of glucose. Aliquots corresponding to 0.2 ml of culture were analyzed for uracil permease by Western immunoblotting. As already mentioned (48), potential dimers can be observed in the upper part of the gel. Bands just above the main signal correspond mostly to ubiquitin-permease conjugates over background (9), their steady-state abundance relative to the main signal is lower in galactose-grown cells (t0) than during further growth in the presence of glucose.
Ubiquitin-permease conjugates are up-regulated in uracil-exposed or stressed cells.
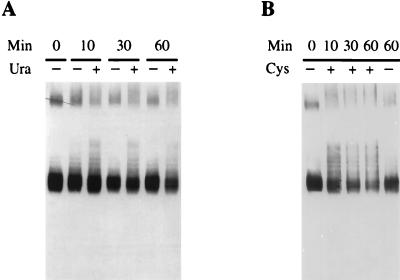
A direct relationship between ubiquitination and efficient removal of permease from the plasma membrane has been suggested (9, 32). We therefore tested whether the presence of uracil stimulated permease ubiquitination. Conditional thermosensitive act1-3 cells were used, as they are defective for the internalization step of endocytosis and thus accumulate ubiquitin-permease conjugates (9). act1-3 cells expressing galactose-driven permease were subjected to the galactose shut-off procedure in the presence or absence of uracil. Extracts from cells withdrawn at various times after transfer to the restrictive temperature 36°C were analyzed by Western blotting (Fig. 5A). Minor permease species were detected just above the main permease signal. These species that are ubiquitin conjugates (9) were more abundant in the presence of added uracil. An enrichment in ubiquitin-conjugates was also observed in response to general stress. Cysteine was found fortuitously to promote accelerated permease turnover (9). The addition of 5 mM cysteine to act1-3 cells, simultaneously with transfer at the restrictive temperature, led to substantial enrichment in ubiquitin-permease conjugates within a few minutes (Fig. 5B). A similar effect, although weaker, was observed when 15 mM 2-mercaptoethanol was added (data not shown). The addition of such thiol compounds alters the balance between reduced and oxidized intracellular glutathione (34) that controls various processes, including the disulfide formation machinery of the endoplasmic reticulum, and thus triggers the unfolding response. These data indicate that the increased turnover of uracil permease, whatever its origin, resulted from an increase in the extent of ubiquitination, which in turn speeded internalization of the permease.
FIG. 5.
Production of ubiquitin-permease conjugates is promoted in stimulated turnover conditions. NY279 (act1) cells transformed with plasmid p195gF were grown at 24°C to an A600 of 0.5, with galactose as the carbon source. Glucose was then added, and 20 min later the cells were shifted to the nonpermissive temperature. Cells were brought rapidly to 36°C by dilution of the culture with an equal volume of medium prewarmed to 48°C and incubated in the absence (−) or presence (+) of 40 μg of uracil/ml (A) or 5 mM cysteine (B). Protein extracts were prepared from cells harvested at the times indicated after the shift to 36°C, and aliquots from 0.3 ml of culture were analyzed for uracil permease by Western immunoblotting. The putative dimers present at the top of the gel produced larger species in the presence of uracil.
Permease may be inhibited before its internalization.
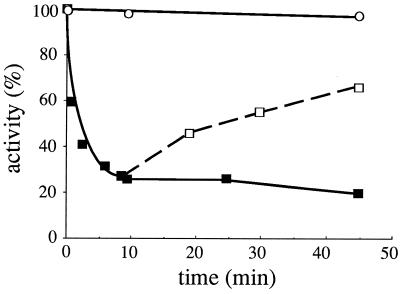
For several transporters negatively controlled by their ligands, inactivation precedes internalization (25, 30). We assessed whether exogenous uracil might have such an effect on its cognate permease. Npi1 cells were used to address this possibility, as they are defective for ubiquitination of uracil permease and hence for its internalization (9). The amount of permease immunodetected in these cells was maintained equally in the absence and presence of uracil (see above). After glucose arrest of galactose-driven permease synthesis, npi1 cells were provided with uracil for various times, collected by filtration, thoroughly washed to eliminate cold uracil, and tested for uracil uptake activity (Fig. 6). The rate of uracil uptake fell very rapidly (half-time, 1 min). The presence of uracil did not completely abolish uptake; a new steady-state level of activity was established. This inhibition could be reversed by removing uracil from the growth medium (Fig. 6). The recovery of uracil uptake activity could not be due to neosynthesized permease, since the addition of glucose had shut off the GAL10 promoter. The kinetics of recovery were slower than those of inactivation and probably involved the removal of excess internal uracil by its further metabolization. Parental cells exposed to uracil under similar conditions displayed a decrease in activity, with the same kinetics (data not shown). This inactivation allows more rapid adjustment of uracil uptake than endocytic internalization of the permease, which is a relatively slow process. Uracil-induced inhibition could not be evidenced in FL200 wild-type cells that expressed only the chromosome-encoded uracil permease, but interestingly, cytosine availability producing an intracellular concentration of uracil higher than that produced by uracil import (22) rapidly inhibited the chromosomal uracil permease (data not shown). These results suggest that the inhibition was due to direct binding of intracellular uracil to a site on the cytoplasmic domain of the permease.
FIG. 6.
Inhibition of uracil uptake activity. 27038a (npi1) cells producing the uracil permease from plasmid p195gF were grown to mid-log phase with galactose. Glucose was then added, and 20 min later 40 μg of uracil/ml was added. At various times, cells were quickly collected by filtration, extensively washed, and resuspended in a prewarmed uracil-free medium. Uracil uptake activity was immediately assayed by incubation with [14C]uracil (■). Complete stability of uracil uptake in npi1 cells incubated in the absence of uracil was verified by assays under the same conditions (○). To check the reversibility of the inhibition, cells incubated for 8 min with uracil were filtered, extensively washed, transferred to prewarmed uracil-free medium, and further incubated for various periods of time. Then uracil uptake was measured (□).
DISCUSSION
We describe the down-regulation of uracil uptake activity in yeast by exogenous pyrimidines. This type of regulation was proposed in pioneering work on pyrimidine uptake and metabolism (13). We showed that the down-regulation is due to events at three levels as follows: the synthesis and degradation rates of the permease and the decrease of its catalytic activity. Note that FUR4 gene transcription is under the control of the dhu1 locus (4). Neither the DHU1 gene nor conditions causing FUR4 derepression have been identified, but we showed that the constitutively derepressed uracil permease in dhu1 cells displayed the same behavior toward exogenous uracil and cytosine as that observed in wild-type cells. FUR4-disrupted cells display no obvious phenotypic defect, at least not under standard laboratory conditions, and thus uracil permease is a nonessential protein. It is unclear why uracil permease is regulated so tightly. Yeast cells, like other organisms, salvage uracil of exogenous or catabolic origin but must avoid high cellular levels of dUTP because the utilization of dUTP produces extensively uracil-substituted DNA that has been shown to be lethal (8). Although overexpression of uracil permease did not impair cell growth, the availability of uracil to such cells resulted in a 20 to 40% increase in doubling time compared to that of control cells that import uracil only by the chromosome-encoded permease. High levels of intracellular uracil and/or its derived nucleotides may be detrimental to cells, and down-regulation of the uracil permease may prevent excessive uracil uptake. This possibility is consistent with the observation of substantial release of uracil into the growth medium by cells impaired in the feedback regulation of the pyrimidine biosynthetic pathway (23).
The reduction in the steady-state abundance of FUR4 mRNA due to exposure to uracil cannot be accounted for by a transcriptional event because the decreases in the steady-state level after the addition of uracil were equal whether permease expression was controlled by its own promoter or by the GAL10 promoter. Moreover, β-galactosidase activity expressed from a FUR4-lacZ fusion was not sensitive to the presence of uracil. Therefore, the negative effect of uracil may involve enhanced instability of the transcript. This effect required a high uracil uptake activity and was associated with a decrease in growth rate. However, there was no simple relationship between growth rate and the abundance of the FUR4 transcript. For example, the abundance of the transcript was the same whether cells were grown on galactose or glucose (doubling time, 4 and 3 h, respectively). Exogenous uracil and cytosine positively control the mRNA level of the FUR1 gene encoding uracil phosphoribosyl transferase, which converts uracil into UMP (27). The opposite effects of exogenous pyrimidine on the abundance of FUR4 and FUR1 transcripts presumably contribute to the maintenance of the intracellular pool of uracil at a homeostatic low level. Indeed, subsequent metabolization of uracil was not required for down-regulation of the FUR4 mRNA abundance, as the effect was observed in FUR1-disrupted cells (data not shown).
Uracil permease mRNA is also sensitive to environmental changes. The synthesis and/or degradation rates of many transcripts change, often transiently, in response to various stresses. Mild heat shock, nutritional deprivation, progression through the growth curve cycle, and an upshift in carbon source can all modulate the level of both ribosomal RNAs and mRNAs for ribosomal proteins (21, 28, 52) and have similar effects upon the FUR4 mRNA and/or protein (this study and reference 49). As uracil-derived nucleotides are found mostly in ribosomes, this is not likely to be fortuitous. Moreover, as stressful conditions that activate permease turnover also lead to degradation of ribosomes, uracil of catabolic origin may signal down-regulation of the permease in cells subjected to stress.
We show that accelerated turnover of the uracil permease resulted from increased ubiquitination efficiency, indicating that the formation of ubiquitin conjugates is indeed the rate-limiting step in the internalization of the permease. Any stress, whatever its origin, may increase the activity of the ubiquitin conjugation system toward a set of target proteins, including uracil permease. Enhanced turnover triggered by uracil is more likely due to a change of the permease itself. The change in its phosphorylation pattern in response to uracil might induce it to change its conformation such that it becomes more susceptible to ubiquitination. By using mutant permeases, it was previously shown that phosphorylation of a PEST-like sequence is a prerequisite for efficient ubiquitination (32). The uracil permease is phosphorylated mainly, but not exclusively, within the PEST-like sequence, since phosphorylation was strongly reduced but not abolished in a mutant permease from which the PEST sequence had been deleted (32). Data presented here that linked uracil-induced underphosphorylation to more efficient ubiquitination might indicate that the phosphorylation of a residue lying outside the PEST-like sequence negatively controls the ubiquitination process. It is interesting that uracil permease was both less phosphorylated and less stable in galactose-grown cells than in glucose-grown cells (48, 49). Thus, a lower phosphorylation level, either in the presence of uracil or in galactose-grown cells, appeared to be correlated with a higher turnover rate for the permease.
In contrast, the underphosphorylation is not involved in the substantial loss of permease activity triggered by the exposure of cells to uracil, since the change of the phosphorylation level (shown in Fig. 1 and 4) did not occur within the short time that was required for the loss of most of the uptake activity (data not shown). In the case of the spermidine transporter, a direct binding of intracellular substrate to an allosteric site has been proposed to account for ligand-induced inactivation (25). Similarly, the feedback inhibition of uracil permease is possibly mediated by the direct binding of excess uracil to a site on the cytoplasmic side of the permease, since we have shown that uracil has to be taken up for the down-regulation of the uracil permease. Our results are thus coherent with a model in which the binding of uracil to the permease first induces a conformational change to an inactive form, ensuring a rapid decrease in uptake before the protein is internalized.
The regulation of inositol permease was previously compared with that of uracil permease (40). The data presented here emphasize the similarities of their behaviors. Both uracil and inositol permeases are down-regulated at several levels upon exposure to their own substrates, and both uracil and inositol can be excreted by yeast cells, indicating that their intracellular concentration must be held down to appropriate levels. Whether common elements regulate these down-regulations remains to be determined.
ACKNOWLEDGMENTS
We thank Rosine Labbe-Bois for invaluable advice, Danièle Urban-Grimal for constructive discussions and critical reading of the manuscript, and Alex Edelman for editorial assistance.
This work was supported by a special grant from CNRS (program Biologie Cellulaire, project no. 96105).
REFERENCES
- 1.Bisson L F, Coons D M, Kruckeberg A L, Lewis D A. Yeast sugar transporters. Crit Rev Biochem Mol Biol. 1993;28:259–308. doi: 10.3109/10409239309078437. [DOI] [PubMed] [Google Scholar]
- 2.Bonneaud N, Ozier-Kalogeropoulos O, Li G Y, Labouesse M, Minvielle-Sebastia L, Lacroute F. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast. 1991;7:609–615. doi: 10.1002/yea.320070609. [DOI] [PubMed] [Google Scholar]
- 3.Cereghino G P, Scheffler I E. Genetic analysis of glucose regulation in Saccharomyces cerevisiae: control of transcription versus mRNA turnover. EMBO J. 1996;15:363–374. [PMC free article] [PubMed] [Google Scholar]
- 4.Chevallier M R. Cloning and transcriptional control of a eucaryotic permease gene. Mol Cell Biol. 1982;2:977–984. doi: 10.1128/mcb.2.8.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dancis A, Haile D, Yuan D S, Klausner R D. The Saccharomyces cerevisiae copper transporter protein (Ctr1p) J Biol Chem. 1994;269:25660–25667. [PubMed] [Google Scholar]
- 6.Didion T, Regenberg B, Jorgensen M U, Kielland-Brandt M C, Andersen H A. The permease homologue Ssy1p controls the expression of amino acid and peptide transporter genes in Saccharomyces cerevisiae. Mol Microbiol. 1998;27:643–650. doi: 10.1046/j.1365-2958.1998.00714.x. [DOI] [PubMed] [Google Scholar]
- 7.Eddy A A. Expulsion of uracil and thymine from the yeast Saccharomyces cerevisiae: contrasting responses to changes in the proton electrochemical gradient. Microbiology. 1997;143:219–229. doi: 10.1099/00221287-143-1-219. [DOI] [PubMed] [Google Scholar]
- 8.Gadsden M H, McIntosh E M, Game J C, Wilson P J, Haynes R H. dUTP pyrophosphatase is an essential enzyme in Saccharomyces cerevisiae. EMBO J. 1993;12:4425–4431. doi: 10.1002/j.1460-2075.1993.tb06127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galan J M, Moreau V, André B, Volland C, Haguenauer-Tsapis R. Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. J Biol Chem. 1996;271:10946–10952. doi: 10.1074/jbc.271.18.10946. [DOI] [PubMed] [Google Scholar]
- 10.Gietz D, St. Jean A, Woods R A, Schiestl R H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gitan R S, Luo H, Rodgers J, Broderius M, Eide D. Zinc-induced inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation. J Biol Chem. 1998;273:28617–28624. doi: 10.1074/jbc.273.44.28617. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez C I, Martin C E. Fatty acid-responsive control of mRNA stability. J Biol Chem. 1996;271:25801–25809. doi: 10.1074/jbc.271.42.25801. [DOI] [PubMed] [Google Scholar]
- 13.Grenson M. The utilization of exogenous pyrimidines and the recycling of uridine-5-phosphate derivatives in Saccharomyces cerevisiae, as studied by means of mutants affected in pyrimidine uptake and metabolism. Eur J Biochem. 1969;11:249–260. doi: 10.1111/j.1432-1033.1969.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 14.Hein C, Springael J Y, Volland C, Haguenauer-Tsapis R, André B. NPI1, an essential yeast gene involved in induced degradation of Gap1 and Fur4 permeases, encodes the Rsp5 ubiquitin-protein ligase. Mol Microbiol. 1995;18:77–87. doi: 10.1111/j.1365-2958.1995.mmi_18010077.x. [DOI] [PubMed] [Google Scholar]
- 15.Herrick D, Parker R, Jacobson A. Identification and comparison of stable and unstable mRNAs in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:2269–2284. doi: 10.1128/mcb.10.5.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herruer M H, Mager W H, Raué H A, Vreken P, Wilms E, Planta R J. Mild temperature shock affects transcription of yeast ribosomal protein genes as well as the stability of their mRNAs. Nucleic Acids Res. 1988;16:7917–7929. doi: 10.1093/nar/16.16.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins P, Chevallier M R, Jund R, Eddy A A. Use of plasmid vectors to show that the uracil and cytosin permeases of the yeast Saccharomyces cerevisiae are electrogenic proton symports. FEMS Microbiol Lett. 1988;49:173–177. [Google Scholar]
- 18.Horak J, Wolf D H. Catabolite inactivation of the galactose transporter in the yeast Saccharomyces cerevisiae: ubiquitination, endocytosis, and degradation in the vacuole. J Bacteriol. 1997;179:1541–1549. doi: 10.1128/jb.179.5.1541-1549.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iraqui I, Vissers S, Bernard F, De Craene J-O, Boles E, Urrestarazu A, André B. Amino acid signaling in Saccharomyces cerevisiae: a permease-like sensor of external amino acids and F-box protein Grr1p are required for transcriptional induction of the AGP1 gene, which encodes a broad-specificity amino acid permease. Mol Cell Biol. 1999;19:989–1001. doi: 10.1128/mcb.19.2.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaquet L, Lollier M, Souciet J L, Potier S. Genetic analysis of yeast strains lacking negative feedback control: a one-step method for positive selection and cloning of carbamoylphosphate synthetase-aspartate transcarbamoylase mutants unable to respond to UTP. Mol Gen Genet. 1993;241:81–88. doi: 10.1007/BF00280204. [DOI] [PubMed] [Google Scholar]
- 21.Ju Q, Warner J R. Ribosome synthesis during the growth cycle of Saccharomyces cerevisiae. Yeast. 1994;10:151–157. doi: 10.1002/yea.320100203. [DOI] [PubMed] [Google Scholar]
- 22.Jund R, Chevallier M R, Lacroute F. Uracil transport in Saccharomyces cerevisiae. J Membr Biol. 1977;36:233–251. doi: 10.1007/BF01868153. [DOI] [PubMed] [Google Scholar]
- 23.Jund R, Lacroute F. Genetic and physiological aspects of resistance to 5-fluoropyrimidines in Saccharomyces cerevisiae. J Bacteriol. 1970;102:607–615. doi: 10.1128/jb.102.3.607-615.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jund R, Weber E, Chevallier M R. Primary structure of the uracil transport protein of Saccharomyces cerevisiae. Eur J Biochem. 1988;171:417–424. doi: 10.1111/j.1432-1033.1988.tb13806.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaouass M, Gamache I, Ramotar D, Audette M, Poulin R. The spermidine transport system is regulated by ligand inactivation, endocytosis, and by the Npr1p Ser/Thr protein kinase in Saccharomyces cerevisiae. J Biol Chem. 1998;273:2109–2117. doi: 10.1074/jbc.273.4.2109. [DOI] [PubMed] [Google Scholar]
- 26.Kern L, de Montigny J, Jund R, Lacroute F. The FUR1 gene of Saccharomyces cerevisiae: cloning, structure and expression of wild-type and mutant alleles. Gene. 1990;88:149–157. doi: 10.1016/0378-1119(90)90026-n. [DOI] [PubMed] [Google Scholar]
- 27.Kern L, de Montigny J, Lacroute F, Jund R. Regulation of the pyrimidine salvage pathway by the FUR1 gene product of Saccharomyces cerevisiae. Curr Genet. 1991;19:333–337. doi: 10.1007/BF00309592. [DOI] [PubMed] [Google Scholar]
- 28.Klein C, Struhl K. Protein kinase A mediates growth-regulated expression of yeast ribosomal protein genes by modulating RAP1 transcriptional activity. Mol Cell Biol. 1994;14:1920–1928. doi: 10.1128/mcb.14.3.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacroute F. Regulation of pyrimidine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1968;95:824–832. doi: 10.1128/jb.95.3.824-832.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai K, Bolognese C P, Swift S, McGraw P. Regulation of inositol transport in Saccharomyces cerevisiae involves inositol-induced changes in permease stability and endocytic degradation in the vacuole. J Biol Chem. 1995;270:2525–2534. doi: 10.1074/jbc.270.6.2525. [DOI] [PubMed] [Google Scholar]
- 31.Lai K, McGraw P. Dual control of inositol transport in Saccharomyces cerevisiae by irreversible inactivation of permease and regulation of permease synthesis by INO2, INO4, and OPI1. J Biol Chem. 1994;269:2245–2251. [PubMed] [Google Scholar]
- 32.Marchal C, Haguenauer-Tsapis R, Urban-Grimal D. A PEST-like sequence mediates phosphorylation and efficient ubiquitination of the yeast uracil permease. Mol Cell Biol. 1998;18:314–321. doi: 10.1128/mcb.18.1.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medintz I, Jiang H, Han E K, Cui W, Michels C. Characterization of the glucose-induced inactivation of maltose permease in Saccharomyces cerevisiae. J Bacteriol. 1996;178:2245–2254. doi: 10.1128/jb.178.8.2245-2254.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 35.Myers A M, Tzagoloff A, Kinney D M, Lusty C J. Yeast shuttle and integrative vectors with multiple cloning sites suitable for construction of lacZ fusions. Gene. 1986;45:299–310. doi: 10.1016/0378-1119(86)90028-4. [DOI] [PubMed] [Google Scholar]
- 36.Ooi C E, Rabinovich E, Dancis A, Bonifacino J S, Klausner R D. Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. EMBO J. 1996;15:3515–3523. [PMC free article] [PubMed] [Google Scholar]
- 37.Ozcan S, Dover J, Johnston M. Glucose sensing and signaling by two glucose receptors in the yeast Saccharomyces cerevisiae. EMBO J. 1998;17:2566–2573. doi: 10.1093/emboj/17.9.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potier S, Lacroute F, Hubert J C, Souciet J L. Studies on transcription of the yeast URA2 gene. FEMS Microbiol Lett. 1990;60:215–219. doi: 10.1016/0378-1097(90)90374-y. [DOI] [PubMed] [Google Scholar]
- 39.Riballo E, Herweijer M, Wolf D H, Lagunas R. Catabolite inactivation of the yeast maltose transporter occurs in the vacuole after internalization by endocytosis. J Bacteriol. 1995;177:5622–5627. doi: 10.1128/jb.177.19.5622-5627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson K S, Lai K, Cannon T A, McGraw P. Inositol transport in Saccharomyces cerevisiae is regulated by transcriptional and degradative endocytic mechanisms during the growth cycle that are distinct from inositol-induced regulation. Mol Biol Cell. 1996;7:81–89. doi: 10.1091/mbc.7.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose M D, Winston F, Hieter P. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 42.Schmitt M E, Brown T A, Trumpower B L. A rapid method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990;18:3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shortle D, Novick P, Botstein D. Construction and genetic characterization of temperature-sensitive mutant allele of the yeast actin gene. Proc Natl Acad Sci USA. 1984;81:4889–4893. doi: 10.1073/pnas.81.15.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silve S, Volland C, Garnier C, Jund R, Chevallier M R, Haguenauer-Tsapis R. Membrane insertion of uracil permease, a polytopic yeast plasma membrane protein. Mol Cell Biol. 1991;11:1114–1124. doi: 10.1128/mcb.11.2.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Springael J-Y, Andre B. Nitrogen regulated ubiquitination of the Gap1 permease of Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:1253–1263. doi: 10.1091/mbc.9.6.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas B J, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- 47.Urban-Grimal D, Pinson B, Chevallier J, Haguenauer-Tsapis R. Replacement of Lys by Glu in a transmembrane segment strongly impairs the function of the uracil permease from Saccharomyces cerevisiae. Biochem J. 1995;308:847–851. doi: 10.1042/bj3080847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volland C, Garnier C, Haguenauer-Tsapis R. In vivo phosphorylation of the yeast uracil permease. J Biol Chem. 1992;267:23767–23771. [PubMed] [Google Scholar]
- 49.Volland C, Urban-Grimal D, Géraud G, Haguenauer-Tsapis R. Endocytosis and degradation of the yeast uracil permease under adverse conditions. J Biol Chem. 1994;269:9833–9841. [PubMed] [Google Scholar]
- 50.Wach A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast. 1996;12:259–265. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C259::AID-YEA901%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 51.Wach A, Brachat A, Pöhlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 52.Woolford J L, Jr, Warner J. The ribosome and its synthesis. In: Jones E W, Pringle J R, Broach J R, editors. The molecular and cellular biology of the yeast saccharomyces. 1. Genome dynamics, protein synthesis and energetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. pp. 587–626. [Google Scholar]