Abstract
Methods
This review was focused on studying the various secondary metabolites in model plants of Iranian herbal medicine known as treatment of kidney diseases in traditional Persian medicine textbooks including Makhzan-ol-Advieh, The Canon of Medicine, and Taghvim al-Abdan fi Tadbir al-Ensan.
Results
Secondary metabolites of 94 medical plants belonging to 42 families were reported with their scientific and family name.
Conclusion
Although herbal medicines are gaining rapid popularity among people and the pharmaceutical industry, the understandings of the phytochemical and therapeutic properties of medicinal plant are important for developing effective nephroprotective medicines. Therefore, the relationship between traditional uses and biological properties should be clearly verified through further studies.
1. Introduction
Since ancient times, natural products, such as parts of plants, animals, and microbes have been utilized in medicine to treat diseases. According to fossil records, human usage of plants as remedies may be dated back at least 60,000 years [1]. Over the past decade, an increasing attention has been focused on the effect of medicinal plants. Traditional herbs from different habitats and geographical locations can be considered as a new strategy for the treatment of injuries and protection against infections.
The kidney, as a vital organ, controls water balance, maintains electrolyte concentrations, removes nitrogenous waste products, and regulates blood pressure in the body [2]. The kidney is very susceptible to damage and injury and it might lose its function and cannot act as it should. Kidney diseases can be made up from inherited mutations [3] and chronic injuries, such as diabetes [4] and inflammatory diseases [5]. Moreover, the most famous renal failure known as kidney stone is appeared by abnormal accumulation of crystalline substances such as calcium. Typically, surgery of kidney stone causes to appear serious difficulties such as urinary tract obstruction, abnormal urine metabolism, and hydronephrosis [6]. This critical organ also goes through the processes of anatomical and physiological changes by aging, obesity, and hypertension. Moreover, a greater number of individuals have been diagnosed with chronic renal failure, which the body is unable to maintain metabolic, fluid, and electrolyte balance, resulting in a retention of urea and other nitrogenous wastes in the blood [7, 8]. The kidney is also remarkably prone to drug-induced toxicity due to exposure to the largest proportion of circulating chemicals and drugs [9]. Despite remarkable advances in diagnostic and treatment techniques of kidney diseases, the prevalence of renal dysfunction has been increasing in recent years. Interestingly, numerous experimental studies have revealed that herbal medicine has a beneficial effect on improving kidney function.
Epidemiological evidence suggests that natural bioactive substances play an essential role in the treatment and control of modern diseases [10]. Natural products, which have evolved over millions of years, have a distinct chemical diversity and result in a wide range of biological activities and drug-like qualities [1]. Plants produce constitutive metabolites known as phytochemicals which play a critical role in their survival and proper function. These chemical components not only protect plants from competitors, pathogens, or predators but also control the growth along with regulating the pollination, fertilization, and the rhizosphere environment [11]. Phytochemicals can be found in various parts of plant, including stems, leaves, roots, seeds, fruits, and flowers. However, many phytochemicals, notably color compounds, are found in high concentrations in the outer layers of plant tissues [12]. Previous investigations have reported that phytochemicals lead to reduction in the risk of some diseases such as coronary heart, diabetes, liver disorders, high blood pressure, as well as reducing the synthesis or absorption of cholesterol [13].
Phytochemicals are classified as primary and secondary metabolites, based on their function in plant metabolism. Primary metabolites are necessary for plant life and include carbohydrates, amino acids, proteins, lipids, purines, and pyrimidines of nucleic acids. On the contrary, secondary metabolites are the remaining plant chemicals produced by the cells through metabolic pathways derived from the primary metabolic pathways [14, 15]. These chemical components have been described as an antiviral, antifungal, and antibiotic, which are responsible for protecting plants from pathogens. Additionally, they are critical UV absorbing chemical factors, preventing severe leaf damage from the light [16]. Due to their great biological activities, plant secondary metabolites have been exerted for centuries in traditional medicine and the medicinal effects of the plants come from these molecules [17]. Moreover, various tissues and organs of medicinal plants could have peculiar medicinal properties at specific developmental phases [18]. These days, they are associated with valuable industries such as pharmaceutics, cosmetics, and fine chemicals [19].
Secondary metabolites in plants are classified into three main groups based on their biosynthetic pathway; (a) nitrogen-containing compounds such as alkaloids, glucosinolates, and cyanogenic glycosides, (b) phenolic compounds such as phenylpropanoids and flavonoids, and (c) terpenes [17, 20]. Alkaloids are a class of nitrogen-containing compounds produced in plants in response to biotic or abiotic environment which endows alkaloids to possess remarkable biological activities and structure diversity [21, 22]. Cyanogenic glycosides are amino acid-derived plant components found in more than 2500 plant species and are widely distributed among 100 families of flowering plants [23, 24]. The toxicity of cyanogenic glycoside derivatives is based on the release of hydrogen cyanide [25]. Glucosinolates contain sulfur and nitrogen produced in some plants and are chemically stable under normal conditions [26]. The nonprotein amino acids are structurally similar to protein amino acids and particularly participate in plant defense against stress and act as essential mediators in response to abiotic factors [27]. Amines as low molecular weight are nitrogenous compounds which are naturally present in plants and are responsible for many biological effects such as acting as important precursors of hormones [28]. Phenolic components are derived from shikimate, pentose phosphate, and phenylpropanoid pathways in plants and have an aromatic ring with one or more hydroxyl groups [29, 30]. Glycosides are usually organic molecules isolated from plant sources and consist of one or more sugars incorporated with phenol, alcohol, or a complex molecule such as a steroid nucleus [31]. Terpenoids are the most abundant group of plant secondary metabolites typically produced in flowers, vegetative tissues, and, roots [32]. They show a broad range of biological activities which result in lower total cholesterol, triglycerides, or LDL‐cholesterol, as well as blood pressure [33]. A variety of toxic proteins are expressed in plants and act as resistance factors against plant pathogens and herbivores. Most of toxic proteins accumulate in the vulnerable parts of the plant, such as vegetative storage tissues and seeds [34]. Carbohydrates are produced through photosynthesis in plants and are a crucial source of energy and carbon skeletons for organic compounds and storage components. In addition, they act as signaling molecules as same as hormones [35, 36]. 6, 9-polyunsaturated fatty acids are produced by plants and are essential to the human diet. These components are of importance increasingly as raw materials for industry [37]. Organic acids are intermediate or end products in various fundamental pathways in plant metabolism and catabolism [38].
The effect of medicinal plant utilizes is global and it has been expanding in numerous countries over the world [39]. Importantly, traditional Persian medicine as a source of alternative therapies has become popular over Iran and some countries globally. Iranian herbal medicine consists of natural compounds with complex active ingredients that cause valuable effects. Traditional Persian medicine has been widely used in treating kidney diseases due to its safety and economic advantages. Because of advances in modern technology, it is now possible to assess the pharmacology and mechanisms related to function of many Iranian herbs. A wide range of these medicinal plants has been studied to further apply of plants' function for agriculture, medicine, and chemical industries. This review was focused on studying the various secondary metabolites in model plants of traditional Persian medicine which they are known as a treatment of kidney diseases and injuries in traditional Persian medicine textbooks. We have given the review based on the most important clinical and pharmaceutical traditional Persian medicine textbooks, including Makhzan-ol-Advieh by Aqili (18th century), The Canon of Medicine by Avicenna (10th and 11th centuries), and Taghvim al-Abdan fi Tadbir al-Ensan (11th century). In this review, we investigated nitrogen-containing compounds including glucosinolates, alkaloids, cyanogenic glycosides, nonprotein amino acids, amines, and toxic proteins. Additionally, compounds including phenolic components, terpenoids, glycosides, carbohydrates, fatty acids, and organic acids were considered as non-nitrogen-containing components of medical plants.
2. Methods
First, we have gathered all information of medical plants which were responsible for the treatment of kidney diseases and introduced in Makhzan-ol-Advieh, The Canon of Medicine, and Taghvim al-Abdan fi Tadbir al-Ensan. Then, we have classified them into their scientific name and discussed their phytochemical composition in the next topic. We have collected reports from scientific articles from journals indexed online in PubMed, Science Direct, and Medline. The main findings are summarized in figures and a table.
3. Result
In the current review, a total of 94 medical plant species belonging to 42 families have been reported to treat kidney diseases in traditional Persian medicine textbooks specifically. Table 1 shows bioactive and secondary metabolites of medicinal plants of traditional Persian medicine with their scientific and family name. Among them, Apiaceae (11 species), Alliaceae (7 species each), Pinaceae, Fabaceae (6 species each), Lamiaceae, Malvaceae, and Asteraceae (5 species) were the dominant families.
Table 1.
Secondary metabolites of medicinal plants are used to treat kidney diseases according to traditional Persian medicine.
| No. | Scientific name | Family name | Glycoside | Organic acid | Carbohydrate | Fatty acid | Phenolic | Terpenoid | Toxic protein | Glucosinolate | Cyanogenic glycoside | Amine | Non-protein amino acid | Alkaloid | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Alisma plantago L. | Alismaceae | Ferulic acid, Rosmarinic acid | Triterpenes, diterpenes, sesquiterpenes |
[40] | ||||||||||
| 2 | Allium ampeloprasum L. | Alliaceae | Pinene, beta-pinene |
Dimethyl tetrasulphide | [41] | ||||||||||
| 3 | Allium ascalonicum L. (Allium minutiflorum) | Alliaceae | β-D-glucopyranoside] | Furostanol saponins | [42] | ||||||||||
| 4 | Allium cepa L. | Alliaceae | Quercetin glucosides | Formic acid | Humulaneand phytosterols | Fluorescent protein | [43] | ||||||||
| 5 | Allium porrum L. | Alliaceae | Sapogenin | [44] | |||||||||||
| 6 | Allium roseum L. | Alliaceae | Tetradecane | Hexadecanoic acid | Tricosane | [45] | |||||||||
| 7 | Allium ursinum L. | Alliaceae | Kaempferol | Lectins | [46] | ||||||||||
| 8 | Allium vineale L. (Allium vegetable) | Alliaceae | β-chlorogeni | [47] | |||||||||||
| 9 | Pistacia lentiscus var. Chia | Anacardiaceae | Limonene | Trans-pinocarveol | Α-pinene, α-terpinolene |
[48, 49] | |||||||||
| 10 | Pistacia lentiscus L. | Anacardiaceae | Cadinene | Oleanonic acid | Phytol, α-cadinol |
α-terpineol | [50] | ||||||||
| 11 | Pistacia vera L. | Anacardiaceae | Palmitic acid | Pinocarveol | [51] | ||||||||||
| 12 | Anethum graveolens L. | Apiaceae |
β-pinene, p-cymene, limonene |
[52] | |||||||||||
| 13 | Apium graveolens L. | Apiaceae | Sucrose | Geraniol | β-amyloid | [53] | |||||||||
| 14 | Carum bulbocastanum—(Boiss.) B. Fedtsch. | Apiaceae | Dillapiole | β-Germacrene- | [54] | ||||||||||
| 15 | Carum carvi L. | Apiaceae | Benzenedicarboxylic acid | Limonene, carvone |
[55] | ||||||||||
| 16 | Carum copticum L. | Apiaceae | Thymol |
β-pinene, α-pinene |
[56, 57] | ||||||||||
| 17 | Ferula assa- foetida L. | Apiaceae | P-Cymene | Thymol | α-pinene, phellandrene | [58] | |||||||||
| 18 | Ferula narthex | Apiaceae | Coumarins | [59] | |||||||||||
| 19 | Lagoecia cuminoides L. | Apiaceae | A-pinene, myrcene, limonene | [60] | |||||||||||
| 20 | Petroselinum crispum | Apiaceae | 1,2 benzene-dicarbonic acid | Α-pinen, β-phellandrene | [61, 62] | ||||||||||
| 21 | Seseli tortuosum L. (Seseli libanotis) | Apiaceae | Coumarin | Myrcene | [63] | ||||||||||
| 22 | Sium latifolium L. | Apiaceae | α-thujene, α-pinene | [64] | |||||||||||
| 23 | Arum italicum L. | Araceae | Guaiacylglycerol-β-coniferyl | 8-O-4′-neolignan glucoside | [65] | ||||||||||
| 24 | Arum maculatum L. | Araceae | α-pinene, β-pinene, terpinolene | Indole | [66] | ||||||||||
| 25 | Colocasia antiquorum (Colocasia esculenta) | Araceae | 10-octadecenoic acid | Trypsin | [67] | ||||||||||
| 26 | Cocos nucifera L. | Arecaceae | Sucrose, glucose, fructose | Ascorbic acid | Carbohydrate | Tryptophan | Alanineβ-Alanine, Aspartic acid | Thiamin | [68] | ||||||
| 27 | Asarum europaeum L. | Aristolochiaceae | α-Asarone, β-Asarone | [69] | |||||||||||
| 28 | Asparagus adscendens | Asparagaceae | Spirostanosides | β-D-glucopyranosyl]-(25S)-spirostan-5-en-3β-ol | [70] | ||||||||||
| 29 | Asparagus officinalis L. | Asparagaceae | Capsanthin, Violaxanthin | [71] | |||||||||||
| 30 | Asparagus racemosus | Asparagaceae | n-Hexadecanic acid, Oleic acid | [72] | |||||||||||
| 31 | Aloe vera L. | Asphodelaceae | Glucomannan | Oxalic acid | Tridecanoic acid | Anthraquinone/Phytol | [73] | ||||||||
| 32 | Artemisia abrotonon L. (Artemisia abyssinica) (Artemisia ketone) (Artemisia annua) | Asteraceae | 1,8-cineole | Methyl eugenol, camphor | Total protein | Alkaloids | [74] | ||||||||
| 33 | Artemisia montana (Artemisia ketone, Artemisia annua) | Asteraceae | Ascorbic acid | Quercetin | Camphor | [75] | |||||||||
| 34 | Chrysanthemum indicum L. | Asteraceae | Naphthaleneboronic acid | β-Myrcene | Bornyl acetate | Camphor | [76] | ||||||||
| 35 | Cichorium intybus L. | Asteraceae | Glycosides | Fatty acids | Lactucin, 8-deoxylactucin | Lactupicrin | Alkaloids | [77] | |||||||
| 36 | Cichorium pumilum | Asteraceae | Lactucin | Anthocyanins | Anthocyanins | [78] | |||||||||
| 37 | Capparis decidua (Capparis cartilaginea, Capparis deserti) | Capparidaceae | Butyl isothiocyanate | Ascorbic acid | Cellulose | Isothiocyanate | Alkaloids | [79] | |||||||
| 38 | Capparis spinosa L. (Capparis sicula) | Capparidaceae | Capparisine | Protocatechuric acid | (z,z)-9,12-octadecadienoic acid | Furfural, Bis(5-for-mylfurfuryl) ether | Capparisine | [80] | |||||||
| 39 | Cucumis colocynthis L. | Cucurbitaceae | Cucurbitacin glucosides | Isosaponarin | [81] | ||||||||||
| 40 | Cyperus longus L. | Cyperaceae | α-longipinene | [82] | |||||||||||
| 41 | Cyperus rotundus L. | Cyperaceae | Myrcene | Isocurcumenol | Α-pinene, P-cymene |
Alkaloids | [83] | ||||||||
| 42 | Equisetum arvense L. | Equisetaceae | Hexahydrofarnesyl acetone, Thymol | [84] | |||||||||||
| 43 |
Acacia catechu (L.)
(Acacia Concinna) |
Fabaceae | Malic acid | Saponins | Alkaloids | [85] | |||||||||
| 44 |
Alhagi mannifera
(Alhagi maorurum) (Alhagi pseudalhagi) |
Fabaceae | Saliylic acidvanillic acid | Quercetin | Salsolidine | [86] | |||||||||
| 45 | Glycryrrhiza glabra L. | Fabaceae | Pectin | Flavonoids | Triterpene | [87] | |||||||||
| 46 |
Phaseolus vulgaris L.
(Phaseolus aureus) |
Fabaceae | Trichloroacetic acid | L-tryptophannone | N-acetyl mannosamine | [88] | |||||||||
| 47 |
Vigna reflexo-pilosa
(Vigna radiata) |
Fabaceae | Oleic acid | Galactosylononitol | [89] | ||||||||||
| 48 | Vigna unguiculata | Fabaceae | Sterols | Triterpene | [90] | ||||||||||
| 49 |
Ajuga chamaepitys (L.)
(Ajuga. Reptans) |
Lamiaceae | (α-1,6-galactosyl sucrose) | Carbohydrate | Iridoid | [91] | |||||||||
| 50 |
Ajuga iva L.
(Ajuga orientalis) (Ajuga bracteosa) |
Lamiaceae | Linalool Methyl salicylate | Limonene | [92] | ||||||||||
| 51 | Melissa officinalis L. | Lamiaceae | Epigallocatechin-3-gallate | Rosmarinic acid | Β-carotene | Anthocyanidin, Curcumin |
Citral | Caffeine, Nicotine | [93] | ||||||
| 52 | Origanum majorana L. | Lamiaceae | 4-terpineol | Sabinene | [94] | ||||||||||
| 53 | Teucrium chamaedrys L. | Lamiaceae | Bicyclo [4.4.0] dec-1-ene | 2-Pentadecanone | α-pinene, β-pinen | [95] | |||||||||
| 54 | Cinnamomum bejolghota | Lauraceae | Terpenes | Phenylpropanoids | [96] | ||||||||||
| 55 | Cinnamomum camphora | Lauraceae | Hexadecy | Α-thujene, α-pinene, camphene | [97] | ||||||||||
| 56 | Laurus nobilis L. | Lauraceous | Lauric acid, Myristic acid | Terpinenol | Camphene sabinene, myrcene | [98] | |||||||||
| 57 | Hyacinthus orientalis | Liliaceae | Anthocyanin 3,5-diglucosides | P-cis-coumaric acid, caffeic acid, malonic acid |
Delphinidin | [99] | |||||||||
| 58 | Linum catharticum L. | Linaceae | Octadecanoic acid | (2-hydroxyethyl)amide | [100] | ||||||||||
| 59 | Linum usitatissimum L. | Linaceae | Vanillic acid | α-linolenic acid | Aspartine, Threonine | [101] | |||||||||
| 60 |
Malva sp.
(Malva mauritiana) |
Malvaceae | D-glucose | [102] | |||||||||||
| 61 | Althaea sp. | Malvaceae | Stearic acids | Trenoids | [103] | ||||||||||
| 62 | Gossypium herbaceum L. | Malvaceae | Linoleic acid | β-bisabolol | Tetrahydrolinaloo | [104] | |||||||||
| 63 | Malva parviflora L. | Malvaceae | Cyclopropene | [105] | |||||||||||
| 64 | Malva sylvestris L. | Malvaceae | α-linolenic acid | Quercetin | [106] | ||||||||||
| 65 | Ficus sycomorus L. | Moraceae | 3-acetyl-citric acid | Ethyl-4-methyltetrahydrofuran-3-ol | [107] | ||||||||||
| 66 | Morus alba L. | Moraceae | Cyanidin-3-glucoside, cyanidin-3-glucosylrhamnoside | Apigenin | [108] | ||||||||||
| 67 | Musa sp. | Musaceae | Ascorbic acid, vitamin A | β-carotene, α-carotene | [109] | ||||||||||
| 68 | Syzygium aromaticum (L.) | Myrtaceae | Hexadecanoic acid | Eugenol, | Thymol, caryophyllene oxide | Proteins p53, protein bcl-2 | [110] | ||||||||
| 69 | Sesamum indicum L. | Pedaliaceae | Sesaminol glucosides | 1-hydroxypinoresinol, antioxidant lignans | α-Globulin | [111] | |||||||||
| 70 |
Pinus cembera L. Pinus cembra L. |
Pinaceae | Limonene, β-phellandrene, α-pinene | [112] | |||||||||||
| 71 | Pinus eldarica | Pinaceae | Flavonoids | Proanthocyanins, Flavonols | [113] | ||||||||||
| 72 | Pinus halepensis | Pinaceae | Carbohydrate | Myristic, oleic and linoleic acids | Terpenes, α-β-pinene, limonene | Protein | [114] | ||||||||
| 73 | Pinus nigra | Pinaceae | α-pinene | [115] | |||||||||||
| 74 | Pinus pinea L. | Pinaceae | Fatty acid | Monoterpene, β-pinene | [116] | ||||||||||
| 75 | Pinus sylvestris L. | Pinaceae | Naphthaleneacetic acid | Sucrose | Monoterpene, α-pinene | Benzyladenine | Phenylalanine | [117] | |||||||
| 76 | Piper cubeba L. | Piperaceae | Linalool, caryophyllene | [118] | |||||||||||
| 77 | Piper nigrum L. | Piperaceae | Carboxylic acids, 2,4 tetradecadienoic acid |
Tetracosanoic acid | Anthraquinones | Cadinene, caryophyllene | Piperine, lkaloids | [119] | |||||||
| 78 | Oryza sativa L. | Poaceae | Oligosaccharide | Glycoproteins, α1-acid glycoprotein | Tryptophan | Indole-alkaloid glucoside | [120] | ||||||||
| 79 | Polyporus officinalis | Polyporaceae | Methyl jasmonate | Hexacosanoic acids, pentadecanoic acid | [121] | ||||||||||
| 80 | Anagallis arvensis L. | Primulaceae | Saponins | [122] | |||||||||||
| 81 |
Zizyphus jujuba
(Zizyphi Spinosi semen) |
Rhamnaceae | Saponins | [123] | |||||||||||
| 82 | Prunus avium L. | Rosaceous | Phenolic acid, protocatechuic acid | Tannic acid, protocatechuic acid | Thymol, carvacrol |
[124] | |||||||||
| 83 | Rubus fruticosus L. | Rosaceous | Lanceol | [125] | |||||||||||
| 84 | Nauclea sp. | Rubiaceae | 9,12-octadecadienoic acid, 2-oxopentanedioic acid | Carbohydrates | 17-octadecynoic acid, ethyl ester | Phenolics, 2-methoxy-4-vinylphenol | Phytol, terpenoids | Alkaloids | [126] | ||||||
| 85 | Citrus aurantium L. | Rutaceae | Geraniol, a-terpineol | Methyl anthranilate | [127] | ||||||||||
| 86 | Physalis alkekengi L. | Solanaceae | Glycosides, physanosides | Luteolin | 6S,9R)-roseoside, (6S,9S)-roseoside, citroside A | Alkaloids, hyoscyamine | [128] | ||||||||
| 87 | Styrax officinalis L. | Styracaceae | Eugenol | Terpenoids, linalool | [129] | ||||||||||
| 88 |
Aquilaria malaccensis (Aquilegia vulgaris L., Aquilegia canadensis L., Aquilegia chrysantha, Aquilegia glandulosa) |
Thymelaeaceae | Fatty acid | Caryophyllene oxide | [130] | ||||||||||
| 89 | Trapa natans L. | Trapaceae | (all E)-squalene | [131] | |||||||||||
| 90 | Nardostachys jatamansi | Valerianaceae | Nardin | Coumarins | Sesquiterpenes | Alkaloids | [132] | ||||||||
| 91 |
Valeriana celtica L Valeriana officinalis L. Valeriana wallichii (Valeriana italica, Valeriana tuberosa(3)) |
Valerianaceae | 3-Methyl valeric acid | Isovaleric acid, tetradecanoic acid | Lignans, caffeic acid derivatives | Valerenic acid, sesquiterpene, monoterpenoids | Amino acids | Alkaloids | [133] | ||||||
| 92 | Viola odorata L. (Viola etrusca) | Violaceae | α-Pinen, xamphene | [134] | |||||||||||
| 93 | Viola tricolor L. | Violaceae | Mucilages | Saponins, carotenoids | [135] | ||||||||||
| 94 | Vitis vinifera L. | Vitaceae | Farnesene | [136] |
Figure 1 represents the ratio of two groups of bioactive components in the medical plant of this study. It is shown that the phytochemical components without nitrogen are the major part of these plants (82%) compared to the nitrogen containing component (18%).
Figure 1.
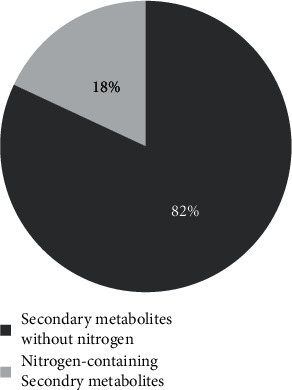
The ratio of phytochemical components of the medicinal plants studied in the current review.
As can be seen in Figure 2, most of the present medicinal plants contained terpenoids (63%) with considerable effects on the treatment of renal failure. 53% of mentioned plants possessed phenolic components. Moreover, organic acids, fatty acids, and glycosides were observed in 26%, 23%, and 22% of medicinal plants, respectively. Among the nitrogen-containing components, alkaloids were seen in 17% of plants and toxic proteins, nonprotein amino acids, amines, cyanogenic glycosides, and glucosinolates were demonstrated in 13%, 8%, 4%, 2%, and 1% of plants, respectively.
Figure 2.
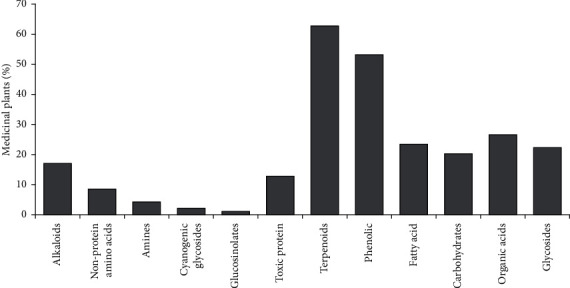
Percentage of the medicinal plants containing secondary metabolites studied in the current review.
4. Discussion
Plants play an essential role in primary health care and treatment of diseases and disorders in traditional medicine. Kidney disorders and urinary infections are common in people over the world and a large number of research works has been done to overcome these challenges. Medicinal plants offer an attractive source for improving kidney function and treating the symptoms of renal disorders. Herein, we have systematically summarized the secondary metabolites of the medical plants introduced in traditional Persian medicine books.
Several studies have shown the kidney treatment properties of some plants presented in the current review on the folk and traditional medicines of the Mediterranean, China, Bulgaria, and Turkey. In Bulgarian traditional and folk medicine, Arum maculatum tuber has been shown to be widely used in cases of kidney stones [137]. Furthermore, aerial parts of Petroselinum crispum impact kidney stones by consuming a decoction of fresh roots as tea in Turkish folk medicine [138]. In European herbal medicine, Cichorium pumilum is known helpful in cleaning the body and stimulating the eliminative process both via intestines and kidneys [139]. The traditional medicine in Algeria believes that Pinus halepensis act as medical plants for healing stomachaches and kidney inflammations [140]. Alisma plantago L. ameliorates hypertension and renal injury based on traditional Chinese medicine [141].
Although these herbal medicines are popular in folk culture, the understandings of the phytochemical and the mode of action of based-plant medicines are of great importance for the development of safe and effective nephroprotective drugs. Over the past years, numerous studies have been performed on some of these traditional medicine plants to investigate their effect on kidney dysfunction. According to the previous papers, Cocos nucifera L. was a urinary antiseptic and coconut water seemed to have protective effects and treated kidney and urethral stones effectively [142, 143]. Aloe vera leaf gel extract showed improvement in the mild damage caused by type2 diabetes on kidney tissue [144]. Aqueous and ethanolic extract powders of dried Syzygium aromaticum buds include adequate gallic acids which are one of the considerable compounds of phenolic. It was shown to have a strong antioxidant impact of gallic acid on kidney dysfunction in rats [145]. Equisetum arvense L. with traces of alkaloids, flavonoids, triterpenoids, phytosterols is the most popular species from the Equisetum genus whose diuretic effects were confirmed in animal models and clinical trials [84]. Camphor is found in roots and stem of the Cinnamomum camphora and is produced for health, medical, and industrial applications. Camphor treatment of diabetic rats reduced the oxidative stress markers in the liver and kidney tissues compared to control rats [146]. It was observed that treatment with Allium porrum L. extract decreased the number of crystals in kidney sections, and creatinine levels in treated animals in comparison with the control group. It suggested that the plant could be an excellent candidate to inhibit the formation of calcium oxalate crystals in the kidney [147].
Due to the active ingredients and active flavonoids of Pistacia vera L., the hydroalcoholic extract was effective on urinary tract (kidney and bladder) disorders by the reducing inflammation and oxidative stress in the kidney. Pistachio extract enhanced creatinine clearance and reduced the urine volume, urine glucose, serum creatinine, blood urea nitrogen levels, and histopathological scores in all doses; however, the highest change was seen at dose of 100 mg/kg [148]. The impact of the extract of Carum copticum seeds was investigated on the urinary stones of 350 patients. 100%, 53%, and 31.25%, of calcium oxalate, calcium oxalate/uric acid, and calcium-oxalate/hydroxyapatite stones, respectively, were treated with the extract [149]. Phytochemical screening showed that Capparis spinosa seed extracts consist of high level of phenolic compounds with individual molecules with high nephroprotective and hepatoprotective activity. Histopathological observation confirmed that pretreatment with extract of C. spinosa improved the damages detected in the kidney [150]. A paper reported that the dose of 200 mg/kg and 400 mg/kg of methanolic extract of Laurus nobilis preserved the functional capacity of the kidney against paracetamol toxicity in treated rat [151]. It was reported that the administration of Malva sylvestris extract not only significantly protected against lithium-induced oxidative damage, histopathological damage, and biochemical changes but also decreased the abnormal features detected in kidney slices of poisoned rats due to the presence of phenolic acids and flavonoids [152, 153]. The Morus alba L. methanolic extract in different mice organs improved the oxidative stress in kidney and consequently, renal functions were modulated. It suggested that the presence of the phenolic groups such as quercetin and naringenin in M. alba could be responsible for OH radical scavenging activity [154]. Supplementing with Sesamum indicum L. oil showed a significant reduction in ALP activities in the kidney with no corresponding increase in the serum, thus suggesting that the benniseed oil appears to attenuate the effect the hypercholesterolemic diet on the kidney [155]. In treatment of calcium oxalate urolithiasis in rats with Piper cubeba L. fruit extract, urinary crystals and histopathological derangement were improved at the doses 35 mg/kg and 60 mg/kg through significant decrease in urinary calcium after 14 days [156]. Oryza sativa L., as a rich source of anthocyanin, was investigated in renal function in obese rats. It was observed to show a reduction in renal injury by the attenuation of either oxidative stress, or apoptosis of renal cells [157].
A paper indicated that Zizyphus jujuba aqueous extract at a concentration of 500 mg/kg had a therapeutic role in reducing nephrotoxicity induced by ibuprofen that is a nonsteroidal anti-inflammatory drug and relieves pain and swelling [158]. The Citrus aurantium L. extract at a dose of 200 mg/kg was treated for a period of 21 days against gentamicin-induced renal damage. According to the results, C. aurantium L. extracts successfully protected renal damage associated with gentamicin due to its flavonoid contents and antioxidant properties [159]. Hydroalcoholic extract of Physalis alkekengi L. at a dose of 420 mg/kg was investigated for its nephroprotective activity against cisplatin-induced acute renal injury in rats of either sex for 10 days. The results showed a significant reduction in the elevated blood urea, serum creatinine, and uric acid and also normalized the histopathological changes [160]. Additionally, the biochemical and histopathological results clarified the role of phenolic-rich Vitis vinifera L. in improving the toxicity of CCl4 in the kidney of rats by suppressing the ROS/NF-κB signaling pathway [161].
Ideally, more investigations on the chemical and pharmacological activities of these medicinal plants are needed to discover their mechanisms and to define the metabolites responsible for their activities. Furthermore, promising chemical compounds should be extracted to find their effects on the treatment of kidney failure and the relationship between biological features and traditional uses should be clearly verified through further studies.
Acknowledgments
This study was carried out without funding and at personal expense.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
References
- 1.Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules . 2016;21(5):p. 559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Little M. H., Combes A. N. Kidney organoids: accurate models or fortunate accidents. Genes & Development . 2019;33(19-20):1319–1345. doi: 10.1101/gad.329573.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soliman N. A. Orphan kidney diseases. Nephron Clinical Practice . 2012;120(4):c194–199. doi: 10.1159/000339785. [DOI] [PubMed] [Google Scholar]
- 4.Lu Z., Zhong Y., Liu W., Xiang L., Deng Y. The efficacy and mechanism of Chinese herbal medicine on diabetic kidney disease. Journal of Diabetes Research . 2019;2019:14. doi: 10.1155/2019/2697672.2697672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W. R., Parikh C. R. Biomarkers of acute and chronic kidney disease. Annual Review of Physiology . 2019;81(1):309–333. doi: 10.1146/annurev-physiol-020518-114605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du H., Lei Y., Gao A. N., et al. Traditional Chinese medicine combined with conventional therapy for female kidney stone: a protocol for systematic review. Medicine (Baltimore) . 2020;99(13) doi: 10.1097/md.0000000000019611.e19611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanif M., Javed H., Jallani U., Ranjha N. M. Prevalence of end stage renal disease in diabetic obese and hypertensive patients and cardiovascular risk in dialysis patients. Pakistan Journal of Pharmaceutical Research . 2016;2(1):42–48. doi: 10.22200/pjpr.2016142-48. [DOI] [Google Scholar]
- 8.Denic A., Glassock R. J., Rule A. D. Structural and functional changes with the aging kidney. Advances in Chronic Kidney Disease . 2016;23(1):19–28. doi: 10.1053/j.ackd.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiong H. Y., Huang P., Xiong S., Li Y., Vathsala A., Zink D. Drug-induced nephrotoxicity: clinical impact and preclinical in vitro models. Molecular Pharmaceutics . 2014;11(7):1933–1948. doi: 10.1021/mp400720w. [DOI] [PubMed] [Google Scholar]
- 10.Xiao J. Handbook of Dietary Phytochemicals . Berlin, Germany: Springer; 2015. [Google Scholar]
- 11.Molyneux R. J., Lee S. T., Gardner D. R., Panter K. E., James L. F. Phytochemicals: the good, the bad and the ugly? Phytochemistry . 2007;68(22-24):2973–2985. doi: 10.1016/j.phytochem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 12.King A., Young G. Characteristics and occurrence of phenolic phytochemicals. Journal of the American Dietetic Association . 1999;99(2):213–218. doi: 10.1016/s0002-8223(99)00051-6. [DOI] [PubMed] [Google Scholar]
- 13.Saxena M., Saxena J., Nema R., Singh D., Gupta A. Phytochemistry of medicinal plants. Journal of Pharmacognosy and Phytochemistry . 2013;1 [Google Scholar]
- 14.Bone K., Mills S. Principles and Practice of Phytotherapy . 2nd. St. Louis, MO, USA: Churchill Livingstone; 2013. 2—principles of herbal pharmacology; pp. 17–82. [Google Scholar]
- 15.Hussein R., El-Anssary A. Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. Herbal medicine . 2019;1:p. 13. doi: 10.5772/intechopen.76139. [DOI] [Google Scholar]
- 16.Bourgaud F., Gravot A., Milesi S., Gontier E. Production of plant secondary metabolites: a historical perspective. Plant Science . 2001;161(5):839–851. doi: 10.1016/s0168-9452(01)00490-3. [DOI] [Google Scholar]
- 17.Jamwal K., Bhattacharya S., Puri S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. Journal of Applied Research on Medicinal and Aromatic Plants . 2018;9:26–38. doi: 10.1016/j.jarmap.2017.12.003. [DOI] [Google Scholar]
- 18.Bartwal A., Mall R., Lohani P., Guru S. K., Arora S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. Journal of Plant Growth Regulation . 2013;32(1):216–232. doi: 10.1007/s00344-012-9272-x. [DOI] [Google Scholar]
- 19.Liu Z., Wang H., Xie J., et al. The roles of cruciferae glucosinolates in disease and pest resistance. Plants . 2021;10(6):p. 1097. doi: 10.3390/plants10061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jan R., Asaf S., Numan M., Kim K.-M., Kim K. M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy . 2021;11(5):p. 968. doi: 10.3390/agronomy11050968. [DOI] [Google Scholar]
- 21.Pong Y. N., Terry C. T., Nancy Y. I. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochemistry International . 2015;89:260–270. doi: 10.1016/j.neuint.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Takooree H., Aumeeruddy M. Z., Rengasamy K. R., et al. A systematic review on black pepper (Piper nigrum L.): from folk uses to pharmacological applications. Critical Reviews in Food Science and Nutrition . 2019;59(sup1):S210–S243. doi: 10.1080/10408398.2019.1565489. [DOI] [PubMed] [Google Scholar]
- 23.Vetter J. Plant cyanogenic glycosides. Toxicon . 2000;38(1):11–36. doi: 10.1016/s0041-0101(99)00128-2. [DOI] [PubMed] [Google Scholar]
- 24.Francisco I. A., Pinotti M. H. P. Cyanogenic glycosides in plants. Brazilian Archives of Biology and Technology . 2000;43(5):487–492. doi: 10.1590/s1516-89132000000500007. [DOI] [Google Scholar]
- 25.Bolarinwa I. F., Oke M. O., Olaniyan S. A., Ajala A. S. Toxicology-New Aspects to This Scientific Conundrum . London, UK: InTech; 2016. A review of cyanogenic glycosides in edible plants; pp. 179–191. [Google Scholar]
- 26.Del Carmen Martínez-Ballesta M., Moreno D. A., Carvajal M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. International Journal of Molecular Sciences . 2013;14(6):11607–11625. doi: 10.3390/ijms140611607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues-Corrêa K. C. D. S., Fett-Neto A. G. Abiotic stresses and non-protein amino acids in plants. Critical Reviews in Plant Sciences . 2019;38(5-6):411–430. doi: 10.1080/07352689.2019.1707944. [DOI] [Google Scholar]
- 28.Ordóñez J. L., Callejón R., Morales M., García-Parrilla M. A survey of biogenic amines in vinegars. Food Chemistry . 2013;141(3):2713–2719. doi: 10.1016/j.foodchem.2013.05.087. [DOI] [PubMed] [Google Scholar]
- 29.Lattanzio V., Kroon P. A., Quideau S., Treutter D. Plant phenolics—secondary metabolites with diverse functions. Recent Advances in Polyphenol Research . 2008;1:1–35. doi: 10.1002/9781444302400.ch1. [DOI] [Google Scholar]
- 30.Ozcan T., Akpinar-Bayizit A., Yilmaz-Ersan L., Delikanli B. Phenolics in human health. International Journal of Chemical Engineering and Applications . 2014;5:393–396. doi: 10.7763/ijcea.2014.v5.416. [DOI] [Google Scholar]
- 31.Hollman A. Plants and cardiac glycosides. Heart . 1985;54(3):258–261. doi: 10.1136/hrt.54.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudareva N., Pichersky E., Gershenzon J. Biochemistry of plant volatiles. Plant Physiology . 2004;135(4):1893–1902. doi: 10.1104/pp.104.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grabmann J. Terpenoids as plant antioxidants. In: Litwack G., editor. Vitamins & Hormones . Cambridge, MA, USA: Academic Press; 2005. pp. 505–535. [DOI] [PubMed] [Google Scholar]
- 34.Dang L., Van Damme E. J. M. Toxic proteins in plants. Phytochemistry . 2015;117:51–64. doi: 10.1016/j.phytochem.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smeekens S. Sugar-induced signal transduction in plants. Annual Review of Plant Physiology and Plant Molecular Biology . 2000;51(1):49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- 36.Rolland F., Moore B., Sheen J. Sugar sensing and signaling in plants. The Plant Cell Online . 2002;14(suppl 1):S185–S205. doi: 10.1105/tpc.010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harwood J. L. Recent advances in the biosynthesis of plant fatty acids. Biochimica et Biophysica Acta (BBA)—Lipids and Lipid Metabolism . 1996;1301(1-2):7–56. doi: 10.1016/0005-2760(95)00242-1. [DOI] [PubMed] [Google Scholar]
- 38.Rivasseau C., Boisson A.-M., Mongélard G., Couram G., Bastien O., Bligny R. Rapid analysis of organic acids in plant extracts by capillary electrophoresis with indirect UV detection: directed metabolic analyses during metal stress. Journal of Chromatography A . 2006;1129(2):283–290. doi: 10.1016/j.chroma.2006.06.099. [DOI] [PubMed] [Google Scholar]
- 39.Yang B., Xie Y., Guo M., Rosner M. H., Yang H., Ronco C. Nephrotoxicity and Chinese herbal medicine. Clinical Journal of the American Society of Nephrology . 2018;13(10):1605–1611. doi: 10.2215/cjn.11571017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X.-Y., Wang G., Wang Y., et al. Chemical constituents from Alisma plantago-aquatica subsp. orientale (sam.) sam and their anti-inflammatory and antioxidant activities. Natural Product Research . 2018;32(23):2749–2755. doi: 10.1080/14786419.2017.1380024. [DOI] [PubMed] [Google Scholar]
- 41.Añides J. A., Dapar M. L. G., Aranas A. T., et al. Phytochemical, antioxidant and antimicrobial properties of the white variety of “Sibujing” (Allium ampeloprasum) Pharmacophore . 2019;10 [Google Scholar]
- 42.Barile E., Bonanomi G., Antignani V., et al. Saponins from Allium minutiflorum with antifungal activity. Phytochemistry . 2007;68(5):596–603. doi: 10.1016/j.phytochem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Lekshmi P., Viveka S., Viswanathan M., Manivannan G., Shobi T. M. GC-MS characterization of volatile odorous compounds in Allium cepa. Proceedings of the Nanobio Pharmaceutical Technology Applications and Perspectives; October 2014; Tiruchirappalli, Tamilnadu. pp. 488–494. [Google Scholar]
- 44.Carotenuto A., Fattorusso E., Lanzotti V., Magno S. Spirostanol saponins of Allium porrum L.fn1. Phytochemistry . 1999;51(8):1077–1082. doi: 10.1016/s0031-9422(98)00712-2. [DOI] [PubMed] [Google Scholar]
- 45.Najjaa H., Neffati M., Zouari S., Ammar E. Essential oil composition and antibacterial activity of different extracts of Allium roseum L., a North African endemic species. Comptes Rendus Chimie . 2007;10(9):820–826. doi: 10.1016/j.crci.2007.03.003. [DOI] [Google Scholar]
- 46.Popova A., Mihaylova D., Alexieva I. GC-MS chemical composition of volatile oil and mineral element content of Allium ursinum and Nectaroscordum siculum. Pakistan Journal of Botany . 2018;50:2351–2354. [Google Scholar]
- 47.Satyal P., Craft J. D., Dosoky N. S., Setzer W. N. The chemical compositions of the volatile oils of garlic (Allium sativum) and wild garlic (Allium vineale) Foods . 2017;6(8):p. 63. doi: 10.3390/foods6080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Assimopoulou A. N., Papageorgiou V. P. GC-MS analysis of penta and tetra cyclic triterpenes from resins of Pistacia species. Part II. Pistacia terebinthus var. Chia. Biomedical Chromatography . 2005;19(8):586–605. doi: 10.1002/bmc.484. [DOI] [PubMed] [Google Scholar]
- 49.Assimopoulou A. N., Papageorgiou V. P. GC-MS analysis of penta and tetra cyclic triterpenes from resins of Pistacia species. Part I. Pistacia lentiscus var. Chia. Biomedical Chromatography . 2005;19(4):285–311. doi: 10.1002/bmc.454. [DOI] [PubMed] [Google Scholar]
- 50.Piccolella S., Nocera P., Carillo P., et al. An apolar Pistacia lentiscus L. leaf extract: GC-MS metabolic profiling and evaluation of cytotoxicity and apoptosis inducing effects on SH-SY5Y and SK-N-BE (2) C cell lines. Food and Chemical Toxicology . 2016;95:64–74. doi: 10.1016/j.fct.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 51.Saitta M., La Torre G. L., Potortì A. G., Di Bella G., Dugo G. Polyphenols of pistachio (Pistacia vera L.) oil samples and geographical differentiation by principal component analysis. Journal of the American Oil Chemists’ Society . 2014;91(9):1595–1603. doi: 10.1007/s11746-014-2493-3. [DOI] [Google Scholar]
- 52.Li H., Zhou W., Hu Y., Mo H., Wang J., Hu L. GC-MS analysis of essential oil from Anethum graveolens L (dill) seeds extracted by supercritical carbon dioxide. Tropical Journal of Pharmaceutical Research . 2021;18(6):1291–1296. doi: 10.4314/tjpr.v18i6.21. [DOI] [Google Scholar]
- 53.Awad H. A., Awda J. M., Abd-Alssirag M., Allaalfalahi D. GC-mass analysis of (Apium graveolens) leaf extracts obtained with aqueous and methanol extraction and study its antimicrobial activity. Asian Journal of Microbiology, Biotechnology & Environmental Sciences . 2019;21 [Google Scholar]
- 54.Kapoor I. P. S., Singh B., Singh G., De Heluani C. S., De Lampasona M. P., Catalan C. A. N. Chemistry and antioxidant activity of essential oil and oleoresins of black caraway (Carum bulbocastanum) fruits: Part 69. Journal of the Science of Food and Agriculture . 2010;90(3):385–390. doi: 10.1002/jsfa.3824. [DOI] [PubMed] [Google Scholar]
- 55.Fang R., Jiang C. H., Wang X. Y., et al. Insecticidal activity of essential oil of Carum carvi fruits from China and its main components against two grain storage insects. Molecules . 2010;15(12):9391–9402. doi: 10.3390/molecules15129391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kazemi M. GC/MS analyses for detection and identification of antioxidant constituents of Carum copticum essential oil. Thai Journal of Agricultural Science . 2014;47:141–145. [Google Scholar]
- 57.Sahaf B. Z., Moharramipour S., Meshkatalsadat M. H. Chemical constituents and fumigant toxicity of essential oil from Carum copticum against two stored product beetles. Insect Science . 2007;14(3):213–218. doi: 10.1111/j.1744-7917.2007.00146.x. [DOI] [Google Scholar]
- 58.Samadi N., Shahani S., Akbarzadeh H., et al. Essential oil analysis and antibacterial activity of ferula Assa-foetida L. aerial parts from neishabour mountains. Research Journal of Pharmacognosy . 2016;3 [Google Scholar]
- 59.Amin A., Tuenter E., Cos P., et al. Antiprotozoal and antiglycation activities of sesquiterpene coumarins from Ferula narthex exudate. Molecules . 2016;21(10):p. 1287. doi: 10.3390/molecules21101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baser K. H., Tümen G. Composition of the essential oil of Lagoecia cuminoides L. from Turkey. Journal of Essential Oil Research . 1994;6(5):545–546. doi: 10.1080/10412905.1994.9698448. [DOI] [Google Scholar]
- 61.Intirach J., Junkum A., Lumjuan N., et al. Antimosquito property of Petroselinum crispum (Umbellifereae) against the pyrethroid resistant and susceptible strains of Aedes aegypti (diptera: culicidae) Environmental Science and Pollution Research . 2016;23:23994–24008. doi: 10.1007/s11356-016-7651-8. [DOI] [PubMed] [Google Scholar]
- 62.Gruszecki R. Effects of harvest time and drying on essential oil properties in lavandin (Lavandula x intermedia Emeric ex Loisel.) Journal of Acta Horticulturae . 2017;826 [Google Scholar]
- 63.Cabral C., Lemos M., Cavaleiro C., Cruz M., Salgueiro L. Essential oil of Seseli tortuosum L. from Portugal: safety and anti-inflammatory potential evaluation. Arabian Journal of Medicinal and Aromatic Plants . 2015;1:31–43. [Google Scholar]
- 64.Letchamo W., Korolyuk E. A., Tkachev A. V. Chemical screening of essential oil bearing flora of Siberia III. composition of the essential oil of Sium latifolium L. tops from Altai Region. Journal of Essential Oil Research . 2005;17(4):396–397. doi: 10.1080/10412905.2005.9698940. [DOI] [Google Scholar]
- 65.Gibernau M., Favre C., Talou T., Raynaud C. Floral odor of Arum italicum. Aroideana . 2004;27:142–147. [Google Scholar]
- 66.Kochmarov V., Marinov L., Kozuharova E., et al. Exploration of collagenase, cyclooxigenases, angiogenesis and free radical processes as the putative pharmacological targets of Arum maculatum L. Biotechnology & Biotechnological Equipment . 2020;34(1):126–134. doi: 10.1080/13102818.2020.1722239. [DOI] [Google Scholar]
- 67.Eleazu C. O. Characterization of the natural products in cocoyam (Colocasia esculenta) using GC–MS. Pharmaceutical Biology . 2016;54(12):2880–2885. doi: 10.1080/13880209.2016.1190383. [DOI] [PubMed] [Google Scholar]
- 68.Soumya V., Muzib Y. I., Venkatesh P., Hariprasath K. GC-MS analysis of Cocus nucifera flower extract and its effects on heterogeneous symptoms of polycystic ovarian disease in female wistar rats. Chinese Journal of Natural Medicines . 2014;12(9):677–684. doi: 10.1016/s1875-5364(14)60103-5. [DOI] [PubMed] [Google Scholar]
- 69.Wilczewska A. Z., Ulman M., Chilmończyk Z., et al. Comparison of volatile constituents of Acorus calamus and Asarum europaeum obtained by different techniques. Journal of Essential Oil Research . 2008;20(5):390–395. doi: 10.1080/10412905.2008.9700038. [DOI] [Google Scholar]
- 70.Jadhav A., Bhutani K. Steroidal saponins from the roots of Asparagus Adscendens Roxb and Asparagus racemosus Willd. Indian Journal of Chemistry . 2006;45B [Google Scholar]
- 71.Zhang H., Birch J., Pei J., et al. Identification of six phytochemical compounds from Asparagus officinalis L. root cultivars from New Zealand and China using UAE-SPE-UPLC-MS/MS: effects of extracts on H2O2-induced oxidative stress. Nutrients . 2019;11(1):p. 107. doi: 10.3390/nu11010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.SR J., Singaravadivel K. Screening of phytochemical and GC-MS analysis of some bioactive constituents of Asparagus racemosus. Screening . 2014;6:428–432. [Google Scholar]
- 73.Lakshmi P., Rajalakshmi P. Identification of phyto components and its biological activities of aloe vera through the gas chromatography-mass spectrometry. International Research Journal of Pharmacy . 2011;2:247–249. [Google Scholar]
- 74.Obistioiu D., Cristina R. T., Schmerold I., et al. Chemical characterization by GC-MS and in vitro activity against Candida albicans of volatile fractions prepared from Artemisia dracunculus, Artemisia abrotanum, Artemisia absinthium and Artemisia vulgaris. Chemistry Central Journal . 2014;8:6–11. doi: 10.1186/1752-153x-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kunihiro K., Myoda T., Tajima N., et al. Volatile components of the essential oil of Artemisia Montana and their sedative effects. Journal of Oleo Science . 2017;66(8):843–849. doi: 10.5650/jos.ess16006. [DOI] [PubMed] [Google Scholar]
- 76.Wu L.-Y., Gao H.-Z., Wang X.-L., Ye J.-H., Lu J., Liang Y.-R. Analysis of chemical composition of Chrysanthemum indicum flowers by GC/MS and HPLC. Journal of Medicinal Plants Research . 2010;4:421–426. [Google Scholar]
- 77.Singh R., Chahal K. K. Cichorium intybus from India: GC-MS profiling, phenolic content and in vitro antioxidant capacity of sequential soxhlet extracted roasted roots. Brazilian Archives of Biology and Technology . 2019;62 doi: 10.1590/1678-4324-2019180370. [DOI] [Google Scholar]
- 78.Al-Akhras M. A. H., Aljarrah K., Al-Khateeb H., et al. Introducing cichorium pumilum as a potential therapeutical agent against drug-induced benign breast tumor in rats. Electromagnetic Biology and Medicine . 2012;31(4):299–309. doi: 10.3109/15368378.2012.662193. [DOI] [PubMed] [Google Scholar]
- 79.Devki G. S., Sisodia R. Screening of the potential phytochemicals from the capparis decidua fruit extract using GC-MS. Journal of Biomedical Sciences . 2020;2 [Google Scholar]
- 80.Altameme H. J. M. GC-MS and FTIR analysis Phytocomponents on different parts of Capparis spinosa L. (Capparidaceae) in Iraq. Journal of Chemical and Pharmaceutical Sciences . 2016;9:3269–3282. [Google Scholar]
- 81.Singh S., Devi B. Estimation of phytoconstituents from Citrullus colocynthis (L.) schrad roots extract by GC-MS spectroscopy. International Journal of Science and Research . 2016;7 [Google Scholar]
- 82.Memariani T., Hosseini T., Kamali H., et al. Evaluation of the cytotoxic effects of Cyperus longus extract, fractions and its essential oil on the PC3 and MCF7 cancer cell lines. Oncology Letters . 2016;11(2):1353–1360. doi: 10.3892/ol.2015.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peerzada A. M., Ali H. H., Naeem M., Latif M., Bukhari A. H., Tanveer A. Cyperus rotundus L.: traditional uses, phytochemistry, and pharmacological activities. Journal of Ethnopharmacology . 2015;174:540–560. doi: 10.1016/j.jep.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 84.Boeing T., Tafarelo Moreno K. G., Gasparotto Junior A., Mota Da Silva L., De Souza P. Phytochemistry and pharmacology of the genus Equisetum (equisetaceae): a narrative review of the species with therapeutic potential for kidney diseases. Evidence-Based Complementary and Alternative Medicine . 2021;2021:17. doi: 10.1155/2021/6658434.6658434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramesh B., Jayabharathi V. Phytochemical screening, HPTLC and GCMS profile of Acacia catechu (lf) willd hydroethanolic leaf extract. International Journal of Current Microbiology and Applied Sciences . 2017;6(1):82–94. doi: 10.20546/ijcmas.2017.601.011. [DOI] [Google Scholar]
- 86.Samejo M. Q., Memon S., Bhanger M. I., Khan K. M. Chemical composition of essential oils from Alhagi maurorum. Chemistry of Natural Compounds . 2012;48(5):898–900. doi: 10.1007/s10600-012-0417-8. [DOI] [Google Scholar]
- 87.Akhtar R., Shahzad A. Alginate encapsulation in Glycyrrhiza glabra L. with phyto-chemical profiling of root extracts of in vitro converted plants using GC-MS analysis. Asian Pacific Journal of Tropical Biomedicine . 2017;7(10):855–861. doi: 10.1016/j.apjtb.2017.09.010. [DOI] [Google Scholar]
- 88.Pascale R., Bianco G., Cataldi T. R., et al. Mass spectrometry-based phytochemical screening for hypoglycemic activity of Fagioli di Sarconi beans (Phaseolus vulgaris L.) Food Chemistry . 2018;242:497–504. doi: 10.1016/j.foodchem.2017.09.091. [DOI] [PubMed] [Google Scholar]
- 89.Ishan U., Suresh M., Suresh M., Bagh R. Volatile phyto-chemical compounds screening in alcoholic extract of western Rajasthan growing mung-bean (Vigna radiata) by gas chromatography-mass spectroscopy. Trends Biosciences . 2017;10:1588–1595. [Google Scholar]
- 90.Aja P., Ugwu O., Okoro C., Nweke O., Ali I. A., Ogbu P. N. Gas chromatographic-mass spectrometric (GC-MS) analysis of ethanol leaf-extract of Vigna unguiculata (cowpea) International Journal of Research and Reviews in Pharmacy and Applied Sciences . 2016;6:1284–1289. [Google Scholar]
- 91.Yilmaz B., Yildiz B. O. Endocrinology of hirsutism: from androgens to androgen excess disorders. Frontiers of Hormone Research . 2019;53:108–119. doi: 10.1159/000494907. [DOI] [PubMed] [Google Scholar]
- 92.Khemkham A., Belhadj S., Meddour R., et al. HS-SPME-GC/MS analysis of 3 lamiaceae plants: ajuga iva (L.) schreb., salvia verbenaca L. and thymus algeriensis BOISS. & reut. Journal of Fundamental and Applied Sciences . 2020;12:700–711. [Google Scholar]
- 93.Rehman S. u., Latief R., Bhat K. A., Khuroo M. A., Shawl A. S., Chandra S. Comparative analysis of the aroma chemicals of Melissa officinalis using hydrodistillation and HS-SPME techniques. Arabian Journal of Chemistry . 2017;10:S2485–S2490. doi: 10.1016/j.arabjc.2013.09.015. [DOI] [Google Scholar]
- 94.Prakash N. K. U., Sripriya N. S., Raj D. D., Deepa S., Bhuvaneswari S. Antioxidant potency and GC-MS composition of Origanum majorana Linn. Pakistan Journal of Pharmaceutical Sciences . 2019;32(5):2117–2122. [PubMed] [Google Scholar]
- 95.Faiku F., Buqaj L., Haziri A. Phytochemicals and antioxidant study of Teucrium chamaedrys (L.) plant. Journal of Agriculture and Forestry . 2019;65(1):137–145. doi: 10.17707/agricultforest.65.1.14. [DOI] [Google Scholar]
- 96.Baruah A., Nath S. C., Hazarika A. K., Sarma T. C. Essential oils of the leaf, stem bark and panicle of Cinnamomum bejolghota (Buch-Ham.) sweet. Journal of Essential Oil Research . 1997;9(2):243–245. doi: 10.1080/10412905.1997.9699472. [DOI] [Google Scholar]
- 97.Guo S., Geng Z., Zhang W., et al. The chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. International Journal of Molecular Sciences . 2016;17(11):p. 1836. doi: 10.3390/ijms17111836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peris I., Blázquez M. A. Comparative GC-MS analysis of bay leaf (Laurus nobilis L.) essential oils in commercial samples. International Journal of Food Properties . 2015;18(4):757–762. doi: 10.1080/10942912.2014.906451. [DOI] [Google Scholar]
- 99.Hosokawa K., Fukunaga Y. Production of essential oils by flowers of Hyacinthus orientalis L. regenerated in vitro. Plant Cell Reports . 1995;14(9):575–579. doi: 10.1007/bf00231941. [DOI] [PubMed] [Google Scholar]
- 100.Karaulov A. E., Rybin V. G., Kuklev D. V., Akulin V. N. Synthesis of fatty-acid ethanolamides from Linum catharticum oils and Cololabis saira fats. Chemistry of Natural Compounds . 2004;40(3):222–226. doi: 10.1023/b:conc.0000039128.78645.a8. [DOI] [Google Scholar]
- 101.Thamilmarai Selvi B., Sathammai Priya N., Steffi P. F., Priyadarshni S. Phytochemical evaluation, GC-MS analysis of phytoactive compounds, and antibacterial activity studies from linum usitatissimum. Asian Journal of Pharmaceutical and Clinical Research . 2019;12:141–149. doi: 10.22159/ajpcr.2019.v12i18.34126. [DOI] [Google Scholar]
- 102.Cecotti R., Bergomi P., Carpana E., Tava A. Chemical characterization of the volatiles of leaves and flowers from cultivated Malva sylvestris var. mauritiana and their antimicrobial activity against the aetiological agents of the European and American foulbrood of honeybees (Apis mellifera) Natural Product Communications . 2016;11(10) doi: 10.1177/1934578x1601101026.1934578X1601101026 [DOI] [PubMed] [Google Scholar]
- 103.Valiei M., Shafaghat A., Salimi F. Chemical composition and antimicrobial activity of the flower and root hexane extracts of Althaea officinalis in Northwest Iran. Journal of Medicinal Plants Research . 2011;5:6972–6976. [Google Scholar]
- 104.Al-Snafi A. E. Chemical constituents and pharmacological activities of Gossypium herbaceum and Gossypium hirsutum-A. IOSR Journal of Pharmacy . 2018;8:64–80. [Google Scholar]
- 105.Al-Qarawi K. K., Al-Obaidi H. M. R. Detection of the active compounds in the leaves of the common mallow plant Malva parviflora L. using GC-MS and HPLC technology. Kufa Journal For Agricultural Sciences . 2018;10 [Google Scholar]
- 106.Jabbari H., Shendabadizad R. GC-MS analysis of essential oils of Humulusn lupulus, Malva Sylvestris and thymus plants in water solvent. Journal of Advanced Pharmacy Education & Research . 2020;10 [Google Scholar]
- 107.El-Beltagi H. S., Mohamed H. I., Abdelazeem A. S., Youssef R., Safwat G. GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from ficus sycomorus fruits and leaves. Notulae Botanicae Horti Agrobotanici Cluj-Napoca . 2019;47 [Google Scholar]
- 108.Natić M. M., Dabić D. Č., Papetti A., et al. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chemistry . 2015;171:128–136. doi: 10.1016/j.foodchem.2014.08.101. [DOI] [PubMed] [Google Scholar]
- 109.Pareek S. Nutritional Composition of Fruit Cultivars . Amsterdam, Netherlands: Elsevier; 2016. Nutritional and biochemical composition of banana (Musa spp.) cultivars. [Google Scholar]
- 110.Teles A. M., Silva-Silva J. V., Fernandes J. M. P., et al. GC-MS characterization of antibacterial, antioxidant, and antitrypanosomal activity of Syzygium aromaticum essential oil and eugenol. Evidence-Based Complementary and Alternative Medicine . 2021;2021:12. doi: 10.1155/2021/6663255.6663255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mili A., Das S., Nandakumar K., Lobo R. A comprehensive review on Sesamum indicum L.: botanical, ethnopharmacological, phytochemical, and pharmacological aspects. Journal of Ethnopharmacology . 2021;281 doi: 10.1016/j.jep.2021.114503.114503 [DOI] [PubMed] [Google Scholar]
- 112.Apetrei L. C., Spac A., Brebu M., Tuchilus C., Miron A. Composition, antioxidant and antimicrobial activity of the essential oils of a full grown tree of Pinus cembra L. from the Calimani mountains (Romania) Journal of the Serbian Chemical Society . 2013;78(1):27–37. doi: 10.2298/jsc120409075a. [DOI] [Google Scholar]
- 113.Kaundun S. S., Fady B., Lebreton P. Genetic differences between Pinus halepensis, Pinus brutia and Pinus eldarica based on needle flavonoids. Biochemical Systematics and Ecology . 1997;25(6):553–562. doi: 10.1016/s0305-1978(97)00049-5. [DOI] [Google Scholar]
- 114.Aloui F., Baraket M., Jedidi S., et al. Chemical composition, anti-radical and antibacterial activities of essential oils from needles of Pinus halepensis mill., P. Pinaster aiton, and P. Pinea L. Journal of Essential Oil Bearing Plants . 2021;24(3):453–460. doi: 10.1080/0972060x.2021.1943541. [DOI] [Google Scholar]
- 115.Amri I., Hanana M., Jamoussi B., Hamrouni L. Essential oils of Pinus nigra JF arnold subsp. laricio maire: chemical composition and study of their herbicidal potential. Arabian Journal of Chemistry . 2017;10:S3877–S3882. doi: 10.1016/j.arabjc.2014.05.026. [DOI] [Google Scholar]
- 116.Tumen I., Hafizoglu H., Kilic A., Dönmez I. E., Sivrikaya H., Reunanen M. Yields and constituents of essential oil from cones of Pinaceae spp. natively grown in Turkey. Molecules . 2010;15(8):5797–5806. doi: 10.3390/molecules15085797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Judzentiene A., Kupcinskiene E. Chemical composition on essential oils from needles of Pinus sylvestris L. grown in northern Lithuania. Journal of Essential Oil Research . 2008;20(1):26–29. doi: 10.1080/10412905.2008.9699413. [DOI] [Google Scholar]
- 118.Andriana Y., Xuan T. D., Quy T. N., Tran H.-D., Le Q.-T. Biological activities and chemical constituents of essential oils from Piper cubeba Bojer and Piper nigrum L. Molecules . 2019;24(10):p. 1876. doi: 10.3390/molecules24101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sleem F. M. A. Insecticidal effect of Piper Nigrum L. (Pipeaceae) and Prunus Cerasus L. (rosaceae) seeds extract against rhyzopertha dominica f. (coleoptera: bostrichidae) International Journal of Advance and Innovative Research . 2021;9 [Google Scholar]
- 120.Takashima S., Abe T., Yoshida S., et al. Analysis of sialyltransferase-like proteins from Oryza sativa. Journal of Biochemistry . 2006;139(2):279–287. doi: 10.1093/jb/mvj029. [DOI] [PubMed] [Google Scholar]
- 121.Ćosović Č., Proštenik M. Lipids of higher fungi. V: the occurrence of long-chain erythro-2, 3-dihydroxy fatty acids in Polyporus officinalis. Chemistry and Physics of Lipids . 1979;23(4):349–353. doi: 10.1016/0009-3084(79)90012-4. [DOI] [Google Scholar]
- 122.Soberón J. R., Sgariglia M. A., Pastoriza A. C., et al. Antifungal activity and cytotoxicity of extracts and triterpenoid saponins obtained from the aerial parts of Anagallis arvensis L. Journal of Ethnopharmacology . 2017;203:233–240. doi: 10.1016/j.jep.2017.03.056. [DOI] [PubMed] [Google Scholar]
- 123.Wang L. Characterization of volatile and non-volatile compounds and classification of different cultivars of chinese Ziziphus jujuba Mill. Fruits . Clemson, SC, USA: Clemson University; 2017. [Google Scholar]
- 124.Tsao R., Zhou T. Interaction of monoterpenoids, methyl jasmonate, and Ca2+ in controlling postharvest brown rot of sweet cherry. HortScience . 2000;35(7):1304–1307. doi: 10.21273/hortsci.35.7.1304. [DOI] [Google Scholar]
- 125.Afif C. T., Bendahou M., Arab K. Antibacterial activity of two extracts from Rubus fruticosus L. against resistant pathogens and their antioxidant potential. African Journal of Microbiology Research . 2015;9(18):1255–1262. doi: 10.5897/ajmr2015.7437. [DOI] [Google Scholar]
- 126.Iheagwam F. N., Ogunlana O. O., Chinedu S. N. Model optimization and in silico analysis of potential dipeptidyl peptidase iv antagonists from GC-MS identified compounds in Nauclea latifolia leaf extracts. International Journal of Molecular Sciences . 2019;20(23):p. 5913. doi: 10.3390/ijms20235913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mirzaei-Najafgholi H., Tarighi S., Golmohammadi M., Taheri P. The effect of citrus essential oils and their constituents on growth of Xanthomonas citri subsp. citri. Molecules . 2017;22(4):p. 591. doi: 10.3390/molecules22040591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rahimi Shokooh A., Naghdi Badi H., Abdossi V., Mehrafarin A. Overview on the agronomic, phytochemical and therapeutic traits of bladder cherry (Physalis alkekengi L.) Journal of Medical Internet Research . 2019;18:1–13. [Google Scholar]
- 129.Robert D., Karim M., Rita M. Preliminary bioactivity investigation of Styrax officinalis fruit extract as potential biopesticide. Journal of Pharmacognosy and Phytotherapy . 2016;8(12):209–213. doi: 10.5897/jpp2016.0422. [DOI] [Google Scholar]
- 130.Bylka W., Szaufer Hajdrych M., Matławska I., Goślińska O. Antimicrobial activity of isocytisoside and extracts of Aquilegia vulgaris L. Letters in Applied Microbiology . 2004;39(1):93–97. doi: 10.1111/j.1472-765x.2004.01553.x. [DOI] [PubMed] [Google Scholar]
- 131.Radojevic I. D., Vasic S. M., Dekic M. S., et al. Antimicrobial and antibiofilm effects of extracts from trapa natans L., evaluation of total phenolic and flavonoid contents and gc-ms analysis. Acta Poloniae Pharmaceutica . 2016;73(6):1565–1574. [PubMed] [Google Scholar]
- 132.Razack S., Kumar K. H., Nallamuthu I., Naika M., Khanum F. Antioxidant, biomolecule oxidation protective activities of Nardostachys jatamansi DC and its phytochemical analysis by RP-HPLC and GC-MS. Antioxidants . 2015;4(1):185–203. doi: 10.3390/antiox4010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feng Y.-X., Wang Y., Chen Z.-Y., Guo S.-S., You C. X., Du S. S. Efficacy of bornyl acetate and camphene from Valeriana officinalis essential oil against two storage insects. Environmental Science and Pollution Research . 2019;26(16):16157–16165. doi: 10.1007/s11356-019-05035-y. [DOI] [PubMed] [Google Scholar]
- 134.Jasim S. F., Baqer N. N., Alraheem E. A. Detection of phytochemical constituent in flowers of Viola odorata by gas chromatography-mass spectrometry. Asian Journal of Pharmaceutical and Clinical Research . 2018;11(5) doi: 10.22159/ajpcr.2018.v11i5.24288. [DOI] [Google Scholar]
- 135.Mousavi S. H., Naghizade B., Pourgonabadi S., Ghorbani A. Protective effect of Viola tricolor and Viola odorata extracts on serum/glucose deprivation-induced neurotoxicity: role of reactive oxygen species. Avicenna Journal of Phytomedicine . 2016;6(4):434–441. [PMC free article] [PubMed] [Google Scholar]
- 136.Petretto G. L., Mercenaro L., Urgeghe P. P., Fadda C., Valentoni A., Del Caro A. Grape and wine composition in Vitis vinifera L. Cv. Cannonau explored by GC-MS and sensory analysis. Foods . 2021;10(1):p. 101. doi: 10.3390/foods10010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kochmarov V., Kozuharova E., Naychov Z., Momekov G., Mincheva I. Ethnobotany and ethnopharmacology of Arum maculatum L.(Araceae) in Bulgaria with an emphasis on its effect against haemorrhoids. International Journal of Pharmaceutical, Chemical and Biological Sciences . 2015;5:394–402. [Google Scholar]
- 138.Uzun E., Sariyar G., Adsersen A., et al. Traditional medicine in Sakarya province (Turkey) and antimicrobial activities of selected species. Journal of Ethnopharmacology . 2004;95(2-3):287–296. doi: 10.1016/j.jep.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 139.Saad B., Azaizeh H., Said O. Arab herbal medicine. Botanical Medicine in Clinical Practice . 2008;4:31–39. doi: 10.1079/9781845934132.0031. [DOI] [Google Scholar]
- 140.Senouci F., Ababou A., Chouieb M. Ethnobotanical survey of the medicinal plants used in the southern mediterranean. Case study: the region of bissa (northeastern dahra mountains, Algeria) Pharmacognosy Journal . 2019;11(4):647–659. doi: 10.5530/pj.2019.11.103. [DOI] [Google Scholar]
- 141.Yan D., Yue B., Qian M., et al. JYYS granule mitigates renal injury in clinic and in spontaneously hypertensive rats by inhibiting NF-κB signaling-mediated microinflammation. Evidence-Based Complementary and Alternative Medicine . 2018;2018:13. doi: 10.1155/2018/8472963.8472963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.DebMandal M., Mandal S. Coconut (Cocos nucifera L.: arecaceae): in health promotion and disease prevention. Asian Pacific Journal of Tropical Medicine . 2011;4(3):241–247. doi: 10.1016/s1995-7645(11)60078-3. [DOI] [PubMed] [Google Scholar]
- 143.Lima E., Sousa C., Meneses L., et al. Cocos nucifera (L.) (Arecaceae): a phytochemical and pharmacological review. Brazilian Journal of Medical and Biological Research . 2015;48(11):953–964. doi: 10.1590/1414-431x20154773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bolkent S., Akev N., Ozsoy N., et al. Effect of Aloe Vera (L.) burm. fil. leaf gel and pulp extracts on kidney in type-ii diabetic rat models. Indian Journal of Experimental Biology . 2004;42 [PubMed] [Google Scholar]
- 145.Lee J. H., Park M., Jung K., et al. Identification of gallic acid as a active ingredient of Syzygium aromaticum against tacrolimus-induced damage in renal epithelial LLC-PK1 cells and rat kidney. Bioorganic & Medicinal Chemistry Letters . 2021;41 doi: 10.1016/j.bmcl.2021.128012.128012 [DOI] [PubMed] [Google Scholar]
- 146.Drikvandi P., Bahramikia S., Alirezaei M. Modulation of the antioxidant defense system in liver, kidney, and pancreas tissues of alloxan‐induced diabetic rats by camphor. Journal of Food Biochemistry . 2020;44(12) doi: 10.1111/jfbc.13527.e13527 [DOI] [PubMed] [Google Scholar]
- 147.Hajian E., Eidi M., Abbaspour H. Effect of hydromethanolic extract of Allium porrum L. seed on treatment of ethylene glycol-induced kidney stone in male rats N. Journal of Sabzevar University of Medical Sciences . 2018;24 [Google Scholar]
- 148.Ehsani V., Amirteimoury M., Taghipour Z., et al. Protective effect of hydroalcoholic extract of Pistacia vera against gentamicin-induced nephrotoxicity in rats. Renal Failure . 2017;39(1):519–525. doi: 10.1080/0886022x.2017.1326384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.G Sabar A. Lithotripsy of different urinary tract stones by using seeds of Carum copticum. Iraqi Journal of Pharmaceutical Sciences . 2017;19(2):38–41. doi: 10.31351/vol19iss2pp38-41. [DOI] [Google Scholar]
- 150.Tir M., Feriani A., Labidi A., et al. Protective effects of phytochemicals of Capparis spinosa seeds with cisplatin and CCl4 toxicity in mice. Food Bioscience . 2019;28:42–48. doi: 10.1016/j.fbio.2019.01.002. [DOI] [Google Scholar]
- 151.Ravindran C. A., Murugaiyah V., Khiang P., Xavior R. Hepatoprotective activity of leaf of methanol extract of Laurus nobilis L. against paracetamol induced hepatotoxicity in rats. Asian Journal of Pharmaceutical and Clinical Research . 2013;6:153–157. [Google Scholar]
- 152.Marouane W., Soussi A., Murat J.-C., Bezzine S., El Feki A. The protective effect of Malva sylvestris on rat kidney damaged by vanadium. Lipids in Health and Disease . 2011;10:65–68. doi: 10.1186/1476-511x-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ben Saad A., Rjeibi I., Brahmi D., et al. Malva sylvestris extract protects upon lithium carbonate-induced kidney damages in male rat. Biomedicine & Pharmacotherapy . 2016;84:1099–1107. doi: 10.1016/j.biopha.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 154.Dkhil M. A., Bauomy A. A., Diab M. S., Al-Quraishy S. The antioxidant effect of Morus alba leaves extract on kidney, testes, spleen and intestine of mice. Pakistan Journal of Zoology . 2015;47 [Google Scholar]
- 155.Ajayi Olubumi B., Akomolafe S., Malachi Oluwaseyi I., Oyerinde Adebowale S. Effect of Sesamum indicum L. seed oil supplementation on the kidney function parameters of hypercholesterolemic rats. Journal of Nutrition & Food Sciences . 2014;4:306–309. [Google Scholar]
- 156.Bano H., Jahan N., Makbul S. A. A., Kumar B. N., Husain S., Sayed A. Effect of Piper cubeba L. fruit on ethylene glycol and ammonium chloride induced urolithiasis in male Sprague Dawley rats. Integrative Medicine Research . 2018;7(4):358–365. doi: 10.1016/j.imr.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Laorodphun P., Arjinajarn P., Thongnak L., et al. Anthocyanin rich fraction from black rice, Oryza sativa L. var. indica “Luem Pua,” bran extract attenuates kidney injury induced by high fat diet involving oxidative stress and apoptosis in obese rats. Phytotherapy Research . 2021;35 doi: 10.1002/ptr.7188. [DOI] [PubMed] [Google Scholar]
- 158.Awad D. S., Ali R. M., Mhaidat N. M., Shotar A. M. Zizyphus jujuba protects against ibuprofen-induced nephrotoxicity in rats. Pharmaceutical Biology . 2014;52(2):182–186. doi: 10.3109/13880209.2013.821665. [DOI] [PubMed] [Google Scholar]
- 159.Ullah N., Khan M. A., Khan T., Ahmad W. Nephroprotective potentials of Citrus Aurantium: a prospective pharmacological study on experimental models. Pakistan Journal of Pharmaceutical Sciences . 2014;27(3):505–510. [PubMed] [Google Scholar]
- 160.Sabahatullah A., Aslam M., Javed K., Siddiqui W. Nephroprotective activity of hydroalcoholic extract of Physalis alkekengi (solanaceae) fruit against cisplatin induced nephrotoxicity in rats. Planta Medica . 2010;76(5):p. P101. doi: 10.1055/s-0030-1251863. [DOI] [Google Scholar]
- 161.Habashy N. H., Kodous A. S., Abu-Serie M. M. Targeting ROS/NF-κB signaling pathway by the seedless black vitis vinifera polyphenols in CCl4-intoxicated kidney, lung, brain, and spleen in rats. Scientific Reports . 2021;11:16575–16617. doi: 10.1038/s41598-021-96008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


