Abstract
Neurodegenerative disorders such as Alzheimer's disease (AD) and Parkinson's disease (PD) are becoming more frequent as the age increases. Contemporary therapies provide symptom resolution instead of targeting underlying pathological pathways. Consequently, there is considerable heterogeneity in response to treatment. Research has elucidated multiple potential of pathophysiological mechanisms contributing to neurodegenerative conditions, among which oxidative stress pathways appear to be suitable drug targets. The oxidative stress pathway has given rise to numerous novel pharmacological therapies that may provide a new avenue for neurodegenerative diseases. For example, SKQ (plastoquinone), MitoVitE, vitamin E, SOD mimic, MitoTEMPO (SOD mimetic), and bioactive molecules like curcumin and vitamin C have indeed been examined. To better understand how oxidative stress contributes to neurodegenerative diseases (such as Alzheimer's and Parkinson's), we analyzed the medicinal qualities of medicines that target markers in the cellular oxidative pathways. The specific pathway by which mitochondrial dysfunction causes neurodegeneration will require more investigation. An animal study should be carried out on medications that tackle cellular redox mechanisms but are not currently licensed for use in the management of neurodegenerative conditions.
1. Introduction
Since they are both neurological diseases, Alzheimer's disease (AD) and Parkinson's disease (PD) remain untreated [1]. PD affects approximately one percent of the overall of the individuals over the age of 60, while Alzheimer's disease (AD) affects approximately four percent of the overall of the people above the age of 65 [2, 3].
Oxidative stress, which is a hallmark of aging, is the major trigger for both disorders. Mitochondrial dysfunction is the redox condition that results from an imbalance between the production and elimination of reactive oxygen species (ROS) (Figure 1). It is impossible to avoid ROS even though they metabolize all significant cellular components, especially DNA, RNA, protein, and triglycerides [4]. ROS have been used in cell signaling pathways when they are present in high concentrations.
Figure 1.

Defining the role of free radicals and antioxidants in cellular oxidative development.
Neuropathologically, PD is distinguished by
a reduction in the amount of dopaminergic currently offered for neurotransmission in the substantia nigra pars compacta (SNpc) resulting in a loss of dopamine pathways; and
there is a development of Lewy bodies, neurofibrillary tangles aggregates that incorporate microfibrils synuclein [5]
Disruption of circuitry that controls movement and posture is caused by deficit in the dopamine neurotransmitter in the SNpc, which results in symptoms including slowness of movement and relaxation trembling. Parkinson's disease is also known to cause nonmotor signs such as sleep disorders, anxiety, memory impairment, and malfunctions of the autonomous nervous system and the senses [6, 7]. The neurodegenerative disorders expression of AD is substantially more extensive, with functional decline occurring in conjunction with amyloid plaques, neurofibrillary tangles, and cerebral amyloid angiopathy [8]. The final outcome of degenerative progressions that have yet to be defined in AD and PD is oxidative stress as a result of cellular malfunction.
It is still not obvious how oxidative stress plays a role in either disease's onset or progression, but this is one theory that has been floated by some researchers. Previous studies have attempted to disseminate the role of oxidative stress in the pathophysiology of neurodegenerative diseases [9].
Treatments aiming cellular oxidative pathways may be beneficial in the management of neurodegenerative disorders, particularly those associated with endothelial dysfunction recently. Oxidative stress is a major contributor to neurodegeneration in general and to the development of both Parkinson's disease and Alzheimer's disease (AD). There will also be an examination of present and upcoming treatments for oxidative stress. For these new medicines, future outlooks are presented to discuss their possibility for disease-modifying.
2. The Role of Oxidative Stress in Neurodegeneration
2.1. Mechanisms of Oxidative Stress
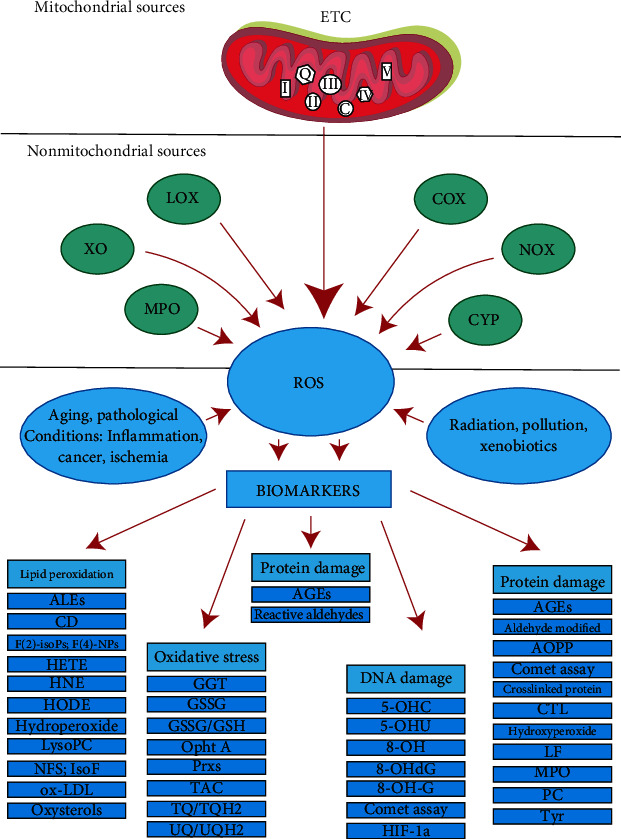
The electrons in the outermost electron shells of reactive species (subatomic particles, molecules, or ions) are unpaired, and this gives them a high degree of responsiveness. Endogenous reactive oxygen species (also known as ROS) have the ability to metabolize macromolecules since they are capable of doing so. Numerous metabolic reactions, such as oxidative phosphorylation, generate ROS. Mitochondrial-derived reactive oxygen species (mtROS) (Figures 2 and 3) include singlet oxygen (O2), superoxide anion (O2•−), hydrogen peroxide (H2O2), nitric oxide (NO•), hydroxyl radical (OH•), and hydroxyl ion (OH−). Xanthine oxidase (XO) or mitochondrial respiratory chain complexes I (NADH dehydrogenase) and III (bc1 complex) first convert oxygen to superoxide anion [3, 10] (Figure 2). In both the matrix and the intermembranous region, complex III generates a superoxide anion (Figure 2) [11].
Figure 2.
Reactive oxygen species (ROS) production in mitochondria (mtROS).
Figure 3.

Radical species development.
A patient's condition, age, and hormonal status all affect how much of these complexities are present in IMM. In order to produce hydrogen peroxide, the superoxide anion must first be converted by SOD. Hydrogen peroxide can be detoxicated to water and oxygen using glutathione peroxidase, catalase (CAT), or thioredoxin peroxidase (TPx) [12, 13]. It can also be converted to hydroxyl radicals and hydroxyl ions via the Fenton reaction (Figure 3) [14].
At physiological levels, ROS are sequestered by endogenous antioxidants such as glutathione peroxidase and superoxide dismutase. The central nervous system (CNS) is especially vulnerable to oxidative stress [15]. The rapid energy level of cerebral cortex, the greater amount of polyunsaturated fatty acids (PUFA) in the biological synapses, and the native autooxidative pathways engaged in neurotransmitters are all factors that contribute to this sensitivity.
Despite unsaturated fatty acids comprising 20% of the brain's total fatty acid content, only around 3% of the total glutathione peroxidase in the human liver is found in the brain. Superoxide dismutase levels in brain tissue are comparable to those found in the heart and liver. Iron, often found as iron-neuromelanin complexes in dopaminergic neurons, under pathological conditions may act as a cofactor for producing ROS [16]. All the above contribute to the greater susceptibility of CNS to ROS. Locally increased levels of ROS can precipitate mitochondrial dysfunction by damaging membrane proteins and may cause adverse mutations in mitochondrial DNA [17, 18].
The endpoint of this cascade is neuroinflammation and neuronal dysfunction as seen in PD and AD [19]. Currently biological studies posit that such consequences are primarily the result of mitochondrial dysfunction, secondary from oxidative stress, leading to neurodegeneration [20–22].
2.2. Reactive Species as Modulators of the Neurological Function
Reactive oxygen species (ROS) modulate many aspects of neurological function by acting as secondary messengers in several pathways (Figure 4). There are two types of synaptic plasticity: LTP and LTD, which refer to an improvement in synaptic performance as well as a decrease in signal transduction [23].
Figure 4.
Cellular redox indicators' derivation [27].
ROS are relevant in the hippocampus and spinal cord where they partake in LTP [24]. Secondly, ROS activates microglia and astrocytes causing the ongoing release of proinflammatory cytokines and chemokines analogous to the systemic, low-grade inflammation that occurs secondary to malignancy. Thirdly, ROS play a crucial role in the differentiating of neurons by influencing the multiplication of brain progenitor and epigenetics.
Fourthly, ROS inhibits sodium currents needed for action potentials via the oxidation of thiol groups (Figure 4). Interestingly the opening of voltage-gated calcium channels is enhanced by ROS [25, 26].
Complex I: NADH dehydrogenase; II: succinate dehydrogenase; III: bc1 complex; IV: cytochrome C oxidase; V: ATP synthase; Q: Ubiquinone; Cyt C: cytochrome C; Cyclo D: Cyclophilin D; mPTP: mitochondrial permeability transition pore; SOD: superoxide dismutase; GPxs: glutathione peroxidase; TPx: thioredoxin peroxidase. See text below for details [27].
2.3. Oxidative Stress in the Pathogenesis of PD and AD
Postmortem frontal brain samples from people aged 26 to 126 years showed a decline in genes linked to synapse formation, vesicular transport, and mitochondrial activity beyond the age of 40. The response to stress and antioxidants and DNA fix genes were then upregulated as a result of these modifications [28].
PD patients' nigral neurons, which are particularly vulnerable to mitochondrial malfunction due to high levels of oxidative metabolism, have been the subject of extensive investigation since the 1980s [2]. Regarding AD, though its pathophysiology has been explained through the amyloid and the neurofibrillary tangles, research has also implicated other mechanisms in its development and progression; among these mechanisms is mitochondrial dysfunction and oxidative stress [2], though, most authors agree that mitochondrial dysfunction precedes amyloid plaque deposition and is thus not the underlying cause.
In postmortem tissues, elevated levels of ATP were found in patients with AD both in cerebral structures and in peripheral tissues, signifying the presence of mitochondrial dysfunction. Complex I, III, and IV inadequacies have been observed in postmortem dissections thus far. Apart from mitochondrial dysfunction changes, there have also been changes in morphology and distribution of mitochondria, with research describing the length reduction and increase in numbers [2].
2.4. Novel Therapies Targeting Oxidative Stress Pathways
The capacity of these medications to pass the blood-brain barrier (BBB) is a critical hurdle when developing innovative therapeutics for neurodegenerative illness. According to research, the disease associated with complex 1 is the major source of mitochondrial dysfunction [29]; treatments that address this are being explored, including substances like SKQ (plastoquinone), MitoVitE (vitamin E), MitoTEMPO (SOD mimic), MCAT (catalase), MitoPBN (CoQ), and phenyl tert butylnitrone conjugation, as well as other chemicals.
Others are lipophilic cation-based tetrapeptide compounds or choline esters of glutathione and N-acetyl l-cysteine that can penetrate cells. There has been evidence that MitoQ slows down the onset of Alzheimer's disease by decreasing A-induced neurodegeneration in neuronal cells, reducing free radical generation [30, 31].
Intracellular enzymes such as superoxide dismutase and glutathione peroxidase provide some protection from ROS. Synthetic molecules such as butylated hydroxytoluene, butylated hydroxyanisole, and ethoxyquin PAPLAL (mixture of Pd and Pt NPs) have been designed to mimic these enzymes but may produce adverse effects resulting from systemic administration [31].
An erythropoietin (Neuro-EPO) intranasally administered shields from inflammation and neurological assaults, restoring impairments in recollection, acquisition, and recognition of new images while also reducing antioxidant activity [32, 33].
Due to the Nrf2–NF-B signaling axis, Cyclo (His-Pro) seems to have some anti-inflammatory and stress activities, as well as ability to repair neuronal functionality Antioxidant defenses can be activated and apoptosis inducing and inflammation response can be reduced by increasing the nuclear level of Nrf2 and preventing IB degradation [34].
Studies suggest Glial activation-mediated inflammation has some role in Parkinson's disease (PD), via the Glia maturation factor (GMF) [35]. Modified GMF articulation directly influences the creation of reactive oxygen species by 1-methyl-4-phenylpyridinium (MPP +). GMF suppression correlated with a decline in reactive oxygen species and resulted in downregulation of NF-kappaB-induced creation of TNFalpha and IL-1b. Thus, it decreased lipid peroxidation levels and increased levels of glutathione [35].
Metal-protein attenuating compounds (MPACs) interrupt the abnormal metal-protein interaction and normalize its distribution by competing with target proteins for metal ions [36].
Autopsies performed on brain tissue of PD and AD patients show involvement of transition metals during the formation of cytotoxic tissue aggregates, and metal chelating therapy has demonstrated significant efficacy in certain PD models by preventing lipid peroxidation, although long term use might interfere with normal physiological function [37]. Although these compounds show great results, they are still at the experimental phase, and their clinical application is being investigated.
2.5. Oxidative Stress Drug Targets in Anti-Alzheimer's Disease Therapy
Antioxidant properties of medications used to treat Alzheimer's disease show considerable differences based on dose and AD model. Tacrine, which was the first anticholinesterase inhibitor approved by the Food and Drug Administration (FDA), showed reduction in overall survival (OS) in an animal Alzheimer's disease model [38] and at a dose of 50–800 g/kg i.m. increased FRAP, and hence “antioxidant efficacy” [39], without raising any sign of OS-associated damage in brain tissue. Tacrine has a favorable effect when provided in doses that improve the antioxidant system without increasing oxidative stress-induced damage in brain tissue [40].
Donepezil, another cholinesterase inhibitor used in Alzheimer's disease patients, resulted in dose-dependent effects on antioxidant capacity and reduced lipid peroxidation when administered in mice AD models at modest doses. In the APPswe/PS1 transgenic mice AD model, donepezil did not decrease OS biomarkers or show significant antioxidant activity [41]. Hence, discrepancy in results from different studies suggests that adaptations to its use may be the source of the observed discrepancy in outcomes in transgenic and nontransgenic AD mice models.
Rivastigmine, another drug used to treat of Alzheimer's disease, does not show to decrease lipid peroxidation or replenish GSH in an AD rat brain model [42], despite a previous report indicating antioxidant capabilities for rivastigmine when Alzheimer's disease was produced in rats by aluminum chloride treatment [43].
A single study found that another AChE inhibitor, galantamine, might lower OS, leading to decreased lipid peroxidation, nitrate, and GSSG levels, as well as increased SOD activity and lower GSH levels, while also restoring cognitive impairments [44].
Studies performed on Alzheimer's disease preclinical models demonstrate Memantine to decrease oxidative-stress induced damage to cortex and hippocampus proteins, improving age-related recognition memory in senior rats [45]. Memantine also reduced the frequency of inducible forms of NOS in an A25–35 AD model [46] and, in addition, ROS and nitrate levels in the hippocampus and cortex in a streptozotocin AD model [47] as well as a kainic acid-induced model of dementia [47]. However, this effect was not observed in the striatum [45].
Immunomodulatory agents such as Fingolimod or FTY720, Tanshinone I, Lenalidomide, Thalidomide, Ginsenoside Rg1, CNI-1493, Pycnogenol, and C5aR antagonist DF3016A demonstrate anti-inflammatory action, thereby reducing OS and lipid peroxidation products and decreasing microglial, astrocytic, and T cell activities. They might be used as a preventative measure, and there is some evidence that they help with motor impairments and nigral dopaminergic neurotoxicity [48].
Over time, innovations in the field of nanomedicine have garnered widespread interest in the scientific community. Some compounds of interest are cerium oxide NPs, ceria/polyoxometalates hybrid, manganese tetroxide and manganese ferrite nanoparticles, yttrium oxide nanoparticles, iron oxide nanoparticles, copper nanoparticle clusters, cobalt oxide (Co3O4 NPs), and cobalt ferrite nanoparticles (CoFe2O4 NPs). Some compounds have antioxidant properties and other compounds like ceria mimic enzymes [49]. Nanoceria mimics SOD and CAT activity. Due to the mixed valency state of cerium oxide, it reacts with free radicals and detoxifies ROS and therefore may be neuroprotective.
Apart from metal and metal oxide nanoparticles described above, inorganic nanoparticles such as mesoporous silica nanoparticles (MSNs) can have potential applications as explained by their large surface area, structural tunability, and easy functionalization [50]. Therapeutic trials of these drugs and therapies in animal and human models are discussed below [38, 41, 42, 51–65]. In patients: (i) vitamin E (α-tocopherol, 800 IU/day) + vitamin C (500 mg/day) + α-lipoic acid (900 mg/day) ↓F2-isoprostane in CSF [51]; (ii) Coenzyme Q10 (400 mg ×3 times/day) NO CHANGE F2-isoprostane in CSF [51]; (iii) ω-3 (3 g/day contained 675 mg DHA and 975 mg EPA) NO CHANGE F2-isoprostane in urine, NO CHANGE PC in plasma [52]; (iv) ω-3 + α-lipoic acid (ω-3, 3 g/day contained 675 mg DHA and 975 mg EPA + α-lipoic acid, 600 mg/day in one tablet) NO CHANGE F2-isoprostane in urine NO CHANGE PC in plasma [52]; (v) vitamin C (1,000 mg/day) + vitamin E (400 IU/day) ↓oxidation of CSF [53]; (vi) Curcumin (1 or 4 g/day) NO CHANGE F2-isoprostane in plasma [54]; (vii) curcuminoids (2 or 4 g/day) NO CHANGE F2-isoprostane in CSF [55]. In animal models: (i) Schisantherin A 0.1 mg/kg for 5 days i.c.v., injection started after 3 days from Aβ1–42 injection (↓MDA in cerebral CTX, ↑SOD, ↑GPx, ↑GSH in HIP and cerebral CTX) [38]; (ii) vitamin E 150 mg/kg, p.o. for 27 days, administration began 7 days before Aβ1–42 i.c.v. (↓MDA, ↓PC, ↓Mn-SOD, ↓Zn, Cu-SOD, ↑GPx, Ø GR in cerebral CTX and HIP) [57]; (iii) Piperine 5 or 10 mg/kg p.o. 2 weeks before and 1 week after AF64A (↓MDA in HIP) [58]; (iv) S-allyl cysteine 30 mg/kg i.p. for 15 days pretreatment before streptozocin (↓TBARS, ↑GSH, ↑GPx, ↑GR in HIP) [59]; (v) Imperatorin 2×/day for 7 days with scopolamine injection (↓MDA, ↑SOD in CTX and HIP, ↑GPx in CTX and HIP, ↑GR in CTX) [42]; (vi) α-lipoic acid 30 mg/kg/day enriched diet for 10 months (↓HNE, Ø 3-NT in brain homogenates) [60]; (vii) vitamin C 125 mg/kg i.p. for 12 days (no change MDA in HIP) [61]; (viii) vitamin C low diet content 0.099 g/L of drinking water (↑MDA in CTX) [62]; (ix) melatonin 5 mg/kg p.o. for 5.5 months (↓MDA, ↓PC in HIP) [63]; (x) melatonin 10 mg/kg/day for 4 months intragastrically (↓TBARS, ↑GSH, ↑SOD in the brain homogenate) [64]
2.6. Oxidative Stress Drug Targets in Anti-Parkinson's Disease Therapy
Drugs like valproic acid, melatonin, ceftriaxone, and N-acetylcysteine have been shown in research to have oxidative impacts on human health. Antioxidative defense enzyme activity in mice was recovered after ceftriaxone treatment (glutathione, catalase, and SOD). Prior to taking ceftriaxone, Ropinirole considerably increased the protective effects [66].
Using 6-OHDA-induced SNpc dopaminergic neuronal damage in rat models, serofendic acid plays a protective role against 6-OHDA-induced oxidative stress parameters, such as 3-nitrotyrosine and 4-hydroxy-2-nonenal (4-HNE). When N-acetylcysteine was administered to animals, it induced an upsurge in the efficiency of lipid peroxylase, superoxide dismutase (SOD), and g-glutamyl transpeptidase (g-GTP) and a big decline in glutathione (GSH) levels and glutathione peroxidase function in the SNpc [67].
Ropinirole, a dopaminergic stimulant, increased GSH levels and CAT action, according to a new analysis. These PLGA nanoparticles (NPs) were developed to enhance the drug's effectiveness and distribution [55].
Parkinson's disease may be treated with the nootropic centrophenoxine (CPH). Catalase and superoxide dismutase (SOD) upregulated, whereas nitric oxide (NO) and citrulline levels decreased, according to one study [68]. These compounds seem to be promising; further studies are required to understand their efficacy in a natural setting because in the above studies, to produce selective DAergic neuronal degeneration in PD, rotenone-induced neurotoxicity was used as a preclinical model PD in mice. Many therapeutic trials of these drugs and therapies in animal and human models are conducted until date [66, 69–71]. These included (i) L-DOPA (200 mg/kg i.p. 2 injections/day for 4 weeks, coadministration with MPTP (no change GSH in SN) [69], (ii) Ropinirole 1, 5, or 3 mg/kg i.p. for 14 days, after MPTP (↑GSH, ↑CAT, ↓nitrate (only 1.5 mg/kg) in STR and CTX) [66]; (iii) Pramipexole 1 mg/kg i.p. 2 injections/day for 4 weeks, coadministration with MPTP (↑GSH in SN) [69]; and (iv) Deferoxamine 50 mg/kg p.o. for 14 days, coadministration with 6-OHDA (↓PC, ↑GSH, and ↑SOD in STR) [71].
3. Conclusions and Future Perspectives
Fundamentally, it is necessary to do extensive study on the inclusionary practices of ROS in the neurodegenerative process and AD, in order to analyze therapeutic targets that are noteworthy. Current medications, though effective, should not be considered optimal management of neurodegenerative disease. The drugs discussed above should undergo further evaluation for feasibly in human subjects. The effectiveness of pharmacological drugs that target the cellular oxidative system in reducing neurodegenerative should next be thoroughly tested in medical tests. Despite the fact that mitochondrial dysfunction is an important role in the development of AD and PD, little is known about the extent of this impact, which makes it difficult to put hypothetical pharmacological targets into reality in the real world.
Contributor Information
Wireko Andrew Awuah, Email: andyvans36@yahoo.com.
Athanasios Alexiou, Email: alextha@yahoo.gr.
Conflicts of Interest
The authors of this research disclose that they have no competing interests in its manuscript.
Authors' Contributions
Abdullahi Tunde Aborode, Wireko Andrew Awuah, and Manas Pustake conceptualized the concept for this review. Manas Pustake, Mariam Alwerdani, Parth Shah, Rohan Yarlagadda, Shahzaib Ahmad, Ayush Chandra, Esther Patience Nansubuga, Omar Ali, and Aashna Mehta wrote the first draft. Inês F Silva Correia, Toufik Abdul-Rahman, Aashna Mehta, Shekinah Obinna Amaka, Yves Miel H. Zuñiga, Anastasiia D. Shkodina, Oko Christian Inya, Bairong Shen, and Athanasios Alexiou edited the second draft. Athanasios Alexiou, Bairong Shen, Abdullahi Tunde Aborode, and Wireko Andrew Awuah provided suggestions for improvement and adjustments. The final manuscript was proofread by all of the authors, and they all gave their approval.
References
- 1.Reddy A. P., Ravichandran J., Carkaci-Salli N. Neural regeneration therapies for Alzheimer’s and Parkinson’s disease-related disorders. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease . 2020;1866(4, article 165506) doi: 10.1016/J.BBADIS.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald R., Barnes K., Hastings C., Mortiboys H. Mitochondrial abnormalities in Parkinson’s disease and Alzheimer’s disease: can mitochondria be targeted therapeutically? Biochemical Society Transactions . 2018;46(4):891–909. doi: 10.1042/BST20170501. [DOI] [PubMed] [Google Scholar]
- 3.Qiu C., Kivipelto M., Von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues in Clinical Neuroscience . 2009;11(2):111–128. doi: 10.31887/DCNS.2009.11.2/CQIU. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrer M. D., Sureda A., Mestre A., Tur J. A., Pons A. The double edge of reactive oxygen species as damaging and signaling molecules in HL60 cell culture. Cellular Physiology and Biochemistry . 2010;25(2–3):241–252. doi: 10.1159/000276558. [DOI] [PubMed] [Google Scholar]
- 5.Spillantini M. G., Schmidt M. L., Lee V. M. Y., Trojanowski J. Q., Jakes R., Goedert M. α-Synuclein in Lewy bodies. Nature . 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 6.Perez F. A., Palmiter R. D. Parkin-deficient mice are not a robust model of parkinsonism. Proceedings of the National Academy of Sciences . 2005;102(6):2174–2179. doi: 10.1073/PNAS.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi W.-S., Kruse S. E., Palmiter R. D., Xia Z. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proceedings of the National Academy of Sciences . 2008;105(39):15136–15141. doi: 10.1073/PNAS.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serrano-Pozo A., Frosch M. P., Masliah E., Hyman B. T. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine . 2011;1(1, article a006189) doi: 10.1101/CSHPERSPECT.A006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X., Guo C., Kong J. Oxidative stress in neurodegenerative diseases. Neural Regeneration Research . 2012;7(5):376–385. doi: 10.3969/j.issn.1673-5374.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St-Pierre J., Buckingham J. A., Roebuck S. J., Brand M. D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. The Journal of Biological Chemistry . 2002;277(47):44784–44790. doi: 10.1074/JBC.M207217200. [DOI] [PubMed] [Google Scholar]
- 11.Li X., Fang P., Mai J., Choi E. T., Wang H., Yang X. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. Journal of Hematology & Oncology . 2013;6:1–19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. Journal of Bioenergetics and Biomembranes . 1999;31(4):347–366. doi: 10.1023/A:1005427919188. [DOI] [PubMed] [Google Scholar]
- 13.Murphy M. P. How mitochondria produce reactive oxygen species. The Biochemical Journal . 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Förstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nature Clinical Practice Cardiovascular Medicine . 2008;5(6):338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- 15.Cobley J. N., Fiorello M. L., Bailey D. M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biology . 2018;15:490–503. doi: 10.1016/J.REDOX.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suárez A., Ramírez-Tortosa M., Gil A., Faus M. J. Addition of vitamin E to long-chain polyunsaturated fatty acid-enriched diets protects neonatal tissue lipids against peroxidation in rats. European Journal of Nutrition . 1999;38(4):169–176. doi: 10.1007/S003940050058. [DOI] [PubMed] [Google Scholar]
- 17.Hauck A. K., Huang Y., Hertzel A. V., Bernlohr D. A. Adipose oxidative stress and protein carbonylation. The Journal of Biological Chemistry . 2019;294(4):1083–1088. doi: 10.1074/JBC.R118.003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plascencia-Villa G., Perry G. Preventive and therapeutic strategies in Alzheimer’s disease: focus on oxidative stress, redox metals, and ferroptosis. Antioxidants & Redox Signaling . 2021;34(8):591–610. doi: 10.1089/ARS.2020.8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elfawy H. A., Das B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: etiologies and therapeutic strategies. Life Sciences . 2019;218:165–184. doi: 10.1016/J.LFS.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 20.Tobore T. O. On the central role of mitochondria dysfunction and oxidative stress in Alzheimer’s disease. Neurological Sciences . 2019;40(8):1527–1540. doi: 10.1007/S10072-019-03863-X. [DOI] [PubMed] [Google Scholar]
- 21.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules . 2019;24(8, article 1583) doi: 10.3390/MOLECULES24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walczak-Jedrzejowska R., Wolski J. K., Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Central European Journal of Urology . 2013;66(1, article 60) doi: 10.5173/CEJU.2013.01.ART19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudryavtseva A. V., Krasnov G. S., Dmitriev A. A., et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget . 2016;7(29, article 44879) doi: 10.18632/ONCOTARGET.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckhauser T. F., Francis-Oliveira J., De Pasquale R. Reactive oxygen species: physiological and physiopathological effects on synaptic plasticity. Journal of Experimental Neuroscience . 2016;10, article JEN-S39887(Suppl 1) doi: 10.4137/JEN.S39887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramírez A., Vázquez-Sánchez A. Y., Carrión-Robalino N., Camacho J. Ion channels and oxidative stress as a potential link for the diagnosis or treatment of liver diseases. Oxidative Medicine and Cellular Longevity . 2016;2016:17. doi: 10.1155/2016/3928714.3928714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsatmali M., Walcott E. C., Makarenkova H., Crossin K. L. Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Molecular and Cellular Neuroscience . 2006;33(4):345–357. doi: 10.1016/J.MCN.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niedzielska E., Smaga I., Gawlik M., et al. Oxidative stress in neurodegenerative diseases. Molecular Neurobiology . 2016;53(6):4094–4125. doi: 10.1007/S12035-015-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin M. T., Beal M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature . 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 29.Abramov A. Y., Angelova P. R. Cellular mechanisms of complex I-associated pathology. Biochemical Society Transactions . 2019;47(6):1963–1969. doi: 10.1042/BST20191042. [DOI] [PubMed] [Google Scholar]
- 30.Reddy P. H., Tripathi R., Troung Q., et al. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease . 2012;1822(5):639–649. doi: 10.1016/J.BBADIS.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Augustyniak A., Bartosz G., Čipak A., et al. Natural and synthetic antioxidants: an updated overview. Free Radical Research . 2010;44(10):1216–1262. doi: 10.3109/10715762.2010.508495. [DOI] [PubMed] [Google Scholar]
- 32.Rama R., Garzón F., Rodríguez-Cruz Y., Maurice T., García-Rodríguez J. C. Neuroprotective effect of Neuro-EPO in neurodegenerative diseases: ‘Alea jacta est. Neural Regeneration Research . 2019;14(9, article 1519) doi: 10.4103/1673-5374.255968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsiao K., Chapman P., Nilsen S., et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science . 1996;274(5284):99–103. doi: 10.1126/SCIENCE.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 34.Grottelli S., Ferrari I., Pietrini G., Peirce M. J., Minelli A., Bellezza I. The role of cyclo (His-Pro) in neurodegeneration. International Journal of Molecular Sciences . 2016;17(8, article 1332) doi: 10.3390/IJMS17081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan M. M., Zaheer S., Nehman J., Zaheer A. Suppression of glia maturation factor expression prevents 1-methyl-4-phenylpyridinium (MPP+)-induced loss of mesencephalic dopaminergic neurons. Neuroscience . 2014;277:196–205. doi: 10.1016/J.NEUROSCIENCE.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenche V. B., Barnham K. J. Alzheimer’s disease & metals: therapeutic opportunities. British Journal of Pharmacology . 2011;163(2):211–219. doi: 10.1111/J.1476-5381.2011.01221.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deas E., Cremades N., Angelova P. R., et al. Alpha-synuclein oligomers interact with metal ions to induce oxidative stress and neuronal death in Parkinson’s disease. Antioxidants & Redox Signaling . 2016;24(7):376–391. doi: 10.1089/ARS.2015.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jhoo J. H., Kim H. C., Nabeshima T., et al. β-Amyloid (1–42)-induced learning and memory deficits in mice: involvement of oxidative burdens in the hippocampus and cerebral cortex. Behavioural Brain Research . 2004;155(2):185–196. doi: 10.1016/J.BBR.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Kracmarova A., Bandouchova H., Pikula J., Pohanka M. Tacrine is implicated in oxidative stress in the laboratory guinea pig model. Neuroendocr. Letters . 2012;33:136–144. [PubMed] [Google Scholar]
- 40.Benzie I. F. F., Strain J. J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Analytical Biochemistry . 1996;239(1):70–76. doi: 10.1006/ABIO.1996.0292. [DOI] [PubMed] [Google Scholar]
- 41.Wang D., Liu L., Zhu X., Wu W., Wang Y. Hesperidin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress in a mouse model of Alzheimer’s disease. Cellular and Molecular Neurobiology . 2014;34(8, article 98):1209–1221. doi: 10.1007/s10571-014-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Budzynska B., Boguszewska-Czubara A., Kruk-Slomka M., et al. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology . 2015;232(5):931–942. doi: 10.1007/s00213-014-3728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahdy K., Shaker O., Wafay H., Nassar Y., Hassan H., Hussein A. Effect of some medicinal plant extracts on the oxidative stress status in Alzheimer’s disease induced in rats. European Review for Medical and Pharmacological Sciences . 2012;16(3):31–42. [PubMed] [Google Scholar]
- 44.Kumar A., Prakash A., Pahwa D. Galantamine potentiates the protective effect of rofecoxib and caffeic acid against intrahippocampal Kainic acid-induced cognitive dysfunction in rat. Brain Research Bulletin . 2011;85(3–4):158–168. doi: 10.1016/J.BRAINRESBULL.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Pietá Dias C., Martins de Lima M. N., Presti-Torres J., et al. Memantine reduces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscience . 2007;146(4):1719–1725. doi: 10.1016/J.NEUROSCIENCE.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Chi L., Ke Y., Luo C., Gozal D., Liu R. Depletion of reduced glutathione enhances motor neuron degeneration in vitro and in vivo. Neuroscience . 2007;144(3):991–1003. doi: 10.1016/J.NEUROSCIENCE.2006.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rai S., Kamat P. K., Nath C., Shukla R. Glial activation and post-synaptic neurotoxicity: the key events in Streptozotocin (ICV) induced memory impairment in rats. Pharmacology, Biochemistry, and Behavior . 2014;117:104–117. doi: 10.1016/J.PBB.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 48.Martinez B., Peplow P. Neuroprotection by immunomodulatory agents in animal models of Parkinson’s disease. Neural Regeneration Research . 2018;13(9):1493–1506. doi: 10.4103/1673-5374.237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eleftheriadou D., Kesidou D., Moura F., Felli E., Song W. Redox-responsive nanobiomaterials-based therapeutics for neurodegenerative diseases. Small . 2020;16(43, article 1907308) doi: 10.1002/SMLL.201907308. [DOI] [PubMed] [Google Scholar]
- 50.Narayan R., Nayak U. Y., Raichur A. M., Garg S. Mesoporous silica nanoparticles: a comprehensive review on synthesis and recent advances. Pharmaceutics . 2018;10(3, article 118) doi: 10.3390/PHARMACEUTICS10030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galasko D. R., Peskind E., Clark C. M., et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Archives of Neurology . 2012;69(7):836–841. doi: 10.1001/ARCHNEUROL.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shinto L., Quinn J., Montine T., et al. A randomized placebo-controlled pilot trial of Omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. Journal of Alzheimer's Disease . 2014;38(1):111–120. doi: 10.3233/JAD-130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arlt S., Müller-Thomsen T., Beisiegel U., Kontush A. Effect of one-year vitamin C-and E-supplementation on cerebrospinal fluid oxidation parameters and clinical course in Alzheimer’s disease. Neurochemical Research . 2012;37(12):2706–2714. doi: 10.1007/s11064-012-0860-8. [DOI] [PubMed] [Google Scholar]
- 54.Baum L., Lam C. W. K., Cheung S. K. K., et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. Journal of Clinical Psychopharmacology . 2008;28(1):110–113. doi: 10.1097/JCP.0B013E318160862C. [DOI] [PubMed] [Google Scholar]
- 55.Ringman J. M., Frautschy S. A., Teng E., et al. Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer's Research & Therapy . 2012;4(5):1–8. doi: 10.1186/ALZRT146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thal L. J., Grundman M., Berg J., et al. Idebenone treatment fails to slow cognitive decline in Alzheimer’s disease. Neurology . 2003;61(11):1498–1502. doi: 10.1212/01.WNL.0000096376.03678.C1. [DOI] [PubMed] [Google Scholar]
- 57.Li X., Zhao X., Xu X., et al. Schisantherin A recovers Aβ-induced neurodegeneration with cognitive decline in mice. Physiology & Behavior . 2014;132:10–16. doi: 10.1016/J.PHYSBEH.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 58.Chonpathompikunlert P., Wattanathorn J., Muchimapura S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food and Chemical Toxicology . 2010;48(3):798–802. doi: 10.1016/J.FCT.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Javed H., Khan M. M., Khan A., et al. S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Research . 2011;1389:133–142. doi: 10.1016/J.BRAINRES.2011.02.072. [DOI] [PubMed] [Google Scholar]
- 60.Siedlak S. L., Casadesus G., Webber K. M., et al. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer’s disease. Free Radical Research . 2009;43(2):156–164. doi: 10.1080/10715760802644694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrison F. E., Hosseini A. H., McDonald M. P., May J. M. Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacology, Biochemistry, and Behavior . 2009;93(4):443–450. doi: 10.1016/J.PBB.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrison F. E., May J. M., McDonald M. P. Vitamin C deficiency increases basal exploratory activity but decreases scopolamine-induced activity in APP/PSEN1 transgenic mice. Pharmacology, Biochemistry, and Behavior . 2010;94(4):543–552. doi: 10.1016/J.PBB.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otalora B. B., Popovic N., Gambini J., et al. Circadian system functionality, hippocampal oxidative stress, and spatial memory in the APPswe/PS1dE9 transgenic model of Alzheimer disease: effects of melatonin or ramelteon. Chronobiology International . 2012;29(7):822–834. doi: 10.3109/07420528.2012.699119. [DOI] [PubMed] [Google Scholar]
- 64.Feng Z., Qin C., Chang Y., Zhang J. T. Early melatonin supplementation alleviates oxidative stress in a transgenic mouse model of Alzheimer’s disease. Free Radical Biology & Medicine . 2006;40(1):101–109. doi: 10.1016/J.FREERADBIOMED.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 65.Duffy A. M., Hölscher C. The incretin analogue D-Ala 2GIP reduces plaque load, astrogliosis and oxidative stress in an APP/PS1 mouse model of Alzheimer’s disease. Neuroscience . 2013;228:294–300. doi: 10.1016/J.NEUROSCIENCE.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 66.Bisht R., Kaur B., Gupta H., Prakash A. Ceftriaxone mediated rescue of nigral oxidative damage and motor deficits in MPTP model of Parkinson’s disease in rats. Neurotoxicology . 2014;44:71–79. doi: 10.1016/J.NEURO.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Barcia E., Boeva L., García-García L., et al. Nanotechnology-based drug delivery of ropinirole for parkinson’s disease. Drug Delivery . 2017;24(1):1112–1123. doi: 10.1080/10717544.2017.1359862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verma R., Nehru B. Effect of centrophenoxine against rotenone-induced oxidative stress in an animal model of Parkinson’s disease. Neurochemistry International . 2009;55(6):369–375. doi: 10.1016/J.NEUINT.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Shin J. Y., Park H. J., Ahn Y. H., Lee P. H. Neuroprotective effect of l-dopa on dopaminergic neurons is comparable to pramipexol in MPTP-treated animal model of Parkinson’s disease: a direct comparison study. Journal of Neurochemistry . 2009;111(4):1042–1050. doi: 10.1111/J.1471-4159.2009.06381.X. [DOI] [PubMed] [Google Scholar]
- 70.Khurana N., Gajbhiye A. Ameliorative effect of Sida cordifolia in rotenone induced oxidative stress model of Parkinson’s disease. Neurotoxicology . 2013;39:57–64. doi: 10.1016/J.NEURO.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Haleagrahara N., Siew C. J., Ponnusamy K. Effect of quercetin and desferrioxamine on 6-hydroxydopamine (6-OHDA) induced neurotoxicity in striatum of rats. The Journal of Toxicological Sciences . 2013;38(1):25–33. doi: 10.2131/JTS.38.25. [DOI] [PubMed] [Google Scholar]


