Abstract
Background
Current guidelines recommend the use of potent antiplatelet agents in patients undergoing percutaneous coronary intervention (PCI) following an acute coronary syndrome (ACS). However, data about optimal platelet inhibition in severe renal insufficiency patients are scarce. The purpose of this study is to determine if ticagrelor is more effective than clopidogrel in patients with ACS and severe renal insufficiency treated with PCI.
Methods
We retrospectively enrolled patients with ACS and severe renal insufficiency (eGFR ≤ 30 ml/min·1.73 m2 or dialysis) who underwent PCI at our hospital between January 2015 and March 2020. We used the adjusted Cox proportional hazards models to analyze the 1-year outcome endpoints, including the primary endpoint (the composite of cardiovascular death, recurrence of MI, or nonfatal ischemic stroke), death from any cause, and bleeding events (Bleeding Academic Research Consortium, BARC criteria).
Results
A total of 276 patients with ACS and severe renal insufficiency who were treated with PCI with ticagrelor (n = 108) or clopidogrel (n = 168) were included in the study. After adjustment, there was no statistical difference in risk of the primary endpoint (HR, 0.78; 95% CI, 0.46–1.33; P=0.367) and death from any cause (HR, 0.86; 95% CI, 0.38–1.89; P=0.708) in the ticagrelor group against the clopidogrel group. However, the risk of total bleeding was significantly higher in the ticagrelor group (HR, 3.01; 95% CI, 1.81–5.62; P=0.01). Subgroup analysis according to the confounders did not identify any significant subgroup heterogeneity.
Conclusion
Ticagrelor did not improve the major adverse cardiovascular events and all-cause mortality when compared to clopidogrel, but significantly increased the risk of bleeding in Chinese patients with ACS and severe renal insufficiency undergoing PCI.
1. Introduction
Chronic kidney disease (CKD) affects between 20% and 40% of patients hospitalized with the acute coronary syndrome (ACS), and these individuals have a greater death rate than those with normal renal function [1]. Studies have shown that percutaneous coronary intervention (PCI) improves the prognosis of patients with ACS and CKD, but patients with renal insufficiency have a higher risk of thrombosis and bleeding after the procedure [2]. Current guidelines recommend dual antiplatelet therapy (DAPT) with aspirin and P2Y12 receptor inhibitors for 12 months after PCI in patients with ACS [3].
Ticagrelor or prasugrel, both of which are potent oral P2Y12 receptor inhibitors, are recommended for the treatment of patients with ACS and CKD due to their rapid onset of action and high rate of platelet inhibition compared to clopidogrel [3–5]. Clopidogrel has a delayed onset of effect and wide interindividual variability in biological efficacy leading to a 40% rate of high on-treatment platelet reactivity (HTPR) [6, 7]. Importantly, CKD patients have a greater risk of HTPR than the overall population, which was linked to thrombotic events and death [8, 9]. Ticagrelor is more powerful and reproducible in its platelet inhibition than clopidogrel, even in CKD patients [10–12].
Numerous studies have demonstrated that ticagrelor improves clinical outcomes such as all-cause mortality and major cardiovascular adverse events in patients with ACS and CKD when compared to clopidogrel [13–17]. However, it has also been shown that ticagrelor is not superior to clopidogrel and may increase the risk of bleeding [17–19]. Since most of the previous studies were conducted in patients with mild to moderate renal insufficiency, the efficacy and safety of DAPT with potent P2Y12 inhibitors in patients with severe renal insufficiency is unclear [15, 20]. This study was designed to evaluate the 1-year clinical outcomes of ticagrelor vs. clopidogrel in patients with severe renal insufficiency (eGFR ≤ 30 ml/min·1.73 m2 or dialysis) undergoing PCI, including cardiovascular death, death from any cause, MI, stroke, and bleeding events.
2. Methods
2.1. Study Design and Patient Population
This was a single-center retrospective study. We consecutively selected patients with ACS and severe renal function (eGFR < 30 ml/min·1.73 m2), including patients on hemodialysis treatment who underwent successful PCI in our hospital between January 2015 and March 2020. We excluded all patients on anticoagulant therapy at discharge, patients with missing creatinine measurements, patients not receiving dual antiplatelet therapy at discharge, and patients lost to follow-up within 1 year. Patients were divided into the ticagrelor group or the clopidogrel group according to the dual antiplatelet regimen during hospitalization and after hospital discharge. All patients' baseline characteristics, past medical history, clinical test results, hospital medications, and angiographic data should be documented. The Ethics Committee of Yuebei People's Hospital approved the study protocol, and all study subjects provided informed consent (Registration Number: ChiCTR2100043135). All procedures were carried out in accordance with the applicable guidelines and regulations.
2.2. Definitions
ACS, including unstable angina, non-ST-segment elevation myocardial infarction, and ST-segment myocardial infarction, were defined according to the diagnostic criteria established by the European Society of Cardiology [5]. According to the Bleeding Academic Research Consortium (BARC) classification [21], we defined mild to moderate bleeding as BARC 1 or 2 and severe bleeding as BARC 3 or 5. The estimated glomerular filtration rate (eGFR) was calculated from creatinine measurements on admission and using the Chronic Kidney Disease Epidemiology Collaboration equation [22]. Cardiovascular death was defined as death because of AMI, heart failure, cardiogenic shock, ventricular arrhythmia, or cerebrovascular events.
2.3. Interventional Procedures and In-Hospital Medications
PCI procedures were performed by three experienced surgeons, and all patients were implanted with a Firebird2 coronary rapamycin-eluting cobalt-based alloy stent (MicroPort Scientific Corporation, China) [23]. All patients underwent revascularization of the culprit's vessel. The decision to perform complete revascularization depended on the site of the vascular lesion, its severity, the patient's general condition, and the surgeon's strategy. Because of the health insurance policy, there were only two new oral antiplatelet drugs at our hospital, clopidogrel and ticagrelor, and no prasugrel. Clinicians chose different antiplatelet agents for treatment based on guidelines, personal experience, and the patient's condition. Before the intervention, all patients received antiplatelet agents, including aspirin 300 mg loading dose (LD) and clopidogrel 300–600 mg LD or ticagrelor 180 mg LD. Following the intervention, the patients were given aspirin 100 mg once daily indefinitely, as well as clopidogrel 75 mg once daily or ticagrelor 90 mg twice daily for at least a year. Other drugs, such as glycoprotein IIb/IIIa inhibitors, antithrombotic drugs, ACEI/ARB, beta-blockers, and so on, were chosen based on the clinical situation of the patient.
2.4. Study Endpoint
During a 1-year follow-up period, adverse clinical outcomes were recorded. We used major adverse cardiovascular events (MACEs, the composite of cardiovascular death, recurrence of MI, or nonfatal ischemic stroke) as a primary endpoint. The secondary endpoint included the individual components of the primary outcome described separately as well as death from any cause and bleeding events (BARC classification).
2.5. Statistical Analysis
Continuous data were expressed as a median ± standard or an interquartile range (IQR), while categorical data were expressed as percentages. The variables were compared using the chi-square tests or Fisher's exact tests (categorical variables), the Student's t (continuous variables), and Kruskal–Wallis (skewed distribution) tests, respectively. Kaplan–Meier analysis was used to assess the event-free status of the patients using the log-rank test. Multivariable Cox regression analyses were adopted to assess the independent association between DAPT regimens and 1-year outcomes after adjusting for clinical characteristics. Subgroup analyses were stratified by some relevant effect covariates. All the analyses were performed with the statistical software packages R (https://www.R-project.org, The R Foundation) and Free Statistics software version 1.3. A two-tailed test was performed and p 0.05 was considered statistically significant.
3. Results
3.1. Population and Baseline Characteristics
Between January 2015 and March 2020, our hospital treated 8210 consecutive patients with ACS who underwent PCI. In total, 322 of these patients had severe renal function (eGFR < 30 ml/min·1.73 m2). We excluded 20 patients who were on anticoagulant therapy at discharge, 12 patients with missing dates, and 14 patients lost to follow-up, resulting in a study population of 276 patients. The flow chart of the study patients' selection is shown in Figure 1. Enrolled patients were divided into 2 groups: ticagrelor group (N = 108) and clopidogrel group (N = 168). There was no statistical difference between the 2 groups on age, sex, diagnosis at discharge, traditional cardiovascular risk, previous medical history, dialysis, creatine clearance, treated vessel, or medications at admission. The baseline characteristics of all participants are given in Table 1.
Figure 1.
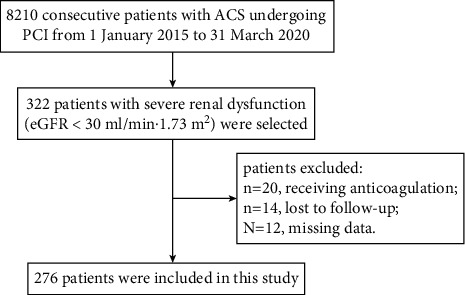
Flowchart of patient selection.
Table 1.
Baseline characteristics of the study participants.
| Variables | Total (n = 276) | Ticagrelor (n = 108) | Clopidogrel (n = 168) | P value |
|---|---|---|---|---|
| Male, no.(%) | 192 (69.6) | 79 (73.1) | 113 (67.4) | 0.473 |
|
| ||||
| Age(years),mean ± SD | 67.5 ± 11.6 | 66.3 ± 10.8 | 68.2 ± 12.1 | 0.24 |
|
| ||||
| Diagnosis at discharge, n (%) | 0.085 | |||
| STEMI | 117 (42.4) | 48 (44.4) | 69 (41.1) | |
| NSTEMI | 86 (31.2) | 40 (37.0) | 46 (27.4) | |
| Unstable angina | 73 (26.4) | 20(18.5) | 53 (31.5) | |
|
| ||||
| Risk factors, n (%) | ||||
| Hypertension | 210 (76.1) | 78 (72.2) | 132 (78.6) | 0.367 |
| Diabetes | 109 (39.5) | 36 (33.3) | 73 (43.5) | 0.187 |
| Current smoker | 70 (25.4) | 32 (29.6) | 38 (22.6) | 0.326 |
|
| ||||
| Previous medical history, n (%) | ||||
| Myocardial infarction | 28 (10.1) | 12(11.1) | 16 (9.5) | 0.798 |
| Congestive heart failure | 63 (22.8) | 20 (18.5) | 43 (25.6) | 0.289 |
| COPD | 20 (6.9) | 5 (4.6) | 15 (8.9) | 0.204 |
| Nonhemorrhagic stroke | 22 (10.2) | 14 (12.9) | 16 (9.5) | 0.466 |
|
| ||||
| Dialysis, n (%) | 50 (18.1) | 18 (16.7) | 32 (19.0) | 0.83 |
|
| ||||
| SBP (mmHg), mean ± SD | 134.8 ± 31.5 | 132.9 ± 30.9 | 135.9 ± 31.9 | 0.494 |
|
| ||||
| DBP (mmHg), mean ± SD | 79.5 ± 16.4 | 78.6 ± 16.1 | 80.0 ± 16.5 | 0.57 |
|
| ||||
| HR (bpm), mean ± SD | 86.3 ± 14.6 | 87.1 ± 13.4 | 85.9 ± 15.3 | 0.566 |
|
| ||||
| Hemoglobin (g/L), mean ± SD | 106.2 ± 14.4 | 108.3 ± 10.4 | 105.0 ± 16.1 | 0.104 |
|
| ||||
| eGFR (ml/min·1.73 m2), median (IQR) | 17.3 (10.6, 24.3) | 18.0 (12.3, 24.4) | 17.0 (9.1, 24.2) | 0.281 |
|
| ||||
| Creatinine (μmol/L), mean ± SD | 404.2 ± 267.4 | 369.0 ± 227.9 | 424.1 ± 286.3 | 0.147 |
|
| ||||
| Hs-cTn T (pg/mL), Mean ± SD | 1544.5 ± 2497.3 | 1263.4 ± 2095.7 | 1703.3 ± 2692.3 | 0.214 |
|
| ||||
| CK-MB (U/L), Mean ± SD | 137.5 ± 192.2 | 130.4 ± 180.0 | 141.4 ± 199.4 | 0.688 |
|
| ||||
| NT-proBNP(pg/mL), mean ± SD | 16372.1 ± 11914.7 | 14931.3 ± 11720.8 | 17186.5 ± 11988.4 | 0.182 |
|
| ||||
| EF (%), mean ± SD | 50.4 ± 6.8 | 50.5 ± 7.0 | 50.4 ± 6.8 | 0.936 |
|
| ||||
| BMI (kg/m2), mean ± SD | 23.8 ± 2.2 | 24.1 ± 2.2 | 23.7 ± 2.1 | 0.155 |
|
| ||||
| Cholesterol (mmol/L),mean ± SD | 4.3 ± 1.1 | 4.4 ± 1.3 | 4.2 ± 1.0 | 0.217 |
|
| ||||
| LDL-C (mmol/L), mean ± SD | 2.4 ± 1.0 | 2.5 ± 1.1 | 2.3 ± 0.9 | 0.248 |
|
| ||||
| Glucose (mmol/L), mean ± SD | 8.2 ± 3.3 | 8.3 ± 3.2 | 8.1 ± 3.3 | 0.808 |
|
| ||||
| Radial access, n (%) | 265 (96.0) | 104 (96.3) | 161 (95.8) | 0.714 |
|
| ||||
| Number of diseased vessels, n (%) | 0.144 | |||
| One | 43 (15.6) | 19 (17.6) | 24 (14.3) | |
| Two | 115 (41.6) | 36 (33.3) | 79 (47.0) | |
| Three | 118 (42.8) | 53 (49.1) | 65 (38.7) | |
|
| ||||
| Complete revascularization, n (%) | 182 (66.0) | 78 (72.2) | 102 (62.0) | 0.387 |
|
| ||||
| Medication on arrival, n (%) | ||||
| Aspirin | 276 (100.0) | 108 (100) | 168 (100) | 1 |
| GP IIb/IIIa inhibitors | 55 (19.9) | 28 (25.9) | 27 (16.1) | 0.121 |
| LMWH | 131 (47.5) | 47 (43.5) | 84 (50.0) | 0.445 |
| PPI | 215 (77.9) | 87 (80.6) | 128 (76.1) | 0.532 |
| β-blocker | 232 (84.1) | 95 (88.0) | 137 (81.5) | 0.28 |
| ACE inhibitor and/or ARB | 142 (51.4) | 56 (51.9) | 86 (51.2) | 0.987 |
| Stain, n (%) | 276 (100.0) | 108 (100) | 168 (100) | 1 |
| CCB, n (%) | 152 (55.1) | 58 (53.7) | 94 (56.0) | 0.752 |
STEMI, ST-elevation myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction; COPD, chronic obstructive pulmonary disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; eGFR, estimated glomerular filtration rate calculated by the chronic kidney disease epidemiology equation; hs-cTn T, high sensitivity cardiac troponin T; CK-MB, heart-type isoenzyme of creatine kinase; NT-proBNP, N-terminal pro-brain natriuretic peptide; EF, ejection fraction; LDL-C, low-density lipoprotein cholesterol; LMWH, low-molecular-weight heparin; PPI, proton pump inhibitors; CCB, calcium channel blockers.
3.2. Cardiovascular Outcomes
During the 1-year observation period, 39 (36.1%) patients in the ticagrelor group and 69 (41.1%) patients in the clopidogrel group met the primary endpoint. After adjustment, there were no significant differences in the risk of the primary endpoint (HR, 0.78; 95% CI, 0.46–1.33; P = 0.367), death from any cause (HR, 0.86; 95% CI, 0.38–1.89; P = 0.708), cardiovascular death (HR, 0.80; 95% CI, 0.35–2.12; P = 0.521), recurrent MI (HR, 0.72; 95% CI, 0.31–2.36; P = 0.485), or stroke (HR, 0.46; 95% CI, 0.24–3.07; P = 0.172) between the clopidogrel and ticagrelor groups in any of the three extended multivariable Cox models (Table 2). Kaplan–Meier analysis showed no difference between the two groups in the 1-year primary endpoint (Log-rank test: P = 0.35, Figure 2(a)). Subgroup analyses were performed according to confounding factors including gender, ACS staging, hemodialysis, use of glycoprotein IIb/IIIa inhibitors, and low-molecular heparin, and we did not observe any significant interactions in the subgroups (P value for interaction >0.05 for all, Figure 3(a)).
Table 2.
The association between use of different antiplatelet agents and cardiovascular outcomes using an extended cox model.
| Outcome | Events (n) | Nonadjusted model | Model I | Model 2 | ||||
|---|---|---|---|---|---|---|---|---|
| Ticagrelor (n = 108) | Clopidogrel (n = 168) | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Primary end point (composite of CV death, MI and stroke) | 39 (36.1) | 69 (41.1) | 0.83 (0.56∼1.23) | 0.353 | 0.81 (0.50∼1.28) | 0.378 | 0.78 (0.46∼1.33) | 0.367 |
| Death from any cause | 21 (19.4) | 38 (22.6) | 0.83 (0.45∼1.55) | 0.563 | 0.86 (0.47∼1.61) | 0.645 | 0.86 (0.38∼1.89) | 0.708 |
| Cardiovascular death | 15 (13.9) | 30 (17.9) | 0.72 (0.35∼1.46) | 0.356 | 0.73 (0.36∼1.49) | 0.385 | 0.80 (0.35∼2.12) | 0.521 |
| Myocardial infarction | 10 (9.3) | 23 (13.7) | 0.59 (0.25∼1.4) | 0.231 | 0.59 (0.25∼1.4) | 0.233 | 0.72 (0.31∼2.36) | 0.485 |
| Ischemic stroke | 8 (7.4) | 13 (7.7) | 0.91 (0.34∼2.46) | 0.852 | 0.94 (0.35∼2.57) | 0.909 | 0.46 (0.24∼3.07) | 0.172 |
Figure 2.
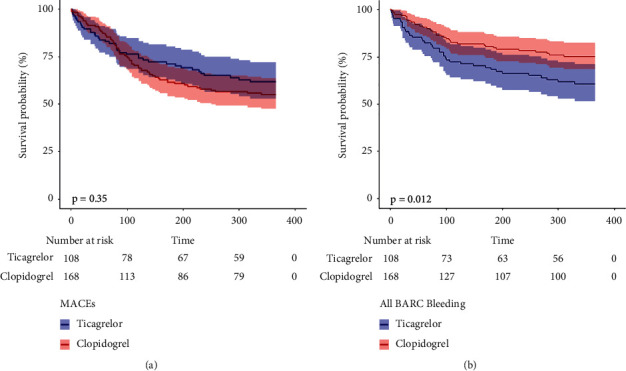
Kaplan–Meier curve for the 1-year primary endpoint (a) and total BARC bleeding (b) between clopidogrel and ticagrelor group.
Figure 3.
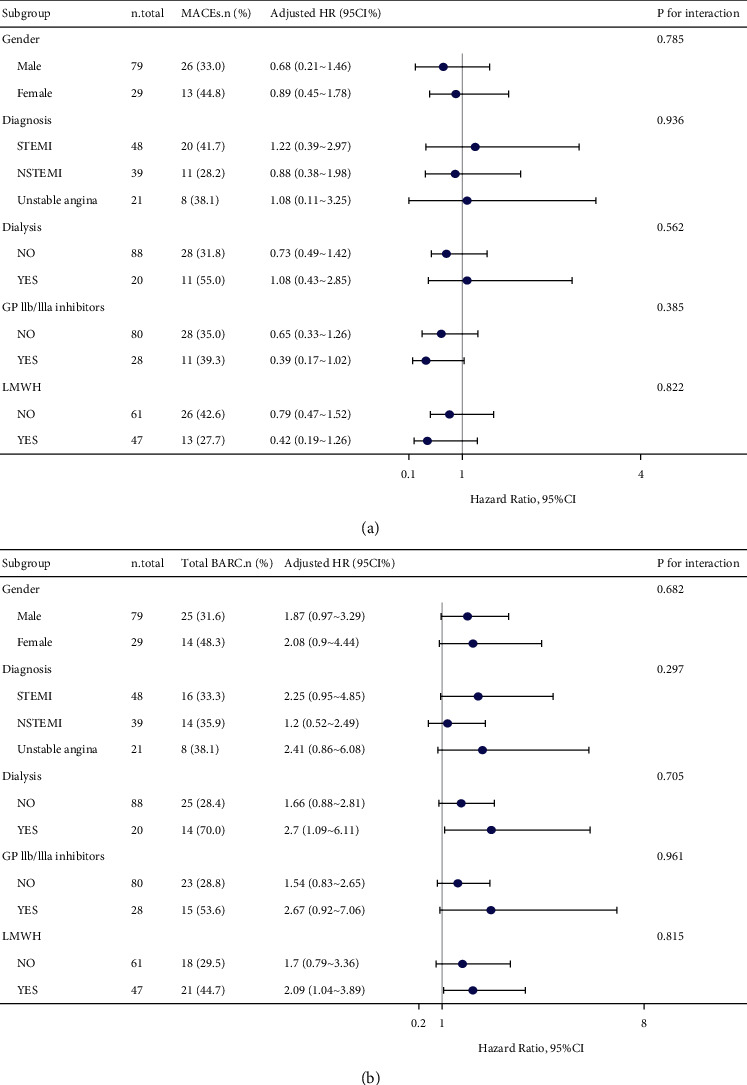
Forest plot illustrating the adjusted HR for primary outcomes (a) and total BARC bleeding (b) stratified on confounding factors (including gender, ACS staging, hemodialysis, use of glycoprotein IIb/IIIa inhibitors, and low-molecular heparin). STEMI, ST-elevation myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction; LMWH, low-molecular-weight heparin.
3.3. Bleeding
During the 1-year observation period, 39 (36.1%) patients in the ticagrelor group and 37 (22.0%) patients in the clopidogrel group occurred any bleeding events. After adjusted, ticagrelor was associated with a significantly higher risk of total BARC bleedings (HR 3.01, 95% CI 1.81–5.62, P=0.010), BARC 1 or 2 bleedings (HR 3.14, 95% CI 1.52–5.76, P=0.018) and BARC 3 or 5 bleedings (HR 2.87, 95% CI 1.12–7.03, P=0.045) when compared to clopidogrel (Table 3). Kaplan–Meier curve showed a significantly higher risk of total BARC bleeding in the ticagrelor group than in the clopidogrel group (Log-rank test: P=0.012, Figure 2(b)). Subgroup analysis also did not identify any significant subgroup heterogeneity (P value for interaction >0.05 for all, Figure 3(b)).
Table 3.
The association between use of different antiplatelet agents and bleeding outcomes using an extended cox model.
| Outcome | Events (n) | Nonadjusted model | Model I | Model 2 | ||||
|---|---|---|---|---|---|---|---|---|
| Ticagrelor (n = 108) | Clopidogrel (n = 168) | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Total BARC bleedings | 39 (36.1) | 37 (22.0) | 1.88 (1.12∼3.15) | 0.026 | 1.96 (1.17∼3.28) | 0.021 | 3.01 (1.81∼5.62) | 0.010 |
| BARC 1 or 2 bleedings | 25 (23.1) | 24 (14.3) | 1.83 (0.97∼3.46) | 0.064 | 2.06 (1.08∼3.92) | 0.038 | 3.14 (1.52∼5.76) | 0.018 |
| BARC 3 or 5 bleedings | 14 (13.0) | 13 (7.7) | 1.98 (0.83∼4.77) | 0.116 | 1.9 (0.79∼4.56) | 0.101 | 2.87 (1.12∼7.03) | 0.045 |
Notes. data presented are HRs and 95% CIs. Adjust I model adjusts for age and gender; adjust II model adjust for variables that, when added to this model, changed the matched odds ratio by at least 10 percent, including adjusting for adjust I + smoking status, diabetes, diagnosis, congestive heart failure, COPD, stroke history, heart rate, hemoglobin, ejection fraction, hs-cTn T, Nt-proBNP, eGFR, dialysis, GP IIb/IIIa inhibitors, 3-vessel disease.
4. Discussion
Patients with CKD have a higher incidence of ACS, as well as a higher risk of thrombosis and bleeding, with worse outcomes than the general population [24]. The clear recommendation regarding the selection of P2Y12 inhibitors in CKD patients including those with severe CKD (eGFR < 30 mL/min) is unavailable [5, 25]. We conducted this study to compare the 1-year clinical outcomes between the clopidogrel and the ticagrelor groups in patients with severe renal insufficiency undergoing PCI. The main findings of our study were that ticagrelor did not improve the primary endpoint (the composite of cardiovascular death, recurrence of MI, or nonfatal ischemic stroke) and all-cause mortality when compared to clopidogrel, but did increase bleeding events in these patients.
4.1. Effect of Ticagrelor and Clopidogrel on Cardiovascular Outcomes in ACS Patients with Severe Renal Insufficiency
Numerous studies, including the well-known PLATO randomized controlled clinical trial, have demonstrated that ticagrelor reduces the risk of vascular death, all-cause death, myocardial infarction, stent thrombosis, or stroke in patients with ACS receiving or not receiving PCI [13, 15, 26]. These findings are supported by some basic research. Patients with ACS and CKD have a high thrombotic risk related to 3 main factors: alteration of the coagulation cascade, endothelial injury, and platelet alteration [4]. Patients with CKD have greater ADP-induced platelet aggregation, and the degree of reduction in clopidogrel response increases with renal insufficiency [7]. From a biological point of view, ticagrelor has a more rapid onset of action and induces a more potent and reproducible platelet inhibition than clopidogrel [12, 27].
There may be several reasons why our findings differed from these studies. First, our study population is different from the current studies in that we enrolled patients with an estimated glomerular filtration rate of less than 30 ml/min·1.73 m2, whereas most other studies enrolled patients with a creatinine clearance of less than 60 ml/min [4, 16, 17]. Furthermore, in some other trials, the number of stage 4 CKD and end-stage kidney failure was limited. For example, in the PLATO trial, the proportion of CKD patients was 21.3%, whereas only 214 patients had a calculated clearance of less than 30 mL/min and dialysis was excluded [13]. While in PLATO more than one-third of patients were treated conservatively, all patients in our study were treated with PCI. Second, it is possible that Asians have a lower rate of thrombosis and fewer ischemic events than Western patients [28]. Several clinical studies from Asia have shown that ticagrelor is not superior to clopidogrel in patients with ACS with normal renal function and may increase the risk of bleeding [18, 19, 29, 30]. In a separate endpoint analysis, our data suggested that cardiovascular death (4% absolute risk difference) and myocardial infarction (4.4% absolute risk difference) were lower in the ticagrelor group than in clopidogrel, though this difference was not statistically significant. This could imply that ticagrelor may still reduce cardiovascular adverse events in ACS patients with severe renal insufficiency. However, we were unable to reach this conclusion at this time. This was because ticagrelor patients may be in the better physical condition and so had a better prognosis. More prospective randomized controlled trials are needed to validate this conclusion. In our study, the 1-year cardiovascular events rate was high for both ticagrelor and clopidogrel. In addition, patients suffering from severe renal insufficiency have a poor prognosis and are more likely to die from infection, heart failure, respiratory failure, or other complications [31]. This suggests that antiplatelet therapy may only be part of the treatment for ACS patients with severe renal insufficiency.
4.2. Effect of Ticagrelor and Clopidogrel on Bleeding Outcomes in ACS Patients with Severe Renal Insufficiency
Our study showed a high risk of bleeding in patients with severe renal insufficiency and that ticagrelor increased the risk of bleeding in patients compared to clopidogrel. This was consistent with the findings of other Asian studies. In Korean acute coronary syndrome patients intended to receive early invasive management, standard-dose ticagrelor as compared with clopidogrel was associated with a higher incidence of clinically significant bleeding [19]. Among real-world Chinese patients with ACS treated by PCI, ticagrelor only showed superior efficacy in patients with low bleeding risk but lost its advantage in patients with moderate-to-high bleeding potential [32]. A review found that ticagrelor and clopidogrel had comparable efficacies in East Asian patients with ACS. Ticagrelor therapy also displays some side effects including an increased risk of major bleeding [29]. It was crucial to note that our study found that ticagrelor increased the risk of mild to moderate hemorrhage more than severe bleeding.
Studies have shown that low body weight, anemia, and chronic kidney disease were risk factors for major bleeding after ticagrelor therapy [33, 34]. In the real world, patients with severe renal insufficiency often have chronic anemia, so these patients are at a much higher risk of bleeding [17]. It should be underlined that significant bleeding events have an impact on total patient mortality, and this is especially true for ACS patients undergoing PCI. Some studies in recent years have shown that early de-escalation, including de-escalation from a potent P2Y12 inhibitor to clopidogrel or low-dose ticagrelor, was an effective strategy for ACS treatment, resulting in fewer bleeding events without increasing ischemic events [35–37]. In ACS patients with severe renal insufficiency, early de-escalation of antiplatelet therapy may be considered as an alternate strategy.
4.3. Limitations
This study has several limitations. First, it was a retrospective study and some confounding bias was difficult to exclude. Second, all data were collected from a single medical center. Due to the small sample size, we did not perform statistical adjustments including propensity scores matching, which may lead to less representative results. Third, there was a possibility of selection bias in our study since clinical practitioners may choose a less potent antiplatelet drug for patients with bleeding tendencies. Lastly, we categorized patients based on their DAPT regimen throughout their hospitalization and did not take into account differences in DAPT regimen duration. There was no investigation into whether patients changed antiplatelet medicines following a cardiovascular or bleeding incident.
5. Conclusion
Our study shows that the use of ticagrelor did not improve the composite of cardiovascular death, recurrence of MI, or nonfatal ischemic stroke and all-cause mortality when compared to clopidogrel, however, significantly increased bleeding events in Chinese patients with ACS and severe renal insufficiency undergoing PCI. Large-scale, long-term, randomized trials should be required to find the efficacy and safety dose of ticagrelor in East Asian patients with ACS and severe renal insufficiency.
Acknowledgments
The authors thank Dr. Liu Jie (People's Liberation Army of China General Hospital, Beijing, China) and Dr. Yang Qilin (The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China) for helping in this revision.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This study protocol was reviewed and approved by the Ethics Committee of our institution (Yue Bei People's Hospital), Approval Reference: No.2019-28. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of Interest
The authors declare that they have no conflicts of interest to disclose with respect to this manuscript.
Authors' Contributions
CYX and TLQ contributed to the conception and design of the study. CYX, TSW, CZX, XJ, CBF, and CJF participated in the clinical practice, including diagnosis, treatment, consultation, and follow-up of patients. LJR and CXY contributed to the analysis of data. CYX wrote the manuscript. TLQ revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Lamprea-Montealegre J. A., Shlipak M. G., Estrella M. M. Chronic kidney disease detection, staging and treatment in cardiovascular disease prevention. Heart . 2021;107(16):1282–1288. doi: 10.1136/heartjnl-2020-318004. [DOI] [PubMed] [Google Scholar]
- 2.Davlouros P., Xanthopoulou I., Goudevenos J., et al. Contemporary antiplatelet treatment in acute coronary syndrome patients with impaired renal function undergoing percutaneous coronary intervention. Cardiology . 2017;138(3):186–194. doi: 10.1159/000477798. [DOI] [PubMed] [Google Scholar]
- 3.Levine G. N., Bates E. R., Bittl J. A., et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Journal of the American College of Cardiology . 2016;68(10):1082–1115. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 4.Bonello L., Angiolillo D. J., Aradi D., Sibbing D. P2Y12-ADP receptor blockade in chronic kidney disease patients with acute coronary syndromes. Circulation . 2018;138(15):1582–1596. doi: 10.1161/circulationaha.118.032078. [DOI] [PubMed] [Google Scholar]
- 5.Collet J. P., Thiele H., Barbato E., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Heart Journal . 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 6.Bonello L., Tantry U. S., Marcucci R., et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. Journal of the American College of Cardiology . 2010;56(12):919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Ilardi F., Gargiulo G., Paolillo R., et al. Impact of chronic kidney disease on platelet aggregation in patients with acute coronary syndrome. Journal of Cardiovascular Medicine . 2020;21(9):660–666. doi: 10.2459/jcm.0000000000000981. [DOI] [PubMed] [Google Scholar]
- 8.Angiolillo D. J., Bernardo E., Capodanno D., et al. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. Journal of the American College of Cardiology . 2010;55(11):1139–1146. doi: 10.1016/j.jacc.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 9.Li Q., Chen Y., Liu Y., Yu L., Zheng J., Sun Y. Impact of renal function on residual platelet reactivity and clinical outcomes in patients with acute coronary syndrome treated with clopidogrel. Clinical Cardiology . 2021;44(6):789–796. doi: 10.1002/clc.23588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y., Dong W., Wan Z., et al. Ticagrelor versus clopidogrel in Chinese patients with acute coronary syndrome: a pharmacodynamic analysis. International Journal of Cardiology . 2015;201:545–546. doi: 10.1016/j.ijcard.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Franchi F., James S. K., Ghukasyan Lakic T., et al. Impact of diabetes mellitus and chronic kidney disease on cardiovascular outcomes and platelet P2Y12 receptor antagonist effects in patients with acute coronary syndromes: insights from the PLATO trial. Journal of American Heart Association . 2019;8(6) doi: 10.1161/jaha.118.011139.e011139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanderson N. C., Parker W. A. E., Storey R. F. Ticagrelor: clinical development and future potential. Reviews in Cardiovascular Medicine . 2021;22(2):373–394. doi: 10.31083/j.rcm2202044. [DOI] [PubMed] [Google Scholar]
- 13.Wallentin L., Becker R. C., Budaj A., et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. New England Journal of Medicine . 2009;361(11):1045–1057. doi: 10.1056/nejmoa0904327. [DOI] [PubMed] [Google Scholar]
- 14.James S., Budaj A., Aylward P., et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the platelet inhibition and patient outcomes (PLATO) trial. Circulation . 2010 2010-09-14;122(11):1056–1067. doi: 10.1161/circulationaha.109.933796. [DOI] [PubMed] [Google Scholar]
- 15.Bonello L., Laine M., Lemesle G., et al. Meta-analysis of potent P2Y12-ADP receptor antagonist therapy compared to clopidogrel therapy in acute coronary syndrome patients with chronic kidney disease. Thrombosis & Haemostasis . 2018 2018-10-01;118(10):1839–1846. doi: 10.1055/s-0038-1669426. [DOI] [PubMed] [Google Scholar]
- 16.De Filippo O., D’Ascenzo F., Raposeiras-Roubin S., et al. P2Y12 inhibitors in acute coronary syndrome patients with renal dysfunction: an analysis from the RENAMI and BleeMACS projects. European Heart Journal-Cardiovascular Pharmacotherapy . 2020;6(1):31–42. doi: 10.1093/ehjcvp/pvz048. [DOI] [PubMed] [Google Scholar]
- 17.Park S., Choi Y. J., Kang J. E., et al. P2Y12 antiplatelet choice for patients with chronic kidney disease and acute coronary syndrome: a systematic review and meta-analysis. Journal of Personalized Medicine . 2021;11(3):p. 222. doi: 10.3390/jpm11030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park K. H., Jeong M. H., Ahn Y., et al. Comparison of short-term clinical outcomes between ticagrelor versus clopidogrel in patients with acute myocardial infarction undergoing successful revascularization; from Korea acute myocardial infarction registry-national institute of health. International Journal of Cardiology . 2016;215:193–200. doi: 10.1016/j.ijcard.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 19.Park D. W., Kwon O., Jang J. S., et al. Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation . 2019;140(23):1865–1877. doi: 10.1161/circulationaha.119.041766. [DOI] [PubMed] [Google Scholar]
- 20.Rymer J. A., Kaltenbach L. A., Doll J. A., Messenger J. C., Peterson E. D., Wang T. Y. Safety of dual-antiplatelet therapy after myocardial infarction among patients with chronic kidney disease. Journal of American Heart Association . 2019;8(10) doi: 10.1161/jaha.119.012236.e012236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehran R., Rao S. V., Bhatt D. L., et al. Standardized bleeding definitions for cardiovascular clinical trials. Circulation . 2011;123(23):2736–2747. doi: 10.1161/circulationaha.110.009449. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y. C., Zuo L., Chen J. H., et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. Journal of the American Society of Nephrology . 2006;17(10):2937–2944. doi: 10.1681/asn.2006040368. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F., Yang J., Qian J., et al. Long-term performance of the second-generation cobalt-chromium sirolimus-eluting stents in real-world clinical practice: 3-year clinical outcomes from the prospective multicenter FOCUS registry. Journal of Thoracic Disease . 2016;8(7):1601–1610. doi: 10.21037/jtd.2016.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dusing P., Zietzer A., Goody P. R., et al. Vascular pathologies in chronic kidney disease: pathophysiological mechanisms and novel therapeutic approaches. Journal of Molecular Medicine (Berlin) . 2021;99(3):335–348. doi: 10.1007/s00109-021-02037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konishi A., Shinke T., Otake H., et al. Impact of residual platelet reactivity under clopidogrel treatment for lesions and the clinical outcome after drug-eluting stent implantation in patients with hemodialysis. Journal of Cardiology . 2016;67(6):531–537. doi: 10.1016/j.jjcc.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Wang D., Yang X. H., Zhang J. D., Li R. B., Jia M., Cui X. R. Compared efficacy of clopidogrel and ticagrelor in treating acute coronary syndrome: a meta-analysis. BMC Cardiovascular Disorders . 2018;18(1):p. 217. doi: 10.1186/s12872-018-0948-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laine M., Toesca R., Berbis J., et al. Platelet reactivity evaluated with the VASP assay following ticagrelor loading dose in acute coronary syndrome patients undergoing percutaneous coronary intervention. Thrombosis Research . 2013;132(1):e15–8. doi: 10.1016/j.thromres.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 28.Levine G. N., Jeong Y. H., Goto S., et al. Expert consensus document: world heart federation expert consensus statement on antiplatelet therapy in east Asian patients with ACS or undergoing PCI. Nature Reviews Cardiology . 2014;11(10):597–606. doi: 10.1038/nrcardio.2014.104. [DOI] [PubMed] [Google Scholar]
- 29.Wu B., Lin H., Tobe R. G., Zhang L., He B. Ticagrelor versus clopidogrel in east-Asian patients with acute coronary syndromes: a meta-analysis of randomized trials. Journal of Comparative Effectiveness Research . 2018 2018-03-01;7(3):281–291. doi: 10.2217/cer-2017-0074. [DOI] [PubMed] [Google Scholar]
- 30.Ahn K. T., Seong S. W., Choi U. L., et al. Comparison of 1-year clinical outcomes between prasugrel and ticagrelor versus clopidogrel in type 2 diabetes patients with acute myocardial infarction underwent successful percutaneous coronary intervention. Medicine (Baltimore) . 2019;98(11) doi: 10.1097/md.0000000000014833.e14833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webster A. C., Nagler E. V., Morton R. L., Masson P. Chronic kidney disease. The Lancet . 2017;389(10075):1238–1252. doi: 10.1016/s0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 32.Wang H. Y., Li Y., Xu X. M., Li J., Han Y. L. Impact of baseline bleeding risk on efficacy and safety of ticagrelor versus clopidogrel in Chinese patients with acute coronary syndrome undergoing percutaneous coronary intervention. Chinese Medical Journal . 2018;131(17):2017–2024. doi: 10.4103/0366-6999.239306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho J. Y., Lee S. Y., Yun K. H., et al. Factors related to major bleeding after ticagrelor therapy: results from the TICO trial. Journal of American Heart Association . 2021;10(7) doi: 10.1161/jaha.120.019630.e019630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samuelsen P. J., Eggen A. E., Steigen T., et al. Incidence and risk factors for major bleeding among patients undergoing percutaneous coronary intervention: findings from the Norwegian coronary stent trial (NORSTENT) PLoS One . 2021;16(3) doi: 10.1371/journal.pone.0247358.e0247358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sibbing D., Aradi D., Jacobshagen C., et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet . 2017;390(10104):1747–1757. doi: 10.1016/S0140-6736(17)32155-4. [DOI] [PubMed] [Google Scholar]
- 36.Park M. W., Kim C. J., Kim M. C., et al. A prospective, multicentre, randomised, open-label trial to compare the efficacy and safety of clopidogrel versus ticagrelor in stabilised patients with acute myocardial infarction after percutaneous coronary intervention: rationale and design of the TALOS-AMI trial. EuroIntervention . 2021;16(14):1170–1176. doi: 10.4244/eij-d-20-00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoji S., Kuno T., Fujisaki T., et al. De-escalation of dual antiplatelet therapy in patients with acute coronary syndromes. Journal of the American College of Cardiology . 2021;9 doi: 10.1016/j.jacc.2021.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.


